Open Access Article
Open Access ArticleUnexpected and divergent mechanosynthesis of furanoid-bridged fullerene dimers C120O and C120O2†
Gang
Shao‡
a,
Yuan-Yuan
Liu‡
a,
Chuang
Niu
 b,
Zheng-Chun
Yin
b,
Zheng-Chun
Yin
 b,
Shi-Qi
Ye
a,
Yang-Rong
Yao
d,
Muqing
Chen
b,
Shi-Qi
Ye
a,
Yang-Rong
Yao
d,
Muqing
Chen
 e,
Jun-Shen
Chen
a,
Xu-Ling
Xia
a,
Shangfeng
Yang
e,
Jun-Shen
Chen
a,
Xu-Ling
Xia
a,
Shangfeng
Yang
 *d and
Guan-Wu
Wang
*d and
Guan-Wu
Wang
 *abc
*abc
aHefei National Research Center for Physical Sciences at Microscale and Department of Chemistry, University of Science and Technology of China, Hefei, Anhui 230026, P. R. China. E-mail: gwang@ustc.edu.cn
bKey Laboratory of Functional Molecular Solids, Ministry of Education, Anhui Laboratory of Molecule-Based Materials, and School of Chemistry and Materials Science, Anhui Normal University, Wuhu, Anhui 241002, P. R. China
cState Key Laboratory of Applied Organic Chemistry, Lanzhou University, Lanzhou, Gansu 730000, P. R. China
dHefei National Research Center for Physical Sciences at the Microscale, CAS Key Laboratory of Materials for Energy Conversion, and Department of Materials Science and Engineering, University of Science and Technology of China, Hefei, Anhui 230026, P. R. China. E-mail: sfyang@ustc.edu.cn
eSchool of Environment and Civil Engineering, Dongguan University of Technology, Dongguan, Guangdong 523808, P. R. China
First published on 16th August 2024
Abstract
An unexpected, divergent and efficient approach toward furanoid-bridged fullerene dimers C120O and C120O2 was established under different solvent-free ball-milling conditions by simply using pristine C60 as the starting material, water as the oxygen source and FeCl3 as the mediator. The structures of C120O and C120O2 were unambiguously established by single-crystal X-ray crystallography. A plausible reaction mechanism is proposed on the basis of control experiments. Furthermore, C120O2 has been applied in organic solar cells as the third component and exhibits good performance.
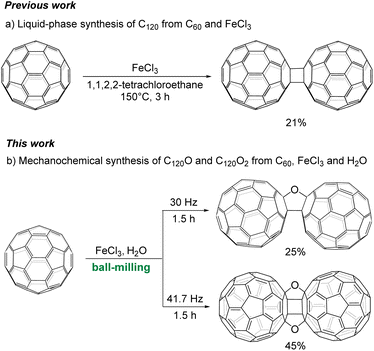
Over the past three decades, [60]fullerene (C60) and its derivatives have attracted significant attention due to their remarkable applications in numerous fields of materials science, biological applications and nanotechnology.1 Fullerene dimers, including C120, C120O and C120O2, are molecular structures linking two fullerene skeletons directly or via bridge(s) and are the fundamental subunits of fullerene polymers. Fullerene dimers exhibit many unique properties and thus have enormous potential for various applications.2
The selective formation of the dumbbell-shaped dimer C120 under solvent-free and mechanical milling conditions was first disclosed in 1997 by Wang et al.3a Since then, more solvent-free mechanochemical methods have been reported to generate fullerene dimer C120via different promoters, such as inorganic salts, organic compounds and alkali metals.3b,c Later, the FeCl3-mediated solution-phase synthesis of C120 was reported, providing a new approach for the synthesis of fullerene dimers (Scheme 1a).4 To date, there are few methods for synthesizing furanoid-bridged dimers C120O and C120O2, and their syntheses are generally limited by the use of preprepared fullerene derivatives as the starting material, high temperatures, long reaction times and inert atmospheres.5,6 C120O with one furan bridge was previously prepared by heating a mixture of C60O and 5–6 equiv. of C60 in the solid state at 200 °C for 1 h (19–20% yield)5a or in 1,2-dichlorobenzene (1,2-C6H4Cl2) at 180 °C for 3 days (26% yield).5b C120O2 with cages bis-linked by adjacent furanoid bridges was formed in ca. 15% yield by heating solid C120O to 400 °C for 1 h in an argon atmosphere.6 C60O was alternatively prepared by photooxygenation,7a oxidation with dimethyldioxirane,7bm-chloroperoxybenzoic acid,7c cytochrome P450 chemical models,7d methyltrioxorhenium–hydrogen peroxide7e or methyl (trifluoromethyl)dioxirane7f in yields ranging from 4% to 35%. Therefore, the overall yields of furanoid-bridged C120O and C120O2 starting from C60 are estimated to be only 0.8–9% and 0.1–1%, respectively. The identities of both C120O and C120O2 were deduced from their spectral data and theoretical calculations,5,6,8 but confirmation by single-crystal X-ray structures is lacking. The harsh reaction conditions and use of preprepared C60O and C120O as the starting materials in these methodologies encounter obvious limitations and prevent their practical applications. Therefore, a more straightforward and efficient approach for accessing C120O and C120O2, particularly from pristine C60, is highly desirable.
Mechanochemistry has gained increasing interest in recent years. Apart from making various chemical reactions possible under solvent-free conditions, mechanochemical reactions feature many unique advantages, such as shorter reaction times, cleaner and safer reaction conditions, lower energy consumption, higher product yields and even different product selectivities.9 C60 is barely soluble in common organic solvents, and its poor solubility somewhat restricts the exploration of its chemical reactions. Thus, mechanochemistry has emerged as an attractive alternative to conventional solution-based reactions in the field of fullerene chemistry.10
Due to the potential application of fullerene dimers and our continuous interest in fullerene mechanochemistry,10,11 herein we disclose the unexpected, divergent and straightforward mechanosynthesis of furanoid-bridged fullerene dimers C120O and C120O2 directly from easily available pristine C60 (Scheme 1b). This highly efficient mechanochemical method will provide more possibilities for practical application of these fullerene dimers and new avenues for other bridged fullerene dimers.
In attempts to mechanochemically synthesize C120 from C60 and FeCl3, it was intriguing to discover that the addition of H2O could alter the reaction product from C120 to furanoid-bridged fullerene dimers. Therefore, the mechanochemical reaction of C60 with FeCl3 and H2O was systematically investigated. The results of the reaction optimizations for the furanoid-bridged dimer C120O are shown in Table 1. Initially, C60 (0.05 mmol), 6 equiv. of FeCl3 and 15 equiv. of H2O were added into a stainless steel jar (5 mL) together with 4 stainless steel balls (5 mm in diameter) under solvent-free and ambient conditions and milled vigorously at 1800 cycles per minute (30 Hz) in a GT 300 mixer mill at room temperature for 90 min. The reaction mixture was dissolved in 1,2-C6H4Cl2 and monitored by high-performance liquid chromatography (HPLC) on a Cosmosil Buckyprep-D column with toluene as the mobile phase. It was found that the reaction mixture consisted of the furanoid-bridged dimer C120O, dumbbell-shaped dimer C120 and unreacted C60. The percentage of each component was calculated by quantitative analysis based on HPLC peak areas. The yield of C120O was thus determined to be 30%, in addition to the 33% yield of C120 (Table 1, entry 1). By increasing the amount of FeCl3 to 7 equiv., the yield of C120O was enhanced to 33% (Table 1, entry 2), yet further increasing the amount of FeCl3 to 8 equiv. resulted in a substantially decreased yield of 20% due to the formation of C120O2 (Table 1, entry 3). In addition, no benefit to the product yield could be achieved by shortening or prolonging the reaction time (Table 1, entries 4 and 5). When the reaction was attempted in the absence of H2O, only trace amounts of C120 and C120O were detected, highlighting the pivotal role of H2O in this mechanochemical reaction (Table 1, entry 6). Increasing or reducing the amount of H2O resulted in a slightly lower yield (Table 1, entries 7 and 8 vs. entry 2). Furthermore, the product yield of C120O was lowered as the milling frequency was increased or decreased (Table 1, entries 9 and 10). On the basis of the results presented above and the isolated yield of C120O for each entry, the optimized conditions to afford C120O were determined as shown in entry 2 of Table 1: C60 (0.05 mmol), FeCl3 (0.35 mmol), H2O (0.75 mmol), a milling frequency of 30 Hz and a milling time of 90 min. The HPLC chromatogram on a Cosmosil Buckyprep-D (10 × 250 mm) column with toluene as the eluent at a flow rate of 1 mL min−1 and the detector wavelength at 326 nm is shown in Fig. 1. The retention times of C60, C120, C120O and C120O2 were 10.6 min, 16.1 min, 17.3 min and 20.0 min, respectively. The isolated yield of C120O by recycling HPLC on a Cosmosil Buckyprep-D (10 × 250 mm) column was 25%, which was significantly higher than the 0.8–9% for the two-step procedure starting from C60 (vide supra).
| Entry | Ratiob | Yield of C120Oc (%) | Yield of C120c (%) | Recovered C60c (%) |
|---|---|---|---|---|
| a Unless otherwise noted, all reactions were performed with 0.05 mmol of C60, FeCl3, and H2O together with 4 stainless steel balls (5 mm in diameter) in a stainless steel jar (5 mL) and milled vigorously (30 Hz) at room temperature for 90 min. b The molar ratio refers to C60/FeCl3/H2O. c Based on HPLC area ratios on a Cosmosil Buckyprep-D (10 × 250 mm) column. d The reaction time was 60 min. e The reaction time was 120 min. f The reaction frequency was 25 Hz. g The reaction frequency was 35 Hz. | ||||
| 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
30 | 33 | 37 |
| 2 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
33 | 27 | 33 |
| 3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
20 | 11 | 28 |
| 4d | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
19 | 33 | 48 |
| 5e | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
29 | 16 | 32 |
| 6 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
Trace | Trace | 36 |
| 7 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
28 | 34 | 34 |
| 8 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
30 | 26 | 43 |
| 9f | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
20 | 30 | 50 |
| 10g | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
26 | 38 | 29 |
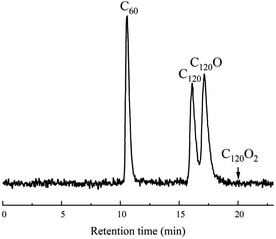 | ||
| Fig. 1 HPLC chromatogram for the synthesis of C120O from C60, H2O and FeCl3 under optimal ball-milling conditions. | ||
During the optimization process for the synthesis of the furanoid-bridged C120O, it was found that increasing the amount of FeCl3 as well as the milling frequency would favour the formation of the fullerene dimer C120O2 with bis-linked furanoid bridges. Therefore, we further modified our reaction conditions with the expectation of obtaining C120O2 dominantly or even selectively. The results from HPLC analyses are summarized in Table 2. A reaction mixture of C60 (0.05 mmol), 9 equiv. of FeCl3 and 15 equiv. of H2O was milled at 2500 cycles per minute (41.7 Hz) in a GT 600 mixer mill at room temperature for 90 min. To our delight, C120O2 was obtained in 57% yield along with a small amount of C120O (7%) and a trace amount of C120 (Table 2, entry 1). By increasing the amount of FeCl3 to 10 equiv., the yield was enhanced to 69% (Table 2, entry 2). Further increasing the amount of FeCl3 could improve the selectivity and relative yield of C120O2 (Table 2, entries 3 and 4), but led to a decrease in the HPLC intensity, indicating that the amount of C120O2 was actually decreased and would lead to a lower isolated yield. It is believed that more insoluble oligomers were generated in the presence of more FeCl3. Reducing the reaction time led to a lower yield, while increasing the reaction time had no beneficial effect (Table 2, entries 5 and 6). When this reaction was performed without H2O, only a trace amount of C120O2 was observed, indicating that H2O was crucial for promoting this reaction (Table 2, entry 7). In addition, no benefit to this reaction could be achieved by varying the amount of H2O (Table 2, entries 8 and 9). Decreasing the milling frequency to 35 Hz resulted in more C120O and more recovered C60, and increasing the milling frequency to 45 Hz did not improve the yield of C120O2 (Table 2, entries 10 and 11). In efforts to achieve more efficient synthesis of the peculiar C120O2, various Lewis acids, including AlCl3, FeCl2 and NiCl2, and oxidizing agents, such as Oxone, m-chloroperbenzoic acid (m-CPBA) and H2O2, were explored. However, all of these additives were detrimental to the formation of C120O2 and afforded C120 exclusively (Table 2, entries 12–17). To compare the present solvent-free reaction with its liquid-phase counterpart, the reaction of C60 (0.05 mmol) with FeCl3 (0.50 mmol) and H2O (0.75 mmol) was performed in 0.5 mL of 1,1,2,2-tetrachloroethane at 150 °C for 48 h. Neither the desired product C120O2 nor C120O could be isolated, and C120 was instead formed in 15% yield (Table 2, entry 18). Other solvents, including toluene, chlorobenzene or 1,2-C6H4Cl2, were also examined. However, the generation of C120O2 could not be observed in any of these solvents. Thus, it is obvious that the present mechanochemical solvent-free protocol shows advantages and uniqueness compared to the corresponding liquid-phase reaction. On the basis of the results presented above, the optimized conditions to afford the bisfuranoid-bridged dimer C120O2 were determined as shown in entry 2 of Table 2: C60 (0.05 mmol), FeCl3 (0.50 mmol), H2O (0.75 mmol), a milling frequency of 41.7 Hz and a milling time of 90 min. The HPLC chromatogram on a Cosmosil Buckyprep (4.6 × 250 mm) column with toluene as the eluent at a flow rate of 1 mL min−1 and the detector wavelength at 326 nm is shown in Fig. 2. The retention times of C60, C120O and C120O2 were 7.5 min, 18.1 min and 20.7 min, respectively. The isolated yield of C120O2 by HPLC on a Cosmosil Buckyprep-D (10 × 250 mm) column was 45%, which was dramatically higher than the overall yield of 0.1–1% for the three-step process starting from C60 (vide supra).
| Entry | Additive | Ratiob | Yield of C120O2c (%) | Yield of C120Oc (%) | Yield of C120c (%) | Recovered C60c (%) |
|---|---|---|---|---|---|---|
| a Unless otherwise noted, all reactions were performed with 0.05 mmol of C60, FeCl3, and H2O together with 4 stainless steel balls (5 mm in diameter) in a stainless steel jar (5 mL) and milled vigorously (41.7 Hz) at room temperature for 90 min. b The molar ratio refers to C60/FeCl3/H2O. c Based on HPLC area ratios on a Cosmosil Buckyprep (4.6 × 250 mm) and/or Buckyprep-D (10 × 250 mm) column. d The reaction time was 60 min. e The reaction time was 120 min. f The reaction frequency was 35 Hz. g The reaction frequency was 45 Hz. h The reaction was performed in 1,1,2,2-tetrachloroethane (0.5 mL) at 150 °C for 48 h. i Under a N2 atmosphere. j C120O was used instead of C60. | ||||||
| 1 | FeCl3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
57 | 7 | Trace | 36 |
| 2 | FeCl 3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
69 | 9 | Trace | 22 |
| 3 | FeCl3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
71 | Trace | Trace | 26 |
| 4 | FeCl3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
80 | 0 | 0 | 20 |
| 5d | FeCl3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
52 | 10 | Trace | 32 |
| 6e | FeCl3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
67 | 7 | Trace | 26 |
| 7 | FeCl3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
Trace | Trace | Trace | 50 |
| 8 | FeCl3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
57 | 7 | Trace | 36 |
| 9 | FeCl3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
63 | 8 | Trace | 29 |
| 10f | FeCl3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
50 | 13 | Trace | 36 |
| 11g | FeCl3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
60 | 0 | 0 | 40 |
| 12 | AlCl3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
0 | Trace | 50 | 50 |
| 13 | FeCl2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
0 | Trace | 54 | 45 |
| 14 | NiCl2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
0 | Trace | 44 | 55 |
| 15 | Oxone | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
Trace | Trace | 38 | 42 |
| 16 | m-CPBA | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
Trace | Trace | 41 | 45 |
| 17 | H2O2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
Trace | Trace | 39 | 56 |
| 18h | FeCl3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
0 | Trace | 15 | 83 |
| 19i | FeCl3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
54 | 17 | Trace | 27 |
| 20j | FeCl3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
15 | 49 | 16 | 20 |
 | ||
| Fig. 2 HPLC chromatogram for the synthesis of C120O2 using FeCl3 under optimal ball-milling conditions. | ||
The molecular structures of C120O and C120O2 were unambiguously established by single-crystal X-ray crystallography and are shown in Fig. 3. The single-crystal structure of C120O reveals that it has a furanoid ring linking two fullerene cages via [6,6]-ring junctions, clarifying the uncertainty of the oxygen connectivity to fullerenes.12 The single-crystal structure of C120O2 shows that the two fullerene cages are connected by adjacent furanoid rings via [6,6]-ring junctions as in the case of C120O, resulting in a central four-membered-ring bridge via [5,6]-ring junctions. The obtained single-crystal structure of our C120O2 confirms that it has C2v symmetry, is the most stable and plausible structure predicted by theoretical calculations8 and is neither the minor byproduct C120O2 with C1 symmetry accompanying C120O from the solid–state reaction of C60 and C60O at 200 °C13 nor the C120O2 isomer with C2 symmetry from the dimerization of [5,6]-C60O.14 C120O and C120O2 were stable, and their thermogravimetric analyses (TGA) showed obvious weight loss above 370 °C (Fig. S5 and S6†).
To explore whether O2 in the air atmosphere played a role during the milling process, the milling jar containing the reaction mixture was filled with nitrogen (N2) in a glovebox and milled under the optimal conditions, which did not inhibit the production of C120O2 (Table 2, entry 19). When C120O was used to replace C60 under the optimal conditions, the yield of C120O2 decreased dramatically, and a considerable amount of C60 was produced (Table 2, entry 20). This result suggested that C120O2 was more likely to be generated directly from C60 rather than from C120O as a precursor. Therefore, C120O and C120O2 should be formed independently from C60via different reaction pathways.
The mechanochemical 17O/18O labelling using H217O/H218O has been reported.15 To further identify the source of the bridging oxygen atom in C120O2, H218O was introduced to the reaction system, and the reaction process was monitored by HRMS. When H218O (10 atom % 18O) was used, both C12016O2 and C12016O18O were observed (Scheme 2a and Fig. S7†). Next, the addition of H218O (95 atom % 18O) under the optimal reaction conditions provided C12016O18O and C12018O2 (Scheme 2b and Fig. S8†). These results reinforced the conclusion that the bridging oxygen atom in C120O2 originated from H2O. Furthermore, when potassium ferricyanide was added to the reaction mixture, a dark blue precipitate was generated (Fig. S9†), indicating the presence of ferrous ions. More importantly, the X-ray photoelectron spectroscopy (XPS) measurement of the reaction mixture revealed that an Fe(II) species was generated after the reaction was completed (Fig. S10†).
On the basis of the above experimental results and the literature,16 a plausible reaction mechanism is outlined in Scheme 3. First, the initial coordination of C60 with FeCl3 provides the complex FeCl2(η2-C60) (A),16 which is followed by the nucleophilic addition of H2O to afford intermediate B in a 1,4-addition pattern. Loss of H+ from intermediate B gives intermediate C. The hydroxy group in C directly attacks another molecule of pristine C60 to form an oxonium-bridged zwitterionic dimer D. Then, intramolecular cyclization with the removal of FeCl2 and H+ generates the furanoid-bridged dimer C120O. With an increased amount of FeCl3 and a higher milling frequency, the amount of the generated intermediate C can surpass that of the pristine C60, and self-dimerization of C would dominate to give doubly oxonium-bridged intermediate E. Finally, E undergoes dual intramolecular cyclization with elimination of FeCl2 and H+ to provide the bisfuranoid-bridged dimer C120O2.
Given that fullerene derivatives have been applied in organic solar cells (OSCs) as the third component,17 preliminary results showed that C120O2 could be employed in OSCs with the configuration of ITO/PEDOT:PSS/D18-Cl:N3:fullerene (1:1.4:0.12)/PDIN/Ag (Fig. 4). The device with C120O2 as the third component showed a PCE of 17.94% with a JSC of 28.05 mA cm−2, a VOC of 0.86 V and an FF of 74.08%. The control device without a fullerene additive showed a lower PCE of 17.27% with a JSC of 26.41 mA cm−2, a VOC of 0.87 V and an FF of 75.03%. The device with pristine C60 as the third component showed an inferior PCE of 15.77% with a JSC of 26.68 mA cm−2, a VOC of 0.85 V and an FF of 69.24%. These results revealed that pristine C60 had an adverse effect, while the bisfuranoid-bridged dimer C120O2 was a promising third-component material in the active layer of the OSCs. Unfortunately, the synthesized C120O has limited solubility and could not be used as a third component in the current OSCs.
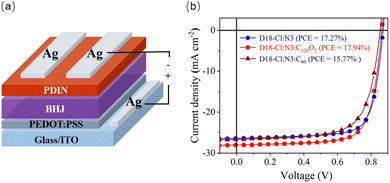 | ||
| Fig. 4 (a) Schematic illustration of the OSC structure used in this work. (b) J–V curves of D18-Cl:N3 (blue line), D18-Cl:N3:C120O2 (red line) and D18-Cl:N3:C60 (brown line)-based OSCs. | ||
Conclusions
In summary, we have disclosed the unexpected, divergent and highly efficient synthesis of furanoid-linked C120O and C120O2 from the FeCl3-mediated mechanochemical reaction of C60 with H2O as the bridging oxygen source at room temperature under an air atmosphere. The present protocol avoids the usage of preprepared C60O and C120O. The selective synthesis of C120O with one furanoid bridge and C120O2 with two furanoid bridges directly from pristine C60 can be achieved by altering the amount of FeCl3 and the milling frequency. The excellent yields, simplicity of this synthetic process and solvent-free and ambient conditions compared to those of previous multistep processes make this mechanochemical protocol an efficient method for the divergent synthesis of C120O and C120O2. The specific structures of C120O and C120O2 have been unequivocally established by single-crystal X-ray crystallography. A plausible reaction mechanism has been proposed based on the control experiments. The bisfuranoid-bridged dimer C120O2 has also been utilized as the third component in organic solar cells and has shown improved performance in OSC devices.Data availability
The data supporting the findings of this study are available within the article and its ESI.†Author contributions
G.-W. W. and S. Y. supervised the project. G. S. and J.-S. C. performed experiments and data analysis and wrote original manuscript, Y.-Y. L. fabricated the organic solar cell devices and analyzed data. G. S., C. N., Z.-C. Y., Y.-R. Y. and M. C. characterized the X-ray structures. S.-Q. Y. performed XPS test and data analysis. X.-L. X. assisted in data analysis and original manuscript preparation. G.-W. W. acquired funding and wrote the manuscript. All the authors contributed with comments and revisions to the manuscript and gave approval to the final version.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for financial support from the National Natural Science Foundation of China (22071231, 21372211).Notes and references
- For reviews, see: (a) A. L. Balch and K. Winkler, Chem. Rev., 2016, 116, 3812–3882 CrossRef CAS PubMed; (b) M. Chen, R. Guan and S. Yang, Adv. Sci., 2019, 6, 1800941 CrossRef PubMed; (c) K. Harano and E. Nakamura, Acc. Chem. Res., 2019, 52, 2090–2100 CrossRef CAS PubMed; (d) T. Umeyama and H. Imahori, Acc. Chem. Res., 2019, 52, 2046–2055 CrossRef CAS PubMed; (e) L. Jia, M. Chen and S. Yang, Mater. Chem. Front., 2020, 4, 2256–2282 RSC; (f) Z. Xing, S.-H. Li and S. Yang, Small Struct., 2022, 3, 2200012 CrossRef CAS.
- J. L. Segura and N. Martín, Chem. Soc. Rev., 2000, 29, 13–25 RSC.
- (a) G.-W. Wang, K. Komatsu, Y. Murata and M. Shiro, Nature, 1997, 387, 583–586 CrossRef CAS; (b) K. Komatsu, G.-W. Wang, Y. Murata, T. Tanaka, K. Fujiwara, K. Yamamoto and M. Saunders, J. Org. Chem., 1998, 63, 9358–9366 CrossRef CAS; (c) K. Komatsu, K. Fujiwara, T. Tanaka and Y. Murata, Carbon, 2000, 38, 1529–1534 CrossRef CAS.
- M. Hashiguchi, H. Inada and Y. Matsuo, Carbon, 2013, 61, 418–422 CrossRef CAS.
- (a) S. Lebedkin, S. Ballenweg, J. Gross, R. Taylor and W. Krätschmer, Tetrahedron Lett., 1995, 36, 4971–4974 CrossRef CAS; (b) A. B. Smith III, H. Tokuyama, R. M. Strongin, G. T. Furst, W. J. Romanow, B. T. Chait, U. A. Mirza and I. Haller, J. Am. Chem. Soc., 1995, 117, 9359–9360 CrossRef.
- A. Gromov, S. Lebedkin, S. Ballenweg, A. G. Avent, R. Taylor and W. Krätschmer, Chem. Commun., 1997, 209–210 RSC.
- (a) K. M. Creegan, J. L. Robbins, W. K. Robbins, J. M. Millar, R. D. Sherwood, P. J. Tindall, D. M. Cox, A. B. Smith, III, J. P. McCauley, Jr, D. R. Jones and R. T. Gallagher, J. Am. Chem. Soc., 1992, 114, 1103–1105 CrossRef CAS; (b) Y. Elemes, S. K. Silverman, C. Sheu, M. Kao, C. S. Foote, M. M. Alvarez and R. L. Whetten, Angew. Chem., Int. Ed. Engl., 1992, 31, 351–353 CrossRef; (c) A. L. Balch, D. A. Costa, B. C. Noll and M. M. Olmstead, J. Am. Chem. Soc., 1995, 117, 8926–8932 CrossRef CAS; (d) T. Hamano, T. Mashino and M. Hirobe, J. Chem. Soc., Chem. Commun., 1995, 1537–1538 RSC; (e) R. W. Murray and K. Iyanar, Tetrahedron Lett., 1997, 38, 335–338 CrossRef CAS; (f) C. Fusco, R. Seraglia, R. Curci and V. Lucchini, J. Org. Chem., 1999, 64, 8363–8368 CrossRef CAS PubMed.
- P. W. Fowler, D. Mitchell, R. Taylor and G. Seifert, J. Chem. Soc., Perkin Trans. 2, 1997, 1901–1905 RSC.
- For reviews, see: (a) G.-W. Wang, Chem. Soc. Rev., 2013, 42, 7668–7700 RSC; (b) J. G. Hernández and C. Bolm, J. Org. Chem., 2017, 82, 4007–4019 CrossRef PubMed; (c) M. Leonardi, M. Villacampa and J. C. Menéndez, Chem. Sci., 2018, 9, 2042–2064 RSC; (d) J. Andersen and J. Mack, Green Chem., 2018, 20, 1435–1443 RSC; (e) T. Friščić, C. Mottillo and H. M. Titi, Angew. Chem., Int. Ed., 2020, 59, 1018–1029 CrossRef PubMed; (f) K. J. Ardila-Fierro and J. G. Hernández, Angew. Chem., Int. Ed., 2024, 63, e202317638 CrossRef CAS PubMed.
- For reviews, see: (a) S. -E Zhu, F. Li and G.-W. Wang, Chem. Soc. Rev., 2013, 42, 7535–7570 RSC; (b) G.-W. Wang, Chin. J. Chem., 2021, 39, 1797–1803 CrossRef CAS.
- For recent examples, see: (a) H.-W. Liu, H. Xu, G. Shao and G.-W. Wang, Org. Lett., 2019, 21, 2625–2628 CrossRef CAS PubMed; (b) G. Shao, C. Niu, H.-W. Liu, H. Yang, J.-S. Chen, Y.-R. Yao, S. Yang and G.-W. Wang, Org. Lett., 2023, 25, 1229–1234 CrossRef CAS PubMed.
- M. M. Olmstead, D. A. Costa, K. Maitra, B. C. Noll, S. L. Phillips, P. M. Van Calcar and A. L. Balch, J. Am. Chem. Soc., 1999, 121, 7090–7097 CrossRef CAS.
- A. Gromov, S. Lebedkin, W. E. Hull and W. Krätschmer, J. Phys. Chem. A, 1998, 102, 4997–5005 CrossRef CAS.
- D. Tsyboulski, D. Heymann, S. M. Bachilo, L. B. Alemany and R. B. Weisman, J. Am. Chem. Soc., 2004, 126, 7350–7358 CrossRef CAS PubMed.
- J. Špačková, C. Fabra, G. Cazals, M. Hubert-Roux, I. Schmitz-Afonso, I. Goldberga, D. Berthomieu, A. Lebrun, T.-X. Métro and D. Laurencin, Chem. Commun., 2021, 51, 6812–6815 RSC.
- (a) N. Malic, P. J. Nichols, M. Makha and C. L. Raston, Cryst. Growth Des., 2009, 9, 863–866 CrossRef CAS; (b) D. V. Konarev, S. S. Khasanov, M. A. Faraonov and R. N. Lyubovskaya, CrystEngComm, 2012, 14, 4350–4356 RSC; (c) D. A. Garcia-hernandez, F. Cataldo and A. Manchado, Fullerenes, Nanotub. Carbon Nanostruct., 2016, 24, 225–233 CrossRef CAS; (d) M. A. Faraonov, A. V. Kuzmin, S. S. Khasanov, A. F. Shestakov, A. Otsuka, H. Yamochi, H. Kitagawa and D. V. Konarev, Inorg. Chem., 2022, 61, 20144–20149 CrossRef CAS PubMed.
- For examples, see: (a) R. Yu, H. Yao, Y. Cui, L. Hong, C. He and J. Hou, Adv. Mater., 2019, 31, 1902302 CrossRef PubMed; (b) M.-A. Pan, T.-K. Lau, Y. Tang, Y.-C. Wu, T. Liu, K. Li, M.-C. Chen, X. Lu, W. Ma and C. Zhan, J. Mater. Chem. A, 2019, 7, 20713–20722 RSC; (c) B. Qiu, S. Chen, C. Sun, J. Yuan, X. Zhang, C. Zhu, S. Qin, L. Meng, Y. Zhang, C. Yang, Y. Zou and Y. Li, Sol. RRL, 2020, 4, 1900540 CrossRef CAS; (d) D. Hu, H. Tang, J. Lv, Z. Liao, Q. Chen, H. Chen and S. Lu, Sustain. Energy Fuels, 2021, 5, 3593–3597 RSC; (e) Z. Liu and H.-E. Wang, Sol. Energy, 2021, 230, 549–557 CrossRef CAS; (f) Z.-C. Yin, M. Li, C. Niu, W.-F. Wang, W.-R. Liu, Q.-W. Zhang and G.-W. Wang, Angew. Chem., Int. Ed., 2023, 62, e202304321 CrossRef CAS PubMed; (g) T.-X. Liu, X. Wang, S. Xia, M. Chen, M. Li, P. Yang, N. Ma, Z. Hu, S. Yang, G. Zhang and G.-W. Wang, Angew. Chem., Int. Ed., 2023, 62, e202313074 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures and characterization data, NMR spectra of C120O and C120O2, X-ray crystallographic data for C120O and C120O2. CCDC 2289163, 2289161. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc04167d |
| ‡ Authors with equal contribution. |
| This journal is © The Royal Society of Chemistry 2024 |