Open Access Article
Open Access ArticlePreparation of intrinsically fragile bent crystals†
Tomohiro
Seki
 *a,
Shiori
Kobayashi
a,
Rintaro
Ishikawa
a,
Keigo
Yano
b,
Takumi
Matsuo
*a,
Shiori
Kobayashi
a,
Rintaro
Ishikawa
a,
Keigo
Yano
b,
Takumi
Matsuo
 bc and
Shotaro
Hayashi
bc and
Shotaro
Hayashi
 bc
bc
aDepartment of Chemistry, Faculty of Science, Shizuoka University, Shizuoka City, Shizuoka 422-8017, Japan. E-mail: seki.tomohiro@shizuoka.ac.jp
bSchool of Engineering Science, Kochi University of Technology, 185 Miyanokuchi, Tosayamada, Kami, Kochi 782-8502, Japan
cResearch Institute, Kochi University of Technology, 185 Miyanokuchi, Tosayamada, Kami, Kochi 782-8502, Japan
First published on 11th July 2024
Abstract
Although molecular crystals have long been considered to be intrinsically brittle, a study of molecular crystals that are capable of plastic or elastic bending upon applying mechanical stress recently attracted significant attention. Malleable molecular crystals often need to meet specific criteria regarding the intermolecular interaction patterns within the crystal structure. Accordingly, examples have been reported where one polymorph shows bending, while other polymorphs of the same compound exhibit fracturing upon exposure to mechanical force. Here, we have succeeded in preparing bent crystals of an intrinsically fragile polymorph. Methylated flufenamic acid (1) can form three different polymorphs, i.e., 1α, 1β, and 1γ, of which 1β is difficult to isolate. Under mechanical force, the crystals of 1α exhibit remarkable plastic deformation, while those of 1γ are readily broken. Similar to the mechanical properties, the emission properties of 1 differ depending on the polymorph, i.e., 1γ exhibits a shorter-wavelength emission maximum and much higher emission quantum yield than 1α. Remarkably, both the unbent and bent forms of the 1α crystals can undergo a phase transition to the 1γ phase upon exposure to ethyl acetate. In this manner, phase transitions of the mechanically bent crystals of polymorph 1α afforded bent crystals of the intrinsically fragile polymorph 1γ. These findings may lead to a potential post-modification method for the preparation of functional flexible materials with enhanced emission properties in order to expand their applications.
Introduction
Molecular crystals have long been considered to be intrinsically brittle. Although they often show unique optoelectronic properties that stem from their anisotropy and dense packing, their brittle nature has hampered their applications in the development of advanced materials.1 However, the past few decades have witnessed numerous reports of molecular crystals that are capable of plastic or elastic bending upon applying mechanical stress. In particular, functional bendable crystals have provided new applications in flexible optoelectronic materials,2 such as optical waveguides,3 conductivity,4 ferro/piezoelectricity,5 and hybridization with a polymer.6Several studies have found that intermolecular interaction patterns within molecular crystals are important for their bending behavior.7 For example, bendable molecular crystals often adopt molecular arrangements that exhibit anisotropy in terms of the strength of the intermolecular interactions. Typically, molecules in plastically bendable crystals exhibit stacking arrangements to form tightly packed column motifs. The columns exhibit strong intermolecular interactions with neighboring columns along one of the two directions orthogonal to the column axis, and weak interactions along the other orthogonal direction.7a,b,e When the intermolecular interactions manifest along the two orthogonal directions with respect to the column axis are energetically comparable, elastic bending behavior is often observed.7c,f,g These results indicate that control over the molecular arrangement is required to achieve bending in molecular crystals.
Polymorphism-dependent crystal-bending behavior has also been reported. Generally, different polymorphs exhibit different properties, such as emission or semiconducting properties, despite the constituent molecule being the same.8 Similarly, the mechanical properties of molecular crystals can differ among their polymorphs.9 Desiraju et al. reported that three polymorphs of 4-bromophenyl-4-bromobenzoate show plastic deformation, elastic deformation, and fracture under mechanical stress.9a In addition, the mechanical properties of crystals can be controlled through phase transitions between polymorphs. For example, Zhang reported a thermal phase transition from polymorph A to polymorph B of 9,10-bis(phenylethynyl)anthracene; these polymorphs exhibit plastic bending and fracture, respectively, when subjected to mechanical force under ambient conditions.9c However, reports of phase transitions in bent crystals remain limited so far.10 Such phase transitions should enable “post-modification” of the properties or the shape of mechanically processed bent crystals.
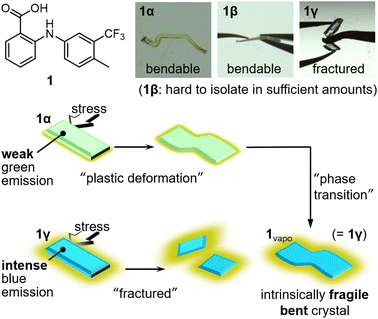
Here, we report methylated flufenamic acid 1,11 which can be used to prepare an intrinsically fragile bent crystal based on its polymorph-dependent mechanical and phase-transition properties (Fig. 1). 1 forms three different polymorphs, i.e., 1α, 1β, and 1γ, although 1β is difficult to isolate in synthetically meaningful amounts. 1α is plastically deformed under mechanical stress, while 1γ is fractured. Crystal-structure analyses confirmed that the packing arrangement of 1α exhibits characteristics typical of plastically bendable molecular crystals. In contrast, 1γ does not exhibit this type of crystal structure. Vapor treatment resulted in an interpolymorph phase transition from 1α to 1γ. Remarkably, the bent crystals of 1α also undergo phase transitions by vapor treatment to give bent crystals of the intrinsically brittle polymorph 1γ.
 | ||
| Fig. 1 Chemical structure of 1. Photographs of 1α, 1β, and 1γ. Schematic representation of polymorphism and mechanical properties of the crystals of 1. | ||
Results and discussion
Compound 111e forms three different polymorphs, i.e., 1α, 1β, and 1γ, under different crystallization conditions. Weakly green-emitting polymorph 1α was obtained by slow evaporation of 1 in methanol for three days. Using the same solution, evaporation over seven days afforded polymorph 1β. However, the concomitant formation of 1α and 1β made the isolation of 1β in synthetically meaningful amounts difficult, which limited our ability to perform several of the experiments for 1β. 1γ was obtained from an ethanol/H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution of 1 and NH4Cl, as reported by Byrn for the crystallization of form V of flufenamic acid.11a A mixture of 1 and NH4Cl in ethanol/H2O was filtered and left to stand for three days under ambient conditions to give crystals of 1γ.
1) solution of 1 and NH4Cl, as reported by Byrn for the crystallization of form V of flufenamic acid.11a A mixture of 1 and NH4Cl in ethanol/H2O was filtered and left to stand for three days under ambient conditions to give crystals of 1γ.
Upon applying mechanical stress, the crystals of 1α and 1β bend plastically, while the crystal of 1γ breaks. Applying mechanical stress to the (001) plane of 1α resulted in a dramatic change in its shape (Fig. 2a and S1 as well as ESI Movie S1†). After removing the stress, 1α retains its deformed shape, confirming the occurrence of plastic deformation. When mechanical stress was applied to the other faces of the crystal of 1α, the crystals fractured (Fig. S2†). The crystals of 1β also displayed plastic deformation upon applying mechanical stress to the (00−1) face (main face, Fig. 2c), but broke when the other crystal faces (minor faces) were pushed (Fig. S3†). Repeated tests revealed that the bending behavior of 1α is much more dramatic than that of 1β (Fig. 2b and d, respectively). Unlike the crystals of 1α and 1β, those of 1γ were found to be mechanically brittle. Irrespective of the face to which the stress was applied, the crystals of 1γ fractured under mechanical stress (Fig. 2e).12 These differences in the mechanical properties of 1 should originate from their different crystal structures.
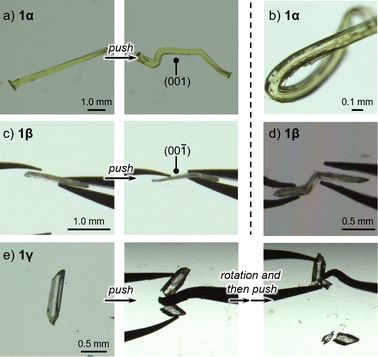 | ||
| Fig. 2 Photographs of pieces of crystals of (a) 1α, (c) 1β, and (e) 1γ before and after applying mechanical stress. Photographs of the most extremely bent crystals of (b) 1α and (d) 1β. | ||
A single-crystal X-ray diffraction (XRD) analysis of 1α indicated the presence of slide planes responsible for plastic bending. 1α crystalized in the Pna21 space group (Fig. 3 and S5 as well as Table S1†). The dihedral angles of the two benzene rings of each molecule (θd) were determined to be 136.77° and 138.82° (Z′ = 2). As typically observed for molecular crystals that contain benzoic-acid groups,13 double hydrogen bonding interactions were observed (O⋯O distance: 2.640 Å; Fig. 3a). The resulting flat planes formed by two benzoic-acid moieties within the dimer stack with the neighboring flat planes of other dimers with an offset result in the formation of a column structure (Fig. 3b, c and S5†). These columns interact tightly with each other along the a axis through CH–π interactions (3.845 or 3.869 Å for the carbon–π plane distance; dashed lines in Fig. 3d) to form sheet-like architectures along the (001) plane. In contrast, no clear “strong” intermolecular interactions are present between the columns along the c direction, where the CF3 and Me groups are oriented (highlighted in green, Fig. 3d). These moieties should act as slide planes along which the molecules within the sheet motif move upon the plastic bending of the crystal.14 Such molecular movements along slide planes have been reported for plastically bending crystals.7a,b,e An energy-framework analysis based on the crystal structure of 1α corroborated a pronounced anisotropy of the strength of the intermolecular interactions (Fig. S6†).15
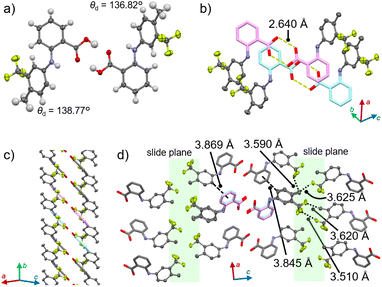 | ||
| Fig. 3 Crystal structure of 1α showing (a) the dimer, (b) the stacked dimers, (c) the column, and (d) the interactions among the columns. | ||
1γ also forms a column motif through the stacking of doubly hydrogen-bonded dimers, but unlike 1α, it does not feature slide planes. Polymorph 1γ crystallizes in the P21/n space group (Fig. 4 and S8 as well as Table S1†). Similar to 1α, the conformation of 1 is twisted (θd = 45.91°; Fig. 4a). The difference between the θd of 1α and 1γ is ∼90°, indicating that the orientation of the CF3 groups is the main difference between the monomer conformations in the two polymorphs (Fig. S9†). The hydrogen-bonded dimers in 1γ (O⋯O distance: 2.649 Å; Fig. 4a) stack to form a column along the a axis through the parallel orientation of the planes formed by the benzoic-acid domains (Fig. 4b, c and S8†). These columns interact along both the b and c directions via weak CH⋯F interactions (dotted lines in Fig. 4d). This is different from the intercolumnar interactions in 1α, in which the columns form sheets through relatively strong CH–π interactions. The energy-framework analysis of 1γ also showed a less pronounced anisotropy of the strength of the intermolecular interactions along the b and c axes (Fig. S10†) compared to that of 1α. Thus, 1γ does not have slide planes, which are often observed in the crystal structures of plastically bendable molecular crystals such as 1α.7a,b,e This is in agreement with the absence of plastic deformation of 1γ upon applying mechanical stress (Fig. 2e).
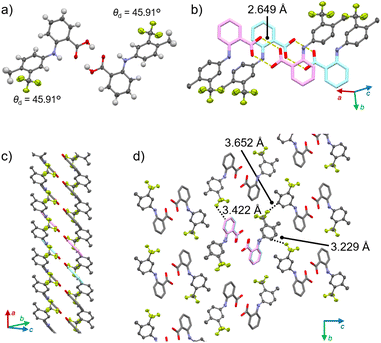 | ||
| Fig. 4 Crystal structure of 1γ showing (a) the dimer, (b) the stacked dimers, (c) the column, and (d) the interactions among the columns. | ||
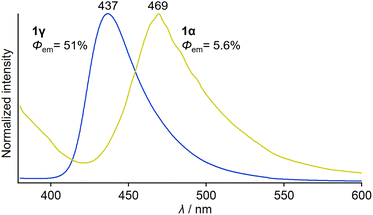
An investigation into the photophysical properties of 1 revealed that mechanically brittle 1γ exhibits a much higher absolute emission quantum yield (Φem) than 1α. Under photoexcitation at 365 nm, the crystal of green-emitting 1α showed broad emission with a maximum wavelength (λem,max) of 469 nm and an Φem of 5.6% (Fig. 5 and Table S2†). The emission–decay profile of 1α provides an average emission lifetime (τav) of 0.66 ns (Fig. S11 and Table S2†), which indicates that the emission of 1α can be assigned to fluorescence, similar to the case of flufenamic acid.11c,d In contrast, blue-emitting 1γ displays a broad emission spectrum (λem,max = 437 nm) that is hypsochromically shifted relative to that of 1α (Fig. 5). The τav of 1γ was determined to be 3.9 ns (Fig. S11 and Table S2†), indicating that the emission of 1γ should also be attributed to fluorescence. The absorption spectral onset wavelength of 1γ is also located in a shorter wavelength region (∼430 nm) than that of 1α (∼470 nm; Fig. S12†).16 Remarkably, the Φem value of 1γ was determined to be 51%, which is roughly nine times that of 1α (Fig. 5 and Table S2†). The low Φem of 1α could be due to the presence of the slide planes, given that the crystal-structure domains with weak intermolecular interactions in the slide planes could promote the nonradiative quenching pathway. These results indicate that the photophysical properties of 1 depend on the polymorph, similar to their mechanical properties, which suggests that if phase transitions between the polymorphs of 1 could be achieved, these properties could be controlled.
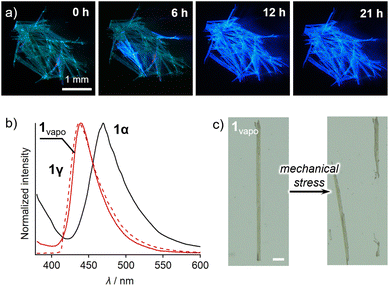
Vapor treatment of 1α resulted in a phase transition to 1γ, accompanied by changes in the mechanical and emission properties. After exposure to ethyl acetate for 24 h, the solid sample of 1α changed color from yellow to white (Fig. S15†). Simultaneously, the emission color changed from blue-green to blue with intensified emission intensity (Fig. 6a). The resulting samples are hereafter called 1vapo.17 Interestingly, the absorption and emission spectra and the emission–decay profiles of 1vapo were different from those of 1α and resemble those of 1γ (Fig. S12, 6b and S11,† respectively). Moreover, single-crystal XRD analysis of the 1vapo crystal revealed that the crystallographic parameters of 1vapo were essentially identical to those of 1γ obtained from recrystallization (Table S3†). Similarly, the DSC traces of 1vapo resemble those of 1γ (Fig. S17†). These results indicate that the phase transition of 1α to a phase similar to that of 1γ occurs upon exposure to ethyl-acetate vapor. Other good solvents, such as acetone and chloroform, can also promote the phase transition from 1α to 1γ (Fig. S18†). As a result of the phase transition from 1α to 1vapo (=1γ), the emission color changed (Fig. 6b), accompanied by a marked increase in emission intensity (Φem: 5.6 → 46%; Fig. S19 and Table S2†). Similarly, the mechanical properties of 1 were modulated, i.e., the bendable 1α became brittle upon vapor treatment when 1vapo is formed (Fig. 6c). The thermal phase transition from 1α to 1γ phases also occurred, as confirmed by spectroscopic and powder XRD analyses (Fig. S20†).18 These results indicate that the vapor treatment of 1 induces a phase transition from 1α to the thermodynamically favorable phase 1γ.
By utilizing the phase transition behavior of 1, bent crystals of intrinsically brittle 1γ can be obtained. A pristine crystal of 1α was bent via mechanical force and then exposed to ethyl-acetate vapor (Fig. 7). This bent crystal of 1α also underwent an emission color change (Fig. 7). The resulting emission spectrum essentially overlaps with that of 1γ (Fig. S21†), similar to the spectrum of the unprocessed crystal of 1α (Fig. 6b). This result indicates that the bent crystal 1α underwent the phase transition to 1vapo (=1γ). The transition was also supported by a single-crystal XRD study of a small piece of a bent 1vapo crystal, which was cut off from a larger crystal (Table S3†). The resulting bent 1vapo crystal showed intense green emission (Fig. 7). Importantly, 1γ is intrinsically brittle under mechanical force (Fig. 2e). Thus, the “intrinsically brittle” bent crystal 1vapo (=1γ) can only be obtained through a vapor-induced phase transition from the bent crystal of malleable 1α. This method will possibly lead to new applications that require both a bent crystal shape and novel emission properties.
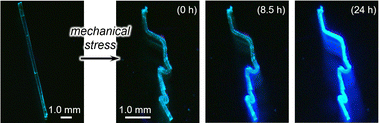 | ||
| Fig. 7 Photographs of unbent and bent 1α and bent 1vapo recorded under irradiation with UV light. The exposure time to ethyl-acetate vapor is shown in parentheses. | ||
The slow phase transition from 1α to 1γ could be the key to retaining the bent shape of the crystals. In previously reported studies that investigated the phase transitions of the mechanically bent crystals, the phase transitions induce the crystal-shape changes to recover the original straight shape or to further bend the shape.10a–d Importantly, these phase transitions occur in a time frame of minutes or less.10a–d Such rapid phase changes often result in accumulated internal strain within the crystalline lattice, especially at interfaces between the parent and the converted domains.10a The crystal-shape changes then occur as a result of releasing this strain. On the other hand, the vapor-induced phase transition of 1 takes 20 h or more, most likely due to the relatively large conformational change including the ∼90° rotation of the CF3-substituted benzene ring of 1 (Fig. S9†). Such slow crystal-structure changes prevent the accumulation of residual strain within the interface. Thus, molecular-arrangement changes in 1 do not lead to a change in the shape of the crystal morphology.
The pristine polymorphs 1α and 1γ were both found to exhibit optical waveguide behavior. Molecular crystals with optical waveguide properties have potential for various applications in optoelectronics and flexible materials.3,19 To assess the waveguide properties of 1, their pristine crystals were photoexcited via irradiation with focused laser light (λex = 405 nm) at specific positions, and the emission spectra were monitored at the edge positions (Fig. 8a, b and S22†). The ratio of the emission intensity at the excitation point (IEx) and crystal edge (IWG) was plotted as a function of the distance (D) between the excitation and detection points (Fig. 8 and S23†). Both polymorphs 1α and 1γ exhibited decreasing IWG/IEx values with increasing D (Fig. 8c and d, respectively), indicating their waveguide activity. Furthermore, the plots were fitted using the relationship IWG/IEx = A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−αD), where A is a constant and α is the loss coefficient.19c The results suggest α values of 0.013 ± 0.005 dB μm−1 and 0.044 ± 0.003 dB μm−1 for 1α and 1γ, respectively. The smaller α value of 1α indicates its superior waveguide behavior compared to that of 1γ. Unfortunately, subjecting 1α to bending or phase transitions led to a loss of its waveguide properties, i.e., the bent crystals of 1α and the crystal of 1vapo did not show waveguide properties (data not shown). This result indicates that the current process of changing the shape or molecular arrangement of the crystals causes a deterioration of the molecular order, which severely affects their specific optical properties, i.e., their waveguide activity. Ideally, the application of external stimulation in an optimized manner, i.e., gentle pushing to bend 1α or pressure/concentration control during vapor exposure for the transformation of 1α into 1vapo, could prevent the deterioration of the molecular arrangement within the resulting crystals, allowing the waveguide activity to be retained. The achievement of waveguide activity in bent 1vapo is our future goal.
exp(−αD), where A is a constant and α is the loss coefficient.19c The results suggest α values of 0.013 ± 0.005 dB μm−1 and 0.044 ± 0.003 dB μm−1 for 1α and 1γ, respectively. The smaller α value of 1α indicates its superior waveguide behavior compared to that of 1γ. Unfortunately, subjecting 1α to bending or phase transitions led to a loss of its waveguide properties, i.e., the bent crystals of 1α and the crystal of 1vapo did not show waveguide properties (data not shown). This result indicates that the current process of changing the shape or molecular arrangement of the crystals causes a deterioration of the molecular order, which severely affects their specific optical properties, i.e., their waveguide activity. Ideally, the application of external stimulation in an optimized manner, i.e., gentle pushing to bend 1α or pressure/concentration control during vapor exposure for the transformation of 1α into 1vapo, could prevent the deterioration of the molecular arrangement within the resulting crystals, allowing the waveguide activity to be retained. The achievement of waveguide activity in bent 1vapo is our future goal.
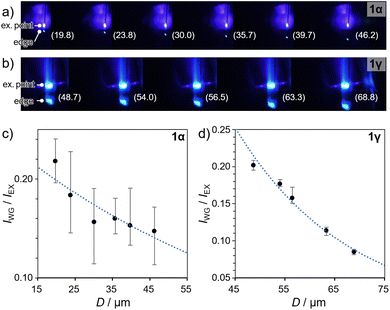 | ||
| Fig. 8 (a) Fluorescence images of (a) pristine 1α and (b) pristine 1γ with laser excitation (λex = 405 nm) applied at different positions along the long-axis direction. The distance between the excitation and detection points (D in μm) is denoted in parentheses. Magnified images are shown in Fig. S22.† (c) Ratios of the emission intensity IWG/IEx plotted as a function of D for (c) 1α and (d) 1γ, corresponding to the images in (a) and (b), respectively. | ||
Conclusions
In conclusion, we have reported the mechanical and emission properties, as well as the phase-transition behavior of two predominant polymorphs of 1, i.e., 1α and 1γ. The crystals of 1α with a low Φem of 5.6% exhibit dramatic plastic bending upon exposure to mechanical stress. In contrast, the crystals of 1γ, which exhibit pronounced emission properties (Φem = 51%), are brittle and break upon exposure to mechanical stress. Treatment of crystals of 1α with ethyl-acetate vapor induces a phase transition, even after these crystals had undergone mechanical deformation, thus allowing the preparation of bent-shaped 1γ crystals (1vapo). This indicates that through exploitation of the polymorph-dependent emission and mechanical properties in conjunction with the phase-transition capability, we successfully achieved the preparation of a novel optically active bent crystal of an intrinsically fragile polymorph. This method can be used as a post-modification technique for the preparation of functional flexible materials to enhance the emission properties in order to expand their application portfolio.Data availability
The data supporting this article have been included as part of the ESI.† Crystallographic data for 1α, 1β, and 1γ has been deposited at the CCDC with CCDC# of 2350732, 2350733, and 2350734, respectively, and can be obtained from https://www.ccdc.cam.ac.uk/.Author contributions
T. S. designed the project; all experiments except for the waveguide experiments were performed and analyzed mostly by S. K. and partially by R. I. with guidance from T. S.; K. Y. and S. H. performed and analyzed the nanoindentation experiments; T. M. and S. H. performed and analyzed the waveguide experiments; T. S. wrote the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by JSPS KAKENHI grants JP22H021550, JP22K190580, and JP24K01574 as well as by JST PRESTO (JPMJPR21AB) and JST FOREST Program (JPMJFR211W) (Japan).Notes and references
- (a) S. Hayashi, Polym. J., 2019, 51, 813–823 CrossRef CAS; (b) S. K. Park and Y. Diao, Chem. Soc. Rev., 2020, 49, 8287–8314 RSC; (c) W. M. Awad, D. W. Davies, D. Kitagawa, J. M. Halabi, M. B. Al-Handawi, I. Tahir, F. Tong, G. Campillo-Alvarado, A. G. Shtukenberg, T. Alkhidir, Y. Hagiwara, M. Almehairbi, L. Lan, S. Hasebe, D. P. Karothu, S. Mohamed, H. Koshima, S. Kobatake, Y. Diao, R. Chandrasekar, H. Zhang, C. C. Sun, C. Bardeen, R. O. Al-Kaysi, B. Kahr and P. Naumov, Chem. Soc. Rev., 2023, 52, 3098–3169 RSC; (d) M. Hasegawa and K. Ishii, Chem.–Eur. J., 2019, 25, 5105 CrossRef PubMed.
- (a) A. J. Thompson, A. I. Chamorro Orué, A. J. Nair, J. R. Price, J. McMurtrie and J. K. Clegg, Chem. Soc. Rev., 2021, 50, 11725–11740 RSC; (b) S. Ghosh and M. K. Mishra, Cryst. Growth Des., 2021, 21, 2566–2580 CrossRef CAS; (c) T. Seki, N. Hoshino, Y. Suzuki and S. Hayashi, CrystEngComm, 2021, 23, 5686–5696 RSC; (d) C. Wei, L. Li, Y. Zheng, L. Wang, J. Ma, M. Xu, J. Lin, L. Xie, P. Naumov, X. Ding, Q. Feng and W. Huang, Chem. Soc. Rev., 2024, 53, 3687–3713 RSC.
- W. Wu, K. Chen, T. Wang, N. Wang, X. Huang, L. Zhou, Z. Wang and H. Hao, J. Mater. Chem. C, 2023, 11, 2026–2052 RSC.
- Y. Chen, Z. Chang, J. Zhang and J. Gong, Angew. Chem., Int. Ed., 2021, 60, 22424–22431 CrossRef CAS PubMed.
- M. Owczarek, K. A. Hujsak, D. P. Ferris, A. Prokofjevs, I. Majerz, P. Szklarz, H. Zhang, A. A. Sarjeant, C. L. Stern, R. Jakubas, S. Hong, V. P. Dravid and J. F. Stoddart, Nat. Commun., 2016, 7, 13108 CrossRef CAS PubMed.
- For the hybridization of elastic crystals and polymeric materials in order to endow bendable molecular crystals with vapor responsivity, see: L. Lan, X. Yang, B. Tang, X. Yu, X. Liu, L. Li, P. Naumov and H. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202200196 CrossRef CAS PubMed.
- (a) C. M. Reddy, M. T. Kirchner, R. C. Gundakaram, K. A. Padmanabhan and G. R. Desiraju, Chem.–Eur. J., 2006, 12, 2222–2234 CrossRef CAS PubMed; (b) C. M. Reddy, K. A. Padmanabhan and G. R. Desiraju, Cryst. Growth Des., 2006, 6, 2720–2731 CrossRef CAS; (c) S. Ghosh, M. K. Mishra, S. B. Kadambi, U. Ramamurty and G. R. Desiraju, Angew. Chem., Int. Ed., 2015, 54, 2674–2678 CrossRef CAS PubMed; (d) T. Seki and H. Ito, Chem.–Eur. J., 2016, 22, 4322–4329 CrossRef CAS PubMed; (e) M. K. Panda, K. B. Pal, G. Raj, R. Jana, T. Moriwaki, G. D. Mukherjee, B. Mukhopadhyay and P. Naumov, Cryst. Growth Des., 2017, 17, 1759–1765 CrossRef CAS; (f) S. Hayashi, T. Koizumi and N. Kamiya, Cryst. Growth Des., 2017, 17, 6158–6162 CrossRef CAS; (g) M. Đaković, M. Borovina, M. Pisačić, C. B. Aakeröy, Ž. Soldin, B. M. Kukovec and I. Kodrin, Angew. Chem., Int. Ed., 2018, 57, 14801–14805 CrossRef PubMed.
- (a) L. Yu, Acc. Chem. Res., 2010, 43, 1257–1266 CrossRef CAS PubMed; (b) J. Bernstein, Cryst. Growth Des., 2011, 11, 632–650 CrossRef CAS.
- (a) S. Saha and G. R. Desiraju, Chem. Commun., 2018, 54, 6348–6351 RSC; (b) B. Tang, B. Liu, H. Liu and H. Zhang, Adv. Funct. Mater., 2020, 30, 2004116 CrossRef CAS; (c) J. Cao, H. Liu and H. Zhang, CCS Chem., 2020, 2, 2569–2575 Search PubMed; (d) L. Lan, Q. Di, L. Li, B. Liu, X. Yu, P. Naumov and H. Zhang, Cryst. Growth Des., 2022, 22, 3435–3441 CrossRef CAS.
- For phase transitions of mechanically bent crystals with a shape-memory effect, see: (a) D. P. Karothu, J. Weston, I. T. Desta and P. Naumov, J. Am. Chem. Soc., 2016, 138, 13298–13306 CrossRef CAS PubMed; (b) S. Takamizawa and Y. Takasaki, Chem. Sci., 2016, 7, 1527–1534 RSC; (c) C. Feng, T. Seki, S. Sakamoto, T. Sasaki, S. Takamizawa and H. Ito, Chem. Sci., 2022, 13, 9544–9551 RSC; (d) E. Ahmed, D. P. Karothu, M. Warren and P. Naumov, Nat. Commun., 2019, 10, 3723 CrossRef PubMed. For another example, see: (e) A. C. Maahs, M. G. Ignacio, M. Ghazzali, D. V. Soldatov and K. E. Preuss, Cryst. Growth Des., 2017, 17, 1390–1395 CrossRef CAS.
- Numerous studies regarding e.g., polymorphism, emission properties, and mechanical bending of flufenamic acid have already been reported; for selected examples, see: (a) E. H. Lee, S. X. M. Boerrigter, A. C. F. Rumondor, S. P. Chamarthy and S. R. Byrn, Cryst. Growth Des., 2008, 8, 91–97 CrossRef CAS; (b) V. López-Mejías, J. W. Kampf and A. J. Matzger, J. Am. Chem. Soc., 2012, 134, 9872–9875 CrossRef PubMed; (c) X. Wang, Y. Chen, J. Gong and J. Hou, Org. Biomol. Chem., 2019, 17, 3409–3415 RSC; (d) Y. Liu, P. Yang, K. Zhang, J. Xu, S. Wu and J. Gong, Cryst. Growth Des., 2022, 22, 1312–1318 CrossRef CAS; (e) L. Waterloo, H. Hübner, F. Fierro, T. Pfeiffer, R. Brox, S. Löber, D. Weikert, M. Y. Niv and P. Gmeiner, J. Med. Chem., 2023, 66, 3499–3521 CrossRef CAS PubMed.
- Nanoindentation tests of 1α, 1β, and, 1γ were performed (Fig. S4†).
- T. Seki, K. Kobayashi, T. Mashimo and H. Ito, Chem. Commun., 2018, 54, 11136–11139 RSC.
- The crystal structure of the bendable 1β polymorph has similar characteristics to that of 1α. The columns of 1β are formed by the stacking of double hydrogen-bonded benzoic-acid moieties. Columns interact with neighboring columns via weak and strong intermolecular interactions (Fig. S7†).
- (a) M. J. Turner, S. P. Thomas, M. W. Shi, D. Jayatilaka and M. A. Spackman, Chem. Commun., 2015, 51, 3735–3738 RSC; (b) K. B. Raju, S. Ranjan, V. S. Vishnu, M. Bhattacharya, B. Bhattacharya, A. K. Mukhopadhyay and C. M. Reddy, Cryst. Growth Des., 2018, 18, 3927–3937 CrossRef CAS; (c) P. R. Spackman, M. J. Turner, J. J. McKinnon, S. K. Wolff, D. J. Grimwood, D. Jayatilaka and M. A. Spackman, J. Appl. Crystallogr., 2021, 54, 1006–1011 CrossRef CAS PubMed; (d) M. Yoshida, Y. Makino, T. Sasaki, S. Sakamoto, S. Takamizawa, A. Kobayashi and M. Kato, CrystEngComm, 2021, 23, 5891–5898 RSC.
- The DFT calculations based on the single-crystal structures of 1α and 1γ indicate that the longer-wavelength emission of 1α is most likely caused by the more pronounced intermolecular charge-transfer character of the excited state of 1α compared to that of 1γ (Fig. S13 and S14†).
- NMR studies indicate that the crystalline lattice of 1vapo does not contain ethyl acetate (Fig. S16†).
- The thermal phase transition of 1α is rather complicated compared to the vapor-induced phase transition (Fig. S17 and S20†).
- (a) L. Catalano, D. P. Karothu, S. Schramm, E. Ahmed, R. Rezgui, T. J. Barber, A. Famulari and P. Naumov, Angew. Chem., Int. Ed., 2018, 57, 17254–17258 CrossRef CAS PubMed; (b) S. Hayashi, S. Yamamoto, D. Takeuchi, Y. Ie and K. Takagi, Angew. Chem., Int. Ed., 2018, 57, 17002–17008 CrossRef CAS PubMed; (c) M. Nakabayashi, T. Matsuo and S. Hayashi, Chem.–Eur. J., 2023, 29, e202302351 CrossRef CAS PubMed; (d) D. Tian and Y. Chen, Adv. Opt. Mater., 2021, 9, 2002264 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: X-ray crystallographic data, optical microscopy images, thermal data, emission spectra and other additional information. CCDC 2350732–2350734. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc02918f |
| This journal is © The Royal Society of Chemistry 2024 |