Open Access Article
Open Access ArticleCrafting 1,4-diaryl spirobifluorene hosts in OLEDs via interannular C–H arylation: synergistic effects of molecular linearity and orthogonality†
Qian
Li
,
Zhiqian
Yu
,
Qianhui
Liu
,
Yusong
Guo
,
Zhangyi
Fu
,
Yudong
Yang
 ,
Zhengyang
Bin
,
Zhengyang
Bin
 ,
Di
Wu
,
Di
Wu
 * and
Jingbo
Lan
* and
Jingbo
Lan
 *
*
Key Laboratory of Green Chemistry and Technology of Ministry of Education, College of Chemistry, Sichuan University, 29 Wangjiang Road, Chengdu 610064, People's Republic of China. E-mail: wood@scu.edu.cn; jingbolan@scu.edu.cn
First published on 4th June 2024
Abstract
In this work, we present a design concept of introducing linear structures into the orthogonal configuration of 9,9′-spirobifluorene (SBF), aiming to enhance carrier mobilities while maintaining high triplet energies (ET), which are two critical parameters for optimizing host materials in organic light-emitting diodes (OLEDs). To validate our proposed design, four pivotal model molecules of 1,4-diaryl SBFs were synthesized via interannular C–H arylation of bi(hetero)aryl-2-formaldehydes, a task challenging to accomplish using previous synthetic methodologies. The orthogonal configuration and the steric hindrance of SBF lead to high ET through the conjugation breaking at C1 and C4 positions, rendering 1,4-diaryl SBFs suitable as universal pure hydrocarbon (PHC) hosts for red, green, and blue (RGB) phosphorescent OLEDs (PhOLEDs). Meanwhile, the linearity and relatively good planarity of the para-quaterphenyl structure promote high carrier mobilities through orderly intermolecular packing. The synergistic effects of linearity and orthogonality in 1-(para-biphenyl)-4-phenyl-SBF result in exceptional device performance with external quantum efficiencies (EQEs) of 26.0%, 26.1%, and 22.5% for RGB PhOLEDs, respectively. Notably, the green PhOLED exhibits minimal efficiency roll-off, positioning its device performances among the state-of-the-art in PHC hosts.
Introduction
9,9′-Spirobifluorene (SBF) comprises two fluorene units connected by a shared spiro carbon atom, and the orthogonal configuration between two fluorenyl planes endows SBF derivatives with good solubilities and thermal stabilities.1 Of particular interest are multi-aryl SBFs, especially in applications involving electroluminescent devices such as organic light-emitting diodes (OLEDs) and solid-state lasers.2–5 Multi-aryl SBFs with more modifiable sites provide possibilities for the structural diversity of organic luminescent materials, therefore attracting much attention in recent years.4,5 The synthesis of multi-aryl SBFs typically involves the electrophilic bromination of fluorene, fluorenone and SBF, followed by Suzuki coupling with arylboronic acid.1,2 Notably, the C2 position exhibits the highest electrophilic reactivity.1,2 As a result, there has been a considerable body of research dedicated to exploring SBFs with C2-aryl substitution.1–5 In contrast, multi-aryl SBFs lacking C2-aryl group have received less attention, primarily due to synthetic challenges, limiting the exploration of new materials based on SBFs.2 Typically, the synthesis of unsymmetrical multi-aryl SBFs poses additional complexities, necessitating the introduction of multiple halogen atoms with different reactivities on aromatic rings to accomplish sequential Suzuki coupling.Multi-aryl SBFs are also an important kind of pure hydrocarbon (PHC) hosts for phosphorescent OLEDs (PhOLEDs).6,7 Recent studies have shown that incorporating various aryl substituents at specific positions of SBFs enables precise tuning of triplet energies (ET), concurrently enhancing carrier mobility of target molecules, which are two crucial factors in optimizing universal host materials for red, green, and blue (RGB) PhOLEDs.8,9 The incorporation of C2-aryl substituent has been noted to significantly reduce the ET of SBFs due to electronic coupling (Scheme 1a, 2-Ph-SBF and 1-pbp-7-p-SBF). The introduction of an aryl substituent at C1 position exerts a minimal impact on ET because the large dihedral angle between the fluorene plane and C1-aromatic ring disrupts the π-conjugation (Scheme 1a, 1-mtp-SBF).6,10–12 Additionally, the presence of a C4-aryl substituent has a slight effect on ET, attributed to the steric hindrance-induced partial disruption of conjugation at the C4 position (Scheme 1a, 4-Ph-SBF).13–15 Research reported by Jiang, Liao, and Poriel suggests that incorporating meta-biphenyl (mbp), and meta-terphenyl (mtp) at the C1 position substantially enhances carrier mobility without causing significant alteration to the ET of host materials.12 Our recent investigation also demonstrates that the introduction of para-biphenyl (pbp) at the C1 position contributes significantly to the carrier mobility.6 Based on these findings, we envision the simultaneous introduction of aryl substituents at both C1 and C4 positions of SBFs would result in a linear para-terphenyl or para-quaterphenyl structure, and a good linear structure might hold the potential to facilitate organized packing between molecules, thereby promoting high carrier mobilities while keeping high ET. Density functional theory (DFT) calculations indicate that the highest occupied molecular orbitals (HOMO) of 1,4-diaryl SBFs are distributed over the whole spirobifluorene skeleton. Meanwhile, their lowest unoccupied molecular orbitals (LUMOs) are mainly localized on the substituted fluorene with slight delocalization to the C4-aryl substituent. This result suggests a partial planarization between the fluorene and the C4-aromatic ring in excited states (Scheme 1b). Calculations demonstrate that these 1,4-diaryl SBFs exhibit high ET exceeding 2.90 eV, providing potential for their application as universal hosts in RGB PhOLEDs (Scheme 1b).16,17 In this study, we present a palladium-catalyzed interannular selective ortho-C–H arylation of bi(hetero)aryl-2-formaldehydes, serving as a molecular engineering strategy for generating a diverse array of aryl fluorenone derivatives, encompassing 4-aryl, 1,4-diaryl, 2,4-diaryl, 3,5-diaryl, 1,4,6-triaryl, 2,4,6-triaryl, and 1,4,5,7-tetraaryl fluorenones (Scheme 1c). Leveraging this efficient and concise synthetic pathway, we synthesized various 1,4-diaryl SBFs with distinct structural features (Scheme 1d). Subsequently, we investigated their electronic and physical properties as well as performance as host materials in PhOLEDs.
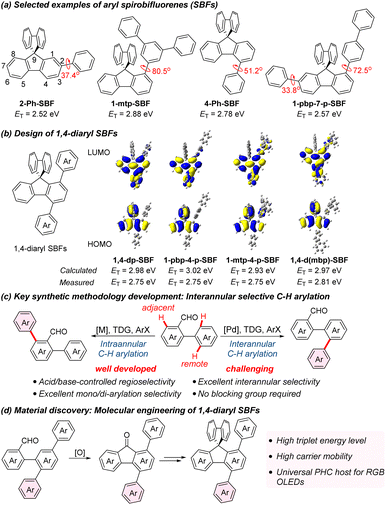 | ||
| Scheme 1 Design and synthesis of 1,4-diaryl spirobifluorenes (1,4-diaryl SBFs) via interannular selective C–H arylation. For the structures of 1,4-diaryl SBFs see Scheme 4. TDG = transient directing group. | ||
Results and discussion
Synthetic methodology development
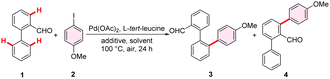
Transition metal-catalyzed C–H arylation has emerged as a pivotal strategy for accomplishing aryl–aryl coupling and constructing fluorene structures.18–25 Recently, our research group successfully developed an Ir(III)-catalyzed intraannular ortho-C–H diarylation/annulation of benzoic acids, leading to the construction of SBFs with C1-aryl substitution.6 However, it is not suitable to construct 4-aryl SBFs or 1,4-diaryl SBFs via this Ir(III)-catalyst system owing to product substitution patterns and/or generation of inseparable regioisomeric products. In principle, the interannular ortho-C–H arylation of biphenyl-2-carboxylic acids or biphenyl-2-formaldehydes can offer a convenient route to synthesize 4-aryl SBFs. In recent years, tremendous efforts have been devoted to the remote functionalization of biaryl substrates through an interannular directed C–H activation strategy.26–31 Nevertheless, the selective interannular over intraannular ortho-C–H arylation of biphenyl-2-formaldehydes as well as biphenyl-2-carboxylic acids remains a challenging task (Scheme 1c). The current compromise solution to this issue relied on (i) incorporating blocking groups at both competitive reaction sites and (ii) utilizing an alkene insertion/β-oxygen elimination/protonolysis/dehydration cascade process with strained 7-oxabenzonorbornadienes as the arylation reagents.32,33 However, the former cannot access 4-aryl SBFs because blocking groups of biphenyl-2-formaldehyde substrates are difficult to remove. The latter only affords benzaldehydes containing binaphthyl units as the main product, which are unable to be transformed into the SBF structure.To tackle the aforementioned synthetic problem, the interannular ortho-C–H arylation of biphenyl-2-carboxylic acid was initially explored. However, despite attempts with various catalyst systems, only the intraannular ortho-C–H arylated product was observed. Next, the interannular C–H arylation of biphenyl-2-formaldehyde (1) was studied instead, employing 1-iodo-4-methoxybenzene (2) as the arylation reagent, L-tert-leucine as the TDG, Pd(OAc)2 as the catalyst and Ag2CO3 as the additive (Tables 1 and S1†). Irrespective of the use of HFIP/HOAc or TFE/HOAc, the primary product identified was the intraannular ortho-C–H arylated product 4 (Table 1, entries 1 and 2). Notably, the TFE/TFA system also predominantly exhibited intraannular ortho-C–H arylated reactivity (Table 1, entry 3). From these observations, we concluded that the addition of acid to the reaction system did not effectively promote the formation of the interannular arylated product. When HFIP or TFE was used as solvent alone, the desired product 3 could be obtained but in low yield (Table 1, entries 4 and 5). Surprisingly, the introduction of alkaline additives into the reaction system resulted in an increased conversion rate while maintaining excellent selectivity for interannular arylation (Table 1, entries 6 and 7). Specifically, the inclusion of ZnCO3 as an additive and TFE as the solvent yielded a favorable outcome, enabling the isolation of the interannular arylated product 3 in a yield of 42% (Table 1, entry 7). Given the crucial role of silver salts in arylating reactions with iodobenzene substrates, we conducted a screening of various silver salts.34 The results indicated that silver trifluoroacetate (AgTFA) exhibited the best reaction efficiency. The replacement of Ag2CO3 with AgTFA significantly improved the reaction yield, reaching 72% (Table 1, entry 8). No arylation reaction was detected in the absence of a TDG, indicating the necessity of a TDG (Table S1,† entry 17). Further optimization of the reaction parameters including palladium catalysts, oxidants, TDGs, and reaction temperatures could not significantly improve the reaction yield (Table S1†). Consequently, the optimal conditions comprised of Pd(OAc)2 as the catalyst, L-tert-leucine as the TDG, AgTFA and ZnCO3 as the additives, and TFE as the solvent.
| Entry | Additive I | Additive II | Solvent | Yield of 3 (%) | Yield of 4 (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1 (0.20 mmol), 2 (0.30 mmol), Pd(OAc)2 (10 mol%), L-tert-leucine (30 mol%), additives and solvent at 100 °C for 24 h under air. HFIP = 1,1,1,3,3,3-hexafluoro-2-propanol; TFE = 2,2,2-trifluoroethanol; TFA = trifluoroacetic acid. | |||||
| 1 | Ag2CO3 | HOAc | HFIP | 12 | 27 |
| 2 | Ag2CO3 | HOAc | TFE | 0 | 22 |
| 3 | Ag2CO3 | TFA | TFE | Trace | 43 |
| 4 | Ag2CO3 | — | HFIP | 25 | 0 |
| 5 | Ag2CO3 | — | TFE | 28 | 0 |
| 6 | Ag2CO3 | K2CO3 | TFE | 36 | 0 |
| 7 | Ag2CO3 | ZnCO3 | TFE | 42 | 0 |
| 8 | AgTFA | ZnCO 3 | TFE | 72 | 0 |
Scope of substrates
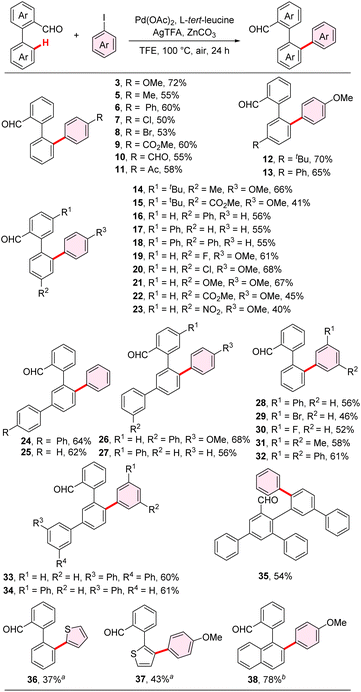
Under optimal conditions, we explored the substrate scope of aryl iodides (Scheme 2). Various functional groups, including methoxy (3), methyl (5), phenyl (6), halogen (7 and 8, 29 and 30), ester (9), formyl (10), and acetyl groups (11), were well-tolerated, affording the interannular ortho-C–H arylated products with excellent selectivity in moderate to good yields. Iodobiaryl substrates, such as iodo biphenyl and iodo terphenyl, worked well under the standard condition, offering possibilities for the follow-up preparation of PHC host materials (6 and 32). We also explored the generality of aryl aldehyde substrates. Biphenyl-2-formaldehyde substrates featuring both electron-donating groups, such as alkyl (14), and methoxy (21), and electron-withdrawing groups, such as ester (15 and 22), and nitro (23) and halogens (19 and 20), demonstrated successful engagement in the interannular arylation reaction. Selective arylation of 3′-aryl substituted biphenyl-2-formaldehydes yielded the desired products, which are crucial intermediates for the preparation of 1,4-diaryl SBFs (24, 25, 33, 34). Their scale-up syntheses were also performed under the same conditions (Fig. S2†). Additionally, 2-(thiophen-2-yl)benzaldehyde was compatible, achieving the arylation at β-rather than α-sites of thiophene unit in a 43% isolated yield (37). The relatively low yield was attributed to inferior conversion rather than side reactions. Notably, 2-(naphthalen-1-yl)benzaldehyde gave the interannular arylation product in 78% yield, when increasing the amount of the iodide substrate to two equivalents (38).Synthesis of multi-aryl fluorenones and 1,4-diaryl SBFs
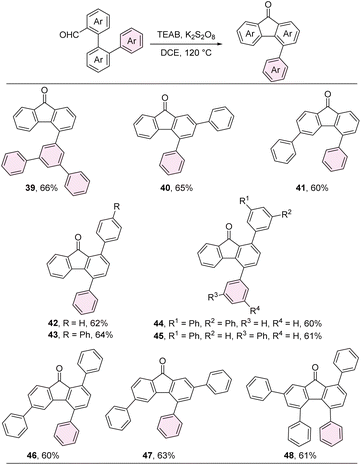
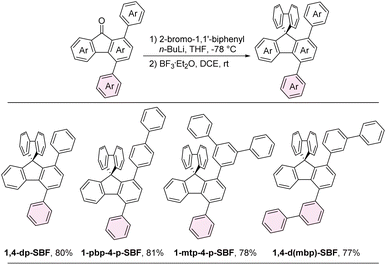
A series of aryl fluorenones, including 4-aryl, 1,4-diaryl, 2,4-diaryl, 3,5-diaryl, 1,4,6-triaryl, 2,4,6-triaryl, and 1,4,5,7-tetraaryl fluorenones were synthesized through the intramolecular dehydrogenative arylation of aryl-substituted biphenyl-2-formaldehyde products. This transformation was achieved in the presence of potassium persulfate and tetraethylammonium bromide (TEAB), as illustrated in Scheme 3.35 Subsequently, 1,4-diaryl SBFs were prepared through the nucleophilic addition of in situ generated [1,1′-biphenyl]-2-yllithium to the carbonyl group of fluorenones, followed by intramolecular Friedel–Crafts alkylation promoted by BF3 (Scheme 4).Single crystal structures
The single crystal X-ray structures and packing patterns of 1-pbp-4-p-SBF, 1-mtp-4-p-SBF and 1,4-d(mbp)-SBF are depicted in Fig. 1.36 The crystal structures reveal that the introduction of aryl group at C4 position results in a relatively large dihedral angle between the fluorene plane and its pendant aromatic ring, with angle degrees of 47.4° for 1-pbp-4-p-SBF, 63.5° for 1-mtp-4-p-SBF, and 67.8° for 1,4-d(mbp)-SBF, due to the repulsive effect between adjacent aromatic rings. Owing to the orthogonal configuration between two fluorenyl cores of SBF leading to a considerable steric hindrance at the C1 site, a large dihedral angle was observed between the C1-aromatic ring and its connected fluorene plane of SBF, estimated to be 73.6°, 78.7°, and 86.2° for 1-pbp-4-p-SBF, 1-mtp-4-p-SBF, and 1,4-d(mbp)-SBF, respectively. Notably, the dihedral angles of 1-pbp-4-p-SBF at both C1 and C4 positions are smaller than those in 1-mtp-4-p-SBF or 1,4-d(mbp)-SBF. Moreover, the dihedral angle of the para-biphenyl group at the C1 position in 1-pbp-4-p-SBF (11.8°) is much smaller than those of meta-terphenyl in 1-mtp-4-p-SBF (35.1° and 36.7°) and meta-biphenyl in 1,4-d(mbp)-SBF (30.8°). The good planarity of the para-biphenyl structure contributes to the π⋯π conjugation (electronic coupling), leading to a decrease in free energy. This is a powerful driving force, which may be a reasonable explanation for the overall smaller dihedral angles of the para-quaterphenyl structure in 1-pbp-4-p-SBF. By comparison, the electronic decoupling of the meta-connection mode may result in the overall larger dihedral angles in 1-mtp-4-p-SBF and 1,4-d(mbp)-SBF. In a word, the para-quaterphenyl structure in 1-pbp-4-p-SBF features not only the best linearity but also the best planarity among the three molecules, possibly favorable towards organized intermolecular packing and carrier mobilities. 1-pbp-4-p-SBF, 1-mtp-4-p-SBF, and 1,4-d(mbp)-SBF exhibit short distances of 3.07 Å, 3.10 Å, and 3.10 Å between the carbon atom connected to the C1 position and the non-substituted fluorene plane, respectively. The shorter distance of 3.07 Å between the intramolecular π-planes in 1-pbp-4-p-SBF is beneficial to the through-space charge transfer,37,38 which might further improve the carrier mobility. All three crystals adopt an edge-to-face packing mode stabilized by intermolecular multiple C–H⋯π interaction networks.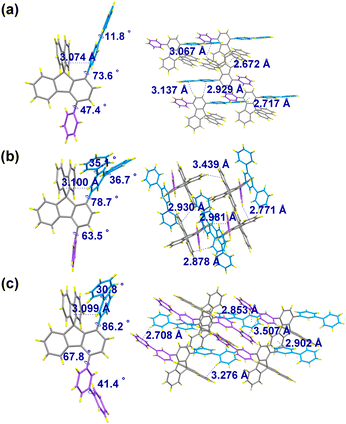 | ||
| Fig. 1 Single crystal X-ray structures and packing patterns of (a) 1-pbp-4-p-SBF, (b) 1-mtp-4-p-SBF, and (c) 1,4-d(mbp)-SBF. | ||
Electronic and physical properties
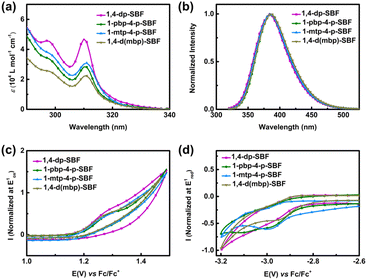
As depicted in Fig. 2a and summarized in Table 2, all 1,4-diaryl spirobifluorenes exhibit similar UV/vis absorption spectra. The absorption bands at 298 and 310–311 nm are closely consistent with the characteristic band of non-substituted SBF, indicating that the aryl substituents at both C1 and C4 positions have little effect on the absorption spectra. Their optical band gaps (Eg) were calculated from the onset of the absorption spectra and found to be 3.92, 3.90, 3.88, and 3.89 for 1,4-dp-SBF, 1-pbp-4-p-SBF, 1-mtp-4-p-SBF, and 1,4-d(mbp)-SBF, respectively. These 1,4-diaryl SBFs show red-shifted emission bands compared to 1-aryl substituted spirobifluorenes,12 yet their spectra closely resemble that of the 4-Ph-SBF.13 These findings demonstrate that the aryl substituent attached at C1 position exerts very little influence on the emission property, attributed to the conjugation breaking at C1 position in both the ground and excited states. In contrast, C4-aryl substituents have a slight impact on the emission spectra owing to partial planarization between the C4-aromatic ring and its connected fluorene plane of SBF in the first excited state.13 The quantum yields of these 1,4-diaryl SBFs range from 43% to 55%. The singlet energies (ES1) of 1,4-dp-SBF, 1-pbp-4-p-SBF, 1-mtp-4-p-SBF, and 1,4-d(mbp)-SBF were estimated to be 3.68, 3.65, 3.65, and 3.60 eV, respectively. These four 1,4-diaryl SBFs exhibit high ET. Calculated from the first phosphorescence peak at 77 K in toluene, the corresponding ET1 values are 2.75, 2.75, 2.75, and 2.81 eV for 1,4-dp-SBF, 1-pbp-4-p-SBF, 1-mtp-4-p-SBF, and 1,4-d(mbp)-SBF, respectively (Table 2 and Fig. S3a†). 1,4-dp-SBF, 1-pbp-4-p-SBF, and 1-mtp-4-p-SBF containing the same phenyl substituent at their C4 positions, exhibit identical ET1 values. This result clearly demonstrates that the C1-aryl substituent, regardless of phenyl, para-biphenyl, or meta-terphenyl group, has almost no effect on ET owing to the conjugation breaking. The 1,4-d(mbp)-SBF with the meta-biphenyl group at C4 position shows higher ET1 value than the other three 1,4-diaryl SBFs, perhaps because that the electronic decoupling of the meta-connection mode leads to a large dihedral angle, weakening the conjugation degree of molecules in the excited state. All four 1,4-diaryl SBFs in the pure film show slightly redshifted phosphorescence emission compared to those in toluene solutions, owing to stronger intermolecular interactions in the aggregated state (Fig. S3b†). Utilizing cyclic voltammogram (CV) measurements (Fig. 2c and d), the HOMO energy levels for 1,4-dp-SBF, 1-pbp-4-p-SBF, 1-mtp-4-p-SBF, and 1,4-d(mbp)-SBF were determined to be −5.77, −5.79, −5.77, and −5.78 eV, respectively. Their LUMOs were measured at −1.74, −1.75, −1.77, and −1.77 eV, respectively.| Property | 1,4-dp-SBF | 1-pbp-4-p-SBF | 1-mtp-4-p-SBF | 1,4-d(mbp)-SBF |
|---|---|---|---|---|
| a Measured in toluene solution (1.0 × 10−5 M), where λabs is the absorption peak, λem is the photoluminescence peak at room temperature, Φ is the fluorescence quantum yield, and ε is the molar absorption coefficient in parentheses. b Calculated from the onset of emission at rt. c Calculated from the first phosphorescence peak at 77 K in toluene. d Calculated from the onset of the absorption spectra. e From CVs (CH2Cl2 in oxidation and DMF in reduction). f Hole mobility (μh). g Electron mobility (μe). | ||||
| λ abs (ε)a [nm] | 298 (4.58) | 298 (3.35) | 298 (3.59) | 298 (2.53) |
| (104 L mol−1 cm−1) | 310 (4.70) | 310 (2.83) | 311 (3.09) | 311 (2.23) |
| λ em [nm] | 384 | 385 | 386 | 386 |
| E S1 [eV] | 3.68 | 3.65 | 3.65 | 3.60 |
| E T1 [eV] | 2.75 | 2.75 | 2.75 | 2.81 |
| E g [eV] | 3.92 | 3.90 | 3.88 | 3.89 |
| T g [°C] | 78 | 97 | — | 100 |
| T d [°C] | 304 | 356 | 380 | 394 |
| Φ [%] | 43 | 52 | 47 | 55 |
| LUMOe [eV] | −1.74 | −1.75 | −1.77 | −1.77 |
| HOMOe [eV] | −5.77 | −5.79 | −5.77 | −5.78 |
| μ h (10−5)f [cm2 V−1 S−1] | 1.29 | 3.83 | 1.45 | 1.84 |
| μ e (10−5)g [cm2 V−1 S−1] | 2.74 | 4.06 | 3.15 | 1.63 |
The thermal and morphological stabilities of host materials play a crucial role in determining the stability of OLEDs. Thermogravimetric analysis (TGA) indicates that 1,4-dp-SBF, 1-pbp-4-p-SBF, 1-mtp-4-p-SBF, and 1,4-d(mbp)-SBF possess high decomposition temperatures at 5% mass loss (Td), ranging from 304 to 394 °C, ensuring the stability of the compounds under vacuum evaporation (Table 2 and Fig. S4†). Differential scanning calorimetry (DSC) measurements were performed for the four 1,4-diaryl SBFs between 30 and 250 °C (Table 2 and Fig. S4†). At the first heating curve, 1,4-dp-SBF, 1-pbp-4-p-SBF, and 1,4-d(mbp)-SBF exhibited sharp endothermic peaks at 214 °C, 233 °C, and 221 °C, respectively, and then completely melted. These molten liquids, upon cooling to room temperature, solidified into amorphous states without undergoing recrystallization. During the second heating cycle at the same rate, the glass transition temperatures (Tg) were measured to be 78 °C, 97 °C, and 100 °C for 1,4-dp-SBF, 1-pbp-4-p-SBF, and 1,4-d(mbp)-SBF, respectively. However, 1-mtp-4-p-SBF did not exhibit melting even beyond 250 °C, and consequently, its glass transition phenomenon was not observed.
The carrier mobility and charge balance capacity of host materials have a significant impact on the performance of OLED devices.39 Therefore, prior to assessing the electroluminescence (EL) performance of these host materials, hole-only devices (HODs) and electron-only devices (EODs) were fabricated to investigate their charge transport properties (Fig. S5† and Table 2). All four 1,4-diaryl SBFs exhibit relatively high carrier mobilities for both hole and electron, among which 1-pbp-4-p-SBF displays the highest mobility and excellent charge balance with a hole mobility of 3.83 × 10−5 cm2 V−1 s−1 and an electron mobility of 4.06 × 10−5 cm2 V−1 s−1, demonstrating that the para-biphenyl substituent at the C1 position contributes to carrier mobility, consistent with our earlier speculation.
To gain deeper insight into the charge transport properties of 1,4-diaryl SBFs, the transfer integrals of 1-pbp-4-p-SBF, 1-mtp-4-p-SBF, and 1,4-d(mbp)-SBF were estimated by density functional theory (DFT) calculation at the B3LYP/DZ level using the Multiwfn (Fig. 3).40 In all crystals, 1-pbp-4-p-SBF possesses the most orderly arrangement, because of multiple strong C–H⋯π interactions between substituted and non-substituted fluorenes in adjacent molecules. The relatively weak intermolecular C–H⋯π interactions between the para-biphenyl at C1 position and non-substituted fluorene further strengthen the orderly arrangement. Therefore, 1-pbp-4-p-SBF displays the largest transfer integrals with t1 = 44 meV, t2 = 38 meV, and t3 = 10 meV for holes, as well as t1 = 14 meV, t2 = 4 meV, and t3 = 5 meV for electrons, consistent with its high carrier mobilities. In contrast, the intermolecular arrangements of 1-mtp-4-p-SBF and 1,4-d(mbp)-SBF are less orderly, therefore resulting in smaller transfer integrals and lower carrier mobilities. These results suggest that the better linear and planar para-quaterphenyl structure of 1-pbp-4-p-SBF significantly facilitates more orderly intermolecular packing compared to the para-terphenyl structures of 1-mtp-4-p-SBF and 1,4-d(mbp)-SBF, therefore leading to higher carrier mobilities, which further indicates that the molecular design strategy herein proposed for PHC hosts was successful.
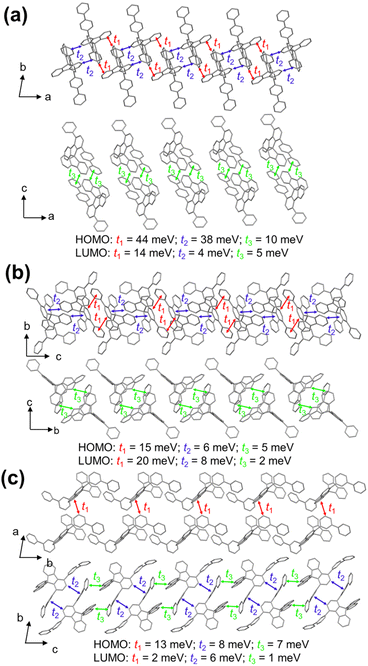 | ||
| Fig. 3 HOMO and LUMO transfer integral spatial distributions in the equilibrium crystal structures of (a) 1-pbp-4-p-SBF, (b) 1-mtp-4-p-SBF, and (c) 1,4-d(mbp)-SBF. | ||
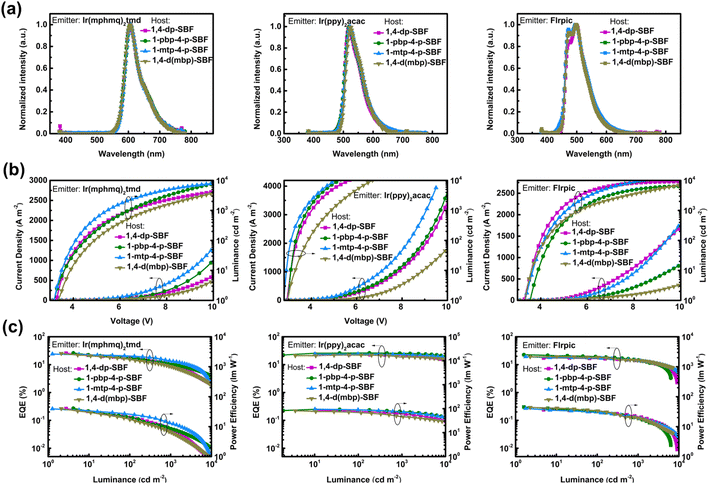
Electroluminescence performances
We assembled RGB PhOLEDs employing widely used bis[4-methyl-2-(3,5-dimethylphenyl)quinoline)] tetramethyl heptadionate iridium(III) (Ir(mphmq)2(tmd)) as a red phosphor, bis(2-phenylpyridine) iridium(III)-acetylacetonate (Ir(ppy)2(acac)) as a green phosphor, and bis(3,5-difluoro-2-(2-pyridyl)phenyl) 2-carboxypyridyl iridium(III) (FIrpic) as a blue phosphor (Fig. 4 and Table 3). Each of the RGB devices exhibited identical EL emission spectra, affirming effective energy transfer from host to emitter. The electroluminescence of Firpic in the four hosts are slightly different, due to its dual-emission characteristic.41 In the blue device, the best performance was achieved using 1-pbp-4-p-SBF as the host material, attaining a maximum external quantum efficiency (EQEmax) of 22.5%. 1-pbp-4-p-SBF also showed excellent performance as the host of green PhOLEDs with EQEmax of 26.1% and minimal efficiency roll-off. At luminance levels of 1000 cd m−2 and 5000 cd m−2, the EQE values are well-preserved at 25.4% and 23.0%, respectively (Tables 3 and S5†). These favorable outcomes could be attributed to its high carrier mobilities and excellent charge balance. Furthermore, the red PhOLED using 1-pbp-4-p-SBF as the host also demonstrated superior performance, achieving an EQEmax of 26.0%.| Emitter | Host | ELpeak [nm] | V on [V] | CIEb [x, y] | EQEmax/100/1000c [%] | PEmax/100/1000d [lm W−1] |
|---|---|---|---|---|---|---|
| a Turn-on voltage. b Commission internationale de I'Eclairage (CIE). c Maximum external quantum efficiency and external quantum efficiency at the luminance of 100 cd m−2 and 1000 cd m−2. d Maximum power efficiency and power efficiency at the luminance of 100 cd m−2 and 1000 cd m−2. | ||||||
| Ir(mphmq)2tmd | 1,4-dp-SBF | 606 | 3.4 | [0.63, 0.36] | 25.7/16.8/10.6 | 40.1/20.2/8.3 |
| 1-pbp-4-p-SBF | 605 | 3.4 | [0.63, 0.37] | 26.0/17.5/10.2 | 40.8/20.7/8.5 | |
| 1-mtp-4-p-SBF | 608 | 3.3 | [0.64, 0.36] | 24.0/20.3/13.3 | 39.2/26.0/12.7 | |
| 1,4-d(mbp)-SBF | 606 | 3.5 | [0.63, 0.37] | 25.1/15.2/8.6 | 39.8/17.2/6.6 | |
| Ir(ppy)2acac | 1,4-dp-SBF | 520 | 3.0 | [0.30, 0.63] | 23.6/22.8/21.4 | 86.4/76.3/60.8 |
| 1-pbp-4-p-SBF | 521 | 3.0 | [0.31, 0.63] | 26.1/26.0/25.4 | 93.8/89.7/76.0 | |
| 1-mtp-4-p-SBF | 522 | 2.9 | [0.32, 0.63] | 24.0/24.0/22.8 | 94.8/89.2/70.9 | |
| 1,4-d(mbp)-SBF | 523 | 3.0 | [0.31, 0.64] | 22.0/21.9/18.9 | 81.2/69.5/46.3 | |
| Flrpic | 1,4-dp-SBF | 497 | 3.4 | [0.18, 0.42] | 17.5/16.5/13.9 | 36.4/30.7/21.6 |
| 1-pbp-4-p-SBF | 498 | 3.5 | [0.18, 0.41] | 22.5/19.2/14.5 | 45.1/31.6/18.8 | |
| 1-mtp-4-p-SBF | 495 | 3.4 | [0.19, 0.39] | 19.2/17.0/14.0 | 40.4/29.8/19.7 | |
| 1,4-d(mbp)-SBF | 498 | 3.6 | [0.18, 0.41] | 19.3/18.3/14.2 | 43.6/33.6/19.6 | |
Conclusions
In summary, we have developed a highly efficient palladium catalyst system for achieving the interannular selective C–H arylation of bi(hetero)aryl-2-formaldehydes, facilitating the synthesis of various aryl fluorenone derivatives, including 4-aryl, 1,4-diaryl, 2,4-diaryl, 3,5-diaryl, 1,4,6-triaryl, 2,4,6-triaryl, and 1,4,5,7-tetraaryl fluorenones. Leveraging this streamlined pathway, we have prepared four 1,4-diaryl SBF derivatives and investigated their potential as universal PHC hosts for RGB PhOLEDs. The design concept of introducing a linear structure into the orthogonal configuration of SBF is proposed. The four 1,4-diaryl SBFs as the pivotal model molecules successfully verify this concept. The coordination of molecular linearity and orthogonality endows 1-pbp-4-p-SBF with excellent performances as a universal PHC host. This work not only underscores the great potential of 1,4-diaryl SBFs as PHC hosts but also presents an expedited route to access these hosts.Data availability
All data supporting the findings of this study are available within the article and its ESI file.†Author contributions
Q. Li carried out most parts of the experiments. Z. Yu analyzed the partial data. Q. Liu and Y. Guo performed some of the synthesis. Z. Fu performed DFT calculations. J. Lan designed and directed the project. J. Lan, Z. Bin, Y. Yang, Z. Yu and Q. Li wrote the manuscript. All authors contributed to discussions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge financial support from National Key R&D Program of China (No. 2021YFA1500100), National Natural Science Foundation of China (No. 22371196, 22071162, 22171188, and 22031007), Natural Science Foundation of Sichuan, China (2022NSFSC0029), and Fundamental Research Funds for the Central Universities. We also thank the support of Prof. Pengchi Deng from the Analytical and Testing Center of Sichuan University and Comprehensive Training Platform Specialized Laboratory, College of Chemistry, Sichuan University.Notes and references
- T. P. I. Saragi, T. Spehr, A. Siebert, T. Fuhrmann-Lieker and J. Salbeck, Chem. Rev., 2007, 107, 1011–1065 CrossRef CAS PubMed
.
- R. Pudzich, T. Fuhrmann-Lieker and J. Salbeck, Adv. Polym. Sci., 2006, 199, 83–142 CrossRef CAS
.
- Y. Tao, C. Yang and J. Qin, Chem. Soc. Rev., 2011, 40, 2943–2970 RSC
.
- C. Poriel and J. Rault-Berthelot, J. Mater. Chem. C, 2017, 5, 3869–3897 RSC
.
- Y. Jiang, Y.-Y. Liu, X. Liu, H. Lin, K. Gao, W.-Y. Lai and W. Huang, Chem. Soc. Rev., 2020, 49, 5885–5944 RSC
.
- Y. Luo, Z. Liu, G. Yang, T. Wang, Z. Bin, J. Lan, D. Wu and J. You, Angew. Chem., Int. Ed., 2021, 60, 18852–18859 CrossRef CAS PubMed
.
- Q. Wang, F. Lucas, C. Quinton, Y.-K. Qu, J. Rault-Berthelot, O. Jeannin, S.-Y. Yang, F.-C. Kong, S. Kumar, L.-S. Liao, C. Poriel and Z.-Q. Jiang, Chem. Sci., 2020, 11, 4887–4894 RSC
.
- C. Poriel, L. Sicard and J. Rault-Berthelot, Chem. Commun., 2019, 55, 14238–14254 RSC
.
- C. Poriel and J. Rault-Berthelot, Acc. Mater. Res., 2022, 3, 379–390 CrossRef CAS
.
- S. Thiery, C. Declairieux, D. Tondelier, G. Seo, B. Geffroy, O. Jeannin, R. Métivier, J. Rault-Berthelot and C. Poriel, Tetrahedron, 2014, 70, 6337–6351 CrossRef CAS
.
- L. Sicard, C. Quinton, J.-D. Peltier, D. Tondelier, B. Geffroy, U. Biapo, R. Métivier, O. Jeannin, J. Rault-Berthelot and C. Poriel, Chem.–Eur. J., 2017, 23, 7719–7727 CrossRef CAS PubMed
.
- F.-C. Kong, Y.-L. Zhang, C. Quinton, N. McIntosh, S.-Y. Yang, J. Rault-Berthelot, F. Lucas, C. Brouillac, O. Jeannin, J. Cornil, Z.-Q. Jiang, L.-S. Liao and C. Poriel, Angew. Chem., Int. Ed., 2022, 61, e202207204 CrossRef CAS PubMed
.
- S. Thiery, D. Tondelier, C. Declairieux, G. Seo, B. Geffroy, O. Jeannin, J. Rault-Berthelot, R. Métivier and C. Poriel, J. Mater. Chem. C, 2014, 2, 4156–4166 RSC
.
- L.-S. Cui, Y.-M. Xie, Y.-K. Wang, C. Zhong, Y.-L. Deng, X.-Y. Liu, Z.-Q. Jiang and L.-S. Liao, Adv. Mater., 2015, 27, 4213–4217 CrossRef CAS PubMed
.
- X. Tang, Y. Li, Y.-K. Qu, C.-C. Peng, A. Khan, Z.-Q. Jiang and L.-S. Liao, Adv. Funct. Mater., 2020, 30, 1910633 CrossRef CAS
.
- Y. Wang, J. H. Yun, L. Wang and J. Y. Lee, Adv. Funct. Mater., 2021, 31, 2008332 CrossRef CAS
.
- C. Poriel and J. Rault-Berthelot, Adv. Funct. Mater., 2021, 31, 2010547 CrossRef CAS
.
- C. Liu, J. Yuan, M. Gao, S. Tang, W. Li, R. Shi and A. Lei, Chem. Rev., 2015, 115, 12138–12204 CrossRef CAS PubMed
.
- Q.-Z. Zheng and N. Jiao, Chem. Soc. Rev., 2016, 45, 4590–4627 RSC
.
- Y. Yang, J. Lan and J. You, Chem. Rev., 2017, 117, 8787–8863 CrossRef CAS PubMed
.
- Y. Wei, P. Hu, M. Zhang and W. Su, Chem. Rev., 2017, 117, 8864–8907 CrossRef CAS PubMed
.
- Y.-F. Zhang and Z.-J. Shi, Acc. Chem. Res., 2019, 52, 161–169 CrossRef CAS PubMed
.
- B. Li, A. I. M. Ali and H. Ge, Chem, 2020, 6, 2591–2657 CAS
.
- T. Dalton, T. Faber and F. Glorius, ACS Cent. Sci., 2021, 7, 245–261 CrossRef CAS PubMed
.
- Y. Yang, Y. Wu, Z. Bin, C. Zhang, G. Tan and J. You, J. Am. Chem. Soc., 2024, 146, 1224–1243 CrossRef CAS PubMed
.
- G. Liao, T. Zhang, Z.-K. Lin and B.-F. Shi, Angew. Chem., Int. Ed., 2020, 59, 19773–19786 CrossRef CAS PubMed
.
- C.-X. Liu, W.-W. Zhang, S.-Y. Yin, Q. Gu and S.-L. You, J. Am. Chem. Soc., 2021, 143, 14025–14040 CrossRef CAS PubMed
.
- G. Liao, B. Li, H.-M. Chen, Q.-J. Yao, Y.-N. Xia, J. Luo and B.-F. Shi, Angew. Chem., Int. Ed., 2018, 57, 17151–17155 CrossRef CAS PubMed
.
- G. Liao, Q.-J. Yao, Z.-Z. Zhang, Y.-J. Wu, D.-Y. Huang and B.-F. Shi, Angew. Chem., Int. Ed., 2018, 57, 3661–3665 CrossRef CAS PubMed
.
- U. Dhawa, C. Tian, T. Wdowik, J. C. A. Oliveira, J. Hao and L. Ackermann, Angew. Chem., Int. Ed., 2020, 59, 13451–13457 CrossRef CAS PubMed
.
- H.-M. Chen, G. Liao, C.-K. Xu, Q.-J. Yao, S. Zhang and B.-F. Shi, CCS Chem., 2021, 3, 455–465 CrossRef CAS
.
- J. Zhang, J. Fan, Z. Guo, Y. Wu, J. Wu and M. Xie, Adv. Synth. Catal., 2022, 364, 3589–3599 CrossRef CAS
.
- G. Liao, H.-M. Chen, Y.-N. Xia, B. Li, Q.-J. Yao and B.-F. Shi, Angew. Chem., Int. Ed., 2019, 58, 11464–11468 CrossRef CAS PubMed
.
- R. L. de Carvalho, E. B. T. Diogo, S. L. Homölle, S. Dana, E. N. da Silva Júnior and L. Ackermann, Chem. Soc. Rev., 2023, 52, 6359–6378 RSC
.
- Z. Shi and F. Glorius, Chem. Sci., 2013, 4, 829–833 RSC
.
- CCDC 2303140 (1,4-d(mbp)-SBF), 2303142 (1-mtp-4-p-SBF) and 2303143 (1-pbp-4-p-SBF) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre.
- X. Tang, L.-S. Cui, H.-C. Li, A. J. Gillett, F. Auras, Y.-K. Qu, C. Zhong, S. T. E. Jones, Z.-Q. Jiang, R. H. Friend and L.-S. Liao, Nat. Mater., 2020, 19, 1332–1338 CrossRef CAS PubMed
.
- X.-Q. Wang, S.-Y. Yang, Q.-S. Tian, C. Zhong, Y.-K. Qu, Y.-J. Yu, Z.-Q. Jiang and L.-S. Liao, Angew. Chem., Int. Ed., 2021, 60, 5213–5219 CrossRef CAS PubMed
.
- A. Yoshii, Y. Onaka, K. Ikemoto, T. Izumi, S. Sato, H. Kita, H. Taka and H. Isobe, Chem.–Asian J., 2020, 15, 2181–2186 CrossRef CAS PubMed
.
- T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed
.
- E. Baranoff and B. F. E. Curchod, Dalton Trans., 2015, 44, 8318–8329 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, crystallographic data, photophysical performances, thermal of the compounds. CCDC 2303140 (1,4-d(mbp)-SBF), 2303142 (1-mtp-4-p-SBF), and 2303143 (1-pbp-4-p-SBF). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc02178a |
| This journal is © The Royal Society of Chemistry 2024 |