Open Access Article
Open Access ArticleEmerging high voltage V4+/V5+ redox reactions in Na3V2(PO4)3-based cathodes for sodium-ion batteries
Meng
Zhou†
a,
Xunzhu
Zhou†
bc,
Lin
Li
 *bcd,
Xiang
Chen
e,
Zhenan
Qiao
*bcd,
Xiang
Chen
e,
Zhenan
Qiao
 *d and
Shulei
Chou
*d and
Shulei
Chou
 *bc
*bc
aCollege of Chemical Engineering and Technology, Yantai Nanshan University, Yantai, Shandong 265713, China
bInstitute for Carbon Neutralization Technology, College of Chemistry and Materials Engineering, Wenzhou University, Wenzhou, Zhejiang 325035, China. E-mail: linli@wzu.edu.cn; chou@wzu.edu.cn
cWenzhou Key Laboratory of Sodium-Ion Batteries, Wenzhou University Technology Innovation Institute for Carbon Neutralization, Wenzhou, Zhejiang 325035, China
dState Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry, Jilin University, Changchun 130012, P. R. China. E-mail: qiaozhenan@jlu.edu.cn
eCollege of Textile Science and Engineering (International Institute of Silk), Zhejiang Sci-Tech University, Hangzhou 310018, PR China
First published on 1st May 2024
Abstract
Na3V2(PO4)3 (NVP) cathode materials with the advantages of long cycle life and superior thermal stability have been considered promising cathode candidates for SIBs. However, the unsatisfactory energy density derived from low theoretical capacity and operating voltage (3.35 V vs. Na+/Na, based on the V3+/V4+ redox couple) inevitably limits their practical application. Therefore, the activation of the V4+/V5+ redox couple (∼4.0 V vs. Na+/Na) in NVP-based cathode materials to boost the energy density of SIBs has attracted extensive attention. Herein, we first analyze the challenges of activation of the V4+/V5+ redox couple in NVP-based cathode materials. Subsequently, the recent achievement of NVP-based cathode materials with activated V4+/V5+ redox reactions for SIBs is overviewed. Finally, further research directions of high voltage V4+/V5+ redox reactions in NVP-based cathodes are proposed. This review provides valuable guidance for developing high energy density NVP-based cathode materials for SIBs.
1. Introduction
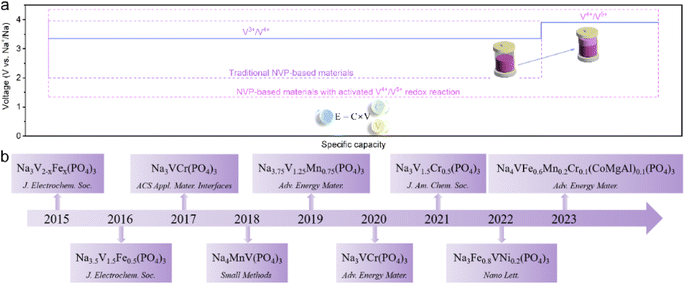
Since lithium-ion batteries (LIBs) were successfully commercialized by Sony in 1991, they have been widely applied in portable/mobile electronics (e.g. laptops, cell phones, etc.) and electric vehicle fields and exhibited a promising application in large-scale energy storage systems.1–3 However, an unfortunate fact is, the element lithium (Li) is rare (20 ppm) and unevenly distributed on our planet.4 Large-quantity applications of LIBs in EVs and large-scale energy storage systems will inevitably cause the depletion of lithium resources and will cause a steep increase in costs, which is unfavorable both ecologically and economically. Sodium, which shows similar physical and chemical properties to lithium, is the fifth-most abundant element in the earth's crust. The earth-abundant properties of sodium make sodium-ion batteries (SIBs) ideal supplements for LIBs, especially with great prospects for application in large-scale energy storage systems.5–9The working principle of SIBs is similar to that of LIBs. During the charging process, Na+ is released from the cathode and embedded into the anode through the electrolyte. In order to balance the charge, the electrons move from the external circuit to the anode. While during discharging, it is completely the opposite. The electrode material plays a key role in the electrochemical performance of SIBs. For the anode materials, hard carbons (HCs) with a low void, highly disordered structure, large layer space and achievable reversible capacity above 300 mA h g−1, are considered to be the most promising anode materials for SIBs and extensively applied both in laboratory research and the market.10,11 As for the cathode side, the widely studied materials of SIBs at present are layered transition metal (TM) oxides,12–15 polyanionic materials,16–19 Prussian Blue,20–22 and organic materials.23–25 Polyanionic materials, with a general formula of NaxMy(XaOb)zZw (where M represents one or more transition metals such as Ti, V, Cr, Mn, Fe, Co, Ni, etc.; X = Si, B, S, P, etc.; Z = F, OH, etc.), have been regarded as potential cathode materials for high performance SIBs due to the advantages of stable structure frameworks and superior safety.16,26,27
Among the reported polyanionic materials, NASICON-structured Na3V2(PO4)3 (NVP) became the most typical polyanionic material and received extensive attention in SIBs.28,29 Typically, the multivalent vanadium atom allows a consecutive redox reaction of V2+/V3+/V4+/V5+, with a considerable energy output. According to the previous reports, NVP generally undergoes a two-electron reaction (V3+/V4+) during the charge/discharge process, obtaining an operating voltage of 3.35 V (vs. Na+/Na) and a reversible capacity of 110 mA h g−1.30 It seems impossible for NVP to extract more than 2 Na+ to realize the reversible transition of the V4+/V5+ redox couple, accompanied by the phase transition from NaV2(PO4)3 to V2(PO4)3.31 Therefore, it is worth noting that the successful activation of the V4+/V5+ redox couple is of vital importance in the electrochemical performance of SIBs, for it not only gives an elevated electrochemical platform (∼4.0 V), but also offers additional capacity, and thus, a significantly enhanced energy density for NVP-based cathode materials (Fig. 1a).32,33 Until now, multiple advancements in the activation of the V4+/V5+ redox couple in NVP-based cathode materials have been achieved (Fig. 1b). To the best of our knowledge, there are currently no relevant reviews in this field.
In this review, we will focus on the cation substituted NVP-based cathodes with triggered V4+/V5+ redox reactions to improve the energy density of SIBs. We first introduced the challenges of NVP-based cathodes to achieve reversible activation of the V4+/V5+ redox couple. Then, we overviewed the recent achievements of NVP-based cathodes with activated V4+/V5+ redox reactions. Finally, future perspectives on activating V4+/V5+ redox reactions in NVP-based cathode materials for SIBs will also be highlighted.
2. Challenges of activation of the V4+/V5+ redox couple
According to the density functional theory (DFT) calculations, pure NVP exhibits a high V4+/V5+ redox potential of 4.64 V, which is beyond the electrochemical window of most electrolytes.34 Therefore, the V4+/V5+ redox reaction can barely be activated in Na3V2(PO4)3. In general, transition metal substitution was demonstrated as an effective strategy to activate the V4+/V5+ redox reaction in NVP-based cathode materials. However, for NASICON-structured NVP-based cathode materials with the activated V4+/V5+ redox couple, there still exist some challenges that limit their practical application, including low electronic conductivity and unsatisfactory structure reversibility at high voltage.35,36 In general, NASICON-structured NVP-based materials exhibit acceptable Na+ ionic conductivity due to their unique 3D open framework for fast ionic transfer. However, the intrinsically low electronic conductivity inevitably leads to sluggish electrochemical reaction kinetics, causing inferior rate performance. In addition, more Na+ intercalation/deintercalation into/from NVP-based cathode materials with the activated V4+/V5+ redox couple results in a relatively large volume change during the charge/discharge process, which leads to structure collapse and poor cycling stability. Therefore, it's important to explore NVP-based cathode materials with stable structure frameworks and high electronic conductivity for highly reversible activation of the V4+/V5+ redox couple.3. Recent achievement of activation of the V4+/V5+ redox couple
3.1 Single metal substitution
In 2015, Tirado's group first disclosed the V4+/V5+ redox reaction in NVP-based cathode materials.37 A series of Fe3+ substituted NVP (Na3V2−xFex(PO4)3, 0 ≤ x ≤ 0.5) materials was prepared through a simple sol–gel method. Fe-substituted materials (x ≥ 0.2) showed an obvious redox peak at ca. 4 V, corresponding to the V4+/V5+ redox reaction, which is demonstrated using the XPS and 57Fe Mössbauer spectra. Attempt on substitution of the V site with aliovalent Fe2+ to obtain sodium-rich compounds was first proposed by Yamada's group.38 The Na-rich composite Na3.5V1.53+Fe0.52+(PO4)3 delivers a capacity retention of 80% after 300 cycles. Subsequently, a novel sodium-deficient NASICON material Na3.41£0.59FeV(PO4)3 was applied in SIBs.39 Na3.41£0.59FeV(PO4)3 shows a high initial discharge capacity of 170 mA h g−1 with three electron reactions in the wide voltage of 1.5–4.4 V. In addition, Na3.41£0.59FeV(PO4)3 shows a single-phase mechanism with a small volume change of 3.44% during the charge/discharge process, which is responsible for its superior cycling stability. Recently, Hu's group prepared various carbon-coated Na-rich Na3+xV2−xFex(PO4)3 (NVFP-x, where x = 0, 0.2, 0.4, 0.6, 0.8 and 1.0) cathodes for SIBs; Fe2+/Fe3+ (2.45 V), V3+/V4+ (3.35 V), and V4+/V5+ (3.95 V) redox reactions were observed (Fig. 2a and b).40 As shown in Fig. 2c, NVFP-0.4 has a highest actual capacity of ∼133 mA h g−1 due to its highly reversible V4+/V5+ (3.95 V) redox reactions. Noticeably, they uncovered that the relative contents of Na1 and Na2 in the structure play an important role in the reversibility of V4+/V5+ redox couples, and enough Na2 content is necessary to enable reversible activation (Fig. 2d and e). The Fe substitution is closely related to the Na content at the Na2 site, and the Na1/Na2 ratio reached a minimum value when x = 0.4. Moreover, they proposed that the Na+ ion extraction from NVFP-0.4 during the Fe2+/Fe3+ redox reaction at low-voltage can act as the Na compensation agent in the NVFP-0.4‖HC full cells. As a result, the NVFP-0.4‖HC full cells show an improved energy density and rate performance, compared with NVP‖HC full cells (Fig. 2f and g). The high efficiency, simple operation, and easily scalable feature of the Na self-compensation strategy can effectively promote the practical application of SIBs.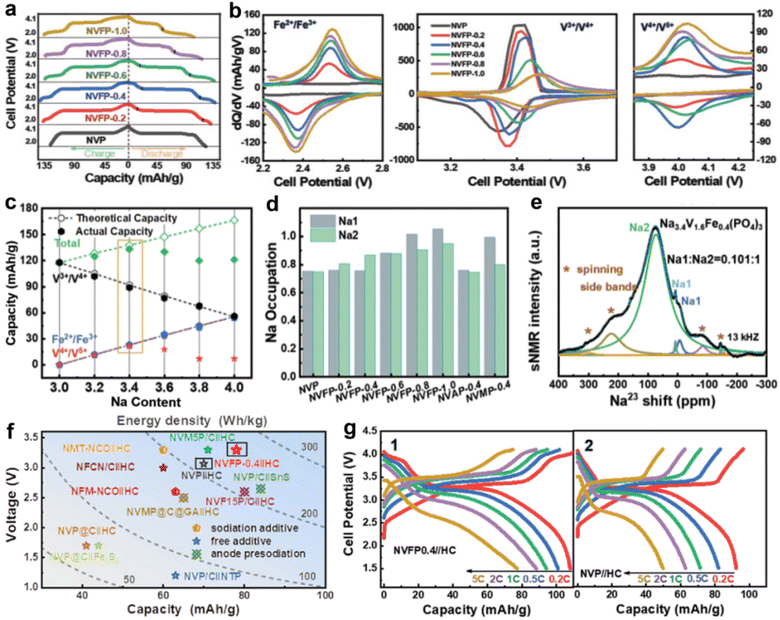 | ||
| Fig. 2 (a) The charge/discharge curves of pure NVP and NVFP-x (0 < x ≤ 1). (b) dQ/dV curves of Fe2+/Fe3+, V3+/V4+, and V4+/V5+ redox reactions in pure NVP and Na-rich NVFP-x samples. (c) Comparison of the actual capacity and theoretical capacity of NVFP-x samples with different Na contents. (d) The Na occupations of the NVFP-x family according to XRD refinement. (e) The fitted 23Na ss-NMR spectra of NVFP-0.4. (f) Comparison of the energy density of the state-of-the-art full cells for NIBs. (g) The charge/discharge curves of NVFP-0.4‖HC (g1) and NVP‖HC (g2) at different rates from 0.2 to 5C. Reproduced with permission.40 Copyright 2022, Wiley-VCH. | ||
Most recently, Na3.5V1.5Fe0.5(PO4)3 was reported by Liang's group, in which the reversible V4+/V5+ redox reaction was activated with a capacity of 148.2 mA h g−1 and enabled an improved energy density.41 A highly reversibly chemical state evolution of V and Fe (Fe2+/Fe3+, V3+/V4+, and V4+/V5+) during the charge/discharge process was demonstrated by XPS (Fig. 3a). Noticeably, they proposed that the reversible V4+/V5+ redox reaction was dependent on the unpaired electrons of Fe in the 3d orbital (Fig. 3b and c). As shown in Fig. 3d and e, Na3.5V1.5Fe0.5(PO4)3 shows a lower forbidden bandgap of 1.05 eV than that of Na3V2(PO4)3, indicating its improved electronic conductivity. In addition, Na3.5V1.5Fe0.5(PO4)3 exhibits a low Na+ migration energy barrier and fast Na+ diffusion kinetics. Therefore, Na3.5V1.5Fe0.5(PO4)3 shows an obvious advantage in terms of energy density (501 W h kg−1) and rate performance. More importantly, the superior electrochemical performance of Na3.5V1.5Fe0.5(PO4)3‖HC demonstrated the potential of Na3.5V1.5Fe0.5(PO4)3 for practical application (Fig. 3f). This work could significantly accelerate the commercialization of sodium-ion batteries.
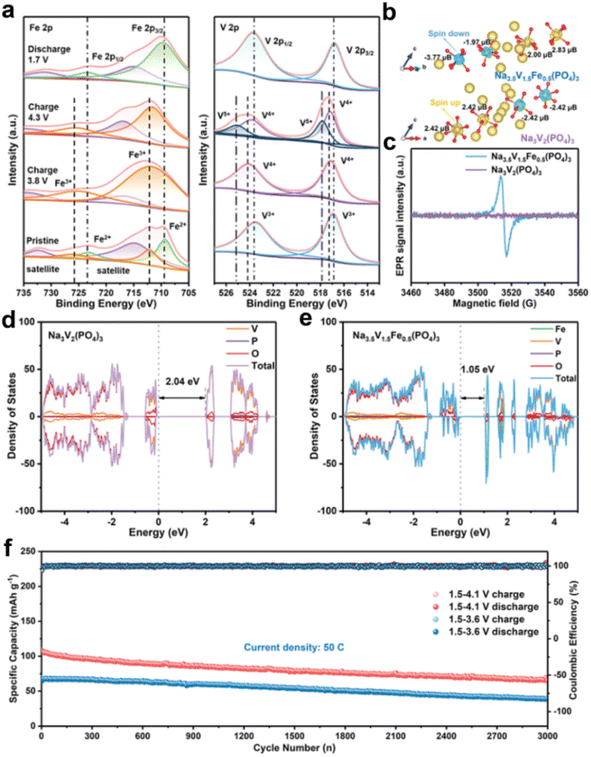 | ||
| Fig. 3 (a) XPS spectra of Fe and V at different electrochemical states for the Na3.5V1.5Fe0.5(PO4)3 electrode. (b) The comparative electronic spin states and (c) EPR spectra of Na3.5V1.5Fe0.5(PO4)3 and NVP. (d and e) DFT calculations of total DOS and the selected element DOS of (d) NVP and (e) Na3.5V1.5Fe0.5(PO4)3. (f) Long-term cycling stability of the HC‖NVFP full cells. Reproduced with permission.41 Copyright 2023, Wiley-VCH. | ||
The electrochemical performance of sodium-rich compounds of Na4MnV(PO4)3 and Na4NiV(PO4)3 was investigated by Goodenough's group.42 However, no V4+/V5+ redox reaction was observed in Na4MnV(PO4)3 due to the low cut-off voltage of 3.8 V, which needs to be further explored in a wide voltage range. Noticeably, the Ni2+ and V4+ in Na4NiV(PO4)3 were partially oxidized to Ni3+ and V5+ after charging to 4.2 V. Subsequently, Masquelier's group investigated the electrochemical behavior of Na4MnV(PO4)3 at a high cutoff voltage of 4.3 V.36 Na4MnV(PO4)3 delivers a high charge capacity of ∼156 mA h g−1 with electrochemical oxidation of the V4+ state to the V5+ state. Unfortunately, operando X-ray diffraction revealed a irreversible structure evolution during the charge/discharge process, which resulted in an unsatisfactory cycling stability (Fig. 4a). Subsequently, Ghosh et al. performed a comprehensive study to understand the role of Mn2+ in NVP.43 They found that the electrochemical performance of Mn-substituted NVP can be significantly improved by modulating electronic and crystal structures. Na3.75V1.25Mn0.75(PO4)3 with an optimized bottleneck size (≈5 Å2) and modulated V- and Mn-redox centers show highly reversible redox processes that enable a better rate performance and cycling stability than that of Na4MnV(PO4)3 (Fig. 4b–e).
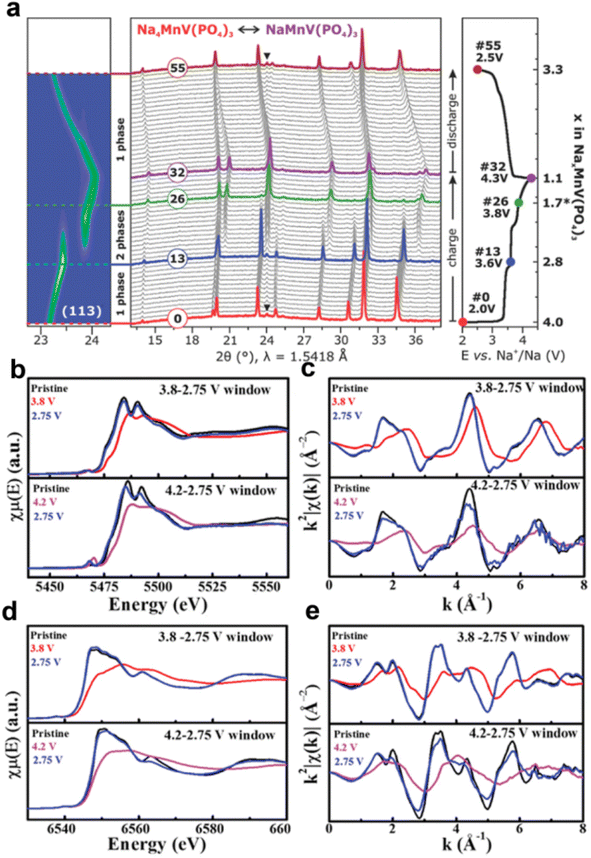 | ||
| Fig. 4 (a) Operando XRD patterns of Na4MnV(PO4)3 during the first cycle. Reproduced with permission.36 Copyright 2019, Wiley-VCH. (b and d) Ex situ XAS of Na3.75V1.25Mn0.75(PO4)3 cathodes at different states and the normalized absorption spectra at V- and Mn-K edges. (c and e) k2-Weighted χ(k) signals collected at V-K and Mn-K edges. Reproduced with permission.43 Copyright 2020, Wiley-VCH. | ||
The Mg-substitution strategy was proposed by Okada and coworkers; Na3+xV2−xMgx(PO4)3 (x = 0.1 to 0.7) was synthesized as a cathode material for SIBs.44 Among these, Na3.2V1.8Mg0.2(PO4)3 demonstrated a higher capacity (122 mA h g−1) than undoped Na3V2(PO4)3 (117.6 mA h g−1) because of the successful activation of the V5+/V4+ redox couple. In contrast, Na3.5V1.5Mg0.5(PO4)3 shows a low discharge capacity due to the poor reversibility of the V5+/V4+ redox couple, which derived from the irreversible change in the crystal structure when charged to 4.3 V.
The activation of the V4+/V5+ redox couple in Cr3+ substituted NVP was first revealed by Lavela's group.45 Subsequently, Yang's group investigated the electrochemical sodium storage behaviors of Na3VCr(PO4)3 (NVCP) by a series of advanced characterization techniques such as ex situ51V solid-state nuclear magnetic resonance, X-ray absorption near-edge structure, and in situ X-ray diffraction.46 They proposed that the poor cycling stability of NVCP at a high cutoff voltage of 4.3 V at 30 °C originates from the irreversible structure evolution and unrecovered local environment of vanadium atoms. Noticeably, decreasing the testing temperature was demonstrated as an effective way to suppress the irreversible structure evolution and boost the electrochemical performance. NVCP exhibits improved cycling stability (a capacity retention of 92% after 200 cycles) at −15 °C. The structural degradation mechanism of NVCP was further explored by combining with in situ XRD, ex situ X-ray absorption fine structure (XAFS) at the V-K edge, ex situ soft X-ray absorption spectroscopy (sXAS) at the V-L edge and atomically resolved electron microscopy.35 They found that the V migration during deep Na+ extraction induces irreversible structural transformation of NVCP at 30 °C (Fig. 5a). Therefore, relieving diffusion of V atoms by decreasing the operating temperature is an effective method to improve the structural stability of NVCP (Fig. 5b). In addition, they proposed a more practical strategy to boost the sodium storage performance of NVCP. The collapsed structure can be restored by room temperature low voltage discharging (<1.7 V), which can make dislocated V atoms return to their original lattice. Therefore, the capacity decay of NVCP was suppressed in a wide voltage range of 1.7–4.3 V (Fig. 5c).
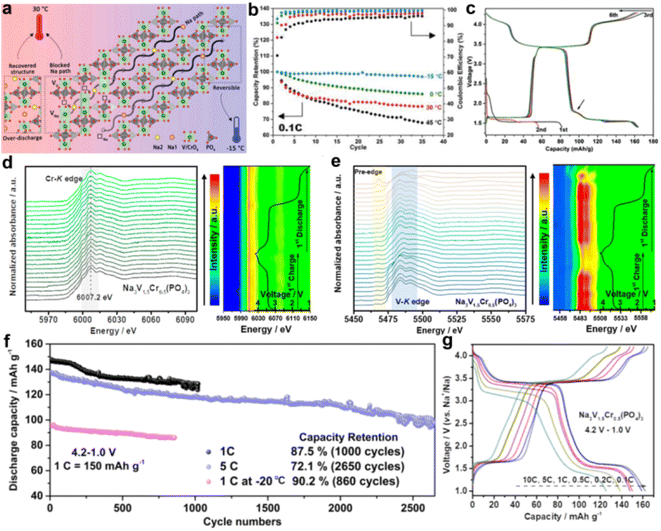 | ||
| Fig. 5 (a) Schematic graphical illustration of the V migration in Na2−xVCP at 30 °C and −15 °C. (b) Charge/discharge capacity of Na3VCr(PO4)3 (NVCP) electrodes cycled between 2.5 and 4.3 V at different temperatures at 0.1C. (c) Cycling results of the over-discharge method at 0.1C at 30 °C. First discharged to 1.4 V, cycled between 1.4 and 2.5 V in the second cycle, and then 1.4–4.3 V in subsequent cycles. Reproduced with permission.35 Copyright 2021, Wiley-VCH. (d) Operando XANES spectra and 2D contour plot at the Cr-K edge and (e) V-K edge of the Na3V1.5Cr0.5(PO4)3 electrode for the first cycle. (f) Long-term cycling stability of the Na3V1.5Cr0.5(PO4)3 electrode at 1C (at room temperature and at −20 °C) and 5C. (g) Galvanostatic charge/discharge curves of the Na3V1.5Cr0.5(PO4)3 electrode at different rates. Reproduced with permission.47 Copyright 2021, American Chemical Society. | ||
Na3V1.5Cr0.5(PO4)3 was reported by Goodenough and co-workers, which delivers a high capacity of 150 mA h g−1 with reversible three-electron redox reactions (V2+/V3+ at 1.6 V, V3+/V4+ at 3.6 V, and V4+/V5+ at 4.1 V) and an excellent cycling stability (96% capacity retention after 400 cycles).32 Benefiting from the potential difference between the three redox couples in Na3V1.5Cr0.5(PO4)3, a symmetric sodium-ion battery was assembled. The symmetric cell maintained a specific capacity of ∼100 mA h g−1 after 200 cycles and a capacity of 75 mA h g−1 at 1 A g−1. The structural and electrochemical behaviors of Na3V1.5Cr0.5(PO4)3 were comprehensively investigated by Kang's group.47 The unique solid-solution reaction of Na3V1.5Cr0.5(PO4)3 was demonstrated by operando XRD. As shown in Fig. 5d and e, the V4+/V5+ redox couple was activated in Na3V1.5Cr0.5(PO4)3, and the Cr is maintained electrochemically inactive during the charge/discharge process. The Cr substitution achieves a lower sodium diffusion barrier of 0.311 eV and a smaller forbidden bandgap of 1.98 eV, compared with NVP. Benefiting from the above merits, Na3V1.5Cr0.5(PO4)3 delivers a high reversible capacity (163.2 mA h g−1 at 0.1C), long cycle life (a capacity retention of 72.1% even after 2650 cycles, Fig. 5f), superior rate performance (∼127 mA h g−1 at 10C, Fig. 5g) and good low-temperature performance (a reversible capacity of 97.5 mA h g−1 at −20 °C). More importantly, they uncovered that the unpaired 3d orbital electrons in Cr play a key role in triggering the V4+/V5+ redox couple. The fast-charging performance of Na3V1.5Cr0.5(PO4)3 was explored by Lai's group.48 They designed a reduced graphene oxide supported Na3V1.5Cr0.5(PO4)3 cathode (VC/C-G) for fast sodium storage, which delivers a high specific capacity of 176 mA h g−1 and long cycle life. Noticeably, VC/C-G also exhibits an outstanding fast-charging performance (a short time of ∼11 min to realize 80% state of charge).
Recently, V/Cr solid-solution MXene was employed as a precursor to prepare Cr-substituted NVP (Na3V1.625Cr0.375(PO4)3) by a facile in situ reactive transformation strategy (Fig. 6a).49 An extra voltage plateau of the V4+/V5+ redox couple at 4.02/3.98 V was observed in Na3V1.625Cr0.375(PO4)3. They found that the Na+ ordering at Na2 sites was changed by Cr substitution, which induces an intermediate phase during the charge/discharge process and decreases the Na+ migration energy barriers. The Na+ diffusion coefficient of Na3V1.625Cr0.375(PO4)3 was significantly improved. As a result, the Na3V1.625Cr0.375(PO4)3 electrode delivers smaller polarization (40 mV, Fig. 6b), outstanding rate performance (78.0 mA g−1 at 200C, Fig. 6c), long cycle life (a capacity retention of 71.5% after 1500 cycles, Fig. 6d), and high energy density (410 W h kg−1) and power density (68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 975 W kg−1). Most recently, a Na3V4/3Cr2/3(PO4)3 hollow microsphere was prepared by spray drying, which shows a high discharge capacity of 175 mA h g−1 and excellent cycling performance.50
975 W kg−1). Most recently, a Na3V4/3Cr2/3(PO4)3 hollow microsphere was prepared by spray drying, which shows a high discharge capacity of 175 mA h g−1 and excellent cycling performance.50
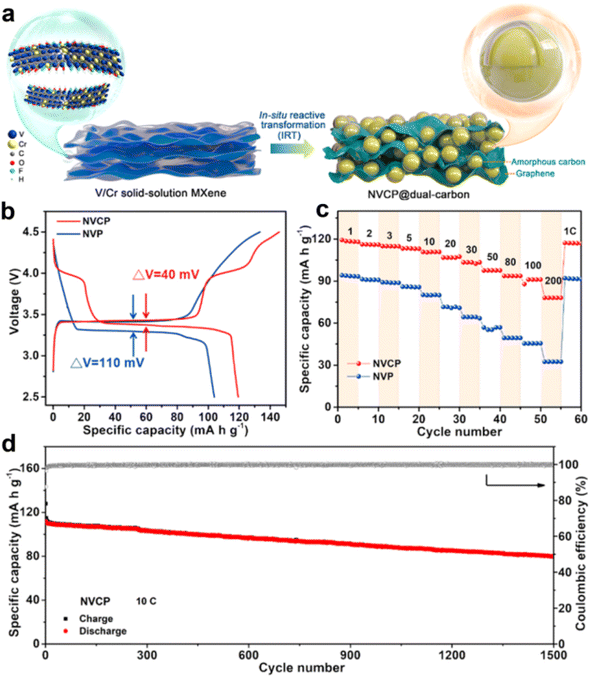 | ||
| Fig. 6 (a) Schematic representation of the in situ reactive transformation of MXene to Na3V1.625Cr0.375(PO4)3@dual-carbon architectures. (b) The charge/discharge curves of NVP and Na3V1.625Cr0.375(PO4)3. (c) Comparison of rate capability of Na3V1.625Cr0.375(PO4)3 and NVP. (d) Long-term cycling stability of NVCP. Reproduced with permission.49 Copyright 2022, American Chemical Society. | ||
The aluminum substituted NVP with the activated V4+/V5+ redox couple was first reported by Masquelier's group.51 Benefiting from the lighter molecular weight of Al and high operating potential of the V4+/V5+ redox couple, the Na3Al0.5V1.5(PO4)3 achieves an improved energy density of 424.6 W h kg−1. Subsequently, Lavela designed a carbon-loaded Na3V1.8Al0.2(PO4)3 as a cathode material for SIBs.52 The GITT result reveals the fast electrochemical reaction kinetics of Na3V1.8Al0.2(PO4)3. Therefore, Na3V1.8Al0.2(PO4)3 delivers a superior rate performance. Similarly, Criado et al. prepared a carbon-coated Na3VAl(PO4)3 by a single and easily scalable sol–gel route.53 Unfortunately, the V4+/V5+ redox couple in Na3VAl(PO4)3 showed an unsatisfactory reversibility.
In 2019, Chen et al. synthesized a series of graphene-like graphitic layer decorated Na3V2−xGax(PO4)3 (x = 0, 0.1, 0.2, 0.4, and 0.6) materials via a solid-state reaction method.54 Noticeably, an impurity of Ga(PO3)3 was generated when x reached 0.6. Na3V2−xGax(PO4)3 (x = 0.1, 0.2 and 0.4) exhibited two redox peaks at 3.4 V and 4 V, which were assigned to V3+/V4+ and V4+/V5+ redox couples. Na3V1.6Ga0.4(PO4)3 shows dramatically improved rate performance and cycling stability, a reversible capacity of 80.6 mA h g−1 at 50C, and a capacity retention of 97.3% after 1000 cycles. More importantly, Na3V1.6Ga0.4(PO4)3 delivers a high energy density (355.5 W h kg−1) and power density (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 075 W kg−1 at 50C). They disclosed that the Ga3+ substitution also benefits the enhancement of the proportion of sp2-type carbon and boost the rate performance. Similarly, Zheng's group also demonstrated that the reversible activation of V4+/V5+ can be realized by introducing Ga3+ in NVP.55
075 W kg−1 at 50C). They disclosed that the Ga3+ substitution also benefits the enhancement of the proportion of sp2-type carbon and boost the rate performance. Similarly, Zheng's group also demonstrated that the reversible activation of V4+/V5+ can be realized by introducing Ga3+ in NVP.55
The reason for the different potentials of the V4+/V5+ redox couple in Na3VM(PO4)3 (M = Ga or Al) and Na3V2(PO4)3 was investigated by Jin's group.31 They reveal that the potentials of the V4+/V5+ redox couple were closely related to the crystallographic sites of sodium ion extraction/insertion. Sodium ions were extracted from the Na2 site in Na3VM(PO4)3, while it was from the Na1 site in Na3V2(PO4)3. Therefore, Na3VM(PO4)3 showed lower potentials of the V4+/V5+ redox couple than that of Na3V2(PO4)3. In addition, they found that the excessive diffusion activation energy in Na3VM(PO4)3 was responsible for the limited V4+/V5+ redox reaction, therefore, a breakthrough can be realized by smaller-sized cation substitution for V3+.
Noticeably, the effect of alkali metal substitution (Li, Na, and K) on the electrochemical performance of NVP-based materials was recently investigated by Wu's group.56 They found that the trace of alkali metal substitute V can effectively improve electronic conductivity and speed up ionic diffusion. Meanwhile, the V4+/V5+ redox reaction was partially activated with an improved reversible capacity. Compared with Na3V1.92Li0.08(PO4)3 and Na3V1.92Na0.08(PO4)3, Na3V1.94K0.06(PO4)3 (NVP-K0.06) shows a higher reversible capacity, superior cycling stability and outstanding rate performance. More importantly, NVP-K0.06 shows a good temperature tolerance, and can operate in a wide temperature range of −30–55 °C.
3.2 Bimetal substitution
The bimetal substitution strategy for NVP-based materials in SIBs was reported by Wu's group; Fe and Ni were substituents for V to obtain Na3Fe0.8VNi0.2(PO4)3.57 Na3Fe0.8VNi0.2(PO4)3 with the activated V4+/V5+ redox reaction showed a high initial discharge capacity of 163.6 mA h g−1, but suffer from rapid capacity fading (only 22.9 mA h g−1 after 50 cycles) due to its poor structural reversibility in a wide voltage range. They also proposed that the Na+ at the Na2 site determines the reversibility of the V4+/V5+ redox reaction and the cycling stability of Na3Fe0.8VNi0.2(PO4)3 can be improved by adjusting the charging–discharging mode in order to have enough Na+ in the Na2 site. Subsequently, Wang et al. designed a double-carbon-layer hierarchical structure Na3.5VMn0.5Cr0.5(PO4)3@C/rGO (VMC@C/rGO) for SIBs.58 The unique double-carbon-layer hierarchical structure significantly improves the electronic conductivity and structural stability of VMC@C/rGO, enabling a reversible V4+/V5+ redox reaction. The hierarchical VMC@C/rGO electrode realized a 2.4-electron redox reaction with a high reversible capacity (136 mA h g−1), capacity retention (81% after 8000 cycles at 20C) and energy density (472 W h kg−1). The VMC@C/rGO‖HC full cells also exhibited superior electrochemical performance, suggesting that the VMC@C/rGO can be a promising cathode candidate for SIBs.Recently, Liu's group reported a low-strain Na3.5Fe0.5VCr0.5(PO4)3 (NFVCP) for SIBs, which achieves a multi-electron reaction (2.61 electrons) based on Fe2+/Fe3+, V3+/V4+/V5+, and Cr3+/Cr4+ redox couples.59 As shown in Fig. 7a and b, an unexpected reversible release/uptake of the Na+ in the Na1 site was observed during the charge/discharge process. In addition, in situ XRD reveals that the NFVCP undergoes a highly reversible bi-phase and solid-solution reaction with a small volume change of 3.87%. NFVCP also exhibits a low migration energy barrier of 0.23–0.46 eV and a narrow band gap of 0.917 eV (Fig. 7c–e). Therefore, NFVCP shows a high reversible capacity of 148.5 mA h g−1 and good capacity retention of 95.1% after 2000 cycles. The potential of NFVCP for practical application was further demonstrated by the superior electrochemical performance of NFVCP‖HC full cells. NFVCP‖HC full cells delivered a good rate capability (70.9 mA h g−1 at 30C), low-temperature performance (77.2% of its room-temperature capacity at −20 °C) and cycling stability (a capacity retention of 90.9% after 100 cycles, Fig. 7f). More importantly, NFVCP‖HC full cells exhibit a high energy density of ∼318.2 W h kg−1 (Fig. 7g).
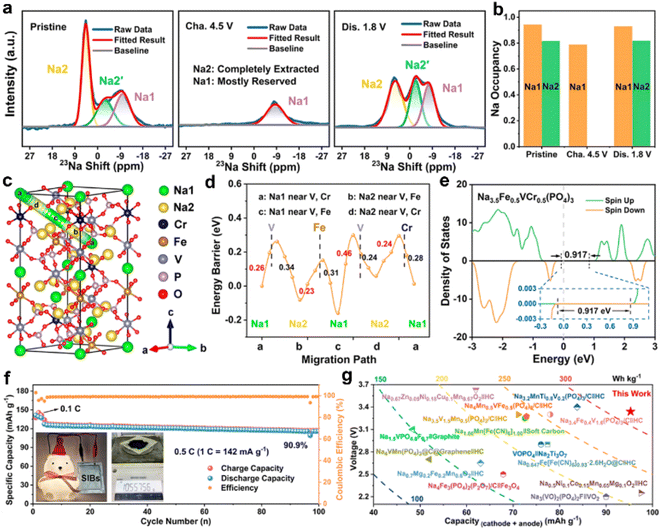 | ||
| Fig. 7 (a) Ex situ23Na ss-NMR spectra and (b) Na occupancy results of NFVCP at various electrochemical states. (c) DFT calculations of the schematic diagram of the optimum Na+ migration path in the NFVCP lattice and (d) corresponding migration energy barriers. (e) The total density of states of NFVCP. (f) Cycling stability of the NFVCP‖HC full cell. Insets show the mass production of the NFVCP cathode material and the LED bulb powered by the pouched full cell. (g) Comparison of voltage, capacity, and energy density between the NFVCP‖HC full cell and those of the state-of-the-art Na-ion full cells. Reproduced with permission.59 Copyright 2023, American Chemical Society. | ||
3.3 Regulation of conformational entropy
In general, the increase of conformational entropy induces many possible combinations of interaction that can boost the crystal structure stability and electrochemical performance.60–63 The conformational entropy ≥1.5R (where R is the molar gas constant) is referred to as high-entropy.64 Materials with conformational entropy ranging from 1R to 1.5R are classified as medium-entropy, while those below 1R are termed low-entropy. Zhao et al. applied the concept of high entropy and constructed NASICON-type Na3VAl0.2Cr0.2Fe0.2In0.2Ga0.2(PO4)3 (NVMP) as a cathode material for SIBs (Fig. 8a and b).65 A highly reversible V4+/V5+ redox reaction and structural evolution with a volume change of 1.1% during the charge/discharge process were realized in NVMP. Accordingly, the NVMP cathode offers a high reversible specific capacity of 102 mA h g−1 and a good rate performance (71 mA h g−1 at 20C). In addition, the NVMP cathode delivers superior cycling stability in a wide temperature range (capacity retention of 86.8% after 5000 cycles at room temperature (Fig. 8c) and a capacity retention of ∼94.2% after 1000 cycles at −20 °C). The NVMP‖HC full cells showed a capacity retention of 93% after 50 cycles, indicating the potential of NVMP cathode materials for practical application. Similarly, Hou's group realized excellent rate capability at high voltage, and good ionic and electronic conductivity by the intrinsic effect of multiple metals in the as-prepared cathode material Na4VFe0.6Mn0.2Cr0.1(CoMgAl)0.1(PO4)3 (NVFP-HE).66 In addition, in situ XRD revealed that the high entropy NVFP-HE showed a highly reversible structure evolution at high voltage, therefore enabling NVFP-HE to deliver superior cycling stability (a capacity retention of 66.7% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles).
000 cycles).
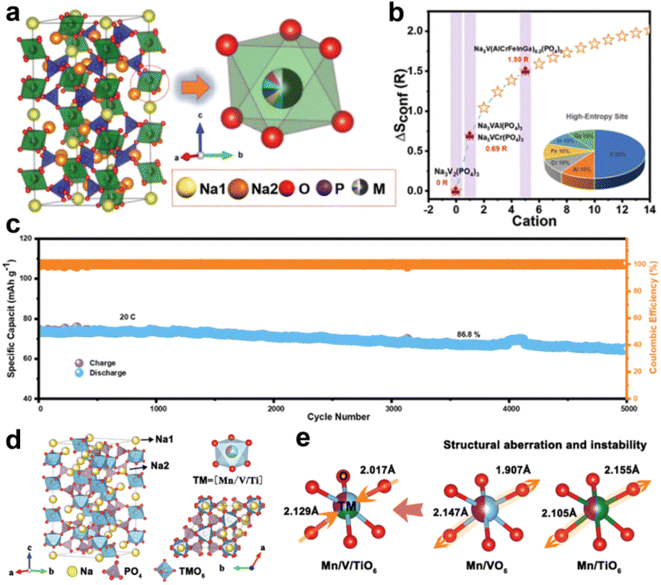 | ||
| Fig. 8 (a) Schematic sketch of the NVMP crystal structure. (b) Dependence of configurational entropy on the number of cation elements. (c) Long-term cycling performance of the NVMP cathode electrode. Reproduced with permission.65 Copyright 2023, Wiley-VCH. (d) Visualization of the crystal structure and atomic occupation of ME-NMVTP. (e) Magnifications of the TMO6 octahedron structure in ME-NMVTP cathode materials. Reproduced with permission.67 Copyright 2023, Wiley-VCH. | ||
The medium-entropy Na3Mn2/3V2/3Ti2/3(PO4)3/C@CNTs (ME-NMVTP) cathode for SIBs was reported by Wang's group (Fig. 8d and e).67 Various redox reactions of V2+/V3+, Ti3+/Ti4+, V3+/V4+, Mn2+/Mn3+, V4+/V5+, and Mn3+/Mn4+ were observed in ME-NMVTP, which achieved a high reversible capacity of 147.9 mA h g−1. Benefiting from the synergistic effect of the three transition-metal elements, ME-NMVTP showed improved electronic conductivity, accelerated Na+ diffusion coefficient and superior structural stability with a small volume change. Both the half cell and ME-NMVTP‖HC full cell exhibited superior rate performance and cycling stability, suggesting that ME-NMVTP‖HC is a promising cathode candidate for SIBs. Similarly, Zhao's group applied a medium-entropy NASICON-structure cathode Na3.5V0.5Mn0.5Fe0.5Ti0.5(PO4)3 (Me-NVMP) for SIBs.68In situ XRD discloses that the Me-NVMP shows a solid-solution-type Na+ storage behavior. In addition, Me-NVMP can effectively inhibit the undesirable Jahn–Teller distortion and irreversible structural evolution at high voltage. Therefore, Me-NVMP shows a high reversible capacity (165.8 mA h g−1) and superior cycling stability (a high capacity retention of 83.5% even after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles).
000 cycles).
Most recently, Chou's group proposed a strategy that combines high-entropy substitution and electrolyte optimization to boost the reversible multielectron reactions of NVP.69In situ X-ray absorption near-edge structure spectra and in situ XRD demonstrated the high reversibility of the V5+/V4+ redox couple and crystalline structure evolution. Therefore, Na3.32V1.6Cr0.08Fe0.08Mn0.08Mg0.08Ca0.08(PO4)3 (HE-V1.6) delivers a high capacity retention of 93.1% after 2000 cycles. Meanwhile, HE-V1.6 shows a good temperature tolerance; it maintains a stable operation in a wide temperature range (−20 to 60 °C). More importantly, the HE-V1.6‖natural graphite full cell was assembled to demonstrate the potential of HE-V1.6 for practical application, which shows a high power density and long cycle life. These results reveal that HE-V1.6 is a promising candidate for high-performance SIBs.
4. Conclusions and perspectives
NVP-based cathode materials with activated V4+/V5+ redox reactions have been considered as promising candidates for high energy density SIBs. In this review, we systematically discuss the emerging high voltage V4+/V5+ redox reaction in NVP-based cathodes, including their faced challenges and recent achievements. In general, the NVP-based cathode material with the activated V4+/V5+ redox reaction suffers from low electronic conductivity and inferior structure reversibility at high voltage. Meanwhile, transition metal substitution was demonstrated as an effective strategy to activate the V4+/V5+ redox reaction in NVP-based cathode materials. To better understand the superiority of the NVP-based cathode material with the activated V4+/V5+ redox reaction, the electrochemical performance of some representative materials is summarized in Table 1.| Classification | Materials | Voltage range (V versus Na+/Na) | Initial discharge capacity (mA h g−1)/current density (mA g−1) | V4+/V5+ contribution in total capacity/% | Rate performance (mA h g−1)/current density (mA g−1) | Cycling stability (capacity retention %/cycles) | Ref. |
|---|---|---|---|---|---|---|---|
| Single metal substitution (Fe) | Na3.5V1.5Fe0.5(PO4)3 | 2.0–4.2 | ∼90/0.5C | ∼11.1 | ∼30/10C | 80/300 | 38 |
| Na3.4V1.6Fe0.4(PO4)3 | 2.0–4.1 | 133/0.5C | ∼16.9 | ∼113/20C | 96/2000 | 40 | |
| Na3.5V1.5Fe0.5(PO4)3 | 1.7–4.3 | 148.2/0.5C | ∼6.7 | 73.5/100C | 72/500 | 41 | |
| Single metal substitution (Cr) | Na3V1.5Cr0.5(PO4)3 | 1.0–4.4 | ∼150/30 | ∼20 | ∼35/1000 | 96/400 | 32 |
| Na3V1.5Cr0.5(PO4)3 | 1.0–4.2 | 163.2/0.1C | ∼9.2 | ∼120/10C | 72.1/2650 | 47 | |
| Na3Cr0.5V1.5(PO4)3 | 176/0.2C | ∼8.5 | 94.8/50C | ∼50/1000 | 48 | ||
| Na3V1.625Cr0.375(PO4)3 | 2.5–4.5 | 122.9/1C | ∼16.3 | 78.0/200C | 93.4/300 | 49 | |
| Na3V4/3Cr2/3(PO4)3 | 1.5–4.4 | 175/100 | ∼14.3 | 94/5000 | 86/2000 | 50 | |
| Single metal substitution (Ga) | Na3V1.6Ga0.4(PO4)3 | 2.3–4.3 | 105.8/0.1C | ∼14.2 | 80.6/50C | 97.3/1000 | 54 |
| Na3V1.25Ga0.75(PO4)3 | 1.4–4.2 | 152.3/1C | 58.9/40C | 84.52/600 | 55 | ||
| Single metal substitution (Ni) | Na4NiV(PO4)3 | ∼80/1C | 70/5C | ∼83/500 | 42 | ||
| Single metal substitution (K) | Na3V1.94K0.06(PO4)3 | 2.0–4.0 | 120.3/0.1C | 100.5/20C | 99.1/3000 | 56 | |
| Single metal substitution (Mn) | Na3.75V1.25Mn0.75(PO4)3 | 2.75–4.2 | 100/1C | 95/2C | 61/50 | 43 | |
| Na3.5VMn0.5Cr0.5(PO4)3 | 2.0–4.2 | 136/0.2C | 61.4/20C | 94.7/1600 81/8000 | 58 | ||
| Bimetal substitution | Na3.5Fe0.5VCr0.5(PO4)3 | 1.8–4.5 | 148.5/0.1C | ∼10.1 | 88.3/50C | 95.1/2000 | 59 |
| Na3Mn2/3V2/3Ti2/3(PO4)3 | 1.5–4.3 | 147.9/50 | 57.7/500 | 88.3/1000 | 67 | ||
| Na3Fe0.8VNi0.2(PO4)3 | 1.3–4.2 | 161.8/0.1C | ∼9.3 | 63.1/20C | 71.2/200 | 57 | |
| Regulation of conformational entropy | Na4VFe0.6Mn0.2Cr0.1(CoMgAl)0.1(PO4)3 | 1.5–4.1 | 141.98/1C | 85.77/50C | 66.7/10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
66 | |
| Na3VAl0.2Cr0.2Fe0.2 In0.2Ga0.2(PO4)3 | 2.5–4.4 | 102/0.1C | ∼22 | 71/20C | 86.8/5000 | 65 | |
| Na3.32V1.6Cr0.08Fe0.08Mn0.08Mg0.08Ca0.08(PO4)3 | 1.2–4.2 | 152.3/50 | 122.3/5000 | 93.1/2000 | 69 | ||
| Na3.5V0.5Mn0.5Fe0.5Ti0.5(PO4)3 | 1.8–4.4 | 165.8/0.1C | 69/100C | 83.5/10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
68 |
To achieve the practical application of NVP-based cathode materials with activated V4+/V5+ redox reactions, we suggested that further research should be focused on the following aspects. First, the inert or low redox potential of transition metal substitution inevitably leads to a decrease in the number of active redox centers or operating voltage, resulting in an unsatisfactory energy density. Artificial intelligence can be used as a suitable tool to screen novel high-performance NVP-based cathode materials with activated V4+/V5+ redox reactions to boost the energy density of SIBs. Second, the electrode/electrolyte interphase plays a key role in the electrochemical performance of high-voltage electrode materials, exploring an electrolyte with good compatibility to construct a robust electrode/electrolyte interphase and improve sodium storage performance is very meaningful. Third, the intrinsic low electronic conductivity and poor structural reversibility of NVP-based cathode materials inevitably limit their electrochemical performance. Therefore, in order to elevate electronic conductivity and alleviate volume change, the development of NVP-based cathode materials with high conductivity coating layers and unique morphology might be a significant approach. Fourth, exploring the effects of composition on electrochemical performance is of vital importance. Fifth, the mechanism of activating V4+/V5+ redox reactions in NVP-based electrode materials is not clear; it is necessary to get further understanding of the mechanism by both advanced in situ characterization techniques and theoretical calculations. Finally, the potential of cathode materials for SIBs should be evaluated in full cells and pouch cells.
Author contributions
M. Z. and X. Z. performed the literature search, analyzed the published results, and wrote the manuscript. X. C. structured this review. L. L., Z. Q. and S. C. provided key advice and supervised the preparation of the text.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (52202286, 22309002, 52250710680, and 52171217), Natural Science Foundation of Zhejiang Province (LY24B030006), High-end Foreign Experts Recruitment Plan of China (G2023016009L), Key Research and Development Program of Zhejiang Province (2023C011232, 2024C01057), Science and Technology Project of State Grid Corporation of China (5419-202158503A-0-5-ZN), Basic Research Project of Wenzhou City (G20220016), and Science and Technology Plan Project of Wenzhou Municipality (ZG2022032).References
- M. Li, J. Lu, Z. Chen and K. Amine, Adv. Mater., 2018, 30, 1800561 CrossRef PubMed.
- Z. Fan, X. Zhou, J. Qiu, Z. Yang, C. Lei, Z. Hao, J. Li, L. Li, R. Zeng and S. Chou, Angew. Chem., Int. Ed., 2023, 62, e202308888 CrossRef CAS PubMed.
- T. Jin, H. Li, K. Zhu, P. Wang, P. Liu and L. Jiao, Chem. Soc. Rev., 2020, 49, 2342–2377 RSC.
- Y. You and A. Manthiram, Adv. Energy Mater., 2017, 8, 1701785 CrossRef.
- X. Zhou, Y. Huang, B. Wen, Z. Yang, Z. Hao, L. Li, S. Chou and F. Li, Proc. Natl. Acad. Sci. U. S. A., 2024, 121, e2316914121 CrossRef CAS PubMed.
- J. Y. Hwang, S. T. Myung and Y. K. Sun, Chem. Soc. Rev., 2017, 46, 3529–3614 RSC.
- X. Zhou, X. Chen, Z. Yang, X. Liu, Z. Hao, S. Jin, L. Zhang, R. Wang, C. Zhang, L. Li, X. Tan and S. Chou, Adv. Funct. Mater., 2024, 34, 2302281 CrossRef CAS.
- Y. Liao, L. Yuan, Y. Han, C. Liang, Z. Li, Z. Li, W. Luo, D. Wang and Y. Huang, Adv. Mater., 2024, 36, 2312287 CrossRef CAS PubMed.
- Z. Yang, X. Zhou, Z. Hao, J. Chen, L. Li, Q. Zhao, W. Lai and S. Chou, Angew. Chem., Int. Ed., 2024, 63, e202313142 CrossRef CAS PubMed.
- Z. Tang, R. Zhang, H. Wang, S. Zhou, Z. Pan, Y. Huang, D. Sun, Y. Tang, X. Ji, K. Amine and M. Shao, Nat. Commun., 2023, 14, 6024 CrossRef CAS PubMed.
- Y. Yang, C. Wu, X.-X. He, J. Zhao, Z. Yang, L. Li, X. Wu, L. Li and S.-L. Chou, Adv. Funct. Mater., 2024, 34, 2302277 CrossRef CAS.
- S. Chu, D. Kim, G. Choi, C. Zhang, H. Li, W. Pang, Y. Fan, A. M. D'Angelo, S. Guo and H. Zhou, Angew. Chem., Int. Ed., 2023, 62, e202216174 CrossRef CAS PubMed.
- T. Song, C. Wang and C.-S. Lee, Carbon Neutralization, 2022, 1, 68–92 CrossRef.
- A. K. Paidi, W. B. Park, P. Ramakrishnan, S. H. Lee, J. W. Lee, K. S. Lee, H. Ahn, T. Liu, J. Gim, M. Avdeev, M. Pyo, J. I. Sohn, K. Amine, K. S. Sohn, T. J. Shin, D. Ahn and J. Lu, Adv. Mater., 2022, 34, 2202137 CrossRef CAS PubMed.
- H. Fu, Y. Wang, G. Fan, S. Guo, X. Xie, X. Cao, B. Lu, M. Long, J. Zhou and S. Liang, Chem. Sci., 2022, 13, 726–736 RSC.
- Z. Hao, X. Shi, Z. Yang, X. Zhou, L. Li, C. Ma and S. Chou, Adv. Mater., 2024, 36, 2305135 CrossRef CAS.
- J. Guo, H. Zhang, Z. Gu, M. Du, H. Lü, X. Zhao, J. Yang, W. Li, S. Kang, W. Zou and X. Wu, Adv. Funct. Mater., 2022, 32, 2209482 CrossRef CAS.
- Y. Qi, Z. Tong, J. Zhao, L. Ma, T. Wu, H. Liu, C. Yang, J. Lu and Y. Hu, Joule, 2018, 2, 2348–2363 Search PubMed.
- M. Xu, F. Zhang, Y. Zhang, C. Wu, X. Zhou, X. Ai and J. Qian, Chem. Sci., 2023, 14, 12570–12581 RSC.
- C. Xu, Y. Ma, J. Zhao, P. Zhang, Z. Chen, C. Yang, H. Liu and Y.-S. Hu, Angew. Chem., Int. Ed., 2023, 62, e202217761 CrossRef CAS PubMed.
- H. Zhang, J. Peng, L. Li, Y. Zhao, Y. Gao, J. Wang, Y. Cao, S. Dou and S. Chou, Adv. Funct. Mater., 2023, 33, 2210725 CrossRef CAS.
- E. J. Kim, T. Hosaka, P. R. Kumar, Z. T. Gossage, R. Tatara, S. Komaba and K. Kubota, Chem. Sci., 2022, 13, 6121–6158 RSC.
- C. Wang, Y. Fang, Y. Xu, L. Liang, M. Zhou, H. Zhao and Y. Lei, Adv. Funct. Mater., 2016, 26, 1777–1786 CrossRef CAS.
- G. Zhou, Y. Miao, Z. Wei, L. Mo, F. Lai, Y. Wu, J. Ma and T. Liu, Adv. Funct. Mater., 2018, 28, 1804629 CrossRef.
- Y. Lu, Q. Zhang, L. Li, Z. Niu and J. Chen, Chem, 2018, 4, 2786–2813 CAS.
- Y. Niu, Y. Zhao and M. Xu, Carbon Neutralization, 2023, 2, 150–168 CrossRef CAS.
- H. Pan, Y. Hu and L. Chen, Energy Environ. Sci., 2013, 6, 2338–2360 RSC.
- Y. Liu, X. Wu, A. Moeez, Z. Peng, Y. Xia, D. Zhao, J. Liu and W. Li, Adv. Energy Mater., 2023, 13, 2203283 CrossRef CAS.
- Y. Jiang, X. Zhou, D. Li, X. Cheng, F. Liu and Y. Yu, Adv. Energy Mater., 2018, 8, 1800068 CrossRef.
- G. Chen, Q. Huang, T. Wu and L. Lu, Adv. Funct. Mater., 2020, 30, 2001289 CrossRef CAS.
- Q. Wang, H. Gao, J. Li, G. Liu and H. Jin, ACS Appl. Mater. Interfaces, 2021, 13, 14312–14320 CrossRef CAS PubMed.
- Y. Zhao, X. Gao, H. Gao, H. Jin and J. B. Goodenough, Adv. Funct. Mater., 2020, 30, 1908680 Search PubMed.
- T. I. Perfilyeva, A. M. Alekseeva, O. A. Drozhzhin and E. V. Antipov, Russ. J. Electrochem., 2023, 59, 481–488 CrossRef CAS.
- S. Y. Lim, H. Kim, R. A. Shakoor, Y. Jung and J. W. Choi, J. Electrochem. Soc., 2012, 159, A1393–A1397 CrossRef CAS.
- R. Liu, S. Zheng, Y. Yuan, P. Yu, Z. Liang, W. Zhao, R. Shahbazian-Yassar, J. Ding, J. Lu and Y. Yang, Adv. Energy Mater., 2020, 11, 2003256 CrossRef.
- F. Chen, V. M. Kovrugin, R. David, O. Mentré, F. Fauth, J. N. Chotard and C. Masquelier, Small Methods, 2018, 3, 1800218 CrossRef.
- M. J. Aragón, P. Lavela, G. F. Ortiz and J. L. Tirado, J. Electrochem. Soc., 2015, 162, A3077–A3083 CrossRef.
- B. M. de Boisse, J. Ming, S. Nishimura and A. Yamada, J. Electrochem. Soc., 2016, 163, A1469–A1473 CrossRef.
- M. Hadouchi, N. Yaqoob, P. Kaghazchi, M. Tang, J. Liu, P. Sang, Y. Fu, Y. Huang and J. Ma, Energy Storage Mater., 2021, 35, 192–202 CrossRef.
- C. Xu, J. Zhao, Y. Wang, W. Hua, Q. fu, X. Liang, X. Rong, Q. Zhang, X. Guo, C. Yang, H. Liu, B. Zhong and Y. Hu, Adv. Energy Mater., 2022, 12, 2200966 CrossRef CAS.
- Y. Zhou, G. Xu, J. Lin, Y. Zhang, G. Fang, J. Zhou, X. Cao and S. Liang, Adv. Mater., 2023, 35, 2304428 CrossRef CAS PubMed.
- W. Zhou, L. Xue, X. Lu, H. Gao, Y. Li, S. Xin, G. Fu, Z. Cui, Y. Zhu and J. B. Goodenough, Nano Lett., 2016, 16, 7836–7841 Search PubMed.
- S. Ghosh, N. Barman, M. Mazumder, S. K. Pati, G. Rousse and P. Senguttuvan, Adv. Energy Mater., 2019, 10, 1902918 CrossRef.
- A. Inoishi, Y. Yoshioka, L. Zhao, A. Kitajou and S. Okada, ChemElectroChem, 2017, 4, 2755–2759 Search PubMed.
- M. J. Aragón, P. Lavela, G. F. Ortiz and J. L. Tirado, ChemElectroChem, 2015, 2, 995–1002 CrossRef.
- R. Liu, G. Xu, Q. Li, S. Zheng, G. Zheng, Z. Gong, Y. Li, E. Kruskop, R. Fu, Z. Chen, K. Amine and Y. Yang, ACS Appl. Mater. Interfaces, 2017, 9, 43632–43639 CrossRef CAS PubMed.
- M. Chen, W. Hua, J. Xiao, J. Zhang, V. W. Lau, M. Park, G. H. Lee, S. Lee, W. Wang, J. Peng, L. Fang, L. Zhou, C. K. Chang, Y. Yamauchi, S. Chou and Y. M. Kang, J. Am. Chem. Soc., 2021, 143, 18091–18102 CrossRef CAS PubMed.
- W. Zhang, Y. Wu, Z. Xu, H. Li, M. Xu, J. Li, Y. Dai, W. Zong, R. Chen, L. He, Z. Zhang, D. J. L. Brett, G. He, Y. Lai and I. P. Parkin, Adv. Energy Mater., 2022, 12, 2201065 CrossRef CAS.
- H. Yu, X. Ruan, J. Wang, Z. Gu, Q. Liang, J. Cao, J. Kang, C. Du and X. Wu, ACS Nano, 2022, 16, 21174–21185 CrossRef CAS PubMed.
- B. Mai, B. Xing, Y. Yue, N. Cai, C. Cai, S. Lian, H. Fan, M. Yan, T. Zhu, P. Hu, X. Wang and L. Mai, J. Mater. Sci. Technol., 2023, 165, 1–7 CrossRef CAS.
- F. Lalère, V. Seznec, M. Courty, R. David, J. N. Chotard and C. Masquelier, J. Mater. Chem. A, 2015, 3, 16198–16205 Search PubMed.
- M. J. Aragón, P. Lavela, R. Alcántara and J. L. Tirado, Electrochim. Acta, 2015, 180, 824–830 CrossRef.
- A. Criado, P. Lavela, J. L. Tirado and C. Perez-Vicente, ACS Appl. Mater. Interfaces, 2020, 12, 21651–21660 Search PubMed.
- Q. Hu, J. Liao, X. He, S. Wang, L. Xiao, X. Ding and C. Chen, J. Mater. Chem. A, 2019, 7, 4660–4667 RSC.
- Y. Chen, X. Liao, P. Wang, J. Chen, X. Zhang, X. Wu, S. C. Smith, D. Lin, X. Tan and Q. Zheng, J. Colloid Interface Sci., 2024, 653, 1–10 Search PubMed.
- X. Shen, M. Han, Y. Su, M. Wang and F. Wu, Nano Energy, 2023, 114, 108640 CrossRef CAS.
- Q. Zhao, J. Li, M. Chen, H. Wang, Y. Xu, X. Wang, X. Ma, Q. Wu, X. Wu and X. Zeng, Nano Lett., 2022, 22, 9685–9692 CrossRef CAS PubMed.
- J. Wang, L. Zhao and F. Lu, Adv. Mater., 2023, 4, 1998–2007 RSC.
- H. Li, Y. Wang, X. Zhao, J. Jin, Q. Shen, J. Li, Y. Liu, X. Qu, L. Jiao and Y. Liu, ACS Energy Lett., 2023, 8, 3666–3675 CrossRef CAS.
- K. Tian, H. He, X. Li, D. Wang, Z. Wang, R. Zheng, H. Sun, Y. Liu and Q. Wang, J. Mater. Chem. A, 2022, 10, 14943–14953 RSC.
- Y. Ma, Y. Ma, S. L. Dreyer, Q. Wang, K. Wang, D. Goonetilleke, A. Omar, D. Mikhailova, H. Hahn, B. Breitung and T. Brezesinski, Adv. Mater., 2021, 33, e2101342 Search PubMed.
- H. Li, M. Xu, H. Long, J. Zheng, L. Zhang, S. Li, C. Guan, Y. Lai and Z. Zhang, Adv. Sci., 2022, 9, e2202082 CrossRef PubMed.
- K. Wang, Z. Zhang, S. Cheng, X. Han, J. Fu, M. Sui and P. Yan, eScience, 2022, 2, 529–536 CrossRef.
- Y. Yao, Q. Dong, A. Brozena, J. Luo, J. Miao, M. Chi, C. Wang, I. G. Kevrekidis, Z. J. Ren, J. Greeley, G. Wang, A. Anapolsky and L. Hu, Science, 2022, 376, eabn3103 CrossRef CAS PubMed.
- M. Li, C. Sun, Q. Ni, Z. Sun, Y. Liu, Y. Li, L. Li, H. Jin and Y. Zhao, Adv. Energy Mater., 2023, 13, 2203971 CrossRef CAS.
- M. T. Ahsan, D. Qiu, Z. Ali, Z. Fang, W. Zhao, T. Shen and Y. Hou, Adv. Energy Mater., 2024, 14, 2302733 CrossRef CAS.
- L. Zhu, M. Wang, S. Xiang, D. Sun, Y. Tang and H. Wang, Adv. Energy Mater., 2023, 13, 2302046 CrossRef CAS.
- M. Li, C. Sun, X. Yuan, Y. Li, Y. Yuan, H. Jin, J. Lu and Y. Zhao, Adv. Funct. Mater., 2024 DOI:10.1002/adfm.202314019.
- Z. Hao, X. Shi, W. Zhu, Z. Yang, X. Zhou, C. Wang, L. Li, W. Hua, C. Ma and S. Chou, ACS Nano, 2024, 18, 9354–9364 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally: Meng Zhou and Xunzhu Zhou. |
| This journal is © The Royal Society of Chemistry 2024 |