Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Efficient cytosolic delivery of luminescent lanthanide bioprobes in live cells for two-photon microscopy†
Kyangwi P.
Malikidogo‡§
 ab,
Thibault
Charnay‡
ab,
Daouda
Ndiaye
a,
Ji-Hyung
Choi
a,
Lucile
Bridou
c,
Baptiste
Chartier
a,
Sule
Erbek
de,
Guillaume
Micouin
c,
Akos
Banyasz
ab,
Thibault
Charnay‡
ab,
Daouda
Ndiaye
a,
Ji-Hyung
Choi
a,
Lucile
Bridou
c,
Baptiste
Chartier
a,
Sule
Erbek
de,
Guillaume
Micouin
c,
Akos
Banyasz
 c,
Olivier
Maury
c,
Olivier
Maury
 c,
Véronique
Martel-Frachet
c,
Véronique
Martel-Frachet
 de,
Alexei
Grichine
de,
Alexei
Grichine
 d and
Olivier
Sénèque
d and
Olivier
Sénèque
 *a
*a
aUniv. Grenoble Alpes, CNRS, CEA, IRIG, LCBM (UMR 5249), F-38000 Grenoble, France. E-mail: olivier.seneque@cea.fr
bUniv. Grenoble Alpes, CNRS, DCM (UMR 5250), F-38000 Grenoble, France
cUniv. Lyon, ENS de Lyon, CNRS UMR 5182, Laboratoire de Chimie, Lyon F-69342, France
dUniv. Grenoble Alpes, INSERM U1209, CNRS UMR 5309, Institute for Advanced Biosciences, F-38000 Grenoble, France
eEPHE, PSL Research University, 4-14 rue Ferrus, 75014 Paris, France
First published on 17th May 2024
Abstract
Lanthanide(III) (Ln3+) complexes have desirable photophysical properties for optical bioimaging. However, despite their advantages over organic dyes, their use for microscopy imaging is limited by the high-energy UV excitation they require and their poor ability to cross the cell membrane and reach the cytosol. Here we describe a novel family of lanthanide-based luminescent probes, termed dTAT[Ln·L], based on (i) a DOTA-like chelator with a picolinate moiety, (ii) a two-photon absorbing antenna to shift the excitation to the near infrared and (ii) a dimeric TAT cell-penetrating peptide for cytosolic delivery. Several Tb3+ and Eu3+ probes were prepared and characterized. Two-photon microscopy of live cells was attempted using a commercial microscope with the three probes showing the highest quantum yields (>0.15). A diffuse Ln3+ emission was detected in most cells, which is characteristic of cytosolic delivery of the Ln3+ complex. The cytotoxicity of these three probes was evaluated and the IC50 ranged from 7 μM to >50 μM. The addition of a single positive or negative charge to the antenna of the most cytotoxic compound was sufficient to lower significantly or suppress its toxicity under the conditions used for two-photon microscopy. Therefore, the design reported here provides excellent lanthanide-based probes for two-photon microscopy of living cells.
Introduction
Fluorescence microscopy is an essential tool in biological research1 and among the various techniques, Laser Scanning Confocal Microscopy (LSCM), which allows imaging of a cell section and 3D reconstruction of cells, is one of the most popular techniques today.2,3 LSCM has prompted the development of genetically encoded fluorescent proteins or synthetic cell-permeable molecular fluorescent dyes to reveal subcellular components or monitor biological processes.4–6 The first fluorophores used for LSCM required high-energy excitation in the UV-blue optical window, which damages cells. This led to the development of two-photon microscopy (2PM),7 a technique based on two-photon (2P) excited fluorescence, in which excitation of the molecule is caused by simultaneous absorption of two photons of equal energy, half the energy required for single photon (1P) excitation. Consequently, instead of being excited in the UV-blue window, 2P-absorbing dyes are excited with near-infrared (NIR) light, which is less energetic and therefore less damaging to cells. In addition, NIR light penetrates deeper into the biological tissues.8 Since 2P excitation happens only in a very small volume corresponding to the focal point of the pulsed laser source, 2PM is comparable to LSCM in its ability to image cell sections, but superior to LSCM for imaging live cells or thick biological samples due to the NIR excitation. Over the past two decades, numerous organic fluorescent dyes have been developed that are suitable for 1P or 2P excitation in the red-NIR window, but in many cases they suffer from a lack of photostability, poor water solubility and low cell penetration capacity.9–11Complexes of trivalent lanthanides (Ln3+) exhibit interesting luminescence properties for biological applications, making them an attractive alternative to organic fluorescent dyes.12–17 Each Ln3+ exhibits a fingerprint-like emission consisting of sharp atom-like emission bands at fixed wavelengths, independent of the environment, making them easily recognizable. Depending on the Ln3+, these bands cover the visible and NIR. Ln3+ luminescence is characterized by long lifetimes (in the microsecond to millisecond range), allowing background fluorescence (in the nanosecond range) to be suppressed by time-gated detection. In addition, luminescent Ln3+ complexes are highly photostable compared to organic dyes. They have been widely used in biological assays based on homogenous time-resolved technology.
Despite these advantages, Ln3+ complexes are not routinely used by biologists for cell microscopy as compared to organic dyes. There are two reasons for this. First, due to the low extinction coefficient of the f–f transitions, Ln3+ luminescence must rely on the antenna effect to be effective: a light-harvesting chromophore located close to the Ln3+ cation is excited and transfers energy to the Ln3+ to bring it into its emitting excited state. However, the antenna chromophore usually requires UV excitation which is damaging to cells. To overcome this problem, it is possible to use Ln3+ complexes with 2P-absorbing push–pull antennas.18–20 Importantly, it was demonstrated that 2PM with such Ln3+ complexes is possible with commercial microscopes commonly used by biologists.21 However, most of these 2P-absorbing Ln3+ complexes fail to penetrate live cells and cell fixation was required prior to incubation with the Ln3+ probe in order to permeabilize the cell membrane and let the probe go into the cell.
The second reason against using Ln3+ complexes as dyes for LCSM or 2PM is their cell penetration and their cellular localization properties. Parker and co-workers have studied the cell uptake and localization of various Ln3+ complexes with cyclen-based macrocyclic chelator with appended antenna. Many of them are able to penetrate cells, either neutral, negatively or positively charged. Indeed, cell penetration seems not to depend on charge but rather on antenna nature and linkage.22–26 Nevertheless, this cell uptake property does not apply to all Ln3+ complexes since other negatively charged cyclen-based Ln3+ complexes that we have recently described are unable to penetrate cell.27 Parker and co-workers have performed LSCM of cells using numerous Ln3+ complexes with 1P excitation in the UV.26,28,29 Most often, however, Ln3+ complexes end up in lysosomes or stick to the mitochondrial membrane.26 They are not properly delivered to the cytosol. Furthermore, it is difficult to predict the subcellular localization of a Ln3+ complex on the basis of its molecular structure, as localization can be influenced by minor changes in the antenna or chelator moieties. A convenient solution to these problems would be to conjugate the Ln3+ complex to a cell-penetrating peptide (CPP). CPPs are short peptides, usually cationic, that enter cells either by direct membrane translocation or by endocytosis.30 However, the CPP has to be chosen carefully, as many CPPs, whether natural such as TAT (transactivator of transcription of human immunodeficiency virus) or synthetic such as R8 (octa-arginine), do not effectively reach the cytosol.31
Ten years ago, Pellois and co-workers described a dimer of the TAT CPP, called dfTAT, with a (tetramethyl)rhodamine dye grafted onto each TAT monomer and dimerized by a disulfide bond (Fig. 1A).32,33 dfTAT is internalized into living cells by endocytosis and promotes endosomal leakage so that the rhodamine fluorophore reaches the cytosol and spreads throughout the entire cell. Inspired by this work, we describe here a family of luminescent bioprobes, called dTAT[Ln·L], consisting of Ln3+ complex with 2P absorbing push–pull antennas conjugated to a TAT dimer (Fig. 1B). We demonstrate that they enable 2PM imaging of living cells thanks to the appropriate photophysical properties of the Ln3+ complexes and the efficient cytosolic delivery with the TAT dimer. In addition, we show that toxicity issues can be easily controlled by modulation of the antenna.
 | ||
| Fig. 1 Chemical structure of (A) dfTAT and (B) Ln3+-based probes dTAT[Eu·L] and mTAT[Eu·L]. L denotes the DO3Apic macrocycle. | ||
Results and discussion
Design of the probes
Two factors are important for efficient cytosolic delivery of the dfTAT system (Fig. 1A): (i) the high number and high density of arginines in the dimer and (ii) the hydrophobic aromatic core of the rhodamine dye. Therefore, we decided to evaluate whether replacing the rhodamine dye with a Ln3+ complex could provide a probe that reaches the cytosol, with the aromatic sensitizing antenna playing the role of the rhodamine aromatic core in endosomal escape. As a Ln3+ ligand, we chose DO3Apic, a DOTA-type ligand in which one of the acetate arms has been replaced by a picolinate one. It forms stable complexes with Ln3+ that exhibit interesting luminescence properties, including long luminescence lifetimes and moderate to high quantum yields for Eu3+ and Tb3+.34 The addition of a π-conjugated system with an electron donating group to the picolinate antenna allows a red-shift of the excitation wavelength and an increase in the extinction coefficient for superior luminescence properties27,35 and converts the picolinate into a push–pull antenna suitable for 2P absorption.35 From a synthetic point of view, it is easy to distinguish the carboxylate of the picolinate from those of the acetates by playing with tert-butyl/methyl ester protection (see ESI†) in order to use it as a unique conjugation site for coupling to a peptide lysine side chain. Therefore, we decided to use the DO3Apic macrocycle as a Ln3+ ligand in the TAT dimer system. From our experience, we know that aryl-alkynyl-picolinate antennas, which are excellent for sensitizing Eu3+ luminescence, are not very stable under the conditions required for peptide synthesis (especially under the acidic conditions used for resin cleavage and deprotection steps) due to the addition of nucleophiles on the triple bond.27 Therefore, we opted for aryl-picolinate antennas and three of them were evaluated (Fig. 1B). The first one has an ortho-methyl group and a para-methoxy, which acts as an electrodonating group in the push–pull system. It was previously shown that such an antenna sensitizes Tb3+ better than Eu3+.36 In the second antenna, the methoxy group is replaced by an acetamide moiety and in the third antenna, the ortho-methyl group is deleted compared to the second one. In order to characterize the luminescence properties of the corresponding Tb3+ and Eu3+ complexes, the six conjugates mTAT[Ln·L-MeAr-OMe], mTAT[Ln·L-MeAr-NHAc] and mTAT[Ln·L-Ar-NHAc] with Ln = Tb and Eu (Fig. 1B) were prepared. The Gd3+ analogues were also prepared to determine the energy of their excited triplet state. Their synthesis is described in the ESI.†Photophysical properties
The luminescence properties of the probes were evaluated in PBS (pH 7.4). Absorption, excitation and emission spectra are shown in Fig. 2 or in the ESI† and spectroscopic data are summarized in Table 1. Absorption spectra of mTAT[Ln·L] compounds show two bands at ca. 285 and 315 nm, which are attributed to locally excited (LE) and intra-ligand charge transfer (ILCT) transitions within the antenna, respectively.27,36 In the case of twisted antennas, i.e. with an ortho-methyl group, the LE band dominates and the ILCT band appears as a shoulder while in the case of the freely rotating antenna, i.e. in the absence of ortho-methyl, the ILCT band dominates the absorption. The ILCT band extends up to ca. 365 nm (λcut-off in Table 1).| Compound | λ max; λcut-off/nm | E(S1); E(T1)b/cm−1 | ε at λmax/M−1 cm−1 | σ 2P at 700 nm/GM | Φ Ln | τ Ln/ms | IC50/μM |
|---|---|---|---|---|---|---|---|
a Error on ε value is estimated ±5% on ε values and ±10% on ΦLn and σ2P. Error on τLn is estimated ±0.03 ms.
b Energy of the excited triplet state is estimated from the wavelength at half-maximum on the onset of the time-gated phosphorescence spectrum of the Gd3+ analogue in PBS/glycerol 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v recorded at 77 K.
c Extinction coefficient ε of dimers are estimated twice that of the monomer with the same antenna. 1 v/v recorded at 77 K.
c Extinction coefficient ε of dimers are estimated twice that of the monomer with the same antenna.
|
|||||||
| mTAT[Ln·L-MeAr-OMe] | 285; 363 | S1: 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
5.7 | Tb: 0.55 | Tb: 2.11 | |
T1: 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
Eu: 0.040 | Eu: 1.05 | |||||
| mTAT[Ln·L-MeAr-NHAc] | 287; 361 | S1: 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4.2 | Tb: 0.20 | Tb: 0.83 | |
T1: 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
Eu: 0.048 | Eu: 1.05 | |||||
| mTAT[Ln·L-Ar-NHAc] | 315; 366 | S1: 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
11.1 | Tb: 0.027 | Tb: 0.07 | |
T1: 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Eu: 0.15 | Eu: 1.05 | |||||
| dTAT[Tb·L-MeAr-OMe] | 285, 366 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000a 000a |
Tb: 0.38 | Tb: 1.40 | 18 ± 3 | ||
| dTAT[Tb·L-MeAr-NHAc] | 288, 364 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000c 000c |
Tb: 0.12 | Tb: 0.46 (98%), 1.63 (2%) | 7.6 ± 1.6 | ||
| dTAT[Eu·L-Ar-NHAc] | 317; 370 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000c 000c |
Eu: 0.15 | Eu: 1.08 | >50 | ||
| dTAT[Tb·L-MeAr-NHβAla(+)] | 289, 366 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Tb: 0.14 | Tb: 0.34 (99%), 1,43 (1%) | 22 ± 5 | ||
| dTAT[Tb·L-MeAr-NHSuc(−)] | 286, 368 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Tb: 0.17 | Tb: 0.46 (97%), 2.07 (3%) | >50 | ||
Excitation into these bands yields characteristic Tb3+ 5D4 → 7FJ (J = 6–0) or Eu3+ 5D0 → 7FJ (J = 0–4) emissions in the 450–750 nm range. The excitation spectra match the absorption spectra, which confirms sensitization of Ln3+ luminescence by antenna effect. Interestingly, with respect to quantum yields of Ln3+ emission, the antenna with the ortho-methyl group sensitizes Tb3+ better while the one without the ortho-methyl group sensitizes Eu3+ better.36 Quantum yields and lifetimes of Tb3+ emission decrease in the order L-MeAr-OMe > L-MeAr-NHAc > L-Ar-NHAc, following the T1 excited state energy (Table 1). Indeed, it seems that back-energy transfer from the Tb3+ 5D4 state to the antenna T1 state is responsible for this trend (the energy gap between antenna T1 and Tb3+ 5D4 states is particularly low for L-Ar-NHAc, ca. 1600 cm−1), which is confirmed by longer lifetimes in deoxygenated PBS (ESI†). For all these compounds, comparison of Ln3+ luminescence lifetimes measured in H2O and D2O indicates that the hydration number q is zero, in agreement with the saturated coordination sphere expected for these ligands (ESI†).27,34
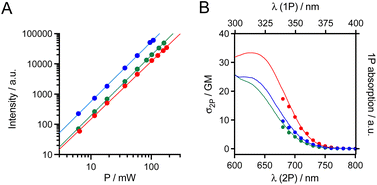
The 2P absorption cross-sections (σ2P) in the NIR, between 680 and 800 nm, were determined for mTAT[Tb·L-MeAr-OMe], mTAT[Tb·L-MeAr-NHAc] and mTAT[Eu·L-Ar-NHAc] using the 2P excited fluorescence method (ESI†). For the three compounds, upon excitation at 720 nm, the characteristic Ln3+ emission is obtained and it displays a quadratic variation of intensity with respect to the incident laser power (Fig. 3A), the signature of a 2P-antenna effect. The 2P absorption spectrum was in good agreement with the wavelength-doubled 1P absorption one (Fig. 3B), indicating that the low-energy ILCT transition responsible for the sensitization of Ln3+ luminescence is one- and two-photon allowed. The values of σ2P are low (Table 1), ca. 4–11 GM at 700 nm, and the compound with the freely rotating antenna, mTAT[Eu·L-Ar-NHAc], shows the highest cross-section. However, regarding the 2P brightness B2P = σ2P × ΦLn, which quantifies the efficiency of the whole absorption/emission process, mTAT[Tb·L-MeAr-OMe] appears to be the best compound with a B2P value of 3.1 at 700 nm compared to 0.85 and 1.7 for mTAT[Tb·L-MeAr-NHAc] and mTAT[Eu·L-Ar-NHAc], respectively. In cells, the main contributors to 2P-excited autofluorescence are NAD(P)H and FAD.37,38 They show emission bands with maxima at ca. 465 and 530 nm,38 respectively, and quantum yields of ca. 0.02 and 0.03 at physiological pH, respectively.39 Their 2P cross section under excitation around 700 nm is in the range 0.03–0.1 GM.37 Their 2P brightness is therefore lower than that of the Ln3+-based probes but the latter has to accumulate in sufficient amount in the cell to be detected efficiently, i.e. above the autofluorescence level.
Given the better luminescence properties of mTAT[Tb·L-MeAr-OMe], mTAT[Tb·L-MeAr-NHAc] and mTAT[Eu·L-Ar-NHAc] compared to the other three compounds, we have synthesized and characterized their dimeric analogues dTAT[Tb·L-MeAr-OMe], dTAT[Tb·L-MeAr-NHAc] and dTAT[Eu·L-Ar-NHAc] (ESI† and Table 1). Dimeric dTAT[Eu·L-Ar-NHAc] and monomeric mTAT[Eu·L-Ar-NHAc] exhibit identical photophysical properties (Table 1). However, for the Tb3+ compounds, the luminescence quantum yields and lifetimes of the dimeric compounds are lower than their monomeric counterparts, probably due to additional deactivation by the T1 state of the second Tb3+ complex of the dimer. However, should these compounds manage to reach the cell cytosol, the disulphide will be reduced due to its high glutathione content (ca. 4–5 mM).40–42 Consequently, the monomeric form is likely to better represent the species present inside cells with associated photophysical properties.
Two-photon microscopy imaging of live cells
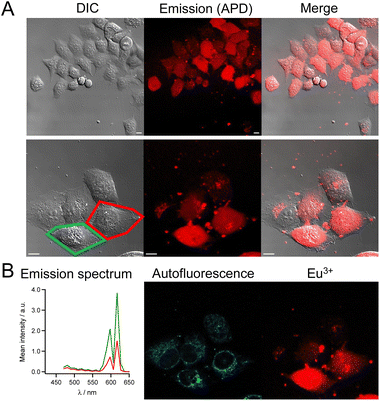
The cell imaging properties of the dTAT[Ln·L] conjugates were investigated by microscopy using 2P excitation at 720 nm. Live HeLa cells were incubated for 1 h with the dTAT[Ln·L] conjugates then washed and imaged. Images obtained with dTAT[Tb·L-MeAr-OMe] (10 μM) are shown in Fig. 4. In the DIC image (Fig. 4A, left), the cells phenotype is consistent with that of live cells. Luminescence emission under 2P excitation at 720 nm was collected with an avalanche photodiode (APD; Fig. 4A, middle panel) with a 420–650 nm bandpass (bp) filter. It was detected in all cells, but its intensity varied from cell to cell. Both punctate and diffuse emission signals are detected within cells, the latter covering in the entire cell. These features are reminiscent of the fluorescence pattern detected with dfTAT32 and, more generally, are characteristic of fluorophores internalized with CPP. A single APD does not allow to identify the emissive species based on its emission spectrum. Therefore, spectral detection was performed using a photomultiplier (PMT array of Quasar detectors). The left panel of Fig. 4B shows the emission spectra averaged within the entire cell arising from the two cells outlined in red and green in panel 4A, left. These emission spectra are dominated by the Tb3+ emission but a weaker contribution from autofluorescence background is also detected. Deconvolution of the luminescence images acquired in spectral mode resulted in the autofluorescence and Tb3+ emission maps showed in the middle and right panels of Fig. 4B, respectively. The autofluorescence localization is characteristic of the typical distribution of mitochondria, consistent with 2P excitation of NADH fluorescence. Tb3+ emission is detected in all cells, whether diffuse or punctate. The diffuse emission, which is observed in the entire cell, including nucleus, indicates successful cytosolic delivery of the Tb3+ complex, whereas the punctate emission may correspond to Tb3+ complexes still trapped in endosomes. After 4 h, the punctate distribution is still present. The luminescence decay of diffuse Tb3+ emission was measured in cells using TSLIM measurements21 and could be fitted bi-exponentially with lifetimes of 0.75 and 0.15 ms (Fig. S12†), which are both shorter than the lifetime measured in PBS solution with a spectrometer (Table 1). Nevertheless, these millisecond values confirm emission arising from the Tb3+ ion.Similar images and cell distributions were obtained with dTAT[Tb·L-MeAr-NHAc] (Fig. S13†) and dTAT[Eu·L-Ar-HNAc] after 1 h incubation at 10 μM (Fig. 5). In the former case, Tb3+ emission was clearly detected in cells by spectral detection although it was weaker than for dTAT[Tb·L-MeAr-OMe] compared to autofluorescence emission, consistent with the lower 2P brightness of the former. Nevertheless, the deconvolution of autofluorescence and Tb3+ emission (Fig. S13†) shows a Tb3+ signal that is both diffuse and punctate, with the diffuse component attesting to cytosolic delivery. A bi-exponential luminescence decay with lifetimes of 0.84 and 0.15 ms was measured in cells for the diffuse Tb3+ signal.
In the case of dTAT[Eu·L-Ar-HNAc], a large number of cells shows diffuse Eu3+ emission above the autofluorescence as shown in the spectrum in Fig. 5, with peaks at 590 and 615 nm. This confirms again the cytosolic delivery of the Eu3+ complex. Some cells show also punctate Eu3+ emission inside the cell but outside the nucleus, which may correspond to the Eu3+ probe trapped in endosomes or lysosomes. Small clumps of the Eu3+ probe are also observed in areas where no cell is present or sticked to the cell membrane. This may correspond to probe aggregates that form in the culture medium (not observed in drop of water solution of the probe, even at 100 μM). Within cells, the Eu3+ luminescence decay lifetime of diffuse emission was 0.65 ms. As for Tb3+ compounds, the lifetime measured in the living cell is shorter than in PBS solution. The lifetime measured in a drop of Ln3+ conjugate with the microscope is within margin error equal to the one determined in solution with a spectrometer, ruling out problems due to measurement procedure with the microscope. The reason is unclear but we can envisage that energy transfer from the excited Ln3+ to cell components (aromatic co-factors) happens. The reason of the bi-exponential decay of the Tb3+ needs further studies to be clarified.
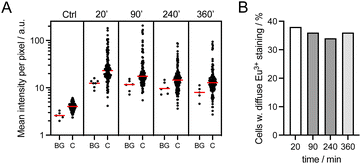
Cell staining with dTAT[Eu·L-Ar-HNAc] was also studied in a concentration dependent manner. At 2.5 μM (1 h incubation), cells only show punctate Eu3+ around the nucleus (Fig. S14†). Counter-staining with LysoView488™ (Biotium), a lysosome marker, shows excellent overlap with Eu3+ distribution (Fig. S15†), confirming that the punctate probe emission corresponds to endosomal/lysosomal entrapment. Next we examined the time dependence of the staining after 1 h incubation of HeLa cells with dTAT[Eu·L-Ar-HNAc] at 5 μM. The mean intensity of Eu3+ emission recorded using a 590–680 nm bp filter was measured within cells 20, 90, 240 and 360 min after washing (ca. 120 cells analysed at each time point) and compared to non-incubated HeLa cells as a control (Fig. 6A). The background emission was higher in incubated cells than in control, especially close to the surface. This may be attributed to dTAT[Eu·L-Ar-HNAc] adhered to the surface, as already described for CPP-rhodamine conjugates.43 The distribution of mean intensity within cells does not vary significantly with time up to 6 h. The number of cells showing cytosolic staining, i.e. diffuse Eu3+ emission was also determined. It remains constant (34–38%) over 6 hours after washing (Fig. 6B). Punctate Eu3+ emission trapped in endosomes is still observed after 6 h in some cells.
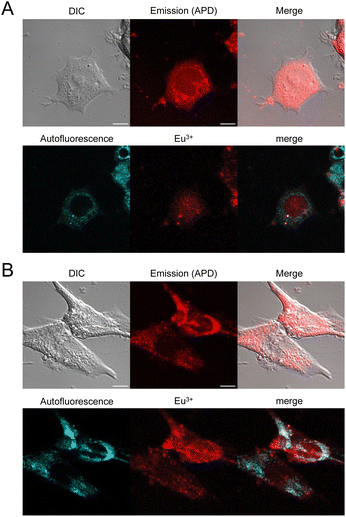
Finally, internalization was confirmed in other cell types such as HEK293T or MRC5 cells with dTAT[Eu·L-Ar-HNAc] with successful cytosolic delivery demonstrated by the characteristic diffuse Eu3+ localization (Fig. 7). Interestingly, no significant internalization was observed with mTAT compounds in HeLa cells, demonstrating the importance of TAT dimerization for cell penetration efficiency and cytosolic delivery.
Cytotoxicity
The cytotoxicity of dTAT[Ln·L] conjugates was evaluated using the MTT proliferation assay. The compound concentration causing a 50% reduction in proliferation (IC50) is given in Table 1 and plots of cell viability as a function of compound concentration are shown in Fig. 8 dTAT[Eu·L-Ar-HNAc] is the least toxic (IC50 > 50 μM), showing no toxicity at the 10 μM concentration used for 2PM. The IC50 of the two Tb3+ compounds carrying the ortho-methyl on the antenna are lower, with dTAT[Tb·L-MeAr-HNAc] being the most toxic with an IC50 below 10 μM. We also observed stronger toxicity (IC50 = 2.5 μM) with a related compound bearing a carbazole instead of a methoxy or acetamide electrodonating group (this compound will be described in detail elsewhere). Suspecting that the toxicity might be related to the hydrophobicity of the antenna and its possible interaction with membrane lipids, we synthesized two analogues of the more toxic Tb3+ compound but with charged pendants on the amide electrodonating group: dTAT[Tb·L-MeAr-NHβAla(+)] and dTAT[Tb·L-MeAr-NHSuc(−)] with an additional positive and negative charge, respectively. Their luminescence properties (Table 1) are similar to those of their parent compound dTAT[Tb·L-MeAr-NHAc], indicating that the introduction of the charge has little effect on the emission properties. Interestingly, however, the additional charge greatly reduces the toxicity (Table 1), increasing the IC50 values above 10 μM, which is the highest concentration used for 2P imaging experiments. It should be noted that the MTT assays were carried out with a 2 hour incubation instead of the 1 hour incubation for 2PM. Consequently, toxicity might be overestimated compared with 2PM conditions. 2PM imaging of HeLa cells showed that the additional charge does not alter the cell penetration properties: both dTAT[Tb·L-MeAr-NHβAla(+)] and dTAT[Tb·L-MeAr-NHSuc(−)] are efficiently delivered to the cytosol with characteristic diffuse emission (Fig. S16 and S17†). This demonstrates that the cytotoxicity of these compounds can be easily modulated by reducing the lipophilicity of the antenna, without affecting the photophysical, penetration and imaging properties. | ||
| Fig. 8 MTT proliferation assay for dTAT[Ln·L] conjugates. (A) Red: dTAT[Eu·L-Ar-NHAc]; blue: dTAT[Tb·L-MeAr-OMe]; green: dTAT[Tb·L-MeAr-NHAc]. (B) Pink: dTAT[Tb·L-MeAr-NHSuc(−)]; black: dTAT[Tb·L-MeAr-NHβAla(+)]; green: dTAT[Tb·L-MeAr-NHAc]. Symbols correspond to experimental data and solid lines to the fit that yielded IC50 values given in Table 1. Error bars correspond to the SEM. The dotted line indicates the highest concentration used for 2P microscopy in this article (10 μM). | ||
Toward multiplex imaging
Finally, since these compounds have similar 2P brightness, we attempted cell imaging with a Tb3+/Eu3+ mixture. HeLa cells were incubated for 1 h with dTAT[Tb·L-MeAr-NHβAla(+)] (10 μM) and dTAT[Eu·L-Ar-NHAc] (5 μM), rinsed and imaged by 2P microscopy. A luminescence emission could be detected within the cells, the intensity of which again varied from cell to cell (Fig. 9). However, the diffuse emission was detected in many cells. The emission spectrum arising from cells under 2P excitation at 720 nm clearly shows fingerprint peaks of Tb3+ (490 and 540 nm) and Eu3+ (590 and 615 nm) emission (Fig. 9B). This indicates that both Tb3+ and Eu3+ complexes were co-internalized in the cells. Spectral deconvolution (Fig. 9B, right) showed that they were delivered to the cytosol because of the diffuse signal and co-localized well throughout the entire cell. A similar Tb3+/Eu3+ co-internalization could be obtained with dTAT[Tb·L-MeAr-NHSuc(−)] (Fig. S18†). This opens the way to multiplex imaging with multiple Ln3+ probes in living cells.Conclusion
In the present work we report a family of luminescent lanthanide bioprobes, named dTAT[Ln·L], that are efficiently delivered into the cytosol of living cells and allow two-photon microscopy imaging. These probes are based on a TAT dimer functionalized by Ln3+ complexes, which exhibit 2P absorption properties thanks to a push–pull antenna. As a Ln3+ chelator (L), we choose DO3Apic but equipped with different π-extended picolinate moieties. It can be easily conjugated to peptides via the picolinate group and forms stable Tb3+ and Eu3+ complexes with good to excellent photophysical properties. Optical properties identified three candidates for 2PM imaging of cells, i.e. two Tb3+ and one Eu3+ complexes with quantum yields ≥15% and featuring 2P absorption. 2PM of live HeLa cells incubated for 1 h with these complexes conjugated to TAT dimers showed intense Ln3+ emission under 2P excitation at 720 nm despite their low 2P cross sections. Successful cytosolic delivery of the Ln3+ complex was observed in most cells with characteristic diffuse Tb3+ or Eu3+ emission within the entire cell. The conjugates can be used to image other cell types as demonstrated with HEK293T and MRC5 cells. Interestingly, we have shown that the potential toxicity of these conjugates can be easily reduced to acceptable levels by adding charges onto the antenna to reduce its lipophilicity. In contrast to the dimeric dTAT[Ln·L] compounds, the monomeric analogues mTAT[Ln·L] are unable to penetrate cells sufficiently for 2PM imaging. Hence, this article shows that the TAT dimer is a suitable scaffold for the design of Ln3+-based luminescent probes that have to be delivered into the cytosol of living cells.The controlled delivery of dTAT[Ln·L] is really innovative compared to previous Ln3+ luminescent probes that required cell fixation to permeabilize the cell membranes for probe internalization or that accumulated in an uncontrolled manner in either lysosomes or mitochondria of living cells. Importantly, 2PM imaging with the dTAT[Ln·L] conjugates was performed using a commercial confocal microscope routinely used in biology laboratories and Ln3+ could be fully characterized inside cells by recording the emission spectrum and the millisecond luminescence decay lifetime.
Finally, given the design of these probes and the versatility offered by their synthetic pathway, there is room to improve their photophysical properties. The design described here opens the way to more sophisticated responsive probes with ratiometric detection based on emission intensity using mixtures of Ln3+ or emission lifetime.
Data availability
The datasets supporting this article have been uploaded as part of the ESI.† They are also available from the authors on reasonable request.Author contributions
Conceptualization: O. S., O. M.; investigation: K. P. M., T. C., D. N., J.-H. C., L. B., B. C., G. M., S. E., V. M.-F., A. G., O. S.; validation: O. S., A. B., O. M., V. M.-F., A. G.; witting (original draft): O. S.; writing (review & editing): all authors. Visualization: O. S.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Authors acknowledge the Labex ARCANE, CBH-EUR-GS (ANR-17-EURE-0003), the Agence Nationale de la Recherche (ANR-18-CE06-0022), the CEA FOCUS Biomarqueurs program and the Aviesan ITMO Cancer PCSI program (ONCOVIEW project) for financial support. Imaging experiments were done on Microcell core facility of the Institute for Advanced Biosciences (UGA - Inserm U1209 - CNRS 5309). This facility belongs to the IBISA-ISdV platform, member of the national infrastructure France-BioImaging supported by the French National Research Agency (ANR-10-INBS-04).Notes and references
- E. Haustein and P. Schwille, HFSP J., 2007, 1, 169–180 CrossRef CAS PubMed
.
- K.-J. Halbhuber and K. König, Ann. Anat., 2003, 185, 1–20 CrossRef PubMed
.
- A. D. Elliott, Curr. Protoc. Cytom., 2020, 92, e68 CrossRef PubMed
.
- T. Ueno and T. Nagano, Nat. Methods, 2011, 8, 642–645 CrossRef CAS PubMed
.
- L. D. Lavis and R. T. Raines, ACS Chem. Biol., 2008, 3, 142–155 CrossRef CAS PubMed
.
- M. Wang, Y. Da and Y. Tian, Chem. Soc. Rev., 2023, 52, 1189–1214 RSC
.
- W. Denk, J. H. Strickler and W. W. Webb, Science, 1990, 248, 73–76 CrossRef CAS PubMed
.
- R. J. Gillies, J. Cell. Biochem., 2002, 231–238 CrossRef CAS PubMed
.
- J. B. Grimm, B. P. English, J. Chen, J. P. Slaughter, Z. Zhang, A. Revyakin, R. Patel, J. J. Macklin, D. Normanno, R. H. Singer, T. Lionnet and L. D. Lavis, Nat. Methods, 2015, 12, 244–250 CrossRef CAS PubMed
.
- C. Ding and T. Ren, Coord. Chem. Rev., 2023, 482, 215080 CrossRef CAS
.
- L. Xu, J. Zhang, L. Yin, X. Long, W. Zhang and Q. Zhang, J. Mater. Chem. C, 2020, 8, 6342–6349 RSC
.
-
J.-C. G. Bünzli and S. V. Eliseeva, in Lanthanide Luminescence, ed. P. Hänninen and H. Härmä, Springer Berlin Heidelberg, 2011, pp. 1–45 Search PubMed
.
- J.-C. G. Bünzli, J. Lumin., 2016, 170, 866–878 CrossRef
.
- M. Sy, A. Nonat, N. Hildebrandt and L. J. Charbonnière, Chem. Commun., 2016, 52, 5080–5095 RSC
.
- E. Mathieu, A. Sipos, E. Demeyere, D. Phipps, D. Sakaveli and K. E. Borbas, Chem. Commun., 2018, 54, 10021–10035 RSC
.
- G.-Q. Jin, Y. Ning, J.-X. Geng, Z.-F. Jiang, Y. Wang and J.-L. Zhang, Inorg. Chem. Front., 2020, 7, 289–299 RSC
.
- D. Parker, J. D. Fradgley and K.-L. Wong, Chem. Soc. Rev., 2021, 50, 8193–8213 RSC
.
- A. Picot, A. D'Aleo, P. L. Baldeck, A. Grichine, A. Duperray, C. Andraud and O. Maury, J. Am. Chem. Soc., 2008, 130, 1532–1533 CrossRef CAS PubMed
.
- A. D’Aléo, A. Bourdolle, S. Brustlein, T. Fauquier, A. Grichine, A. Duperray, P. L. Baldeck, C. Andraud, S. Brasselet and O. Maury, Angew. Chem., Int. Ed., 2012, 51, 6622–6625 CrossRef PubMed
.
- N. Hamon, A. Roux, M. Beyler, J.-C. Mulatier, C. Andraud, C. Nguyen, M. Maynadier, N. Bettache, A. Duperray, A. Grichine, S. Brasselet, M. Gary-Bobo, O. Maury and R. Tripier, J. Am. Chem. Soc., 2020, 142, 10184–10197 CrossRef CAS PubMed
.
- A. Grichine, A. Haefele, S. Pascal, A. Duperray, R. Michel, C. Andraud and O. Maury, Chem. Sci., 2014, 5, 3475–3485 RSC
.
- R. A. Poole, C. P. Montgomery, E. J. New, A. Congreve, D. Parker and M. Botta, Org. Biomol. Chem., 2007, 5, 2055–2062 RSC
.
- R. Pal and D. Parker, Org. Biomol. Chem., 2008, 6, 1020–1033 RSC
.
- C. P. Montgomery, B. S. Murray, E. J. New, R. Pal and D. Parker, Acc. Chem. Res., 2009, 42, 925–937 CrossRef CAS PubMed
.
- E. J. New and D. Parker, Org. Biomol. Chem., 2009, 7, 851–855 RSC
.
- E. J. New, A. Congreve and D. Parker, Chem. Sci., 2010, 1, 111–118 RSC
.
- J.-H. Choi, G. Fremy, T. Charnay, N. Fayad, J. Pécaut, S. Erbek, N. Hildebrandt, V. Martel-Frachet, A. Grichine and O. Sénèque, Inorg. Chem., 2022, 61, 20674–20689 CrossRef CAS PubMed
.
- C. P. Montgomery, D. Parker and L. Lamarque, Chem. Commun., 2007, 3841–3843 RSC
.
- S. J. Butler, L. Lamarque, R. Pal and D. Parker, Chem. Sci., 2014, 5, 1750–1756 RSC
.
- L. Peraro and J. A. Kritzer, Angew. Chem., Int. Ed., 2018, 57, 11868–11881 CrossRef CAS PubMed
.
- F. Nadal-Bufí and S. T. Henriques, Pept. Sci., 2020, 112, e24168 CrossRef
.
- A. Erazo-Oliveras, K. Najjar, L. Dayani, T.-Y. Wang, G. A. Johnson and J.-P. Pellois, Nat. Methods, 2014, 11, 861–867 CrossRef CAS PubMed
.
- J. K. Allen, D. J. Brock, H. M. Kondow-McConaghy and J.-P. Pellois, Biomolecules, 2018, 8, 50 CrossRef PubMed
.
- M. Regueiro-Figueroa, B. Bensenane, E. Ruscsak, D. Esteban-Gomez, L. J. Charbonniere, G. Tircso, I. Toth, A. de Blas, T. Rodriguez-Blas and C. Platas-Iglesias, Inorg. Chem., 2011, 50, 4125–4141 CrossRef CAS PubMed
.
- A. D’Aléo, M. Allali, A. Picot, P. L. Baldeck, L. Toupet, C. Andraud and O. Maury, C. R. Chim., 2010, 13, 681–690 CrossRef
.
- A. T. Bui, A. Roux, A. Grichine, A. Duperray, C. Andraud and O. Maury, Chem.–Eur. J., 2018, 24, 3408–3412 CrossRef CAS PubMed
.
- Y. Qin and Y. Xia, Front. Phys., 2021, 9, 642302 CrossRef
.
- R. Cao, H. K. Wallrabe and A. Periasamy, J. Biomed. Opt., 2020, 25, 014510 CAS
.
- I. A. Gorbunova, M. K. Danilova, M. E. Sasin, V. P. Belik, D. P. Golyshev and O. S. Vasyutinskii, J. Photochem. Photobiol., A, 2023, 436, 114388 CrossRef CAS
.
- R. C. Cumming, N. L. Andon, P. A. Haynes, M. Park, W. H. Fischer and D. Schubert, J. Biol. Chem., 2004, 279, 21749–21758 CrossRef CAS PubMed
.
- C. D. Austin, X. Wen, L. Gazzard, C. Nelson, R. H. Scheller and S. J. Scales, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 17987–17992 CrossRef CAS PubMed
.
- Y.-J. Lee, S. Datta and J.-P. Pellois, J. Am. Chem. Soc., 2008, 130, 2398–2399 CrossRef CAS PubMed
.
- M. Serulla, P. Anees, A. Hallaj, E. Trofimenko, T. Kalia, Y. Krishnan and C. Widmann, Eur. J. Pharm. Biopharm., 2023, 184, 116–124 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available: Procedures for the synthesis of protected macrocyclic ligands, peptide and conjugates and Ln3+ complexes; characterizations by 1P and 2P spectroscopy; protocols for cell culture, 2P microscopy and MTT proliferation assays. See DOI: https://doi.org/10.1039/d4sc00896k |
| ‡ These authors contributed equally to this work. |
| § Present address: Faculté des Sciences et Technologies, Université de Goma, B.P. 204 Goma, R. D. Congo. |
| This journal is © The Royal Society of Chemistry 2024 |