Open Access Article
Open Access ArticleUnleashing the high energy potential of zinc–iodide batteries: high-loaded thick electrodes designed with zinc iodide as the cathode†
Jingkang
Ma
ab,
Alireza
Azizi
c,
Erhuan
Zhang
d,
Hong
Zhang
*b,
Anqiang
Pan
 *ce and
Ke
Lu
*ce and
Ke
Lu
 *a
*a
aInstitutes of Physical Science and Information Technology, Key Laboratory of Structure and Functional Regulation of Hybrid Materials of Ministry of Education, Anhui University, Hefei, Anhui 230601, China. E-mail: luke@ahu.edu.cn
bSchool of Chemistry and Chemical Engineering, Harbin Institute of Technology, Harbin, Heilongjiang 150001, China. E-mail: zhanghonghit@hit.edu.cn
cSchool of Materials Science and Engineering, Central South University, Changsha 410083, Hunan, China. E-mail: pananqiang@csu.edu.cn
dGlobal Institute of Future Technology, Shanghai Jiao Tong University, Shanghai 200240, China
eSchool of Physics and Technology, Xinjiang University, Urumqi, Xinjiang 830046, China
First published on 23rd February 2024
Abstract
The realization of high energy is of great importance to unlock the practical potential of zinc–iodine batteries. However, significant challenges, such as low iodine loading (mostly less than 50 wt%), restricted iodine reutilization, and severe structural pulverization during cycling, compromise its intrinsic features. This study introduces an optimized, fully zincified zinc iodide loaded onto a hierarchical carbon scaffold with high active component loading and content (82 wt%) to prepare a thick cathode for enabling high-energy Zn–I2 batteries. The synergistic interactions between nitrogen heteroatoms and cobalt nanocrystals within the porous matrix not only provide forceful chemisorption to lock polyiodide intermediates but also invoke the electrocatalytic effects to manipulate efficient iodine conversion. The ZnI2 cathode could effectively alleviate continuous volumetric expansion and maximize the utilization of active species. The electrochemical examinations confirm the thickness-independent battery performance of assembled Zn–I2 cells due to the ensemble effect of composite electrodes. Accordingly, with a thickness of 300 μm and ZnI2 loading of up to 20.5 mg cm−2, the cathode delivers a specific capacity of 92 mA h gcathode−1 after 2000 cycles at 1C. Moreover, the Zn–I2 pouch cell with ZnI2 cathode has an energy density of 145 W h kgcathode−1 as well as a stable long cycle life.
Introduction
Aqueous zinc–iodine (Zn–I2) batteries are promising candidates for large-scale energy storage devices due to their notable attributes, such as high energy/power density, enhanced safety, low cost, and wide availability.1–5 However, the scalable application potentials of Zn–I2 batteries are still compromised by some key inherent problems associated with iodine cathodes, such as limited utilization of active components and the significant volumetric expansion of iodine during repeated discharging/recharging processes.6–10 While engineering the composition and/or structure of composite iodine cathodes provides possible solutions to increase iodine utilization, there is a trade-off in cathode materials between specific capacity and active materials content, thereby compromising the available specific energy of Zn–I2 cells.11–21 According to the latest findings in the available research, the introduction of inactive host materials usually decreases the content of active components (mostly less than 60 wt%), which is significantly different from the necessary conditions for high content in practical composite cathodes (>80 wt%, Fig. 1a).22–26 Furthermore, the structural stability of the iodine cathode plays a pivotal role in determining the cycling life of the assembled practical cells. However, the conventional iodine cathode based on elemental iodine species suffers from a volume expansion of 30.84% in the first zincification. This imposes substantial stress on the host materials, which can lead to cracking and structural pulverization and/or collapse of the cathodes (Fig. 1b).27–30 For this purpose, replacing iodine with fully zincified zinc iodide (ZnI2) paves an attractive pathway for solving the above problem because it experiences a volume shrinkage of 23.52% during dezincification, providing space for subsequent volume expansion of the zincification. Such merit minimizes the structural destruction of the composite cathode and may allow higher loading of ZnI2 compared with I2. Moreover, apart from improving the utilization and stability of the cathode, a thick electrode design has been considered a direct and promising solution to elevate the energy density of cells to reach the practical level this is attributed to its ability to provide much space to enhance the energy density of given electrochemically coupled cells via maximizing the volume ratio of active to inactive materials.31–33 However, based on our current knowledge, the construction of thick electrodes for Zn–I2 batteries has not been reported. Therefore, compared with conventional thin I2 electrodes, it is hypothesized that using well-designed high-loading ZnI2 cathodes with high content and iodine utilization to fabricate the thick electrodes is a feasible approach to achieve high-energy-density Zn–I2 batteries (Fig. 1c). Herein, we report the realization of high-energy Zn–I2 batteries utilizing hierarchical cobalt nanocrystals decorated carbon as the bifunctional matrix for supporting fully zincified ZnI2 with high content up to 82 wt% as novel thick cathodes. The synergistic interactions between heteroatoms and metal nanocrystals not only allow strong physicochemical immobilization of intermediate iodine species but also invoke the electrocatalytic effect to manipulate efficient iodine reversible conversion. Significantly, unlike I2 cathodes, benefiting from the initial shrinkage of the fully zinced ZnI2 cathode during dezincification, the critical issue of continuous volumetric expansion directed irreversible structural pulverization and/or collapse during long cycling can be alleviated within the thick electrode. Notably, the electrochemical examinations confirm the thickness-independent battery performance of assembled Zn–I2 cells due to the ensemble effect of composite electrodes. Accordingly, these features enable the ZnI2 cathode, with an areal mass loading of up to 20.5 mg cm−2 and a thickness of 300 μm, to achieve a high capacity of 92 mA h gcathode−1 after 2000 cycles at 1C. Meanwhile, a proof-of-concept pouch cell using the ZnI2 cathode delivers an energy density of 145 W h kgcathode−1 along with a stable long cycle life. Our work offers a novel insight for developing advanced cathodes to realize practical zinc–iodine electrochemistry.Results and discussion
Structural characterization of the functional matrix
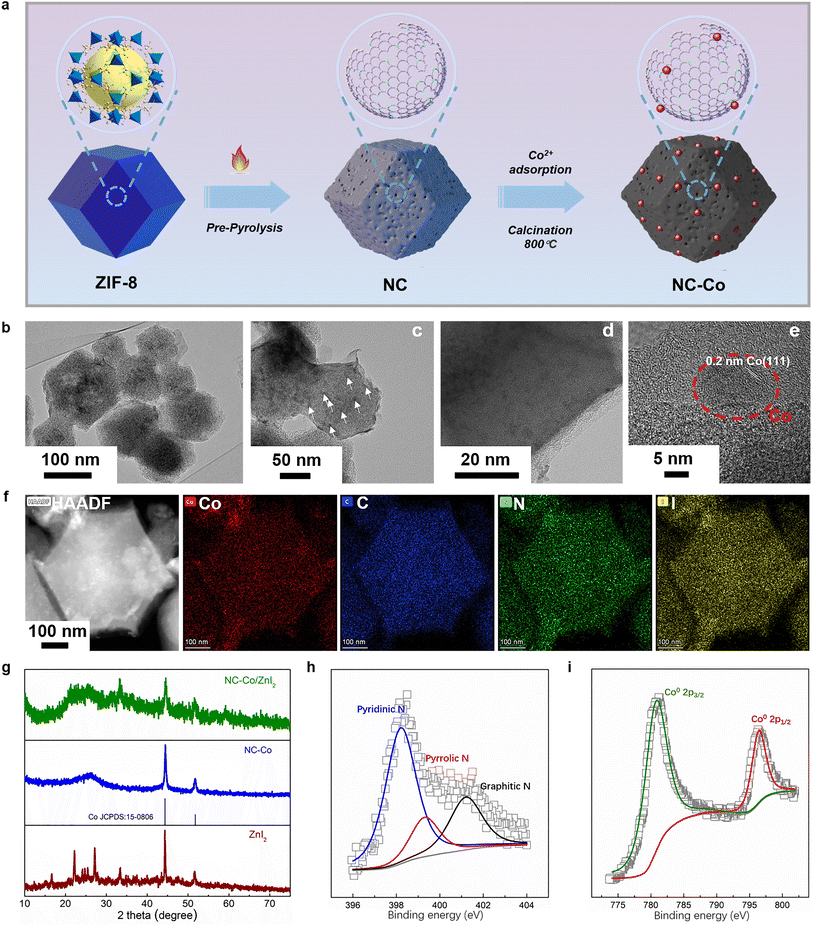
Fig. 2a shows the schematic illustration of the preparation process of NC-Co. Initially, the ZIF-8 nanoparticles were synthesized at room temperature through a standard method.34,35 Subsequently, the porous N-doped carbon (NC) was prepared through the direct pyrolysis of ZIF-8 under the N2 atmosphere. Following that, the NC powder was dispersed in Co(NO3)2 solution to gain the precursor that filled with Co(II) in nanopores.36 After the second thermal activation of this precursor in nitrogen/hydrogen flow, the final product named NC-Co was obtained. The transmission electron microscope (TEM) images of the NC-Co are shown in Fig. 2b–e, where it still maintains a regular rhombic dodecahedral morphology. The highly porous structure and uniformly loaded nanoparticles are clearly observed (Fig. 2c, arrows represent the positions of nanoparticles). High-resolution TEM (HRTEM) shows lattice stripes with a spacing of 0.2 nm, which is consistent with the spacing of the (111) plane of Co, further suggesting that these particles are in the form of monolithic Co nanocrystals and anchored on the NC framework.37–39 As shown in Fig. 2f, HAADF-STEM images and the corresponding EDX elemental maps reveal the uniform distribution of C, N, Co, I elements in the NC-Co composites after ZnI2 loading and further confirm that the ZnI2 was loaded in the pores of the NC-Co rather than randomly attached on the interface. The X-ray powder diffraction (XRD) patterns of ZnI2, NC-Co, and NC-Co/ZnI2 samples are presented in Fig. 2g. Notably, the NC-Co/ZnI2 composite exhibits the crystal peaks of ZnI2, which is consistent with the fact that zinc iodide is uniformly dispersed in the nanopores of the NC-Co framework. In addition, the valence states and compositions of the elements of NC-Co have been studied by X-ray photoelectron spectroscopy (XPS). The resolved N 1s spectrum (Fig. 2h) displays the coexistence of graphite (401.2 eV), pyrrole (400.1 eV), and pyridine (398.6 eV), implying that N has been successfully doped into the carbon matrix.40,41 The two prominent peaks at 780.9 and 796.5 eV in the Co 2p spectrum (Fig. 2i) are assigned to metallic Co, suggesting that the Co species in NC-Co are present as metal states rather than oxidized states.42Investigation of the bifunctional matrix manipulated reversible iodine conversion
High loading of active substances is a prerequisite for realizing high-energy Zn–I2 batteries. The nitrogen adsorption–desorption isotherms (Fig. 3a) are used to investigate the structural information of NC-Co, NC-Co/I2, and NC-Co/ZnI2. As shown, the NC-Co exhibits typical type IV isotherms, hysteresis returns, as well as the presence of high nitrogen adsorption at low pressures, suggesting the coexistence of micropores and mesopores in its structure.43,44 Following iodine impregnation, there is a significant decrease in the N2 adsorption characteristics in the low-pressure region, while the hysteresis return line still exists in the high-pressure region. Comparatively, the minimization of the nitrogen adsorption profile characteristics at both low and high pressures is almost achieved with loaded ZnI2. In addition, calculations performed by Brunauer–Emmert–Teller (BET) show that the specific surface area of NC-Co/ZnI2 (34 m2 g−1) is significantly lower than that of NC-Co/I2 (186 m2 g−1) and NC (988 m2 g−1), suggesting that the ZnI2 is fully encapsulated within the micropores/mesopores of NC-Co compared to I2. The corresponding pore size distributions in Fig. 3b further indicate that the multistage pore structure of NC-Co provides more space for loading high contents of ZnI2 and enables complete infiltration of ZnI2 into its pores, which is consistent with the nitrogen adsorption isotherm. Moreover, NC-Co with abundant polar nitrogen and cobalt sites can enhance the intermolecular interaction force with the same polar molecule ZnI2 through intermolecular dipole–dipole interactions, thus realizing the ZnI2 chemisorption and obtaining a higher content of ZnI2 loading. For these reasons, the zinc iodide realizes loadings of up to 82% (Fig. 3c and S1†).To evaluate the chemical anchoring capability of NC-Co towards polyiodide and the stability of different cathodes during the reaction, the color of the electrolytes for the three electrodes has been compared in the visual vial experiments, as shown in Fig. 3d. It is clearly observed that the color of the electrolyte of the cell with NC-Co/ZnI2 cathode remains colorless after the cycling test, while the electrolyte in the NC-Co/I2 cell turns light yellow, and the color change is even more obvious in the AC/ZnI2 cell. On the one hand, the difference between NC-Co/ZnI2 and AC/ZnI2 is attributed to the chemical affinity of NC-Co towards polyiodides, which can better confine the release of soluble polyiodides. On the other hand, the continuous volume expansion of iodine during cycling and the serious dissolution in the electrolyte result in ineffective locking of polyiodides by the NC-Co/I2 cathode, which exhibits deeper electrolyte color as compared with the NC-Co/I2 cathode. The results of the UV-visible spectra in Fig. 3e further quantified the above speculation.45 As shown, the NC-Co/I2 exhibits much lower absorption peak intensity (0.17) at ∼365 nm compared to AC/ZnI2 (0.41). Encouragingly, the absorption peak of NC-Co/ZnI2 is negligible. This suggests that the synergistic of ZnI2 and NC-Co promise efficient active species confinement. Furthermore, in situ Raman characterization has been conducted to validate the matrix catalytic effect of NC-Co/ZnI2 in Zn–I2 batteries. Initially, the bands at 110 and 164 cm−1 are attributed to the symmetric stretching bands of I3− and I5−, respectively.46–48 For the NC-Co/ZnI2 electrode (Fig. 3f), the peak intensities of I3− and I5− increase with the increase of potential during charging and reach the maximum intensity at 1.6 V. During the discharge process, the peak intensities of I3− and I5− gradually decrease with decreasing the potential, ultimately disappear completely until the discharge potential of approximately 0.4 V. The NC-Co/ZnI2 electrode displays a gradual conversion of iodide (I5− ↔ I3− ↔ I−) as well as excellent reversibility under the charging and discharging potentials. In contrast, during charge/discharge, the incomplete conversion of iodine intermediates within the NC/ZnI2 electrode (Fig. 3g) indicates sluggish kinetics in the reversible conversion of iodine, thus leading to lower conversion efficiency. The strong catalytic effect of the Co catalytic center promotes rapid iodine redox conversion and simultaneously strengthens the inhibition of the polyiodide shuttle (Fig. 3h).
Electrochemical investigation on the cathodic component dependent battery performance
The electrochemical measurements were performed to estimate the effect of the cathodic component on the electrochemical performance of Zn–I2 cells. Note that the majority of composite iodine cathodes in existing investigations incorporate significant amounts of inactive substrates (even up to 50 wt%) to improve the iodine utilization, but diminish the specific energy. Therefore, to obtain practically relevant results, all the specific capacities and current densities are normalized based on the mass of composite cathodes in cells. As shown in Fig. 4a, the AC/ZnI2 delivers a specific capacity of 84 mA h g−1 at 1C (1C = 167 mA g−1), while the introduction of heteroatom catalytic centers can achieve a 28.6% capacity increase (108 mA h g−1 of NC/ZnI2). The implantation of Co hotspots intensifies this pattern, as verified by the notably higher capacity exhibited in NC-Co/ZnI2 (130 mA h g−1) when compared to control electrodes. It is noteworthy that the NC-Co/I2 presents a high specific capacity of 180 mA h g−1 based on the mass of the active component, which is higher than the NC-Co/ZnI2 (150 mA h g−1). However, when calculating specific capacity based on the composite cathode, the capacity sharply decreases to 90 mA h g−1 and is only comparable with the AC/ZnI2 (Fig. 4b). It should be noted that the NC-Co matrix alone without ZnI2 loading have neglectable contribution to the total capacity (Fig. S2†). This significant difference not only highlights the importance of evaluating specific capacity based on the mass of the overall cathode but also confirms the high active component content in maximizing battery performance toward the practical application.The improved cycling stability has been a persistent challenge for Zn–I2 cells, whose structural pulverization of the cathode is caused by continuous volumetric variation during the charging/discharging. Interestingly, using the ZnI2 as the cathodic material addresses this concern. As a proof-of-concept experiment, both the NC-Co/ZnI2 and NC-Co/I2 electrodes, with the same active materials loading (∼3 mg cm−2), performed 100 cycles at 1C. The corresponding SEM analysis was conducted on the cathodes before and after cycling. As shown in Fig. 4c and d, the thickness of the NC-Co/ZnI2 electrode remains nearly unchanged after the cycle, whereas the electrode thickness of NC-Co/I2 experiences an expansion rate of about 52.4%. Such differences reveal that the initial shrinkage of the ZnI2 cathode during dezincification could minimize the continuous volumetric expansion and maintain the high stability of the electrode. Accordingly, the NC-Co/ZnI2 indeed exhibits enhanced cycling stability compared to the NC-Co/I2. Specifically, the NC-Co/ZnI2 cathode allows high-capacity retention of 85.4% after ultra-long cycling over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 1C, which is better than the limited capacity retention of 72.1% for the NC-Co/I2 electrode (Fig. 4e), highlighting the cathodic component-dependent battery performance. By comparison with some representative reports, the NC-Co/ZnI2 cathode demonstrates an outstanding cycling life and competitive specific capacity based on the mass of the composite cathode (Table S1†). Meanwhile, visually, the coin cell with NC-Co/I2 cathode exhibits significant swelling, as depicted in Fig. 4f after the cycling test, while in contrast, there is no observable change in the NC-Co/ZnI2//Zn cell (Fig. 4g), confirming the superiority of ZnI2 as the cathodic material. Moreover, the superior rate capability indicates that the active ZnI2 not only could stabilize the cathode structure but also endow the faster redox kinetics. As shown in Fig. S3,† when the current density is increased 10 times to 10C, the NC-Co/ZnI2 still realizes a high-capacity retention of 79.2%, which is far more than the 47.1% retention observed in NC-Co/I2.
000 cycles at 1C, which is better than the limited capacity retention of 72.1% for the NC-Co/I2 electrode (Fig. 4e), highlighting the cathodic component-dependent battery performance. By comparison with some representative reports, the NC-Co/ZnI2 cathode demonstrates an outstanding cycling life and competitive specific capacity based on the mass of the composite cathode (Table S1†). Meanwhile, visually, the coin cell with NC-Co/I2 cathode exhibits significant swelling, as depicted in Fig. 4f after the cycling test, while in contrast, there is no observable change in the NC-Co/ZnI2//Zn cell (Fig. 4g), confirming the superiority of ZnI2 as the cathodic material. Moreover, the superior rate capability indicates that the active ZnI2 not only could stabilize the cathode structure but also endow the faster redox kinetics. As shown in Fig. S3,† when the current density is increased 10 times to 10C, the NC-Co/ZnI2 still realizes a high-capacity retention of 79.2%, which is far more than the 47.1% retention observed in NC-Co/I2.
Thickness-independent battery performance of the Zn–I2 cells using high-loading electrodes
To improve the energy efficiency of Zn–I2 cells for practical applications, the electrochemical performance of NC-Co/ZnI2 electrodes with different thicknesses is also investigated. Fig. 5a–c show cross-sectional SEM images of NC-Co/ZnI2 cathodes compacted with ZnI2 loadings of 6.8 mg cm−2, 12.8 mg cm−2, and 20.5 mg cm−2, respectively, which correspond to electrode thicknesses of 100, 200 and 300 μm. As shown in Fig. 5d, even at such high area loading, the capacity of NC-Co/ZnI2 still exhibits initial capacities of 120, 115, and 108 mA h gcathode−1, which are much higher than those of NC-Co/I2 under the same conditions (79, 56, and 45 mA h gcathode−1). In the cycling performance (Fig. 5e and f), after 2000 cycles, NC-Co/ZnI2 exhibits a reversible capacity of 107 mA h gcathode−1 for the area mass loading of 6.8 mg cm−2, 102 mA h gcathode−1 for 12.8 mg cm−2, 92 mA h gcathode−1 for 20.5 mg cm−2. In contrast, NC-Co/I2 experiences severe degradation during cycling, providing only the capacity of 52, 27, and 16 mA h gcathode−1. Meanwhile, NC-Co/ZnI2 can maintain almost the same capacity retention at different area loads, whereas the capacity retention of NC-Co/I2 consistently decreases with increasing mass loading (Fig. 5g), which indicates that the increased thickness of the NC-Co/ZnI2 electrode does not influence cell performance. In addition, NC-Co/I2 cells at different area loads exhibit severe swelling after cycling (Fig. 5h). In contrast, cells with NC-Co/ZnI2 display minimal volume changes, underscoring the fact that ZnI2 facilitates the mitigation of volume expansion during cycling. Finally, a pouch cell with NC-Co/ZnI2 cathode is assembled further to demonstrate its practical application in the Zn–I2 system (Fig. 5i). The initial coulombic efficiency is about 94.7% for the first cycle. After the first cycle, the coulombic efficiency reaches 98.3% and a record-high average CE of 99.1% after 100 cycles. Note that the pouch cell exhibits an initial energy density of 145 W h kgcathode−1, and provides a reversible capacity of 90 mA h g−1 after 100 cycles, and it also displays the capability to power a red LED bulb (Fig. 5i, inset), further demonstrating the viability of the NC-Co/ZnI2 cathode for utilization in realistic devices. Actually, the influence of the weight of the anode and electrolyte on the calculation of the energy density of full cells was considered. Lowering the mass loading of metal anode (thin metal anode and low N/P ratio) and E/S ratio are very important for the real application of batteries. Accordingly, the pouch cells assembled with different E/S ratios and the same cathodes were further tested and the results are shown in Table S2.† In accordance, the stability decreases with lower E/S and N/P ratios. Based on all of these aspects, we believe the content of electrolyte determine the upper limit of overall energy density of pouch cell. For future research, lean-electrolyte cells and/or novel electrolyte that could realize the tunable/efficient iodine conversion are need to be explored. Fig. 5j provides a comprehensive comparison of the Zn–I2 cells (coin and pouch) produced in this work with those previously reported studies. The cells in this study exhibit the highest energy density and discharge capacity, confirming the reliability and practicability of the reported system.Conclusion
In summary, we present a practical high-energy Zn–I2 battery that utilizes fully zinced iodide as active components. Hierarchically, cobalt nanocrystals and nitrogen heteroatom decorated carbon were fabricated and used as polar matrix to realize an extremely high active component loading and reaching up to 82 wt%. The introduction of dual catalytic hotspots not only facilitates strong physicochemical immobilization of poly(iodide) intermediates but also manipulates deep and reversible iodine conversion, even after a long cycling test. Experiment results confirm that the ZnI2-based cathodic materials could minimize the continuous volumetric expansion/shrinkage and permit the high reutilization of the active species simultaneously. The ensemble effect of composite electrodes enables the thickness-independent battery performance of as-prepared Zn–I2 cells. Accordingly, the thick cathodes with high ZnI2 loading of up to 20.5 mg cm−2 and thickness of 300 μm exhibit a high capacity of 92 mA h gcathode−1 after 2000 cycles at 1C. Furthermore, the stable Zn–I2 pouch cell with an energy density of 145 W h kgcathode−1 was also demonstrated, highlighting its potential for practical applications.Data availability
Data available on request.Author contributions
K. L. conceived and designed the project. J. M. and H. Z. performed all experimental and mechanism studies and wrote the required scripts. All authors were involved in the analysis of the results and further editing and reviewing process.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
This work was financially supported by the Natural Scientific Foundation of China (22109001, 22208335), Postdoctoral Fellowship Program of CPSF (GZB20230950), and Heilongjiang Postdoctoral Science Foundation (LBH-Z23187).References
- M. L. Wang, J. K. Ma, H. Zhang, L. Fu, X. L. Li and K. Lu, Small, 2023,(1613–6810), 2307021 CrossRef PubMed.
- L. Q. Zhang, H. L. Guo, W. Zong, Y. P. Huang, J. J. Huang, G. J. He, T. X. Liu, J. Hofkens and F. L. Lai, Energy Environ. Sci., 2023, 16, 4872–4925 RSC.
- J. Z. Ma, M. M. Liu, Y. L. He and J. T. Zhang, Angew. Chem., Int. Ed., 2021, 60, 12636–12647 CrossRef CAS PubMed.
- Y. Lyu, J. A. Yuwono, P. T. Wang, Y. Y. Wang, F. H. Yang, S. L. Liu, S. L. Zhang, B. F. Wang, K. Davey, J. F. Mao and Z. P. Guo, Angew. Chem., Int. Ed., 2023, 62, e202303011 CrossRef CAS PubMed.
- Y. H. Kang, G. H. Chen, H. M. Hua, M. H. Zhang, J. Yang, P. X. Lin, H. Y. Yang, Z. H. Lv, Q. L. Wu, J. B. Zhao and Y. Yang, Angew. Chem., Int. Ed., 2023, 62, e202300418 CrossRef CAS PubMed.
- L. T. Ma, Y. R. Ying, S. M. Chen, Z. D. Huang, X. L. Li, H. T. Huang and C. Y. Zhi, Angew. Chem., Int. Ed., 2021, 60, 3791–3798 CrossRef CAS PubMed.
- C. Prehal, H. Fitzek, G. Kothleitner, V. Presser, B. Gollas, S. A. Freunberger and Q. Abbas, Nat. Commun., 2020, 11, 4838 CrossRef CAS PubMed.
- Z. Cheng, H. Pan, F. Li, C. Duan, H. Liu, H. Y. Zhong, C. C. Sheng, G. J. Hou, P. He and H. S. Zhou, Nat. Commun., 2022, 13, 6788 CrossRef PubMed.
- Z. G. Li, X. H. Wu, X. Y. Yu, S. Y. Zhou, Y. Qiao, H. S. Zhou and S. G. Sun, Nano Lett., 2022, 22, 2538–2546 CrossRef CAS PubMed.
- W. W. Zhang, M. L. Wang, H. Zhang, L. Fu, W. L. Zhang, Y. P. Yuan and K. Lu, Chem. Sci., 2023, 14, 12730–12738 RSC.
- D. L. Yu, A. Kumar, T. A. Nguyen, M. T. Nazir and G. Yasin, ACS Sustain. Chem. Eng., 2020, 8, 13769–13776 CrossRef CAS.
- L. J. Yan, T. F. Liu, X. M. Zeng, L. Sun, X. H. Meng, M. Ling, M. Q. Fan and T. L. Ma, Carbon, 2022, 187, 145–152 CrossRef CAS.
- Q. Guo, H. Z. Wang, X. T. Sun, Y. N. Yang, N. Chen and L. T. Qu, ACS Mater. Lett., 2022, 4, 1872–1881 CrossRef CAS.
- H. L. Pan, B. Li, D. H. Mei, Z. M. Nie, Y. Y. Shao, G. S. Li, X. H. S. Li, K. S. Han, K. T. Mueller, V. Sprenkle and J. Liu, ACS Energy Lett., 2017, 2, 2674–2680 CrossRef CAS.
- S. Chen, Y. L. He, S. Y. Ding and J. T. Zhang, J. Phys. Chem. C, 2023, 127, 7609–7617 CrossRef CAS.
- K. Zhang, Q. Yu, J. Sun, Z. Tie and Z. Jin, Adv. Mater., 2023, 36, 2309838 CrossRef PubMed.
- L. Ma, G. Zhu, Z. Wang, A. Zhu, K. Wu, B. Peng, J. Xu, D. Wang and Z. Jin, Nano Lett., 2023, 23, 5272–5280 CrossRef CAS PubMed.
- Y. Zhang, L. Wang, Q. Li, B. Hu, J. Kang, Y. Meng, Z. Zhao and H. Lu, Nano-Micro Lett., 2022, 14, 208 CrossRef CAS PubMed.
- J. Wei, P. Zhang, T. Shen, Y. Liu, T. Dai, Z. Tie and Z. Jin, ACS Energy Lett., 2022, 8, 762–771 CrossRef.
- Y. Feng, Y. Wang, L. Sun, K. Zhang, J. Liang, M. Zhu, Z. Tie and Z. Jin, Small, 2023, 2302650 CrossRef CAS PubMed.
- X. Guo, S. Liu, X. Wan, J. Zhang, Y. Liu, X. Zheng, Q. Kong and Z. Jin, Nano Lett., 2022, 22, 4879–4887 CrossRef CAS PubMed.
- A. Bhargav, J. R. He, A. Gupta and A. Manthiram, Joule, 2020, 4, 285–291 CrossRef.
- K. Lu, Z. Y. Hu, J. Z. Ma, H. Y. Ma, L. M. Dai and J. T. Zhang, Nat. Commun., 2017, 8, 527 CrossRef PubMed.
- X. Y. Yang, H. Q. Fan, F. L. Hu, S. M. Chen, K. Yan and L. T. Ma, Nano-Micro Lett., 2023, 15, 126 CrossRef CAS PubMed.
- G. H. Chen, Y. H. Kang, H. Y. Yang, M. H. Zhang, J. Yang, Z. H. Lv, Q. L. Wu, P. X. Lin, Y. Yang and J. B. Zhao, Adv. Funct. Mater., 2023, 33, 2300656 CrossRef CAS.
- J. He, H. Hong, S. Hu, X. Zhao, G. Qu, L. Zeng and H. Li, Nano Energy, 2024,(119), 109096 CrossRef CAS.
- C. Bai, F. S. Cai, L. C. Wang, S. Q. Guo, X. Z. Liu and Z. H. Yuan, Nano Res., 2018, 11, 3548–3554 CrossRef CAS.
- L. Q. Zhang, M. J. Zhang, H. L. Guo, Z. H. Tian, L. F. Ge, G. J. He, J. J. Huang, J. T. Wang, T. X. Liu, I. P. Parkin and F. L. Lai, Adv. Sci., 2022, 9, 2105598 CrossRef CAS PubMed.
- H. Chen, X. Li, K. Q. Fang, H. Y. Wang, J. Q. Ning and Y. Hu, Adv. Energy Mater., 2023, 13, 2302187 CrossRef CAS.
- X. L. Li, N. Li, Z. D. Huang, Z. Chen, G. J. Liang, Q. Yang, M. Li, Y. W. Zhao, L. T. Ma, B. B. Dong, Q. Huang, J. Fan and C. Y. Zhi, Adv. Mater., 2021, 33, 2006897 CrossRef CAS PubMed.
- Z. L. Han, S. P. Li, R. Y. Xiong, Z. P. Jiang, M. J. Sun, W. Hu, L. F. Peng, R. J. He, H. M. Zhou, C. Yu, S. J. Cheng and J. Xie, Adv. Funct. Mater., 2022, 32, 2108669 CrossRef CAS.
- S. B. Tu, Z. H. Chen, B. Zhang, X. C. Wang, R. M. Zhan, C. H. Li and Y. M. Sun, Nano Lett., 2022, 22, 5982–5989 CrossRef CAS PubMed.
- J. Y. Wu, X. Zhang, Z. Y. Ju, L. Wang, Z. Y. Hui, K. Mayilvahanan, K. J. Takeuchi, A. C. Marschilok, A. C. West, E. S. Takeuchi and G. H. Yu, Adv. Mater., 2021, 33, 2101275 CrossRef CAS PubMed.
- Z. C. Yan, Y. R. Liang, W. B. Hua, X. G. Zhang, W. H. Lai, Z. Hu, W. L. Wang, J. Peng, S. Indris, Y. X. Wang, S. L. Chou, H. K. Liu and S. X. Dou, ACS Nano, 2020, 14, 10284–10293 CrossRef CAS PubMed.
- X. F. Zhou, Z. X. Yu, Y. Yao, Y. Jiang, X. H. Rui, J. Q. Liu and Y. Yu, Adv. Mater., 2022, 34, 2200479 CrossRef CAS PubMed.
- J. Z. Li, H. G. Zhang, W. Samarakoon, W. T. Shan, D. A. Cullen, S. Karakalos, M. J. Chen, D. M. Gu, K. L. More, G. F. Wang, Z. X. Feng, Z. B. Wang and G. Wu, Angew. Chem., Int. Ed., 2019, 58, 18971–18980 CrossRef CAS PubMed.
- Y. Y. Xue, Y. B. Guo, Q. M. Zhang, Z. J. Xie, J. P. Wei and Z. Zhou, Nano-Micro Lett., 2022, 14, 162 CrossRef CAS PubMed.
- W. Y. Du, K. Q. Shen, Y. R. Qi, W. Gao, M. L. Tao, G. Y. Du, S. J. Bao, M. Y. Chen, Y. M. Chen and M. W. Xu, Nano-Micro Lett., 2021, 13, 50 CrossRef CAS PubMed.
- J. R. Mou, Y. J. Li, T. Liu, W. J. Zhang, M. Li, Y. T. Xu, L. Zhong, W. H. Pan, C. H. Yang, J. L. Huang and M. L. Liu, Small Methods, 2021, 5, 2100455 CrossRef CAS PubMed.
- T. T. Liu, H. J. Wang, C. J. Lei, Y. Mao, H. Q. Wang, X. He and X. Liang, Energy Storage Mater., 2022, 53, 544–551 CrossRef.
- J. T. Zhang, Z. H. Zhao, Z. H. Xia and L. M. Dai, Nat. Nanotechnol., 2015, 10, 444–452 CrossRef CAS PubMed.
- S. H. Liu, J. Li, X. Yan, Q. F. Su, Y. H. Lu, J. S. Qiu, Z. Y. Wang, X. D. Lin, J. L. Huang, R. L. Liu, B. N. Zheng, L. Y. Chen, R. W. Fu and D. C. Wu, Adv. Mater., 2018, 30, 1706895 CrossRef PubMed.
- H. Zhang, B. Song, W. W. Zhang, B. W. An, L. Fu, S. T. Lu, Y. W. Cheng, Q. W. Chen and K. Lu, Angew. Chem., Int. Ed., 2023, 62, e202217009 CrossRef CAS PubMed.
- Y. R. Qi, Q. J. Li, Y. K. Wu, S. J. Bao, C. M. Li, Y. M. Chen, G. X. Wang and M. W. Xu, Nat. Commun., 2021, 12, 6347 CrossRef CAS PubMed.
- W. Z. Gao, S. T. Cheng, Y. X. Zhang, E. R. Xie and J. C. Fu, Adv. Funct. Mater., 2023, 33, 2211979 CrossRef CAS.
- S. J. Zhang, J. N. Hao, H. Li, P. F. Zhang, Z. W. Yin, Y. Y. Li, B. K. Zhang, Z. Lin and S. Z. Qiao, Adv. Mater., 2022, 34, 2201716 CrossRef CAS PubMed.
- H. J. Yang, Y. Qiao, Z. Chang, H. Deng, P. He and H. S. Zhou, Adv. Mater., 2020, 32, 2004240 CrossRef CAS PubMed.
- M. M. Liu, Q. W. Chen, X. Y. Cao, D. X. Tan, J. Z. Ma and J. T. Zhang, J. Am. Chem. Soc., 2022, 144, 21683–21691 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc00276h |
| This journal is © The Royal Society of Chemistry 2024 |