Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Highly selective, reversible water activation by P,N-cooperativity in pyridyl-functionalized phosphinines†‡
Richard O.
Kopp§
a,
Sabrina L.
Kleynemeyer§
 a,
Lucie J.
Groth
a,
Moritz J.
Ernst
a,
Susanne M.
Rupf
a,
Lucie J.
Groth
a,
Moritz J.
Ernst
a,
Susanne M.
Rupf
 a,
Manuela
Weber
a,
Laurence J.
Kershaw Cook
b,
Nathan T.
Coles
a,
Manuela
Weber
a,
Laurence J.
Kershaw Cook
b,
Nathan T.
Coles
 *ac,
Samuel E.
Neale
*ac,
Samuel E.
Neale
 *d and
Christian
Müller
*d and
Christian
Müller
 *a
*a
aInstitute of Chemistry and Biochemistry, Freie Universität Berlin, Fabeckstr. 34/36, 14195 Berlin, Germany. E-mail: c.mueller@fu-berlin.de
bDepartment of Chemistry and Materials Innovation Factory, University of Liverpool, Crown Street, Liverpool L69 7ZD, UK
cSchool of Chemistry, University of Nottingham, University Park, Nottingham NG7 2RD, UK. E-mail: Nathan.Coles@nottingham.ac.uk
dDepartment of Chemistry, University of Bath, Claverton Down, Bath BA2 7AY, UK. E-mail: sen36@bath.ac.uk
First published on 14th March 2024
Abstract
Tetrapyridyl-functionalized phosphinines were prepared and structurally characterized. The donor-functionalized aromatic phosphorus heterocycles react highly selectively and even reversibly with water. Calculations reveal P,N-cooperativity for this process, with the flanking pyridyl groups serving to kinetically enhance the formal oxidative addition process of H2O to the low-coordinate phosphorus atom via H-bonding. Subsequent tautomerization forms 1,2-dihydrophosphinine derivatives, which can be quantitatively converted back to the phosphinine by applying vacuum, even at room temperature. This process can be repeated numerous times, without any sign of decomposition of the phosphinine. In the presence of CuI·SMe2, dimeric species of the type ([Cu2I2(phosphinine)]2) are formed, in which each phosphorus atom shows the less common μ2-bridging 2e−-lone-pair-donation to two Cu(I)-centres. Our results demonstrate that fully unsaturated phosphorus heterocycles, containing reactive P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds, are interesting candidates for the activation of E–H bonds, while the aromaticity of such compounds plays an appreciable role in the reversibility of the reaction, supported by NICS calculations.
C double bonds, are interesting candidates for the activation of E–H bonds, while the aromaticity of such compounds plays an appreciable role in the reversibility of the reaction, supported by NICS calculations.
Introduction
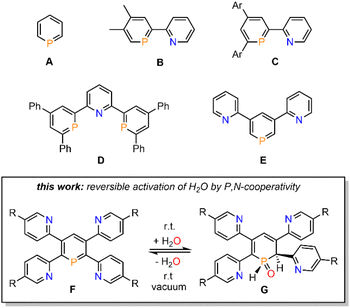
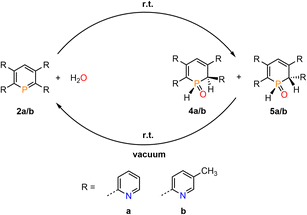
Phosphinines (A, Fig. 1), the fully unsaturated six-membered phosphorus heterocycles, are currently undergoing a renaissance as they provide new directions in the fields of coordination chemistry, homogeneous catalysis, activation of small molecules and photoluminescent molecular materials.1Functionalized phosphinines are of particular interest, as the incorporation of additional substituents within the aromatic framework allows for a modification of the stereo-electronic properties of the resulting phosphorus heterocycles. These alterations enable interesting coordination motifs, optical properties, and pronounced reactivities towards small molecules, such as CO2, alkynes, alkenes, esters, alcohols or amines.1d,e,f,l The development of pyridyl-functionalized phosphinine derivatives, that contain both hard (N-atom) and soft (P-atom) donor atoms (P,N-hybrid ligands), allows generation of novel coordination compounds, which would not be accessible with monodentate phosphinines due to their overall poor net-donor properties.2 However, such compounds are rare, as their synthesis can be rather tedious and usually requires multiple steps. The only examples of pyridyl-substituted phosphinines reported to date are summarized in Fig. 1. NIPHOS, a phosphorus derivative of 2,2′-bipyridine (B), was reported by Mathey and co-workers in 1982.3 Based on the modular pyrylium-salt route, developed by Märkl in 1966, our group has accessed the pyridyl-phosphinine C and extensively explored its coordination chemistry and reactivity, including metal–ligand-cooperativity to activate small molecules.1a,l,4
In a similar way, a pyridyl-bridged diphosphinine (D) could be prepared, which shows unusual coordination behaviour towards Cu(I) compared to the structurally related terpyridine.5 Avarvari, Le Floch and Mathey, who pioneered the 1,3,2-diazaphosphinine route, reported on the polyfunctionalized phosphinine E, containing two remote pyridyl-groups.6 During the course of our investigations on the synthesis, coordination chemistry and reactivity of functionalized λ3-phosphinines, we found that tetrapyridyl-substituted phosphinines (F) readily and quantitatively react with H2O to the corresponding 1,2-dihydrophosphinine oxide derivatives (G). Notably, this reaction turned out to be fully reversible, even at room temperature, and prompted us to explore the H2O-addition to the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond and follow-up chemistry in more detail.
C double bond and follow-up chemistry in more detail.
Results and discussion
We recently started to investigate the reactivity of λ3-phosphinines in small molecule activations. Through this, we uncovered that the nature of the substituent(s) within the aromatic phosphorus heterocycle plays a crucial role in its reactivity towards alcohols and primary amines.1d,2b Based on our intriguing results in the past on the diverse chemistry of the mono-pyridyl-functionalized phosphinines C and D (Fig. 1), we strived to develop tetrapyridyl-substituted phosphinines 2a/b. For comparative reasons, the structurally related and literature known 2,3,5,6-tetraphenyl-substituted phosphinine 3 was also prepared (Scheme 1).6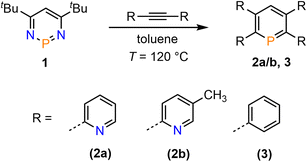 | ||
| Scheme 1 Double [4 + 2]-cycloaddition-cycloreversion reaction for the synthesis of phosphinines 2a/b and 3. | ||
Compounds 2a/b and 3 were synthesized starting from diazaphosphinine 1.6 While the commercially available diphenylacetylene was used for the synthesis of 3, the pyridyl-substituted acetylenes 1,2-bis(pyridin-2-yl)ethyne and 1,2-bis(5-methylpyridin-2-yl)ethyne, needed for the preparation of 2a/b, were obtained via Stille-coupling from 1,2-bis(tributyltin)-ethyne and the corresponding bromo-pyridines.7 Phosphinines 2a/b and 3 were obtained as off-white solids in moderate to good yields and show the typical downfield resonances in their 31P{1H} NMR spectra (2a: δ (ppm) = 213.9; 2b: δ (ppm) = 211.3; 3: δ (ppm) = 206.2). Single crystals of 2a, suitable for X-ray diffraction, were obtained by layering a solution of 2a in CH2Cl2 with acetonitrile. Crystals of 2b could also be obtained from slow evaporation of a concentrated toluene solution (Fig. S4.1‡). The results of the X-ray structural analysis of 2a are depicted in Fig. 2. The crystallographic characterization of 2a shows the expected connectivity of the atoms, with the phosphinine core exhibiting inversion disorder across a special position, typical for species which show high symmetry.8
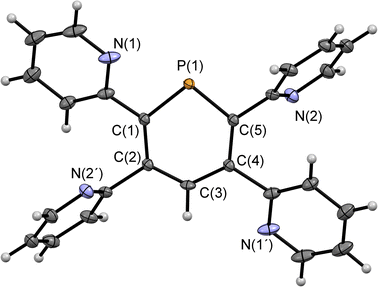 | ||
| Fig. 2 Molecular structure of 2a in the crystal. Thermal ellipsoids given at 50% probability. Atoms marked with ‘are symmetry generated using the following equation: 1−X, 1−Y, 1−Z. | ||
While working up the reaction depicted in Scheme 1, a slight colour change was noticed, when a solution of 2a/b in CDCl3 was kept open to air. Interestingly, it turned out that the phosphorus heterocycles are very sensitive towards water. This is surprising, as phosphinines typically do not react with H2O. The diazaphosphinine 1 is a highly reactive, very air- and moisture sensitive compound and forms a 1,2-dihydro-1,3,2-diazaphosphinine oxide in the presence of traces of H2O.6a Compound 1 also reacts with HCl under formation of a chlorodiazaphosphacycle.9 However, so far only a single example was reported by Le Floch and co-workers, in which an SPS-based pincer-type “classical” phosphinine was found to react with H2O.10 Initial, yet inconclusive calculations revealed, that the sulfur-functionality of the additional Ph2P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S-groups in 2- and 6-position of the phosphorus heterocycle plays an important role in the addition of H2O across the P
S-groups in 2- and 6-position of the phosphorus heterocycle plays an important role in the addition of H2O across the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond (vide infra). Indeed, we observed that neither the pyridyl-functionalized phosphinine C (Fig. 1), nor the reference compound 3 (Scheme 1) react with H2O, even at elevated temperatures.
C double bond (vide infra). Indeed, we observed that neither the pyridyl-functionalized phosphinine C (Fig. 1), nor the reference compound 3 (Scheme 1) react with H2O, even at elevated temperatures.
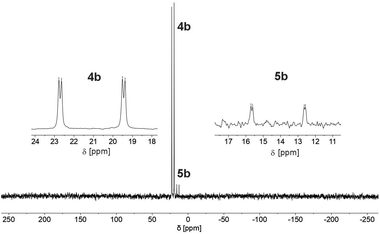
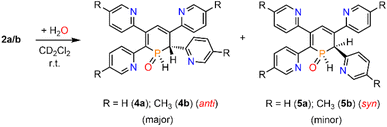
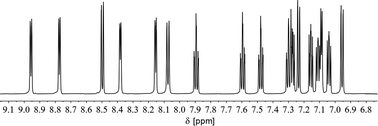
We therefore decided to investigate this rather intriguing reaction of an aromatic phosphorus heterocycle with H2O in more detail. After addition of 10 equiv. of H2O to a solution of 2b in CD2Cl2, the quantitative conversion of the starting material to a new species (4b) was observed after approximately 18 hours. The main product shows a singlet in the 31P{1H} NMR spectrum at δ (ppm) = 21.1 (Fig. 3).
A second, minor resonance (5b) is formed, which displays a signal in the 31P{1H} NMR spectrum at δ (ppm) = 14.1. In the proton coupled 31P NMR spectrum of the reaction mixture, both signals consist of a doublet of doublet, with coupling constants of 1JP–H = 530 Hz and 2JP–H = 22.0 Hz for the major product, and 1JP–H = 498 Hz and 2JP–H = 16.5 Hz for the minor one (Fig. 3). The final ratio between both species of 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 remains constant, even after prolonged monitoring the sample for up to two months. This observation, along with 1H and 13C{1H} NMR spectroscopic data (vide infra), is consistent with the formation of a diastereomeric pair of 1,2-dihydrophosphinine oxide derivates (4b/5b), depicted in Scheme 2. While a chemical shift difference of Δδ (ppm) = 7 in the 31P NMR spectra is rather large when compared to non-cyclised phosphine oxide diastereomers it is comparable to what is observed for diasteromeric phosphole heterocycles, for which chemical shift differences of Δδ (ppm) = ∼10 have been observed in the corresponding 31P NMR spectra.11,12
3 remains constant, even after prolonged monitoring the sample for up to two months. This observation, along with 1H and 13C{1H} NMR spectroscopic data (vide infra), is consistent with the formation of a diastereomeric pair of 1,2-dihydrophosphinine oxide derivates (4b/5b), depicted in Scheme 2. While a chemical shift difference of Δδ (ppm) = 7 in the 31P NMR spectra is rather large when compared to non-cyclised phosphine oxide diastereomers it is comparable to what is observed for diasteromeric phosphole heterocycles, for which chemical shift differences of Δδ (ppm) = ∼10 have been observed in the corresponding 31P NMR spectra.11,12
For a better understanding of the structural characteristics of the obtained products, additional spectroscopic and crystallographic studies were performed. The assignment of the recorded signals in the 1H and 1H{31P} NMR spectra, in combination with 13C{1H} NMR spectroscopy and the respective 2D NMR spectroscopic data, led finally to the successful identification of both diastereomers.
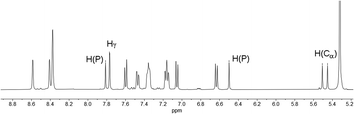
In fact, the 1H NMR spectrum of the reaction mixture reveals the P–H proton as a doublet resonance with a chemical shift of δ (ppm) = 7.16 and a coupling constant of 1JH–P = 528 Hz (Fig. 4, H(P)/H(P)). This coupling constant is consistent with the coupling constant detected for the major diastereomer signal in the 31P NMR spectrum.
The hydrogen atom which shows a 2JP–H coupling in the 31P NMR spectrum can be assigned to the doublet with a chemical shift of δ (ppm) = 5.48 and a coupling constant of 2JH–P = 21.9 Hz in the 1H NMR spectrum. This hydrogen atom couples via1JC–H coupling to a tertiary carbon atom at δ (ppm) = 46.7 with a coupling constant of 1JC–P = 64 Hz in the corresponding HMQC spectrum and can therefore be assigned to a Cα atom (H(Cα)). The hydrogen atom in the 4-position of the heterocycle (Hγ) shows a chemical shift of δ (ppm) = 7.77 and a coupling constant of 4JH–P = 1.8 Hz in the 1H NMR spectrum. The chemical inequivalence of the four pyridyl moieties is also observed with chemical shifts of δ (ppm) = 2.36, 2.29, 2.23 and 2.22 for the methyl groups in the 1H NMR spectrum. These assignments are also in accordance with the recorded phosphorus decoupled 1H{31P} NMR spectra. Based on these findings, the major species can be assigned to the 1,2-dihydrophosphinine oxide 4b. It is worth noting that the corresponding H(Cα) cannot be clearly located in the 1H and 13C{1H} spectra for 5b. However, three small methyl signals can be detected, suggesting asymmetry of the methyl groups as expected for 5b (Fig. S2.19‡).
Similar observations also arise for the formation of 4a/5a. The H(Cα) of 4a is found at δ (ppm) = 5.62 in the 1H NMR spectrum with a coupling constant of 2JH–P = 22 Hz. The corresponding carbon signal was found at δ (ppm) = 47.4 in the 13C{1H} spectrum with a coupling of 1JC–P = 64 Hz. For this species a second set of signals for the minor diastereomer can also be observed. This compound shows a minor doublet-of-doublets at δ (ppm) = 5.42 with couplings of 2JH–P = 17 Hz and 3JH–H = 3 Hz in the 1H NMR spectrum (Fig. S2.13‡). The 13C{1H} signal appears also as a doublet at δ (ppm) = 44.9 with a coupling constant of 1JC–P = 65 Hz (Fig. S2.14‡).
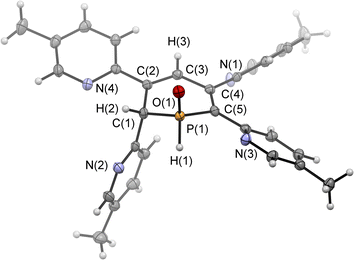
Single crystals of the major diastereomer 4b, suitable for X-ray diffraction analysis, were finally obtained from layering a dried sample of 4b in dichloromethane with acetonitrile and subsequent slow evaporation of the solvent. The molecular structure of 4b in the crystal is depicted in Fig. 5, along with selected bond lengths and angles.
The solid-state structure is indeed in accordance with the proposed structure of a 1,2-dihydrophosphinine oxide and related to the reported data of Le Floch et al. on their 1,2-dihydrophosphinine oxide.10 The major diastereomer shows an anti-orientation of the two hydrogen atoms arising from the water addition across the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond (H–Cα/H–P) The phosphorus atom adopts the expected tetrahedral geometry with a C(1)–P(1)–C(5) angle of 103.6(5)°. The alternation between the C–C single and double bonds in the heterocyclic ring is evident from the smaller and larger bond lengths between C(1)–C(2)/C(3)–C(4) and C(2)–C(3)/C(4)–C(5), respectively. The P(1)–O(1) bond length of 1.482(2) Å is in accordance with other reported phosphine oxides found in the literature (Ph3P
C double bond (H–Cα/H–P) The phosphorus atom adopts the expected tetrahedral geometry with a C(1)–P(1)–C(5) angle of 103.6(5)°. The alternation between the C–C single and double bonds in the heterocyclic ring is evident from the smaller and larger bond lengths between C(1)–C(2)/C(3)–C(4) and C(2)–C(3)/C(4)–C(5), respectively. The P(1)–O(1) bond length of 1.482(2) Å is in accordance with other reported phosphine oxides found in the literature (Ph3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O: 1.46(1) Å).13 The minor diastereomer 5b is consequently a species, in which the two hydrogen atoms arising from the water addition are oriented in a syn-fashion with respect to one another.
O: 1.46(1) Å).13 The minor diastereomer 5b is consequently a species, in which the two hydrogen atoms arising from the water addition are oriented in a syn-fashion with respect to one another.
The products of the water addition are stable in the solid state and can be stored under inert conditions for approximately two weeks at room temperature. Much to our surprise, however, we found that upon warming a sample of 4b/5b to T = 95 °C, the aromatic phosphinine 2b reforms. At this temperature a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 was detected spectroscopically (2b:4b/5b). A similar observation was made for 4a/5a and phosphinine 2a.
9 was detected spectroscopically (2b:4b/5b). A similar observation was made for 4a/5a and phosphinine 2a.
This indicates that the water addition to the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond is reversible and a slightly exergonic process. Most strikingly, it was possible to render the H2O addition to phosphinine 2b fully reversible. When removing the solvent of a freshly prepared solution of 4b/5b under vacuum, a gradual loss of H2O and formation of 2b is observed. With additional gentle heating to T = 40 °C, the recorded 31P and 1H NMR spectra of the residue show the exclusive presence of phosphinine 2b (Fig. 6 and Scheme 3).
C double bond is reversible and a slightly exergonic process. Most strikingly, it was possible to render the H2O addition to phosphinine 2b fully reversible. When removing the solvent of a freshly prepared solution of 4b/5b under vacuum, a gradual loss of H2O and formation of 2b is observed. With additional gentle heating to T = 40 °C, the recorded 31P and 1H NMR spectra of the residue show the exclusive presence of phosphinine 2b (Fig. 6 and Scheme 3).
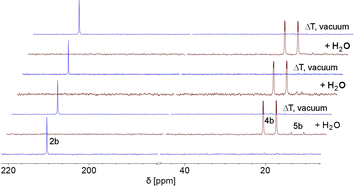 | ||
| Fig. 6 Reversible reaction of 2b with H2O to 4b and traces of 5b, monitored by 31P NMR spectroscopy in CD2Cl2 (3 cycles). | ||
Measuring the 31P NMR spectrum against an internal standard revealed a quantitative spectroscopic yield. The procedure can be repeated numerous times (Fig. 6 and Scheme 3), without the formation of any detectable side or decomposition products. A similar situation was observed for the re-formation of 2a from 4a/5a. However, after several cycles, the formation of traces of impurities could be detected by means of 31P NMR spectroscopy for this particular case.
To review the sensitivity of the water addition, the reaction between 2a and varying equivalents of H2O was monitored via UV/vis spectroscopic measurements (ESI,‡ Chapter 3). Despite the extremely low concentrations of these samples, the formation of 4a/5a could still be observed in form of a new absorption signal at λ = 355 nm when using more than five equivalents of water (Fig. S3.1–3‡).
Apart from these spectroscopic insights, we further found a correlation between the time needed for full conversion of 2b with H2O and the nature of the solvent. While the total reaction time both in dichloromethane and ethanol amounted to 18 h (vide supra), it could be significantly reduced when changing the solvent for DME, DMF, DMSO, (4 h each) pyridine (5 h) and methanol (8 h). Interestingly, the use of chloroform resulted in a complete conversion after only 30 min. A correlation between the relative solvent polarities and the reaction times was, on the other hand, not found.14 A change in the final product ratio between the diastereomers 4b/5b and (97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) was not observed either. Analogous observations were made for the reaction of 2a with H2O.
3) was not observed either. Analogous observations were made for the reaction of 2a with H2O.
We could demonstrate here for the first time, that a phosphinine can fully reversibly react with H2O, even at room temperature. Neither a reversible H2O-addition to the diazaphosphinine 1, nor to the Ph2P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S-substituted phosphinine have been reported (vide supra). We believe, that our findings are particularly interesting, as reports on the reversible activation of small molecules by main group compounds are still extremely rare. Typically, geometrically constrained phosphorus(III) compounds, such as those reported by Radosevich, Goicoechea and others, have proven to be excellent candidates for the activation of E–H bonds. However, in most cases, these transformations are usually irreversible.15 Nevertheless, a reversibility of such processes is a key to the development of catalytic reactions based on elements other than transition metals.
S-substituted phosphinine have been reported (vide supra). We believe, that our findings are particularly interesting, as reports on the reversible activation of small molecules by main group compounds are still extremely rare. Typically, geometrically constrained phosphorus(III) compounds, such as those reported by Radosevich, Goicoechea and others, have proven to be excellent candidates for the activation of E–H bonds. However, in most cases, these transformations are usually irreversible.15 Nevertheless, a reversibility of such processes is a key to the development of catalytic reactions based on elements other than transition metals.
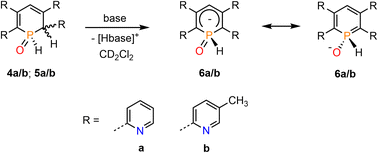
We have recently shown that water-addition products of cationic Rh(III) and Ir(III) complexes, containing the 2,4-diphenyl-6-pyridylphosphinine (C, Fig. 1, Ar![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Ph) as ligand, can undergo deprotonation reactions upon addition of a base.16 We were therefore interested whether 4a/b (5a/b) can be converted into the related λ5-phosphinin-1-olates 6a/b as well (Scheme 4).
Ph) as ligand, can undergo deprotonation reactions upon addition of a base.16 We were therefore interested whether 4a/b (5a/b) can be converted into the related λ5-phosphinin-1-olates 6a/b as well (Scheme 4).
We first applied relatively weak bases such as pyridine and imidazole in excess. As this led to no conversion of the starting material 4b/5b, the bases were changed for the considerably stronger isopropylamine and triethylamine. The attempt with iPrNH2 resulted in 80% conversion to 6b after 1 h but the product started to decompose shortly after. Reaction of 4b/5b with stoichiometric amounts of NEt3 at room temperature gave only 20% conversion of the starting material after 3 days, while gentle heating to T = 40 °C led to decomposition of the sample. When using an excess of 10 equiv. of NEt3, the conversion could be increased to 40% after 2 h but did not further proceed over the next few days. The base was therefore changed again, now using the slightly more basic piperidine. In a first attempt an excess of 10 equiv. of base immediately led to decomposition of the starting material. When only 2.0 equiv. of piperidine were used, an instantaneous conversion of the starting material to phosphininolate 6b was observed in the proton-coupled 31P NMR spectrum, also indicated by an immediate colour change from yellow to dark red. The product depicted a doublet for the P–H bond with a chemical shift of δ = 7.74 ppm and a P–H coupling constant of 1JP–H = 527 Hz. However, the NMR spectra showed the formation of at least three side-products, whose amounts increased over time relative to 6b. After 5 h, 6b was completely decomposed.
Finally, the use of lithium diisopropyl amide (LDA) resulted in the complete conversion of 4b/5b and sole formation of phosphininolate 6b, as evidenced by 31P NMR spectroscopy. The newly observed doublet with a chemical shift of δ (ppm) = 7.7 exhibits only a 1JP–H coupling constant of 528 Hz, confirming the successful elimination of the proton at the α-carbon atom. More importantly, only one product signal was obtained in the recorded NMR spectrum (Fig. 7).
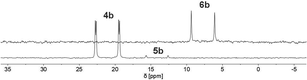 | ||
| Fig. 7 31P NMR spectra (CD2Cl2) of 1,2-dihydrophosphinine oxide 4b (bottom) and its deprotonation product, phosphinine-1-olate 6b, after reaction with LDA (top). | ||
A strong bright orange fluorescence of the sample, which could be stored at T = −21 °C for several days, was also noticed. It should be noted here that λ5-phosphinines often show strong fluorescence, while their electronic structure has been addressed computationally.17 An analogous reaction of 4b/5b with 1.1 equiv. of n-BuLi allowed for isolation of the product as a bright red powder in 90% yield. The product signals could be completely assigned by means of 2D NMR spectroscopy (1H/13C HMQC/HMBC). The spectroscopic analysis confirmed the proposed structure of 6b. Additionally, the formation of the symmetrical λ5-phosphinine 6b compared to the starting material leads to the overall reduced number of signals in the recorded 1H NMR spectrum of 6b, indicating a larger number of chemically equivalent nuclei. More importantly, when comparing the aromatic region of the 1H NMR spectrum of 6b to the one of 4b/5b, the lack of a signal for the hydrogen atom in the 2-position is indeed noticeable. Simultaneously, the signal belonging to the phosphorus-bound hydrogen atom is significantly down-field shifted in 6b, while the C(4)-bound hydrogen atom Hγ is strongly shifted upfield compared to the one in 4b/5b. Similar observations were made in the deprotonation of 4a/5a.
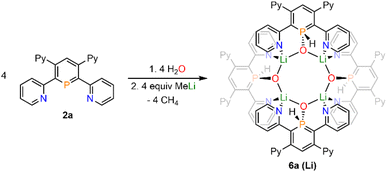
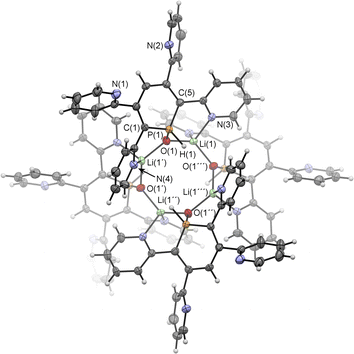
Upon adding first H2O and subsequently MeLi/Et2O to a solution of 2a in CD2Cl2 we could finally obtain needle-shaped orange, slightly fluorescent crystals of 6a(Li) after a few hours from the reaction mixture. The crystallographic representation of this compound shows that 6a(Li) exists as a tetramer in the solid state, which accounts for its very low solubility in common solvents (Scheme 5 and Fig. 8).
Having investigated the reaction of 2a/b with H2O in detail, we anticipated that other small molecules, such as alcohols, could be activated in a similar manner. Phosphinine 2b was dissolved in methanol, serving as the reactant and the solvent at the same time, and the reaction was monitored by means of 31P NMR spectroscopy at room temperature over several days. Much to our surprise, no reaction between 2b and methanol was observed. The same result was obtained under similar conditions for the attempted reaction of 2b with ethanol, as well as with the more acidic phenol in DMSO. Phosphinine 2b thus turned out to exclusively activate H2O. As mentioned above, this is in clear contrast to most phosphinine metal complexes and phosphinine sulfides/selenides, which generally react with a multitude of protic reagents. To probe the differences in H2O addition reactivity between the tetrapyridyl-functionalized phosphinine (2b), 2,4-diphenyl-6-pyridylphosphinine (C), and the reference compound 2,3,5,6-tetraphenylphosphinine (3), a computational mechanistic study was undertaken using density functional theory (at the M052X-D3(CPCM![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2Cl2)/def2-QZVPP//TPSS/def2-TZVPP level of theory). The resulting characterized free energy profile (in kcal mol−1) for the addition and subsequent formal oxidative addition of the O–H bond to the phosphorus atom of 2b and 2,4-diphenyl-6-pyridylphosphinine (C) is presented in Fig. 9.
CH2Cl2)/def2-QZVPP//TPSS/def2-TZVPP level of theory). The resulting characterized free energy profile (in kcal mol−1) for the addition and subsequent formal oxidative addition of the O–H bond to the phosphorus atom of 2b and 2,4-diphenyl-6-pyridylphosphinine (C) is presented in Fig. 9.
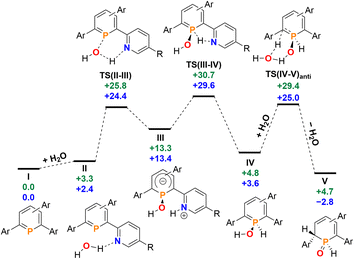 | ||
Fig. 9 Free energy profile (calculated with DFT at the M052X-D3(CPCM![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2Cl2)/def2-QZVPP//TPSS/def2-TZVPP level of theory, energies in kcal mol−1) of H2O addition at 2b (blue) and at 2,4-phenyl-6-pyridylphosphinine (C, green). Note: each structure is labelled with a subscript “2b” (i.e.I2b) and “C” (IC) in the ESI‡ to distinguish between the identity of the phosphinine. CH2Cl2)/def2-QZVPP//TPSS/def2-TZVPP level of theory, energies in kcal mol−1) of H2O addition at 2b (blue) and at 2,4-phenyl-6-pyridylphosphinine (C, green). Note: each structure is labelled with a subscript “2b” (i.e.I2b) and “C” (IC) in the ESI‡ to distinguish between the identity of the phosphinine. | ||
The mechanistic study focussed first on the initial interaction of H2O with the low-coordinated phosphorus centre via one of the oxygen lone-pairs. We anticipated that a subsequent formal oxidative addition of the O–H bond to the phosphorus atom occurs at the tetramethyl-tetrapyridyl-phosphinine I (2b). The corresponding phosphinin-1-ol then undergoes fast tautomerization, leading to the experimentally observed 1,2-dihydrophosphinine oxide derivatives.9 A direct oxidative addition at the phosphorus centre via a three-membered transition state was first considered and modelled, but subsequently discounted given the excessively high energy of the corresponding transition state, TS(OA) (+62.8 kcal mol−1, Fig. S2d‡). This led to the consideration that the flanking pyridyl groups serve to kinetically enhance the formal oxidative addition process via H-bonding, given that pyridine, and its related analogues, are competent H-bond acceptors. We indeed found that the approaching H2O to I can form the H-bonded adduct II (+2.4 kcal mol−1, Fig. 9). Subsequently, deprotonation of H2O along with the transfer of the hydroxyl group to the phosphorus atom and formation of a zwitterionic species occurs viaTS(II–III) (+25.8 kcal mol−1, Fig. 10a). This is supported by the fact, that nucleophilic attack at the low-coordinate phosphorus atom in phosphinines (energetically low-lying LUMO) is well documented in the literature.1d,18 Thus, this characterised mode of H2O addition by P,N cooperativity via complementary participation of the formal electrophilic (Lewis acidic) phosphorus centre, and the nucleophilic (Lewis basic) pyridyl-nitrogen centre, appears somewhat resemblant of reported modes of H2O activation by intramolecular Frustrated Lewis Pairs (FLPs).19
Participation of further equivalents of H2O via H-bonded networks of two (TS(II–III)2: +26.7 kcal mol−1), or three equivalents of H2O (TS(II–III)3: +34.1 kcal mol−1) to further accelerate this step were also considered and modelled (Fig. S1‡).
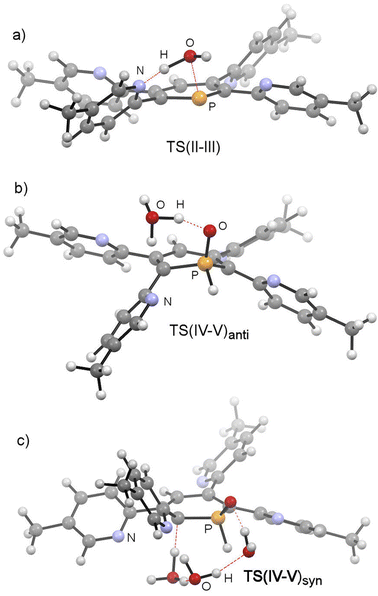
This was, however, found to be kinetically disfavoured with respect to TS(II–III). From III, H+ transfer from the protonated pyridyl group to the lone-pair at the phosphorus atom takes place viaTS(III–IV) (+29.6 kcal mol−1) to form IV (+3.6 kcal mol−1), which is a λ5-phosphinin-1-ol and the formal oxidative addition product of one O–H bond of H2O to the phosphorus atom. This step is feasible, as the phosphorus atom in III becomes much more nucleophilic after addition of OH−, and the remaining phosphorus lone-pair can now easily take up a proton to form IV. Moreover, the dominant displacement of the proton in the large imaginary mode (1267i cm−1) upon frequency analysis of TS(III–IV), suggests a degree of proton tunnelling that can also serve to accelerate this process and account for the observed reactivity already at room temperature. From IV, a final and fast de-aromatizing H-transfer process occurs viaTS(IV–V)anti (+25.0 kcal mol−1, Fig. 10) to form the secondary 1,2-dihydro-phosphine oxide (SPO) species V (−2.8 kcal mol−1), which is the experimentally observed 1,2-dihydrophosphinine oxide 4b as the major species However, unlike the initial formal oxidative addition process, this step was identified to be favoured in the presence of an additional H2O, thus forming a network to facilitate proton shuttling through an ordered six-membered transition state. An alternatively considered four-membered transition-state was kinetically disfavoured (TS(4M), +37.4 kcal mol−1, Fig. S1.2e‡). Moreover, TS(IV–V)anti (Fig. 10b) rationalizes the observed anti-selectivity (H–Cα/H–P) of the addition, where the second water molecule is situated perfectly to allow hydrogen bonding to the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O moiety and subsequent proton transfer. To confirm the favourability of anti-selectivity, a competing process of syn-selective product formation via proton-shuttling mechanism with a network of three water molecules was considered and computed (Fig. 10c). The corresponding transition state (TS(IV–V)syn, +36.9 kcal mol−1) and product (Vsyn, +0.1 kcal mol−1) were found to be 11.9 kcal mol−1 and +3.1 kcal mol−1 higher in energy than TS(IV–V)anti, and so further supports the experimentally observed anti-selectivity for the preferred formation of the secondary phosphine oxide species 4b (see ESI‡ for further details), while the diastereomer 5b is formed as the minor species.
O moiety and subsequent proton transfer. To confirm the favourability of anti-selectivity, a competing process of syn-selective product formation via proton-shuttling mechanism with a network of three water molecules was considered and computed (Fig. 10c). The corresponding transition state (TS(IV–V)syn, +36.9 kcal mol−1) and product (Vsyn, +0.1 kcal mol−1) were found to be 11.9 kcal mol−1 and +3.1 kcal mol−1 higher in energy than TS(IV–V)anti, and so further supports the experimentally observed anti-selectivity for the preferred formation of the secondary phosphine oxide species 4b (see ESI‡ for further details), while the diastereomer 5b is formed as the minor species.
To survey the influence of phosphinine aromaticity on the reversibility of water addition at 2b, NICS(0) and NICS(+1/−1) calculations were performed for I2b, IV2b, and V2b (B3LYP/def2-TZVPP level, see the ESI‡ for full details). For I2b, appreciable negative NICS(+1/−1) values are found (−7.3/−7.3), identifying the starting phosphinine species as aromatic (cf. C6H6, NICS(1) = −10.0), which supports other computational studies of aromatic properties of phosphinines.20 Calculations also indicate that upon H2O addition to form λ5-phosphinin-1-ol IV2b some partial loss of aromaticity occurs (NICS(+1/−1) = −2.4/−3.9), which is in line with computational studies on λ5-phosphinines.17 As expected, no discernible aromaticity is quantified for the ultimate 1,2-dihydrophosphinine oxide product 5b/V2b (NICS(+1/−1) = +0.5/−0.3), indicating the reversibility of P(III)/P(V) redox driven water activation is at least partially influenced by an aromaticity loss/recovery sequence.
Re-computation of these reaction steps with 2,4-diphenyl-6-pyridylphosphinine (C) indicates that the initial formal oxidative addition viaTS(II–III) is accessible at +25.8 kcal mol−1. While only +2 kcal mol−1 in energy higher than calculated for 2b, the rate-limiting H+ transfer from the nitrogen atom to the phosphorus donor viaTS(III–IV) lies at +30.7 kcal mol−1. Moreover, the formation of the final 1,2-dihydrophosphinine oxide V (+4.7 kcal mol−1) indicates that the overall reaction is now endergonic, as opposed to the exergonic one related to the reaction I → VI in case of H2O. Based on these calculations we currently propose that the experimentally observed lack of reactivity of H2O with 2,4-phenyl-6-pyridyl-phosphinine can be explained by two factors. Firstly, the slightly higher rate-limiting barrier for this phosphinine results in a kinetic inhibition, even if a pyridyl group is still present to accelerate the initial formal oxidative addition process. Secondly, the overall endergonicity associated with the reaction of I to VI renders the reaction thermodynamically unfavourable. For the reference compound 2,3,5,6-tetraphenylphosphinine (3), an analogous deprotonation-oxidative addition process can obviously not be postulated as no ortho-pyridyl groups are present. The only oxidative addition process successfully computed with this species was via a 3-coordinate saddle point, analogous to TS(OA) (Fig. S1.2c‡). However, the corresponding transition state (+65.1 kcal mol−1), was found to be kinetically disfavoured. This rationalizes why the formation of 1,2-dihydrophosphinine oxides is not observed.
Given that only the ortho pyridyl groups appear required to facilitate the water activation reaction via H-bonding, re-computation of the reaction steps with a hypothetical 2,6-dipyridylphosphinine (labelled I2c) was undertaken to discern the role of the rear meta-pyridyl groups in 2a/2b (see the ESI‡ for full details).21 The kinetics of H2O addition at I2c are almost quantitatively consistent with the tetrapyridyl-substituted phosphinine 2b (where rate-limiting TS(III–IV)2c rests at +29.5 kcal mol−1 above reactants I2c and H2O, cf. TS(III–IV)2b = +29.6 kcal mol−1). However, the relative free energy of V2c (+2.9 kcal mol−1) indicates that its formation is thermodynamically disfavoured under equilibrating conditions, and thus identifies the importance of the rear 3,5-pyridyl groups to stabilize the formation of 5a/5b over 2a/2b. We anticipate that in the presence of both meta-pyridyl groups, the {H}-transfer to form 5a/5b alleviates some steric clashing between ortho and meta substituents which gives the process further exergonicity that is otherwise not present without them.
It should be mentioned here that our mechanistic studies on the activation of H2O by phosphinines are completely different compared to the commonly accepted mechanisms for O–H cleavage reactions by other main group-based compounds, such as carbenes or silylenes.22 In these systems, the strong nucleophilic character of the main-group element can cause the deprotonation of H2O first, followed by reaction with the remaining OH−. Based on our DFT calculations, the mode of H2O activation by 2a/b is therefore a concerted deprotonation of H2O by the basic pyridyl arm, along with P–OH formation.
Finally, the addition and splitting of MeOH was computed at 2b to probe why this reactivity is not observed (Fig. 11). We found, that an initial O–H cleavage process viaTS(II–III) (+21.7 kcal mol−1) takes place to form III (+11.5 kcal mol−1), with subsequent rate-limiting H+ transfer viaTS(III–IV) (+26.8 kcal mol−1) to yield the formal oxidative addition product IV (+1.9 kcal mol−1). Consequently, the calculations indicate that the overall reaction is endergonic and reversible, despite the fact that this process is highly kinetically accessible with a low overall energetic span. Under equilibrating conditions, only a minor amount of IV should be observed experimentally as a consequence.
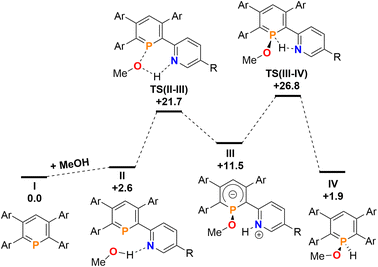 | ||
Fig. 11 Free energy profile (calculated with DFT at the M052X-D3(CPCM![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2Cl2)/def2-QZVPP//TPSS/def2-TZVPP level of theory, energies in kcal mol−1) of MeOH addition at 2b. CH2Cl2)/def2-QZVPP//TPSS/def2-TZVPP level of theory, energies in kcal mol−1) of MeOH addition at 2b. | ||
Having synthesized the novel tetrapyridyl-functionalized phosphinines and having explored in detail their reactivity towards H2O, we were also interested in the coordination chemistry of 2a/b and the reactivity of the corresponding complexes. These heterocycles possess multiple coordination sites displaying both hard (N) and soft (P) donor atoms, according to Pearson's HSAB concept. In fact, 2a/b can also be regarded as a phosphorus derivative of terpyridine (inverse of D, Fig. 2), with two additional remote pyridyl-groups in the meta-position. Because coinage metal complexes of phosphinines are well known, we focussed on investigated the coordination chemistry of 2a/b towards Cu(I).23
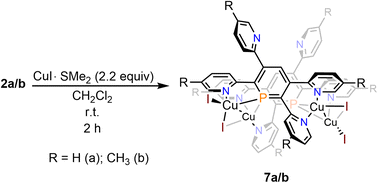
Reaction of 2a with CuI·SMe2 in dichloromethane leads to a red solution, which shows a sharp, single resonance at δ (ppm) = 154.3 in the 31P{1H} NMR spectrum (Scheme 6).
The upfield-shift of approximately Δδ (ppm) = 60 in the 31P{1H} NMR spectrum compared to the starting material 2a indicates the presence of a phosphinine-Cu(I) complex (7a), with coordination via the phosphorus atom to the metal centre. The 1H NMR spectrum shows a complex, yet very distinct splitting pattern of the signals in the aromatic region (vide infra).
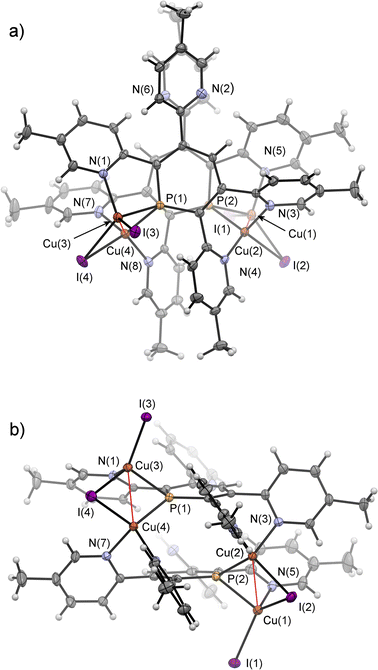
Crystals of 7b, suitable for single crystal X-ray diffraction, were obtained by slow diffusion of pentane into a dichloromethane solution of 7b. The molecular structure of 7b in the crystal, along with selected bond lengths and angles, is depicted in Fig. 12. Remarkably, the crystallographic characterization reveals the presence of a dimeric sandwich-type structure for 7b with the general composition [Cu2I2(2b)]2. In 7b each phosphorus atom shows the less common μ2-bridging coordination mode to two Cu(I)-centres, which are additionally linked by an I− ligand.1b,g,2a,23a,26 One of the Cu(I) centres of each Cu2-core carries a terminal I− ligand. The edistorted tetrahedral coordination environment of each Cu(I) centre is completed by the phosphinine pyridyl moieties, one pyridyl group for the Cu bearing the terminal I− and two coordinated pyridyl groups for the remaining Cu centres. One meta-pyridyl group of each phosphorus heterocycle remains uncoordinated.
Fig. 13 shows the 1H NMR spectrum of the aromatic region of 7a in CD2Cl2. It is possible to assign 16 resonances to the protons of both coordinated and uncoordinated pyridyl-groups as well as the remaining doublet at δ (ppm) = 7.09 to the proton in para-position of the phosphorus heterocycle (5JH–P = 5.4 Hz).
This indicates, that the dimeric structure is present both in solution and in the solid state. Similar NMR spectra were obtained from the reaction of 2b with CuI·SMe2.
Interestingly, coordination compounds 7a/b are remarkably stable towards air and moisture. This contrasts most phosphinine-based metal complexes as well as phosphinine-sulfides/selenides, which normally react readily with traces of protic reagents at the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond.24,25 To the best of our knowledge, reactivity studies on phosphinine-metal complexes, in which the phosphorus atom shows the less common μ2-coordination mode have not yet been performed. We anticipate, that the reactivity of such complexes towards protic solvents might be completely different compared to complexes, in which the phosphinine shows the more common η1-as well as the π-coordination mode.1b,g,2a,23a,26 Studies in this direction will be part of future investigations.
C double bond.24,25 To the best of our knowledge, reactivity studies on phosphinine-metal complexes, in which the phosphorus atom shows the less common μ2-coordination mode have not yet been performed. We anticipate, that the reactivity of such complexes towards protic solvents might be completely different compared to complexes, in which the phosphinine shows the more common η1-as well as the π-coordination mode.1b,g,2a,23a,26 Studies in this direction will be part of future investigations.
Conclusions
We have developed novel tetrapyridyl-substituted λ3-phosphinines by performing a double cycloaddition–cycloreversion reaction of diazaphosphinine with 1,2-bis(pyridyn-2-yl)ethyne and 1,2-bis(5-methylpyridyn-2-yl)ethyne, respectively. In contrast to other substituted phosphinines, the title compounds are highly sensitive towards H2O and selectively form the corresponding 1,2-dihydrophosphinine oxides, with an anti-arrangement of the two newly implemented hydrogen atoms as the major compound. In the presence of lithium diisopropyl amide, n-BuLi or MeLi, the water addition products can be quantitatively deprotonated, generating the corresponding strongly fluorescent λ5-phosphininolates, which show a tetrameric structure in the solid state. Most strikingly, however, the addition of H2O to the novel phosphinines is fully reversible, as loss of H2O and complete re-generation of the phosphorus heterocycles is observed by gentle heating and placing the samples under vacuum. This process can be repeated numerous times without the formation of any detectable side or decomposition products. Moreover, phosphinines 2a/b serve as a water-sensor, as only H2O is activated. Computational mechanistic studies using density functional theory suggest that the selective and reversible water addition to the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond of the phosphinine most likely occurs via P,N-cooperativity, as the initial formal oxidative addition of the O–H bond to the phosphorus atom at the tetrapyridyl-phosphinine is supported by the flanking pyridyl groups serving to kinetically enhance this process via H-bonding. Thus, this complementary participation of the formal electrophilic (Lewis acidic) phosphorus centre and the nucleophilic (Lewis basic) pyridyl-nitrogen centre resembles reported modes of H2O activation by intramolecular Frustrated Lewis Pairs (FLPs). Subsequently, an H-transfer process occurs to form the 1,2-dihydrophosphinine oxide in an overall slightly exergonic reaction. This step was identified to be favoured in the presence of an additional H2O molecule, thus forming a network to facilitate proton shuttling through an ordered six-membered transition state. This also rationalizes the observed anti-selectivity of the addition. The calculations are also in line with the fact, that neither the reference compound 2,3,5,6-tetraphenylphosphinine reacts with water, nor do alcohols react with the tetrapyridyl-functionalized phosphinines. Our here-presented results show yet another example of the fascinating reactivity of functionalized phosphinines towards small molecules, showcasing the intriguing properties of these aromatic, low-coordinate phosphorus compounds for the activation of E–H bonds. As a matter of fact, the re-aromatization of the heterocycle is a key aspect for maintaining a fully reversible reaction, also from a conceptual point of view. Despite the presence of multiple coordination sites, we could further show that a dimeric sandwich-type structure with the general composition [Cu2I2(phosphinine)]2 is formed selectively upon reaction of the starting material with CuI·SMe2.
C double bond of the phosphinine most likely occurs via P,N-cooperativity, as the initial formal oxidative addition of the O–H bond to the phosphorus atom at the tetrapyridyl-phosphinine is supported by the flanking pyridyl groups serving to kinetically enhance this process via H-bonding. Thus, this complementary participation of the formal electrophilic (Lewis acidic) phosphorus centre and the nucleophilic (Lewis basic) pyridyl-nitrogen centre resembles reported modes of H2O activation by intramolecular Frustrated Lewis Pairs (FLPs). Subsequently, an H-transfer process occurs to form the 1,2-dihydrophosphinine oxide in an overall slightly exergonic reaction. This step was identified to be favoured in the presence of an additional H2O molecule, thus forming a network to facilitate proton shuttling through an ordered six-membered transition state. This also rationalizes the observed anti-selectivity of the addition. The calculations are also in line with the fact, that neither the reference compound 2,3,5,6-tetraphenylphosphinine reacts with water, nor do alcohols react with the tetrapyridyl-functionalized phosphinines. Our here-presented results show yet another example of the fascinating reactivity of functionalized phosphinines towards small molecules, showcasing the intriguing properties of these aromatic, low-coordinate phosphorus compounds for the activation of E–H bonds. As a matter of fact, the re-aromatization of the heterocycle is a key aspect for maintaining a fully reversible reaction, also from a conceptual point of view. Despite the presence of multiple coordination sites, we could further show that a dimeric sandwich-type structure with the general composition [Cu2I2(phosphinine)]2 is formed selectively upon reaction of the starting material with CuI·SMe2.
Data availability
All data can be found in the ESI.†Author contributions
C. M., N. T. C. and S. E. N.: conceptualization and supervision of the project, writing, reviewing and editing of the manuscript. S. M. R., M. J. E. and N. T. C. collected XRD data and solved and refined the crystal structures. N. T. C. and L. J. K. C. were involved in the validation of the XRD data. R. O. K., S. L. K., L. J. G. and N. T. C. contributed to the experimental work, formal analysis and data curation. S. E. N. performed the computational work.Conflicts of interest
There are no conflicts to declare.Acknowledgements
C. M. and R. O. K. thank the German research foundation (DFG) for financial support. The authors gratefully acknowledge the core facility BioSupraMol supported by the German research foundation (DFG). We thank Dr Simon Steinhauer for his support on variable temperature NMR spectroscopy.References
- (a) G. Märkl, Angew. Chem., 1966, 78, 907 CrossRef; (b) K. Masada, K. Okabe, S. Kusumoto and K. Nozaki, Chem. Sci., 2023, 14, 8524 RSC; (c) E. Yue, A. Petrov, D. S. Frost, L. Dettling, L. Conrad, F. Wossidlo, N. T. Coles, M. Weber and C. Müller, Chem. Commun., 2022, 58, 6184 RSC; (d) N. T. Coles, L. J. Groth, L. Dettling, D. S. Frost, M. Rigo, S. E. Neale and C. Müller, Chem. Commun., 2022, 58, 13580 RSC; (e) J. Lin, F. Wossidlo, N. T. Coles, M. Weber, S. Steinhauer, T. Böttcher and C. Müller, Chem.–Eur. J., 2022, 28, e202104135 CrossRef CAS PubMed; (f) J. Saltó, D. Tarr, A. Petrov, D. Reich, M. Weber, M. Biosca, O. Pàmies, C. Müller and M. Diéguez, Adv. Synth. Catal., 2024, 366, 813 CrossRef; (g) N. T. Coles, A. Sofie Abels, J. Leitl, R. Wolf, H. Grützmacher and C. Müller, Coord. Chem. Rev., 2021, 433, 213729 CrossRef CAS; (h) S. Giese, K. Klimov, A. Mikeházi, Z. Kelemen, D. S. Frost, S. Steinhauer, P. Müller, L. Nyulászi and C. Müller, Angew. Chem., Int. Ed., 2021, 133, 3625 CrossRef; (i) G. Pfeifer, F. Chahdoura, M. Papke, M. Weber, R. Szűcs, B. Geffroy, D. Tondelier, L. Nyulászi, M. Hissler and C. Müller, Chem.–Eur. J., 2020, 26, 10534 CrossRef CAS PubMed; (j) J. Leitl, P. Coburger, D. J. Scott, C. G. P. Ziegler, G. Hierlmeier, R. Wolf, N. P. van Leest, B. de Bruin, G. Hörner and C. Müller, Inorg. Chem., 2020, 59, 9951 CrossRef CAS PubMed; (k) J. Leitl, A. R. Jupp, E. R. M. Habraken, V. Streitferdt, P. Coburger, D. J. Scott, R. M. Gschwind, C. Müller, J. C. Slootweg and R. Wolf, Chem.–Eur. J., 2020, 26, 7788 CrossRef CAS PubMed; (l) J. Leitl, M. Marquardt, P. Coburger, D. J. Scott, V. Streitferdt, R. M. Gschwind, C. Müller and R. Wolf, Angew. Chem., Int. Ed., 2019, 58, 15407 CrossRef CAS PubMed.
- (a) P. Le Floch, Coord. Chem. Rev., 2006, 250, 627 CrossRef CAS; (b) F. Wossidlo, D. S. Frost, J. Lin, N. T. Coles, K. Klimov, M. Weber, T. Böttcher and C. Müller, Chem.–Eur. J., 2021, 27, 12788 CrossRef CAS PubMed.
- J. M. Alcaraz, A. Breque and F. Mathey, Tetrahedron Lett., 1982, 23, 1565 CrossRef CAS.
- C. Müller, D. Wasserberg, J. J. M. Weemers, E. A. Pidko, S. Hoffmann, M. Lutz, A. L. Spek, S. C. J. Meskers, R. A. J. Janssen, R. A. van Santen and D. Vogt, Chem.–Eur. J., 2007, 13, 4548 CrossRef PubMed.
- C. Müller, E. A. Pidko, M. Lutz, A. L. Spek and D. Vogt, Chem.–Eur. J., 2008, 14, 8803 CrossRef PubMed.
- (a) N. Avarvari, P. Le Floch and F. Mathey, J. Am. Chem. Soc., 1996, 118, 11978 CrossRef CAS; (b) G. Frison, A. Sevin, N. Avarvari, F. Mathey and P. Le Floch, J. Org. Chem., 1999, 64, 5524 CrossRef CAS PubMed.
- A. E. Brown and B. E. Eichler, Tetrahedron Lett., 2011, 52, 1960 CrossRef CAS.
- L. Fischer, F. Wossidlo, D. Frost, N. T. Coles, S. Steinhauer, S. Riedel and C. Müller, Chem. Commun., 2021, 57, 9522 RSC.
- P. A. Cleaves, B. Gourlay, R. J. Newland, R. Westgate and S. M. Mansell, Inorganics, 2022, 10, 17 CrossRef CAS.
- M. Doux, N. Mézailles, L. Ricard and P. Le Floch, Eur. J. Inorg. Chem., 2003, 3878 CrossRef CAS.
- (a) P.-Y. Renard, P. Vayron, E. Leclerc, A. Valleix and C. Mioskowski, Angew. Chem., Int. Ed., 2003, 42, 2389 CrossRef CAS PubMed; (b) S. Sowa, M. Stankevič, A. Szmigielska, H. Małuszyńska, A. E. Kozioł and K. Michal Pietrusiewicz, J. Org. Chem., 2015, 80, 1672 CrossRef CAS PubMed; (c) R. W. Ware, C. S. Day and S. Bruce King, J. Org. Chem., 2002, 67, 6174 CrossRef CAS PubMed; (d) A. Brandi and P. Cannavò, J. Org. Chem., 1989, 54, 3073 CrossRef CAS.
- L. Hintermann, M. Schmitz, O. V. Maltsev and P. Naumov, Synthesis, 2013, 45, 0308 CrossRef CAS.
- G. Bandoli, G. Bortolozzo, D. A. Clemente, U. Croatto and C. Panattoni, J. Chem. Soc. A, 1970, 2778 RSC.
- T. Welton and C. Reichardt, Solvents and Solvent Effects in Organic Chemistry, John Wiley & Sons, New York, NY, edn 3, 2011, chp. 7, pp. 418–424 Search PubMed.
- See for example (a) W. Zhao, S. M. McCarthy, T. Yi Lai, H. P. Yennawar and A. T. Radosevich, J. Am. Chem. Soc., 2014, 136, 17634 CrossRef CAS PubMed; (b) T. P. Robinson, D. M. De Rosa, S. Aldridge and J. M. Goicoechea, Angew. Chem., Int. Ed., 2015, 54, 13758 CrossRef CAS PubMed; (c) T. Chu and G. I. Nikonov, Chem. Rev., 2018, 118, 3608 CrossRef CAS PubMed; (d) F. Buß, C. Mück-Lichtenfeld, P. Mehlmann and F. Dielmann, Angew. Chem., Int. Ed., 2018, 57, 4951 CrossRef PubMed; (e) J. Abbenseth, O. P. E. Townrow and J. M. Goicoechea, Angew. Chem., Int. Ed., 2021, 60, 23625 CrossRef CAS PubMed; (f) A. J. King, J. Abbenseth and J. M. Goicoechea, Chem.–Eur. J., 2023, 29, e202300918D CrossRef PubMed; (g) D. Roth, A. T. Radosevich and L. Greb, J. Am. Chem. Soc., 2023, 145(44), 24184 CrossRef CAS PubMed; (h) N. Beims, T. Greven, M. Schmidtmann and J. I. van der Vlugt, Chem.–Eur. J., 2023, 29, e202302463 CrossRef CAS PubMed; (i) A. J. King, J. Abbenseth and J. M. Goicoechea, Chem.–Eur. J., 2023, 29, e202300818 CrossRef CAS PubMed.
- I. de Krom, E. A. Pidko, M. Lutz and C. Müller, Chem.–Eur. J., 2013, 19, 7523 CrossRef CAS PubMed.
- (a) K. Dimroth, Top. Curr. Chem., 1973, 38, 1 Search PubMed; (b) L. Nyulászi and T. Veszprémi, J. Phys. Chem., 1996, 100, 6456 CrossRef; (c) L. Nyulászi, Chem. Rev., 2001, 101, 1229 CrossRef PubMed; (d) Z. Benkő and L. Nyulászi, Top. Heterocycl. Chem., 2009, 19, 27 Search PubMed; (e) Z.-X. Wang and P. von Ragué Schleyer, Helv. Chim. Acta, 2001, 84, 1578 CrossRef CAS; (f) H. S. Rzepa and K. Y. Taylor, J. Chem. Soc., Perkin Trans. 2, 2002, 1499 RSC; (g) G. Pfeifer, F. Chahdoura, M. Papke, M. Weber, R. Szűcs, B. Geffroy, D. Tondelier, L. Nyulászi, M. Hissler and C. Müller, Chem.–Eur. J., 2020, 26, 10534 CrossRef CAS PubMed.
- (a) G. Märkl and C. Martin, Angew. Chem., Int. Ed., 1974, 13, 408 CrossRef; (b) A. Moores, N. Mézailles, L. Ricard and P. Le Floch, Organometallics, 2005, 24, 508 CrossRef CAS; (c) M. Bruce, G. Meissner, M. Weber, J. Wiecko and C. Müller, Eur. J. Inorg. Chem., 2014, 2014, 1719 CrossRef CAS.
- See for example: S. Sinha and S. Giri, Chem. Phys. Lett., 2022, 799, 139634 CrossRef CAS.
- (a) Y. Hou, Z. Li, Y. Li, P. Liu, C.-Y. Su, F. Puschmann and H. Grützmacher, Chem. Sci., 2019, 10, 3168 RSC; (b) S. Giese, K. Klimov, A. Mikeházi, Z. Kelemen, D. S. Frost, S. Steinhauer, P. Müller, L. Nyulászi and C. Müller, Angew. Chem., Int. Ed., 2021, 60, 3581 CrossRef CAS PubMed.
- A 2,6-dipyridyl-substituted phosphinine cannot be synthesized from diazaphosphinine and pyridyl-acetylene, as the 3,5-dipyridyl-substituted phosphinine will be formed. See ref. 6.
- (a) W. Kirmse and J. Kilian, J. Am. Chem. Soc., 1990, 112, 6399 CrossRef CAS; (b) J. R. Pliego and W. B. De Almeida, J. Phys. Chem. A, 1999, 103, 3904 CrossRef CAS; (c) P. Costa and W. Sander, Angew. Chem., 2014, 126, 5222 CrossRef; (d) J. Knorr, P. Sokkar, S. Schott, P. Costa, W. Thiel, W. Sander, E. Sanchez-Garcia and P. Nuernberger, Nat. Commun., 2016, 7, 12968 CrossRef PubMed; (e) Z. Mo, T. Szilvási, Y.-P. Zhou, S. Yao and M. Driess, Angew. Chem., Int. Ed., 2017, 56, 3699 CrossRef CAS PubMed; (f) S. Yao, Y. Xiong and M. Driess, Organometallics, 2011, 30, 1748 CrossRef CAS.
- (a) P. Le Floch and F. Mathey, Coord. Chem. Rev., 1998, 179–180, 771 CrossRef; (b) N. Mézailles, F. Mathey and P. Le Floch, Prog. Inorg. Chem., 2001, 455 Search PubMed; (c) M. Rigo, L. Hettmanczyk, F. J. L. Heutz, S. Hohloch, M. Lutz, B. Sarkar and C. Müller, Dalton Trans., 2017, 46, 86 RSC; (d) P. Roesch, J. Nitsch, M. Lutz, J. Wiecko, A. Steffen and C. Müller, Inorg. Chem., 2014, 53, 9855 CrossRef CAS PubMed.
- (a) B. Schmid, L. M. Venanzi, A. Albinati and F. Mathey, Inorg. Chem., 1991, 30, 4693 CrossRef CAS; (b) L. E. E. Broeckx, A. Bucci, C. Zuccaccia, M. Lutz, A. Macchioni and C. Müller, Organometallics, 2015, 34, 2943 CrossRef CAS; (c) X. Chen, Z. Li and H. Grützmacher, Chem.–Eur. J., 2018, 24, 8432 CrossRef CAS PubMed; (d) R. J. Newland, A. Smith, D. M. Smith, N. Fey, M. J. Hanton and S. M. Mansell, Organometallics, 2018, 37, 1062 CrossRef CAS; (e) R. J. Newland, M. P. Delve, R. L. Wingad and S. M. Mansell, New J. Chem., 2018, 42, 19625 RSC.
- G. Pfeifer, P. Ribagnac, X.-F. Le Goff, J. Wiecko, N. Mézailles and C. Müller, Eur. J. Inorg. Chem., 2015, 240 CrossRef CAS.
- J. Zhang, Y. Hou, S. Liu, J. Lin and Z. Li, Dalton Trans., 2024 10.1039/D4DT00228H.
Footnotes |
| † Dedicated to Prof. Dr Hansjörg Grützmacher on the occasion of his 65th birthday. |
| ‡ Electronic supplementary information (ESI) available. CCDC 2302172, 2304990–2304992, 2305048 and 2305850. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3sc05930h |
| § The authors have contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |