Turkish science high school students’ mental models of the electron cloud
Sevgül
Çalış

Faculty of Education, Department of Mathematics and Science Education, Bursa Uludag University, Bursa, Turkey. E-mail: scalis@uludag.edu.tr
First published on 18th June 2024
Abstract
This study focuses on examining the mental models of 11th and 12th-grade students attending a science high school in Turkey regarding the concept of the electron cloud. The study involved 72 students and employed the case study method. The precondition for selecting the sample was that the students had covered the unit on modern atomic theory in their chemistry classes. The concept of the electron cloud chosen for the study is integrated into the units of “Atom and Periodic System” and “Modern Atomic Theory.” To guide the research questions, the progression of the lessons and activities within the unit were observed in three-week intervals across different classes taught by the participating teacher. Research data were collected using a data collection tool consisting of 7 open-ended questions, considering the high school chemistry course objectives. The research questions were prepared in three categories: conceptual, relational, and visual. A rubric was developed for data analysis, and codes corresponding to levels of understanding were determined. At the end of the evaluation, three mental models were identified: the electron cloud model, the hybrid/synthesis electron cloud model, and the primitive model. For these models, eight mental model categories have been determined: fully scientific, partially scientific, conceptual, relational, conceptual–relational, conceptual-visual, relational-visual, and incompatible. At the conclusion of the study, only 5.56% of students provided answers at the scientific understanding level for all categories, placing them in the full scientific model category under the electron cloud model. 16.67% of students fell into the partial scientific model category, while 75.29% demonstrated a hybrid/synthesized electron cloud model. A small portion, 2.78%, adopted a primitive model.
Introduction
In the realm of chemistry, the comprehension of concepts related to atoms and atomic models presents various challenges for learning. Consequently, issues pertaining to the teaching and learning of concepts related to atomic models have become the focus of numerous research endeavors (Taber, 1997; Nakiboglu, 2003; Tsaparlis and Papaphotis, 2009; Chen et al., 2015; Akaygun, 2016; Eryılmaz Muştu, 2021). Throughout the historical timeline, diverse models have been proposed for the structure of the atom. However, research findings indicate that among these models, the Bohr atomic model stands out as the most prevalent in terms of knowledge retention and durability within students' minds (Zarkadis et al., 2017). Bohr's elucidation that electrons occupy specific orbits at a designated distance from the nucleus, coupled with the model's ease of expression and visual representation, has proven instrumental in facilitating students' facile and enduring comprehension or acceptance of this model. In history, the refutation of the Bohr atomic model led to the proposal of quantum theory. This theory, despite predicting that the exact location of an electron within an atom cannot be determined, can explain where an electron might be found within a certain time frame. According to this theory, the probability of finding electrons in a specific region of an atom is expressed with the concept of an “electron cloud,” where regions with a high electron density indicate a high probability of finding electrons, and vice versa for regions with low density. In this theory, the most probable location of an electron around an atom is described as an orbital, which is a three-dimensional representation (Chang, 2009). Each type of orbital has a unique shape of probability density. According to this theory, quantum numbers are necessary to explain the distribution of electrons in atoms. Quantum numbers can also be thought of as a way to address electrons around the nucleus. While the Bohr atomic model, which explains that electrons move in circular orbits at certain distances from the nucleus with specific energies, finds a solid place in many students' minds, the explanation according to quantum mechanics that the speed and position of electrons cannot be determined at the same time and the expression of probability conditions fail to form a concrete correspondence in students' minds. Consequently, concepts such as uncertainty about the position of electrons, quantized energy levels, electron density, matter waves, and probability appear more challenging to grasp. Furthermore, these concepts in modern theory, being more abstract than in the Bohr theory, constitute the basis for the difficulty students encounter in conceptualizing or visualizing them. Within the chemistry course, which encompasses many abstract concepts such as the particulate nature of matter, molecules, and atoms, conceptual understanding in chemistry education is gaining importance day by day.Liu et al. (2014) noted that when students enter the science classroom to learn scientific concepts, they possess different mental states, and these mental states may encompass their emotions, learning intentions, and mental representations related to scientific concepts. Özcan (2015) asserted that understanding some concepts in chemistry is possible because they appear in daily life, while others may be challenging to comprehend without prior knowledge. For instance, experiencing or visualizing concepts related to the atom or the particulate nature of matter clearly is not feasible, and when teaching such concepts, it is necessary to draw parallels with everyday life or a known event. Trindade and Fiolhais (2003) highlighted that a series of studies have been conducted on students' misconceptions and difficulties in understanding quantum mechanics. For example, Chen et al. (2015) stated that atoms and molecules in chemistry are invisible and abstract to students, making it challenging for them to learn these concepts, especially the atomic orbital concept involving quantum mechanics. Taber (1997) in his study highlighted that students struggle to understand the differences between the concepts of orbitals, shells, subshells, and energy levels. Similarly, Nakiboglu (2003) found that chemistry teacher candidates confuse and interchange the concepts of orbitals, shells, and orbits. Therefore, as Airey and Linder (2009) and Schönborn and Anderson (2006) also pointed out, the importance of the visual representation of abstract concepts in teaching is crucial, and this is particularly significant for visualizing concepts related to quantum chemistry. In this regard, studies determining mental models play a pivotal role in understanding the reasons for difficulties in comprehending such concepts.
Theoretical framework related to mental models
Although there is a fundamental consensus about the nature of mental models, there are different definitions emphasizing specific features (Hong and O'Neil, 1992). For instance, mental models are individualized representations of elements existing in individuals' minds, systematically structured within a framework (Coll and Treagust, 2003). According to Harrison and Treagust (2000), mental models are expressed as the mental representations of ideas or events that individuals construct through their mental thinking skills. Wang and Barrow (2013) have stated that mental models have characteristics of both visual-virtual and mature explanations made by an individual. According to Akaygun (2016), mental models are often represented with words or images, each having different possibilities, and they are referred to as representations of mental models. According to Coll and Treagust (2001), mental models are unstable because they develop during a student's learning process. Therefore, it is important in the educational process to take this characteristic into account by assessing the knowledge students possess and identifying their mental models, as it also helps in preventing possible misconceptions. Considering these definitions, it can be argued that learners' mental models can accurately structure information as long as they are scientific (Nakiboglu, 2019).The development of students' mental models occurs through their use of models, making predictions, receiving feedback, and adjusting their understanding accordingly (Chittleborough and Treagust, 2009). Therefore, the models created in students' minds provide clarity to educators about how students comprehend concepts and can also inform teachers about the students' knowledge structure (Wittmann, 2002). The emerging structure of the mental model represents a more abstract part of the mental model and provides information about the relationships established between concepts (Vogt et al., 2021). Supasorn (2015) has emphasized the importance of mental models in learning chemistry. Albaiti and Lepa (2022) highlighted that if the three chemical levels expressed as symbolic, macroscopic, and submicroscopic are not fully conveyed to students, they may fail to establish connections between levels, experience conceptual misunderstandings, and cannot develop mental models. However, many educators often focus on two of these three levels, namely macroscopic and symbolic levels. Nevertheless, understanding the microscopic level is particularly important for students because the nature of chemical processes can only be explained by the movement and behavior of particles (Akaygun, 2016). Taber (2013) stated that modern chemistry develops comprehensive theoretical models based on the nature of entities much smaller than what can be observed with an optical microscope. According to them, a significant part of chemistry that can be taught in high school and university is related to molecules, ions, electrons, orbitals, and energy levels. Therefore, the submicroscopic explanations of chemistry are crucial for teaching these concepts. Consequently, it is essential to investigate the levels of understanding and mental models of students due to the abstract nature of the submicroscopic world formed by subatomic particles.
There are several studies in the literature that investigate high school students' mental models of atomic structures. For example, Petri and Niedderer (1998) examined the learning process of a 13th-grade student in a German sports school in a quantum physics course. They found that the student's learning process progressed from a planetary model to a series of various concepts related to the atom. However, the post-instructional situation exhibited a combination of three parallel concepts: the planetary model, the stationary electron model, and the electron cloud model. Papaphotis and Tsaparlis (2008) conducted studies with 12th-grade students in Greece on basic quantum chemistry concepts. They found that the Bohr model and the language of the old quantum theory prominently stood out in many students' responses, while other students had hybrid models that mixed the planetary model with quantum mechanics. In another of their studies with 12th-grade students, Tsaparlis and Papaphotis (2009) noted strong support for the planetary Bohr model. However, many students did not understand the probabilistic nature of the orbital concept, some had hybrid models, and in some cases, students did not accept that the electron cloud corresponded to the picture of the atom. They also stated that most students did not understand the fundamental nature of the uncertainty principle. In a study conducted by Akaygun (2016) with 10th and 11th-grade students, she aimed to investigate and compare the static and dynamic representations of students' mental models related to the atomic structure. Static representations of mental models were expressed through drawings and explanations on paper, while dynamic representations were created using animation development software. The study concluded that animations created by students could positively influence their learning. In addition to these studies, Budde et al. (2002) developed the electronium model within the scope of the quantum atomic model for secondary school students with the aim of eliminating existing misconceptions. Vieira and Morais (2022) used a music analogy in their study with 50 middle school students in a music class regarding the quantum model of the atom. They concluded that difficulties in understanding the model could be alleviated with such analogies. In their study examining the consistency of mental models regarding atomic structure with 10th and 11th-grade students, Zarkadis et al. (2017) found that many students were influenced by the Bohr model while describing the electron cloud or created a hybrid model between the Bohr model and the quantum model. Similar confusion or hybrid structures have been mentioned in studies by Treagust (2000), Taber (2002, 2005), Trindade and Fiolhais (2003), Park and Light (2009), Stevens et al. (2010), Harrison and Allred and Bretz (2019). Moreover, in studies on the structure of the atom with high school students (e.g., Demirci et al., 2016), the use of 3D representations in students' formation of mental models about atomic orbitals (e.g., Chen et al., 2015), and quantum atomic models (e.g., Budde et al., 2002) suggests that students have different mental models.
In the development of mental model structures, a form of knowledge architecture, three distinct knowledge types are taken into account: content knowledge, structural knowledge, and procedural knowledge (Hill, 2006). Content knowledge defines a student's understanding of concepts, processes, or events. Structural knowledge allows the student to articulate the relationships and connections pertaining to scenarios, while procedural knowledge facilitates the demonstration of how a student utilizes content and structural knowledge in a given situation. Mental models are internal representations of knowledge and thoughts that students create in their minds. Students use these models for reasoning, problem-solving, making predictions, and explaining skills that are crucial for learning science (Harrison and Treagust, 2000). Therefore, they are significant for science education.
An important issue in mental model studies is how to measure a mental model. To answer this question, a detailed examination of the obtained data is required (Vogt et al., 2021). Coll and Treagust (2003) have categorized mental models into two groups: physical and conceptual. Mental models created regarding the atom topic are included in the conceptual mental models group. In studies aiming to determine mental models, it is observed that a single model is not used (Didiş et al., 2014). This is because mental models have an individualized and complex structure, and therefore, various methods are used in mental model determination studies. The essential aspect of determining students' mental model structures is the written or verbal narratives provided by the students. Therefore, in studies, various techniques are used for data collection purposes, including interview techniques (Coll and Treagust, 2003), open-ended questions, drawings (Demirçalı, 2016), multiple-choice questions including drawings and explanations (Demirkol, 2017; İyibil Durukan, 2019), or a combination of these.
Purpose and significance
There are numerous studies aimed at identifying mental models related to atomic models, generally exploring mental models concerning atomic theories. However, there are fewer studies focusing on the concept of the electron cloud, which holds significant importance in modern atomic theory, particularly in understanding what it represents and its characteristics for high school students. This study targets the electron cloud model directly, unlike existing studies. The study aims to identify the mental models that will emerge regarding the concept of the electron cloud among students. It will help educators by addressing the erroneous models that may occur during the teaching of related subjects in high school and by revealing the reasons why the Bohr model predominates in students' minds, as encountered in many studies. Identifying the non-scientific mental models that students hold is crucial for educators as it guides them in planning their lessons. Understanding this concept is also vital for making topics related to quantum numbers and orbitals, which are central to modern theory, more comprehensible.In Turkey, there are different types of high schools, such as Science High Schools, Anatolian High Schools, and vocational high schools. The transition to these schools is based on the exam scores of students at the end of the 8th grade. Among these, Science High Schools are the type of high school with the highest entrance scores, and their curriculum is more focused on science compared to other high schools. In the Chemistry curriculum of Science High Schools in Turkey, atom models have a crucial place. The unit titled “Atom and Periodic Table” provides a detailed explanation of atomic models and the structure of the atom. Under the unit “Modern Atomic Theory,” detailed information is provided about the limitations of the Bohr atomic model, modern atomic theory, the concepts of orbits and orbitals, quantum numbers, and energy levels of orbitals (MEB, 2023). This research aims to investigate the mental models of Science High School students regarding the concept of the “electron cloud,” which is one of the fundamental concepts in modern atomic theory and quantum mechanics. By exploring how the concept of the “electron cloud” is understood or misunderstood, the study aims to contribute clarity to the existing problem in the field. The perspectives of Science High School students regarding the research problem will be collected to determine their mental models related to the concept of the electron cloud. Therefore, to identify students' mental models, questions have been developed that allow for defining concepts, revealing inter-conceptual relationships, and displaying information about subjects through visual representations. These questions focus on conceptual, relational, and visual types of knowledge, providing a comprehensive approach to understanding and evaluating students' cognitive frameworks.
Research problem
The research problem is defined as “What are the mental models of high school students regarding the electron cloud concept?” and the sub-problem of the research is determined as “What are the conceptual, relational, and visual levels of mental models that high school students have regarding the electron cloud concept?”Method
A case study was chosen for the research for two reasons: (i) it provides an opportunity to describe and explain the real-life situation of chemistry teaching in detail, and (ii) it helps in identifying the nature of mental models of students using how and why questions (Creswell, 2012; Kilinc et al., 2017). The unit of analysis defines what the case is—for example, an event, a process, an individual, a group, or an organisation (Yin, 2003). In this study, the unit of analysis has been students’ mental models about the electron cloud. The students’ teacher's chemistry teaching orientation has also helped in the descriptions of these models.Topics related to atoms and atomic models are covered in the 9th and 11th-grade curricula in high school. During the study, the researcher was present as an observer alongside the chemistry teacher during the weeks the units were taught to 9th and 11th graders. These units are taught over a three-week period, with 9th graders receiving two class hours per week and 11th graders receiving four class hours per week. In the 9th-grade science high school program, under the title “Atom and Periodic System”, atomic models (Dalton, Thomson, Rutherford, Bohr, and modern atomic model), the structure of the atom (subatomic particles), and the periodic system are taught. In the 9th grade, the teacher first explained the models verbally, then elaborated on them using visuals with the support of a smart board. The teacher devoted more time to the Bohr atomic model, explaining that electrons are located in circular orbits at specific radii and energy levels around the nucleus. To reinforce what was taught in class and to help students remember the chronological order of the atomic models, the teacher gave examples of rhymes and used various analogies. After presenting the example of a hydrogen atom and explaining the characteristics of the model using visuals, the teacher then discussed the limitations of the model. Since the modern atomic theory is not covered in detail in the 9th-grade science high school curriculum, the teacher briefly mentioned it, indicating it would be covered in detail in the 11th grade. Observations during the lectures revealed that most students disliked verbal descriptions and showed little interest in the class; however, when visuals related to the lesson were used, participation and interest in the class increased. In the 11th grade at science high schools, under the unit “Modern Atomic Theory,” topics such as the limitations of the Bohr atomic model, the quantum model of the atom, the concepts of orbits and orbitals, quantum numbers, and the energy levels of orbitals are covered. At the beginning of the lesson, the teacher created a discussion atmosphere in the class by asking questions like “Why was there a need for the quantum model and as a result of which scientific studies did this model emerge?” and started the lesson by evaluating student responses. As the topics progressed, the teacher stated that the exact location of an electron in an atom cannot be determined, but its probability of being found can be discussed. In subsequent lessons, the concept of orbitals, types of orbitals, and the teaching of orbital numbers were covered. The teacher explained that quantum numbers are used in quantum theory to explain the distribution of electrons in atoms and described the quantum numbers and their functions. To help better understand quantum numbers, the teacher used analogies, such as “Just as an address gives an idea of where a person can be found, quantum numbers indicate where an electron can be found.” The teacher made analogies like, “Just as we cannot be sure that a person will be at the given address, we cannot be certain where an electron will be.” Describing a person's location by first mentioning the city, district, and neighborhood they live in, the teacher explained that similarly, the principal quantum number, azimuthal quantum number, and magnetic quantum number would be needed to indicate the region where an electron is likely to be found. Through such addressing, just as the region where a person is likely to be found can be described, attention was drawn to the probability of an electron being found in an orbital. This analogy example was seen to be effective in helping students understand quantum numbers. The teacher used everyday life analogies to explain the types and number of orbitals depending on the energy level. For example, the teacher compared the energy levels in an atom to rows of seats in an amphitheater, associating the increase in energy levels and the number of orbitals in an atom with moving away from the stage, where higher and more distant rows have more seats. The researcher's participation as an observer in the lessons also contributed to the formulation of research questions.
Participants
Throughout the entire process of this research, from planning to implementation, from data collection to data analysis, all rules stated under the “Scientific Research and Publication Ethics Directive of Higher Education Institutions” have been adhered to. Permission was obtained from the research and publication ethics committee presidency of the University (Ethics evaluation document number: 39) before conducting this study during the spring semester of the 2022–2023 academic year with a total of 72 students who volunteered to participate in the study at a Science High School in Turkey, including 54 students in the 11th grade and 18 students in the 12th grade. All students included in the sample had completed at least one semester since covering the relevant subjects. When evaluating the data obtained from the study, no significant differences were observed between classes that would affect the results of the study; therefore, the groups have been considered together for analysis. These students were presumed to have a high interest in topics related to the electron cloud since they were attending a Science High School and were expected to have the competencies defined by the Turkish Ministry of National Education (MEB, 2023). Purposeful sampling was employed in selecting the sample, with the prerequisite that students had covered the unit on modern atomic theory in their chemistry classes. Prior to the research, discussions were held with researchers and the chemistry teacher at the Science High School to gather insights on the content. Subsequently, the researchers actively followed the teaching process during three-week periods when the units on the atom and the periodic system, as well as modern atomic theory, were covered by the teacher. Before the research commenced, students were informed about the research objectives and how the data would be evaluated. To maintain confidentiality, coding such as S1, S2, etc., was used for the students' expressions.Data collection tool
The research data were collected using a data collection tool consisting of 7 open-ended questions, developed in consideration of the achievements of high school chemistry classes. The questions were prepared with consideration of class observations and the curriculum of the Turkish Ministry of National Education Science High School program (MEB, 2023). The developed questions were given to students during the chemistry class, and they were given one class period to respond. Necessary explanations were provided for students who experienced any confusion. Following the categories specified in İyibil Durukan's, (2019) study, the research questions were organized into three categories: “conceptual, relational, and representational”. The questions and categories of the data collection tool are presented in Table 1. For conceptual questions, the criterion is that the theoretical knowledge includes scientific information. For relational questions, it is based on the relationships between concepts being scientifically adequate. And for visual questions, the criteria are that the drawings are accurate and sufficient. The levels of understanding for each category created using these criteria are provided in Table 2.| Question number | Category | Questions |
|---|---|---|
| 1 | Conceptual | Explain the concept of the electron cloud? |
| 2 | Relational | How can you explain the electron cloud in modern atomic theory by making analogies with everyday examples? |
| 3 | Relational | What shortcomings in atomic models led to the development of the concept of the electron cloud in modern atomic theory? |
| 4 | Conceptual | What is the meaning of the concept of the orbital in modern atomic theory? |
| 5 | Relational | Why were new quantum numbers introduced in modern atomic theory in place of the orbit concept in the Bohr atomic model, and what are their names? |
| 6 | Visual | Draw a figure representing the modern atomic model (cloud model)? |
| 7 | Visual | Draw the probability of finding an electron in the 1S orbital as a function of the distance from the nucleus. |
| Question category | Levels | Explanations |
|---|---|---|
| Conceptual | [0] | Unanswered, unclear, and ambiguous responses |
| [1] | Responses containing alternative concepts (conceptual misconceptions) and/or non-scientific information | |
| [2] | Responses containing basic level knowledge or information along with an alternative concept | |
| [3] | Responses with acceptable levels of scientific knowledge without alternative concepts (conceptual misconceptions) | |
| [4] | Responses with scientifically accurate knowledge | |
| Relational | [0] | Responses with unanswered, unclear, and ambiguous relationships |
| [1] | Responses with incorrect and non-scientific relationships | |
| [2] | Responses with incorrect relationships and basic-level established relationships | |
| [3] | Responses with correct and acceptable-level established relationships | |
| [4] | Responses with scientifically established relationships | |
| Visual | [0] | Responses with unanswered, unclear, and ambiguous drawings |
| [1] | Drawings containing non-scientific visual elements | |
| [2] | Responses that include non-scientific visual elements and drawings made at a basic level | |
| [3] | Drawings with correct and acceptable-level constructions | |
| [4] | Drawings with scientifically constructed elements | |
Data analysis
For the analysis of the obtained data, a rubric was created using İyibil Durukan's (2019) framework. Codes corresponding to levels of understanding, such as [0], [1], [2], [3], and [4], were assigned for each category. Explanations for these codes are provided in Table 2.The questions in the measurement tool for understanding levels have been organized taking into account these categories. Levels [3] and [4] indicate that the student possesses a scientific level of understanding, level [2] indicates that the student has a basic level of understanding but may also have misconceptions in their expressions, and levels [0] and [1] show that the student does not have an understanding at a scientific level.
For the analysis of mental models, student responses were independently coded by two academics, one specializing in chemistry education and the other in physics education. They conducted coding independently and reached a consensus to determine the final results. The agreement rate between the codings of the researchers was found to be 94% (Miles and Huberman, 1994). In codes where there was no agreement, researchers came together to re-evaluate the student responses and discussed them until a consensus was reached. Subsequently, mental model matrices were created following the frameworks of Saglam (2004) and İyibil Durukan (2019). These matrices were used to identify mental models held by students. The distribution of understanding levels given in Table 2 was considered during the matrix creation. Matrix templates corresponding to the answers given by the students were used to obtain three mental models: the electron cloud model, the hybrid/synthesis electron cloud model, and the primitive model, along with eight mental model categories for these models, in accordance with the descriptions by Vosniadou and Brewer (1992), İyibil Durukan (2019), and Franco and Colinvaux (2000). Finally, Table 3 was created utilizing İyibil Durukan (2019).
| Mental model name | Mental model category | The characteristics of the mental model | Mental model matrix | ||
|---|---|---|---|---|---|
| {Conceptual | Relational | Visual} | |||
| Electron cloud model (ECM) | Full scientific (FS) | The understandings of all question types (conceptual, relational, diagrammatic) are scientific in nature. | 4 | 4 | 4 |
| 3 | 3 | 3 | |||
| 4 | 4 | 0 | |||
| 3 | 3 | ||||
| Partial scientific (PS) | Except for any one level of understanding, all other levels of understanding in the categories are scientific or closely related to science. | 4 | 0 | 4 | |
| 3 | 3 | ||||
| 0 | 4 | 4 | |||
| 3 | 3 | ||||
| Hybrid/synthesized electron cloud model (HECM) | Conceptual (C) | The meanings associated with conceptual question types are scientific or closely related to science; the meanings associated with relational and visual question types are non-scientific in nature. | 0 | 0 | |
| 4 | 1 | 1 | |||
| 3 | 2 | 2 | |||
| Relational (R) | The meanings associated with relational question types are scientific or closely related to science; the meanings associated with conceptual and visual question types are non-scientific in nature. | 0 | 0 | ||
| 1 | 4 | 1 | |||
| 2 | 3 | 2 | |||
| Conceptual-relational (CR) | The meanings associated with conceptual and relational question types are scientific or closely related to science; the meanings associated with visual question types are not of a scientific nature. | 0 | |||
| 4 | 4 | 1 | |||
| 3 | 3* | 2 | |||
| Conceptual-visual (CV) | The meanings associated with conceptual and visual question types are scientific or closely related to science; the meanings associated with relational question types are not of a scientific nature. | 0 | |||
| 3 | 1 | 3* | |||
| 4 | 2 | 4 | |||
| Relational-visual (RV) | The meanings associated with relational and visual question types are scientific or closely related to science; the meanings associated with conceptual question types are not of a scientific nature. | 0 | |||
| 1 | 3* | 3 | |||
| 2 | 4 | 4 | |||
| Primitive model (PM) | Incompatible (I) | The meanings associated with conceptual, relational, and visual question types are not of a scientific nature. | 0 | 0 | 0 |
| 1 | 1 | 1 | |||
| 2 | 2 | 2 | |||
For instance, to be classified in the fully scientific category in the electron cloud model, a student must provide responses to all three types of questions at levels [3] or [4]. To be classified in the partially scientific category, the student's responses must be at levels [3] and [4] for the other two categories, excluding any level of understanding. For the hybrid electron cloud model, the responses to questions in that category must be at levels [3] or [4], while the understanding levels for the other two categories should be [0], [1], or [2]. For the primitive model, understanding levels for all categories must be [0], [1], or [2]. Table 3 provides a detailed explanation of these criteria.
The use of matrix templates
A matrix template that corresponds to the answers given by each student to all the questions was determined, and the mental models of the student were identified using these determined matrix templates. Out of the 7 questions asked to the students, 2 are conceptual, 3 are relational, and 2 are visual question types. For example, for a student to be placed in the fully scientific category, the answers to all 7 questions must be either all (3), all (4), or a combination of understanding levels (3) and (4). This means the participant should not answer any of the 7 questions at levels (0), (1), or (2). On the other hand, to be placed in the partially scientific category, a student may answer questions from any one category at level (0), while the answers to questions from the other two categories must consist of either all (3), all (4), or a combination of understanding levels (3) and (4).As another example, for a student to be placed in the hybrid/synthesis electron cloud model (HSECM) in the CR (conceptual–relational) category, the understandings for the conceptual and relational question types must be (4) or (3), while the understandings for the visual question types are at (0), (1), or (2). However, a student presumed to have this model and placed in the HSECM CR category has answered the two conceptual questions as (4), (4), the two visual questions as (1), (0), and the three relational questions as [(1), (3), (3)]. This student has answered two of the three relational questions at a (3) level of understanding and one at a (1) level, which is an undesirable situation. However, the most general model that this student is compatible with has been accepted as the HSECM in the CR category. The matrix templates in Table 3 have been used in this way to determine the mental model, and an asterisk (*) sign has been used to prevent overlap between categories and to determine the appropriate category.
Results and discussion
In this section, findings obtained from questions aimed at solving the research problem and results are presented. In the study, each of the 72 students was presented with a total of 7 open-ended questions, including 2 conceptual, 3 relational, and 2 visual questions. The understanding levels for each question type in the three categories related to the electron cloud concept were determined according to Table 3, and the quantitative findings are presented in Table 4.| Question number | Question category | Level of understanding | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| [0] | [1] | [2] | [3] | [4] | |||||||
| N | % | N | % | N | % | N | % | N | % | ||
| 1 | Conceptual | 2 | 2.78 | 10 | 13.89 | 11 | 15.78 | 15 | 20.83 | 34 | 47.22 |
| 2 | Relational | 16 | 22.22 | 10 | 13.89 | 23 | 31.94 | 9 | 12.5 | 14 | 19.44 |
| 3 | Relational | 4 | 5.55 | 2 | 2.78 | 23 | 31.94 | 42 | 58.33 | 1 | 1.39 |
| 4 | Conceptual | 4 | 5.55 | 7 | 9.72 | 7 | 9.72 | 9 | 12.5 | 45 | 62.5 |
| 5 | Relational | 3 | 4.17 | 9 | 12.50 | 12 | 16.67 | 45 | 62.5 | 3 | 4.16 |
| 6 | Visual | 6 | 8.33 | 31 | 43.05 | 24 | 33.33 | 5 | 6.94 | 6 | 8.33 |
| 7 | Visual | 15 | 20.83 | 40 | 55.55 | 6 | 8.33 | 4 | 5.55 | 7 | 9.72 |
Conceptual category
In the conceptual category, as the first question, students were asked to explain the concept of the “electron cloud,” and the levels of understanding were determined based on the received answers. When examining Table 4 for this question, it was found that the total percentage of students with understanding levels of [3] and [4] is 68.05%, indicating that they provided responses containing acceptable scientific knowledge and information at a scientific level. Some examples of student responses for each level of understanding regarding the explanation of this concept are provided in Table 5.| Question 1 | Level of understanding | Sample responses |
|---|---|---|
| Explain the concept of the electron cloud. | [4] | S2: “Regions where, according to modern atomic theory, the probability of finding electrons is high.” |
| S15, S16, S17: “Regions where the probability of finding electrons around the nucleus is high.” | ||
| [3] | S1, S8, S36: “Region where electrons are dense.” | |
| S44: “Section where electrons are densely located.” | ||
| [2] | S10: “Predicted electron orbits where the speeds and positions of electrons cannot be determined.” | |
| S30: “The rotation of electrons in orbitals around the atomic nucleus.” | ||
| [1] | S13: “Indicates the place where electrons move; electrons move independently.” | |
| S40, S57: “Regions or orbits where electrons settle around the nucleus.” | ||
| S41: “Where multiple electrons are found together.” | ||
| [0] | S49: “Refers to substances moving around the nucleus.” | |
| S67: “A system involving orbitals and the atomic nucleus.” |
In the first question within the conceptual category, when defining the electron cloud concept, students at the [4] level of understanding, as can be seen from Table 5, referred to regions where electrons are likely to be found and to probability concepts emphasized in modern theory, and thus have been assessed at the [4] level of understanding (S2, S15, S16, S17). As seen in Table 4, the percentage of students at this level is 47.22%. It can be said that a considerable number of students express the concept of the electron cloud at a conceptual level. Students at the [3] level of understanding made definitions without mentioning the probability concept (S1, S8, S36, S44), and the percentage of students at this level is 20.83%.
In the responses of students at the [4] level of understanding, the inclusion of the phrase “regions where the probability of finding electrons is high” in the definition of the electron cloud concept was considered important. As Zarkadis et al. (2017) pointed out, the most advanced and abstract student models are those that take into account quantum theory and approach atomic structure with probabilistic logic. Because in modern theory, concepts such as orbitals, the electron cloud, uncertainty principle, energy quantization, wave function, and probability exist. In the study, as seen from Table 5, students with a low level of understanding have typically made definitions by conflating the electron cloud model with the Bohr model. It has been observed that students used the concept of orbit instead of orbital (S30, S40, S57).
In the fourth question within the conceptual category, students were asked to explain the meaning of the orbital concept in modern atomic theory. The results, as depicted in Table 4, reveal that 75% of students demonstrated understanding at [3] and [4] levels. Among them, 62.5% provided responses containing scientifically substantial content, while only 12.5% delivered responses deemed acceptable in terms of scientific knowledge. Sample student responses at different levels of understanding for this question are presented in Table 6.
| Question 4 | Level of understanding | Sample responses |
|---|---|---|
| What is the meaning of the concept of the orbital in modern atomic theory? | [4] | S1, S10, S11, S13, S15, S17: “The region where the probability of finding the electron is highest.” |
| [3] | S31, S36, S67: “The region where electrons are most densely located.” | |
| [2] | S3, S9, S25: “Energy levels around the nucleus of an atom where electrons can be found.” | |
| [1] | S16, S21: “It is a function that defines the position relative to the nucleus and wave properties of the atom.” | |
| S60: “Specifies the energy levels.” | ||
| S66: “The predicted paths followed by electrons.” | ||
| [0] | S6, S49: “The distance of electrons from the atomic nucleus.” | |
| S55: “The direction of rotation and the level at which it is.” |
Despite the presence of a definition in students' textbooks for orbitals as “the space region where electrons are most likely to be found and which has the highest charge density,” none of the students used the concept of charge density in their definitions. However, as seen from Table 6, even though they did not mention charge density, these responses have been accepted at the [4] level of understanding (S1, S10, S13, S15, S17). The reason for such an assessment is that students made explanations using the concept of probability and indicated from their expressions in the previous conceptual question that they believe electrons are more densely found in regions close to the nucleus.
As seen from Table 6, it has been observed that some students, influenced by the Bohr model (such as S3, S9, S25), have misconceptions by describing “energy levels around the nucleus where electrons can be found.” Tsaparlis and Papaphotis (2009) have mentioned that due to conceptual difficulties, learning the concept of orbitals at the high school level is challenging. Similarly, in this study, it has been found that while the phrase “regions where electrons are most likely to be found” in the definition of orbitals is more firmly established in students' minds, the phrase “the space region that has the highest charge density” is not understood by students. Taber (2005) also noted that even though students preparing for university entrance exams might embrace the concept of orbitals, some still tend to understand and use this term as the path that electrons travel around the nucleus. Nakiboglu (2003), in his study, identified the misconception among students that “orbitals are the orbits around the nucleus where electrons revolve. “When reviewing the literature, many studies (Taber, 2002; Nakiboglu, 2003; Dangur et al., 2014; Özcan, 2015; Sunyono et al., 2016; Zarkadis et al., 2017; Allred and Bretz, 2019) indicate that students are generally influenced by the Bohr model, thereby using the concepts of shells and orbits interchangeably or synonymously. For instance, Tsaparlis and Papaphotis (2002) stated that high school students could not understand the probabilistic nature of atomic orbitals, were confused among various atomic and orbital representations, and were able to maintain a deterministic perspective. Similar to the conceptual misunderstandings mentioned in the literature, similar results have been seen in this study. For example, as seen in Table 6, the student coded S60 was shown to have a misconception for the orbital concept by stating “indicates energy layers,” and student coded S66 by saying “the orbits that electrons follow.”
Researchers expected the responses to the two conceptual questions in this study to be prepared in a mutually supportive way, anticipating consistency in students' answers to these questions. It was expected that a student who defined the “electron cloud” concept as the region where the probability of finding electrons is high in the first question would also clearly express the orbital concept in the second question. The study found that only 52% of students provided consistent responses at [3] or [4] understanding levels for both questions, indicating a lower level of consistency than expected. This finding indicates that the concept of the electron cloud has not yet formed meaningfully in the minds of many students.
Relational category
In the relational category, the second question prompted students to explain the concept of the electron cloud using everyday examples and analogies. Upon examining Table 4, it is seen that the total percentage of students with understanding levels of [3] and [4] in the second question is 31.9%. In other words, the majority of students did not offer responses that included scientifically valid and acceptable relationships, indicating a struggle in drawing successful analogies. Examples of student responses at different levels of understanding for this question are presented in Table 7.| Question 2 | Level of understanding | Sample responses |
|---|---|---|
| How can you analogize the electron cloud in modern atomic theory to everyday examples, explaining it through comparisons with daily life? | [4] | S22: “The probability of all students being in their classrooms during school hours is high.” |
| S23: “When you travel westward in our country, you expect to encounter more people, but it is not certain.” | ||
| S44: “The probability of encountering students around the school is high, but as you move away from the school, this probability decreases.” | ||
| [3] | S4, S5: “Airplane routes most frequently taken in the sky.” | |
| S59: “Traffic conditions on roads in a city.” | ||
| S38: “The light spread around a street lamp.” | ||
| [2] | S11, S30: “Like the movement of a cloud.” | |
| S17: “Cars turning at an intersection.” | ||
| [1] | S49, S52: “The solar system.” | |
| S71: “Particle-filled liquid inside a blender.” | ||
| [0] | S39: “I couldn't find an example.” |
As can be seen from Table 4, the percentage of students who answered at the [4] level is 19.44%, and at the [3] level is 12.5%, while it is observed that the rates of students at lower levels are high. Additionally, 22% of the responses were left unanswered or contained incomprehensible expressions.
According to Duit (1991), analogy is defined as the cognitive representation of relationships between objects when transitioning from source information to new information. Evaluating the answers at the [4] understanding level based on this definition, it is gratifying that students (S22, S23, S38, S44) consider the probability concept, incorporate correct relationships, and include creative and qualified analogies, albeit in small numbers. As Vieira and Morais (2022) express, analogies are described as a powerful tool to explain complex scientific concepts such as abstract or unconventional ones like the quantum atomic model using familiar terms, encouraging positive attitudes towards learning. The results reveal that students face difficulty in making analogies and establishing scientific relationships with everyday life examples. The failures in students' analogy-making also suggest a close association with insufficient conceptual understanding.
In the analysis of the analogies prepared by Derman and Tufan (2021), when evaluating our students' analogies according to the categorical framework used, it is observed that the examples of students who make correct analogies, such as student coded S22 with “The likelihood of all students being in their classrooms during class hours at school is high.” as seen in Table 7, exhibit verbal and functional characteristics in terms of their representation.
In the relational category, question number 3 asked students to explain which shortcomings in atomic models led to the emergence of the electron cloud concept. Upon reviewing Table 4, it was observed that, for this question in the relational category, only 1.39% of students demonstrated an understanding at [4] level, while 58.33% were at [3] level. The majority of responses contained relationships that were correctly established and considered scientifically valid. Examples of student responses at different levels of understanding for this question are provided in Table 8.
| Question 3 | Level of understanding | Sample responses |
|---|---|---|
| What shortcomings in atomic models led to the development of the concept of the electron cloud in modern atomic theory? | [4] | S45: “The Bohr atomic model can only explain the emission of particles with a single electron. It cannot describe multi-electron atoms, the behavior of atoms in a magnetic field, and the simultaneous knowledge of both the speed and position of an electron.” |
| [3] | S3, S4, S14, S15: “The speed and position of the electron cannot be known simultaneously.” | |
| S27: “Due to the absence of the concept of orbits.” | ||
| [2] | S29: “The absence of definite positions for atoms.” | |
| S37: “The inability to determine the location of the electron.” | ||
| [1] | S57: “Electrons rotate in a three-dimensional space; the electron cloud is a two-dimensional concept.” | |
| [0] | S28: “I can't remember.” |
For this question, examples of student responses for each level of understanding have been provided in Table 8. For example, students with codes S3, S27, S14, S15 stated that the Bohr atomic model successfully explains line spectra of single atoms and ions but falls short in explaining the line spectra of multi-electron atoms. However, many students could not provide an explanation for the behavior of atoms in magnetic fields. The study shows that while students mention some factors leading to the birth of modern theory, they cannot express all of them together. Consistent with the literature findings by Nakiboglu (2003), Taber (2005), Stevens et al. (2010), quantum mechanics is difficult for students to understand due to its abstract and complex nature, and concepts are often used interchangeably. Examining student responses, for instance, the response of student S27, linking the lack of the concept of orbits to the emergence of EBM, implies that the reasons for the emergence of ECM are not fully understood.
In question number 5, located in the relational category, students were asked to specify the reasons for using new quantum numbers in modern atomic theory and to identify what these quantum numbers are. Examples of student responses at different levels of understanding for this question are presented in Table 9.
| Question 5 | Level of understanding | Sample responses |
|---|---|---|
| In modern atomic theory, new quantum numbers have been used instead of the orbit concept in the Bohr atomic model for several reasons. What are these reasons, and what are the names of these new quantum numbers? | [4] | S28, S40: “Wave mechanics explains multi-electron atoms with quantum numbers. There are four of them. The principal quantum number is n, the angular quantum number is l, the magnetic quantum number is m, and the spin quantum number is s.” |
| [3] | S2, S3: “Principal quantum number, angular quantum number, magnetic quantum number, spin quantum number.” | |
| [2] | S5: “The uncertainty of the electron's position and the inability to model atoms with more than 20 electrons.” | |
| [1] | S49: “Used to explain multi-atomic molecules.” | |
| S55: “To determine the direction of rotation.” | ||
| S52: “Principal quantum numbers: s, p, d, f.” | ||
| [0] | S47: “No response” |
As seen in student responses in question number 5 under the relational category (S2, S3, S5), although a large majority of students (62.5%) correctly define quantum numbers, they did not mention that quantum numbers are used to explain multi-electron atoms in wave mechanics. From the responses, it is observed that students, having learned information at a symbolic level, can more easily write the symbols and names of the four quantum numbers correctly but fall short in explaining the rationale for their usage (S2, S3, S5). Only a few students mentioned that wave mechanics can explain multi-electron atoms using quantum numbers (S28, S40). According to Papaphotis and Tsaparlis (2008), students do not have a comprehensive understanding of orbitals and quantum numbers. This may be due to the necessity of understanding various abstract, complex, and symbolic concepts involved in the quantum model of atomic structure, as mentioned by Zarkadis et al. (2022). Additionally, according to Zarkadis et al. (2022), studies on this topic (for example, Sunyono et al. 2016; Temel and Özcan 2018; Papaphotis and Tsaparlis 2008) support the view that students often try to understand quantum numbers with a simple, deterministic, or mechanistic approach. The results obtained in the study are consistent with these literature findings. While in the Bohr model, electrons are thought to follow circular paths at specific energy levels, in the modern theory, electrons are found in orbitals with high probability of existence, designated by the letters s, p, d, f. The exact distinction between these orbitals does not seem to be clear in the minds of students.
Visual category
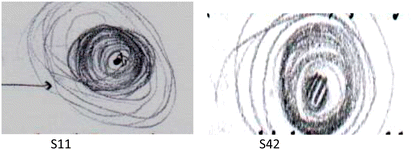
In the visual category, the sixth question asked students to draw a figure explaining the electron cloud model, and their understanding levels were determined based on their responses. Upon examining Table 4, it is observed that only 15.28% of students drew the model at understanding levels [3] and [4]. The majority of students demonstrated understanding at levels [1] and [2], indicating drawings that incorporate non-scientific visual elements or are at a basic level. Examples of student responses at different understanding levels for this question are provided in Table 10.One way to uncover students' mental models is to allow them to create their own models. As Akaygun (2016) pointed out, these drawings reveal how students visualize specific events and the differences in their mental models. Thus, in question number 6 in the visual category, students were asked to draw diagrams for the electron cloud model. When examining the drawings, it was observed that 8.33% of students responded at the [4] understanding level, and 6.94% at the [3] understanding level, while the percentage of students at lower understanding levels was high. In the drawings of students at the [0] and [1] understanding levels, non-scientific visual elements are predominant (S9, S1, S21, S20).
Although we have considered the drawings by students who answered at the [4] level of understanding (S11, S42) as scientifically accepted drawings, they made two-dimensional drawings without using a three-dimensional axis system. The reason these drawings are accepted at the [4] level of understanding is because, in the conceptual question, the students used the concept of region while defining the electron cloud concept. This acceptance can be seen as a limitation of the study. In their drawings, these students represented the nucleus at the center with a dot and depicted the orbital representation as expected with a cloud-like depiction, darker in regions close to the nucleus and lighter as it moves away from the nucleus. Students who answered at the [3] level of understanding (S19, S43) have made drawings at an acceptable level. In these drawings, students represented the nucleus with a central point and depicted the orbital representation either as a cloudy representation or with dots, but they could not clearly show the relationship between the distance from the nucleus and electron density. Students who responded at the [2] understanding level (S6, S57) were influenced by the Bohr model, producing drawings that did not align with the cloud model. In these drawings, it is evident that students represented electrons with dots and tended to view electrons as particles. They drew continuous and circular orbits representing the Bohr atomic model. Student S (21) is observed to intertwine the orbits of the Bohr model with a wavy pattern, suggesting a combination of the cloud model and the Bohr model in their mind. As seen in the drawings, students tend to perceive electrons as particles moving in specific orbits.
In the study, it was observed that many students could not translate the conceptually defined electron cloud model into drawings. For example, as seen in the figures of students S21 and S9, despite drawing non-scientific shapes for the cloud model, they correctly explained the electron cloud concept in conceptual questions. This may be attributed to the fact that although concepts are expressed verbally, meaningful learning does not occur in the student's mind. Additionally, it could be considered that students may lack sufficient spatial abilities or that visual explanations with adequate clarity are not used in teaching, or three-dimensional visual materials are not employed. Another significant consideration is the necessity for students to use their imagination to create mental designs to form their understanding of the electron cloud model (Yang et al., 2003). Consistent with our study results, Cascarosa Salillas et al. (2022) stated that most students do not have sufficient spatial vision and abstraction ability to create a consistent mental atomic model with the atomic model. Furthermore, Park and Light (2009) and Dangur et al. (2014) explained that students' illustrated representations of the structure of the atom are not consistent with their corresponding verbal explanations. In contrast, Tsaparlis and Papaphotis (2009) stated that although students can draw an electron cloud defined with a specific quantum number, they cannot use concepts such as electron probability density, point cloud, or cloudy structure in their explanations of the drawings.
In the visual category, the seventh question asked students to draw a figure explaining the probability of finding an electron in the “1s” orbital as a function of the distance from the nucleus. Upon examining Table 4, it is observed that only 15.28% of students drew the model at understanding levels [3] and [4]. The majority of students demonstrated understanding at level [1], indicating drawings that incorporate non-scientific visual elements or are at a basic level. Additionally, 20.83% of students did not draw anything for this question. Examples of student responses at different understanding levels for this question are provided in Table 11.
Student drawings were evaluated based on their ability to use spatial representation correctly by referencing the x, y, and z axes of the Cartesian coordinate system. For this question, 9.72% of students responding at the [4] level drew diagrams showing that the probability of finding the electron decreases as it moves away from the nucleus (S18, S51). Students at the [3] level, accounting for 5.55%, drew the x, y coordinate system but, as in the case of S19, illustrated a shape showing that the electron density decreases as it moves away from the nucleus without aligning the drawing to the coordinate system. A high percentage of students at other levels, including S1, S55, S3 and S57, were determined to lack the ability to create scientifically valid drawings by not using spatial qualities. In the conceptual category, students described the electron cloud concept by defining it as the place where electrons are densely located, stating that this density decreases as they move away from the nucleus. However, it is apparent from the figures that they were not successful in transferring this information onto a graph. Considering Rau's (2015) suggestion that students' learning success depends on their ability to establish connections between graphical representations, it can be seen that students could not meaningfully learn or internalize the concept.
In conclusion, each student was evaluated using the matrix patterns determined in Table 3, and the types of mental models attributed to students were identified. The quantitative data resulting from the assessment are provided in Table 12.
| Mental model name | Mental model category | f | % |
|---|---|---|---|
| Electron cloud model (ECM) | Full scientific model (FSM) | 4 | 5.56 |
| Partial scientific model (PSM) | 12 | 16.67 | |
| Hybrid/synthesized electron cloud model (HECM) | Conceptual model (CM) | 24 | 33.33 |
| Relational model (RM) | 5 | 6.95 | |
| Conceptual relational model (CRM) | 16 | 22.22 | |
| Conceptual visual model (CVM) | 6 | 8.33 | |
| Relational visual model (RVM) | 3 | 4.16 | |
| Primitive model (PM) | Incompatible model (IM) | 2 | 2.78 |
Upon examining Table 12, it is evident that 8 mental model categories have emerged under the umbrella of the three identified mental models. Of the students, 5.56% provided sufficiently scientific answers to questions related to the electron cloud model, placing them in the fully scientific model category. Additionally, 2.78% were categorized into the incompatible model category associated with the primitive model due to non-scientific responses. The category in which students are most prominently represented is the conceptual model category under the hybrid/synthesized electron cloud model, constituting 33.33% of the responses. Due to the research sample consisting of Science High School students, the 5.56% rate obtained in the fully scientific model category is below the expected level. It has been determined that the majority of the students (84.7%) in the partially scientific model category are placed in this category because they failed in the research questions of the visual category. As Zarkadis et al. (2022) noted, despite visual representations being present in textbooks, students are known to struggle with drawing shapes. In this study, some students (e.g., S21 and S9) were able to define the electron cloud concept in the conceptual category with scientific terms. However, they struggled to accurately depict this knowledge in a drawing, resulting in a lower-than-expected number of students in the full scientific category. As visual representations are crucial in teaching concepts in chemistry, students learn through the visual language of shapes (Schönborn and Anderson, 2006; Airey and Linder, 2009). Therefore, the obtained result reflects a significant deficiency. The low success rate in the shape-related category is crucial, as noted by Chi (2009), as it indicates that students hold conflicting ideas and highlights the presence of conceptual misconceptions that need to be addressed. For instance, students like S6, S57, and S24 still demonstrate dominance of the Bohr atomic model in their drawings of the electron cloud model.
According to the study results, the majority of students (75.49%) fall within the hybrid/synthesized ECM. Among these models, the largest percentage (33.33%) is conceptual models. Students with this mental model exhibit understanding at [3] and [4] levels only in conceptual questions, while their understanding at other levels is non-scientific and contains conceptual misconceptions. While only a small number of students (6.95%) possess the relational model, 22.22% have the conceptual–relational model. The findings for each category are discussed separately below.
In the mental models emerging from Table 12, despite Modern Atomic Theory emphasizing the expression of energy shells at certain energy levels instead of the orbits at specific energy levels in the Bohr atomic model, and stating that these shells are divided into subshells which contain orbitals occupied by electrons, the prevalence of a hybrid structure among students is still observed. This is because, in the Bohr atomic model, electrons are particles that orbit the nucleus at a certain distance in circular orbits. This theory is easily explained to students through animations and is clearly embraced by them. However, in quantum theory, the concept of charge density is the probability of electrons being within a certain volume and unfortunately, even if this situation is transformed into a visual dimension, the existence and properties of a cloud-like structure conflict with the particle concept in the student's mind. Thus, using concepts such as probability, charge density, and orbitals becomes challenging, and the use of a hybrid model is thought to be predominant. These findings align with the notion of a hybrid structure mentioned by Tsaparlis and Papaphotis (2009), and similar results are found in studies by Harrison and Treagust (2000), Taber (2002, 2005), Trindade and Fiolhais (2003), Park and Light (2009), Stevens et al. (2010), and others in the literature. Similar results are also reported in other studies in the literature. For example, Allred and Bretz (2019) found that, despite being taught the quantum model of the atom, many first-year university students still preferred to think in terms of the Bohr model and struggled to understand the meaning of the electron cloud concept. Papageorgiou et al. (2016) indicated that students faced difficulty in adopting modern atomic theory due to the probability concept and had conceptual misconceptions. Additionally, Budde et al. (2002) mentioned that students tend to maintain their existing biases when teaching the probability model, even returning to these biases after education, with no long-term solution.
Conclusions and implications
This study has been conducted with the aim of uncovering the mental models related to the electron cloud concept among science high school students. What sets this study apart from other studies in the same field is the use of open-ended question types prepared in three categories—conceptual, relational, and visual—at the same time to determine the mental model for the electron cloud concept among science high school students.The results of this study indicate that there are still problems with the comprehensibility of modern theory. One such issue is the persistent nature of misconceptions and the difficulty in eliminating them. Another is the inclusion of a series of abstract concepts in modern theory, such as the concept of probability, the uncertainty principle, and charge density. Besides, it is considered that the probabilistic nature of these concepts creates a significant gap in the student's mind, hindering the full conceptualization of the concept. Moreover, it is observed that the persuasive nature of pioneering models related to atomic models has a lasting effect in terms of persistence. Therefore, we believe that it is particularly important for teachers to emphasize in their lectures that the modern atomic theory represents the current model and that adopting this model will be effective in reducing students' conceptual misconceptions about the atom.
The participation of science high school students in the study, the more detailed program of the science high school in comparison to other high school programs regarding the unit on modern atomic theory, the greater number of class hours allocated to the unit, and the teacher being an experienced chemistry teacher might have led us to achieve better results in the conceptual category. Yet, a higher level of achievement was expected for science high school students. As Papageorgiou et al. (2016) have indicated, several factors such as the chemistry curriculum, class, or individual differences are known to influence students' adoption of one model over another. The electron cloud concept in modern theory is a crucial cornerstone for understanding the structure of the atom. Furthermore, the role of concepts as a springboard in the learning process, allowing students to make connections with their previous learning and to structure concepts healthily in their minds, will also affect the future teaching process. Therefore, unveiling how the electron cloud concept is structured in students' minds, and the constructions and thoughts forming the concept is deemed significant. One reason for the low number of students falling into the fully scientific and partially scientific model categories in the study could be the submicroscopic nature of the electron cloud concept. Previous research has shown that even university students might not have developed the mental models necessary to effectively think about the submicroscopic world (Chittleborough et al., 2002). This is because, as Gabel (1993) pointed out, concepts are taught in chemistry lessons with little emphasis on microscopic and macroscopic levels. However, as observed from classroom observations on the teaching of the modern atomic theory, the teacher has made an effort to relate concepts with everyday life examples and use analogies instead of presenting them in a symbolic dimension. Indeed, the effectiveness of the analogies made by the teacher in explaining quantum numbers in understanding by students has been observed. Similarly, we believe that developing and applying creative and effective analogies for other concepts will be beneficial. Allowing students to make analogies with the teacher's support in class will, in a sense, lay the groundwork for revealing the structures in their minds. The teacher's feedback and reinforcement to the analogies will enrich the association of concepts, aiding in mental structuring. In addition to known outcomes related to modern atomic theory, it becomes apparent that some educational adjustments related to this theory need to be made in science programs. It may be considered necessary to updated curricula and textbooks to address issues related to the teaching of atomic models, by making new adjustments in the content of programs and textbooks. For example, enabling students to access 3D visuals during lesson explanations through quick response (QR) codes placed in textbooks will be effective in understanding related concepts and making more scientific drawings. Moreover, providing concrete examples with computer support for understanding and relating the concept of probability and the uncertainty principle could also be beneficial.
However, due to the university entrance exam system in Turkey and the anxieties related to this exam, the use of analogies, models, visuals, and computer-assisted education in teaching is limited. As a result, there is a tendency to reinforce topics primarily through multiple choice-type questions, which is thought to have an impact on the findings. Considering the results obtained, it can be suggested to curriculum developers that chemistry lessons in K-12 programs should be delivered using an integrated model of knowledge and skills.
Finally, it is recommended that researchers conduct studies examining the contribution of teaching processes using 3D materials to the teaching of modern atomic theory, specifically looking at their impact on the development of students' mental models.
Limitation of the study
The generalizability of the study results has some limitations. The primary limitation of the study is that it evaluates students' two-dimensional drawings as scientifically accepted representations, even though they did not use a three-dimensional coordinate system. Additionally, the study was conducted with a small sample group and focused on a limited topic. Even though the study is designed qualitatively and not intended for generalization, repeating the study may be necessary for a robust generalization. Another limitation is the determination of codes, which could be considered as the assessment tools created and used by researchers for the purpose of analyzing verbal explanations and figures.Conflicts of interest
There are no conflicts to declare.Acknowledgements
I would like to thank Prof. Dr N. Remziye Ergül, who assisted in data analysis, and the reviewers, who provided insightful feedback.References
- Airey J. and Linder C., (2009), A disciplinary discourse perspective on university science learning: achieving fluency in a critical constellation of modes, J. Res. Sci. Teach., 46(1), 27–49.
- Akaygun S., (2016), Is the oxygen atom static or dynamic? The effect of generating animations on students' mental models of atomic structure, Chem. Educ. Res. Pract., 17(4), 788–807.
- Albaiti A. J., and Lepa A. A., (2022), Solubility and solubility product phenomena: papua senior high school students mental model, J. Turkish Sci. Educ., 19(2), 481–495 DOI:10.36681/tused.2022.132.
- Allred Z. D. R., and Bretz S. L., (2019), University chemistry students’ interpretations of multiple representations of the helium atom, Chem. Educ. Res. Pract., 20(2), 358–368 10.1039/C8RP00296G.
- Budde M., Niedderer H., Scott P. and Leach J.,(2002), ‘Electronium’: a quantum atomic teaching model, Phys. Educ., 37, 197 DOI:10.1088/0031-9120/37/3/303.
- Cascarosa Salillas E., Pozuelo Muñoz J., Jiménez M. and Fernández Álvarez F. J., (2022), Analysis of the mental model about the atom concept in Spanish 15-to 18-years old students, Educ. Química, 33(2), 181–193 DOI:10.22201/fq.18708404e.2022.2.79895.
- Chang R., (2009), Genel Kimya (4.Baskı) (T. Uyar, S. Aksoy, R. İnam, Çev.). Palme Yayıncılık (Originally published in 2006), 5th edn.
- Chen S. C., Hsiao M. S. and She H. C., (2015), The effects of static versus dynamic 3D representations on 10th grade students’ atomic orbital mental model construction: evidence from eye movement behaviors, Comput. Human Behav., 53, 169–180 DOI:10.1016/j.chb.2015.07.003.
- Chi M. T., (2009), Three types of conceptual change: Belief revision, mental model transformation, and categorical shift, International handbook of research on conceptual change, Routledge, pp. 89–110.
- Chittleborough G. D., Treagust D. F. and Mocerino M., (2002), Constraints to the development of first year university chemistry students’ mental models of chemical phenomena, in Bunker A. and Swan G. (ed.), Focusing on the student, Perth, pp. 43–50.
- Chittleborough G. D. and Treagust D. F., (2009), Why models are advantageous to learning science, Educación química, 20(1), 12–17.
- Coll R. K. and Treagust D. F., (2001), Learners' mental models of chemical bonding. Res. Sci. Educ., 31(3), 357–382.
- Coll R. K. and Treagust D. F., (2003), Investigation of secondary school, undergraduate, and graduate learners' mental models of ionic bonding. J. Res. Sci. Teach., 40, 464–486.
- Creswell J. W., (2012), Qualitative inquiry and research design: Choosing among five approaches, 3rd edn, London: Sage Publications.
- Dangur V., Avargil S., Peskin U. and Dori Y. J., (2014), Learning quantum chemistry via a visual-conceptual approach: students' bidirectional textual and visual understanding, Chem. Educ. Res. Practice, 15(3), 297–310 10.1039/C4RP00025K.
- Demirçalı S., (2016), The effect of modeling-based science teaching on students' academic achievement, scientific process skills and mental model development: an example of the 7th grade “Solar System and Beyond – Space Riddle” unit, MPhil thesis, Ankara: Gazi University.
- Demirci S., Yılmaz A. and Şahin E., (2016), Lise ve üniversite öğrencilerinin atomun yapısı ile ilgili zihinsel modellerine genel bir bakış, Türkiye Kimya Derneği Dergisi Kısım C: Kimya Eğitimi, 1(1), 87–106.
- Demirkol H., (2017), Determination of 6th grade students' mental models and cognitive structures regarding physical and chemical changes, MPhil thesis, Balıkesir: Balıkesir Üniversitesi.
- Derman A. and Tufan M., (2021), Kimya Öğretmenlerinin öğretimlerinde analoji kullanım durumlarının incelenmesi, OPUS Int. J. Soc. Res., 18(44), 7749–7776 DOI:10.26466/opus.957650.
- Didiş N., Eryılmaz A. and Erkoç Ş., (2014), Investigating students’ mental models about the quantization of light, energy, and angular momentum, Phys. Rev. Phys. Educ. Res., 10(2), 020127 DOI:10.1103/PhysRevSTPER.10.020127.
- Duit R., (1991), On the role of analogies and metaphors in learning science, Sci. Educ., 75(6), 649–672.
- Eryılmaz Muştu Ö., (2021), Qualitative evaluation of prospective science teachers' concept maps about the atom, Int. J. Progressive Educ., 17(1), 158–171 DOI:10.29329/ijpe.2021.329.11.
- Franco C. and Colinvaux D., (2000), Grasping Mental Models, in Developing Models in Science Education, J. K. Gilbert and C. J. Boulter (ed.), Kluwer Academic Publishers.
- Gabel D. L., (1993), Use of the particle nature of matter in developing conceptual understanding, J. Chem. Educ., 70(3), 193–194.
- Harrison A. G. and Treagust D. F., (2000), Learning about atoms, molecules, and chemical bonds: a case study of multiple-model use in grade 11 Chemistry, Sci. Educ., 84(3), 352–381.
- Hill B. R., (2006), Eliciting, analyzing, and comparing mental models of complex audio recording systems between professional and novice recording engineers, Unpublished doctoral dissertation, Pennsylvania State University, Pennsylvania.
- Hong E. and O'Neil H. F., (1992), Instructional strategies to help learners build relevant mental models in inferential statistics, J. Educ. Psychol., 84(2), 150 DOI:10.1080/09500690801891908.
- İyibil Durukan Ü. G., (2019), Elektrik akım konusuna yönelik tasarlanan adidaktik öğrenme ortamlarının lisans öğrencilerinin zihinsel modellerinin gelişimine etkisi, MPhil thesis, Trabzon: Trabzon Üniversitesi.
- Kilinc A., Demira, U. and Kartal T., (2017), Resistance to dialogic discourse in SSI teaching: the effects of an argumentation-based workshop, teaching practicum, and induction on a preservice science teacher. J. Res. Sci. Teach., 54(6), 764–789 DOI:10.1002/tea.21385.
- Liu C. J., Hou I. L., Chiu H. L. and Treagust D. F., (2014), An exploration of secondary students’ mental states when learning about acids and bases, Res. Sci. Educ., 44, 133–154.
- MEB, (2023), T.C. Millî Eğitim Bakanlığı Talim Terbiye Kurulu Başkanlığı https://mufredat.meb.gov.tr/programlar adresinden 20.04.2024 tarihinde alınmıştır.
- Miles M. B. and Huberman M. A., (1994), Qualitative data analysis: An expanded sourcebook, London: Sage.
- Nakiboglu C., (2003). Instructional misconceptions of Turkish prospective chemistry teachers about orbitals and hybridization, Chem. Educ. Res. Pract., 4(2), 171–188 10.1039/B2RP90043B.
- Nakiboglu C., (2019), Kimya öğretmen adaylarının metalik yapı ile ilgili zihinsel modelleri ve metalik bağ ile ilgili kavramaları, Karaelmas Eğitim Bilimleri Dergisi, 7(1), 133–144.
- Özcan Ö., (2015). Investigating students’ mental models about the nature of light in different contexts. Eur. J. Phys., 36(6), 065042 DOI:10.1088/0143-0807/36/6/065042.
- Papageorgiou G., Markos A. and Zarkadis N., (2016). Students' representations of the atomic structure–the effect of some individual differences in particular task contexts, Chem. Educ. Res. Pract., 17(1), 209–219 10.1039/C5RP00201J.
- Papaphotis G. and Tsaparlis G., (2008), Conceptual versus algorithmic learning in high school chemistry: the case of basic quantum chemical concepts. Part 2. Students’ common errors, misconceptions and difficulties in understanding, Chem. Educ. Res. Pract., 9(4), 332–340 10.1039/B818470B.
- Park E. J. and Light G., (2009), Identifying Atomic Structure as a Threshold Concept: studentmental models and troublesomeness, Int. J. Sci. Educ., 31(2), 233–258 DOI:10.1080/09500690701675880.
- Petri J. and Niedderer H., (1998), A learning pathway in high-school level quantum atomic physics, Int. J. Sci. Educ., 20(9), 1075–1088 DOI:10.1080/0950069980200905.
- Rau M. A., (2015), Enhancing undergraduate chemistry learning by helping students make connections among multiple graphical representations, Chem. Educ. Res. Pract., 16(3), 654–669 10.1039/C5RP00065C.
- Saglam A., (2004), Les équations différentielles en mathématiques et en physique: étude des conditions de leur enseignement et caractérisation des rapports personnels des étudiants de première année d’université à cet objet de savoir, (Unpublished doctoral dissertation), Universite Joseph Fourier, Grenoble.
- Schönborn K. J. and Anderson T. R., (2006), The importance of visual literacy in the education of biochemists, Biochem. Mol. Biol. Educ., 34(2), 94–102 DOI:10.1002/bmb.2006.49403402094.
- Stevens, S. Y., Delgado C. and Krajcik J. S., (2010), Developing a hypothetical multi-dimensional learning progression for the nature of matter, J. Res. Sci. Teach., 47(6), 687–715 DOI:10.1002/tea.20324.
- Sunyono S., Tania L. and Saputra A., (2016), A Learning Exercise using Simple and Real-Time Visualization Tool to Counter Misconceptions about Orbitals and Quantum Numbers, J. Baltic Sci. Educ., 15(4), 452–463. https://scientiasocialis.lt/jbse/.
- Supasorn S., (2015), Grade 12 students' conceptual understanding and mental models of galvanic cells before and after learning by using small-scale experiments in conjunction with a model kit. Chem. Educ. Res. Pract., 16(2), 393–407 10.1039/C4RP00247D.
- Taber K. S., (1997), Understanding chemical bonding – the development of A level students’ understanding of the concept of chemical bonding, PhD thesis, University of Surrey.
- Taber K. S., (2002), Conceptualizing quanta illuminating the ground state of student understanding of atomic orbitals, Chem. Educ. Res. Pract., 3(2), 145–158 10.1039/B2RP90012B.
- Taber K. S., (2005), Learning quanta: barriers to stimulating transitions in student understanding of orbital ideas, Sci. Educ., 89(1), 94–116 DOI:10.1002/sce.20038.
- Taber K. S., (2013), Revisiting the chemistry triplet: drawing upon the nature of chemical knowledge and the psychology of learning to inform chemistry education, Chem. Educ. Res. Practice, 14(2), 156–168 10.1039/C3RP00012E.
- Temel S. and Özcan Ö., (2018), Students’ Understanding of Quantum Numbers: A Qualitative Study, in SHS Web of Conferences, EDP Sciences, vol. 48, p. 01002.
- Trindade J. and Fiolhais C., (2003), Students' visualization and conceptual understanding of atomic orbitals using a virtual environment, Proceedings 3rd IEEE International Conference on Advanced Technologies, IEEE, pp. 298–299 DOI:10.1109/ICALT.2003.1215092.
- Tsaparlis G. and Papaphotis G., (2002), Quantum-chemical concepts: are they suitable for secondary students? Chem. Educ. Res. Pract., 3(2), 129–144 10.1039/B2RP90011D.
- Tsaparlis G. and Papaphotis G., (2009), High-School Students’ Conceptual Difficulties and Attempts at Conceptual understanding of atomic orbitals, Chem. Educ. Res. Pract., 3(2), 145–158 DOI:10.1080/09500690801891908.
- Vieira H. and Morais C., (2022), Musical Analogies to Teach Middle School Students Topics of the Quantum Model of the Atom, J. Chem. Educ., 99(8), 2972–2980 DOI:10.1021/acs.jchemed.2c00289.
- Vogt A., Babel F., Hock P., Baumann M. and Seufert T., (2021), Prompting in-depth learning in immersive virtual reality: impact of an elaboration prompt on developing a mental model, Comput. Educ., 171, 104235 DOI:10.1016/j.compedu.2021.104235.
- Vosniadou S. and Brewer W. F., (1992), Mental models of the earth: a study of conceptual change in childhood, Cognitive Psychol., 24(4) 535–585 DOI:10.1016/0010-0285(92)90018-W.
- Wang C. Y. and Barrow L. H., (2013), Exploring conceptual frameworks of models of atomic structures and periodic variations, chemical bonding, and molecular shape and polarity: a comparison of undergraduate general chemistry students with high and low levels of content knowledge, Chem. Educ. Res. Pract., 14(1), 130–146 10.1039/C2RP20116J.
- Wittmann M. C., (2002). The object coordination class applied to wavepulses: analysing student reasoning in wave physics, Int. J. Sci. Educ., 24(1), 97–118 DOI:10.1080/09500690110066944.
- Yang E. M., Andre T., Greenbowe T. J. and Tibell L., (2003), Spatial ability and the impact of visualization/animation on learning electrochemistry, Int. J. Sci. Educ., 25(3), 329–349 DOI:10.1080/09500690210126784.
- Yin R. K., (2003), Case study research: Design and methods, 3rd edn, London: Sage.
- Zarkadis N., Papageorgiou G. and Markos A., (2022), Incorporating Quantum Number Characteristics in the Pictorial Representations of the Atomic Structure: Consistency Issues and Students' Relevant Profiles, Sci. Educ. Int., 33(1), 93–101 DOI:10.33828/sei.v33.i1.10.
- Zarkadis N., Papageorgiou G. and Stamovlasis D., (2017). Studying the consistency between and within the student mental models for atomic structure, Chem. Educ. Res. Pract., 18(4), 893–902 10.1039/C7RP00135E.
| This journal is © The Royal Society of Chemistry 2024 |