STEM-PT Traveler, a game-based approach for learning elements of the periodic table: an approach for enhancing secondary school students’ motivation for learning chemistry
Mohammad Najib
Mohammed Naaim
and
Mageswary
Karpudewan
 *
*
School of Educational Studies, Universiti Sains Malaysia, 11800 Penang, Malaysia. E-mail: kmageswary@usm.my
First published on 25th June 2024
Abstract
The COVID-19 pandemic has significantly impacted students' motivation for learning. As students return to schools in the post-pandemic era, their motivation for learning continues to deteriorate due to challenges in adapting to the new educational norms. This study aimed to enhance the motivation of secondary school students towards learning chemistry, particularly during the period when their motivation has tended to be low upon returning to regular schooling after the pandemic. To achieve this objective, the researchers developed and implemented a self-designed game-based learning approach called STEM-PT Traveler during lessons focused on the periodic table. STEM-PT Traveler incorporated elements of enjoyable learning and play, introducing an interdisciplinary perspective to periodic table lessons. The effectiveness of STEM-PT Traveler in improving motivation was compared to an alternative student-centred, non-game-based learning approach using an explanatory mixed-method design. Two intact classes from a public secondary school were randomly assigned to two groups—one group utilized the game-based learning approach (N = 45), while the other group employed the non-game-based approach (N = 46). The multivariate analysis of covariance (MANCOVA) findings from pre-test and post-test questionnaires administered before and after treatment revealed significant differences in overall motivation and in the subscales of intrinsic motivation, career motivation, and self-efficacy. Non-significant differences were observed for grade motivation and self-determination. Qualitative interviews conducted with both groups after the treatment provided additional insights into the questionnaire outcomes. Specifically, during the interviews, students highlighted that the game facilitated engagement with the periodic table elements due to their intrinsic value. Additionally, the game provided a career perspective and instilled a belief that excelling in chemistry is instrumental. This study suggests that a game-based approach is an effective alternative to the predominantly used teacher-centred teaching of the periodic table and advocates for the integration of interdisciplinary perspectives into lessons on the Periodic Table.
Introduction
The definition of motivation in the literature varies according to the perspectives established by the motivational proponents. Energy, direction and perseverance to sustain any activities are named as motivation (Deci and Ryan, 2000). Glynn et al. (2011), in the context of learning science, viewed motivation as students' efforts to learn science. This encompasses the reasons behind their efforts, the duration and intensity of their efforts, and the emotions they experience as they strive. Extending this concept to learning chemistry—including the reasons, duration, intensity, and emotional aspects—infers the motivation for learning chemistry (Ardura and Pérez-Bitria, 2018; De Souza et al., 2022; Zhang and Zhou, 2023).Currently, the pressing global concern is the shrinking number of STEM graduates to support the demand for the STEM workforce, which is consistently increasing with advancements in science and technology. According to a Harvard University report, STEM occupations have grown to 79% in the past three decades, and STEM jobs are projected to grow an additional 11% from 2020 to 2030 (O’Rouke, 2021). The report further said that to stay competitive in the STEM field, STEM education should be revitalized beginning from the high school level, because only 20% of high school graduates major in STEM at the college level. The insufficient number of STEM graduates is a serious problem in Malaysia, the country where this study was conducted (Academy of Science Malaysia, 2018). The academy has indicated that the country needs eight million STEM workers by 2050. The Education Ministry's 2020 annual report reveals that 47.18% of secondary students are in a STEM stream. This percentage is still far from meeting the need for eight million STEM workers by 2050. Chemistry is a core subject for students enrolled in the STEM field. The decline in the percentage of STEM students implies fewer students are learning chemistry in schools in Malaysia.
The COVID 19 pandemic greatly affected students' motivation for learning. Globally, secondary school students in many countries have experienced a high level of stress and anxiety due to the sudden transition to online teaching and learning, and students are continuing to struggle to stay motivated and engaged (Chiu et al., 2021; Pelikan et al., 2021; Chiu, 2022). Secondary school students in Malaysia encountered similar experiences (Tan, 2021). At this time, researchers globally introduced a range of context-based online learning interventions targeting improving engagement, efficacy, and intrinsic motivation to empower students to self-regulate their learning to enhance motivation. Broadly, the attempts to improve motivation centred around undergraduate students. For instance, Kahoot is recommended to be integrated into chemical engineering education (Martín-Sómer et al., 2021); there are reports of an augmented-reality escape activity for the topics of inorganic stereochemistry (Elford et al., 2022) and a virtual laboratory using videos for pharmaceutical students (Díez-Pascual and Jurado-Sánchez, 2022); a combination of asynchronous and synchronous teaching methods has been used to increase student's engagement for organic chemistry (Sunasee, 2020). However, there has been a dearth of studies on improving motivation to learn chemistry among secondary schools, both during the pandemic and at the advent of the post-pandemic period. The World Bank called for a more tailor-made curriculum to encourage knowledge sharing and peer learning to reverse the learning loss that occurred at the time of the pandemic (World Bank, 2022).
Game-based learning (GBL) is an approach that promotes affective, behavioural, cognitive and sociocultural engagement (Plass et al., 2015) while students have fun interacting with the game to achieve the learning objectives (Gupta, 2019). GBL is the most germane approach to be implemented in schools at the time of the post-pandemic period to promote social skills and to address the learning crisis (Li et al., 2022). Nonetheless, a systematic literature review reveals a dearth of studies implementing game-based learning in chemistry education (Hu et al., 2022). Since students lack of learning motivation and specifically motivation for learning chemistry is the fundamental concern at the time of the post-pandemic period, GBL is worthwhile to implement within chemistry education. While addressing the learning loss, the necessity for bringing interdisciplinary perspectives to equip students with knowledge to prepare them for their future careers and solve complex everyday problems should not be neglected. Considering both the concerns of the post-pandemic learning crisis and the need for interdisciplinary perspectives, through this study, we designed and implemented a STEM-integrated board game for lessons on the periodic table (known as STEM-PT Traveler), one of the fundamental chemistry topics that students learn at the early stage when they start learning chemistry for the first time. The study aims to seek answer for the research question: what is the effectiveness of STEM-PT Traveler in improving intrinsic, career, extrinsic, grade, and self-determination motivation? and how does motivation change as a result of using STEM-PT Traveler during lessons on the periodic table?
Background of the study
Motivation for learning chemistry
Motivation is dynamic, evolves consistently and is not directly visible. Perceived value and expectation of success are measured as a motivation in the expectancy-value theory (Wigfield and Eccles, 2000). Attribution theory explains motivation as the personal and environmental factors that drive students to sustain the activity (Weiner, 1985). Motivations to master the content or to perform better than others are the latent constructs of motivation from the perspective of goal-directed theory (Elliot et al., 2011). Deci and Ryan (1985), based on self-determination theory (SDT), iterated that satisfying the three basic psychological needs—autonomy, competence and relatedness—necessarily has a direct effect on motivation.Focussing on social cognitive theory, Bandura (1986) employed a reciprocal interaction model to interpret human functioning. According to Bandura, individuals stay motivated to achieve targeted goals by self-regulating the ever-changing processes that happen at the personal level, which affect the social and physical environment the individual occupies and the person's behaviour. The environment and behaviour, subsequently, influence the personal process that aids in the development of motivation. In a later study based on Bandura's social cognitive theory, Glynn et al. (2011) advanced motivation for learning science as intrinsic motivation, self-efficacy, self-determination, and extrinsic motivation, which is constituted by career and grade motivation (Glynn et al., 2011). The perspective of motivation for learning science introduce by Glynn et al. (2011) has been translated to motivation for learning chemistry in various other studies (Ardura and Pérez-Bitria, 2018; De Souza et al., 2022; Zhang and Zhou, 2023). Motivation for learning chemistry in the above studies perceived the inherent satisfaction experienced by students from learning chemistry as the intrinsic motivation; having control over learning chemistry as self-determination; the belief that they perform well in chemistry as self-efficacy; and learning chemistry to accomplish the goal to attain a better grade (grade motivation) and secure a better job (career motivation) collectively as extrinsic motivation.
The breadth of literature documents shows that many efforts have been undertaken to improve secondary students’ motivation for learning chemistry. The efforts include using a flipped approach with peer-led team learning in organic chemistry (Liu et al., 2018); using visualization to develop motivation for learning chemistry in electrochemistry lessons (Lin and Wu, 2021); student-centred learning in quantum chemistry (Partanen, 2020); and augmented-reality-supported educational escape activities in inorganic and general courses (Elford et al., 2022). The primary distinctions in the above studies are the invariable measures for motivation used in the studies. Lin and Wu (2021) used an instrument designed by Tuan et al. (2005), which measures motivation as self-efficacy, active learning strategies, achievement goals, and learning environment stimulation. Liu et al. (2018) used a more general motivation scale called the academic motivation scale and Elford et al. (2022) used an intrinsic motivation inventory. While overlap between the measures is obvious, the five constructs of motivations dictated by Glynn et al. (2011) are more conclusive, encompassing all the components. For this reason, Glynn’s conceptualization of motivation is widely employed to survey chemistry learning motivation but limited experimental research has opted for using Glynn's measure of motivation.
Game-based learning and motivation
Game-based learning is a thoughtfully designed learning method in which students engage in a game following the instructions, rules and steps set in the game to achieve the learning objectives or outcomes (Plass et al., 2015). The rules, steps and rewards in the game, known as the mechanics of the game, draw students into an artificial conflict in the process of attaining the goals of the game and being the winner (Salen and Zimmerman, 2004). The struggle players experience at the point of conflict urges them to stay motivated, cooperating and competing, adhering to the game mechanics towards the goal. Plass et al. (2015) described the game mechanics as requiring repeated movement in a loop of feedback, challenges, and response (known as a magic circle) for achieving the learning objectives. A GBL design centred on a learning context that generates different types of challenges, which engages students to respond to the challenges with various kinds of feedback (the magic circle), encourages affective, cognitive and social engagements, which essentially determine the quality of learning including improving motivation (Plass et al., 2015).The presence of artificial conflict and the magic circle in studies aimed at enhancing both intrinsic and extrinsic motivation is widely acknowledged in the literature. Collaboration among team members and intergroup competition, as exemplified in a scientific game named SumMagic, has been found to significantly boost students' intrinsic motivation (Chen, 2019). Games that seamlessly integrate competition with collaboration within a continuous feedback loop, involving challenges and responses, serve to foster intrinsic motivation. In a distinct study, the intrinsic motivation of seventh-grade students exhibited improvement through engagement with a game named Carrot Land, which focused on the force and motion curriculum (Chen and Law, 2016). Despite the extensive literature linking GBL and motivation, there is a notable scarcity of studies examining the relationship between GBL and intrinsic motivation in the field of science, particularly in the domain of chemistry (Hu et al., 2022).
GBL is also employed as a strategy to enhance extrinsic motivation. A meta-analysis investigating the effects of GBL has demonstrated a positive impact on students' science achievement, suggesting that students learn significantly more through GBL compared to traditional instruction (Li et al., 2022). Consequently, GBL is widely utilized to improve students' performance in chemistry. An illustrative example is CheMakers, a team-based board game designed to formulate strategies for solving organic chemistry problems and achieving specific objectives. This game encourages meaningful discussions that contribute to a better understanding of organic reaction mechanisms (Zhang et al., 2021). Another instance involves a group of high school students choosing to use games as a tool for learning the periodic table in chemistry. The engagement facilitated by games leads to spontaneous learning (Alvarez-Herrero and Cristina, 2021). Educational escape-room games, adhering to elements such as the game as a system, player involvement, artificial conflict, rules, and quantifiable outcomes, are widely adopted to motivate students towards achieving better grades (Peleg et al., 2019).
The collaborative play of the Crystal Island game among 5th graders, following lessons on landforms and ecosystems, led to a significant improvement in the science learning self-efficacy of the participants when compared to individual gameplay (Meluso et al., 2012). In a study conducted by Lu and Lien (2020), differences in self-efficacy were investigated among students with varying levels of efficacy perceptions—strong, moderate, and weak—related to learning and playing. Despite these initial differences, students exhibited enhanced science learning self-efficacy when engaging in collaborative problem-solving through playing a game called Formosa Hope, focusing on science concepts associated with daily life. Wang and Zheng (2021) employed Lazer and Lazer Maze to elevate the self-efficacy of middle school students in science learning. However, a noticeable gap exists in the literature concerning the relationship between GBL and self-efficacy in chemistry learning.
Self-determination refers to students' assumption of control over their learning, mirroring the demonstrated learning autonomy within the lesson (Black and Deci, 2000). The exploration of self-determination as autonomy is prevalent in science education (Karpudewan and Chong, 2020). However, limited attention has been given to the autonomy of students in chemistry education, especially in endeavours utilizing GBL to support student autonomy, as evidenced by the scarcity of studies on this subject (Hu et al., 2022). A study by Elford et al. (2022) highlights the careful design of escape activities, incorporating features that foster student autonomy, such as limiting difficulty levels and options for exploration. According to Plass et al. (2015), incorporation of these features within GBL—limiting difficulty levels and exploration options—enhances player engagement and adaptability to the game while operating in a continuous loop of the magic cycle: feedback, challenges, and responses. The resulting heightened engagement and adaptation to the game contribute to autonomous learning.
Learning elements of the periodic table (PT) using a board game and interdisciplinary approach
The PT holds a central position in chemistry education across various academic levels. Within this subject, students explore the systematic arrangement of elements, considering their chemical and physical properties. The PT serves as a framework that reveals patterns and trends in the properties of elements across groups and periods, enabling students to make predictions about the properties of other elements. Existing literature highlights the prevalent use of traditional teacher-centred approaches, where students are often required to memorize element properties without gaining a deep understanding of the reasons behind their specific positions in the table (Bierenstiel and Snow, 2019). Traver et al. (2021) underscore the drawbacks of this traditional method, emphasizing its tendency to create a tedious and unengaging learning experience for students. The application of uninspiring approaches at the secondary level has been identified as a significant factor contributing to the numerous learning challenges faced by high school students in comprehending the intricacies of the PT. Despite these challenges, there exists an unexplored landscape in the domain of secondary-level learning about the PT (Girón-Gambero and Franco-Mariscal, 2023).GBL is increasingly capturing the attention of educators as an alternative approach to replace the traditional teacher-centred method in teaching PT (Bernando and Gonzalez, 2021). Among various games, board games have been identified as a suitable learning tool that facilitates effective understanding of chemistry concepts (Li et al., 2022), especially the PT. This is particularly crucial because mastering the PT demands a heightened level of commitment and intellectual engagement (Franco-Mariscal et al., 2016), which resonates with the assertion that GBL played on a board deeply involves students in behavioural, cognitive, and sociocultural engagement (Plass et al., 2015). Through interactive and enjoyable experiences with the game on the board, students actively work towards achieving the specified learning objectives (Gupta, 2019). Consequently, GBL played on physical media, such as cards or boards, has gained popularity in teaching the PT.
Franco-Mariscal et al. (2016), for instance, adopted a socio-constructivist framework to create GBL for PT. The GBL incorporated various games and tasks, such as playing games involving drawing and building models related to daily life, as well as answering questions and creating summaries. The implementation of this educational game yielded multifaceted positive outcomes, including improved knowledge acquisition, enhanced intrinsic and extrinsic motivation, and increased classroom participation. The Elemental Periodica game, fashioned as a Bingo game, was designed by Bayir (2014) to educate students on the placements of elements in the s- and p-blocks, as well as common elements in the d-block of the periodic table. The game aimed to establish connections between an element's name, symbol, atomic number, and common properties relevant to daily life. Secondary students, exposed to the game for two days, found it to be an entertaining means of learning about the PT.
Another game, ChemMend, described as being akin to UNO, was introduced by Martí-Centelles and Rubio-Magnieto (2014) to address the limitations of existing games that focus solely on significant elements and neglect the correlation between names and symbols. In contrast, ChemMend engages students in mentally reviewing the periods and groups of elements while matching cards drawn from the pile with reference cards of the same period or group. Failure to make a match results in missing a turn. Participating students perceived the game as entertaining, aiding in the consolidation of knowledge about the periodic table, and providing practical experience in placing elements accurately. Post-game, the average quiz scores improved compared to pre-game performance.
In the diverse array of games highlighted above, the predominant objective is to actively engage students in play, aligning with the learning objectives embedded within the games to foster affective, behavioural and cognitive engagement. Consequently, the games not only provided an entertaining experience but also facilitated knowledge construction pertaining to the arrangement of elements in periods and groups, comprehension of chemical and physical properties, and the ability to predict the properties of various elements. However, the affective outcome frequently is not well understood.
Franco-Mariscal et al. (2016) specifically underscored the pivotal role of motivation derived from the use of educational games, contributing to a heightened level of engagement. In the same study, the authors succinctly asserted that the board game employed for teaching the periodic table improved both extrinsic and intrinsic motivation. However, apart from this concise information, no further details regarding how board games designed for the specific purpose of teaching the PT influence motivation in chemistry learning can be found in the literature.
The imperative to educate students with an integrated understanding of Science, Technology, Engineering, and Mathematics (STEM) has become increasingly crucial. This preparation is deemed essential for the future job market, enabling students to tackle complex challenges such as shifts in weather patterns and food scarcity. The demands of 21st-century jobs emphasize the necessity for students to possess interdisciplinary knowledge rather than specializing in a single subject (English and King, 2018). Integrating the disciplines of Science, Technology, Engineering, and Mathematics in the teaching of scientific concepts marks a shift from the traditional, discipline-specific approach to a more interdisciplinary perspective (Moore et al., 2016). In response to this educational shift, our previous study embraced an interdisciplinary perspective during lessons on electrolysis (Huri and Karpudewan, 2019; Karpudewan and Huri, 2023). The careful design of five electrolysis lessons aimed at facilitating the crossing of STEM disciplines. These efforts align with the fundamental characteristic of Integrated STEM, which emphasizes an increasing level of boundary crossing between disciplines for greater interconnection among them (English, 2016).
Despite the elements of the periodic table bearing high relevance to real-world issues, the traditional teacher-centred lecture-style approach tends to impede the comprehension of these elements concerning their practical applications. Bierenstiel and Snow (2019) have proposed a model known as the ‘periodic-universe model’ as an attempt to introduce an interdisciplinary perspective to lessons on PT. According to the authors, transdisciplinary aspects are integral to this model, necessitating an understanding of the elements of the periodic table in relation to the cultural and historical context of the learners. This model significantly enhances the relevance to the learning context. In a similar vein, with a decline in STEM graduates, it becomes imperative to transform chemistry education to highlight the direct applicability of chemistry to industrial manufacturing processes. Specifically, this transformation could be achieved through lessons on the PT of elements, given the extensive use of these elements in various industries and engineering applications. One potential approach to actualizing this transformation is by facilitating interdisciplinary connections within STEM disciplines while teaching the PT.
Methods
Research design
Following Creswell and Plano's (2011) recommendation of mixed-method research as a robust approach that mitigates limitations associated with relying solely on quantitative or qualitative methods, the study utilized an explanatory mixed-method design to answer the research questions. In the instructional setting, the GBL group received guidance through the utilization of a board game known as STEM-PT Traveler, whereas the non-game-based group (non-GBL) was instructed using an alternative student-centred approach. To assess chemistry learning motivation, the Chemistry Motivation Questionnaire II (CMQ II), comprising 25 items, was administered as a pre- and post-test for both groups, one week before and one week after the treatment. Additionally, post-treatment interviews were conducted to delve into students' motivation subsequent to the instructional interventions.Samples
In the context of Malaysia, where this study took place, students are introduced to chemistry concepts starting from the primary level and progress through secondary and tertiary education. In primary school (ages 7 to 12) and lower secondary school (ages 13 to 15), chemistry concepts are typically integrated into the general science curriculum. As students advance to upper secondary school (ages 16 to 17), they have the option to specialize in the science stream, where chemistry is taught as a distinct subject, or pursue the humanities stream, where chemistry concepts remain embedded within the broader science curriculum.The sample for this study consisted of Form Four science stream students, equivalent to grade 11, who were 16 years old and attending a secondary school. Typically, students in the science stream continue their education at the tertiary level in STEM fields, where chemistry is a mandatory subject. The study was conducted at this particular school for convenience; it is conveniently situated, and the researchers obtained necessary permissions from the District Education Office, the school principal, and the chemistry teacher who facilitated the study. Malaysia operates under a centralized education system, overseen by the Ministry of Education, which ensures uniformity in the curriculum across schools nationwide. Teachers are provided with the Document Standard Chemistry Curriculum and Evaluation (MOE, 2018) to guide their teaching practices. Given this centralized structure, convenience sampling was justified for this research (Creswell and Plano, 2011).
The research design employed a quasi-experimental approach for the quantitative segment, with two classes randomly designated as the GBL and non-GBL groups. The GBL group comprised 46 students, while the non-GBL group consisted of 45 students. Gender distribution across the samples was nearly balanced, with 21 girls and 25 boys in the GBL group, and 22 girls and 23 boys in the non-GBL group. The results of the chemistry test, administered to students from both groups, indicated that the majority of students scored within the 45–55% range, suggesting a moderate level of proficiency.
The sample size used in this study aligns with recommendations stated in the literature. According to Stevens (1980), factors such as statistical power, significance, and effect size influence sample size determination. Cohen (1988) notes that small and medium effect sizes are common in social science research; hence, a medium effect size guides this study. For multivariate analysis involving two groups and five dependent variables, at a significance level of 0.05, achieving a statistical power of 0.8—a common power level in social science analysis (Hair et al., 2010)—requires a sample size ranging between 45 to 50 per group (Lauter, 1978). The sample sizes of 45 for the non-GBL group and 46 for the GBL group adhere to the recommendations for performing multivariate analysis as cited in the literature.
To ensure sample representativeness, 15 students were purposively selected from each group based on their performance in previous chemistry tests for the post-treatment interview. The interviewees consisted of 5 high performers with an achievement of more than 80% in previous chemistry tests, 5 mediocre with scores between 50 and 79%, and low achievers with grades below 50%. This selection process facilitated the inclusion of students with varying academic abilities in the study.
To mitigate potential bias, the same chemistry teacher, holding a Bachelor of Science Education degree with a major in chemistry from a local university and possessing over 10 years of teaching experience, conducted the PT lessons for both the GBL and non-GBL groups. To control for potential teacher effects, the teacher underwent specialized training to deliver the PT lessons and was provided with detailed lesson plans to guide the execution of the treatment.
Instruments
Chemistry motivation questionnaire II
The current study employed the CMQII, derived from the Science Motivation Questionnaire (SMQ11) developed by Glynn et al. (2011). Grounded in social cognitive theory, Glynn et al. (2011) conceptualized science learning motivation as a multidimensional construct, encompassing intrinsic motivation, career motivation, grade motivation, self-determination motivation, and self-efficacy. Subsequent investigations adapted the SMQ11 by substituting the term “science” with “chemistry” to assess chemistry learning motivation (Salta and Koulougliotis, 2015; Ardura and Pérez-Bitria, 2018; De Souza et al., 2022). This modified questionnaire, referred to as the CMQII, has been translated and applied in diverse contexts, including in Brazil, Indonesia, Germany, Greece, and China. Studies specific to each context (Salta and Koulougliotis, 2015; Ardura and Pérez-Bitria, 2018; De Souza et al., 2022) have indicated that the CMQII items exhibit high levels of validity and reliability.The CMQII used in this study comprised 25 items, with five items dedicated to each of the five constructs. Respondents evaluated the frequency of occurrence of the statements on a five-point Likert scale. The scale is as follows: 1 (never) implies that students do not experience the situation indicated in the statement; 2 (rarely); 3 (sometimes); 4 (usually); and 5 (always). Table 1 below presents descriptions of each construct of motivation and examples of items.
| Constructs | Descriptions | Example of items |
|---|---|---|
| Intrinsic motivation | The degree to which students perceived chemistry learning as interesting, challenging, and enjoyable | Learning chemistry is interesting in school |
| Self-determination | The extent to which students felt that they have control of their chemistry learning | I study hard to learn chemistry |
| Self-efficacy | Measures students’ confidence in their ability to accomplish chemistry tasks and excel in the subject. | I am confident I will do well on chemistry tests |
| Grade | Measures the motivation level to excel in chemistry for obtaining higher grades | It is important that I get an ‘‘A” in chemistry |
| Career | Measures motivation level to pursue careers related to chemistry | Learning chemistry will help me get a good job |
All items in the CMQII underwent initial translation into Malay Language, a process carried out independently by both authors. One author, possessing a decade of experience as a chemistry teacher in secondary schools, collaborated with an experienced chemistry educator with 12 years of expertise in training chemistry teachers at a local university. Subsequently, an English teacher with two decades of English teaching experience performed the back-translation of the translated items into English. This iterative process aimed to unveil any potential disparities in meaning between the original and translated versions. Upon completion of the back-translation, the authors meticulously reviewed the results, implementing necessary corrections and modifications to the Malay version of the questionnaire to ensure the preservation of content and meaning in the items.
Interview questions
The formulation of interview questions was guided by the items in the CMQII, with questions designed to assess students' motivation across the five subscales indicated in the CMQII. Questions such as ‘Why have you chosen to study chemistry?’, ‘Do you believe that learning chemistry will contribute to your career preparedness?’, ‘What approaches do you employ in learning chemistry, and how much time and effort do you invest in the learning process?’, ‘Do you perceive a sense of control in your learning of chemistry?’, and ‘Do you consider achieving high grades in chemistry as significant?’ were utilized to gauge students' intrinsic motivation, career motivation, self-efficacy, self-determination, and grade motivation, respectively. During the interviews, the initial responses from students were further probed by the interviewer through additional questions until the intended responses were obtained. This iterative process allowed for a comprehensive exploration of students' motivations in alignment with the constructs identified by the CMQII.Teaching of the periodic table
Both the GBL-STEM-PT Traveler and non-GBL approaches were meticulously designed and implemented, closely aligning with the nine learning standards outlined for the topic of the PT in the Document Standard Chemistry Curriculum and Evaluation (MOE, 2018). The nine standards aim to equip students with the ability to:1. Predict the position of given elements within the appropriate group and period on the periodic table.
2. Discover the patterns of changes in the chemical and physical properties of elements in group 18 as they progress down the group.
3. Discover the patterns of changes in the chemical and physical properties of elements in group 1 as they move down the group.
4. Write chemical equations for the reactions between metals and water, oxygen, and chlorine.
5. Discover the patterns of changes in the chemical and physical properties of elements in group 17 as they move down the group.
6. Write chemical equations for the reactions between halogens and water, metals, and bases.
7. Understand the trend of physical properties of elements across period 3, considering aspects such as atom size and electronegativity.
8. Identify transition elements, highlighting their unique properties, including their role as catalysts, possession of multiple oxidation states, and ability to form complex compounds and ions.
9. Acquire knowledge about the industrial and real-life applications of these elements.
Game-based approach – STEM-PT traveler
STEM-PT Traveler is a board game that incorporates principles from the snakes and ladders board game commonly played by adolescents. STEM-PT Traveler is played in two phases, each involving a group of four students. Phase one of the game focuses on content related to PT elements, specifically those in groups 1, 17 and 18, and the transition elements, and the variations in the physical and chemical properties of elements as they move across periods and groups. Fig. 1 provides a visual representation of the game board in phase 1.In addition to the board, the game includes components such as a dice, tokens, reward cards (representing protons, neutrons, and electrons), and STEM career cards. Various career options for chemists, accompanied by corresponding salary draws, are also incorporated. The allocation of rewards—protons, electrons and neutrons, and the quantity of rewards—is contingent upon the difficulty level of the questions posed.
Gameplay unfolds in groups of four, with each student assigned a token of a unique colour. All participants roll the dice to determine the initial player, with the student achieving the highest dice roll initiating the game by rolling the dice once more. Following this, the token is moved to a square corresponding to the dice value. Progression in the game requires players to respond to question cards, which are revealed by flipping them over. Successful completion of the questions allows students to earn protons, neutrons, or electrons, with the specific reward indicated on each question card. These rewards can be exchanged for additional benefits, such as using accumulated protons to acquire career cards. Career cards outline the salary and associated perks. Electrons serve to avoid penalties, such as losing turns due to incorrect answers, while neutrons grant students a second opportunity to respond to a question, allowing them to refine their answers. Neutrons can also be accumulated and traded for career cards. During Phase 1, students engage in the game with the objective of answering as many questions correctly as possible and accumulating rewards. These accumulated rewards were subsequently exchanged for career cards. A total of 16 career cards are available, each offering distinct high salary prospects. The player who accumulates the greatest number of salary cards and achieves the highest salary amount is declared the winner of the game. The accumulated rewards were used in the second phase of the game.
The depiction in Fig. 2 illustrates the second phase of the board game. The game board represents the standard PT, featuring images of products that incorporate specific elements. For the second phase of the board game, researchers employed the illustration of the PT using pictures as proposed by Enevoldsen (2016). Players determine their assigned group and period through a drawing of lots. For example, the number obtained from the initial dice roll designates the group, while the number from the subsequent roll designates the period. The token is then placed on the corresponding element based on the determined group and period. Another round of lot-drawing ensues, where participants receive a number accompanied by either a negative or positive sign (e.g., −3 or +3). A negative sign necessitates moving the token in the direction of reducing the size of the element by three steps, while a positive sign indicates an increase in size by three steps. Following the placement of the token on the appropriate square, students are obligated to purchase the elements using the rewards accumulated during Phase 1. These steps are iterated five times to acquire a sufficient number of elements for use in the subsequent STEM activity, which is conducted as an after-school program.
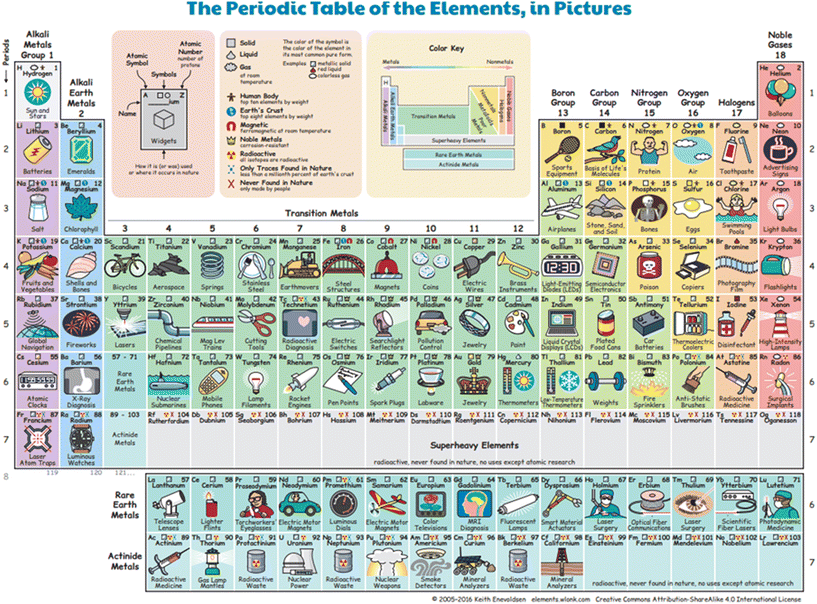 | ||
| Fig. 2 Phase 2 of STEM-PT Traveler obtained from Enevoldsen (2016). | ||
The STEM activity necessitates students to collaborate in groups, investigating the chemical and physical properties of the elements they have gathered, along with products manufactured using these elements. For example, one group gathered elements such as iron, zinc, hydrogen, sulphur, carbon, and oxygen. They subsequently conducted research on how these elements were utilized in the production of various types of batteries. The acquired information was then presented to the entire class, followed by a question-and-answer session. This activity transcends the boundaries of the four STEM disciplines: the exploration of chemical and physical properties addresses the science dimension, engaging in the engineering design process to investigate battery manufacturing represents the engineering dimension, understanding the appropriate elemental ratios in products aligns with the mathematics dimension, and deciding on the combination of elements for proposing a battery prototype embodies the technology dimension.
Non-GBL approach
The comparison group students were subjected to a non-game-based, student-centred instructional approach, wherein they were organized into small groups of 4 to 5 individuals. A total of nine stations were meticulously arranged, with materials pertinent to learning standard 1 allocated to station 1. Subsequently, the remaining stations corresponded to the subsequent learning standards, and the associated materials, including textbooks, reference books, and printed reading materials, were strategically placed at each station.Before the lesson commenced, students were tasked with drafting summaries for the assignments assigned to them, aligning with the learning standards of each station. These summaries were designed to highlight the practical application of the elements in real-life situations. The drafting process occurred outside of regular school hours, with students provided with textbooks, reference materials, and other relevant resources to support their efforts. During the actual lesson, students gathered in groups at each station to discuss and delve deeper into the summaries. Following these group discussions, the summaries were presented to the entire class. Upon completing their activities at one station, students transitioned to the next one.
For example, Group 1 delved into learning standard 1 at station 1, engaging in activities such as reading materials, sharing and discussing their comprehension of the arrangement of elements in the PT in groups and periods. Students identified relationships between atomic number, atomic mass, and neutrons for each element in the PT, along with understanding the proton and electron numbers comprising an element. A summary of the acquired knowledge, in the form of a poster, was prepared and presented. Following the 15-minute interval at station 1, Group 1 progressed to station 2, while Group 2 moved from station 2 to station 3, and so forth. Similarly, at station 2, Group 1 investigated the provided materials to address learning standard 2. After 15 minutes, Group 1 shifted to station 2, and this rotation continued. Groups cycled through the stations until they completed activities at all nine stations. During their time at station 1, Group 1 students delved into the chemical and physical properties of group 18 elements, identifying notable trends in properties as they progressed down group 18. This process was replicated systematically across all nine stations until comprehensive coverage was achieved. Fig. 3 illustrates the summary prepared for the presentation.
Ethical considerations
Before the commencement of the study, students were duly informed about its nature, and their voluntary participation was emphasized. They were explicitly informed of their right to withdraw from the study at any point during its progress. Furthermore, students were apprised that the study's findings would be published, and their consent was sought if they had concerns about the researchers disseminating the results. In order to conduct the study, the researchers obtained formal approval from both the Ministry of Education and the school principal. The participation of the teacher was entirely voluntary, and the teacher briefed on the study's processes and procedures before agreeing to take part.Pilot study
A pilot study involved 40 Form Four students, who were not participants in the main study, along with three chemistry teachers and two chemistry education researchers. Both of the two teachers are expert teachers with more than 15 years of experience teaching chemistry in schools and have doctorates in chemistry education. These teachers have experience of engaging in developing the chemistry curriculum, textbooks and providing professional development courses for chemistry teaching for junior teachers. The chemistry education researchers are professor and associate professors of chemistry education from a local university engaged in training chemistry pre-service teachers and actively doing motivation-related chemistry education research. The pilot study was conducted to assess the validity and reliability of the instruments and the treatments. The students required 25 minutes to complete all the items in the CMQII. Pilot study data were examined to identify evidence for test content and response processes, supporting the validity of the CMQII used in this study (Arjoon et al., 2013; Lewis, 2022). Additionally, a literature review of past studies employing the CMQII was conducted to find evidence supporting its internal structure and the relationship between motivation and other variables (Arjoon et al., 2013; Lewis, 2022). Reliability was assessed by measuring the internal consistency of the items for each construct emerging from the data.In terms of test content, chemistry education researchers with significant expertise in motivation for chemistry learning reviewed the translated items alongside the original ones to examine whether the five subscales of motivation were adequately represented in the translated version of the CMQII. They concluded that “the items echo the exact meaning of the original items, and the number and content of items appropriately measure the intended constructs.” They also stated that “the wording used in the statements mirrors the constructs of motivation in the original version.” For example, the panel explicitly noted that in the translated version, the correct Malay words were used for the construct of intrinsic motivation to ensure the meaning was retained. They further stated, “having five items for intrinsic motivation sufficiently measures the construct.” Similar views were expressed by the panel for other constructs of motivation.
To assess response processes, interviews were conducted with students who participated in the pilot study. For instance, when students were asked to explain their understanding of the item “I enjoy learning chemistry” on a scale ranging from 1 (never) to 5 (always), with the options of 1 (never), 2 (rarely), 3 (sometimes), 4 (usually), and 5 (always), their responses indicated that they could discriminate between the five levels of responses. “Rarely” was interpreted as “hardly,” “sometimes” as “once in a while,” “often” as “enjoying it more times,” “usually” as “only when the lessons were less enjoyable,” and “always” as “never not enjoying chemistry lessons.” Similar responses were observed for other items, providing evidence for response processes.
Given the small sample size in the current study, evidence supporting the structural validity of the CMQII is provided by reviewing studies that employed the CMQII in similar contexts. The original version of the CMQII consisted of 25 items grouped into five constructs, with five items constituting each construct (Glynn et al., 2011). The constructs and items suggested by Glynn have been directly used in studies measuring secondary school students’ motivation. The CMQII, when administered to Brazilian (De Souza et al., 2022), Greek (Salta and Koulougliotis, 2015), and Chinese (Zhang and Zhou, 2023) secondary students, resulted in a five-component factor structure consistent with the original version. These past studies provide evidence supporting the internal structure validity of the five-component structure of motivation and the items associated with the constructs used to measure the motivation of secondary school students.
Evidence supporting the relationship between the construct being measured and other variables indicates that the instrument is functioning as intended (Lewis, 2022). Studies using the CMQII to measure motivation and its relationship with other constructs demonstrate that the CMQII functions as intended. The literature documents that the CMQII has been used to measure motivation and its relationship with understanding (Karpudewan and Chong, 2020). In another study, the CMQII was employed to measure motivation and its relation to causal attributions for choosing or abandoning chemistry (Ardura et al., 2021). Additionally, the CMQII was used in a study to measure the effect of motivation on choosing chemistry (Ardura and Pérez-Bitria, 2018).
Cronbach's alpha values for all five subscales of CMQII, ranging from 0.76 to 0.85, indicate that the items for each subscale exhibit acceptable internal consistency. Specifically, the alpha for the intrinsic motivation subscale is 0.76, for self-determination is 0.79, for self-efficacy is 0.81, for career motivation is 0.88, and for grade motivation is 0.85. These values suggest satisfactory internal consistency for each subscale.
Regarding interview questions, the chemistry teachers recommended focusing on the context of instruction rather than asking general questions. Students and teachers were exposed to both GBL and non-GBL approaches. According to student feedback, the rules of the game were easy to follow, and the underlying mechanisms were not overly complex. The instructions for the game were deemed clear and straightforward. However, non-GBL students recommended more structured instructions at each station, expressing uncertainty about what to do with the materials. Similarly, chemistry teachers suggested providing more structured and clear instructions to guide students in playing the game.
Data analysis
Motivation, being inherently multidimensional, led to the implementation of a one-way multivariate analysis of covariance (MANCOVA) for the quantitative analysis. The MANCOVA analysis commenced with an assessment of whether the data met the necessary assumptions, followed by a descriptive analysis before delving into the multivariate analysis. Quantitative MANCOVA analyses were conducted using SPSS software version 22.For the analysis of interview data, Braun and Clark's (2006) thematic analysis framework was employed. The analysis was conducted manually by the researchers in collaboration with the panels involved in the pilot study, which consisted of two chemistry education researchers and three chemistry expert teachers. Initially, the interview transcripts were read multiple times to enable the experts to familiarize themselves with the data. The interrater reliability value of 0.85 reflects the strong agreement between the outcomes of the analysis from the panels. Subsequently, initial subthemes were identified, and excerpts for each motivation subscale were categorized accordingly. For example, responses to the question ‘Why do you choose to learn chemistry?’ such as ‘Chemistry is a compulsory subject, so I don't have any option’ from the non-GBL group and ‘I enjoyed playing the game and discovering applications of elements’ from the GBL group were categorized into sub-themes ‘compulsory’ and ‘enjoy’. Next, the subthemes were merged to derive the emerging theme. For the non-GBL group, subthemes were merged to form the theme ‘students learning chemistry because of the requirement’. Conversely, the nature of chemistry, engaging them in discovery and leading them to enjoy the lessons for their intrinsic values, forms the theme ‘students were intrinsically motivated’ for the GBL group.
Results
Quantitative CMQII findings
Table 2 presents the descriptive mean and standard deviation (SD) values for each motivation construct in both the GBL and non-GBL groups. The higher mean scores observed for overall motivation and all constructs post-instruction suggest an enhancement in students' motivation for both groups. The disparities in gain scores between pre- and post-test mean scores indicate that the GBL group experienced greater gains compared to the non-GBL group. Specifically, in intrinsic motivation, the GBL group exhibited a gain of 1.56, surpassing the non-GBL group's gain of 0.05. Similarly, for self-efficacy, the GBL group showed a gain of 1.40, while the non-GBL group had a gain of 0.17. Continuing this trend, the GBL group's gain in career motivation was 1.64, whereas the non-GBL group only increased by 0.22. Grade motivation witnessed an improvement of 0.37 in the GBL group, exceeding the non-GBL group's gain of 0.02. Furthermore, for self-determination, the GBL group increased by 0.70, compared to the non-GBL group's increase of 0.15. Overall, these findings underscore the positive impact of the instructional strategies on motivation, with the GBL group consistently outperforming the non-GBL group across all constructs.| Pre test | Post test | |||||||
|---|---|---|---|---|---|---|---|---|
| Non-GBL | GBL | Non-GBL | GBL | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Overall motivation | 3.70 | 0.62 | 3.35 | 0.61 | 3.81 | 0.54 | 4.89 | 0.40 |
| Intrinsic motivation | 3.58 | 0.75 | 3.17 | 0.72 | 3.63 | 0.73 | 4.73 | 0.54 |
| Self-efficacy | 3.42 | 0.82 | 3.31 | 0.82 | 3.59 | 0.74 | 4.71 | 0.65 |
| Career motivation | 3.64 | 0.99 | 3.06 | 0.90 | 3.88 | 1.02 | 4.70 | 0.63 |
| Grade motivation | 4.08 | 0.77 | 3.75 | 0.83 | 4.10 | 0.56 | 4.12 | 0.48 |
| Self determination | 3.76 | 0.73 | 3.44 | 0.67 | 3.91 | 0.61 | 4.14 | 0.45 |
To determine the significance of differences between the comparison and experimental groups, Multivariate Analysis of Covariance (MANCOVA) was employed. Motivation, being a multidimensional variable, consists of various non-observable latent variables that do not naturally exist distinctly. In such a scenario, Tabachnick and Fidell (2007) propose that a multivariate analysis is more suitable than treating each subscale as a univariate dimension to prevent Type One errors. Furthermore, to account for pre-test influences, the authors recommend incorporating pre-test scores as covariates. Therefore, MANCOVA is considered the appropriate statistical approach in this study.
Before conducting MANCOVA, an examination was undertaken to assess the equality of variance–covariance matrices in the data. Box's M value of 30.09, with a p-value of 0.20 (p > 0.05), suggests that the assumption of equal variance–covariance matrices among the groups is valid. Levene's test findings’ homogeneity of the variance was not violated for all the five subscales at a significance level of p > 0.05. The results of Levene's test are displayed in Table 3 below.
| F | Df1 | Df2 | p-Value | |
|---|---|---|---|---|
| *p > 0.05. | ||||
| Intrinsic motivation | 0.003 | 1 | 83 | 0.291* |
| Career motivation | 0.034 | 1 | 83 | 0.422* |
| Self efficacy | 0.363 | 1 | 83 | 0.536* |
| Grade motivation | 1.640 | 1 | 83 | 0.769* |
| Self determination | 2.984 | 1 | 83 | 0.141* |
The results of MANCOVA indicate significant mean differences in the post-test scores, encompassing all five constructs of motivation, between the groups when controlling for pre-test scores at the level p < 0.05: Wilk's lambda = 0.847; F(5,75) = 2.67; p < 0.05. The partial eta squared value of 0.15 implies that the treatment accounts for 15% of the differences in post-test scores between the groups. Following the significant findings obtained from the multivariate test, the univariate main effects were examined by a follow-up ANOVA. The results of the univariate tests are presented in Table 4 below.
| Sum of squares | Df | Mean | F | p-Value | Partial eta squared | |
|---|---|---|---|---|---|---|
| *p < 0.01. | ||||||
| Intrinsic motivation | 0.778 | 1 | 0.778 | 4.457 | 0.000* | 0.054 |
| Self efficacy | 1.030 | 1 | 1.030 | 4.532 | 0.000* | 0.055 |
| Career motivation | 2.927 | 1 | 2.927 | 11.061 | 0.000* | 0.124 |
| Grade motivation | 0.007 | 1 | 0.007 | 0.056 | 0.814 | 0.001 |
| Self determination | 0.256 | 1 | 0.256 | 1.259 | 0.265 | 0.016 |
To mitigate Type 1 errors, Bonferroni adjustments were implemented, setting the adjusted alpha (αadj) at αexp/5 levels. Consequently, the original alpha level of 0.05 was divided by the number of dependent variables (five), resulting in a new adjusted alpha of 0.01. The univariate analysis table above outlines the findings at the significance level of p < 0.01. Significant disparities emerged in post-tests for intrinsic motivation, self-efficacy, and career motivation. Notably, the post-tests for the intrinsic motivation sub-scale in non-GBL (Mnon-GBL = 3.63, SD = 0.73) and GBL groups (MGBL = 3.73, SD = 0.54) demonstrated significance (F(1, 74) = 4.46; p < 0.01). A partial eta squared of 0.05 indicates that the treatment causes 5% of the variances. Similarly, significant differences were observed between post-tests for the non-GBL (Mnon-GBL = 3.593, SD = 0.74) and GBL groups (MGBL = 3.71, SD = 0.65) (F(1.74) = 4.53; p < 0.01). Career motivation also displayed significant distinctions (F(1.74) = 11.06, p < 0.01) between post-test mean scores for the non-GBL (Mnon-GBL = 3.68, SD = 0.74) and GBL (Mnon-GBL = 3.71, SD = 0.65) groups. A partial eta squared of 0.124 indicates that the treatment causes 12.4% of the variances. However, the remaining two subscales, grade motivation and self-determination, exhibited non-significant differences between the post-test scores of the groups. For grade motivation, the non-GBL (Mnon-GBL = 4.10, SD = 0.56) and GBL (MGBL = 4.32, SD = 0.45) groups were not significantly different (F(1,74) = 0.056; p > 0.01). Similarly, the post-test means for self-determination in the non-GBL group (Mnon-GBL = 3.91, SD = 0.61) and GBL group (MGBL = 4.14, SD = 0.45) were also not significant (F(1,74) = 1.259; p > 0.01).
Qualitative interview findings
The qualitative interview findings are presented in accordance with the subscales of motivation outlined in the CMQII. The question “Why do you choose to learn chemistry?” was utilized to assess intrinsic motivation. Responses from the non-GBL group, such as “because since I opted for the science stream, chemistry is a compulsory subject,” “it is one of the science disciplines that is compulsory to be taken,” and “chemistry is a difficult subject, but because it is a compulsory subject, I have no option. I have to take it,” were obtained. From these responses, subthemes emerged, namely “compulsory” and “no option,” which were subsequently merged to form the overarching theme that chemistry is learned as one of the subjects to meet the criteria. Conversely, responses from the GBL group yielded subthemes such as “relevance to real life,” “interesting to learn,” “enjoy learning,” and “discovery.” Participants in this group expressed that “The board game made me realize that chemistry naturally encompasses everything surrounding us.” and “The board game not only made learning attractive, but also interesting, as we got to know about the functions of elements across periods and groups in real life.” Another response highlighted that “The board game allows us to collaboratively play and learn at the same time. For example, in the second phase, I enjoyed discovering about the application of elements in manufacturing products.” The subthemes derived from the GBL group point to the overarching theme that students learn chemistry for its inherent value. Engaging in a task for its intrinsic value aligns with intrinsic motivation. Throughout their responses, GBL group students consistently referenced the board game, indicating that the game has significantly contributed to students comprehending the inherent importance of chemistry.The responses to the second question, “Do you think learning chemistry will help you to prepare yourself for a career?” reflect students' career motivation. Students from both groups concurred with the assertion that studying chemistry prepares them for careers related to the field. Non-GBL students expressed sentiments such as, “I think the reason for us to learn about physical and chemical properties is mainly because this is the kind of knowledge relevant to the industry. Otherwise, I don’t see the reason why we are learning heavy science facts?” and “We learn about the reaction between sodium metal and oxygen and reactions of other metals with oxygen… basically, this kind of knowledge is important for industrial applications.” From these responses, a subtheme highlighting the relevance of chemistry knowledge to industrial applications emerged. This subtheme elucidates the broader theme of utilizing chemistry knowledge in industry. However, whether this knowledge prepares them for future careers was not explicitly stated. In contrast, GBL students made explicit references to the contribution of chemistry to their career advancement, as illustrated in the excerpts from three students below:
“In phase 2, we explored the industrial application of how elements were used to manufacture batteries. This section of the activity learning chemistry is an advantage for me to secure a job in the industry later.”
“We compete among friends, trying to get more protons (as rewards). We solve interesting problems. I will work towards improving problem-solving, which I think is important to secure a good job later.”
“Changing the reward points to a career card is interesting. It gives a picture that jobs related to chemistry and the salary offered.”
From the above excerpts, subthemes such as the relevance of knowledge to industry, career advantages, problem-solving, and the role of learning chemistry in securing better jobs have emerged. Notably, the problem-solving skills and the discovery of new knowledge during the game directly align with job requirements. Specifically, the career card component raised awareness of chemistry-related jobs and potential salaries. Collectively, these subthemes contribute to the overarching theme of career motivation.
Self-efficacy was assessed using the question, ‘Do you think you can do well in the chemistry exam?’ Significant differences were observed between the responses of the non-GBL and GBL groups. Non-GBL group students primarily relied on the ability to memorize and recall chemistry facts, expressing uncertainty and doubt regarding their performance. The subthemes ability to memorize, recalling chemistry facts, expressing uncertainty and doubt regarding their performance emerged from the following excerpts: ‘Not sure. Chemistry is difficult. I spend time memorizing. But I don’t really get good grades,’ and ‘Chemistry involves memorizing facts. And I don’t like memorizing facts. Most of the time I gave up,’ which contribute to the overarching theme that students are less confident in their ability to perform well.
On the other hand, GBL students indicated the following:
“I am quite confident this time. With the board game, I still need to memorize but playing the games many rounds and repeatedly finding answers to the questions on the cards, I felt more confident in learning chemistry.”
“Joining friends, finding solutions for the problem, getting rewards for our effort—it is interesting. I enjoy learning this way. For this reason, I believe I can do well in the exam.”
The above responses from the GBL group suggest that the approach, which involves learning while simultaneously having fun playing, represents a different learning strategy that makes the learning process interesting and joyful, and instils a sense of confidence. The subthemes having fun, a learning strategy that makes the learning process interesting, joyful, and confidence, noticed in the GBL group's students' responses, collectively explain the theme of self-efficacy.
Self-determination pertains to the control students have over their learning. Both groups of students indicated that they dedicate a substantial amount of time to studying chemistry. Responses such as ‘Compared to other subjects, I spend more time on chemistry. Because it involves many concepts, I usually do a lot of exercises to consistently recall facts’ from non-GBL students suggest that they may be less self-determined or autonomous. Upon further inquiry about whether they structure their own learning, they mentioned, ‘After listening to the teacher in school, I will do some exercises, read the textbook, and memorize the facts before the examination.’ These responses imply that students heavily depend on teachers and lessons delivered in class. The subthemes less autonomous, and heavily dependent on the external sources (textbooks and classroom teaching), explain the theme less self-regulated.
Similar sentiments were echoed by the GBL group students. The only distinction lies in their consistent reference to the board game. For instance, one student mentioned, ‘I usually spend a lot of time memorizing facts from the textbook. With the board game, I found another approach. It's not boring. We are motivated to repeatedly play the game.’ This response suggests that students may be less autonomous and lack the freedom to regulate their learning. The board game is used as an alternative to textbooks and classroom teaching, but they still rely on an external tool. Another student's response, ‘besides memorizing, the board game is another alternative in which we can work in groups to learn chemistry,’ further reinforces the assertion they were dependent on the board for learning. Similar to the non-GBL group, from the GBL group's students’ responses, the subthemes less autonomous and dependence on an external source (boardgame in addition to textbooks and classroom) emerged, explaining the theme of reduced self-regulation.
For the question of whether obtaining a good grade in chemistry is important, both groups provided almost similar answers. Regardless of whether they were from the GBL or non-GBL group, students emphasized the significance of obtaining a good grade in chemistry. The difference between the groups lies in their perceptions of chemistry and the strategies employed. From the responses of non-GBL students, subthemes such as viewing chemistry as difficult, relying on memorization of facts, and striving for good grades emerge to explain the theme of grade motivation. This is evident in the excerpt from one student: ‘I need to get an A in chemistry so that I can continue my studies at tertiary in science-related courses. A good grade is a prerequisite for this. Therefore, I spend a lot of time memorizing chemistry facts.’ In contrast, the GBL group enjoyed the lesson and appeared confident in indicating the importance of obtaining a good grade in chemistry, as reflected in the following excerpts: ‘The career card in the board game made me realize the importance of getting a good grade in chemistry. The card also indicated that good grades in chemistry would result in high-paying jobs.’ The subthemes of enjoyable lessons, confidence, and pursuit of good grades elucidate the theme of grade motivation for the GBL group.
Discussion
This study was conducted during the reinstatement of normal schooling, following an extended lockdown prompted by the global pandemic. A prevailing issue affecting students returning to face-to-face learning post-pandemic is a notable lack of motivation (Chiu et al., 2021; Pelikan et al., 2021; Chiu, 2022). To address this concern, numerous concerted efforts have been made globally to reintroduce students to the learning environment. These initiatives involve the implementation of curricula designed to foster engagement, empowering students to take ownership of their learning and enhance their sense of belonging. Despite these efforts, there has been limited focus on encouraging chemistry learning among students, particularly at the secondary school level (Hu et al., 2022). Consequently, the current study aims to bridge this gap by introducing a game-based learning approach. This approach is considered suitable for facilitating a shift from the predominant emphasis on individual learning during the pandemic to a more collaborative team-oriented learning model (Li et al., 2022). The choice of a game-based learning approach is justified by its potential to address challenges associated with the recovery period. Students, having experienced a prolonged break from traditional learning, may encounter difficulties in grasping abstract concepts, such as those in chemistry, using conventional cognitively demanding methods. Hence, the game-based learning strategy is perceived as relevant and effective in aiding students during this transitional phase.The literature on educational interventions consistently reports heightened student motivation following implementation (Liu et al., 2018; Partanen, 2020; Lin and Wu, 2021; Elford et al., 2022). This study aligns with these findings, revealing a notable disparity in mean scores across all five dimensions between students using the STEM-PT Traveler game-based learning approach for periodic table lessons and a comparison group instructed through an alternative student-centred method. Despite both approaches being student-centred, the transparency of the ‘magic cycle’ within STEM-PT Traveler, as described by Plass et al. (2015), is noteworthy. The cyclic process, involving dice rolling, token movement, and response to questions from the provided cards, fosters continuous engagement. This dynamic prompts students to confront challenges, discuss answers, and provide feedback iteratively. The positive emotions experienced during the pursuit of higher reward points, linked to proton, neutron, and electron comprehension in Phase 1 of the game, contribute to a joyful and enduring learning experience. The magic cycle extends into Phase 2, where teams of students explore various resources to understand how elements are utilized in manufacturing. Collaborative efforts, involving questioning, feedback, and resolution of differing opinions during discussions, mark this phase. The magic cycle, evident in both phases of STEM-PT Traveler, intersects with Bandura's (1986) triad reciprocal interaction in social cognitive theory. Throughout the process of providing feedback and addressing challenges, students engage in continuous self-regulation at the individual level. The collective expression of this self-regulation within the team influences the social and environmental context, ultimately manifesting as observable behaviour. The persistent overlap of the magic cycle and triad reciprocal interaction elucidates the higher motivation scores observed in the GBL group.
The quantitative findings, specifically, revealed significant differences in three dimensions—namely, intrinsic motivation, self-efficacy, and career motivation (a subset of extrinsic motivation)—while non-significant differences were observed in two dimensions—grade motivation (a subset of extrinsic motivation) and self-determination. Notably, the GBL group exhibited higher scores in the post-test assessment. Qualitative interviews conducted after the treatment provided insights into the quantitative findings. In response to the interview questions, students from the GBL group consistently alluded to their experiences in playing the board game. In accordance with Deci and Ryan (2000), intrinsic motivation denotes engagement in an activity driven by its inherent interest. The description of intrinsic motivation aligns with the principles advocated in STEM-PT Traveler. The emotions of enjoyment and a positive mood permeated throughout the various phases. This confirms the claim that game-based learning in chemistry induces different types of emotions (Hu et al., 2022). Establishing connections between chemistry and potential career options, a component of the game wherein students exchanged rewards for different chemistry-related careers, heightened their awareness of the intrinsic value of chemistry. This point was made explicit during the interview. Of paramount importance is the interdisciplinary expansion of lessons in Phase 2, prompting students to consider STEM components and compelling them to transcend the confines of the classroom. In the interview, students made it clear that expansion led them to recognize the practical application of chemistry in the manufacturing of products.
Bandura (1986) defines self-efficacy as “an individual's belief in his or her capacity to execute behaviours necessary to produce specific performance attainments.” Within the triadic reciprocal interaction model, self-efficacy holds significance as a personal-level influence (Schunka and DiBenedetto, 2020). Schunk and DiBenedetto further expound that both social interactions and the environment impact self-efficacy, and vice versa. This reciprocal relationship is evident in courses utilizing a service-learning model to teach chemistry at the undergraduate level (Schmidt et al., 2020). Service learning, an active approach wherein students engage in both serving and learning from the community while mastering chemistry, fosters an environment where students communicate with neighbours during service-learning activities. This interaction elucidates the interplay between the environment, behavioural processes, and personal development, subsequently enhancing self-efficacy. A similar principle is applicable to the present study. Active engagement in problem-solving, applying the chemical and physical properties of elements, empowers students to close the loops within the reciprocal relationship. This dynamic elucidates the observed improvement in self-efficacy within the context of this study.
Deci and Ryan (2000) referred to extrinsic motivation as engaging in completing the task as it leads to a separable outcome. Glynn et al. (2011) envision extrinsic motivation as participating and completing chemistry related tasks for getting a better grade in chemistry (grade motivation) and for securing a better job opportunity in future (career motivation). Grade and career motivation gave distinctive findings in this current study with the former exhibiting a non-significant difference between the control and experimental group and the latter revealing a significant difference. Students participated in both approaches with the intention to acquire understanding of elements in the periodic table. Learning occurred in both situations to secure a better grade in chemistry, as is notable in the interview responses. The common reason for students to enrol in chemistry is because they want to further their studies in chemistry-related fields and to secure better job opportunities. This notion is shown more explicitly in the GBL approach than in the non-GBL approach. Exchanging rewards for career cards, which illustrates the nature of a job and salary, in STEM-PT Traveler, compared to a simplistic understanding of the job, explains the significant difference between the two groups.
Students' ability to take control of their learning during lessons reflects the self-determination they possess (Black and Deci, 2000). When students take control of their learning, they actively regulate their actions, participate in decision-making about the next steps, and engage in discussions about the elements of the periodic table. These actions illustrate the self-determination exhibited by the students in this study. The non-significant difference between both groups indicates that both approaches prompted students to take control of their learning to better understand the concepts. A study by Partanen (2020) suggests that an active learning approach with social features, such as student–peer relationships, fosters autonomy in regulating learning. The consistent presence of student–peer relationships throughout participation in both non-GBL and GBL approaches explains the non-significant difference in the self-determination of the students.
Conclusions and limitations
The recognition that students experience a declining trend in motivation for learning upon returning to school after a prolonged lockdown due to the pandemic prompted the researchers to employ approaches encouraging social interaction and enjoyable learning to lift their motivation levels. Both approaches, the student-centred non-GBL and the STEM-PT Traveler GBL approach, were purposefully designed for the topic of the periodic table. Drawing upon past studies highlighting the need to shift from predominantly teacher-centred approaches to delivering lessons on the periodic table, both strategies emphasize student–student interaction and promote discussion. However, the presence of play elements making learning fun and enjoyable resulted in students in the GBL group exhibiting significantly higher motivation in intrinsic motivation, career motivation, and self-efficacy. Non-significant differences were observed for self-determination and grade motivation. Many board games have been specifically designed for teaching and learning the periodic table, considering the context of the students. Likewise, STEM-PT Traveler was designed in a similar manner. However, unlike the board games in the literature, STEM-PT Traveler explicitly extends learning about the properties of elements across the four STEM disciplines. This advances the idea of how to integrate STEM into lessons on the PT.The board game STEM-PT Traveler is an exemplary educational tool. The board game is specifically designed for students to grasp the organization of elements within periods and groups, to facilitate understanding of the physical and chemical properties of elements, and the trends in these properties as they move down groups and across periods, and to foster motivation for learning chemistry. The chemistry content related to periodic table elements covered by STEM-PT Traveler aligns with the standard curriculum taught in most secondary or high school chemistry classes. One notable aspect of STEM-PT Traveler is its adaptability and feasibility for use in various countries where motivation for learning chemistry is typically low. Unlike digital games, STEM-PT Traveler, being a board game, doesn't necessitate any special requirements. This feature makes the game easily implementable by educators, and its rules can be adjusted to suit specific needs.
The study is subject to several limitations that warrant consideration. Firstly, the exploration of motivation in this study is primarily grounded in the perspectives introduced by Glynn et al. (2011) and Bandura's social cognitive theory (Bandura, 1986). To provide a comprehensive understanding, future research should extend the examination to incorporate Self-Determination Theory and other relevant motivational theories. Deci and Ryan (2000) delineated three basic psychological needs influencing motivation—autonomy, relatedness, and competence. Examining how STEM-PT Traveler contributes to student motivation would benefit from assessing whether students perceive autonomy, competence, and the relevance of the topic to their careers and academic performance. Secondly, the study's focus on motivation is limited to the five subscales outlined in Glynn et al.'s model. However, other crucial motivational constructs, such as interest and engagement, were not specifically addressed. Expanding the investigation to encompass these additional constructs would provide a more comprehensive perspective on students' motivation. The third limitation pertains to the study's duration. Students were exposed to both non-GBL and GBL approaches within a limited timeframe. While this timeframe is appropriate for measuring effectiveness of any treatment, extending the duration could potentially enhance self-determination and grade motivation, which exhibited non-significance in this study. Future research should consider a more prolonged exposure to capture potential shifts in these motivational aspects. The fourth limitation concerns the availability of evidence for the internal structure of the CMQII. While we provided justification based on past studies in the literature, it is recommended to conduct exploratory and factor analysis to confirm the internal structure. Finally, the last limitation pertains to the sample size. Although the sample size of the study aligns with recommendations for a mixed-method design, it is recommended to repeat the study with a larger sample size to mitigate statistical biases and enhance generalisability.
Data availability
The data are not publicly available as publicly releasing the data could potentially compromise the privacy of research participants.Conflicts of interest
There are no conflicts of interest to declare.References
- Academy of Sciences Malaysia, (2018), Science outlook 2017: converging towards progressive Malaysia 2050. https://www.akademisains.gov.my/studies/flagship/science-outlook/.
- Álvarez-Herrero J.-F. and Valls-Bautista C., (2021), The game as a strategy of learning chemistry among high school students, Eur. J. Sci. Math. Educ., 9(3), 80–91 DOI:10.30935/scimath/10947.
- Ardura D. and Pérez-Bitria A., (2018), The effect of motivation on the choice of chemistry in secondary schools: adaptation and validation of the Science Motivation Questionnaire II to Spanish students, Chem. Educ. Res. Pract., 19, 905 10.1039/c8rp00098k.
- Ardura D., Zamora A. and Pérez-Bitria A., (2021), The role of motivation on secondary school students’ causal attributions to choose or abandon chemistry, Chem. Educ. Res. Pract., 22, 43 10.1039/D0RP00168F.
- Arjoon A. J., Xu X. and Lewis E. J., (2013), Understanding the state of the art for measurement in chemistry. J. Chem. Educ., 90, 536–545 DOI:10.1021/ed3002013.
- Bandura A., (1986), Social foundations of thought and action: A social cognitive theory, Prentice-Hall.
- Bayir E., (2014), Developing and playing chemistry games to learn about elements, compounds, and the periodic table: elemental periodica, compoundica, and groupica, J. Chem. Educ., 91(4), 531–535 DOI:10.1021/ed4002249.
- Bernardo J. M. M. and Gonzalez F. A., (2021), Chemical Battleship: Discovering and Learning the Periodic Table Playing a Didactic and Strategic Board Game, J. Chem. Educ., 98, 907–914 DOI:10.1021/acs.jchemed.0c00553.
- Bierenstiel M. and Snow K., (2019), Periodic universe: a teaching model for understanding the periodic table of the elements, J. Chem. Educ., 96(7), 1367–1376 DOI:10.1021/acs.jchemed.8b00740.
- Black A. E. and Deci E. L., (2000), The effects of instructors’ autonomy support and students’ autonomous motivation on learning organic chemistry: a self-determination theory perspective, Sci. Educ., 84, 740–756.
- Braun V. and Clarke V., (2006), Using thematic analysis in psychology, Qualitative Res. Psychol., 3(2), 77–101 DOI:10.1191/1478088706qp063oa.
- Chen C. H., (2019) The impacts of peer competition-based science gameplay on conceptual knowledge, intrinsic motivation, and learning behavioral patterns, Educ. Technol. Res. Dev., 67, 179–198 DOI:10.1007/s11423-018-9635-5.
- Chen C. H. and Law V., (2016), Scaffolding individual and collaborative game-based learning in learning performance and intrinsic motivation, Comput. Hum. Behav., 55, 1201–1212.
- Chiu T. K. F., (2022), Applying the self-determination theory (SDT) to explain student engagement in online learning during the COVID-19 pandemic, J. Res. Technol. Educ., 54(1), 14–30 DOI:10.1080/15391523.2021.1891998.
- Chiu T. K. F., Lin T. J. and Lonka K., (2021), Motivating online learning: The challenges of COVID-19 and beyond, Asia Pac. Educ. Rev., 30, 187–190 DOI:10.1007/s40299-021-00566-w.
- Cohen J., (1988), Statistical power analysis for the social science, 2nd edn, Hillsdale, NJ: Lawrence Erlbaum Associates.
- Creswell J. W. and Plano Clark V. L., (2011), Designing and Conducting Mixed Methods Research, 2nd edn, Sage Publications.
- Deci E. L. and Ryan R. M., (1985), Intrinsic motivation and self-determination in human behaviour, New York: Springer Science and Business Media.
- Deci E. L. and Ryan R. M., (2000), Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being, Am. Psychol., 55(1), 68–78 DOI:10.1037/0003-066X.55.1.68.
- De Souza P. M. T. R., da Silva N. S. M., Barbato M. L. D., Guzzi, D. R. and Kasseboehmer, C. A., (2022), Motivation to learn chemistry: a thorough analysis of the CMQ-II within the Brazilian context, Chem. Educ. Res. Pract., 23, 99–810 10.1039/d2rp00107a.
- Díez-Pascual A. N. and Jurado-Sánchez B., (2022), Remote teaching of chemistry laboratory courses during COVID-19, J. Chem. Educ., 99(5), 1913–1922 DOI:10.1021/acs.jchemed.2c00022.
- Elford D., Lancaster S. J. and Jones A. G., (2022), Fostering motivation toward chemistry through augmented reality educational escape activities. A self-determination theory approach, J. Chem. Educ., 99, 3406–3417 DOI:10.1021/acs.jchemed.2c00428.
- Elliot, A. J., Murayama, K. and Pekrun, R., (2011), A 3 × 2 achievement goal model, J. Educ. Psychol., 103(3), 632–648 DOI:10.1037/a0023952.
- Enevoldsen K., (2016), The periodic table of elements in pictures and words, Retrieved March, 20, 221 from https://elements.wlonk.com/index.htm.
- English L. D., (2016), STEM education K-12: perspectives on integration, Int. J. STEM Educ., 3(1), 3.
- English L. D. and King D., (2018), STEM integration in sixth grade: designing and constructing paper bridges, Int. J. Sci.Math. Educ., 17, 863–884.
- Franco-Mariscal A. J., Oliva-Martínez J. M., Blanco-López A. and España-Ramos E., (2016), A game-based approach to learning the idea of chemical elements and their periodic classification, J. Chem. Educ., 93(7), 1173–1190 DOI:10.1021/acs.jchemed.5b00846.
- Girón-Gambero J. R. and Franco-Mariscal A. J., (2023), “Atomizados”: an educational game for learning atomic structure. a case study with grade-9 students with difficulties learning chemistry, J. Chem. Educ., 100(8), 3114–3123 DOI:10.1021/acs.jchemed.2c00614.
- Glynn S. M., Brickman P., Armstrong N. and Taasoobshirazi G., (2011), Science motivation questionnaire II: validation with science majors and nonscience majors, J. Res. Sci. Teach., 48(10), 1159–1176 DOI:10.1002/tea.20442.
- Gupta T., (2019), Game-based learning in chemistry: A game for chemical nomenclature, Technology integration in chemistry education and research (TICER), American Chemical Society, pp. 65–79 DOI:10.1021/bk-2019-1318.ch005.
- Hair J. F., Anderson R. E., Babin B. J. and Black W. C., (2010), Multivariate Data Analysis: A Global Perspective (vol. 7), Upper Saddle River, NJ: Pearson.
- Hu Y., Gallagher T., Wouters P., Schaaf M. V. D. and Kester, L., (2022), Game-based learning has good chemistry with chemistry education: a three-level meta-analysis, J. Res. Sci. Teach., 59, 1499–1543 DOI:10.1002/tea.21765.
- Huri N. H. D. and Karpudewan M., (2019), Evaluating the effectiveness of integrated STEMlab activities in improving secondary school students’ understanding of electrolysis, Chem. Educ. Res. Pract., 20, 495–508 10.1039/c9rp00021f.
- Karpudewan M. and Chong T., (2020), Evaluating radioactivity remote laboratory's effectiveness in learning radioactivity concepts, Res. Sci. Educ., 50, 2243–2268 DOI:10.1007/s11165-018-9772-1.
- Karpudewan M. and Huri N. H. D., (2023), Interdisciplinary electrochemistry stem-lab activities replacing the single disciplinary electrochemistry curriculum for secondary schools, J. Chem. Educ., 100(2), 998–1010 DOI:10.1021/acs.jchemed.2c00469.
- Lauter J., (1978), Sample size requirements for the T2 Test of MANOVA, Biomet. J., 20, 389–406.
- Lewis E. S., (2022), Considerations on validity for studies using quantitative data in chemistry education research and practice, Chem. Educ. Res. Pract., 23, 764 10.1039/d2rp90009b.
- Li C.-T., Huei-Tse H. and Wei-Shen L., (2022), Chemistry education board game based on cognitive mechanism: multi-dimensional evaluation of learners’ knowledge acquisition, flow and playing experience of board game materials, Res. Sci. Technol. Educ. DOI:10.1080/02635143.2022.2125505.
- Lin C. Y. and Wu H. K., (2021), Effects of different ways of using visualizations on high school students’ electrochemistry conceptual understanding and motivation towards chemistry learning, Chem. Educ. Res. Pract., 22, 786 10.1039/d0rp00308e.
- Liu Y., Raker J. R. and Lewis J., (2018), Evaluating student motivation in organic chemistry courses: moving from a lecture-based to a flipped approach with peer-led team learning, Chem. Educ. Res. Pract., 19, 251–264 10.1039/C7RP00153C.
- Lu Y.-L. and Lien C.-J., (2020), Are they learning or playing? students’ perception traits and their learning self-efficacy in a game-based learning environment. J. Educ. Comput. Res., 57(8), 1879–1909 DOI:10.1177/0735633118820684.
- Martí-Centelles V. and Rubio-Magnieto J., (2014), Chemmend: a card game to introduce and explore the periodic table while engaging students’ interest. J. Chem. Educ., 91(6), 868–871 DOI:10.1021/ed300733w.
- Martín-Sómer M., Moreira J. and Casado C., (2021), Use of kahoot! to keep students’ motivation during online classes in the lockdown period caused by Covid 19, Educ. Chem. Eng., 36, 154–159 DOI:10.1016/j.ece.2021.05.005.
- Meluso A., Zheng M., Spires A. H. and Lester J., (2012), Enhancing 5th graders’ science content knowledge and self-efficacy through game-based learning, Comput. Educ., 59, 497–504 DOI:10.1016/j.compedu.2011.12.019.
- Ministry of Education MOE, (2018), Secondary school standard curriculum: From Four Chemistry curriculum and assessment standard document, Curriculum Development Centre.
- Moore T. J., Johnson C. C., Peters-Burton E. E. and Guzey S. S., (2016), The need for a stem road map, in Johnson C. C., Peters-Burton E. E., Moore T. J. and Selcen Guzey S., (ed.), STEM road map: a framework for integrated STEM education, Routledge Taylor & Francis Group, pp. 3–12.
- O’Rouke B., (2021) Growing gap in STEM supply and demand, Retrieved on December 15, 2022, from https://news.harvard.edu/gazette/story/2021/11/increasing-access-and-opportunity-in-stem-crucial-say-experts/.
- Partanen L., (2020), How student-centred teaching in quantum chemistry affects students’ experiences of learning and motivation—a self-determination theory perspective, Chem. Educ. Res. Pract., 21, 79 10.1039/c9rp00036d.
- Peleg R., Yayon M. and Katchevich D. et al., (2019), A lab-based chemical escape room: educational, mobile, and fun, J. Chem. Educ., 96(5),955–960 DOI:10.1021/acs.jchemed.8b00406.
- Pelikan E. R., Korlat S., Reiter J., Holzer J., Mayerhofer M. and Schober B. et al., (2021) Distance learning in higher education during COVID-19: the role of basic psychological needs and intrinsic motivation for persistence and procrastination–a multi-country study, PLoS One, 16(10), e0257346 DOI:10.1371/journal.pone.0257346.
- Plass J. L., Homer B. D. and Kinzer C. K., (2015), Foundations of game-based learning, Educ. Psychol., 50(4), 258–283 DOI:10.1080/00461520.2015.1122533.
- Salen K. and Zimmerman E., (2004), Rules of play: Game design fundamentals, MIT Press.
- Salta K. and Koulougliotis D., (2015), Assessing motivation to learn chemistry: adaptation and validation of Science Motivation Questionnaire II with Greek secondary school students, Chem. Educ. Res. Pract., 16, 237 10.1039/c4rp00196f.
- Schmidt E., Vik R., Brubaker B. W. and Abdulahad S. S. et al., (2020), Increasing student interest and self-efficacy in stem by offering a service-learning chemistry course in new orleans, J. Chem. Educ., 97(11), 4008–4018 DOI:10.1021/acs.jchemed.9b01140.
- Schunka D. H. and DiBenedetto M. K., (2020), Motivation and social cognitive theory, Comput. Educ., 60, 101832 DOI:10.1016/j.compedu.2023.104975.
- Stevens J. P., (1980), Power of the multivariate analysis of variance tests, Psychol. Bull., 88, 728–737.
- Sunasee R., (2020), Challenges of Teaching Organic Chemistry during COVID-19 Pandemic at a Primarily Undergraduate Institution, J. Chem. Educ., 97(9), 3176–3181 DOI:10.1021/acs.jchemed.0c00542.
- Tabachnick B. G. and Fidell L. S., (2007), Using Multivariate Statistics (5th edn), New York Allyn and Bacon.
- Tan C., (2021), The impact of COVID-19 on student motivation, community of inquiry and learning performance, Asian Educ. Dev. Stud., 10(2), 308–321 DOI:10.1108/AEDS-05-2020-0084.
- Traver J. V., Leiva A. L., Marti-Centelles V. and Rubio-Magnieto J., (2021), Educational videogame to learn the periodic table: design rationale and lessons learned, J. Chem. Ed., 98, 2298–2306 DOI:10.1021/acs.jchemed.1c00109.
- Tuan H. L., Chin C. C. and Shieh S. H., (2005), The development of a questionnaire to measure students’ motivation towards science learning, Int. J. Sci. Educ., 27, 639–654.
- Wang M. and Zheng X., (2021), Using game-based learning to support learning science: a study with middle school students, Asia-Pacific Edu Res., 30, 167–176 DOI:10.1007/s40299-020-00523-z.
- Weiner B., (1985), An attributional theory of achievement motivation and emotion, Psychol. Rev., 92(4), 548–573 DOI:10.1037/0033-295X.92.4.548.
- Wigfield A. and Eccles J. S., (2000), Expectancy-value theory of achievement motivation, Contemp. Educ. Psychol., 25(1), 68–81 DOI:10.1006/ceps.1999.1015.
- World Bank, (2022), Learning Recovery for Acceleration, Retrieved December, 14, 2022 from https://www.worldbank.org.
- Zhang Z., Muktar P. and Ong C. I. W. et al., (2021), CheMakers: playing a collaborative board game to understand organic chemistry, J. Chem. Educ., 98(2), 530–534 DOI:10.1021/acs.jchemed.0c01116.
- Zhang J. and Zhou Q., (2023), Chinese chemistry motivation questionnaire II: adaptation and validation of the science motivation questionnaire II in high school students, Chem. Educ. Res. Pract., 24, 369 10.1039/d2rp00243d.
| This journal is © The Royal Society of Chemistry 2024 |


