Flipping the script in organic reaction mechanism instruction: using generative pedagogies instead of lecture to improve learning outcomes
Connor
Haindfield
a,
William
Cerbin
b,
Douglas
Baumann
c and
Heather
Schenck
 *a
*a
aUniversity of Wisconsin-La Crosse, Chemistry and Biochemistry, La Crosse, Wisconsin, USA. E-mail: hschenck@uwlax.edu
bUniversity of Wisconsin-La Crosse, Psychology, La Crosse, Wisconsin, USA
cUniversity of Wisconsin-La Crosse, Mathematics and Statistics, La Crosse, Wisconsin, USA
First published on 19th July 2024
Abstract
Two generative approaches to reaction mechanism instruction for novice students were compared to lecture instruction. In both approaches, students were coached to propose selected reaction mechanisms based on prior knowledge. New instructional methods were correlated with increased skill in representations of electron movements and other gains. Students who saw a larger amount of new pedagogy showed stronger abilities to propose mechanisms for unfamiliar reactions. In the group that saw a larger amount of new pedagogy, first-generation college student (FGS) grades rose to match non-FGS grades. Learning gains were interpreted with respect to cognitive load theory, flagging high element interactivity as a likely obstacle for novice students. Problem solving during mechanism instruction for novice students offers the potential to improve learning outcomes.
Introduction
The titanic struggles of organic chemistry students are well documented. Literature has focused more on characterizing weaknesses in student learning (representations and reasoning) than on testing alternative teaching approaches (Cooper et al., 2019). Recent reports of improved student learning in a variety of instructional formats are encouraging (Bodé et al., 2019; Caspari and Graulich, 2019; Wilson and Varma-Nelson, 2019; Carle et al., 2020; Lipton, 2020; Graulich and Caspari, 2021; Lieber et al., 2022). A recent report on development of student reasoning using scaffolded case comparison provides a promising anti-deficit perspective on skill acquisition (Haas et al., 2024). Nevertheless, most reports continue to document learning difficulties rather than to test potential solutions to learning deficits (Bongers et al., 2019; Farhat et al., 2019; Bongers et al., 2020; Finkenstaedt-Quinn et al., 2020; Dood and Watts, 2022, Watts et al., 2022; Asmussen et al., 2023; Dood and Watts, 2023; Frost et al., 2023Kranz et al., 2023; Gao et al., 2024;).Students from under-resourced educational backgrounds can experience greater obstacles to success in organic chemistry (Lovecchio and Dundes, 2002; Barr et al., 2008). These students experience academic difficulties and exhibit poorer persistence to degrees correlated with the extent of resource deficiency experienced (Bettencourt et al., 2020). The intersection of lower persistence with less complete preparation could lead to disproportionately high attrition. These circumstances apply to many first-generation college students (FGS), who represented about a third of students at the authors’ institution during the study. Little has been reported about FGS success in organic chemistry.
Instructional design for organic chemistry
Cultivation of higher order cognitive skills such as causal mechanistic reasoning in the organic classroom has been encouraged (Zoller, 1999; Eilks and Byers, 2010; National Research Council, 2012; Graulich, 2015). Instructional designs tested include process-oriented, guided-inquiry learning, supplemental instruction and curricular reorganizations emphasizing mechanistic content (Karty et al., 2007; Hein, 2012; Conway, 2014; Flynn and Ogilvie, 2015; Lipton, 2020). Course activities beyond class times have also been examined, including flipped classrooms, framework of 3-D learning, study circles, computer-aided instruction and peer-led team learning (Flynn, 2012; Ravishankar et al., 2013; Mooring et al., 2016; Shattuck, 2016; Webber and Flynn, 2018; Cooper et al., 2019; Wilson and Varma-Nelson, 2019; Carle et al., 2020). Positive outcomes from these efforts include greater mastery of electron movements, higher grades, and increased success in downstream courses.Nevertheless, lecture remains the default in many organic classrooms due to the inflexible volume of curricular content. Non-lecture pedagogies may cover topics more slowly (Zoller, 1999; Bransford et al., 2000; Ravishankar et al., 2013; Conway, 2014; Cooper et al., 2019) and may run out of time for required content.
Whole-course redesigns have been recommended over unit-level revisions (Cooper et al., 2019), but decreased content coverage may render full redesign infeasible. Likewise, course activities beyond class times may be inequitable because students from under-resourced backgrounds tend to have larger out-of-school obligations (Wilson and Kittleson, 2013). A targeted pedagogy change on a most-challenging topic within a predominantly lecture course would be a useful resource.
Learning challenges of organic reaction mechanisms
Reaction mechanisms (representations and reasoning) are challenging for students and have been identified as promising targets for pedagogy revision (Grove et al., 2012a, 2012b; Kranz et al., 2023). A firm grasp of mechanistic theory is crucial to success in organic chemistry. Depiction of electron movements with curved arrows (the electron pushing formalism: EPF) has been called “the single most important technique” in organic coursework but poses major challenges for students (Ferguson and Bodner, 2008; Grove et al., 2012a; Bhattacharyya, 2013; Dood and Watts, 2023). Mechanism study also prompts drastic revision and expansion of the schema (knowledge structure) that students possess for chemical reactions. Instructors have identified a dozen or more areas of content knowledge as well as reasoning and representational skills needed for proficiency in mechanism depictions and problem solving (Bhattacharyya, 2013).Not surprisingly, student mechanism explanations and representations routinely show deficiencies (Kraft et al., 2010; Caspari et al., 2018; Dood and Watts, 2022). Students often see reaction mechanisms as facts to memorize (Bhattacharyya and Bodner, 2005; Graulich, 2015). Instructors hope that over time, students will demonstrate greater abstractness and multi-variate reasoning in problem solving and will successfully transfer mechanistic thought processes to new situations (Kraft et al., 2010; Grove et al., 2012b; Weinrich and Sevian, 2017; Bodé et al., 2019; Cooper et al., 2019; Frost et al., 2023). Such hopes are often unmet at even the graduate level (Bhattacharyya and Bodner, 2005; Kraft et al., 2010).
The cruciality of mechanistic approaches to problem solving, the number of skills required to demonstrate that competency and the legendary difficulties encountered by students in their attempts commend this topic for pedagogy modification. For such a complex topic, a range of instructional approaches may be beneficial.
Theoretical framework
Lecture instruction does not necessarily guide students to activate reasoning skills that support robust learning (Bodé et al., 2019). Instructors have been urged to “carefully reflect on the types of reasoning that different contexts demand to more carefully scaffold learning tasks” (Yan and Talanquer, 2015).Cognitive load in mechanism instruction
Mechanism lectures explicate reasoning in an attempt to endow students with a mature schema for reaction mechanisms and with the ability to apply the schema in problem solving. However, as researchers contend, “it would be a mistake simply to expose novices to expert models and assume that the novices will learn effectively” (Bransford et al., 2000). Students must be able to overcome the cognitive challenges presented during instruction in order to learn (Chew and Cerbin, 2021).Cognitive load is the sum of cognitive resources needed to accomplish a task (Sweller, 1994). During typical mechanism instruction, cognitive load consists of the mental effort involved in tracking novel movements of electrons and atoms as well as in recall and new applications of many earlier chemistry concepts. Students need full and fluent mastery of hybridization, acid–base theories, valency, periodic trends, bond polarization and many other topics (Bhattacharyya, 2013) and must be able to recognize when each topic is relevant. Cognitive overload occurs when the mental effort involved in recalling and applying older concepts while processing new concepts and representations exceeds students’ working memory capacity (Sweller, 1994; Van Merriënboer et al., 2006). The consequences of cognitive overload are confusion, incomplete learning, and a sense of being overwhelmed by too much information.
Evidence suggests that cognitive load is a significant challenge during mechanism instruction. Novice students' tendencies to depict isolated processes of bond breakage and formation have been attributed to overloaded working memory (Galloway et al., 2017). Improved learning outcomes have been reported when aspects of mechanism study are temporally separated (Karty et al., 2007; Penn and Al-Shammari, 2008; Flynn and Ogilvie, 2015; Galloway et al., 2017). A recent study also revealed greater diversity in cognitive challenges than knowledge challenges using a revised Bloom's taxonomy specific for mechanism study (Asmussen et al., 2023). Despite these observations, no study has been located that has considered cognitive load (CL) formally or attempted to quantify CL experienced during mechanism instruction.
Cognitive overload in mechanism instruction is plausibly attributable to the fragmentary nature of novice student knowledge frameworks (Graulich, 2015; Cooper et al., 2017). Activities that support students in activating and connecting pieces of knowledge foster productive learning (Graulich and Caspari, 2021). However, attempting to integrate unlinked pieces of information imposes CL due to “element interactivity”: the extent to which ideas being manipulated are unrelated in the mind of the learner (Sweller, 1994; Pollock et al., 2002). Working memory imposes strict limits when dealing with unrelated concepts. Where element interactivity (EI) is high, novices will struggle to follow the most articulate explanation an expert can provide. Based on literature evidence, our work with students, and results obtained in this study, we posit that excessive EI in lecture instruction on mechanisms is a root cause behind many unmet learning goals.
Experts (instructors) have more highly organized knowledge than novices (students) (Bransford et al., 2000). Specifically, experts link related concepts in a schema that decreases EI and permits activation of multiple areas of knowledge. For example, the initial proton transfer in SN1 reaction of tert-butanol with HBr is underpinned by concepts including bond polarity, valency and acidity. Because experts have linked these concepts to proton transfers, experts represent and understand the process differently from a novice. An instructor knows that oxygen is sufficiently electron rich to be protonated by a strong acid, that HBr is a strong acid, and that electrons to form the new bond must come from oxygen. The instructor also knows that the proton cannot remain bonded to bromine and that electrons from the H–Br bond must travel to bromine. These insights make the electron movements of the elementary step inevitable and logical to the instructor.
Novice students, on the other hand, tend to focus on the visible challenge: capturing the instructor's drawings. The additional cognitive effort required to build links to relevant prior knowledge (which could reveal the logic of the electron movements) likely exceeds working memory capacity for many students. Those students may jettison or fail to absorb links to older concepts, resorting instead to memorizing what the instructor drew.
The expert's linking of concepts (sometimes called “chunking”) decreases working memory overloads and enables integrated application of larger bodies of knowledge (Bransford et al., 2000). Development of schemata reduces the cognitive burden of complex tasks (Pollock et al., 2002). Highly complex topics present a paradox, however: the amount of unrelated information required to understand the topic exceeds working memory capacity, yet the schema that would streamline cognitive processing must be constructed from an understanding of the topic (Pollock et al., 2002). Such topics are challenging to learn and understand, and especially to teach.
The imperative to rapidly reactivate and contextualize a large amount of previously-unlinked prior knowledge can render a mechanism lecture not just challenging, but unintelligible. We believe lecture instruction on reaction mechanisms involves inordinately high EI for novice learners. Researchers have tacitly recognized this issue by calling for development of “chunking” strategies in mechanism instruction (Grove et al., 2012b). It is time to reconsider instructional design for reaction mechanisms: there may be more appropriate and attainable learning goals for novice learners than acquisition of intact schema (Kalyuga and Singh, 2016).
Instructional adaptations
The optimal instructional approach to support learning depends on content complexity, learning goals and learner experience level. Careful consideration of each factor is required for best learning outcomes.CL theory recommends explicit instruction for complex content, especially for novice learners (Kalyuga and Singh, 2016). On the other hand, meta-analysis of discovery learning versus explicit instruction found that assisted discovery (e.g., including scaffolds, elicited explanations, etc.) delivers overall better outcomes than other instructional methods (Alfieri et al., 2011). Moreover, explicit instruction can hinder transfer of learning: inhibiting learners from applying new skills creatively (Van Merriënboer et al., 2006).
For complex topics and novice learners, evidence has accrued in support of separation of knowledge acquisition into multiple goals or processes. Instructional design that initially isolates elements that would otherwise interact (thus decreasing EI), then integrates increasing numbers of elements, has been asserted to decrease CL (Pollock et al., 2002; Van Merriënboer et al., 2006). This “isolated elements” effect can deliver superior results specifically for novice learners on complex tasks (Pollock et al., 2002; Kalyuga and Singh, 2016; Lu et al., 2020).
Pre-instruction activities such as prior knowledge activation can boost learning gains from subsequent direct instruction by providing cognitive groundwork for schema development (Kalyuga and Singh, 2016). Such activities may also support end-game goals of transfer and prediction better than direct instruction (Van Merriënboer et al., 2006; Schwartz et al., 2009; Ashman et al., 2020).
Study design elements
The cornerstone of this study was the hypothesis that if student prior knowledge is sufficient to understand mechanistic rationales, then students should be able to build their own mechanisms with coaching. This approach is consistent with recent perspectives on cultivation of student reasoning skills that avoid a deficit model (Haas et al., 2024). Students were guided to generate their own mechanism proposals by two different methods. The generation effect, in which learners show greater mastery after creating information rather than being presented with it, is one of the strongest outcomes identified in cognitive psychology (deWinstanley and Bjork, 2002). The study design included:• Attempts to reduce and manage negative effects of cognitive load
• Classroom activities targeted to support schema construction
Instructional designs that situate problem solving before direct instruction have not been reported in mechanism instruction for novice students. Chemistry students are rarely engaged to create mechanistic explanations of systems or build models in the classroom (Yan and Talanquer, 2015), yet construction of explanations is a key scientific practice (National Research Council, 2012). Increasing the engagement of students in building and evaluating explanations can support sense-making (Yan and Talanquer, 2015; Meek et al., 2016). Evidence favors approaches that enable students to construct their own learning, such as by use of prior knowledge and contrasting cases (deWinstanley and Bjork, 2002; Schwartz et al., 2011; Bisra et al., 2018).
Guided problem solving converts a reaction mechanism from a series of facts and correlations to be remembered into a series of questions to be answered, such as
• Why does that bond form, and what electrons form it?
• Where do the electrons from this bond go when the bond breaks, and why do the electrons travel that way?
• What pattern of electron movement might we predict in this situation based on earlier mechanisms?
Students may engage in self-explanation, spontaneously or in response to prompts, to identify connections among ideas and build mental models of information. Self-explanation is an effective learning approach in chemistry (Villalta-Cerdas and Sandi-Urena, 2014). Students can learn more by trying to explain new concepts than by listening to an expert explanation, especially when prompted with relevant feedback (Bisra et al., 2018).
Starting instruction on a complex topic with problem solving requires instructional scaffolding, which can be especially beneficial for low-scoring students (Noyes and Cooper, 2019; Kranz et al., 2023). Embedding new knowledge in an existing framework creates a more expert-like knowledge structure (Kalyuga and Singh, 2016; Cooper et al., 2019). Scaffolded frameworks that cue students to consider prior knowledge during mechanism study support more robust argumentation and reasoning (Graulich and Caspari, 2021). The breadth of relevant prior knowledge means that explicit cueing may be needed to guide novices to activate specific earlier learning (Gick and Holyoak, 1980). Such prompts may also flush out recall errors and increase fidelity in application of prior knowledge (Chi and Wylie, 2014).
Two scaffolded generative instructional approaches were compared to lecture instruction. One approach (‘Transfer’) involved transfer of principles from an earlier mechanism to propose a new mechanism; the other approach (‘First-principles’) used student prior knowledge to deduce a most-reasonable mechanism. The six-semester study had three cohorts of students who saw different instructional approaches for reaction mechanisms:
• Lecture
• Lecture plus Transfer pedagogy
• Lecture plus Transfer and First-principles pedagogies
Research questions
We wished to investigate effectiveness of the new instructional designs compared to lecture for novice students. Given the high percentage of unsuccessful students in many organic courses, we were also interested in potential differential effects of pedagogies relative to diversity in students’ backgrounds.(1) How do new pedagogies modulate representational and recall skills for curricular reaction mechanisms?
(2) How do new pedagogies affect students’ ability to transfer mechanistic principles to new reactions?
(3) Will students experiencing new pedagogy be as successful as students from conventional first semester courses in a conventional second semester course?
(4) Do students with diverse backgrounds experience comparable effects from lecture and new pedagogies?
Methods
Instructional methods
The study was conducted in the first semester (OChem1) of a two-semester organic series. OChem1 met three times per week. All study sections were taught by the same instructor between 2011 and 2015. The study does not include sections from Spring 2012 (see below) or 2014 (when the instructor was on sabbatical or assigned to a different lecture). Each study group included one fall and one spring section with enrollments of 60–108 students; median enrollment was 92. Differences in pedagogy were limited to mechanism instruction; all other instruction was in lecture format. Group labels reflect mechanism instruction: lecture (L), Transfer plus lecture (TL), or First-principles and Transfer plus lecture (FTL).OChem1 covers preparation and reactions of alcohols, alkyl halides, alkenes, conjugated dienes, alkynes, and electrophilic aromatic substitution. The curriculum included 21–23 mechanisms; alcohol E2 and hydroboration were not covered in Group FTL for time reasons. Curved arrows were introduced in week 1, prior to reactions. Notes templates were provided and used by the instructor. Study design details are shown in Table 1 and Appendix 1.
| Group L | Group TL | Group FTL | |
|---|---|---|---|
| a Homework. b In-class. c Molecular geometry, valence rules, electron configurations, etc.; final assignment covered OChem1 content. | |||
| Sections | 2 | 2 | 2 |
| Students | 177 | 176 | 142 |
| Mechanisms: total (lecture/transfer/first-principles) | 23 (23/0/0) | 23 (20/3/0) | 21 (9/8/4) |
| Exams (100 points, covering 3–4 chapters) | 4 | 4 | 4 |
| Worksheets (25 points, covering 2–3 chapters) | 4 | 4 | 4 |
| Functional group worksheet (10 points)a | 0 | 1 | 1 |
| Quizzesb (10 points) | 0 | 4 | 4 |
| Review assignmentsac (5 points) | 0 | 0 | 5 |
| Practice exercisesb | 0 | 2 | 2 |
| Lecture videos | No | No | Yes |
The study began after an intervention for a struggling section in Spring 2012 delivered positive results. The unpremeditated nature of the study meant that no direct measures of cognitive load were obtained. In addition, some controls needed for conclusive attribution of learning gains to specific instructional designs could not be incorporated (Table 1). Efforts were made to control other aspects of study design including populations (one fall and one spring section per group to address an institutional trend of more strong students enrolling in fall), uniform non-mechanism instruction materials, pacing and topic sequence, but conclusions from this work are necessarily preliminary.
Textbooks for OChem1 and OChem2 (second semester course) were Carey, Organic Chemistry 7th edition through Spring 2013 and Carey and Giuliano 9th edition starting in Fall 2013; all OChem1 instructors covered the same chapters.
Pedagogy methods are described below and in Appendix 2. The methods are applicable to paired electron processes; student prior knowledge was believed to offer few insights to predict radical processes. By count of mechanisms, percents of lecture vs. non-lecture pedagogy used in Groups L, TL and FTL were 100/0, 87/13 and 43/57, respectively.
In non-lecture instruction, students were prompted to draw their best attempt at mechanism depictions for new reactions. Following each attempt, the instructor illustrated a correct drawing with explanations. In some situations (e.g., changes of molecular geometry due to rehybridization), students made predictable mistakes; the instructor drew attention to these points. Assessment of energetics and energy diagrams occurred after mechanism instruction.
In Transfer pedagogy, students proposed a mechanism (or a portion thereof) by analogy to an earlier mechanism. Analogic examples can be powerful aids to new learning (Gick and Holyoak, 1980): the known mechanism serves as relevant prior knowledge that students use in proposing the new mechanism. Novice students reasoned from a familiar model that was similar to the new mechanism.
Groups TL and FTL used Transfer pedagogy for alkyl halide SN1, SN2 and E1 mechanisms based on corresponding alcohol processes covered four weeks earlier. Students were told that curved arrows and intermediates would be similar to earlier reactions because similar logic applied. Group FTL also used Transfer pedagogy for alcohol SN2, acid-catalyzed alkene hydration (based on electrophilic additions of HBr/HCl) and selected electrophilic aromatic substitutions (based on alkene electrophilic additions).
The First-principles approach was used when students’ existing experience with mechanisms was insufficient to support analogic reasoning (e.g., for the first mechanism). Reasoning from chemical principles for mechanism study can provide improved results through the following semester, especially for lower-scoring students (Lipton, 2020). Conversely, inability to engage correctly with various cognitive processes and knowledge components leads to unmet learning objectives in mechanism study (Asmussen et al., 2023). Group FTL used First-principles pedagogy for the first mechanism (alcohol SN1) as well as for mechanisms of alcohol E1 and electrophilic additions of water and HBr/HCl to alkenes.
First-principles pedagogy isolated key elements of mechanism study in a manner expected to reduce EI. Students began by identifying bonds formed and broken (henceforth, “processes”), as has been recommended (Bhattacharyya, 2019). The instructor shared the number of elementary steps in the mechanism and which step was rate limiting. Students used chemical logic to interpret and draw each process in isolation. Guiding students to represent concepts at levels of abstraction beyond a given context can be highly effective in supporting subsequent transfer (Bransford et al., 2000). The value of separating problem-solving steps within mechanisms to enable overall problem-solving has been discussed and is consistent with the “isolated elements” effect (Pollock et al., 2002; Asmussen et al., 2023).
Students next drew the “intermediate/s” from each isolated process, then assessed reasonableness of outcomes. Invariably, at least one student would identify an unreasonable outcome (e.g., pentavalent carbon), as well as a chemical reason (e.g., carbon's valence shell has only four orbitals). The use of infeasible mechanistic steps as options in contrasting-case examples is proposed to foster more complex mechanistic reasoning (Caspari et al., 2018). Pushing students to assess feasibility of mechanistic steps in terms of chemical principles may help students reason based on conceptual knowledge rather than memorized patterns (Bhattacharyya and Bodner, 2005). In some cases, discussion of a process introduced a new concept, e.g., carbocations as viable intermediates.
When all bond formation and breakage processes were analyzed, a chemically reasonable sequence of elementary steps consistent with experimental data (potentially involving combination of processes) was generated. This exercise was found to resolve earlier issues with implausible/impossible intermediates. Similar principles have been discussed by other authors (Meek et al., 2016; Bhattacharyya, 2019; Lipton, 2020). First-principles pedagogy was used selectively due to its higher time commitment.
Participants
Participants were 495 students enrolled in OChem1 at a public, mid-sized predominantly undergraduate institution in the Midwestern United States. Most enrollees (∼80%) were not chemistry majors. Ethnicity data were not collected for this study; however, during the study period the institution enrolled a population that included ∼89% Caucasian, 2.2–2.9% Hispanic, 2.4–2.9% Asian and <1% African American students. Unless stated otherwise, data reflect students who did not withdraw from OChem1. Enrollees were 48% juniors, 35% sophomores, 15% seniors and 2% freshmen/other status; 32% self-identified as first-generation college students.Ethical considerations
Institutional Review Board approval (protocol 12-HS-01) was obtained for use of data from OChem1 sections after Spring 2012, as well as for use of OChem2 scores. Students in Groups TL and FTL were offered the opportunity to sign informed consents for use of their exam and practice exercise data in aggregate form. The informed consent allowed them to withdraw from the study at any time; no students withdrew. Retroactive permission to use final exam and grade data before Spring 2012 (Group L) was provided by the Institutional Review Board. The fourth author was the instructor for all students.Assessments
The effort to improve learning outcomes from mechanism instruction was assessed by evaluating representational skills (EPF), the ability to reproduce curricular mechanisms (final exam questions) and the extent to which students applied mechanistic principles correctly to new reactions (transfer). Data from OChem2 and from other OChem1 instructors were reviewed to examine longitudinal outcomes. One OChem1 instructor participated in the study; OChem2 instructors did not participate.Distance of transfer is a consideration when using transfer as an assessment tool. Students may propose correct mechanisms without the ability to articulate why a proposal is plausible if relying on memorization (Bhattacharyya and Bodner, 2005). Transfer assessments were designed to appear dissimilar to curricular mechanisms to reduce the likelihood of correct answers being generated from memorized content (Kraft et al., 2010).
Each exercise provided a balanced reaction including an SN2-type process (Fig. 2) as part of a ring-opening or -closing reaction. Students were told that each mechanism had two elementary steps and one intermediate.
Three mechanisms (alcohol SN1 and SN2 and alkane halogenation) were covered before the first exercise (Fig. 2a). For these mechanisms, Group TL saw only lecture; Group FTL saw First-principles, Transfer and lecture, respectively.
By the second exercise (Fig. 2b), Groups TL and FTL had covered 13–15 mechanisms. The three most recent mechanisms were alkyl halide SN1, SN2 and E1; transfer pedagogy was used for all three in both groups. Rubrics for practice exercises are shown in Appendix 4.
First generation student outcomes
Disaggregation of research data has been encouraged to confirm that instructional innovations are equitable and inclusive (Sweeder et al., 2023). The student population at the authors’ institution is predominantly white, but first-generation college students (FGS) are well represented. FGS outcomes were explored to assess equity impacts of new pedagogies.Statistical analysis
A linear regression model was fit to investigate the relationship between longitudinal student performance and pedagogy. Student performance was measured using final course letter grades as these data were available via the university's institutional research department. Course grades were then converted to the 4-point scale used by the university to calculate grade point averages (GPAs; A = 4, A/B = 3.5, B = 3, B/C = 2.5, C = 2, D = 1, F = 0). The difference between OChem1 and OChem2 grade points was used as the response.In addition to pedagogy, student demographic variables with demonstrated effects on student success at the authors’ university (instructor, student level (e.g., Freshman), first-generation status, and semesters between OChem1 and OChem2) were used as covariates to isolate the effect of pedagogy on student success.
Model diagnostics were assessed visually (Aldor-Noiman et al., 2013) though not reported. Bonferroni's correction was applied to chi-square analyses used to characterize final exam answers. Bonferroni's correction was also applied to Welch t-tests used for analysis of practice exercise responses and FGS grade outcomes.
The first and fourth authors worked together to generate consensus scoring on final exam questions. Scoring of practice exercises was done initially by the fourth author and redone by the first author. Krippendorff Alpha values calculated to assess inter-rater reliability for scoring of practice exercises indicated strong agreement between scorers (mean = 0.92, sd = 0.06).
Results and discussion
Final exams
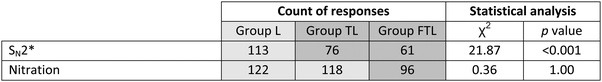
Various types of errors are seen in student work with the EPF (Wilson and Varma-Nelson, 2019). For the SN2 problem (Fig. 1a), curved arrows and structures were examined for weak grasp of concepts and methods such as valency, functional group reactivity, EPF, etc. (Table 2). Errors labeled “illogical” included valence violations, structures that did not follow from curved arrows, and electron movements and reactivity unsupported by the EPF or curriculum. Common specific errors were logged. A few students did not take the final exam, resulting in fewer papers than students.Some attacks drawn on azide and bromine represented a recognized pattern in which one electron-rich species attacks another (Grove et al., 2012a); attack on azide was unique to lecture pedagogy. Illogical errors decreased significantly when alkyl halide SN2 was covered with new pedagogy: students in these groups applied the EPF and assessed reactivity more successfully. Such results are consistent with observations that prior knowledge activation can rectify errors in earlier learning (Chi and Wylie, 2014). No systematic changes were seen in omission of arrows, selection of potential competing processes (SN1, E1, E2) or stereochemical outcomes (data not shown).
The first elementary step of nitration (Fig. 1b) was examined for “illogical” and other errors (Table 3). One group (FTL) experienced new pedagogy during instruction on nitration.
No significant trends were seen in frequency of errors in nitration, possibly reflecting greater difficulty relative to SN2 (i.e., multi-step vs. concerted mechanism).
Mastery of reactivity and regioselectivity in nitration was also assessed (Table 4). Students who showed nitration of any unsubstituted carbon were regarded as grasping reactivity correctly.
While no significant improvement was seen in reactivity predictions, Groups TL and FTL both performed significantly better than Group L in prediction of the correct regio-isomer. This result appears to indicate improvement on a topic peripheral to the mechanism.
Table 5 shows total errors in both final exam problems. Fewer incorrect answers were seen when SN2 was covered with Transfer pedagogy; no change was seen in the percentage of errors in nitration.
Overall, exposure to new pedagogies appeared correlated with equivalent or better mastery of EPF and curricular mechanisms. New pedagogy may also enhance mastery in aspects such as regiochemistry. The latter observation hints at decreased CL, but conclusive analysis on this point was not possible.
Practice exercises
Each exercise (Fig. 2) assessed student skills using the EPF and proposing reasonable mechanisms for unfamiliar reactions. These exercises were given to determine whether new instructional methods supported transfer skills to an extent correlated with activation of prior knowledge (Schwartz et al., 2009; Ashman et al., 2020).The first exercise was based on alcohol SN2 and the second exercise was based on alkyl halide SN2. The alcohol mechanism had been covered with lecture for Group TL and with Transfer pedagogy for Group FTL. The alkyl halide mechanism had been covered with Transfer pedagogy for both groups. Appendix 4 shows scoring rubrics; results are shown in Fig. 3, 4 and Tables 6, 7.
 | ||
| Fig. 3 Practice exercise scoring. Standard errors are shown. (a) First exercise (Fig. 2a); sample sizes were 158 (Group TL) and 129 (Group FTL); (b) second exercise (Fig. 2b); sample sizes were 169 (Group TL) and 129 (Group FTL). | ||
 | ||
| Fig. 4 Difference scores on ES of practice exercises. (a) First exercise (Fig. 2a and 3a); (b) second exercise (Fig. 2b and 3b). | ||
| First practice exercise | Second practice exercise | |||
|---|---|---|---|---|
| ES1 | ES2 | ES1 | ES2 | |
| Group TL | 4.22 (1.1) | 2.42 (1.72) | 2.08 (1.71) | 3.07 (1.16) |
| Group FTL | 4.04 (1.23) | 3.43 (1.53) | 2.90 (1.66) | 3.42 (1.16) |
| First practice exercise | Second practice exercise | |
|---|---|---|
| Group TL | 0.361 (0.383) | −0.198 (0.391) |
| Group FTL | 0.122 (0.301) | −0.102 (0.335) |
No significant difference was seen between groups for the first elementary step (ES1) of the first exercise (t(260) = 1.31, p = 0.38; blue bars, Fig. 3a); however, Group FTL scored on average ∼25% higher than Group TL in the second step (ES2; t(283) = 5.25, p < 0.001; orange bars, Fig. 3a). Stronger performance on ES2 by Group FTL appears to indicate a greater ability to think through the full mechanism, suggesting that students who experienced new pedagogy and/or course structure had stronger transfer skills.
Group FTL scored higher than Group TL on both ES of the second exercise (Fig. 3b). Group FTL scored on average ∼17% higher in ES1 (t(280) = 4.19, p < 0.001; blue bars). A small but significant increase was also observed from TL to FTL on ES2 (t(275) = 2.56, p = 0.022; orange bars). The more substantial difference between groups on the second exercise (despite both groups seeing Transfer pedagogy for the relevant course mechanism) suggests again that new pedagogy and/or course structure provided a stronger foundation for transfer exercises. Table 6 shows means and standard deviations for scores in ES of practice exercises.
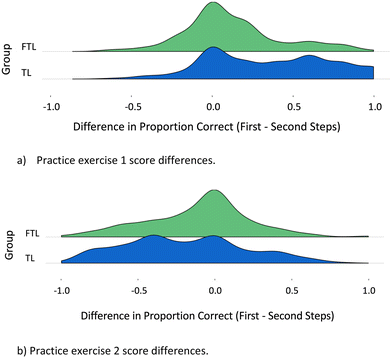
Fig. 4 shows stacked density plots where the horizontal axis represents the difference between each student's ES1 and ES2 scores (as decimal percent). The scale runs from −1 (full credit, second step; zero credit, first step) to 1 (full credit, first step; zero credit, second step). A student scoring the same on both steps appears at zero. Table 7 shows mean and standard deviation for difference scores in Fig. 4.
Group FTL earned the largest percentage of zero difference scores in both exercises; a zero score was the most common result in both exercises only for Group FTL. In the first exercise, the mean difference in scores between ES1 and ES2 was substantially larger for Group TL than for Group FTL (t(295) = 5.9, p < 0.001). The same outcome was seen in the second practice exercise: group TL's mean difference was larger than Group FTL's (t(291) = –2.26, p = 0.049).
Fig. 3 and 4 indicate that Group FTL displayed stronger transfer skills and better ability to work through a two-step mechanism. More students displayed equal proficiency solving both elementary steps in Group FTL than in Group TL. Combination of First-principles and Transfer pedagogies appeared correlated with stronger transfer abilities than Transfer pedagogy. Group FTL's stronger performance after experiencing greater activation of prior knowledge is consistent with past reports (Schwartz et al., 2009; Ashman et al., 2020).
Study groups were not differentiated by meaningful differences in final grade distributions or by percentages of students who withdrew. It was nevertheless necessary to confirm that new pedagogy did not negatively impact student success in OChem2, which was taught in lecture format by instructors who did not participate in the study.
Longitudinal assessment (OChem2)
A comparison of each student's final course grades in OChem 1 and OChem2 is shown in Fig. 5.Fig. 5 shows difference values obtained by assigning numerical values to letter grades (see Statistical analysis). Each student's OChem2 value was subtracted from the student's OChem1 value. A positive value indicates a higher grade in OChem1. The most frequent outcome was for students to earn the same grade in both courses.
Student performance in OChem2 was not significantly associated with pedagogy in OChem1, after accounting for instructor, student level, first generation status, and time between taking OChem1 and OChem2 (F2,1157 = 0.50, p = 0.604; Fig. 5). Group sample sizes range from 142 (Group FTL) to 462 (Group I3).
A significant effect of instructor was observed (F3,1157 = 13.69, p < 0.001). Tukey post hoc comparisons indicate that students in Instructor 1's OChem1 earned between one-third and one-half of a letter grade lower than students from other instructors, among whom no significant differences were observed. Unsurprisingly, increased time between OChem1 and OChem2 had a significantly negative effect on student performance in OChem2. Every additional semester between courses resulted in a decrease of approximately half a letter grade, on average (t = −10.79, p < 0.001).
Longitudinal data indicate that the novel pedagogies used in this study supported equivalent mastery across year-long organic learning goals relative to students in lecture sections.
First generation students (FGS)
Fig. 6 and Table 8 investigate whether new pedagogies impacted FGS outcomes differently from non-FGS. Fig. 6a shows percentages of FGS in each group and in final grade ranges within each group. Fig. 6b shows average grade points earned by FGS and non-FGS, calculated as in Fig. 5. Table 8 lists select statistical parameters for data in Fig. 6b.| Group L | Group TL | Group FTL | |
|---|---|---|---|
| Non-FGS | 2.6 (1.05) | 2.57 (1.02) | 2.5 (0.99) |
| FGS | 2.11 (1.12) | 2.19 (1.15) | 2.55 (0.84) |
Fig. 6a shows that relative to overall percentages of FGS (dotted lines), Groups L and TL had less representation of FGS in A + A/B ranges and more in the F range. For Group FTL, however, upper grade deficits no longer appear: percentages of FGS in grade ranges down to D matched the overall percentage of FGS in the group. FGS earned over half of D grades in Group FTL, but no FGS earned an F.
Fig. 6b and Table 8 show that FGS in Groups L and TL earned lower average grade points than non-FGS (t(208) = 3.83, p = 0.001 and t(91) = 2.07, p = 0.041, respectively). The average grade point of FGS in Group FTL, by contrast, was not different from that of non-FGS (t(112) = −0.32, p = 0.747). For Group FTL alone, first generation status had no relation to grade earned.
All sections in the study had the same syllabus grade cutoffs; end-of-semester variation in cutoffs is reflected in Table 9.
| Letter grades | A (%) | A/B (%) | B (%) | B/C (%) | C (%) | D (%) |
|---|---|---|---|---|---|---|
| Syllabus cutoff | 92 | 89 | 81 | 78 | 70 | 60 |
| Average adjusted cutoff | 91.1 | 88.0 | 80.4 | 77.1 | 69.4 | 58.9 |
| Standard deviation | 1.08 | 1.02 | 0.66 | 0.92 | 0.62 | 2.65 |
FGS data suggest that combined First-principles and Transfer pedagogies may promote improvement in FGS grade outcomes. The pedagogy described herein may thus provide a path to enhance grade equity for first semester organic chemistry students.
Conclusions
Instructional approaches that coach students to build reaction mechanism proposals in the classroom have not been reported for a first semester organic course. Positive results seen in this study add to evidence in favor of generative learning practices, even for highly complex content (deWinstanley and Bjork, 2002). Transfer and First-principles approaches are logistically viable in large lecture settings. The approaches do not require instruction beyond course hours, significant reduction of content or graduate assistant involvement and were obtained by modification of only ∼15% of the curriculum.Cognitive load has received limited formal attention in organic chemistry instruction. Notwithstanding the absence of direct measures of CL, we posit that excessive CL in traditional mechanism instruction, likely due to high element interactivity, is a significant obstacle for novice learners. The instructional designs in this report targeted improved learning outcomes for novice learners consistent with recent research on instruction of complex content, including use of assisted discovery and isolation of elements that would otherwise interact (Pollock et al., 2002; Van Merriënboer et al., 2006; Alfieri et al., 2011; Kalyuga and Singh, 2016; Lu et al., 2020). Modification of instructional design according to principles that manage CL holds potential to deliver improved learning on one of the most stubbornly challenging topics in first semester organic chemistry.
This study prompted students to activate earlier learning to create models of reactive behavior and engage in sense-making in the classroom; such activities are recommended as paths to deeper learning than lecture can provide (Yan and Talanquer, 2015; Meek et al., 2016; Bisra et al., 2018). We posit that this type of design for reaction mechanism instruction can support schema generation more effectively than lecture (Kalyuga and Singh, 2016). Results also align with reports showing greater learning gains when students try to explain a phenomenon than when students hear an explanation (Schwartz et al., 2011; Bisra et al., 2018).
With respect to research questions posed earlier, study results provide preliminary but promising evidence. Use of new instructional approaches was correlated with reductions in some error types, stronger EPF skills and equivalent or greater abilities to reproduce curricular mechanisms. These results suggest that new pedagogies may cultivate representation and recall skills more effectively than lecture. Transfer pedagogy is faster and may thus boost representational skills without increasing time spent on mechanism coverage. A reduction in errors when students activated prior knowledge to propose a second SN2 mechanism is consistent with synergistic correction of errors in earlier learning (Chi and Wylie, 2014). Increased mastery of a tangential topic (regiochemistry) was also seen in both groups that experienced non-lecture pedagogy.
On the question of how students’ transfer abilities were affected by non-lecture pedagogies, exposure to larger amounts of non-lecture pedagogy, including First-principles instruction, was correlated with greater ability to transfer mechanistic concepts effectively to new reactions. This observation is consistent with reported correlations between prior knowledge activation and enhanced learning and transfer abilities (Schwartz et al., 2009; Kalyuga and Singh, 2016; Ashman et al., 2020).
No study results indicated worse learning outcomes. New pedagogies appeared grade-neutral in the second semester course.
Concerning the impact of new pedagogies on organic chemistry students with diverse backgrounds, these results are believed to be a first report of both outcome deficits and instructional design to resolve observed deficits for first-generation students (Lovecchio and Dundes, 2002; Barr et al., 2008; Sweeder et al., 2023). Gains observed for this population may offer additional evidence of the benefits of scaffolded learning for the most challenged or least-resourced students (Kranz et al., 2023).
Taken together, results from the study suggest that the new approaches may help decrease putative cognitive overload experienced during traditional mechanism instruction, increase equity in learning outcomes and support students more effectively than lecture in starting to build a schema for reaction mechanisms.
Implications
Our results recommend instructional designs for novice students that attempt to minimize cognitive overload, activate prior knowledge and provide opportunities for students to construct a schema for reaction mechanisms. Results from lecture settings provide ample evidence that merely hearing about an instructor's knowledge framework is ineffective for many students (Bransford et al., 2000), while activities such as case comparisons can support development of student reasoning skills more effectively (Caspari et al., 2018; Graulich and Caspari, 2021; Haas et al., 2024). An ideal scenario may offer students opportunities to link prior knowledge resources into multiple mechanisms, since mechanisms require non-algorithmic problem-solving (Graulich and Caspari, 2021). Moreover, a skill learned in multiple contexts is more likely to be transferred to new situations (Bransford et al., 2000).The potential for broader utility of generative pedagogy in organic chemistry can be assessed with other student populations. For example, higher-skilled students can experience decreased benefit from some instructional designs due to “expertise reversal” (Kalyuga and Singh, 2016). Increased equity in outcomes for first generation college students in Group FTL also recommends further investigation. It is vital to ascertain whether other under-resourced student populations can experience similar benefits from generative pedagogy (Bettencourt et al., 2020; Sweeder et al., 2023).
Direct or indirect assessment of cognitive load experienced during mechanism study, retrieval practice and transfer exercises is also of significant interest. Comparison of the perceived task difficulty of problem solving by students who experienced lecture or generative pedagogy may be informative. Such studies may help confirm to what extent element interactivity contributes to learning challenges and whether generative pedagogies modulate the CL experienced during instruction. Exploration of the maturity or complexity of students’ schema for reaction mechanisms following different types of instruction may likewise provide insights on student cognitive processes during mechanism study. Practitioners are encouraged to adapt these methods to characterize student skill development and cognitive aspects of organic chemistry instruction in greater detail. Promising results from this initial test of generative approaches to mechanism instruction for novice students suggest that additional improvements are achievable. Further consideration of learning goals and sequencing to precede full schema development may deliver additional benefits (Kalyuga and Singh, 2016).
Limitations
Differential effects of pedagogies cannot be assessed conclusively because no group saw only First-principles pedagogy, groups saw different numbers of mechanisms and experienced different percentages of lecture instruction. Differences in assessment structure and technology support pose additional obstacles to direct comparisons of methods. This report is offered as a preliminary perspective on the nexus between cognitive load and organic chemistry instructional design.The approaches were designed for introduction of reaction mechanisms to novice students. The potential for adaptation of the tools to cultivate mature causal mechanistic reasoning is beyond the scope of this report. Viable approaches to develop such skills have been reported elsewhere (Caspari and Graulich, 2019; Graulich and Caspari, 2021).
Transfer and First-principles pedagogies may not be suitable for every mechanism. Transfer pedagogy must be preceded by a conceptually similar mechanism. Greater time commitment for First-principles pedagogy may preclude its frequent use.
In First-principles pedagogy, care is needed to deconstruct a mechanism into tasks accessible to novice students, sequence tasks for greatest clarity and select cueing questions. Consideration of student skill levels and learning goals (content mastery vs. skill acquisition) can guide instructors to calibrate instruction to the selected goals (Kalyuga and Singh, 2016).
The corresponding author can provide templates of instructional materials used in this study.
Author contributions
Connor Haindfield: formal analysis, writing – review and editing. William Cerbin: investigation, methodology, resources, writing – review and editing. Douglas Baumann: formal analysis, methodology, writing – review and editing. Heather Schenck: conceptualization, formal analysis, methodology, project administration, writing – drafting.Data availability
All data generated or analyzed during this study are included in this article.Conflicts of interest
There are no conflicts to declare.Appendices
Appendix 1
Semester structure for study groups. Each cell = three class days (14 weeks). Symbol placement indicates day 1 (left), day 2 (middle) or day 3 (right). Where timing varied, a box spans the range. Final exams occurred 2–7 days after the fourth exam.Appendix 2
Pedagogy details.| Transfer | |
|---|---|
| Instructional activities | Student activities |
| Tell students to propose a new mechanism or process using analogous reasoning | Recall/review earlier mechanism, draw |
| Coach on chemical points of difference | Edit or correct as needed |
| Draw mechanism | Check work |
| First-principles | |
|---|---|
| Instructional activities | Student activities |
| Tell students to identify reaction processes: bonds formed and broken | Write down analysis; volunteer answers |
| Prompt for each bond broken: | |
| Which way will electrons move? | Point |
| Why? | Identify a relevant chemical concept (electronegativity) and how the concept plays out |
| Draw starting material, curved arrow and “products” | Draw |
| Draw process | Check work |
| Assess reasonableness; remind or share new information (e.g., carbocations, pKas) | Identify concepts implicated in non-viable outcomes |
| Prompt for each bond formed: | |
| What is the most logical source of electrons? | Differentiate between nucleophiles if needed |
| Draw starting materials, curved arrow and “product” | Draw |
| Draw process | Check work |
| Assess reasonableness | Identify concepts implicated in non-viable outcomes |
| Reiterate experimental data (elementary steps, etc.) | |
| Discuss combining and sequencing processes to resolve unreasonableness | Draw elementary steps |
| Is each step reasonable and consistent with experimental data? | Identify concepts or experimental evidence implicated in non-viable outcomes |
| Apply needed corrections (e.g., resequencing) | Edit or correct |
| Draw mechanism | Check work |
Appendix 3
Final exam rubrics with point values. In Appendices 3 and 4, higher point values indicate where partial credit was possible.| SN2 | Nitration |
|---|---|
| ⊖N3 attacks carbon (2) | Ring π attack (2) |
| Br⊖ ejected (2) | Attack on nitrogen (1) |
| Product (2) | N–O π bond broken to O (2) |
| Intermediate (1) |
Appendix 4
Practice exercise rubrics with point values; ES = elementary step.Acknowledgements
The authors gratefully acknowledge funding and mentorship from the Learning by Design program offered by the Center for Advancing Teaching and Learning as well as insightful comments from Dr Scott Cooper, both at the University of Wisconsin – La Crosse. Valuable suggestions from Dr Ryan Stowe (University of Wisconsin – Madison) are also gratefully acknowledged.References
- Aldor-Noiman S., Brown L. D., Buja A., Rolke W. and Stine R. A., (2013), The power to see: a new graphical test of normality, Am. Stat., 67(4), 249–260.
- Alfieri L., Brooks, P. J., Aldrich N. J. and Tenenbaum, H. R., (2011), Does Discovery-Based Instruction Enhance Learning? J. Educ. Psychol., 103(1), 1–18.
- Ashman G., Kalyuga S. and Sweller J., (2020), Problem-solving or explicit instruction: which should go first when element interactivity is high? Ed. Psych. Rev., 32, 229–247.
- Asmussen G., Rodemer M. and Bernhold S., (2023), Blooming student difficulties in dealing with organic reaction mechanisms – an attempt at systematization, Chem. Educ. Res. Pract., 24, 1035–1054.
- Barr D. A., Gonzalez M. E. and Wanat S. F., (2008), The leaky pipeline: factors associated with early decline in interest in premedical studies among underrepresented minority undergraduate students, Acad. Med., 83(5), 503–511.
- Bettencourt G. M., Manly C. A., Kimball E. and Wells R. S., (2020), STEM degree completion and first-generation college students: a cumulative disadvantage approach to the outcomes gap, Rev. High. Ed. 43(3), 753–779.
- Bhattacharyya G., (2013), From source to sink: Mechanistic reasoning using the electron-pushing formalism, J. Chem. Educ., 90(10), 1282–1289.
- Bhattacharyya G., (2019), Construction by deconstruction, J. Chem. Educ., 96(7), 1294–1297.
- Bhattacharyya G. and Bodner G. M., (2005), “It gets me to the product”: How students propose organic mechanisms, J. Chem. Educ., 82(9), 1402–1407.
- Bisra K., Liu Q., Nesbit J. C., Salimi F. and Winne P. H., (2018), Inducing self-explanation: a meta-analysis, Educ. Psychol. Rev., 30, 703–725.
- Bodé N. E., Deng, J. M. and Flynn A. B., (2019), Getting past the rules and to the WHY: causal mechanistic arguments when judging the plausibility of organic reaction mechanisms, J. Chem. Educ., 96(6), 1068–1082.
- Bongers A., Beauvoir B., Streja N., Northoff G. and Flynn A. B., (2020), Building mental models of a reaction mechanism: the influence of static and animated representations, prior knowledge, and spatial ability, Chem. Educ. Res. Pract., 21, 496–512.
- Bongers A., Northoff G. and Flynn A. B., (2019), Working with mental models to learn and visualize a new reaction mechanism, Chem. Educ. Res. Pract., 20, 554–569.
- Bransford, J. D., Brown, A. L., and Cocking, R. R., (2000), How people learn, Washington, DC: National Academy Press.
- Carle M. S., Visser R. and Flynn A. B., (2020), Evaluating students’ learning gains, strategies, and errors using OrgChem101's module: organic mechanisms – mastering the arrows, Chem. Educ. Res. Pract., 21, 582–596.
- Caspari I. and Graulich N., (2019), Scaffolding the structure of organic chemistry students’ multivariate comparative mechanistic reasoning, Int. J. Phys. Chem. Educ., 11(2), 31–43.
- Caspari I., Kranz D. and Graulich N., (2018), Resolving the complexity of organic chemistry students’ reasoning through the lens of a mechanistic framework, Chem. Educ. Res. Pract., 19, 1117–1141.
- Chew S. L. and Cerbin W. J., (2021), The cognitive challenges of effective teaching, J. Econ. Educ., 52(1), 17–40.
- Chi M. T. H. and Wylie R., (2014), The ICAP framework: linking cognitive engagement to active learning outcomes, Educ. Psychol., 49(4), 219–243.
- Conway C. J., (2014), Effects of guided inquiry versus lecture instruction on final grade distribution in a one-semester organic and biochemistry course, J. Chem. Educ., 91(4), 480–483.
- Cooper M. M., Posey L. A. and Underwood S. M., (2017) Core ideas and topics: building up or drilling down? J. Chem. Educ., 94(5), 541–548.
- Cooper M. M., Stowe R. L., Crandell O. M., Klymkowsky M. W., (2019), Organic chemistry, life, the universe and everything (OCLUE): a transformed organic chemistry curriculum, J. Chem. Educ., 96(9), 1858–1872.
- deWinstanley P. A. and Bjork R. A., (2002), Successful Lecturing: presenting information in ways that engage effective processing, in D. F. Halpern and M. D. Hakel (ed.), New Directions for Teaching and Learning, San Francisco: Jossey-Bass, vol. 89, Applying the Science of Learning to University Teaching and Beyond, pp. 19–31.
- Dood A. J. and Watts F. M., (2022), Mechanistic reasoning in organic chemistry: a scoping review of how students describe and explain mechanisms in the chemistry education research literature, J. Chem. Educ., 99(8) 2864–2876.
- Dood A. J. and Watts F. M., (2023), Students’ strategies, struggles, and successes with mechanism problem solving in organic chemistry: a scoping review of the research literature, J. Chem. Educ., 100(1), 53–68.
- Eilks I. and Byers B., (2010), The need for innovative methods of teaching and learning chemistry in higher education – reflections from a project of the European Chemistry Thematic Network, Chem. Educ. Res. Pract., 11, 233–240.
- Farhat N. J., Stanford C. and Ruder S. M., (2019), Assessment of student performance on core concepts in organic chemistry, J. Chem. Educ., 96(5), 865–872.
- Ferguson R. and Bodner G. M., (2008), Making sense of the arrow-pushing formalism among chemistry majors enrolled in organic chemistry, Chem. Educ. Res. Pract., 9, 102–113.
- Finkenstaedt-Quinn S. A., Watts F. M., Petterson M. N., Archer S. R., Snyder-White E. P. and Shultz G. V., (2020), Exploring student thinking about addition reactions, J. Chem. Educ., 97(7), 1852–1862.
- Flynn A. B., (2012), Development of an online, postclass question method and its integration with teaching strategies, J. Chem. Educ., 89(4), 456–464.
- Flynn A. B. and Ogilvie W. W., (2015), Mechanisms before reactions: a mechanistic approach to the organic chemistry curriculum based on patterns of electron flow, J. Chem. Educ., 92(5), 803–810.
- Frost S. J. H., Yik B. J., Dood A. J., Cruz-Ramirez de Arellano D., Fields K. B. and Raker J. R., (2023), Evaluating electrophile and nucleophile understanding: a large-scale study of learners’ explanations of reaction mechanisms, Chem. Educ. Res. Pract., 24, 706–722.
- Galloway K. R., Stoyanovich C. and Flynn A. B., (2017), Students’ interpretations of mechanistic language in organic chemistry before learning reactions, Chem. Educ. Res. Pract., 18, 353–374.
- Gao S., Outlaw T. C., Liang-Lin J. G., Feng A., Shimomura R., Roizen J. L. and Cox, Jr. C. T., (2024), Analysis of resources applied to rationalize elimination mechanisms, Chem. Educ. Res. Pract., 25, 62–78.
- Gick M. L. and Holyoak K. J., (1980), Analogal problem solving, Cogn. Psychol., 12(3), 306–355.
- Graulich N., (2015), Intuitive judgments govern students’ answering patterns in multiple-choice exercises in organic chemistry, J. Chem. Educ., 92(2), 205–211.
- Graulich N. and Caspari I., (2021), Designing a scaffold for mechanistic reasoning in organic chemistry, Chem. Teach. Int., 3(1), 19–30.
- Grove N. P., Cooper, M. M. and Rush K. M., (2012a), Decorating with arrows: toward the development of representational competence in organic chemistry, J. Chem. Ed., 89(7), 844–849.
- Grove N. P., Cooper, M. M. and Cox E. L., (2012b), Does mechanistic thinking improve student success in organic chemistry? J. Chem. Ed., 89(7), 850–853.
- Haas D. B., Watts F. M., Dood A. J. and Shultz G. V., (2024), Analysis of organic chemistry students’ developing reasoning elicited by a scaffolded case comparison activity, Chem. Educ. Res. Pract., 25, 742–759.
- Hein S. M., (2012), Positive impacts using POGIL in organic chemistry, J. Chem. Educ., 89(7), 860–864.
- Kalyuga S. and Singh A.-M., (2016), Rethinking the boundaries of cognitive load theory in complex learning, Educ. Psychol. Rev., 28, 831–852.
- Karty J. M., Gooch G. and Bowman B. G., (2007), Teaching a modified Hendrickson, Cram, and Hammond curriculum in organic chemistry, J. Chem. Educ., 84(7), 1209–1216.
- Kraft A., Strickland A. M. and Bhattacharyya G., (2010), Reasonable reasoning: multi-variate problem-solving in organic chemistry, Chem. Educ. Res. Pract., 11, 281–292.
- Kranz D., Schween M. and Graulich N., (2023), Patterns of reasoning – exploring the interplay of students’ work with a scaffold and their conceptual knowledge in organic chemistry, Chem. Educ. Res. Pract., 24, 453–477.
- Lieber L. S., Krenare I., Caspari-Gnann I. and Graulich N., (2022), Closing the gap of organic chemistry students’ performance with an adaptive scaffold for argumentation patterns, Chem. Educ. Res. Pract., 23, 811–828.
- Lipton M. A., (2020), Reorganization of the organic chemistry curriculum to improve student outcomes, J. Chem. Educ., 97(4), 960–964.
- Lovecchio K. and Dundes L., (2002), Premed survival: understanding the culling process in premedical undergraduate education, Acad. Med., 77(7), 719–724.
- Lu J., Kalyuga S. and Sweller J., (2020), Altering element interactivity and variability in example-practice sequences to enhance learning to write Chinese characters, Appl. Cognit. Psychol., 34, 837–843.
- Meek S. J., Pitman C. L. and Miller A. J. M., (2016), Deducing reaction mechanism: a guide for students, researchers and instructors, J. Chem. Educ., 93(2), 275–286.
- Mooring S. R., Mitchell C. E. and Burrows N. L., (2016), Evaluation of a flipped, large-enrollment organic chemistry course on student attitude and achievement, J. Chem. Educ., 93(12), 1972–1983.
- National Research Council, (2012), A framework for K-12 science education: practices, crosscutting concepts, and core ideas, Washington, DC: The National Academies Press.
- Noyes K. and Cooper M. M., (2019), Investigating student understanding of London dispersion forces: a longitudinal study, J. Chem. Educ., 96(9), 1821–1832.
- Penn J. H. and Al-Shammari A. G., (2008), Teaching reaction mechanisms using the curved arrow neglect (CAN) method, J. Chem. Educ., 85(9), 1291–1295.
- Pollock E., Chandler P. and Sweller J., (2002), Assimilating complex information, Learn. Instr., 12, 61–86.
- Ravishankar L., Ladage S. and Shridhar G., (2013), Exciting undergraduates toward organic chemistry – the study circle approach, Curr. Sci., 105(9), 1227–1229.
- Schwartz D. L., Chase C. C., Oppezzo M. A. and Chin, D. B., (2011), Practicing versus inventing with contrasting cases: the effects of telling first on learning and transfer, J. Educ. Psychol., 103(4), 759–775.
- Schwartz D. L., Lindgren R. and Lewis S., (2009), Constructivism in an age of non-constructivist assessments, in S. Tobias and T. Duffy (ed.), Constructivist instruction: Success or Failure? New York, NY: Routledge, pp. 34–61.
- Shattuck J. C., (2016), A parallel controlled study of the effectiveness of a partially flipped organic chemistry course on student performance, perceptions and course completion, J. Chem. Educ., 93(12), 1984–1992.
- Sweeder R. D., Herrington D. G. and Crandell O. M., (2023), Chemistry education research at a crossroads: Where do we need to go now? J. Chem. Educ., 100, 1710–1715.
- Sweller J., (1994), Cognitive load theory, learning difficulty and instructional design, Learn. Instr.,4(4), 295–312.
- Van Merriënboer, J. J. G., Kester L. and Paas, F., (2006), Teaching complex rather than simple tasks: balancing intrinsic and germane load to enhance transfer of learning, Appl. Cognit. Psychol., 20, 343–352.
- Villalta-Cerdas A. and Sandi-Urena S., (2014), Self-explaining effect in general chemistry instruction: eliciting overt categorical behaviours by design, Chem. Educ. Res. Pract., 15(4), 530–540.
- Watts F. M., Park G. Y., Petterson M. N. and Shultz G. V., (2022), Considering alternative reaction mechanisms: students’ use of multiple representations to reason about mechanisms for a writing-to-learn assignment, Chem. Educ. Res. Pract., 23, 486–507.
- Webber D. M. and Flynn A. B., (2018), How are students solving familiar and unfamiliar organic chemistry mechanism questions in a new curriculum? J. Chem. Educ., 95(9), 1451–1467.
- Weinrich M. L. and Sevian H., (2017), Capturing students’ abstraction while solving organic reaction mechanism problems across a semester, Chem. Educ. Res. Pract., 18, 169–190.
- Wilson R. E. and Kittleson J., (2013), Science as a classed and gendered endeavor: persistence of two white female first-generation college students within an undergraduate science context, J. Res. Sci. Teach., 50(7), 802–825.
- Wilson S. B. and Varma-Nelson P., (2019), Characterization of first-semester organic chemistry peer-led team learning and cyber peer-led team learning students’ use and explanation of electron-pushing formalism, J. Chem. Educ., 96(1), 25–34.
- Yan F. and Talanquer V., (2015), Students’ ideas about how and why chemical reactions happen: mapping the conceptual landscape, Int. J. Sci. Educ., 37(18), 3066–3092.
- Zoller U., (1999), Scaling-up of higher-order cognitive skills-oriented college chemistry teaching: an action-oriented research, J. Res. Sci. Teach., 36(5), 583–596.
| This journal is © The Royal Society of Chemistry 2024 |