Using a combination technique for the assessment of students’ cognitive structures on acid–base chemistry
Ayşegül
Derman
 *a,
Figen
Gunes
*a,
Figen
Gunes
 b,
Ozcan
Gulacar
b,
Ozcan
Gulacar
 c and
Ingo
Eilks
c and
Ingo
Eilks
 de
de
aDepartment of Elementary Science and Math Education, Ahmet Keleşoğlu Education Faculty, Necmettin Erbakan University, Konya, Turkey. E-mail: aderman1977@gmail.com
bEducational Sciences Institute, Necmettin Erbakan University, Meram, Turkey. E-mail: figengunes88@gmail.com
cDepartment of Chemistry, University of California, Davis, CA, USA. E-mail: ogulacar@ucdavis.edu
dDepartment of Biology and Chemistry, Institute for Science Education, University of Bremen, Bremen, Germany. E-mail: ingo.eilks@uni-bremen.de
eFaculty of Mathematics and Natural Sciences, Universitas Negeri Malang, Malang, Indonesia
First published on 5th December 2023
Abstract
This study aims to determine the cognitive structures of students at different educational levels (8th grade and 12th grade) related to acid–base chemistry. The research was designed as a case study and structured in two stages. The first stage analyzed concepts related to acid–base chemistry and their direction and strength in students’ knowledge structures. The second stage determined the descriptive and structural features of students’ knowledge structures related to acid–base chemistry in a more holistic approach. The study was carried out with a total of 160 students, 80 grade 8th and 80 12th grade students. A word association test (WAT) and the free writing technique (FWT) were used together. In the WAT, ten different frequency ranges were determined forming cognitive structure maps of the students. With high-frequency values on the map, it was found that the number of stimulus and response words decreased but the strength of associations increased. In frequency ranges where the frequency values of associations were low, it was found that the number of stimulus and response words increased and the cognitive structure organization was at the most advanced level compared to other frequency ranges, but the strength of associations was weak. In general, it was observed that there were no bidirectional and cross-associations between the concepts in the cognitive structures of the students about chemistry and that there was a static structure that included one-way associations only. Additionally, the concepts in the cognitive structures of students related to acid–base chemistry were analyzed in terms of their structural characteristics. It was found that, in the cognitive structures of the students there were no associations between many concepts that should be related to each other.
Introduction
The content of chemistry is perceived by many students as abstract, difficult, and theoretical (Ebenezer, 2001), especially when it comes to understanding chemical bonding theory (Othman et al., 2008; Levy Nahum et al., 2010) or chemical phenomena at the particulate level of matter (Liu and Lesniak, 2005; Adadan et al., 2010; Eilks, 2013). The literature suggests that students must develop basic knowledge and abstract thinking skills to comprehend chemistry on a theoretical level (Blake and Norland, 1978). Without proper basic knowledge and abstract thinking skills, it is difficult for students to understand advanced chemistry topics such as solution chemistry, acid–base chemistry, electrochemistry, or chemical equilibrium (Hewson and Hewson, 1983; Calık et al., 2005; Uzuntiryaki and Geban, 2005; Adadan and Savasci, 2012). In the case of acid–base chemistry, previous studies have revealed that students have difficulty comprehending the behaviour of acids and bases on a theoretical level and dealing appropriately with related models (Artdej et al., 2011; Cartrette and Mayo, 2011; Tümay, 2016). Some of these challenges determined in students’ learning and applications could be better understood if their cognitive structures are revealed. There have been numerous studies indicating the close interactions between the level of conceptual understanding and cognitive structures (Nakiboğlu, 2008). Although there is a considerable number of studies that investigated students’ cognitive structures concerning different subjects including biology (Sikumbang et al., 2019), mathematics (Yang et al., 2018), and physics (de Jong and Ferguson-Hessler, 1986), the studies on how general chemistry concepts, particularly acid–base chemistry terms, are linked to each other and how they form students’ cognitive structures are still limited.The purpose of this study is to contribute to the existing literature by gaining a deeper understanding of students’ cognitive structures related to acid–base chemistry through the combination of a word associations test (WAT) and the free-writing technique (FWT). The study focuses on the following research question: what are the descriptive and structural features of the 8th- and 12th-grade students’ cognitive structures related to acid–base chemistry?
Background and framework
Students’ knowledge, epistemology, and ability levels influence their learning (Tyson et al., 1997). According to constructivist learning theory, the development of concepts and cognitive structures is influenced by life experience and prior knowledge (Ausubel, 1978 cited in Wilson, 1990). The cognitive structures of the individual influence interaction with new information and how connections within the domain of knowledge are formed. Tsai (2001) claims that students’ cognitive structures acquired from science studies are stored hierarchically and represented in the long-term memory.Even though the term “cognitive structure” does not have a single accepted definition and little information is known about how they develop (Taber, 2008), the term is still used in numerous studies for a long time now (e.g., West and Pines, 1985; West et al., 1985; White, 1985). Cognitive structures are described by different interpretations and terms, such as structural knowledge (Jonassen et al., 1993 cited in Liu and Ebenezer, 2002) or knowledge structures (Gulacar et al., 2022). Structural knowledge is described as how information and concepts in a domain are related to each other (Liu and Ebenezer, 2002). Likewise, a content-specific cognitive structure dealing with the essential elements of students’ conceptual understanding is named structural knowledge (Nakiboğlu, 2008). Both understandings are very near to each other so we decided to use the term cognitive structure in an understanding of how information is stored in the long-term memory and how different information and concepts are related to each other in a certain domain. All these understandings are clearly to be distinguished from declarative knowledge which is understood as a set of isolated information in the memory.
There is a great variety of data collection instruments that have been used in studies about understanding knowledge and understanding acid–base chemistry. The most widely preferred instruments concerning views/opinions, attitudes, and conceptualizations are concept tests as a cognitive measure (Bradley and Mosimege, 1998), interviews (Drechsler and van Driel, 2008; Kala et al., 2013), and attitude or aptitude scales (Kılavuz, 2005).
In the past, studies about students’ understanding of acid–base chemistry focused on problems assessing students’ understanding of acid–base reactions at the submicroscopic level (Drechsler and Schmidt, 2005a) and the use of different acid–base models (Carr, 1984; Atrdej et al., 2011; Tumay, 2016). According to Cartrette and Mayo (2011) and Kousathana et al. (2005), the Brønsted-Lowry model is preferred by students in defining acids and bases, but the Lewis model often is not used properly to describe them. Therefore, Hawkes (1992) suggests that the Brønsted-Lowry model is given priority as students have difficulties in using the Arrhenius model. Hawkes (1992) also argues that the Brønsted–Lowry model, which describes the proton transfer, might be understood clearly while the Lewis model can cause various types of challenges as it includes concepts like electrophilicity and nucleophilicity. Although some other studies (Tümay, 2016) indicate that some students prefer to use the Arrhenius model to classify substances as acids and bases, they encounter problems in differentiating the Arrhenius and Brønsted–Lowry models (Artdej et al., 2011).
In another study, Sheppard (1997) claimed that telling the difference between acid–base models provides difficulty for high school students even when they have extensive discussions on acid–base chemistry. Students could comprehend the scope of chemistry in terms of these models but they have difficulty in understanding the reason why various models are used to explain acid–base reactions (Drechsler and Schmidt, 2005a). One of the possible reasons behind students’ challenges is that teachers are not efficient at differentiating the various acid–base models in their classes (Drechsler and Schmidt, 2005b). But, it is not only the teachers. Drechsler and Schmidt (2005b) examined chemistry textbooks and revealed deficiencies in clarifying the presence of different acid–base models. Carr (1984) also argued that the reason why students usually apply the Arrhenius and Brønsted–Lowry models incorrectly is the fact that these two models are not clearly distinguished in high school and university textbooks.
Other foci in the research on acids and bases were the understanding of bonding theory or the role of protons in acid–base chemistry (Tarhan and Acar Sesen, 2012), the difference between the concentration and strength of acids, alternative conceptions on acids and bases and how to overcome them (Demircioğlu et al., 2005), or any progression in understanding acid–base chemistry (Wilson, 1998).
Overall, there is much literature about students’ difficulties with understanding acid–base chemistry. Much less is known about how the variations in students’ cognitive structures of acid–base chemistry that comprise the focus of this study.
What makes this study specific and significant is that it will present holistic information in a cross-level design by focusing on the concepts in the cognitive structures of students about acid–base chemistry and the structural elements of the relationship between these concepts on two different educational levels, namely 8th grade and 12th grade.
Sample and method
Sample
In Turkey, science courses are taught from 4th to 8th grade by introducing topics from all branches of science; chemistry, biology, earth science, and physics. Acid–base chemistry is first taught in grade 8. The approach at this level is mainly phenomenological. The science education standards suggest learning about acids, bases, pH, acid rain, acidity and alkalinity of substances, and the effects of acids and bases on various materials. Starting in grade 9, chemistry is taught as a self-standing subject throughout the upper secondary level. In grade 10, a unit on acids, bases, and salts focuses, among others, on the concepts of amphoteric metals, acids, bases, indicators, neutralization, pH/pOH, and salt. In grade 11, the standards suggest teaching balancing chemical equations that cover, among others, conjugate acid–base pairs, Brønsted-Lowry acid–base theory, precipitation reaction, solubility product, equivalence point, indicator, chemical equilibrium and its expressions, strong acid/base, Le Chatelier's principle, auto-ionization, pH/pOH, buffer solution, titration, and weak acids/bases and their equilibrium constants.80 students from each grade level, 8 and 12, studying in urban secondary schools located in Eastern Anatolia region in Turkey participated in the study. Data were gathered, during the spring semester, in the academic year of 2018–2019. Tables 1 and 2 provide the basic demographic data for 8th and 12th graders, respectively. The average age of the 8th graders was 13.6, and the gender distribution was 61.25% female and 38.75% male. The mean age of the participants from 12th grade was 17.45 of which 62.5% were female and 37.5% were male. All 12th-grade students participating in the study were students indicated that they plan to major in science when they attend the university.
| f | % | ||
|---|---|---|---|
| Gender | Female | 49 | 61.25 |
| Male | 31 | 38.75 | |
| Total | 80 | 100 | |
| Age | 13 | 34 | 42.5 |
| 14 | 44 | 55 | |
| 15 | 2 | 2.5 | |
| Total | 80 | 100 | |
| f | % | ||
|---|---|---|---|
| Gender | Female | 50 | 62.5 |
| Male | 30 | 37.5 | |
| Total | 80 | 100 | |
| Age | 17 | 48 | 60 |
| 18 | 28 | 35 | |
| 19 | 4 | 5 | |
| Total | 80 | 100 | |
Required permissions were obtained before starting the data collection. All participants were made aware of the study's purpose and methodology. They all consented to take part in the study voluntarily.
Data collection
The research is a case study as it descriptively and structurally discusses the cognitive structures of 8th- and 12th-grade students related to acid–base chemistry and explains the events that are assumed to be causal connections (Yin, 1992).Several methods have been suggested to investigate the cognitive structures in students’ knowledge. One of them is using word association tests (WAT) (Lee, 1986; 1988). This study operates a WAT (Johnson, 1967, 1969) to identify the relationships between students’ knowledge in terms of cognitive structures (Shavelson, 1972; 1974; Bahar et al., 1999; Bahar and Hansell, 2000; Nakiboğlu, 2008; Schizas et al., 2013; Derman and Eilks, 2016).
Although WATs are effective in determining the concepts in cognitive structures and their frequency, power, and direction, Nakiboğlu (2008) and other previous studies (Derman and Eilks, 2016) suggested that a WAT should be used in combination with techniques such as concept mapping or the free writing technique (FWT). This is done to cope with the limitations of WATs by emphasizing the nature of the inter-conceptual relations in the cognitive structure or the limitations it has in determining the structural elements of the cognitive structure. FWT aims to obtain data about hidden thoughts, understandings, and attitudes about concepts. Using FWT allows the determination of, students’ knowledge and misconceptions about any subject area can be determined (White and Gunstone, 2000). Bahar and Hansell (2000) suggest: “It is assumed that the answers given by the student from his/her long-term memory determine the connections between concepts in his/her cognitive structure and show semantic closeness” (p. 353). From this point of view, WAT and FWT were used in combination to increase the reliability of the study as it provides triangulation in terms of data collection.
In this study, the researchers interviewed two experts from the field of analytical chemistry and chemistry education to identify key stimulus words related to acid–base chemistry applicable to grades 8 and 12 to construct the Word Association Test and obtain students’ cognitive structures on this topic. After obtaining the initial list of the key terms, they were checked in the lower and upper secondary science chemistry textbooks and their suitability for the student populations was assured. The final list and the WAT included eleven terms: acid, base, acidity, alkalinity, salt, neutralization, indicator, pH, buffer solution, amphoteric type, and conjugate acid–base pair.
A twelve-page booklet with WAT and FWT sections was created. Each of the 11 stimulus words was written on a separate page. Each student received a booklet, each page provided one of the stimulus words (written each ten times for making associations) (Fig. 1). After the booklets were given to the participants, they were asked to write as many relevant terms as they could associate with the stimulus words. The reason why all stimulus words are written on a single page with enough space around them to write down any thought is to avoid the chain reaction effect that can occur if someone is distracted by impractical information. According to the related literature (Gunstone, 1980; Nakiboğlu, 2008; Ayas, 2011), approximately 70 seconds per item were allotted to each student. Overall, the student had 15 minutes to finish the WAT section in the booklet.
For the FWT section, the twelfth page of the booklet was used. Here, students were asked to write a detailed paragraph using the concepts of “acid, base, acidity, alkalinity, salt, neutralization, indicator, pH, buffer solution, amphoteric type, conjugate acid–base pair”, to reveal further information on the relationship between these concepts. For the students to complete the FWT, they were given 30 minutes.
FWT was used to allow students to put thoughts into connection (Fig. 2). The students were asked to write their thoughts according to their style and to create a unique structure for their writing (Bahar et al., 1999).
Data analysis and reliability measures
Data acquired from the WAT were analyzed with the response frequencies’ map method (Nakiboğlu, 2008; Derman and Eilks, 2016). This method combines response frequencies (Bahar et al., 1999) and relatedness coefficients (Gussarsky and Gorodetsky, 1988). A frequency table with stimulus and response words was created and a cognitive map based on frequency values was obtained. The directions of the arrows and the intensity of the associations shown on the map are specified by the frequency tables. Nakiboğlu (2008) states that this method has more descriptive capacity and is more appropriate for showing both the aspects and the strength of the associations found in students’ knowledge structures. Meaningful and valid answers from the students in the context of acid–base chemistry were then transferred to an Excel sheet. A frequency table was derived from the data by counting the response words for each stimulus word.After this process, frequency maps were created to depict the data. The correlation frequency scores between the stimulus and response words characterized the frequency ranges and vertical directions for weak or strong correlations on the maps. When a stimulus word was acquired from the students as a response word, it was added to a frame. It was not added to a frame in the maps when the response word was a brand-new word distinct from the stimulus words. The width of the frames and arrows are arranged based on the frequency score of the association between a stimulus word and its response word. The power of associations is indicated by width. The first cell has the widest arrows and defines the highest scores. Answers in the first column have arrows from the first row, and the direction of the arrows is parallel to the direction of the associations.
The WAT data set of twenty students was transferred to a chemistry education expert (first author). The data were coded independently by the first author and the second author. Reliability was tested according to Miles and Huberman's Reliability Formula [Reliability = (Agreement/Agreement + Disagreement) × 100]. In studies based on qualitative analysis, 70% and above is considered an acceptable reliability interval (Miles and Huberman, 1994). In the reliability calculation, the focus was on the number of different response words belonging to two independent codings for each stimulus word. Upon reaching a consensus on the codings by discussing the encodings of the stimulus words with a reliability value below 70% (alkalinity 65%, neutralization 62%, buffer solution 50%, conjugate acid–base-pairs 50%), the coding related to these stimulus words was made again and reliability calculations were completed (acid 87%, base 88%, acidity 6%, alkalinity 90%, salt 86%, neutralization 91%, indicator 100%, pH 89%, buffer solution 100%, amphoteric species 100%, conjugate acid–base pairs 85%). After disagreements were resolved the whole data set was coded by second author. In addition to the analysis mentioned above, tables were formed by counting the different meaningful and valid answers given to the stimulus words. Counting the different answers given to each stimulus word is important in terms of summarizing the data obtained (Shavelson, 1974; Bahar et al., 1999; Nakiboğlu, 2008).
In the analysis of the data obtained by the free writing technique, the framework “Types of relationship for describing structural characteristics” was developed by Liu and Ebenezer (2002, p. 115), who adapted it from Holley and Dansereau (1984, p. 85), was used as a coding guide. Every ten paragraphs of the 8th- and 12th-grade students were analyzed independently by the first author and the second author. Again, the reliability formula of Miles and Huberman (1994) was used in the reliability calculations of the coding. Reliability percentages for each category for 8th-grade students’ paragraphs were calculated as Part-of 78%, Type-of/example-of 95%, Lead-to 85%, Analogy 100%, Characteristics 97%, Evidence 100%. For the 12th-grade students, it was calculated as Part-of 92%, Type-of/example-of 87%, Lead-to 94%, Analogy 100%, Characteristics 87%, and Evidence 83%. After solving disagreements, the whole data set was coded by second author.
Results and discussions
Revealing and elucidating 8th-grade students’ cognitive structures on acid–base chemistry
Table 3 documents the frequency of each response word provided by the 8th graders for all the stimulus words utilized in this study based on textbook analysis and expert views. As specified by the analysis of the frequencies, six distinct frequency ranges were identified. Any frequency value less than 10 was ignored in this analysis. The noteworthy ranges include 101–150, 71–80, 41–50, 31–40, 21–30, and 11–20. These ranges then were split into three groups describing the associations between the words as strong (71–150), moderately strong (31–50), and weak (11–30).| Response words | Stimulus words | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acid | Base | Acidity | Alkalinity | Salt | Neutralization | Indicator | pH | Buffer solution | Amphoteric species | Conjugate acid–base pair | |
| Bitter | — | 12 | — | 3 | — | — | — | — | — | — | — |
| Acetic acid | 12 | — | 4 | — | — | — | — | — | — | — | — |
| Reagent | — | — | — | — | — | — | 8 | — | — | — | — |
| Dye | — | 19 | — | 5 | — | — | — | — | — | — | — |
| Value | — | — | — | — | — | — | — | 14 | — | — | — |
| Household cleaning agents | 20 | 72 | 8 | 12 | 24 | — | — | — | — | — | — |
| Folic acid | 14 | — | 3 | — | — | — | — | — | — | — | — |
| Carbonated drinks | 109 | — | 37 | — | — | — | — | — | — | — | — |
| Indicator | — | — | — | — | — | — | 15 | 1 | — | — | — |
| Rock salt | — | — | — | — | 50 | — | — | — | — | — | — |
| Nitric acid | 20 | — | 2 | — | — | — | — | — | — | — | — |
| Personal hygiene agents | — | 46 | — | 16 | — | — | — | — | — | — | — |
| Lemon | 50 | — | 13 | — | — | — | — | — | — | — | — |
| Fruits | 137 | — | 48 | — | — | — | — | — | — | — | — |
| NH3 | — | 12 | — | 2 | — | — | — | — | — | — | — |
| OH− | — | 2 | — | 11 | — | — | — | 1 | — | 1 | — |
| Organic base | — | 30 | — | 7 | — | — | — | — | — | — | — |
| pH | 1 | 2 | 6 | 5 | — | 1 | 2 | — | — | — | — |
| pH meter | — | — | — | — | — | 1 | — | 24 | — | — | — |
| Water | — | — | — | — | 2 | 8 | — | 14 | — | — | 1 |
| Salt | 1 | — | 2 | — | — | 11 | — | — | — | — | — |
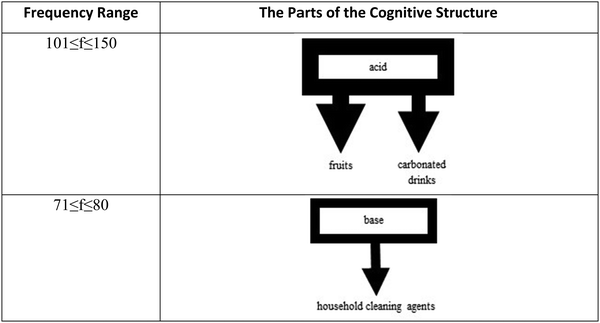
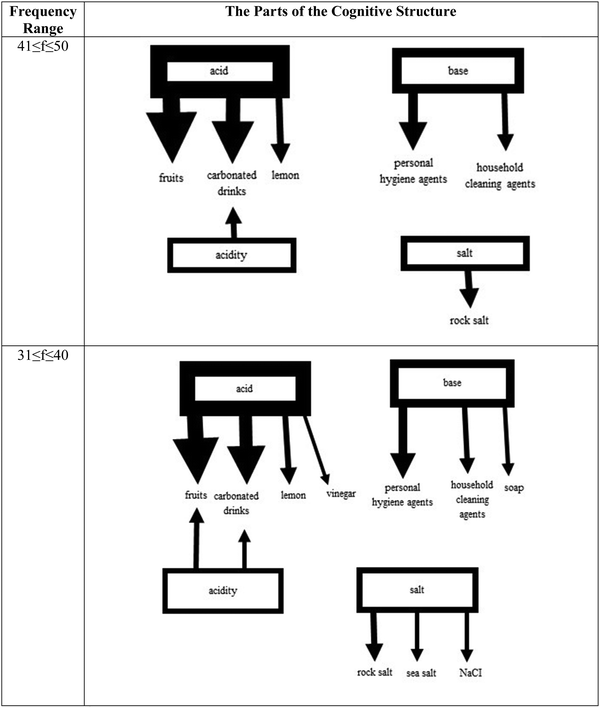
The response words reported in each frequency range were linked to the stimulus words as seen in Fig. 3–5 with arrows having different widths. The strongest word associations were represented with the thickest lines. While forming the cognitive structure maps of the 8th-grade students, 10 different frequency ranges were determined (see Fig. 3–5). No frequency values corresponding to the categories 91 ≤ f ≤ 100, 81 ≤ f ≤ 90, 61 ≤ f ≤ 70, and 51 ≤ f ≤ 60 were identified. There was no correlation made between these frequency ranges. The strongest associations of the students in Fig. 3 are only the stimulus word acid associated with two response words (carbonated drinks and fruits) in the 101 ≤ f ≤ 150 frequency range. It was found that the stimulus word base was associated with a single response word at the 71 < f < 80 level with household cleaning agents.
 | ||
| Fig. 4 8th-grade students’ cognitive structures with the strong and moderately strong word associations. | ||
 | ||
| Fig. 5 8th-grade students’ cognitive structures with all kinds of word associations from the strong to the weak ones. | ||
On the cognitive structure of the 8th graders, it appeared that the stimulus word acid has the strongest associations with two response words, fruits, and carbonated drinks, in the 101–150 frequency range. In the 71–80 frequency level, the next strongest association was observed between the stimulus word base and household cleaning agents as the response word.
When the next two ranges, 41–50 and 31–40, were analyzed, more associations were obtained between the stimulus words acid and base and the response words. Lemon appears as the new response word connected to acid and personal hygiene agents are connected to base as a response word. At the range of 41–50, new stimulus words like acidity and salt emerged. It is interesting to note that acidity and acid share carbonated drinks as a common response word, which links them to each other. However, salt does not share any common response word with the other stimulus words. So, the cognitive structures have three isolated clusters (Fig. 4).
Although at the next range, 31–40, the number of the response words associated with stimulus words acid, base, acidity, and salt increased, the number of clusters remained the same. Due to these disconnected knowledge pieces, it can be argued that students have relatively poor conceptual understanding (Anderson and Schönborn, 2008; Prasetya et al., 2022; He et al., 2023). However, it should be noted that the connection between acidity and acid got stronger with a new common response word.
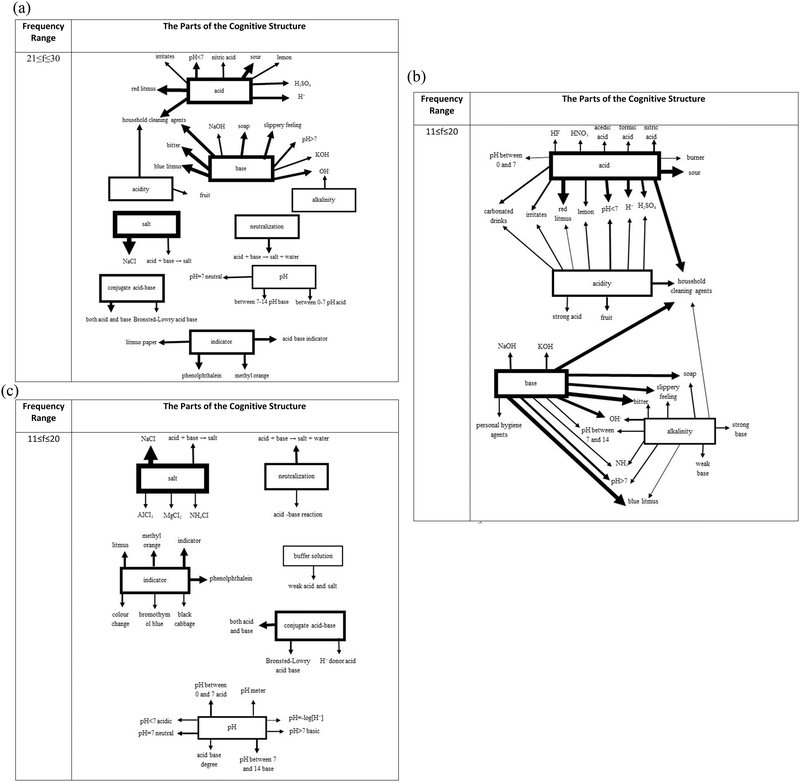
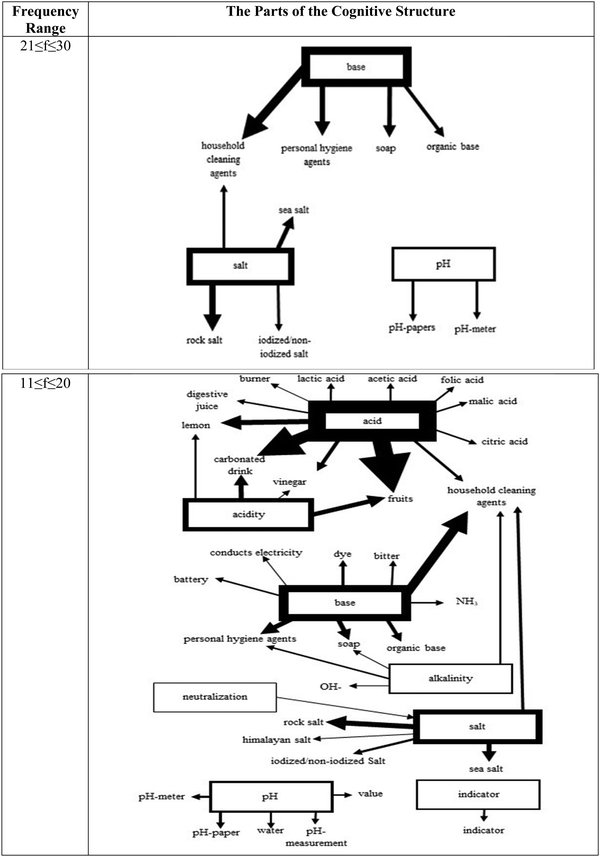
The analysis of the frequency range of 21–30 in Fig. 5 did not reveal any new connections between the stimulus words acid and acidity and the response words. However, it was found that the stimulus words base and salt are weakly connected to the new response words, organic base, and iodized/non-iodized salt, respectively. It also appeared that salt is connected to base through a common response word, house cleaning agents even though it is considered a weak connection. Another interesting finding in this range was that a new stimulus word pH showed up in the cognitive structure of the students in the 8th grade. However, it was not connected to any other cluster and stayed isolated with its own response words. When the lowest frequency range (11–20) of interest was analyzed, eight stimulus words, acid, base, acidity, alkalinity, salt, neutralization, pH, and indicator, were observed in the structure. At this level first time, a stimulus word was mentioned as a response to another stimulus word. Salt was provided as a response word for neutralization.
Since the frequency values (f ≤ 10) of the response words associated with the stimulus words “Conjugate acid–base, buffer solution, and amphoteric species” were low, these stimulus words were not included in the structure.
The stimulus words acid and acidity reappeared in the structure with the new response words and new connections. The stimulus word acid is now connected to other stimulus words like base, alkalinity, and salt in addition to acidity through common response words. The stimulus word base also increased its connections to other clusters. In addition to the previously observed connection with salt, it is connected to alkalinity and salt at this level, which indicates a richer cognitive structure with relatively weak connections. Although the goal should be creating a richer network among all stimulus words as identified as critical for understanding acid–base chemistry well by the experts, the limited lecture time could be used better if teachers prioritize these already existing weak connections and spend extra time on strengthening them.
When the cognitive structure was scrutinized at the lowest level, the richest network with the new response words and, more importantly, with the highest number of connections among the stimulus words was observed even though the associations were considered weak due to the low-frequency values. In general, it was revealed that the cognitive structures of the 8th-grade students about acid–base had a static structure that included one-way associations between concepts, with no bidirectional or cross-associations. Further analysis made clear that the structure includes a big cluster with six stimulus words, acid, acidity, base, alkalinity, salt, and neutralization, and two isolated clusters of stimulus words of pH and indicator. It is possible that students did not consider the conceptual meaning of pH and indicator and how they are related to the reactions at the submicroscopic level. Therefore, they did not link them to the response words commonly associated with the stimulus words acid and base. Teachers possibly introduce these terms in a very strict and narrow context, which should be avoided to minimize their isolation from the rest of the cognitive structure. Moreover, they can consider utilizing the chemistry triplet (Johnstone, 1991) in their explanations to help their students develop a better understanding of phenomena observed or a piece of information learned from textbooks. Students need to realize that macroscopic observations or symbolic representations would not mean much if they are not connected to the submicroscopic level, which is invisible to their naked eyes, but they can have a trip theoretically and connect all these loose ends to construct a strong conceptual understanding of the topics including acid–base chemistry learned in their classrooms regardless of the grade level.
In addition to checking the frequency of response words mentioned by the students, the total number of different responses was examined for each stimulus word to determine which stimulus word(s) are the most associated ones. This analysis helped find out which terms are easier to be connected to a familiar word that 8th graders would know. From the numbers listed in Table 4, it is obvious that students have richer knowledge with regard to the stimulus words acid, acidity, base, alkalinity, and pH.
| Stimulus words | Total number of different response words |
|---|---|
| Acid | 51 |
| Acidity | 43 |
| Base | 31 |
| pH | 30 |
| Alkalinity | 30 |
| Neutralization | 22 |
| Salt | 17 |
| Indicator | 13 |
| Amphoteric species | 8 |
| Buffer solution | 7 |
| Conjugate acid–base | 5 |
Most likely, students come across or learn more about various concepts from different sources including media in their daily lives than from the textbooks and teachers in their formal education. This finding reminds us of the constructive theory that emphasizes the fact that learners constantly gain new knowledge and their mind is not empty vessels to be filled. Rather, it is a precious storage like a hard disk with a wide range of information that needs to be organized to be efficient and beneficial to its holder.
On the other hand, it was noted that three stimulus words conjugate acid–base, buffer solution, and amphoteric species were associated with an exceptionally low number of response words, 5, 7, and 8, respectively. This makes sense because the teachers regularly do not include these concepts in their lessons, given that these concepts are not covered in the 8th-grade science curriculum set by the Ministry of Education (2018) in Turkey; and they are also rarely present in students’ life or the media.
To obtain additional insights into the students’ cognition and capture some hidden characteristics, the Free Writing Technique (FWT) was also used. As highlighted in Table 5, the associations gathered from the FWT paragraphs written by the 8th-grade students were mostly compatible with the characteristics relationship and type-of/example-of relationship category, and the least with the evidence relationship category in terms of structural characteristics. Almost 50% of their responses were considered some kind of statement revealing the students’ understanding concerning characteristic relations, while 35% of them were more about type-of/example-of relations. The remaining statements, which make up about 15% of the entire responses were in the categories of lead-to, part-of, and evidence relations in decreasing order. It can be easily seen that characteristic relations and type-of/example-of relationships are dominant.
| Relationship | Description | Typical words | Examples | Frequency |
|---|---|---|---|---|
| Characteristics | Refers to traits, aspects, qualities, properties, features, attributes, details, characteristics, or usefulness of an object, process, concept, or idea. | Has, characterized, is, feature, property, trait, aspect, attribute | 166 | |
| Type-of/example-of | Refers to members or examples of a class or category, or labelling different groups of things or objects. | Type of, example, kind of | Neutralization is the use of an acid and a base together. | 120 |
| Lead-to | Refers to a causal effect, a change, or a sequential process. | Leads to, results, causes | Basicity = Substances that release hydroxide ions. | 29 |
| Part-of | Refers to parts of an object, composition of something, elements of an idea, or the process of making something. | Part, segment, portion of | Substances that form hydrogen ions (H+) in their aqueous solutions are called acids. Substances that form hydroxide (OH−) ions in their aqueous solutions are called bases. A solution that dissolves in an acid to form hydrogen ions (H+) is acidic, while a solution that a base dissolves into hydroxide ions (OH−) is a basic solution. | 27 |
| Evidence | Refers to evidence, facts, data, supports, proofs, documentation, measures, or confirmation of an object, idea, process, or concept. | Indicates, illustrates, demonstrates, supports, documents, proof, confirms, evidence-of | Acidity, that is drinks containing acid. Bases are those such as guanine, cytosine, and adenine. Salts are found in food and the sea. | 9 |
| Analogy | Refers to logical comparisons. | Similar, analogous, like, corresponds | — |
Typical examples of the category of characteristic relation belonging to 8th-grade students include “Acids taste sour. Bases are bitter.” and “The pH-value of bases is between 7–14.” As for the examples of the type-of/example-of relationship, “Acid = found in colas and sodas. Acidity is the same thing as these.” and “Examples of salt types are rock salt, table salt, sea salt, Himalayan salt.” can be shared. The results show that the structural features of the information in the cognitive structures of the 8th-grade students about acid–base chemistry are basically at the level of descriptive knowledge (Liu and Ebenezer, 2002). Also Morgil et al. (2003) found in their study that acid–base concepts used in daily language are more permanent than scientific concepts and that associations could not be established between concepts that should be related to each other in concept maps created by students. One of the students stated that “Neutralization is zeroing. It is used in atoms.” This quote implies difficulties that students have with interpreting and comprehending the concept of neutralization in the context of acid–base chemistry. If students’ already existing knowledge includes limited or erroneous concepts, it would create obstacles to comprehending and structuring concepts of advanced topics (Adadan, 2014) and incorporating new concepts into their cognitive structures (Derman and Eilks, 2016).
Revealing and elucidating 12th-grade students’ cognitive structures on acid–base chemistry
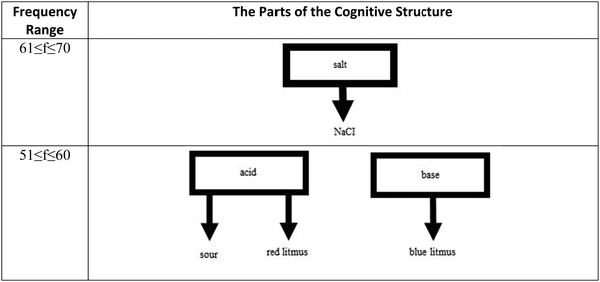
The analysis of response words concerning frequency ranges revealed an interesting difference between the cognitive structures of 8th graders and 12th graders. Although the structure of 8th graders included two stimulus words associated with some response words in the frequency range of 71–150, it was not the case for the 12th graders. The first association was observed in the frequency range of 61–70, which was considered a part of the cognitive structure with the strong word associations together with the frequency range of 51–60. As seen in Fig. 6, the strongest association exists between the stimulus word salt and the response word NaCl, the chemical formula of table salt. Students associate the term with something that they are most familiar with. The information is relevant to them, and they are super familiar with its use and applications. In the next frequency range, two new clusters appear one for the stimulus word acid and one for the stimulus word base. This is also something different from what was determined in the structure of 8th graders. While acid and base were observed in the range of 71–150 in the structure of 8th graders, they only emerged at the range of 51–60 in that of 12th graders.The common thing for both structures is that these stimulus words have their isolated clusters at these ranges. They are not connected to each yet through common response words. When Fig. 7 was examined, the first thing will be noticed that at the range of 41–50, acid and base clusters are now connected as they share household cleaning agents as the common response word for both acid and base. Here, a new cluster for the stimulus word indicator also appears, but it has no connection with the other clusters. In the following range (31–40), two new clusters show up for the stimulus words conjugate acid–base pair and amphoteric species, but they are also isolated from the rest of the structure. It is important to note that these stimulus words appeared in a higher frequency range than the one observed in the 8th graders’ structure. It is possible that this was the case because chemistry teachers at this grade level are supposed to cover upper secondary curriculum, or students have a better understanding of the Brønsted-Lowry acid–base theory.
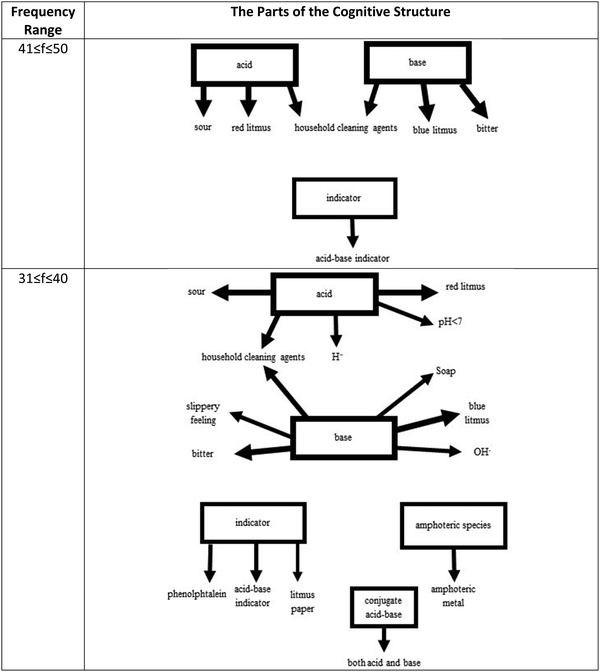 | ||
| Fig. 7 12th-grade students’ cognitive structures with the strong and moderately strong word associations. | ||
Once the remaining frequency values were considered, the cognitive structure became very complicated with several new response words and multiple clusters. Due to this complexity and the range of the associations observed and to make its analysis easier, Fig. 8 was split into three Fig. 8a–c. At the frequency range of 21–30 (Fig. 8a), the cluster that belongs to salt reappeared as some weak associations were made with it. Even more, associations were determined with the stimulus words acid and base, none of them was common between them. However, the stimulus word acidity seemed to be connected to acid through the same response word, household cleaning agents. On the other hand, the stimulus word alkalinity showed up and shared OH− as a response word with the stimulus word base. With the clusters of stimulus words pH and neutralization, the total number of clusters in the cognitive structure at this level increased to six. It should be noted that out of all these clusters, only one cluster consisted of multiple stimulus words, namely acid, base, alkalinity, and acidity.
When the response words are considered at the lowest frequency range (11–20), the total number of clusters increased by one only, going up to seven. However, the most complex cluster observed at one higher level got more complex at this level (Fig. 8b). The increased complexity was not due to an increased number of stimulus words, but because of the newly identified common response words. The stimulus words acid and acidity shared seven response words, while this number was ten between base and alkalinity. The common response word for all these stimulus words was still one, household cleaning agents.
The final figure (Fig. 8c) in this section, highlights the remaining clusters observed at this level. They certainly comprised more response words but none of them consisted of a common response word, leaving them as isolated clusters. Even when the weakest associations were considered, the stimulus words salt, neutralization, buffer solution, indicator, conjugate acid–base pair, and pH did not form a larger cluster representing a stronger conceptual understanding among these concepts.
Even more connections between the stimulus words were obtained in the cognitive structure of the 12th graders, the number of stimulus words in the most complex structure was fewer than that observed in the 8th graders’ structure at the same frequency level. 8th graders somehow were able to connect six clusters while this number was only four for 12th graders. This difference could be attributed to a few factors. The first could be related to the approach teachers take in their classrooms. It is possible that 8th-grade chemistry teachers emphasized conceptual understanding more than the high school chemistry teacher did, which resulted in a better organization and a more complex structure. Another reason could be related to the fact that 12th graders know more content than 8th graders, but they did not internalize them yet. They were able to associate the stimulus words with more response words but could not identify the underlying similarities between the stimulus words at that point. Therefore, more isolated clusters appeared in their structure. Although these differences existed between the two structures, it seemed that like the cognitive structure of 8th graders, this one did not include any bidirectional and cross-associations regarding acid–base chemistry.
Following the examination of frequency values and connections of stimulus words, the total number of different response words for each stimulus word was determined (see Table 6) to enrich the analysis of the 12th-grade students’ cognitive structure as it was completed for the structure of the students in 8th grade.
| Stimulus words | Total number of different response words |
|---|---|
| Acidity | 53 |
| Acid | 51 |
| pH | 48 |
| Salt | 42 |
| Alkalinity | 35 |
| Base | 30 |
| Indicator | 28 |
| Neutralization | 18 |
| Conjugate acid–base | 15 |
| Buffer solution | 12 |
| Amphoteric species | 8 |
Similar to the 8th graders, 12th graders came up with the highest number of different response words for acid and acidity. However, 12th graders produced more response words for pH and salt compared to base and alkalinity. This trend could be about the fact that 12th graders were exposed to calculations involving pH more than were 8th graders. It is reasonable to expect that students had practised more questions on this topic and their familiarity with pH-related concepts increased significantly while moving from 8th through 12th grade. Despite the expectations of higher response words for conjugate acid–base pair, buffer solutions, and amphoteric species, they were determined at the bottom of the list with about a similar number of response words identified for the 8th graders. This could be because these concepts (amphoteric species, buffer solution, and conjugate acid–base) still did not play a prominent role in the teaching of acid–base chemistry at upper secondary school and are not much related to everyday life. The concepts of acid, base, acidity, alkalinity, indicator, pH, salt, and neutralization are included both in the 10th-grade curriculum as well as used in the 11th grade, while the concepts of amphoteric species, buffer solution, and conjugate acid–base are only included in the 11th grade. So, it is not surprising to find out that all these concepts form isolated clusters in the cognitive structure of both cohorts. It could be claimed that students did not develop a good conceptual understanding of concepts involved in acid–base chemistry, especially the five stimulus words listed at the bottom of the list in Table 6.
As the final piece of analysis, the FWT data of 12th-grade students were checked to categorize the associations identified in the sentences. It was determined that most of the associations were compatible with the characteristics relationship and led to the relationship category (Part-of), and the least with the analogy relationship (Table 7).
| Relationship | Description | Typical words | Examples | Frequency |
|---|---|---|---|---|
| Characteristics | It refers to traits, aspects, qualities, properties, features, attributes, details, characteristics, or usefulness of an object, process, concept, or idea | Has, characterized, is, feature, property, trait, aspect, attribute | 1-pH < 7 in acidity, pH > 7 in alkalinity. | 293 |
| 2-There are amphoteric substances that react as both acid and base. | ||||
| 3-Buffer solution shows basic features to balance the acidic environment. It also shows acidic features to balance the basic environment. | ||||
| Lead to | It refers to a causal effect, a change, or a sequential process | That leads to, results, causes | 1-When acids and bases react, they form salt and water. This is called neutralization. | 172 |
| 2-Acids and bases can change colour in the indicator. | ||||
| 3-Acids turn litmus paper red. Bases turn litmus paper blue. | ||||
| Part-of | It refers to parts of an object, composition of something, elements of an idea, or the process of making something | Part, segment, portion of | 1-Acids release H+ when dissolved in water. Bases release OH− when dissolved in water. | 101 |
| 2-The mixture of a weak acid and its salt is a buffer solution. | ||||
| 3-Conjugated acid–base based on the Brønsted-Lowry acid–base definition, the substance that gives H+ in reactions is an acid. | ||||
| Type-of/example-of | It refers to members or examples of a class or category, or labelling different groups of things or objects | Type of category, example, kind of | 1-Acids and bases are found in all areas of our lives. | 98 |
| 2-Amphoteric metals are Zn, Pb, Cr, Sn, and Al. | ||||
| 3-Substances have certain pH. Black cabbage is a natural indicator. | ||||
| Evidence | It refers to evidence, facts, data, supports, proofs, documentation, measures, or confirmation of an object, idea, process, or concept | Indicates, illustrates, demonstrates, supports, documents, proof, confirms, evidence | 1-The indicator allows us to understand whether a substance is an acid or a base. | 33 |
| 2-Calculates pH for acidity and alkalinity. | ||||
| 3-Indicates the pH acidity-basicity range. pH shows acidity and alkalinity. | ||||
| Analogy | It refers to logical comparisons | Similar, analogous, like, corresponds | 1-Amphoteric substances are double-faced substances. | 1 |
In terms of the structural characteristics of the associations in the cognitive structures of 12th-grade students; characteristic relation (293), lead-to relation (172), part-of (101), and type-of/example-of relation (98) were the most common categories. These quotes “Substances that react with both acids and bases are amphoteric species.” and “Buffer solution shows basic properties to balance the acidic environment. It also shows acidic feature to balance the basic environment.” indicated common type examples for the characteristic relationship. Some of the examples of the lead-to relationship were “Acids and bases react with each other to form salt and water. This is called the neutralization reaction.” and “The acid turns the litmus paper red, while the base turns it blue.” These examples together reveal that 12th graders’ cognitive structure was basically at the level of descriptive knowledge. As stated by one of the students “Acid and base are opposite notions. Accordingly, the taste of acids is sweet and that of bases is bitter.” This statement clearly illustrates that students fundamentally misunderstood the concepts of acid and base and do not refer to any theoretical basis of their nature.
Conclusions
In this study, it was found that the organization of the 8th- and 12th-grade students’ cognitive structures in this sample related to acid–base chemistry is static in nature, includes mostly unidirectional associations from the stimulus word to the response word, and forms isolated clusters of knowledge with a low level of reference to theoretical concepts. Despite some similarities between grade-8 and -12 students’ cognitive structures and the number of response words observed, it should be noted that the cognitive structures of the students at a higher grade level are richer in detail and networked with a higher number of response words in their clusters. Also, the association of the same response word with different stimulus words is more common at the 12th-grade level. However, both structures appeared to be the most complex in the lowest frequency range where the strengths of the associations are the weakest. We can clearly state that the structural characteristics of students’ concepts and inter-conceptual associations about acids-bases have diversified and improved from 8th to 12th grade by the spiral curriculum the learners are exposed to. Therefore, their conceptual understanding of acid–base chemistry has developed from middle school to high school level, but only to a certain extent and with few connections between different concepts. Nevertheless, the structural characteristics of students’ explanations show that their knowledge is mostly at the macroscopic level and they rarely provide theory-based explanations at the sub-microscopic level.Based on the findings discussed above, we can conclude that chemistry curriculum developers, textbook authors, and teachers should reflect the operated curriculum to utilize better-prepared teaching resources and activities to promote the growth of highly relevant and reliable cognitive structures. This might contain visualization tools, e.g. concept maps, to better develop interlinked knowledge or the use of chemistry information scales (macroscopic, multiparticle, molecular, atomic, etc.). This might also include reflection on the many potential dimensions (composition/structure, energy, and time) and methodologies (mathematical, conceptual, contextual, and historical) for teaching acid–base chemistry (Talanquer, 2011). Additionally, the teaching approaches must carefully choose and deal with the proper models, explanations, and illustrations while simultaneously taking into account any potential dangers and deceptive information that might be included in textbooks or digital resources (Eilks et al., 2009, 2012). Especially, when it comes to particle descriptions, teaching should focus on creating a coherent curriculum (Eilks, 2013). This can help students to create sufficient cognitive structures for comprehending acid–base chemistry.
Apart from these, we can deduce that it should become part of teachers’ pedagogical content knowledge that they acquire an intimate knowledge of students’ cognitive structures before starting the class. This will enable teachers to better manage the teaching process to build scientifically reliable student knowledge. Knowing their pupils’ prior knowledge can support teachers to guide students effectively through conceptual transformation and encourage their knowledge expansion (Nakiboğlu, 2008). WAT and FWT are practical tools for chemistry teachers to determine students’ knowledge, misconceptions, and cognitive structures and to organize their lessons according to this information. Aside from these, teachers can utilize WAT and FWT to reflect on their teaching practices, and they can monitor students’ conceptual growth both before and after covering any topic in their classes (Bahar et al., 1999; Nakiboğlu, 2008; Derman and Eilks, 2016).
Conflicts of interest
There are no conflicts to declare.References
- Adadan E., (2014), Investigating the influence of preservice chemistry teachers’ understanding of the particle nature of matter on their conceptual understandings of solution chemistry, Chem. Educ. Res. Pract., 15, 219–238.
- Adadan E. and Savasci F., (2012), An analysis of 16–17 year-old students’ understanding of solution chemistry concepts using a two-tier diagnostic instrument, Int. J. Sci. Educ., 34, 513–544.
- Adadan E., Trundle K. C. and Irving K. E., (2010), Exploring grade 11 students’ conceptual pathways of the particulate nature of matter in the context of multirepresentational instruction, J. Res. Sci. Teach., 47, 1004–1035.
- Anderson T. R. and Schönborn K. J., (2008), Bridging the educational research-teaching practice gap: conceptual understanding, Part 1: the multifaceted nature of expert knowledge, Biochem. Mol. Biol. Educ., 36(4), 309–315 DOI:10.1002/bmb.20209.
- Artdej R., Ratanaroutai T., Coll R. K. and Thongpanchang T., (2011), Thai grade 11 students’ alternative conceptions for acid–base chemistry, Res. Sci. Technol. Educ., 28, 167–183.
- Ausubel D. P., Novak J. D. and Hanesian H., (1978), Educational psychology: a cognitive view, New York: Holt, Rinehart & Winston.
- Ayas A., (2011), Kavram Öğrenimi, in S. Çepni (ed.), Kuramdan Uygulamaya Fen ve Teknoloji Öğretimi, Ankara: Pegem Akademi, pp. 126–151.
- Bahar M. and Hansell M. H., (2000), The relationship between some psychological factors and their effect on the performance of grid questions and word association tests, Educ. Psychol., 20, 349–364.
- Bahar M., Johnstone A. H. and Sutcliffe R. G., (1999), Investigation of students’ cognitive structure in elementary genetics through word association tests, J. Biol. Educ., 33, 134–141.
- Blake A. J. and Norland F. H., (1978), Science instruction and cognitive growth in college students, J. Res. Sci. Teach., 15, 413–419.
- Bradley J. D. and Mosimege M. D., (1998), Misconception in acid and bases: a comparative study of student teachers with different chemistry backgrounds, South African J. Chem., 51(3), 137–150.
- Calık M., Ayas A. and Ebenezer J. V., (2005), A review of solution chemistry studies: insights into students’ conceptions, J. Sci. Educ. Technol., 14, 29–50.
- Carr M. J., (1984), Model confusion in chemistry, Res. Sci. Educ., 14, 97–103.
- Cartrette D. P. and Mayo P. M., (2011), Students’ understanding of acids/bases in organic chemistry contexts, Chem. Educ. Res. Pract., 12, 29–39.
- de Jong T. and Ferguson-Hessler M. G. M., (1986), Cognitive Structures of Good and Poor Novice Problem Solvers in Physics, J. Educ. Psych., 78(4), 279–288 DOI:10.1037/0022-0663.78.4.279.
- Demircioğlu G., Ayas A. and Demircioğlu H., (2005), Conceptual change achieved through a new teaching program on acids and bases, Chem. Educ. Res. Pract., 6, 36–51.
- Derman A. and Eilks I., (2016), Using a word association test for the assessment of high school students’ cognitive structures on dissolution, Chem. Educ. Res. Practice, 17(4), 902–913.
- Drechsler M. and Schmidt H. J., (2005a), Upper secondary school students’ understanding of models used in chemistry to define acids and bases, Sci. Educ. Int., 16(1), 39–54.
- Drechsler M. and Schmidt H. J., (2005b), Textbooks’ and teachers’ understanding of acid-base models used in chemistry teaching, Chem. Educ. Res. Pract., 6, 19–35.
- Drechsler M. and van Driel J., (2008), Experienced teachers’ pedagogical content knowledge of teaching acid–base chemistry, Res. Sci. Educ.38, 611–631.
- Ebenezer J. V., (2001), A hypermedia environment to explore and negotiate students’ conceptions: animation of the solution process of table salt, J. Sci. Educ. Technol., 10, 73–91.
- Eilks I., (2013), Teachers’ ways through the particulate nature of matter in lower secondary chemistry teaching: a continued change of different models vs. a coherent conceptual structure? in G. Tsaparlis and H. Sevian (ed.), Concepts of matter in science education, Dordrecht: Springer, pp. 213–230.
- Eilks I., Witteck T. and Pietzner V., (2009), A critical discussion of the efficacy of using visual learning aids from the Internet to promote understanding, illustrated with examples explaining the Daniell voltaic cell, Eurasia J. Math. Sci. Techn. Educ., 5, 145–152.
- Eilks I., Witteck T. and Pietzner V., (2012), The role and potential dangers of visualization when learning about sub-microscopic explanations in chemistry education, Centre Educ. Policy Stud. J., 2, 125–145.
- Gulacar O., Vernoy B., Tran E., Wu A., Huie E., Wadhwa A., Santos E., Sathe R. and Milkey A., (2022), Investigating differences in experts’ chemistry knowledge structures and comparing them to those of general chemistry students, J. Chem. Ed., 99(8), 2950–2963.
- Gunstone F. R., (1980), Word association and the description of cognitive structure. Res. Sci. Educ., 10, 45–53.
- Gussarsky E. and Gorodetsky M., (1988), On the chemical equilibrium concept: constrained word associations and conception, J. Res. Sci. Teachnol., 25, 319–333.
- Hawkes S. J., (1992), Arrhenius confuses students, J. Chem. Educ., 69, 542–543.
- He X., Fang J., Cheng H. N. H., Men Q. and Li Y., (2023), Investigating online learners’ knowledge structure patterns by concept maps: a clustering analysis approach, Educ. Inf. Technol., 28, 11401–11422. DOI:10.1007/s10639-023-11633-8.
- Hewson M. G. and Hewson P. W., (1983), Effect of instruction using students’ prior knowledge and conceptual change strategies on science learning, J. Res. Sci. Teach., 20, 731–743.
- Holley C. D. and Danseraeau D. F., (1984), Networking: the technique and the empirical evidence, in: Holley C. D. and Dansereau D. F. (ed.), Spatial Learning Strategies: techniques, applications, and related issues, Orlando: Academic Press, pp. 81–108.
- Johnson P. E., (1967), Some psychological aspects of subject matter structure, J. Educ. Psych., 58, 75–83.
- Johnson P. E., (1969), On the communication of concepts in science. J. Educ. Psych., 60, 32–40.
- Johnstone A. H., (1991), Why is science difficult to learn? Things are seldom what they seem, J. Comp. Assist. Learn., 7(2), 75–83.
- Jonassen D. H., Beissner K. and Yacci M., (1993), Structural Knowledge: techniques for representing, conveying, and acquiring structural knowledge, Hillsdale: Lawrence Erlbaum.
- Kala N., Yaman F. and Ayas A., (2013), The effectiveness of predict–observe–explain technique ın probing students’ understanding about acid–base chemistry: a case for the concepts of pH, pOH, and strength, Int. J. Sci. Math. Educ., 11, 555–574.
- Kilavuz Y., (2005), The effects of 5e learning cycle model based on constructivist theory on tenth grade students’ understanding of acid-base concepts, Ankara: Unpublished Master Thesis of Middle East Technical University.
- Kousathana M., Demerouti M. and Tsaparlis G., (2005), Instructional misconceptions in acid-base equilibria: An analysis from a history and philosophy of science perspective, Sci. Educ., 14, 173–193.
- Lee K. W., (1986), Problem solving in electrochemistry: variables, strategies and teaching and learning, Unpublished PhD thesis, Australia: Monash University.
- Lee K. W., (1988), Two non-traditional measures of chemistry learning: word association and idea association, Res. Sci. Educ., 18, 169–176.
- Levy Nahum T., Mamlok-Naaman R., Hofstein A. and Taber K. S. (2010), Teaching and learning the concept of chemical bonding, Stud. Sci. Educ., 46, 179–207.
- Liu X. and Ebenezer J., (2002), Descriptive categories and structural characteristics of students’ conceptions: an exploration of the relationship, Res. Sci. Techn. Educ., 20, 111–132.
- Liu X. and Lesniak K., (2005), Students’ progression of understanding the matter concept from elementary to high school, Sci. Educ., 89, 433–450.
- Miles M. B. and Huberman A. M., (1994), Qualitative data analysis: An expanded sourcebook, Thousand Oaks: Sage.
- Ministry of Education (2018), Ortaögretim Kimya Dersi (9, 10, 11 ve 12. Sınıflar) Ogretim Programı (9th, 10th, 11th and 12th Grade Chemistry Course Curriculum). Ankara: M.E.B./TTKB: Retrieved from https://mufredat.meb.gov.tr/ProgramDetay.aspx?PID=350, Accessed 24.06.2019.
- Morgil I., Erdem E. and Yılmaz A., (2003), Misconceptions in chemistry education, Hacettepe Üniversitesi Eğitim Fakültesi Dergisi, 25, 246–255.
- Nakiboğlu C., (2008), Using word associations for assessing non major science students’ knowledge structure before and after general chemistry instruction: the case of atomic structure, Chem. Educ. Res. Pract., 9, 309–322.
- Othman J., Treagust D. F. and Chandrasegaran A. L., (2008), An investigation into the relationship between students’ conceptions of the particulate nature of matter and their understanding of chemical bonding, Int. J. Sci. Educ., 30, 1531–1550.
- Prasetya D. D., Pinandito A., Hayashi Y. and Hirashima T., (2022), Analysis of quality of knowledge structure and students’ perceptions in extension concept mapping, Res. Pract. Technol. Enhanced Learn., 17(1), 14.
- Schizas D., Katrana E. and Stamou G., (2013), Introducing network analysis into science education: methodological research examining secondary school students’ understanding of ’decomposition’, Int. J. Environ. Sci. Educ., 8, 175–198.
- Shavelson R. J., (1972), Some aspects of the correspondence between content structure and cognitive structure in physics instruction, J. Educ. Psychol., 63, 225–234.
- Shavelson R. J., (1974), Methods for examining representations of a subject matter structure in a student's memory, J. Res. Sci. Teachnol., 11, 231–249.
- Sikumbang D., Rakhmawati I. and and Suwandi T. (2019), Investigating the cognitive structure of biology preservice teachers about central dogma of molecular biology through word association test, J. Phys. Conf. Ser., 1155(1), 12047 DOI:10.1088/1742-6596/1155/1/012047.
- Sheppard K., (1997), A qualitative study of high school students’ pre and post instructional conceptions in acid-base chemistry, Ed. D. Thesis, Teachers College, Columbia University, New York.
- Taber K. S., (2008), Conceptual resources for learning science: issues of transience and grain-size in cognition and cognitive structure, Int. J. Sci. Educ., 30, 1027–1053.
- Talanquer V., (2011), Macro, submicro, and symbolic: the many faces of the chemistry “triplet”, Int. J. Sci. Educ., 33, 179–195.
- Tarhan L. and Acar Sesen B., (2012), Jigsaw cooperative learning: acid-base theories, Chem. Educ. Res. Pract., 13, 307–313.
- Tsai C. C., (2001), Probing students’ cognitive structures in science: the use of a flow map method coupled with a meta listening technique, Stud. Educ. Eval., 27, 257–268.
- Tümay H., (2016), Reconsidering learning difficulties and misconceptions in chemistry: emergence in chemistry and its implications for chemical education, Chem. Educ. Res. Pract., 17, 229–245.
- Tyson L. M., Venville G. J., Harrison A. G. and Treagust D. F., (1997), A multidimensional framework for interpreting conceptual change events in the classroom, Sci. Educ., 81, 387–404.
- Uzuntiryaki E. and Geban Ö., (2005), Effect of conceptual change approach accompanied with concept mapping on understanding of solution concepts, Instruct. Sci., 33, 311–339.
- West L. and Pines L., (1985), Cognitive structure and conceptual change, Orlando, FL: Academic Press.
- West L., Fensham P. and Garrand J., (1985), Describing the cognitive structures of learners following instruction in chemistry, in L. West and L. Pınes (eds), Cognitive structure and conceptual change, Orlando, FL: Academic Press, pp. 29–49.
- White R. T., (1985), Interview protocols and dimensions of cognitive structure, in L. West and L. Pınes (ed.), Cognitive structure and conceptual change, Orlando, FL: Academic Press, pp. 51–59.
- White R. and Gunstone R., (2000), Probing understanding, London: Falmer Press.
- Wilson J. M., (1990), Chemistry concepts and group cognitive structure: a study of undergraduate nursing students, Res. Sci. Educ., 20, 292–299.
- Wilson J. M., (1998), Differences in knowledge networks about acids and bases of year-12, undergraduate and postgraduate chemistry students, Res. Sci. Educ., 28, 429–446.
- Yang Z., Zhu M., Qu Z. and Zhang Y., (2018), Research on organization of mathematics knowledge in good mathematical cognitive structure, Eurasia J. Math. Sci. Technol. Educ., 14, 291–302.
- Yin R. K., (1992), The case study method as a tool for doing evaluation, Curr. Sociol., 40, 121–137.
| This journal is © The Royal Society of Chemistry 2024 |