Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Development of a green scalable route toward the synthesis of bio-based 2-pyrones†‡
Grazia Isa C.
Righetti§
*a,
Cristian
Gambarotti§
 *a and
Hans-René
Bjørsvik§
*a and
Hans-René
Bjørsvik§
 b
b
aDepartment of Chemistry, Materials and Chemical Engineering “Giulio Natta”, Politecnico di Milano, Piazza Leonardo da Vinci 32, I-20133 Milano, Italy
bDepartment of Chemistry, University of Bergen, Allegaten 41, N-5007 Bergen, Norway
First published on 24th January 2024
Abstract
2-Pyrones are molecules that gained significant attention in the field of medicine and synthetic chemistry. They are broadly present in nature, where they play an important role in the defense mechanisms of the organisms in which they are present. Due to their unique structure, 2-pyrones hold immense potential both for the development of pharmacologically active compounds and as building blocks in synthetic chemistry; for these reasons those molecules have attracted researcher's attention during the past decade. In this work, we present the synthesis optimization of bio-based 2-pyrones starting from bio sourced galactaric acid by means of a statistical design of experiment, its scale up from 500 mg to 100 g, the solvent recycling to make the reaction greener as well as the synthesis of galactaric acid from galactose.
Introduction
As the world grapples with the challenges of climate change and environmental degradation, industries and scientific communities are increasingly focusing on sustainable practices. In the chemistry field, the development of sustainable chemical routes holds tremendous promise for reducing environmental impact and promoting a greener future. Under this point of view, the exploitation of biomass potential as renewable feedstock is of paramount importance not only in the energy and fuel sector but also in chemistry as a starting material for the production of high value-added bio-sourced molecules.1,2 Carbohydrates represent a major fraction of biomass and are good candidate as renewable raw materials for the synthesis of many molecules that are, nowadays, deriving from a petrochemical route.3,42-Pyrones are unsaturated six-membered cyclic esters able to combine a diene with an aromatic-like reactivity.5 Their peculiar structure makes those molecules highly attractive under a synthetical point of view,6,7 as they can play and important role as building blocks in polymer synthesis and in Diels–Alder reactions.8 2-Pyrone motif is the one more present in nature: it can be found in insects, bacteria, plants and animals where it is involved not only in defense mechanism but it is also a biosynthetic intermediate and metabolite.9 The investigation of the biological activity of those compounds already shown how they can act as antifungal, antibiotic, cytotoxic and neurotoxic agents,10 but most important they have also shown activity against Alzheimer,11 tuberculosis12–14 and HIV.15,16
When performing organic synthesis, the aim is to obtain a new compound by combining the reactants under specific reaction conditions. Achieving high yields and purity while minimizing the waste production and operation cost can be indeed challenging. Design of experiments (DOE) has emerged as a powerful tool given to chemist to implement a process by conducting just few experiments. This statistical approach aims to establish the relationship between multiple variables and a specific response; it allows the simultaneous variation of several parameters with the aim of investigating the reaction space to identify the optimal conditions under which a reaction can be carried out. DOE became quickly a reference method, in place of the traditional one variable at a time (OVAT) approach often proved to be time consuming, to maximize the information gained from each experiment and to fasten the optimization process for chemical reactions.17
Considering the significance of 2-pyrones and the crucial role of aldaric acids, categorized as “Top value-added chemicals from biomass” due to their potential as renewable feedstock for synthesizing a diverse array of molecules, our focus turned to refining the synthetic protocol for converting galactaric acid into 2-pyrones. To the best of our knowledge, there are few examples in the literature related to the synthesis of 2-pyrones from aldaric acids.18 One relies on harsh reaction conditions/pyrolysis, resulting in a final product yield ranging from 11% to 40%. The other involves heating the system at 90 °C in acetic anhydride in the presence of a base, yielding the product up to 80%.
In pursuit of a scalable protocol with minimal waste production, we present the optimization of the bio-based 2-pyrones synthesis from galactaric acid by means of a statistical design of experiments. Additionally, we detail the synthesis of galactaric acid from galactose (Fig. 1).
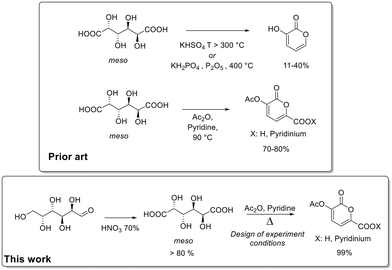 | ||
| Fig. 1 Comparison between state of art and this work for the synthesis of 2-pyrones from renewable sources. | ||
Result and discussion
Galactaric acid synthesis
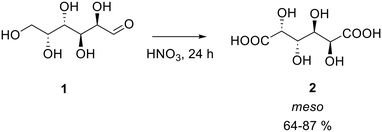
It is already known from the literature that aldaric acids can be obtained from their corresponding sugars by oxidation in the presence of nitric acid, but the reported yields are low.19–21Different nitric acid concentrations and temperature were tried for the synthesis of galactaric acid (2), also known as mucic acid starting from galactose (1) (Scheme 1, Table 1).
| Entry | [HNO3] | V (mL) | T (°C) | Yielda (%) |
|---|---|---|---|---|
| a Yield in isolated product. b Galactose firstly dissolved in 2.5 mL of water; quantity of galactose fixed on 1 g for all the reactions. | ||||
| 1 | 1 M | 10 | 60 | 0 |
| 2 | 8.4 M | 10 | 60 | 0 |
| 3 | 70 (% w/w) | 10 | 60 | 64 |
| 4 | 70 (% w/w) | 5 | 60 | 73 |
| 5 | 70 (% w/w) | 10 | r.t. | 76 |
| 6 | 70 (% w/w) | 5 | r.t. | 61 |
| 7 | 70 (% w/w) | 10 | 0 to r.t. | 82 |
| 8 | 70 (% w/w) | 10b | r.t. | 87 |
Two blank experiments were performed using a diluted solution of nitric acid, proving that no formation of the product was possible under those conditions even under heating. As the concentration of nitric acid resulted to be the most important parameter (entries 1–3, Table 1), the temperature effect was evaluated next. It was found that galactaric acid can be obtained in good yields when the reaction is conducted at a low temperature: from 0 to 20 °C (entries 3–4 vs. 5–7). These protocols, which do not involve heating of nitric acid, result in higher yields of the final product and during the reaction just a negligible quantity of NOx, due to the acid decomposition, are formed making this pathway greener. In these reactions, the system initially appears as a heterogeneous mixture. It was therefore decided to dissolve the sugar in water prior to its oxidation with nitric acid to verify whether a homogeneous system could enhance the final yield. Therefore, galactose was dissolved in 2.5 mL of water prior to the addition of nitric acid (70% w/w) to the solution (entry 8). This procedure brought to obtain 2 in an 87% yield.
The galactaric acid obtained, was then employed in the synthesis of 2-pyrone derivatives.
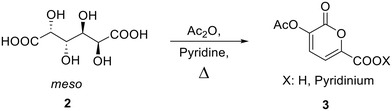
Optimization of the synthetic procedure for pyrone 3
Bio-based 2-pyrones can be easily obtained by reacting galactaric acid in acetic anhydride in the presence of a base.21 This protocol, though, usually brings to 2-pyrone rings with a 70–80% yield. We decided to perform an optimization study on this process to make it more efficient for a possible scale-up through a full factorial two-level design of experiment with three “center” experiments.The synthesis optimization of 3-acetoxy-2-oxo-2H-pyran-6-carboxylic acid pyridinium salt (3, Scheme 2) from galactaric acid (2) was performed on the bases of our previous findings.18
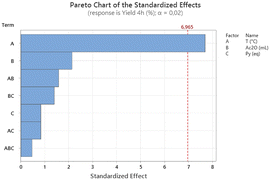
The statistical design of experiment was set up by choosing as the three variables: reaction temperature (x1), volume of acetic anhydride (x2) and quantity of pyridine (x3).
To evaluate the appropriateness of the chosen experimental levels, we conducted experiments no. 1, 8, and 9 (Table 2) first. These three experiments represent the −1, +1, and zero levels, respectively. The results for the ‘zero level’ (depicted by the purple bar in Fig. 2) align with what is reported in the literature. On the other hand, the ‘−1 level’ (pink bar) led to a lower final yield, while the ‘+1 level’ (blue bar) resulted in a higher final yield. The observed variations in yield, when altering all parameters simultaneously, suggest that the experimental parameters are appropriately selected. This increase and decrease in yield provide valuable insights into the sensitivity of the system to parameter adjustments. Then all the other reactions were carried out with a random order to minimize the experimental error distribution.
| Experimental variables | Experimental levels | ||||||
|---|---|---|---|---|---|---|---|
| −1 | 0 | +1 | |||||
| x 1 | Reaction temperature | [°C] | 80 | 90 | 100 | ||
| x 2 | Ac2O volume | [mL] | 5 | 8 | 11 | ||
| x 3 | Quant. of pyridine | [equiv.] | 0.9 | 1.0 | 1.1 | ||
| Experimental variables | Responsea (yield%) | ||||||
|---|---|---|---|---|---|---|---|
| No. | x 1 [°C] | x 2 [mL] | x 3 [eq.] | 1 h | 2 h | 3 h | 4 h |
| a Analytical yield; 500 mg of 2 are employed in all the reactions. | |||||||
| 1 | −1 | −1 | −1 | 14 | 37 | 68 | 78 |
| 2 | +1 | −1 | −1 | 82 | 82 | 90 | 91 |
| 3 | −1 | +1 | −1 | 22 | 53 | 61 | 63 |
| 4 | +1 | +1 | −1 | 70 | 77 | 87 | 87 |
| 5 | −1 | −1 | +1 | 47 | 60 | 70 | 73 |
| 6 | +1 | −1 | +1 | 82 | 85 | 87 | 93 |
| 7 | –1 | +1 | +1 | 31 | 48 | 49 | 68 |
| 8 | +1 | +1 | +1 | 57 | 77 | 79 | 94 |
| 9 | 0 | 0 | 0 | 59 | 73 | 88 | 89.5 |
| 10 | 0 | 0 | 0 | 58 | 78 | 86 | 82 |
| 11 | 0 | 0 | 0 | 53 | 84 | 84 | 87 |
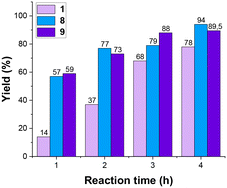 | ||
| Fig. 2 Yields in time for experiments 1, 8 and 9 – Table 2. | ||
The responses (yields in time) obtained were analyzed in order to find a mathematical correlation between the three variables and to establish the relative weight of each parameter on the reaction outcome for a confidence level at 98% (eqn (1)).
| Yield (%) = 80.88 + 10.38x1 − 2.88x2 + 1.13x3 + 2.13x1x2 + 1.12x1x3 + 1.88x2x3 − 0.63x1x2x3 | (1) |
These observations further emphasize the intricate relationship between temperature, solvent, and base in influencing the final product yield, validating the key insights provided by the Pareto chart and reinforcing the importance of temperature control in optimizing the reaction conditions.
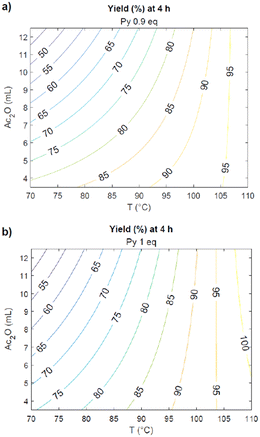
By using the mathematical correlation between all the selected parameters and the final yield (eqn (1)), a projection describing the reaction outcome as a function of those parameters was obtained (Fig. 4).
On the bases of this result, in order to maximize the yield, three more experiments were performed (entries 1–3, Table 3).
This deeper investigation allowed to find the protocol that brings to the full conversion of the substrate to the desired product: this requires heating of galactaric acid in 12 mL of acetic anhydride for 4 hours at 100 °C (entry 2). In light of the parameter effects on the reaction outcome given by the Pareto chart, this latter was tested also using half of the quantity of Ac2O, in order to make the protocol greener and less energy consuming. Also in this case, the product was obtained with a 99% yield. To test the robustness of the process, this synthetic procedure was then scaled-up from 500 mg to 100 g and still gave 99% analytical yield of 3.
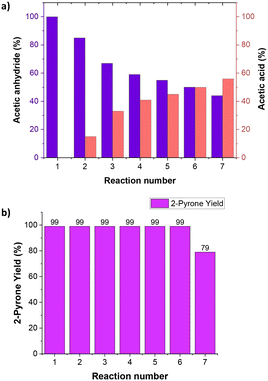
To make the process greener, the recyclability of the solvent was also tested. The acetic anhydride used as solvent for the synthesis of pyrone 3 (Scheme 2) could be recovered and re-employed in a new process. During the course of the reaction, the acetic anhydride plays both the role of solvent and reactant and it is consumed while acetic acid is formed as the only co-product of the reaction. The result obtained showed that after 5 recycling reactions of the solvent, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio between acetic anhydride and acetic acid was reached (Fig. 5a).
1 molar ratio between acetic anhydride and acetic acid was reached (Fig. 5a).
After this point, when the quantity of acetic acid exceeded the amount of acetic anhydride, the yield in the final product decreased from 99% to 79% (Fig. 5b). From this point onwards, the yield in the product continued to decrease as the anhydride is consumed in the processes.
Conclusions
The protocol for the synthesis of bio-sourced 2-pyrones was efficiently optimized by performing a two levels full factorial design of experiment. This study allowed to find a better protocol with full conversion of the substrate: the overall yield of the process passed from 76% to 99% analytical yield reducing the amount of waste produced (unreacted galactaric acid). Furthermore, it was also demonstrated how the acetic anhydride can efficiently be re-employed in the same process up to six times without compromising the final yield of the process (99% yield), making the synthetic protocol more sustainable.The synthesis of galactaric acid itself was also investigated obtaining a higher yield (87%) with respect to the one reported in literature and without the need of heating the system.
Experimental
All reagents and solvents were purchased from Merck and used without any further purification. The 1H-NMR was recorded on a Bruker AV 500 MHz instrument, equipped with a 5 mm multinuclear probe, and 32 scans were acquired with an acquiring time of 3 seconds for each spectrum. The 1H-NMR analysis was performed in the presence of an external standard: 1,4-dinitro benzene for DMSO-d6 and trioxane for D2O. All reagents and solvents were purchased from Merck and used without any further purification.General procedure for the synthesis of galactaric acid (2)
1.0 g of galactose is weighted in a round bottom flask equipped with a magnetic stirrer. Nitric acid is then added and the system is left under stirring (see Table 1 for the quantities and temperature). After 24 hours the system is filtered, the white solid is washed with 3 mL of acetone and analyzed by 1H-NMR in D2O in the presence of NaOH. 1H-NMR (400 MHz D2O) δ = 4.12 (2H, s), 3.79 (2H, s) ppm.General procedure for the synthesis of pyrone 3 as pyridinium salt
500 mg of galactaric acid (2.38 mmol) were placed in a 3 neck round bottom flask equipped with a thermometer and a condenser. Acetic anhydride (8–12 mL) and pyridine (0.9–1.1 equivalents) were added to the reaction flask. The system was heated to the desired temperature (80–100 °C) and kept under stirring for 4 hours. The initial white suspension turns to a purple solution at the end of the reaction. A sample was withdrawn every hour to calculate the analytical yield in 2-pyrone. At the end of the reaction the system was filtered to eliminate the traces of the reactant and the solvent was removed under reduced pressure. The final 2-pyrone is collected as a purple solid. 1H-NMR (400 MHz, DMSO-d6) δ = 7.40 (d, 1H, J = 7.2 Hz), 6.80 (d, 1H, J = 7.2 Hz), 2.26 (s, 3H), 1.90 (s, 6H) ppm. See ESI,‡ Fig. S1.Author contributions
The manuscript was written by contribution of all the authors and all authors approved the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Notes and references
- R. Sakuta and N. Nakamura, Int. J. Mol. Sci., 2019, 20, 1–20 CrossRef PubMed.
- S. Rautiainen, P. Lehtinen, J. Chen, M. Vehkamäki, K. Niemelä, M. Leskelä and T. Repo, RSC Adv., 2015, 5, 19502–19507 RSC.
- J. J. Bozell and G. R. Petersen, Green Chem., 2010, 12, 539–555 RSC.
- S. Hagen, J. V. N. Vara Prasad and B. D. Tait, Adv. Med. Chem., 2000, 5, 159–195 CAS.
- C. W. Bird, Tetrahedron, 1986, 42, 89–92 CrossRef CAS.
- G. A. Kraus, G. R. Pollock, C. L. Beck, K. Palmer and A. H. Winter, RSC Adv., 2013, 3, 12721–12725 RSC.
- J. J. Lee and G. A. Kraus, Green Chem., 2014, 16, 2111–2116 RSC.
- C. Gambarotti, M. Lauria, G. I. C. Righetti, G. Leonardi, R. Sebastiano, A. Citterio and A. Truscello, ACS Sustainable Chem. Eng., 2020, 8, 11152–11161 CrossRef CAS.
- G. P. Mcglacken and I. J. S. Fairlamb, Nat. Prod. Rep., 2005, 22, 369–385 RSC.
- J. S. Lee, Mar. Drugs, 2015, 13, 1581–1620 CrossRef CAS PubMed.
- H. S. Hong, S. Rana, L. Barrigan, A. Shi, Y. Zhang, F. Zhou, L. W. Jin and D. H. Hua, J. Neurochem., 2009, 108, 1097–1108 CrossRef CAS PubMed.
- G. Appendino, E. Mercalli, N. Fuzzati, L. Arnoldi, M. Stavri, S. Gibbons, M. Ballero and A. Maxia, J. Nat. Prod., 2004, 67, 2108–2110 CrossRef CAS PubMed.
- K. Mitra, A. Chadha and M. Doble, Eur. J. Pharm. Sci., 2019, 135, 103–112 CrossRef CAS PubMed.
- R. Mata, I. Morales, O. Pérez, I. Rivero-Cruz, L. Acevedo, I. Enriquez-Mendoza, R. Bye, S. Franzblau and B. Timmermann, J. Nat. Prod., 2004, 67, 1961–1968 CrossRef CAS PubMed.
- M. He, N. Yang, C. Sun, X. Yao and M. Yang, Med. Chem. Res., 2011, 20, 200–209 CrossRef CAS.
- Y. S. Lee, S. N. Kim, Y. S. Lee, J. Y. Lee, C. K. Lee, H. S. Kim and H. Park, Arch. Pharm., 2000, 333, 319–322 CrossRef CAS PubMed.
- S. A. Weissman and N. G. Anderson, Org. Process Res. Dev., 2015, 19, 1605–1633 CrossRef CAS.
- G. Leonardi, J. Li, G. I. C. Righetti, A. M. Truscello, C. Gambarotti, G. Terraneo, A. Citterio and R. Sebastiano, Eur. J. Org. Chem., 2020, 2, 241–251 CrossRef.
- O. Sohst and B. Tollens, Justus Liebigs Ann. Chem., 1888, 245, 1–27 CrossRef.
- T. N. Smith, K. Hash, C.-L. Davey, H. Mills, H. Williams and D. E. Kiely, Carbohydr. Res., 2012, 350, 6–13 CrossRef CAS PubMed.
- C. A. Carpenter, K. I. Hardcastle and D. E. Kiely, Carbohydr. Res., 2013, 376, 29–36 CrossRef CAS PubMed.
Footnotes |
| † This manuscript is dedicated to the memory of Prof. Hans-René Bjørsvik, a great person and a great friend. |
| ‡ Electronic supplementary information (ESI) available: Picture of the 1H NMR spectrum of pyrone 3 as pyridinium salt is reported. See DOI: https://doi.org/10.1039/d3re00667k |
| § The authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |