Open Access Article
Open Access ArticleImproving the energy yield of plasma-based NOx synthesis with in situ adsorption†
Kevin Hendrik Reindert
Rouwenhorst
 abc,
Sybe
Tabak
a and
Leon
Lefferts
abc,
Sybe
Tabak
a and
Leon
Lefferts
 *a
*a
aCatalytic Processes & Materials, MESA+ Institute for Nanotechnology, University of Twente, P.O. Box 217, 7500 AE Enschede, The Netherlands. E-mail: l.lefferts@utwente.nl
bAmmonia Energy Association, 77 Sands Street, 6th Floor, Brooklyn, NY 11201, USA
cKoolen Industries, Europalaan 202, 7559 SC Hengelo, The Netherlands
First published on 17th January 2024
Abstract
Plasma-based NOx synthesis from air is a promising option to electrify nitrogen fixation. However, the energy efficiency of direct plasma-based NOx synthesis in a plasma reactor is severely limited by NOx decomposition in the plasma phase. In situ NOx adsorption on MgO improves the NOx energy yield in a dielectric barrier discharge (DBD) plasma reactor by a factor of 15.
Plasma-based nitrogen fixation via NOx synthesis from air was commercialized in the early 20th century. Kristian Birkeland and Samuel Eyde commercialized the first industrial nitrogen fixation process, the Birkeland–Eyde process, based on an electric arc plasma reactor.1,2 This process was operational in Norway and Canada.3,4 However, the process was eventually outcompeted by the Haber–Bosch process, producing ammonia (NH3) from nitrogen (N2) and hydrogen (H2) derived from steam reforming of hydrocarbons or from water electrolysis.
Plasma-based nitrogen fixation has re-emerged in scientific literature as an option to electrify N2 fixation.5–9 Plasma-based processes have the advantage that the load can be varied quickly, in contrast to thermal processes, so that rapid changes in supply of renewable electricity can be handled.10 Also, absence of the need for intensive energy integration makes local production at relatively small scale feasible. In particular, plasma-N2 fixation in the form of NOx shows promise.5,6
So far, plasma-based NOx synthesis in warm plasma reactors such as gliding arc (GA) and microwave (MW) plasma reactors, shows the best performance,5,6 with NOx formation at an energy cost down to 0.42 MJ per mol NOx,11 which is competitive with the renewable Haber–Bosch process (using electrolysis to produce H2) combined with the Ostwald process (NH3 oxidation) in terms of energy cost (0.6 MJ per mol HNO3).6 However, such low energy consumptions for plasma reactors are only achieved when operating at low NOx concentrations of typically a few hundred ppm,11 which is not practical for NOx absorption in water in an industrial process.12 Concentrations in the order of 5 mol% NOx are required for efficient processing. For those conditions, the energy consumption for NOx formation in plasma reactors is at least in the order of 2 MJ per mol NOx.6 Furthermore, the high temperatures in warm plasmas in the order of 103 K cause thermal NOx decomposition13 and rapid quenching is required to minimize thermal NOx decomposition.
This work introduces a novel concept for energy-efficient NOx formation in a dielectric barrier discharge (DBD) reactor via in situ NOx removal using a solid MgO sorbent. A DBD reactor is a non-thermal plasma reactor operating near room temperature.14 Only a few studies have been done using DBD reactors for NOx synthesis,13,15–18 reporting relatively high energy consumption compared to warm plasma reactors. Nevertheless, a DBD reactor has been used in this work for two reasons. Firstly, thermal NOx decomposition is negligible at mild temperature, and secondly, in situ NOx removal via adsorption on a solid sorbent is easily achievable. Product molecules adsorbed on microporous materials are protected against plasma induced decomposition in a DBD reactor, as we have demonstrated earlier for the case of ammonia synthesis in a DBD reactor.19 Plasma cannot develop in sub-micron pores while plasma activated species are too short-lived to diffuse into these pores; therefore adsorbed molecules are protected against plasma-decomposition.8 Suppressing NOx decomposition is expected to improve both energy efficiency and the single pass conversion in a DBD reactor. As earth-alkali metal oxides are known to sorb NOx,20 we have selected MgO to demonstrate this concept. We will show that both energy efficiency as well as NOx concentrations in the product stream are drastically improved.
High surface area MgO (294 m2 g−1) with a particle size between 250 and 300 μm was prepared and characterized as described in ESI† section S1. The experiments were performed using a packed bed DBD reactor with MgO particles, and the NOx concentration was determined with a mass spectrometer (MS), as described in detail in section S2.1.†
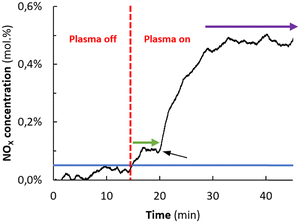
Fig. 1 shows the NOx concentration in the outlet as a function of time on igniting the plasma, operating with an O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N2 ratio of 1
N2 ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, flow rate of 20 mL min−1, plasma power of 6.4 W, implying a SEI (specific energy input) of 19.2 kJ L−1. The corresponding Lissajous plot is shown in Fig. S3 (see ESI†). It is observed that the NOx outlet concentration is about 0.1 mol% during the first 4 min and increases to about 0.5 mol% afterwards. The change in NOx outlet concentration with time in Fig. 1 is attributed to adsorption of NOx on the MgO sorbent, followed by breakthrough of the NOx in the outlet.
1, flow rate of 20 mL min−1, plasma power of 6.4 W, implying a SEI (specific energy input) of 19.2 kJ L−1. The corresponding Lissajous plot is shown in Fig. S3 (see ESI†). It is observed that the NOx outlet concentration is about 0.1 mol% during the first 4 min and increases to about 0.5 mol% afterwards. The change in NOx outlet concentration with time in Fig. 1 is attributed to adsorption of NOx on the MgO sorbent, followed by breakthrough of the NOx in the outlet.
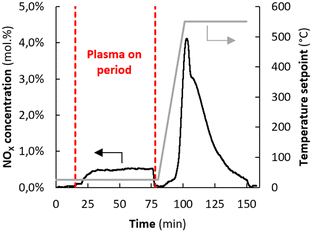
The result shown in Fig. 2 confirms that NOx is indeed adsorbed in situ. After exposure to the plasma, the reactor was heated (25 °C min−1) in N2 (10 mL min−1), inducing desorption of NOx in absence of plasma. The concentration of NO2 is much higher than NO, but we cannot rule out NO formation, as explained in ESI 3.3.† The NOx concentration during desorption is up to 4%, one order of magnitude higher than the NOx concentration during steady-state plasma operation. The amount adsorbed NOx is 0.05 mol per mol MgO.
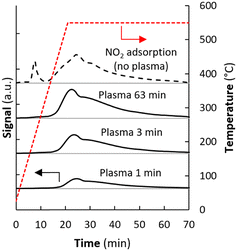
Fig. 3 compares the TPD (temperature programmed desorption) result after NOx synthesis by plasma illumination during different times with TPD after adsorption of NO2 at room temperature. The amount of NOx formed during the plasma experiment obviously varies with plasma time. Fig. 4 shows that the amount of NOx detected with TPD is already saturated after typically 5 minutes of plasma exposure. The amount is slightly lower than after thermal NO2 adsorption in absence of plasma (0.055 mol-NO2 per mol-MgO), in the same order of magnitude as reported by Duong et al.21 for MgO with a slightly lower surface area of 126 m2 g−1. The lower amount observed during NOx synthesis with plasma is likely attributed to mild heating induced by the plasma, causing weakly adsorbed NO2 to desorb. A similar effect was previously reported for in situ NH3 removal by a zeolite for plasma-based NH3 synthesis.19 The effective NH3 capacity on zeolite 4A decreased by 60% due to heating effects.19
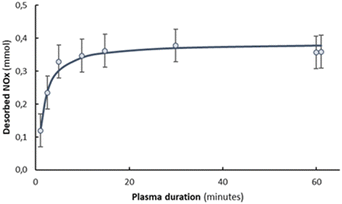 | ||
| Fig. 4 The effect of plasma duration for plasma-based NOx synthesis with in situ adsorption on the amount of adsorbed NOx on 300 mg MgO. | ||
The significance of the in situ adsorption of NOx from the plasma zone is evident from the reduction in energy consumption versus steady-state operation. Steady-state NOx synthesis in this work results in an energy consumption of 93 MJ per mol NOx, in line with literature values reported in the range 16–540 MJ per mol NOx for DBD reactors.13,15–18 In case of in situ product removal during 5 minutes, the energy consumption for the plasma reactor and NOx product desorption decreases to 6.0 MJ per mol NOx as calculated in section S3.2† including the energy cost of the desorption step. Effectively, the energy consumption efficiency is improved by a factor 15. As shown in Fig. 5, in situ adsorption of NOx in a DBD reactor results in the best performance for a non-thermal plasma-reactor, by both decreasing the energy consumption as well as increasing the NOx concentration in the product stream. Importantly, the NOX concentration raised well above 1%, which is considered as the minimum concentration required for nitric acid production by adsorption in water in a commercially viable process.22
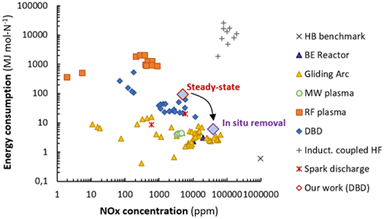 | ||
| Fig. 5 Comparison of energy consumption for NOx production in various plasma reactors. Original figure reproduced from ref. 6, and data from this work added. Steady-state: red lined diamond. In situ removal: purple lined diamond. | ||
Concluding, this work demonstrates that low temperature DBD plasma reactors can produce NOx with similar low energy consumptions obtained with warm plasma reactors like gliding arc reactors and microwave reactors (Fig. 5), by integrating plasma conversion and product separation. Also, the NOx concentration in the product stream is significantly increased. This study demonstrates the concept, but optimization of the (earth-) alkali material as well of the morphology of the adsorbent is likely to result in further improvement.20 Furthermore, addition of a catalyst,23 optimization of the dielectric properties of the adsorbent,18 and optimization of the plasma properties could be considered.24,25
In situ product removal is relevant for the wider scientific community working on plasma(-catalytic) conversion. We demonstrate that a significant improvement for NOx synthesis and it is likely that the concept would also applicable for plasma-based CO2 dissociation and CH4 conversion.26
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project is co-financed by TKI-Energie from Toeslag voor Topconsortia voor Kennis en Innovatie (TKI) from the Ministry of Economic Affairs and Climate Policy, The Netherlands. The authors acknowledge B. Geerdink for technical support, K. Altena-Schildkamp for N2 chemisorption experiments and T. Lubbers for XRF analysis. The authors acknowledge K. Van't Veer and A. M. B. Bogaerts from the University of Antwerp for discussions regarding plasma activation of N2 in DBD reactors.Notes and references
- K. Birkeland, Trans. Faraday Soc., 1906, 2, 98–116 RSC.
- S. Eyde, J. Ind. Eng. Chem., 1912, 4, 771–774 CrossRef CAS.
- A. S. Travis, Nitrogen Capture: The Growth of an International Industry (1900–1940), Springer International Publishing, 2018 Search PubMed.
- K. H. R. Rouwenhorst, A. S. Travis and L. Lefferts, Sustainable Chem., 2022, 3, 149–171 CrossRef CAS.
- B. S. Patil, Q. Wang, V. Hessel and J. Lang, Catal. Today, 2015, 256, 49–66 CrossRef CAS.
- K. H. R. Rouwenhorst, F. Jardali, A. Bogaerts and L. Lefferts, Energy Environ. Sci., 2021, 14, 2520–2534 RSC.
- N. Cherkasov, A. O. Ibhadon and P. Fitzpatrick, Chem. Eng. Process., 2015, 90, 24–33 CrossRef CAS.
- A. Bogaerts, X. Tu, J. C. Whitehead, G. Centi, L. Lefferts, O. Guaitella, F. Azolina-Jury, H.-H. Kim, A. B. Murphy, W. F. Schneider, T. Nozaki, J. C. Hicks, A. Rousseau, F. Thevenet, A. Khacef and M. Carreon, J. Phys. D: Appl. Phys., 2020, 53, 1–51 CrossRef.
- K. H. R. Rouwenhorst, F. Jardali, A. Bogaerts and L. Lefferts, Energy Environ. Sci., 2023, 16, 6170–6173 RSC.
- A. Bogaerts and E. C. Neyts, ACS Energy Lett., 2018, 3, 1013–1027 CrossRef CAS.
- E. Vervloessem, Y. Gorbanev, A. Nikiforov, N. De Geyter and A. Bogaerts, Green Chem., 2022, 24, 916–929 RSC.
- W. A. Dekker, E. Snoeck and H. Kramers, Chem. Eng. Sci., 1959, 11, 61–71 CrossRef CAS.
- X. Pei, D. Gidon, Y. J. Yang, Z. Xiong and D. B. Graves, Chem. Eng. J., 2019, 362, 217–228 CrossRef CAS.
- U. Kogelschatz, Plasma Chem. Plasma Process., 2003, 23, 1–46 CrossRef CAS.
- B. S. Patil, N. Cherkasov, J. Lang, A. O. Ibhadon, V. Hessel and Q. Wang, Appl. Catal., B, 2016, 194, 123–133 CrossRef CAS.
- Q. Sun, A. Zhu, X. Yang, J. Niu and Y. Xu, Chem. Commun., 2003, 1418–1419 RSC.
- A. A. Abdelaziz and H.-H. Kim, J. Phys. D: Appl. Phys., 2020, 53, 114001–114018 CrossRef CAS.
- Y. Ma, Y. Wang, J. Harding and X. Tu, Plasma Sources Sci. Technol., 2021, 30, 105002–105013 CrossRef CAS.
- K. H. R. Rouwenhorst, S. Mani and L. Lefferts, ACS Sustainable Chem. Eng., 2022, 10, 1994–2000 CrossRef CAS.
- C. Verrier, J. H. Kwak, D. H. Kim, C. H. F. Peden and J. Szanyi, Catal. Today, 2008, 136, 121–127 CrossRef CAS.
- T. H. Y. Duong, T. N. Nguyen, H. T. Oanh, T. A. Dang Thi, L. N. T. Giang, H. T. Phuong, N. T. Anh, B. M. Nguyen, V. T. Quang, G. T. Le and T. Van Nguyen, J. Chem., 2019, 2019 DOI:10.1155/2019/4376429.
- K. H. R. Rouwenhorst and L. Lefferts, Catalysts, 2020, 10(9) DOI:10.3390/catal10090999.
- A. ul R. Salman, B. C. Enger, X. Auvray, R. Lødeng, M. Menon, D. Waller and M. Rønning, Appl. Catal., A, 2018, 564, 142–146 CrossRef.
- H.-H. Kim, Y. Teramoto, A. Ogata, H. Takagi and T. Nanba, Plasma Processes Polym., 2017, 14, 1–9 Search PubMed.
- P. Peng, P. Chen, M. Addy, Y. Cheng, E. Anderson, N. Zhou, C. Schiappacasse, Y. Zhang, D. Chen, R. Hatzenbeller and Y. Liu, ACS Sustainable Chem. Eng., 2019, 7, 100–104 CrossRef CAS.
- K. H. R. Rouwenhorst and L. Lefferts, Plasma Processes Polym., 2023, 1–8 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, including materials and preparation, material characterization, plasma characterization, NOx synthesis and adsorption experiments. Results & discussion, including material characterization, plasma characterization, thermal NOx TPD study with MgO, and energy cost for MgO regeneration. See DOI: https://doi.org/10.1039/d3re00593c |
| This journal is © The Royal Society of Chemistry 2024 |