Open Access Article
Open Access ArticleStarch-assisted facile gel formation method for the synthesis of Fe–Mn binary oxide for the simultaneous removal of As(III) and As(V)†
Anwarul Azim a,
Md. Minhajul Alam Khan
a,
Md. Minhajul Alam Khan a,
Shawon Saha
a,
Shawon Saha a,
Hridoy Roy
a,
Hridoy Roy b,
Akter Hossain Reaz
b,
Akter Hossain Reaz ac,
A. K. M. Atique Ullah
ac,
A. K. M. Atique Ullah ac and
Shakhawat H. Firoz
ac and
Shakhawat H. Firoz *a
*a
aDepartment of Chemistry, Bangladesh University of Engineering and Technology (BUET), Dhaka-1000, Bangladesh. E-mail: shfiroz@chem.buet.ac.bd; Tel: +880 1730-713767
bDepartment of Chemical Engineering, Bangladesh University of Engineering and Technology (BUET), Bangladesh
cDepartment of Chemistry, Michigan State University, East Lansing, MI 48824, USA
First published on 2nd December 2024
Abstract
Researchers have significantly focused on eco-friendly methods for nanomaterial synthesis to reduce the reliance on hazardous chemicals. In light of this, this study presents an eco-friendly, straightforward, alkali-free method for synthesizing iron–manganese (Fe–Mn) binary oxide (FMBO) and their single oxides by adopting a direct gel formation approach using starch. The synthesized materials were characterized through FTIR, FESEM, EDX, and XRD. Based on the characteristics of the synthesized materials, the probable formation mechanism of nanomaterials within the starch matrix via gel formation was comprehensively investigated. The presence of MnO4− resulted in faster gelation than Fe3+ owing to its stronger oxidizing properties, leading to a more robust gel network that could stabilize nucleation, control the growth of nanoparticles, and thereby reduce particle agglomeration. The synthesized materials were further employed for the adsorptive removal of arsenic, and the findings demonstrate that FMBO was much more effective than the individual oxides in simultaneously eliminating arsenic species (III and V) with an adsorption capacity of 79 mg g−1 for total arsenic (As(III) + As(V)) owing to its nanostructure, surface characteristics, and synergistic effects of Fe and Mn oxides. Our observation suggests that Mn oxide pre-oxidizes As(III) to As(V), which is then efficiently adsorbed by Fe oxide. This study underscores the potential of starch-based nanomatrices in developing efficient materials with enhanced properties.
1. Introduction
Contamination of groundwater by arsenic (As) is a significant environmental and health issue affecting millions of people around the world. Chronic exposure to low concentrations of arsenic has been linked to a wide range of severe health effects, including cancer of internal organs, skin and cardiovascular diseases, and neurological disorders.1 These health concerns are most acute in areas where groundwater is a major source of drinking water. International organizations such as the World Health Organization (WHO) and the United Nations International Children's Emergency Fund (UNICEF) are actively involved in addressing the crucial concerns of arsenic contamination. However, arsenic contamination is a challenging issue that requires coordinated efforts from governments, researchers, and communities. Researchers around the world continue to work tirelessly to ensure safe drinking water and mitigate the long-term health impacts of arsenic exposure.To date, different approaches have been employed for arsenic removal, including reverse osmosis, adsorption, ion exchange, enhanced coagulation, membrane filtration, and electro-dialysis.2,3 Adsorption is considered the most promising and effective because of its easy operation, high efficiency, and cost-effectiveness compared to other methods.4,5 Adsorbents, such as metal oxide nanoparticles, zeolite, carbon-based materials, and clay minerals, have been used to remove As from water.5 However, apart from metal oxide nanoparticles, most of these adsorbents are not very effective, even at low arsenic concentrations, and often produce harmful sludge.6 Consequently, more research efforts have been directed toward metal oxide nanoparticles owing to their higher efficiency and lower risk of generating harmful byproducts.5,6 Furthermore, two species of arsenic (As) are predominantly found in groundwater: arsenite [As(III)] and arsenate [As(V)]. Both the arsenic species are found in water as oxy-ions in organic and inorganic forms.3,6 Adsorption by metal oxides is typically efficient only for As(V) but not for As(III), which is more prevalent in natural water. Notably, As(III) possesses higher mobility and toxicity compared with As(V). To improve the As(III) removal efficiency, it needs to be converted to As(V) through a pre-oxidation process. This conversion is typically achieved using oxidants like ozone, chlorine, and permanganate.6 However, these oxidation methods are expensive and complex to operate. Thus, researchers are focusing on designing adsorbents that can perform both adsorption and oxidation. Instead of using single metal oxides, binary or bimetallic oxides that offer both properties are being developed. Various binary oxides have been utilized for arsenic removal, including Fe–Mn, Fe–Al, Fe–Ce, Zn–Ce, and Ti–Mn.7–11 Among these, Fe–Mn binary oxide (FMBO) has shown the best efficiency and promising results in effectively adsorbing as well as oxidizing arsenic species. The two components of the binary oxides play different roles. Fe oxides dominate the adsorption process due to their higher affinity to As species, which allows them to form complexes on the surface and readily adsorb As. Meanwhile, Mn oxides can effectively oxidize As(III) to As(V).12–14 These synergistic effects and dual functionality of FMBO enhance the arsenic removal capacity.
Numerous approaches have been employed for FMBO synthesis, including hydrothermal, sol–gel, and precipitation methods. Nowadays, to minimize the use of toxic substances in the synthesis procedure, facile green synthesis approaches are being developed using biomaterials. In this context, several studies have reported the synthesis of FMBO using biomaterials, including starch, tea extract, lignin, and egg white.14–17 Of these, starch is the most convenient and prominent candidate for the synthesis of FMBO. Starch is a complex carbohydrate-based bio-polymer composed of amylose and amylopectin molecules, both of which are glucose units connected by glycosidic linkages. Due to its biodegradability, affordability, and safety, starch is well-studied as a reducing agent, capping agent, and template in various synthesis methods, facilitating the formation and stabilization of nanoparticles. When starch is heated in the presence of water, its granules absorb water and swell, disrupting the semi-crystalline structure and leading to the formation of a thick, viscous gel network, allowing it to serve as a potential gelling agent in gel-based synthesis.18–20 Farhana et al. (2021) utilized this property of starch to synthesize magnetic Fe2O3 via direct starch-assisted gel formation.19 In addition, Hoque et al. (2019) explored the stabilization and capping properties of starch by synthesizing uniformly shaped Fe2O3 nanorods.20 The polymeric structure of starch forms a helical-shaped carbon matrix with multiple polyol groups, acting as a soft template for nanoparticles and helping prevent their aggregation.19 Reports on the synthesis of FMBO using starch have been published by some research groups.14,21 In all these reports, FMBO has been synthesized in a starch solution in the presence of NaOH as the precipitant. However, some studies have identified sodium residue as contamination in the final product when NaOH or Na2CO3 is used as the precipitant. Research has shown that the existence of sodium impurities can adversely affect the performance of mixed metal oxide materials by obstructing their active sites or blocking their active planes.22–24 Einaga et al. (2013) found that when NaHCO3 is used in the synthesis of MnxOy, an impure phase of Na2Mn5O10 is formed.22 Mirzaei et al. (2003) found that the presence of Na impurities has a detrimental effect on the catalytic activity of CuMn2O4.23 As the amount of Na impurity increases, the catalyst activity decreases significantly. In another report, Castañeda et al. (2018) found that Na impurities obstruct the active sites of the catalyst, resulting in a decline in its overall performance.24 Interestingly, this study also discovered that the existence of impurities leads to a decrease in the surface area of the material. To solve these issues, researchers have used organic bases, such as tetramethyl ammonium hydroxide, and other quaternary ammonia bases, but these possess toxicity and require safety precautions.25,26 Therefore, it is necessary to develop a method that does not require a base. For this reason, starch can serve as a viable alternative. As stated previously, starch may serve multiple roles during the synthesis process. An ecofriendly method can be adopted by solely utilizing starch for the synthesis of FMBO. Starch possesses the capacity to form a gel when heated with water. This gel-forming property of starch enables the development of a straightforward direct gel mediated synthesis of FMBO without a base. This method will facilitate the investigation of the growth mechanism of FMBO inside starch, which can also be useful for synthesizing other significant nanoparticles through this facile and environmentally sustainable approach.
Herein, we have developed a facile, green, and base-free synthesis route to fabricate FMBO using starch. The starch matrix provides space for the growth of nanomaterials and also helps avoid agglomeration during particle synthesis. Furthermore, the probable mechanism of nanoparticle formation within the starch matrix is discussed. The arsenic removal efficiency and mechanisms of the single oxides and binary oxides are compared.
2. Experimental methods
2.1. Materials
Iron(III) nitrate (Fe(NO3)3·9H2O; Merck, Germany), potassium permanganate (KMnO4; Merck, Spain), nitric acid (HNO3; 65%; Merck, Germany), starch (Scharlau, Spain), pH buffer capsules (Merck, Germany), sodium hydroxide pellets (NaOH; Merck, Germany), disodium hydrogen arsenate heptahydrate (Na2HAsO4·7H2O; Merck, Germany), and arsenic trioxide (As2O3; Sigma Aldrich, Germany) were purchased. All the chemicals were analytical grade and used without further purification. Deionized water (2 μs cm−1) was used for preparing the solutions needed for the experiments. DI water was obtained from a water purification system (Barnstead nano-pure, Thermo Scientific, USA).2.2. Synthesis of nanoparticles
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.55 molar ratio. This composition was selected via trial and error. In accordance with the ratio, pre-calculated quantities of Fe(NO3)3·9H2O and KMnO4 were dissolved in 100 mL DI water under magnetic stirring. The prepared solution was then dropwise added to 200 mL of a 25% starch solution using a burette. Then the solution was continuously stirred for 80 °C until gel formation and left undisturbed for 24 h. Then it was dried at 60 °C for 48 h and subsequently washed with DI water. The product was annealed at 650 °C for 4 h (Fig. 1).
2.55 molar ratio. This composition was selected via trial and error. In accordance with the ratio, pre-calculated quantities of Fe(NO3)3·9H2O and KMnO4 were dissolved in 100 mL DI water under magnetic stirring. The prepared solution was then dropwise added to 200 mL of a 25% starch solution using a burette. Then the solution was continuously stirred for 80 °C until gel formation and left undisturbed for 24 h. Then it was dried at 60 °C for 48 h and subsequently washed with DI water. The product was annealed at 650 °C for 4 h (Fig. 1).
2.3. Characterization of prepared FMBO
The chemical functional groups of the nanoparticles were evaluated by Fourier Transform Infrared (FTIR) spectral analysis. The spectra were recorded between 4000–400 cm−1 in the transmission mode of an FTIR spectrophotometer (FTIR-8400, Shimadzu, Japan). The surface morphology and elemental composition of the prepared samples were analyzed using a field-emission scanning electron microscope (FESEM; JSM-7600F JEOL, Japan) fitted with an energy-dispersive X-ray spectrometer (EDX). The phase composition and crystallinity of the prepared samples were investigated using an X-ray diffractometer (XRD; Smartlab, Rigaku, Japan).2.4. Surface charge analysis
The surface charge of a material can be analyzed using the point of zero charge (PZC) test, which determines the pH value at which the surface charge components are neutral under specified conditions like temperature, pressure, and aqueous solution composition.27,28 According to the salt addition technique, equal amounts of the substrate were added to a series of solutions with consistent ionic strength but varying pH levels.29 In this procedure, 0.2 g of the sample was mixed with 40 mL of a 0.1 M NaNO3 solution placed in several 50 mL centrifuge tubes. The pH of these solutions was adjusted using 0.1 M HNO3 and 0.1 M NaOH to 2, 4, 6, 7, 8, 10, and 12 (±0.1 pH units). The initial pH values (pHi) of the supernatant in each tube were recorded. The samples were then agitated at 200 rpm for 24 h using an orbital shaker (Stuart-SSL1). After allowing the samples to settle, the final pH values (pHf) of the supernatant were measured. The PZC was determined by plotting the change in pH (ΔpH = pHi − pHf) against pHi. This experiment was also conducted using a 0.05 M NaNO3 solution. All experiments were repeated three times, and the average values were recorded.2.5. Sorption experiments
The As(III) and As(V) adsorption characteristics of the FMBO adsorbent were studied using batch experiments at room temperature at an initial pH value of 7.00 ± 0.1. In brief, As2O3 and Na2HAsO4·7H2O were used to prepare stock solutions of As(III) and As(V), respectively. Typically, 20 mg of adsorbent was added to a 50 mL tube containing 50 ppm of As(III) and As(V) in solution. After adjusting the pH to about 7.0 using the buffer capsules, the mixture was agitated on an orbital shaker at 140 rpm for 24 hours while maintaining a temperature of 25 °C. After 24 hours, the equilibrium pH of the solution was determined, and the concentration of residual arsenic (As) in the solution was analyzed using an atomic absorption spectrometer (Varian AA280Z HGAAS, Australia) after filtration with a Whatman grade 40 ashless filter paper.Initially, the absorbance values of standard solutions with known arsenic concentrations were measured, and a calibration curve was drawn by plotting the absorbance against concentration. Once the calibration curve was established, the absorbance of solutions with unknown arsenic concentrations was measured.
The concentration of the analyte was then calculated using the following formula.30
 | (1) |
The amount of arsenic adsorbed at equilibrium (qe) and the removal efficiency (η) for As(III), As(V) and As(III + V) were calculated using eqn (2) and (3), respectively.
 | (2) |
 | (3) |
3. Results and discussion
3.1. Formation of starch-induced FMBO
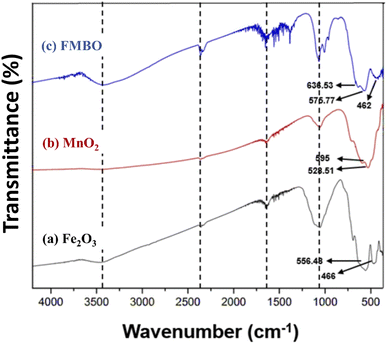
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond;35 additionally, the strong peaks at 1056 cm−1 and 1067 cm−1 indicate the C–O stretching vibrations36 likely due to the adsorption of monomeric glucose units from starch. In Fig. 2(a), the broad peaks at 556.48 cm−1 and 466 cm−1 indicate the Fe–O stretching vibration mode and the bending vibration mode of Fe2O3, respectively.37 The bands at 595 cm−1 and 528.51 cm−1 are attributed to the valency vibrations and indicate the stability of the Mn–O bond.38 In Fig. 2(c), a distinct peak is observed at 636.53 cm−1, which is characteristic of MnO2 and represents the Mn–O–Mn stretching vibration.39–41 The peak observed at 575.77 cm−1 represents the Fe–O stretching vibration, while the small shoulder peak observed at 462 cm−1 corresponds to the Fe–O–Fe stretching vibration.42,43 The peak at 807 cm−1 in Fig. 2(c) may be due to the Fe–O–Mn structure,44 which confirms the formation of the Fe–Mn binary oxide.
O bond;35 additionally, the strong peaks at 1056 cm−1 and 1067 cm−1 indicate the C–O stretching vibrations36 likely due to the adsorption of monomeric glucose units from starch. In Fig. 2(a), the broad peaks at 556.48 cm−1 and 466 cm−1 indicate the Fe–O stretching vibration mode and the bending vibration mode of Fe2O3, respectively.37 The bands at 595 cm−1 and 528.51 cm−1 are attributed to the valency vibrations and indicate the stability of the Mn–O bond.38 In Fig. 2(c), a distinct peak is observed at 636.53 cm−1, which is characteristic of MnO2 and represents the Mn–O–Mn stretching vibration.39–41 The peak observed at 575.77 cm−1 represents the Fe–O stretching vibration, while the small shoulder peak observed at 462 cm−1 corresponds to the Fe–O–Fe stretching vibration.42,43 The peak at 807 cm−1 in Fig. 2(c) may be due to the Fe–O–Mn structure,44 which confirms the formation of the Fe–Mn binary oxide.
The XRD patterns of Fe2O3, MnO2, and FMBO nanoparticles are shown in Fig. 3. The diffraction peaks observed at 2θ = 33.1°, 35.6°, 49.5°, 54.1°, 57.5°, 62.5° and 64.1° in the XRD pattern of Fe2O3 (COD database code: 9000139),45 shown as a black line (a) in Fig. 3, reveals the hexagonal crystal structure of Fe2O3. The red line (b) in Fig. 3 illustrates the XRD pattern of MnO2 (COD database code: 9016667),46 and the diffraction peaks at 2θ = 28.6°, 32.4°, 36.1°, 37.5°, 41.9°, 49.6°, 56.2° and 59.9° correspond to the tetragonal phase of MnO2. The diffraction peaks at 2θ = 30°, 35.4°, 43°, 56.9° and 62.5° in the XRD pattern of FMBO correspond to the Fe/Mn binary oxide Fe2MnO4 (COD database code: 2300618),47 as depicted in blue (c) in Fig. 3. The XRD of FMBO indicates the formation of a cubic Fe2MnO4 structure, indicating a structural change with respect to individually prepared Fe2O3 and MnO2. The crystallite size and lattice parameters of the synthesized materials were calculated using the Scherrer formula and Bragg's law from the base peaks and are summarized in Table S1.†
The morphology of the synthesized products was examined by FESEM. The FESEM image of Fe2O3 illustrates the formation of nanoparticles with uniform orientation, as depicted in Fig. 4(a). The macromolecular structure of starch may possess the ability to suppress the overgrowth and agglomeration of nanoparticles by acting as a stabilizer, leading to uniformity in the shape and size of the particles.19 Fig. 4(b) depicts that MnO2 has a rod-like structure. When the binary oxide was prepared, the surface morphology underwent a complete change compared to the parent oxides. Fig. 4(c) reveals the formation of cubic-shaped FMBO, with a porous network and an irregular and rough surface, which are favorable for efficient adsorption. The formation of pores after calcination at high temperatures is due to the decomposition of complex polymers into CO2 and water. As these molecules evaporate, they leave voids, leading to the creation of a porous and rough surface.
The elemental composition of the products was observed using EDX. The EDX analysis revealed the presence of carbon in all sample surfaces; due to high-temperature calcination, the starch decomposes, and carbon gets deposited on the surface, as shown in Table 1.
| Materials | Fe% | Mn% | O% | C% |
|---|---|---|---|---|
| Fe2O3 | 9.60 | — | 16.01 | 74.39 |
| MnO2 | — | 17.84 | 16.23 | 65.93 |
| FMBO | 5.85 | 16.60 | 25.02 | 52.53 |
In a previous study,20 metal ions in salt solutions were mixed with a starch solution in alkaline conditions, leading to the hydrolysis of metal ions and the subsequent formation of metal hydroxides. These metal hydroxides interacted with starch, resulting in the formation of nanoparticles through hydrogen bond formation. However, in this work, aqueous solutions of potassium permanganate and ferric nitrate were gradually added to a starch solution at a preset concentration, unlike conventional methods that require the addition of alkali in the mixture. This method prevents the formation of metal hydroxides by allowing the metal salts to dissolve and directly form the corresponding ions in the aqueous starch solution. After that, the mixture was stirred vigorously at 80 °C till a gel was formed. The gel formation process involves a highly complex mechanism. Hence, this unexplored mechanism of forming binary metal oxides in a starch solution in the absence of an alkaline medium is an intriguing opportunity for a comprehensive investigation, contributing to the advancement of understanding in this field. Starch, being a polymer composed of interconnected glucose units, undergoes decomposition into smaller structures when heated to specific temperatures. The glucose monomers in starch are typically linked through C-1,4 or C-1,6 linkages, which contribute to the formation of the polymer. It is important to note that the β-C within each glucose unit is in close proximity to the carbonyl group present in the glucose molecule. Consequently, the oxygen atom in β-C becomes polarized and gains the ability to donate or share electrons with electron-deficient sites. Upon the dissolution of metal salts in the aqueous starch solution, Fe3+ and MnO4− ions are formed. The polarized oxygen groups present in the glucose units serve as electron-enriched centers, enabling the donation of electrons to metal cations, which act as electron-deficient centers. Consequently, a redox reaction takes place between the glucose units and metal cations, leading to the formation of a complex metal–glucose structure.
In our study, faster gelation was observed in the case of FMBO compared with iron oxide. This disparity can be attributed to the contrasting oxidizing properties of Fe3+ (a weak oxidizing agent) and MnO4− (a strong oxidizing agent). Slower gel formation with Fe3+ can be explained by the relatively slow rate of its redox reaction with glucose, whereas the rapid reduction of MnO4− leads to the quicker formation of a gel network. Additionally, it is possible that MnO4− interacts with oxygen units other than those present in β-C as it is a potent oxidizing agent, thereby contributing to the formation of a highly robust gel network. The gel network has a highly complex and cross-linked structure, which plays a crucial role in stabilizing nucleation and controlling the growth of nanoparticles. Specific metal ions assume distinct positions within the intricate network, leading to reduced particle agglomeration and enabling precise control of their shape and size.52,53
Subsequently, when the gel is calcined at a high temperature of approximately 650 °C, this complex polymeric structure undergoes decomposition by a combustion process, leading to the generation of carbon dioxide and water; moreover, NO3− from the salts is converted into NO2 and O2, resulting in the formation of pores, voids and defects on the surface and thereby unlocking active sites, as shown in the following equations.50,53
| [C6H10O5]n + 6nO2 → 6nCO2 + 5nH2O |
| [C6H10O5]n + 6(n − x)O2 → 2xC + 6(n − x)CO2 + 5nH2O |
| NO3− → NO2 + 0.5 O2 |
During the calcination process, the generation of oxygen causes the formation of metal oxides, and their oxidation state may vary due to the presence of oxygen. These metal oxides remain in the form of binary oxides. In addition, the presence of starch plays an essential part in controlling the shape as well as textural properties of the composite.54 A possible mechanism of the formation of binary oxide is shown schematically in Fig. 5.
Fig. 5 illustrates the formation of a highly complex metal–starch structure, leading to the formation of a gel. These interactions facilitate controlled nucleation and growth of the nanoparticles. Consequently, these interactions provide stable surface protection and passivation, preventing particle aggregation.55,56
Based on the above discussions, it can be inferred that in this study, FMBO was formed in a well-controlled manner. The FTIR analysis and XRD analysis also confirm the formation of the binary oxide. The XRD analysis suggests that the synthesized FMBO possesses a uniform and compact crystalline structure. The presence of carbon in the synthesized product, as confirmed by the EDX analysis, is possibly due to the above-discussed reaction schemes.
3.2. Arsenic removal using FMBO
The removal of arsenic by the synthesized materials, namely Fe2O3, MnO2, and FMBO, was studied. The sorption capacity of Fe2O3 for As(V) was 5 mg g−1, while there was no sorption of As(III), and the sorption capacity for total arsenic (As(III) + As(V)) was found to be 2.5 mg g−1. The adsorption of As(III) by Fe2O3 is significantly challenging compared to As(V) due to the distinct chemical characteristics of the two species. As(III) predominantly exists in a charge-neutral form, especially in a neutral-pH solution. Since adsorption processes typically rely on electrostatic interactions between the adsorbent surface and the contaminant, the charge neutrality of As(III) prevents any significant electrostatic attraction to the adsorbent. This lack of affinity complicates the adsorption process, which is further hindered by the high mobility and solubility of As(III) in water. In contrast, As(V) exists in a negatively charged form, enabling electrostatic affinity towards the adsorbent. Additionally, As(V) is less mobile in water and hence is easy to adsorb.14 As a result, Fe2O3 was unable to adsorb As(III), while the adsorption of As(V) was fairly possible. Moreover, the sorption study of MnO2 showed that it did not exhibit any sorption capacity for the arsenic species. It has been shown earlier that MnO2 can function as an oxidizing agent and converts As(III) to As(V), while Fe2O3 works as a potential adsorbent for As(V).Consequently, the sorption capacity of FMBO for As(III), As(V), and (As(III) + As(V)) was investigated by the same process. From Fig. 6(a), it is evident that FMBO had the highest sorption capacity for As(III), As(V) and (As(III) + As(V)). The sorption capacity of FMBO was found to be 15 mg g−1, 53 mg g−1 and 79 mg g−1 for As(III), As(V) and (As(III) + As(V)), respectively.
 | ||
| Fig. 6 Arsenic removal by Fe2O3, MnO2, and FMBO. (a) Sorption capacity of As(III), As(V), and (As(III) + As(V)). (b) Sorption efficiency of As(III), As(V) and (As(III) + As(V)). | ||
Fig. 6(b) illustrates the arsenic sorption efficacy of the nanomaterials towards arsenic removal from solutions containing As(V), As(III), and (As(III) + As(V)), respectively. These findings highlight the potential of FMBO as an effective adsorbent for arsenic remediation. Table S2† provides information on the sorption capacity and sorption efficacy of Fe2O3, MnO2 and FMBO for the removal of arsenic from solutions of As(V), As(III) and total arsenic, i.e. (As(III) + As(V)).
The surface charge of FMBO plays a crucial role in facilitating its high adsorption capacity for arsenic species, specifically As(III) and As(V). This relationship is closely tied to the Point of Zero Charge (PZC) of the material. The isoelectric point of FMBO was found to be 9.01, as illustrated in Fig. S2.† PZC is a critical parameter that dictates the surface charge of FMBO under varying pH conditions. When the pH of the solution is below the PZC, the FMBO surface is positively charged due to the adsorption of excess protons. Conversely, when the pH exceeds the PZC, the surface charge turns negative.57 In neutral aqueous media, FMBO remains positively charged because of proton adsorption. This proton-rich surface can act as a Lewis acid.
In aqueous media, As(V) primarily exists as negatively charged species, like H2AsO4− and HAsO4−. These anionic species are effectively adsorbed onto the positively charged FMBO surface driven by coulombic interactions, resulting in its outstanding adsorption capability for As(V).
On the contrary, As(III) predominantly exists as the neutral or weakly charged species H3AsO3 in aqueous media.58 Due to the presence of lone pair electrons on the oxygen atoms, H3AsO3 functions as a Lewis base. The interaction between the Lewis acidic FMBO surface and the Lewis basic As(III) facilitates the adsorption of As(III) onto FMBO.59 Furthermore, As(III) undergoes oxidation to As(V) by donating electrons to Mn(IV) on the FMBO surface, resulting in the formation of As(V) oxyanions. The oxidation of As(III) to As(V) occurs via the sequential reduction of manganese; Mn(IV) is first reduced to Mn(III) and then from Mn(III) to Mn(II) via a two-step electron transfer pathway.14,58 Previous studies by Xiong et al.,60 Ociński et al.61 and Cao et al.62 have explored the arsenic adsorption process, providing significant insights into the oxidation mechanism of As(III) to As(V) facilitated by manganese oxides through a two-step electron transfer pathway, as revealed by XPS analysis. During arsenic adsorption, MnO2 in FMBO undergoes partial reduction to form MnOOH*, indicating that MnO2 accepts an electron, causing the reduction of Mn(IV) to Mn(III) in the first electron transfer step. As MnOOH* accumulates, a subsequent electron transfer step reduces MnOOH* further, forming the lower-valent Mn2+ species. This progression from MnOOH* to more further forms suggests a second electron transfer step. Following the two-step electron transfer pathway, As(III) oxidizes to produce As(V). These newly formed As(V) oxyanions are then adsorbed onto the FMBO surface through electrostatic interactions.
Another factor contributing to the superior adsorption performance of FMBO is the presence of carbon species on its surface, as revealed by the Energy-Dispersive X-ray Spectroscopy (EDX) analysis. It has been reported that carbon has a high adsorption capacity for arsenic.63 The carbon matrix deposited on the FMBO surface during its synthesis from starch enhances the adsorption capacity through a synergistic effect, particularly for the simultaneous removal of As(III) and As(V).
The proposed mechanism of total arsenic removal from water using FMBO is schematically illustrated in Fig. 7.
From the above discussions, superior arsenic adsorption performance of FMBO can be attributed to several factors: (i) the positively charged surface of FMBO effectively interacts with arsenic species, facilitating their adsorption; (ii) MnO2 present within FMBO acts as an oxidizing agent, converting neutral As(III) to negatively charged As(V); (iii) carbon deposited on the FMBO surface synergistically enhances the adsorptive properties of the material; and (iv) the porous nature of the surface and its activation by the formation of surface defects further improve the adsorption efficiency of the adsorbents.
4. Conclusions
A cost-effective and abundant biomaterial, starch, has been used in this work to develop a facile synthetic route for FMBO to achieve total arsenic removal from water. The starch matrix plays a crucial role in the growth of FMBO nanomaterials, leveraging its gel-forming property to stabilize nanoparticle growth and enhance its active surface area. Additionally, starch acts as a carbon source, improving the arsenic adsorption properties of the material. The As(III) and As(V) adsorption capacities of FMBO were higher than those of the individual oxides Fe2O3 and MnO2, exhibiting an arsenic removal capacity of 79 mg g−1. This superior arsenic removal capacity is attributed to the synergistic effect of the binary oxide characteristics. To further understand this synergistic effect, the arsenic removal mechanism was explored, revealing that material composition, morphology and surface charge significantly influence the arsenic removal efficiency. This study opens a new route for the ecofriendly and green synthesis of nanomaterials with unique properties for various applications, including environmental remediation.Data availability
The data that support the conclusions of this research are included in the article and ESI.† Additional data that support the findings of this study are available from the corresponding author upon reasonable request.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors would like to acknowledge the financial support from the Centre for Advanced Scientific Research (CASR), Bangladesh University of Engineering and Technology (BUET).References
- P. L. Smedley and D. G. Kinniburgh, Appl. Geochem., 2002, 17, 517–568 CrossRef CAS.
- S. Alka, S. Shahir, N. Ibrahim, M. J. Ndejiko, D.-V. N. Vo and F. Abd Manan, J. Cleaner Prod., 2021, 278, 123805 CrossRef CAS.
- K. K. Sodhi, M. Kumar, P. K. Agrawal and D. K. Singh, Environ. Technol. Innovation, 2019, 16, 100462 CrossRef.
- R. E. C. Torrejos, G. M. Nisola, H. S. Song, L. A. Limjuco, C. P. Lawagon, K. J. Parohinog, S. Koo, J. W. Han and W.-J. Chung, Chem. Eng. J., 2017, 326, 921–933 CrossRef CAS.
- L. Hao, M. Liu, N. Wang and G. Li, RSC Adv., 2018, 8, 39545–39560 RSC.
- S. I. Siddiqui and S. A. Chaudhry, Process Saf. Environ. Prot., 2017, 111, 592–626 CrossRef CAS.
- G. Zhang, F. Liu, H. Liu, J. Qu and R. Liu, Environ. Sci. Technol., 2014, 48, 10316–10322 CrossRef CAS PubMed.
- C. Meng, Q. Mao, L. Luo, J. Zhang, J. Wei, Y. Yang, M. Tan, Q. Peng, L. Tang and Y. Zhou, Sep. Purif. Technol., 2018, 191, 314–321 CrossRef CAS.
- U. K. Sahu, M. K. Sahu, S. S. Mohapatra and R. K. Patel, J. Environ. Chem. Eng., 2016, 4, 2892–2899 CrossRef CAS.
- A. Dhillon, S. K. Soni and D. Kumar, J. Fluorine Chem., 2017, 199, 67–76 CrossRef CAS.
- W. Zhang, C. Liu, T. Zheng, J. Ma, G. Zhang, G. Ren, L. Wang and Y. Liu, J. Hazard. Mater., 2018, 353, 410–420 CrossRef CAS PubMed.
- J. Li, H. Gyoten, A. Sonoda, Q. Feng and M. Xue, RSC Adv., 2017, 7, 1490–1497 RSC.
- D. Nielsen-Franco and M. Ginder-Vogel, ACS ES&T Water, 2023, 3, 1060–1070 Search PubMed.
- F. Xu, H. Chen, Y. Dai, S. Wu and X. Tang, J. Environ. Manage., 2019, 245, 160–167 CrossRef CAS PubMed.
- W. Chen, J. Wu, X. Weng, G. Owens and Z. Chen, J. Cleaner Prod., 2022, 363, 132406 CrossRef CAS.
- H. Yu, J. Yang, P. Shi, M. Li and J. Bian, ACS Omega, 2021, 6, 16837–16846 CrossRef CAS PubMed.
- S. M. Hashemi, Z. Ataollahi, S. Hasani and A. Seifoddini, J. Sol-Gel Sci. Technol., 2023, 106, 23–36 CrossRef CAS.
- I. Uddin, S. M. Abzal, K. Kalyan, S. Janga, R. Patel and J. K. Dash, J. Electron. Mater., 2023, 52, 1710–1716 CrossRef CAS.
- F. Z. Farhana, M. Umer, A. Saeed, A. S. Pannu, M. Shahbazi, A. Jabur, H. J. Nam, K. Ostrikov, P. Sonar and S. H. Firoz, ACS Appl. Nano Mater., 2021, 4, 1175–1186 CrossRef CAS.
- M. I. U. Hoque, Y. Yamauchi, R. Naidu, R. Holze, R. Saidur, Q. Qu, M. M. Rahman, N. L. Torad, M. S. A. Hossain and M. Kim, ChemistrySelect, 2019, 4, 3730–3736 CrossRef CAS.
- X. Yan, Y. Fei, L. Zhong and W. Wei, Sci. Total Environ., 2020, 707, 136064 CrossRef CAS PubMed.
- H. Einaga, N. Maeda and Y. Teraoka, Appl. Catal., B, 2013, 142, 406–413 CrossRef.
- A. A. Mirzaei, H. R. Shaterian, R. W. Joyner, M. Stockenhuber, S. H. Taylor and G. J. Hutchings, Catal. Commun., 2003, 4, 17–20 CrossRef CAS.
- R. Castañeda, L. Pascual and A. Martínez-Arias, Catal. Commun., 2018, 108, 88–92 CrossRef.
- S. Ding, C. Zhu, H. Hojo and H. Einaga, J. Hazard. Mater., 2022, 424, 127523 CrossRef CAS PubMed.
- C. E. Shuck, K. Ventura-Martinez, A. Goad, S. Uzun, M. Shekhirev and Y. Gogotsi, ACS Chem. Health Saf., 2021, 28, 326–338 CrossRef CAS.
- M. Kosmulski, Adv. Colloid Interface Sci., 2020, 275, 102064 CrossRef CAS PubMed.
- I. A. Jasim, T. S. Mahmood, S. K. Al-Mamoori and L. A. Al-Maliki, J. Urban Regen. Renew., 2021, 14, 264–271 Search PubMed.
- T. Mahmood, M. T. Saddique, A. Naeem, P. Westerhoff, S. Mustafa and A. Alum, Ind. Eng. Chem. Res., 2011, 50, 10017–10023 CrossRef CAS.
- R. Islam, J. Al Foisal, M. Rahman, L. A. Lisa and D. K. Paul, Afr. J. Environ. Sci. Technol., 2016, 10, 9–17 CrossRef CAS.
- W. Liang, S. Wei, L. Lan, J. Chen, Y. Zhou, J. Zhao, H. Wang, R. Gao and F. Zeng, RSC Adv., 2023, 13, 24201–24210 RSC.
- A. Tadjarodi, M. Imani and M. Salehi, RSC Adv., 2015, 5, 56145–56156 RSC.
- B. Sun, Y. Wang, H. Bai, X. Liu, X. Li, L. Yang, H. Liu, X. Li and M. Wei, Surf. Interfaces, 2024, 49, 104402 CrossRef CAS.
- M. Zhang, M. Wu, Z. Wang, R. Cheng, D. Y. Leung, Z. Lu and S. P. Feng, Mater. Sci. Energy Technol., 2020, 3, 734–741 CAS.
- K. M. S. Meera, R. M. Sankar, J. Paul, S. N. Jaisankar and A. B. Mandal, Phys. Chem. Chem. Phys., 2014, 16, 9276–9288 RSC.
- X. Chen, N. Yu, L. Zhang, Z. Liu, Z. Wang and Z. Chen, RSC Adv., 2015, 5, 96888–96895 RSC.
- D. Kumar, H. Singh, S. Jouen, B. Hannoyer and S. Banerjee, RSC Adv., 2015, 5, 7138–7150 RSC.
- M. Qin, H. Zhao, W. Yang, Y. Zhou and F. Li, RSC Adv., 2016, 6, 23905–23912 RSC.
- R. Najjar, R. Awad and A. Abdel-Gaber, J. Supercond. Novel Magn., 2019, 32, 885–892 CrossRef CAS.
- J. Xu, Y.-Q. Deng, X.-M. Zhang, Y. Luo, W. Mao, X.-J. Yang, L. Ouyang, P. Tian and Y.-F. Han, ACS Catal., 2014, 4, 4106–4115 CrossRef CAS.
- W. E. Lee, R. Gadow, V. Mitic and N. Obradovic, III Advanced Ceramics and Applications conference, Springer, Serbia, 2016 Search PubMed.
- K. Song, W. Kim, C.-Y. Suh, D. Shin, K.-S. Ko and K. Ha, Powder Technol., 2013, 246, 572–574 CrossRef CAS.
- N. Chubar, V. Gerda, M. Szlachta and G. Yablokova, Solid State Sci., 2021, 121, 106752 CrossRef CAS.
- M. H. Habibi and V. Mosavi, J. Mater. Sci.: Mater. Electron., 2017, 28, 13643–13648 CrossRef CAS.
- R. Blake, R. Hessevick, T. Zoltai and L. W. Finger, Am. Mineral., 1966, 51, 123–129 CAS.
- D. Taylar, J. Therm. Expansion Data, 1985, 84, 181 Search PubMed.
- E. Solano, C. Frontera, T. Puig, X. Obradors, S. Ricart and J. Ros, J. Appl. Crystallogr., 2014, 47, 414–420 CrossRef CAS.
- X. Sun, C. Zheng, F. Zhang, Y. Yang, G. Wu, A. Yu and N. Guan, J. Phys. Chem. C, 2009, 113, 16002–16008 CrossRef CAS.
- A. A. Ullah, A. F. Kibria, M. Akter, M. Khan, M. Maksud, R. A. Jahan and S. H. Firoz, J. Saudi Chem. Soc., 2017, 21, 830–836 CrossRef.
- H. E. Ali, M. Abdel-Aziz, A. Aboraia, I. Yahia, H. Algarni, V. Butova, A. V. Soldatov and Y. Khairy, Optik, 2021, 227, 165969 CrossRef CAS.
- Y. Chen, Z. Zeng, Y. Li, Y. Liu, Y. Chen, Y. Wu, J. Zhang, H. Li, R. Xu and S. Wang, J. Colloid Interface Sci., 2020, 573, 287–298 CrossRef CAS PubMed.
- S. Wintzheimer, T. Granath, M. Oppmann, T. Kister, T. Thai, T. Kraus, N. Vogel and K. Mandel, ACS Nano, 2018, 12, 5093–5120 CrossRef CAS PubMed.
- A. K. Zak, W. A. Majid, M. Mahmoudian, M. Darroudi and R. Yousefi, Adv. Powder Technol., 2013, 24, 618–624 CrossRef.
- W. Ojok, E. Ntambi, J. Bolender, J. Wasswa, W. Wanasolo and B. Moodley, Carbohydr. Polym. Technol. Appl., 2022, 4, 100241 CAS.
- M. Á. Aguilar-Méndez, T. Espinosa-Solares, F. d. M. Guerrero-Toledo, D. Canseco-González, A. Velázquez-Hernández, G. S. Aguilar-Moreno and E. Navarro-Cerón, IET Nanobiotechnol., 2020, 14, 94–97 CrossRef PubMed.
- H. Dong, Y. Cheng, Y. Lu, K. Hou, L. Zhang, L. Li, B. Wang, Y. Wang, Q. Ning and G. Zeng, Sep. Purif. Technol., 2019, 210, 504–510 CrossRef CAS.
- R. S. Bangari, V. K. Yadav, J. K. Singh and N. Sinha, ACS Omega, 2020, 5, 10301–10314 CrossRef CAS PubMed.
- S. A. Baig, T. Sheng, Y. Hu, J. Xu and X. Xu, Clean:Soil, Air, Water, 2015, 43, 13–26 CAS.
- J. Chen, J. Wang, G. Zhang, Q. Wu and D. Wang, Chem. Eng. J., 2018, 334, 1518–1526 CrossRef CAS.
- Y. Xiong, Q. Tong, W. Shan, Z. Xing, Y. Wang, S. Wen and Z. Lou, Appl. Surf. Sci., 2017, 416, 618–627 CrossRef CAS.
- D. Ociński, I. Jacukowicz-Sobala, P. Mazur, J. Raczyk and E. Kociołek-Balawejder, Chem. Eng. J., 2016, 294, 210–221 CrossRef.
- C.-Y. Cao, J. Qu, W.-S. Yan, J.-F. Zhu, Z.-Y. Wu and W.-G. Song, Langmuir, 2012, 28, 4573–4579 CrossRef CAS PubMed.
- D. Mohan and C. U. Pittman Jr, J. Hazard. Mater., 2007, 142, 1–53 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra06319h |
| This journal is © The Royal Society of Chemistry 2024 |