Open Access Article
Open Access ArticleElectrochemical sensors based on the composite of reduced graphene oxide and a multiwalled carbon nanotube-modified glassy carbon electrode for simultaneous detection of hydroquinone, dopamine, and uric acid†
Wulan Tri Wahyuni ab,
Shafa Aini Hasnawati Ta'aliaa,
Ari Yustisia Akbar
ab,
Shafa Aini Hasnawati Ta'aliaa,
Ari Yustisia Akbar c,
Bunga Rani Elvira
c,
Bunga Rani Elvira c,
Irkham
c,
Irkham d,
Isnaini Rahmawati
d,
Isnaini Rahmawati e,
Ruri Agung Wahyuonof and
Budi Riza Putra
e,
Ruri Agung Wahyuonof and
Budi Riza Putra *c
*c
aAnalytical Chemistry Division, Department of Chemistry, Faculty of Mathematics and Natural Sciences, Kampus IPB Dramaga, Bogor 16680, Indonesia. E-mail: wulantriws@apps.ipb.ac.id
bTropical Biopharmaca Research Center, IPB University, Bogor 16680, Indonesia
cResearch Center for Metallurgy, National Research and Innovation Agency (BRIN), PUSPIPTEK Gd. 470, South Tangerang, Banten 15315, Indonesia. E-mail: budi.riza.putra@brin.go.id
dDepartment of Chemistry, Faculty of Mathematics and Natural Sciences, University of Padjajaran, Bandung 45363, Indonesia
eDepartment of Chemistry, Faculty of Mathematics and Natural Sciences, University of Indonesia, Depok 16424, Indonesia
fDepartment of Engineering Physics, Faculty of Industrial Technology and Systems Engineering, Institut Teknologi Sepuluh Nopember, Jl. Arif Rahman Hakim, Kampus ITS Keputih-Sukolilo, Surabaya 60111, Indonesia
First published on 3rd September 2024
Abstract
Using a simple drop-casting technique, we successfully fabricated a sensitive electrochemical sensor based on the composite of reduced graphene oxide (RGO) and multiwalled carbon nanotubes (MWCNT) deposited on the surface of a glassy carbon electrode (GCE) for individual and simultaneous measurements of hydroquinone (HQ), dopamine (DA), and uric acid (UA). The nanocomposite of RGO/MWCNT was further characterized in terms of its structural properties, surface morphology, and topography using Raman, FT-IR spectroscopy, SEM, HRTEM, and AFM. Then, the proposed sensor for simultaneous measurement of HQ, DA, and UA based on RGO/MWCNT-modified GCE was investigated for its electrochemical behavior and electroanalytical performances using cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS), and differential pulse voltammetry (DPV). In addition, the composition ratio between RGO and MWCT was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 showing the highest electrochemical response for simultaneous detection of HQ, DA, and UA. Owing to the synergistic effect between RGO and MWCNT leading to excellent conductivity properties, the proposed sensor exhibited improved electrochemical response at pH 7 toward the oxidation processes of HQ, DA, and UA on the surface of modified electrode. The proposed sensor demonstrated three well-defined anodic peaks of these analytes with their linear concentrations ranges of 3.0–150.0 μM for HQ, 4.0–100.0 μM for DA, and 2.0–70.0 μM for UA. The limit of detection values for the simultaneous detection of HQ, DA, and UA were found as follows 0.400 ± 0.014, 0.500 ± 0.006, and 0.300 ± 0.016 μM, respectively. The additional features of this proposed sensor are high reproducibility and stability for the simultaneous detection of HQ, DA, and UA with negligible interference effect from interferents such as Mg2+, K+, Cl−, ascorbic acid, and glucose. An acceptable percentage of recovery was also shown by this sensor for simultaneous measurements of HQ, DA, and UA using 6 samples of human urine. In summary, the RGO/MWCNT nanocomposite has been shown to be a promising platform for rapid, simple, and reliable determination of simultaneous measurements of HQ, DA, and UA in practical applications.
1 showing the highest electrochemical response for simultaneous detection of HQ, DA, and UA. Owing to the synergistic effect between RGO and MWCNT leading to excellent conductivity properties, the proposed sensor exhibited improved electrochemical response at pH 7 toward the oxidation processes of HQ, DA, and UA on the surface of modified electrode. The proposed sensor demonstrated three well-defined anodic peaks of these analytes with their linear concentrations ranges of 3.0–150.0 μM for HQ, 4.0–100.0 μM for DA, and 2.0–70.0 μM for UA. The limit of detection values for the simultaneous detection of HQ, DA, and UA were found as follows 0.400 ± 0.014, 0.500 ± 0.006, and 0.300 ± 0.016 μM, respectively. The additional features of this proposed sensor are high reproducibility and stability for the simultaneous detection of HQ, DA, and UA with negligible interference effect from interferents such as Mg2+, K+, Cl−, ascorbic acid, and glucose. An acceptable percentage of recovery was also shown by this sensor for simultaneous measurements of HQ, DA, and UA using 6 samples of human urine. In summary, the RGO/MWCNT nanocomposite has been shown to be a promising platform for rapid, simple, and reliable determination of simultaneous measurements of HQ, DA, and UA in practical applications.
1 Introduction
Electrochemical sensors have shown great promise for the simultaneous detection of multiple analytes in various fields including environmental markers and biological molecules due to their simplicity, rapid, sensitive, and efficient properties.1,2 One of the attractive topics is the development of electrochemical sensors for the detection of several important biological molecules such as hydroquinone (HQ), dopamine (DA), and uric acid (UA) for further analytical applications. These three molecules have been employed in several recent studies of electrochemical sensors with substantial environmental and biological implications for the health of human beings.3–6 HQ or 1,4-dihydroxybenzene is a common constituent of industrial effluents from coal tar production, oil refineries, leather, cosmetics, plastic, paper, steel, and pharmaceutical sectors.7,8 This chemical has been recognized by the European Union (EU) and the United States Environmental Protection Agency (US EPA) as an environmental pollutant that poses health risks at high levels in humans such as dermatitis, irritation, fatigue, liver and kidney disease, and cancer.9,10 In addition, the usage of HQ as skin-lightening cosmetics has been prohibited in many countries worldwide with a 2% permissible limit for topical treatments intended for dermatological purposes.11 According to the Occupational Safety and Health Administration (OSHA) and Threshold Limit Values (TLV), the Permissible Exposure Limit (PEL) of hydroquinone to the human body is 2 mg m−3.12 Another previous work has reported that the HQ level in human urine in the concentration range of 0.6 to 4.2 μg mL−1 equivalent to 5.45–38.2 μM.13Meanwhile, DA and UA are important biomolecules in regulating physiological processes in the human metabolism, central nervous, and renal system.14,15 DA is an essential neurotransmitter that plays a significant role in neurological functions and its abnormal concentrations in the human body may be responsible for diseases such as Parkinson's disease and schizophrenia.16 Several studies have found that the physiological concentrations of dopamine in the human body vary significantly within the urine and cerebral fluid at 5 nM and in blood at less than 0.13 nM.17,18 Human blood also contains UA, a metabolic by-product of purine metabolism, which presents in normal levels at 140–420 μM, and its accumulation causes the blood to become more acidic which can lead to serious diseases such as hyperuricemia, gout, and Lesch-Nyhan syndrome.19,20 Therefore, it is crucial to develop a simple and sensitive analytical method for the simultaneous detection of HQ, DA, and UA in synthetic solutions and real samples. This developed approach will be valuable for early detection of abnormal concentrations of these biological molecules and as a sign of potential disorders for human health.
Several analytical techniques based on high-performance liquid chromatography,21–23 Raman spectroscopy,24–26 fluorescence,27,28 capillary electrophoresis,29–31 gas chromatography-mass spectrometry,32–35 chemiluminescence,36–38 photoelectrochemical,39–41 have been developed for the quantitative analysis of HQ, DA, and UA. These techniques have been widely applied as quality control procedures in many laboratories due to their sensitivity and accuracy in detecting these three biomolecules. Although the above methods have been developed in great advance based on their performances, there are still limitations to be addressed, including high cost, the need for sophisticated instruments, and time-consuming sample pretreatment. Compared to these methods, electrochemical methods have been widely developed for their benefits of simple, low cost, high sensitivity, rapid selectivity, portable, and selectivity in detecting HQ, DA, and UA.42–46 Furthermore, a thorough understanding of the electron transport kinetics of HQ, DA, and UA at the electrode surface is necessary to provide insight into chemical reactions in the biological system of human metabolism.
The morphology of reduced graphene oxide (RGO) as two-dimensional (2D) nanomaterials could provide a large surface area and high conductivity properties which is beneficial to be employed for sensor fabrication.47,48 However, the enhanced conductivity of RGO might be lowered due to the possibility of restacking and aggregation by the presence of oxygen-containing groups on their 2D structures.49,50 One way to improve the RGO conductivity is by incorporating it with multi-walled carbon nanotubes (MWCNT) to produce a stable dispersion of RGO and MWCNT nanocomposite via noncovalent interactions. This nanocomposite has demonstrated good electrical conductivity and high chemical and thermal stability which are advantageous to be employed as a material platform of electrochemical sensors.51,52 The anticipated outcome of the unique properties of RGO and MWCNT could work synergistically to improve the electrochemical oxidation current of HQ, DA, and UA on the electrode surface. In addition, the nanocomposite of RGO and MWCNT has been previously employed as an electrode modifier for the simultaneous detection of nitrite and nitrate,53 ascorbic acid, dopamine, and uric acid,54,55 HQ, catechol, and resorcinol,56,57 hydrogen peroxide, chlorine, and coenzyme,58,59 bisphenol A, 8-hydroxy-2′-deoxyguanosine, and HQ,60 and food dyes (Sunset Yellow and Tartrazine).61
To the author's knowledge, no published studies have reported on the simultaneous measurements of HQ, DA, and UA using nanocomposite consisting of RGO and MWCNT as a material platform of electrode modifiers for the electrochemical sensors. This proposed sensor is developed by modifying the surface of a glassy carbon electrode (GCE) with the RGO/MWCNT composite and employed for the simultaneous determination of HQ, DA, and UA in human urine samples. Based on the results of this work, RGO/MWCNT nanocomposite could improve the conductivity of the modified electrode and thus provide excellent electroanalytical performance for simultaneous detection of HQ, DA, and UA. Under optimum conditions, the proposed sensor shows a remarkable electrochemical response for simultaneous HQ, DA, and UA measurements with low detection limits. This proposed sensor exhibits good reproducibility and repeatability, and negligible effect from common species as potential interferences. In addition, an acceptable recovery percentage is obtained in the practical applications of this proposed sensor for the simultaneous determination of HQ, DA, and UA in 6 samples of human urine.
2 Experimental
2.1. Reagent and apparatus
Graphite powder with carbon content ≥99.95%, multiwalled carbon nanotubes (MWCNT) (>90% carbon basis with diameter versus length: 110–170 nm versus 5–9 μm), N,N-dimethylformamide (DMF) (≥99.8%) (CAS number: 68-12-2), KMnO4 (≥99%), H2SO4 (95–98%), H2O2 30%, NaH2PO4 (≥99%), Na2HPO4 (≥99%), NaNO3 (≥99%), K3[Fe(CN)6] (≥99%), glucose (≥99%), urea (≥99%), ascorbic acid (≥99%), MgSO4 (≥99%), uric acid (≥99%) (CAS number: 1198-77-2), dopamine hydrochloride (≥99%) (CAS number: 62-31-7), and hydroquinone (≥99%) (CAS number: 123-31-9) were obtained from Sigma Aldrich. All chemicals were of analytical grade and employed as received without further purification. Deionized water (conductivity ≈ 0.05 μS cm−1) was used throughout the experiments.The Raman spectra of graphite, graphene oxide (GO), and reduced graphene oxide (RGO) were derived from a Micro Confocal Hyperspectral 3D Imaging Raman Spectrometer (HORIBA LabRAM HR Evolution, Japan). Meanwhile, the infrared (IR) spectra from these materials using a Fourier transform infrared (FTIR) spectrometer (Infrared Bruker Tensor 37, Germany). The photograph of scanning electron microscopy (SEM) for RGO, multiwalled carbon nanotubes (MWCNTs), and its composites were obtained using SEM JEOL JSM-IT 200. Moreover, transmission electron microscope (TEM) images of RGO, MWCNT, and RGO/MWCNT composite were derived from an FEI Tecnai G2 20 S-Twin TEM. The electrochemical impedance spectroscopy (EIS) studies were performed using Sensit BT (PalmSens BV, Houten, The Netherlands). All electrochemical experiments were conducted using PalmSens Emstat3+ Blue (PalmSens BV, Houten, The Netherlands) equipped with 3-electrodes systems. It comprises a glassy carbon electrode (GCE with 3 mm in diameter) from IJ Cambria Scientific, Ag/AgCl as the reference electrode, and platinum wire as the auxiliary electrode. All electrochemical experiments were performed using standard laboratory apparatus such as Pyrex glassware, analytical balance, and micropipettes for solution preparation at ambient temperature.
2.2. Synthesis of reduced graphene oxide (RGO) from graphite precursor
First, graphene oxide (GO) material was synthesized from graphite precursor following the modified Hummer's method.62 Briefly, 1.0 g of graphite was mixed with 0.5 g of NaNO3 in 25 mL of concentrated H2SO4 and subsequently cooled at 0 °C for 1 hour in stirring conditions. Then, 3.0 g of KMnO4 was slowly added to the mixture solution, and the temperature was kept below 20 °C in stirring condition for 1 hour. Next, 50 mL of deionized water was added to the mixture solution to produce an exothermic reaction at 90–95 °C. The obtained solution was stirred for 1 hour and subsequently left for 30 minutes. The reaction in the mixture solution was finally terminated by adding 50 mL of H2O2 30% in stirring conditions for 1 hour. The solution mixture was cooled at room temperature, washed with deionized water, and air-dried to obtain the GO powder.The reduction of GO material was achieved by using ascorbic acid as a reducing agent. Approximately 400 mg of GO powder was diluted in 400 mL of deionized water and added with 4.0 g of ascorbic acid in stirring conditions for 30 minutes at 60 °C. The reduced GO material was centrifuged at 4000 rpm for 40 minutes to remove the supernatant to obtain the black paste. Next, H2O2 30% was added to the black paste in stirring condition for 30 minutes at 60 °C to oxidize the remaining ascorbic acid. The black product containing RGO material was obtained by centrifugation at 4000 rpm with subsequent washing using ethanol and deionized water. The resulting powder was dried in the oven for 24 hours at 120 °C.
The powder of RGO was characterized using Raman and FTIR spectroscopy to observe the changes in functional groups in its chemical structure.
2.3. Fabrication of GCE-modified RGO/MWCNT in different compositions
The stock solution of RGO was prepared with deionized water as a solvent while MWCNT was dissolved in DMF to obtain each concentration as 1 mg mL−1. The composite solution containing RGO and MWCNT was prepared in 3 different compositions at the weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3
1, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, and 7
7, and 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to obtain a total concentration of 1 mg mL−1. Each composite solution was then stirred for 30 min and followed by sonication for 30 min. Next, about 4.0 μL of each composite solution was drop-casted onto the surface of a glassy carbon electrode (GCE). The GCE modified each composite solution was then placed in the oven at 85 °C for 5 min to obtain a stable film of RGO/MWCNT. Fig. 1a illustrates the schematic diagram of the fabrication process of RGO/MWCNT composite and its deposition on the surface of GCE.
3 to obtain a total concentration of 1 mg mL−1. Each composite solution was then stirred for 30 min and followed by sonication for 30 min. Next, about 4.0 μL of each composite solution was drop-casted onto the surface of a glassy carbon electrode (GCE). The GCE modified each composite solution was then placed in the oven at 85 °C for 5 min to obtain a stable film of RGO/MWCNT. Fig. 1a illustrates the schematic diagram of the fabrication process of RGO/MWCNT composite and its deposition on the surface of GCE.
2.4. Investigation of the electrochemical behaviour of GCE-modified RGO/MWCNT in different compositions
The electrochemical behaviour of bare GCE and modified GCE (RGO-modified GCE, MWCNT-modified GCE, and three different weight ratios of (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 3
3, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, and 1
7, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) of RGO/MWCNT-modified GCE) were evaluated in 50 μM hydroquinone (HQ), 30 μM dopamine (DA), and 10 μM uric acid (UA) in 0.1 M of pH 7 phosphate buffer. The phosphate buffer was prepared by mixing 0.1 M NaH2PO4 with 0.1 M Na2HPO4 in certain volume ratio, then its final pH was adjusted into pH 7. Electroanalytical measurements for all modified electrodes were performed using differential pulse voltammetry (DPV) at a potential window of −0.2 V to +0.6 V versus Ag/AgCl, at a scan rate of 25 mV s−1, a potential step of 5 mV, a potential pulse of 50 mV, and a pulse time of 0.1 s. All electroanalytical measurements for simultaneous detection of HQ, DA, and UA using the modified electrodes were carried out in triplicate experiments.
1) of RGO/MWCNT-modified GCE) were evaluated in 50 μM hydroquinone (HQ), 30 μM dopamine (DA), and 10 μM uric acid (UA) in 0.1 M of pH 7 phosphate buffer. The phosphate buffer was prepared by mixing 0.1 M NaH2PO4 with 0.1 M Na2HPO4 in certain volume ratio, then its final pH was adjusted into pH 7. Electroanalytical measurements for all modified electrodes were performed using differential pulse voltammetry (DPV) at a potential window of −0.2 V to +0.6 V versus Ag/AgCl, at a scan rate of 25 mV s−1, a potential step of 5 mV, a potential pulse of 50 mV, and a pulse time of 0.1 s. All electroanalytical measurements for simultaneous detection of HQ, DA, and UA using the modified electrodes were carried out in triplicate experiments.
2.5. Evaluation of the electroanalytical performance of RGO/MWCNT-modified GCE
The analytical performance of the RGO/MWCNT-modified GCE was investigated in several analytical parameters involving linearity, limit of detection (LOD), limit of quantification (LOQ), reproducibility, sensitivity, precision, and stability. The electroanalytical measurement was investigated using the DPV technique at a potential window of −0.2 V to +0.6 V versus Ag/AgCl, at a scan rate of 25 mV s−1, a potential step of 5 mV, a potential pulse of 25 mV, and a pulse time of 0.01 s.2.6. Linearity, LOD, and LOQ
The linearity of individual analytes by gradually decreasing the concentration of one compound while the concentrations of the other two compounds were fixed. LOD and LOQ were calculated based on the ratio of signal to noise (S/N ≈ 3 for LOD and S/N ≈ 10 for LOQ). Linearity was evaluated by measuring HQ solution in the concentration range of 3.0–53.0 μM with the concentrations of DA and UA being fixed at 4.0 μM and 2.0 μM in 0.1 M of pH 7 phosphate buffer. The linearity was also investigated by measuring DA solution in the concentration ranges of 4.0–34.0 μM with the concentrations of HQ and UA fixed at 3.0 μM and 2.0 μM in 0.1 M of pH 7 phosphate buffer. In addition, the linearity for UA was carried out by measuring the UA solution in the concentration range of 2.0–12.0 μM with the concentrations of HQ and DA being fixed at 3.0 μM and 4.0 μM in 0.1 M of pH 7 phosphate buffer. All measurements were performed in triplicate experiments using the DPV technique with the conditions obtained from the previous experiment. Moreover, the HQ, DA, and UA measurements showed the maximum sensitivity when the coefficient of determination (R2) close to 1 was obtained. The sensitivity value can be determined from the slope of the calibration curve which was calculated from triplicate experiments for simultaneous detection of HQ, DA, and UA.2.7. Reproducibility, stability, and selectivity of the proposed sensor
The evaluation of sensor reproducibility was performed by preparing a solution containing 50.0 μM HQ, 30.0 μM DA, and 10.0 μM UA in 0.1 M of pH 7 phosphate buffer and measured with five individual electrodes of RGO/MWCNT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-modified GCE in triplicate experiments. Meanwhile, the sensor stability was evaluated by measuring the solution containing 50.0 μM HQ, 30.0 μM DA, and 10.0 μM UA in 0.1 M of pH 7 phosphate buffer in 7 repetitions using a similar electrode of RGO/MWCNT (1
1)-modified GCE in triplicate experiments. Meanwhile, the sensor stability was evaluated by measuring the solution containing 50.0 μM HQ, 30.0 μM DA, and 10.0 μM UA in 0.1 M of pH 7 phosphate buffer in 7 repetitions using a similar electrode of RGO/MWCNT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-modified GCE. The sensor reproducibility and stability parameters were evaluated as a percentage of the relative standard deviation (%RSD) for simultaneous detection of HQ, DA, and UA. In addition, the selectivity of RGO/MWCNT (1
1)-modified GCE. The sensor reproducibility and stability parameters were evaluated as a percentage of the relative standard deviation (%RSD) for simultaneous detection of HQ, DA, and UA. In addition, the selectivity of RGO/MWCNT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-modified GCE was investigated by simultaneously measuring HQ, DA, and UA in the presence of K+, Mg2+, and Cl−, glucose, urea, and ascorbic acid as interfering compounds. Each interfering compound was prepared at a concentration of 50.0 μM and mixed with the solution containing 50.0 μM HQ, 30.0 μM DA, and 10.0 μM UA in 0.1 M of pH 7 phosphate buffer. The solution was then measured in triplicates using the DPV technique under similar previous experimental conditions.
1)-modified GCE was investigated by simultaneously measuring HQ, DA, and UA in the presence of K+, Mg2+, and Cl−, glucose, urea, and ascorbic acid as interfering compounds. Each interfering compound was prepared at a concentration of 50.0 μM and mixed with the solution containing 50.0 μM HQ, 30.0 μM DA, and 10.0 μM UA in 0.1 M of pH 7 phosphate buffer. The solution was then measured in triplicates using the DPV technique under similar previous experimental conditions.
2.8. Simultaneous detection of HQ, DA, and UA in the sample of human urine using RGO/MWCNT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) 1)-modified GCE
1)-modified GCE
The performance of the RGO/MWCNT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-modified GCE for the simultaneous detection of HQ, DA, and UA was evaluated in the human urine samples using the DPV technique with the standard addition method. The human urine samples were obtained from 6 volunteers of healthy adults and diluted 100 times for simultaneous measurements of HQ, DA, and UA using 0.1 M of pH 7 phosphate buffer. Ethical consent for collecting human urine from adult healthy donors was received from IPB University, Indonesia. Informed written consent was obtained from all volunteers who contributed human urine to this work. The urine samples were collected in clean beakers under instruction to collect midstream urine from healthy volunteers. Samples were then collected and refrigerated within 4 hours of collection. Approximately, a 5 mL diluted sample of human urine was added with a standard solution in successive concentrations for HQ (5.0, 10.0, 15.0, 20.0, and 25.0 μM), for DA (3.0, 6.0, 9.0, 12.0, and 15.0 μM), and for UA (1.0, 2.0, 3.0, 4.0, and 5.0 μM). The human urine samples were then measured using the DPV technique with similar optimized experimental conditions in triplicates. The concentrations of HQ, DA, and UA in the sample of human urine were then calculated as the x-intercept of the standard addition calibration curve as follows in eqn (1):
1)-modified GCE for the simultaneous detection of HQ, DA, and UA was evaluated in the human urine samples using the DPV technique with the standard addition method. The human urine samples were obtained from 6 volunteers of healthy adults and diluted 100 times for simultaneous measurements of HQ, DA, and UA using 0.1 M of pH 7 phosphate buffer. Ethical consent for collecting human urine from adult healthy donors was received from IPB University, Indonesia. Informed written consent was obtained from all volunteers who contributed human urine to this work. The urine samples were collected in clean beakers under instruction to collect midstream urine from healthy volunteers. Samples were then collected and refrigerated within 4 hours of collection. Approximately, a 5 mL diluted sample of human urine was added with a standard solution in successive concentrations for HQ (5.0, 10.0, 15.0, 20.0, and 25.0 μM), for DA (3.0, 6.0, 9.0, 12.0, and 15.0 μM), and for UA (1.0, 2.0, 3.0, 4.0, and 5.0 μM). The human urine samples were then measured using the DPV technique with similar optimized experimental conditions in triplicates. The concentrations of HQ, DA, and UA in the sample of human urine were then calculated as the x-intercept of the standard addition calibration curve as follows in eqn (1):
 | (1) |
3 Results and discussions
3.1. Materials characterization using Raman, IR spectroscopy, SEM, TEM, and AFM
Raman spectroscopy was employed to investigate the structural conversion from graphite into reduced graphene oxide (RGO) in the Raman shift between 50 to 3500 cm−1 as shown in Fig. 1b. Based on this figure, the Raman spectrum of graphite exhibits three prominent peaks at 1346.78, 1577.03, and 2688.57 cm−1, respectively. The vibrations of carbon atoms with dangling bonds in plane termination of disordered graphite are linked to the peak at 1346.78 cm−1 (D-band, the breathing mode of k-point phonons of A1g symmetry).63 Meanwhile, the peak at 1577.03 cm−1 is associated with the vibration of sp2-bonded carbon atoms and represents the E2g mode of graphite.64 In addition, the 2D band depends on the number of layers and stacking order in the graphite structure.65 When graphite was oxidized to graphene oxide (GO), the D band became stronger at 1351.31 cm−1 implying a higher level of disorder of the graphene layers and defects during the chemical oxidation process.66 In addition, the intensity ratio of the D band versus G band (ID/IG) from graphite (0.21) to GO (1.03) is significantly enhanced indicating the formation of sp3 carbon through functionalization processes such as hydroxyl or epoxy causes the structural defects on its structure.67 Furthermore, the intensity ratio of ID/IG is slightly increased after the reduction of GO due to the considerable shrinkage of the in-plane sp2 domains leading to disordered structure in RGO materials.68 Moreover, the highest intensity ratio of ID/IG was obtained in the RGO/MWCNT (1.25) showing an increase in the surface disorder of these nanocomposites. This enhancement might be attributed to the partial insertion of MWCNT into RGO layers which follows previous works on graphene-based nanocomposites.69 FTIR was also performed for material characterization to confirm the changes in functional groups in each material.Infrared (IR) spectroscopy was used to identify and characterize all materials by analyzing the frequency of functional groups related to the molecular or atomic vibrations. Fig. 1c shows the IR spectra obtained from 4 different materials (graphite, GO, RGO, and RGO/MWCNT composite) with no significant peak observed in graphite. Meanwhile, the presence of oxygen functional groups in GO has been revealed at 3400 cm−1 (O–H stretching vibrations), at 2927 cm−1 (C–H stretching vibrations), at 1727 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations), at 1600 cm−1 (C
O stretching vibrations), at 1600 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations), at 1382 and 1190 cm−1 (C–OH stretching vibrations), and 1078 cm−1 (C–OC stretching vibrations). The increase in IR spectrum obtained from GO might be due to the formation of several oxygen functional groups in its structure such as alcohols, carboxylic acids, aldehydes, ketones, ethers, and epoxides.70 In addition, when GO is reduced into RGO, the O–H stretching vibrations at 3400 cm−1 are significantly reduced due to the deoxygenation from the graphene structure. However, several stretching vibrations such as C
C stretching vibrations), at 1382 and 1190 cm−1 (C–OH stretching vibrations), and 1078 cm−1 (C–OC stretching vibrations). The increase in IR spectrum obtained from GO might be due to the formation of several oxygen functional groups in its structure such as alcohols, carboxylic acids, aldehydes, ketones, ethers, and epoxides.70 In addition, when GO is reduced into RGO, the O–H stretching vibrations at 3400 cm−1 are significantly reduced due to the deoxygenation from the graphene structure. However, several stretching vibrations such as C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 1721 cm−1, C
O at 1721 cm−1, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C at 1600 cm−1, and C–OH at 1382 cm−1 become weaker due to the remaining carboxyl groups after the reduction of GO material. Furthermore, the IR spectra of RGO/MWCNT revealed the combination of previously mentioned absorption peaks from each RGO and MWCNT with less intensity than their parent materials. This is due to the co-existence of RGO in the MWCNT network resulting in the successful integration between these two materials. In addition, the IR spectra of this composite indicate the significant reduction of the oxygen as mentioned earlier functionalities upon the GO reduction.
C at 1600 cm−1, and C–OH at 1382 cm−1 become weaker due to the remaining carboxyl groups after the reduction of GO material. Furthermore, the IR spectra of RGO/MWCNT revealed the combination of previously mentioned absorption peaks from each RGO and MWCNT with less intensity than their parent materials. This is due to the co-existence of RGO in the MWCNT network resulting in the successful integration between these two materials. In addition, the IR spectra of this composite indicate the significant reduction of the oxygen as mentioned earlier functionalities upon the GO reduction.
Scanning electron microscope (SEM) analysis was employed to investigate the surface morphology of 3 electrode modifiers (RGO, MWCNT, and RGO/MWCNT composite) with magnifications of 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times. Fig. 1d shows the SEM image of RGO which revealed thin sheets and randomly aggregated layers with distinct edges but crumpled on its surface. This rough surface is the common feature found in RGO materials obtained from the reduction of GO.71 Meanwhile, Fig. 1e shows the characteristics of pristine MWCNT with a netlike structure which may offer a highly permeable region for the affinity adsorption of the analyte. In addition, this porous net structure of MWCNT provides an increased surface area which could offer an excellent ability for the electron transfer processes to the electrode surface. In addition, the incorporation of MWCNT into RGO layers through noncovalent interactions between graphene sheets forms a three-dimensional (3D) network of RGO/MWCNT nanocomposite. As seen in Fig. 1f, the SEM image of this composite shows the unique structure in which the RGO material is coated with the fibrous network MWCNT leading to an enhancement of electrical conductivity. Thus, the synergistic properties in this nanocomposite are expected to be beneficial as a platform of electrochemical sensors for multicomponent simultaneous detection.
000 times. Fig. 1d shows the SEM image of RGO which revealed thin sheets and randomly aggregated layers with distinct edges but crumpled on its surface. This rough surface is the common feature found in RGO materials obtained from the reduction of GO.71 Meanwhile, Fig. 1e shows the characteristics of pristine MWCNT with a netlike structure which may offer a highly permeable region for the affinity adsorption of the analyte. In addition, this porous net structure of MWCNT provides an increased surface area which could offer an excellent ability for the electron transfer processes to the electrode surface. In addition, the incorporation of MWCNT into RGO layers through noncovalent interactions between graphene sheets forms a three-dimensional (3D) network of RGO/MWCNT nanocomposite. As seen in Fig. 1f, the SEM image of this composite shows the unique structure in which the RGO material is coated with the fibrous network MWCNT leading to an enhancement of electrical conductivity. Thus, the synergistic properties in this nanocomposite are expected to be beneficial as a platform of electrochemical sensors for multicomponent simultaneous detection.
A transmission electron microscopy (TEM) study was employed to capture fine-detail images of RGO and MWCNT. Fig. 1g shows the high-resolution transmission electron microscopy (HR-TEM) image obtained from RGO revealing the sheet-like nature with less wrinkles and folding. In addition, the lattice fringes clearly shown in Fig. 1g confirm the interplanar distance for RGO material is 3.81 Å. Moreover, the crystallographic structure of RGO material was characterized by the selected area electron diffraction (SAED) method with the interplanar distance for RGO as 2.28 Å (011) and 1.21 Å (112) plane as shown in the inset of Fig. 1g. Meanwhile, the HR-TEM image of pristine MWCNT shows the regular tubular structure with a lattice fringe of 3.44 Å as shown in Fig. 1h. The selected area electron diffraction (SAED) of this nanomaterial also confirms several interplanar distances (inset Fig. 1h) with corresponding planes as 3.36 Å (002), 2.14 Å (110), 1.63 Å (004), and 1.23 Å (202) planes. Furthermore, the HR-TEM image of rGO/MWCNT nanocomposite (Fig. 1i) displays the lattice fringes for RGO and MWCNT with the corresponding interplanar distance of 3.81 and 3.44 Å, respectively. The SAED pattern of this nanocomposite (inset Fig. 1i) also reveals several number interplanar distances for RGO as 0.87 (011) and 0.70 Å (112) planes and for MWCNT as 3.44 (002), 2.07 (110), 1.65 (004), and 1.21 (202) planes.
The atomic force microscopy (AFM) technique was employed to characterize the surface roughness and topography from three electrode materials (RGO, MWCNT, and RGO/MWCNT composite). Fig. 2 shows the AFM images of 2D (left section) and 3D (right section) obtained from these three different materials in a scan area of 10 × 10 μM with a scan rate of 0.5 Hz. From this investigation, it can be calculated three values of root-mean-square roughness (Rq) for RGO, MWCNT, and RGO/MWCNT composite as follows 37, 39.6, and 70.1 μm, respectively. In addition, the value of peak-to-valley (Rpv) was also derived from RGO, MWCNT, and RGO/MWCNT composite as 86.4, 73.8, and 184.4 μm, respectively. Both increases (Rq and Rpv) from the starting material (RGO and MWCNT) to its composite could be attributed to the strong noncovalent interaction that allows the good dispersion of MWCNT into RGO structures.72 Moreover, the presence of MWCNT could prevent the restacking of graphene layers and act as a spacer in the interlayer spacing of RGO sheets thus increasing the surface area of its composite.73,74 This high surface area of the RGO/MWCNT composite is advantageous to improve its conductivity and adsorption performance since it allows for more surface contact on the surface of modified electrode. Thus, it will be interesting to investigate the employment of this material composite as a platform for the development of electrochemical sensors for the simultaneous detection of electroactive biological molecules particularly for hydroquinone (HQ), dopamine (DA), and uric acid (UA) detection.
3.2. Studies of the charge-transfer behaviour of the modified electrodes
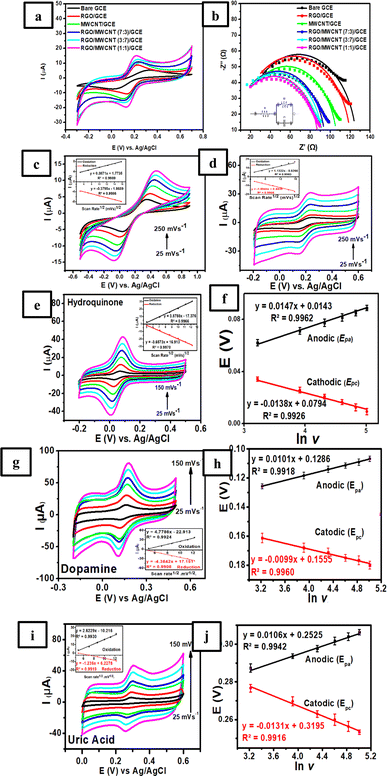
As the initial step to investigate the charge-transfer behaviour of the modified electrodes, cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) techniques were employed on 6 different electrodes (bare GCE, RGO/GCE, MWCNT/GCE, RGO/MWCNT (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3)/GCE, RGO/MWCNT (3
3)/GCE, RGO/MWCNT (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7)/GCE, and RGO/MWCNT (1
7)/GCE, and RGO/MWCNT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
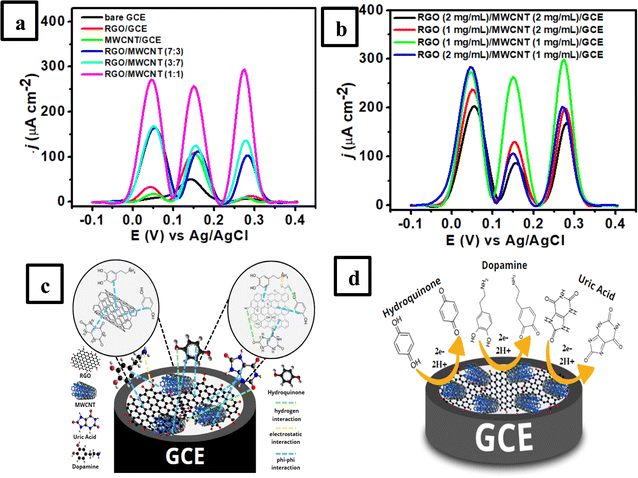
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/GCE). This study was conducted to determine the electrode conductivity of each modified electrode by measuring it using 1.0 mM K3[Fe(CN)6] in 0.1 M KCl solution. Fig. 3a shows the cyclic voltammogram at a scan rate of 50 mV s−1 obtained from 6 different electrodes with the highest sensitivity observed at RGO/MWCNT (1
1)/GCE). This study was conducted to determine the electrode conductivity of each modified electrode by measuring it using 1.0 mM K3[Fe(CN)6] in 0.1 M KCl solution. Fig. 3a shows the cyclic voltammogram at a scan rate of 50 mV s−1 obtained from 6 different electrodes with the highest sensitivity observed at RGO/MWCNT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/GCE for both anodic and cathodic peak currents of Fe3+/Fe2+ redox pair species compared to other electrodes. This result suggests that this electrode possesses the highest conductivity among other modified electrodes for the electron transfer electron process of Fe3+/Fe3+ redox pair species. In addition, the magnitude of both anodic and cathodic peak currents of Fe3+/Fe2+ redox pair species observed at all modified electrodes following the trend as follows: RGO/MWCNT (1
1)/GCE for both anodic and cathodic peak currents of Fe3+/Fe2+ redox pair species compared to other electrodes. This result suggests that this electrode possesses the highest conductivity among other modified electrodes for the electron transfer electron process of Fe3+/Fe3+ redox pair species. In addition, the magnitude of both anodic and cathodic peak currents of Fe3+/Fe2+ redox pair species observed at all modified electrodes following the trend as follows: RGO/MWCNT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/GCE > RGO/MWCNT (3
1)/GCE > RGO/MWCNT (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) > RGO/MWCNT (7
7) > RGO/MWCNT (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) > MWCNT/GCE > RGO/GCE > bare GCE.
3) > MWCNT/GCE > RGO/GCE > bare GCE.
To further justify the result from CV studies, all modified electrodes were subjected to EIS studies to characterize the fundamental properties such as the phenomena of charge transfer resistance at the interface of electrode/electrolyte. The result from EIS studies of each modified electrode will correspond to the electrode conductivity as depicted by the Nyquist plot from 6 different electrodes (Fig. 3b). Based on this figure, the Nyquist plot of each electrode was obtained by measuring 1.0 mM K3[Fe(CN)6] in 0.1 M KCl solution in the frequency range from 105 Hz to 2 × 102 Hz with Edc = 0 V and Eac = 6 × 10−3 V at an open-circuit potential. Based on this figure, the semicircle (Nyquist plot) in the high-frequency region obtained from all electrodes can be attributed to the phenomena of the charge transfer resistance at the interface of electrode/electrolyte.75 In addition, the diameter size of the Nyquist plot could be used to determine the charge transfer resistance (R2) by fitting the experimental result with the corresponding Randles circuit as shown in the inset of Fig. 3b.
According to the calculations from EIS analysis, bare GCE showed the largest semicircle with the R2 values of 118.5 Ω indicating the lowest conductivity among other modified electrodes. However, when the surface of GCE was modified with RGO and MWCNT, the R2 value was decreased to 112.1 Ω for RGO/GCE and 100.6 Ω for MWCNT/GCE. In a further investigation, 3 different compositions of RGO and MWCNT were determined their R2 values which resulted as 94.45 Ω for RGO/MWCNT (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3)/GCE, 92.34 Ω for RGO/MWCNT (3
3)/GCE, 92.34 Ω for RGO/MWCNT (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7)/GCE, and 88.2 Ω for RGO/MWCNT (1
7)/GCE, and 88.2 Ω for RGO/MWCNT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)/GCE. This result revealed that the combination of RGO and MWCNT in the composition ratio 1
1)/GCE. This result revealed that the combination of RGO and MWCNT in the composition ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 showed the lowest resistance for the charge transfer process compared to other evaluated electrodes which agrees with several previous studies.76,77 The heterostructure of a tube-like core of RGO/MWCNT composite, which fills in the gaps between the shell of graphene sheets and creates a continuously conductive network for electron transfer, is responsible for its high conductivity.78 Therefore, RGO/MWCNT (1
1 showed the lowest resistance for the charge transfer process compared to other evaluated electrodes which agrees with several previous studies.76,77 The heterostructure of a tube-like core of RGO/MWCNT composite, which fills in the gaps between the shell of graphene sheets and creates a continuously conductive network for electron transfer, is responsible for its high conductivity.78 Therefore, RGO/MWCNT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-modified GCE was selected for further electrochemical investigations due to its highest electrical conductivity and showed considerable potential to be employed as electrode modifier materials.
1)-modified GCE was selected for further electrochemical investigations due to its highest electrical conductivity and showed considerable potential to be employed as electrode modifier materials.
3.3. The influences of scan rates on the modified electrodes
The effect of scan rate on bare GCE and RGO/MWCNT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-modified GCE was studied by measuring 1.0 mM K3[Fe(CN)6] in 0.1 M KCl at various scan rates (25–250 mV s−1). The current density can be used in this condition to calculate the geometric surface area of the working electrode as 7.06 × 10−2 cm2. The studies of scan rate effect were performed using the CV technique for bare GCE for RGO/MWCNT (1
1)-modified GCE was studied by measuring 1.0 mM K3[Fe(CN)6] in 0.1 M KCl at various scan rates (25–250 mV s−1). The current density can be used in this condition to calculate the geometric surface area of the working electrode as 7.06 × 10−2 cm2. The studies of scan rate effect were performed using the CV technique for bare GCE for RGO/MWCNT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-modified GCE. Based on the inset Fig. 3c, bare GCE was found to be in a linear relationship of both currents of oxidation (Ipa) and reduction (Ipc) versus the square root of scan rates (v1/2) with the corresponding calibration plot as Ipa (μA) = 0.3871v1/2 (mV s−1) + 1.7735, R2 = 0.9989 and Ipc (μA) = −0.3795v1/2 (mV s−1) − 1.8689, R2 = 0.9986. This finding suggests that the electrochemical process on the surface of modified electrode was controlled by diffusion phenomenon. Meanwhile, RGO/MWCNT (1
1)-modified GCE. Based on the inset Fig. 3c, bare GCE was found to be in a linear relationship of both currents of oxidation (Ipa) and reduction (Ipc) versus the square root of scan rates (v1/2) with the corresponding calibration plot as Ipa (μA) = 0.3871v1/2 (mV s−1) + 1.7735, R2 = 0.9989 and Ipc (μA) = −0.3795v1/2 (mV s−1) − 1.8689, R2 = 0.9986. This finding suggests that the electrochemical process on the surface of modified electrode was controlled by diffusion phenomenon. Meanwhile, RGO/MWCNT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-modified GCE also showed a linear relationship for both oxidation and reduction currents with the corresponding calibration plot as Ipa (μA) = 1.1322v1/2 (mV s−1) − 0.8266, R2 = 0.9963 and Ipc (μA) = −1.0944v1/2 (mV s−1) + 0.4638, R2 = 0.9946 as shown in the Fig. 3d. Thus, it can be calculated the electrochemical active surface area (ECSA) for bare GCE and RGO/MWCNT (1
1)-modified GCE also showed a linear relationship for both oxidation and reduction currents with the corresponding calibration plot as Ipa (μA) = 1.1322v1/2 (mV s−1) − 0.8266, R2 = 0.9963 and Ipc (μA) = −1.0944v1/2 (mV s−1) + 0.4638, R2 = 0.9946 as shown in the Fig. 3d. Thus, it can be calculated the electrochemical active surface area (ECSA) for bare GCE and RGO/MWCNT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-modified GCE based on the Randles–Sevcik equation as follows in eqn (2):
1)-modified GCE based on the Randles–Sevcik equation as follows in eqn (2):| Ip = (2.69 × 105)AD1/2n3/2v1/2C | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-modified GCE can be calculated as 2.88 × 10−2 and 4.96 × 10−2 cm2, respectively. The ECSA of both modified electrodes is less than the geometric surface area (7.06 × 10−2 cm2), perhaps due to surface heterogeneities caused by the RGO/MWCNT composite on the GCE surface. Thus, the ECSA of RGO/MWCNT (1
1)-modified GCE can be calculated as 2.88 × 10−2 and 4.96 × 10−2 cm2, respectively. The ECSA of both modified electrodes is less than the geometric surface area (7.06 × 10−2 cm2), perhaps due to surface heterogeneities caused by the RGO/MWCNT composite on the GCE surface. Thus, the ECSA of RGO/MWCNT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-modified GCE was approximately calculated as 2.2 times than obtained at bare GCE. The improved ECSA of RGO/MWCNT (1
1)-modified GCE was approximately calculated as 2.2 times than obtained at bare GCE. The improved ECSA of RGO/MWCNT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-modified GCE compared to bare GCE is expected to enhance the electrocatalytic activity to be utilized as an electrochemical sensing platform for simultaneous detection of HQ, DA, and UA.
1)-modified GCE compared to bare GCE is expected to enhance the electrocatalytic activity to be utilized as an electrochemical sensing platform for simultaneous detection of HQ, DA, and UA.
The relationship between scan rate and the electrochemical behaviors of 50.0 μM HQ, 100.0 μM DA, and 200.0 μM UA in 0.1 M of pH 7 phosphate buffer was also investigated to obtain the electron transfer mechanism at the electrode surface. The cyclic voltammograms obtained at different scan rates (25, 50, 75, 100, 125, and 150 mV s−1) measured using RGO/MWCNT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-modified GCE are shown in Fig. 3e, g and i for HQ, DA, and UA, respectively. Based on these figures, the peak currents showed a linear relationship with the square root of the scan rate, suggesting the diffusion-controlled process predominates for HQ, DA, and UA on the electrode surface due to the fast electron transfer rate towards the RGO/MWCNT nanocomposite. As shown in the inset of Fig. 3e, g and i, the following linear relationship was observed: Ipa (μA) = 3.8788v1/2 (mV s−1) − 17.376, R2 = 0.9966 and Ipc (μA) = −3.6573v1/2 (mV s−1) + 16.913, R2 = 0.9970 for HQ (inset Fig. 3e), Ipa (μA) = 4.7786v1/2 (mV s−1) − 22.913, R2 = 0.9924 and Ipc (μA) = −4.3642v1/2 (mV s−1) + 17.151, R2 = 0.9908 for DA (inset Fig. 3g), and Ipa (μA) = 2.6229v1/2 (mV s−1) − 10.216, R2 = 0.9930 and Ipc (μA) = −1.236v1/2 (mV s−1) + 6.2278, R2 = 0.9919 for UA (inset Fig. 3i). Thus, it can be concluded that all calibration plots showed good linearity with the electrochemical reaction of HQ, DA, and UA on the surface of RGO/MWCNT (1
1)-modified GCE are shown in Fig. 3e, g and i for HQ, DA, and UA, respectively. Based on these figures, the peak currents showed a linear relationship with the square root of the scan rate, suggesting the diffusion-controlled process predominates for HQ, DA, and UA on the electrode surface due to the fast electron transfer rate towards the RGO/MWCNT nanocomposite. As shown in the inset of Fig. 3e, g and i, the following linear relationship was observed: Ipa (μA) = 3.8788v1/2 (mV s−1) − 17.376, R2 = 0.9966 and Ipc (μA) = −3.6573v1/2 (mV s−1) + 16.913, R2 = 0.9970 for HQ (inset Fig. 3e), Ipa (μA) = 4.7786v1/2 (mV s−1) − 22.913, R2 = 0.9924 and Ipc (μA) = −4.3642v1/2 (mV s−1) + 17.151, R2 = 0.9908 for DA (inset Fig. 3g), and Ipa (μA) = 2.6229v1/2 (mV s−1) − 10.216, R2 = 0.9930 and Ipc (μA) = −1.236v1/2 (mV s−1) + 6.2278, R2 = 0.9919 for UA (inset Fig. 3i). Thus, it can be concluded that all calibration plots showed good linearity with the electrochemical reaction of HQ, DA, and UA on the surface of RGO/MWCNT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-modified GCE was controlled by diffusion process.
1)-modified GCE was controlled by diffusion process.
In a further investigation of the scan rate effect on the peak potential of HQ, DA, and UA, it was revealed that the peak potential of oxidation (Epa) shifted positively, and reduction (Epc) shifted negatively with the increasing scan rate from 25 to 150 mV s−1. The shifting of both potential peaks is linearly proportional to the natural logarithmic scan rate (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) for the reversible redox reactions observed in HQ, DA, and UA. The linear regression equations were Epa = 0.0147
v) for the reversible redox reactions observed in HQ, DA, and UA. The linear regression equations were Epa = 0.0147![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v + 0.0143, R2 = 0.9962 and Epc = −0.0138
v + 0.0143, R2 = 0.9962 and Epc = −0.0138![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v + 0.0794, R2 = 0.9926 for HQ (Fig. 3f), Epa = 0.0161
v + 0.0794, R2 = 0.9926 for HQ (Fig. 3f), Epa = 0.0161![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v + 0.1162, R2 = 0.9915 and Epc = −0.0137
v + 0.1162, R2 = 0.9915 and Epc = −0.0137![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v + 0.1825, R2 = 0.9908 for DA (Fig. 3h), and Epa = 0.0101
v + 0.1825, R2 = 0.9908 for DA (Fig. 3h), and Epa = 0.0101![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v + 0.2652, R2 = 0.9936 and Epc = −0.012
v + 0.2652, R2 = 0.9936 and Epc = −0.012![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v + 0.9959, R2 = 0.9926 for UA (Fig. 3j). Based on Laviron's theory for the reversible process of electron transfer on the surface of modified electrode, the relationship between the peak potential of anodic (Epa) and cathodic (Epc) versus the scan rate (v) can be determined using the following eqn (3)–(6):
v + 0.9959, R2 = 0.9926 for UA (Fig. 3j). Based on Laviron's theory for the reversible process of electron transfer on the surface of modified electrode, the relationship between the peak potential of anodic (Epa) and cathodic (Epc) versus the scan rate (v) can be determined using the following eqn (3)–(6):
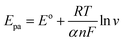 | (3) |
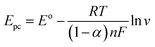 | (4) |
 | (5) |
 | (6) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485.33C mol−1), R is the universal gas constant (8.314 J mol−1 K−1), T is the absolute temperature constant (298 K), v is the scan rate, EΘ is the formal potential, and KΘ is the standard potential. It can be obtained from the above equations the values for n and α are 1.74 ≈ 2 and 0.79 ≈ 1 for HQ, 1.59 ≈ 2 and 0.79 ≈ 1 for DA, and 1.95 ≈ 2 and 1.27 ≈ 1 for UA. Thus, it can be deduced that the redox reactions of HQ, DA, and UA involved 2H+ and 2e− at the electrode/electrolyte interface. Scheme 1 outlines the possible reaction mechanism of HQ, DA, and UA at the electrode surface.
485.33C mol−1), R is the universal gas constant (8.314 J mol−1 K−1), T is the absolute temperature constant (298 K), v is the scan rate, EΘ is the formal potential, and KΘ is the standard potential. It can be obtained from the above equations the values for n and α are 1.74 ≈ 2 and 0.79 ≈ 1 for HQ, 1.59 ≈ 2 and 0.79 ≈ 1 for DA, and 1.95 ≈ 2 and 1.27 ≈ 1 for UA. Thus, it can be deduced that the redox reactions of HQ, DA, and UA involved 2H+ and 2e− at the electrode/electrolyte interface. Scheme 1 outlines the possible reaction mechanism of HQ, DA, and UA at the electrode surface.
 | ||
Scheme 1 The mechanisms of possible electrochemical reactions for (a) HQ, (b) DA, and (c) UA on the surface of RGO/MWCNT(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-modified GCE. 1)-modified GCE. | ||
Accordingly, based on Laviron's theory, the electron transfer rate constant (ket) for HQ, DA, and UA can be calculated as follows 0.58, 0.80, and 4.5 s−1, respectively which are still comparable to the values reported in the previous studies as 0.338 s−1 for HQ (as reported in ref. 79), 0.71 s−1 for DA (as reported in ref. 80), and 6.1 s−1 for UA (as reported in ref. 81). Moreover, the surface coverage of HQ, DA, and UA on the surface of RGO/MWCNT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-modified GCE can be calculated using the following eqn (7):
1)-modified GCE can be calculated using the following eqn (7):
 | (7) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-modified GCE was calculated to be 3.15 × 10−5, 1.71 × 10−5, and 7.76 × 10−6 mmol cm−2, which is comparable to those published previously.82–84
1)-modified GCE was calculated to be 3.15 × 10−5, 1.71 × 10−5, and 7.76 × 10−6 mmol cm−2, which is comparable to those published previously.82–84
3.4. Chronoamperometric studies of HQ, DA, and UA in RGO/MWCNT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) 1)-modified GCE
1)-modified GCE
The chronoamperometric studies were performed to study the performance of RGO/MWCNT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-modified GCE as a working electrode towards the electrocatalytic oxidation of HQ, DA, and UA in 0.1 M of pH 7 phosphate buffer. The potential of RGO/MWCNT (1
1)-modified GCE as a working electrode towards the electrocatalytic oxidation of HQ, DA, and UA in 0.1 M of pH 7 phosphate buffer. The potential of RGO/MWCNT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
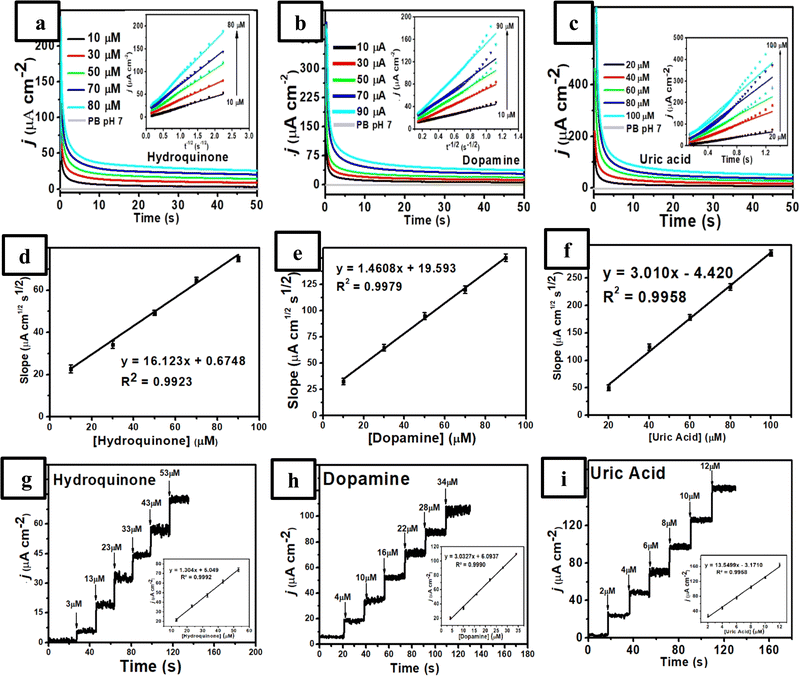
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-modified GCE was fixed at 0.152 V vs. Ag/AgCl for HQ, 0.220 V vs. Ag/AgCl for DA, and 0.360 V vs. Ag/AgCl for UA. As shown in Fig. 4a–c, three chronoamperograms (plot of the current values versus t−1/2) were obtained and the diffusion coefficient value can be calculated using the Cottrell eqn (8).
1)-modified GCE was fixed at 0.152 V vs. Ag/AgCl for HQ, 0.220 V vs. Ag/AgCl for DA, and 0.360 V vs. Ag/AgCl for UA. As shown in Fig. 4a–c, three chronoamperograms (plot of the current values versus t−1/2) were obtained and the diffusion coefficient value can be calculated using the Cottrell eqn (8).| I = nFAD1/2Cπ−1/2t−1/2 | (8) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-modified GCE at different HQ, DA, and UA concentrations.
1)-modified GCE at different HQ, DA, and UA concentrations.
A quantitative analysis was also performed using the chronoamperometric studies by recording amperogram response from the measurements of HQ in the presence of DA and UA at 0.152 V vs. Ag/AgCl, DA in the presence of HQ and UA at 0.220 V vs. Ag/AgCl, and UA in the presence of HQ and DA at 0.360 V vs. Ag/AgCl. Fig. 4g–i display a good linearity corresponding to their increasing concentrations with the successive additions of HQ (3.0–53.0 μM), DA (4.0–34.0 μM), and UA (2.0–12.0 μM) in various concentrations under stirring in 0.1 M of pH 7 phosphate buffer. In addition, from the inset Fig. 4g–i, it was obtained three equations of calibration curve for HQ at the concentration range of 3.0–53.0 μM with Ip = 1.304x + 5.049, R2 = 0.9992, for DA at the concentration range of 4–34 μM with Ip = 3.0327x + 6.0937, R2 = 0.9990, and for UA at the concentration range of 2–12 μM with Ip = 13.5499x − 3.1710, R2 = 0.9958. Furthermore, the proposed sensor exhibits a rapid and stable current response toward HQ, DA, and UA oxidation with a response time of 0.4, 0.2, and 0.2 s, respectively (Fig. S1, ESI†). These results revealed that the independent measurements of three analytes (HQ, DA, and UA) are possible using the chronoamperometric technique without any interference with an excellent linear relationship.
The influence of RGO/MWCNT composition in terms of its concentration in the weight/volume ratios on the intensity of anodic peak current of the modified electrode was also investigated using the DPV technique. Fig. 5b shows the current intensity reaches its maximum with each concentration of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in mg mL−1 unit of RGO/MWCNT when it was employed for the simultaneous measurement of 50 μM HQ, 30 μM DA, and 10 μM UA in 0.1 M of pH 7 phosphate buffer. Above the concentration of RGO/MWCNT (1
1 in mg mL−1 unit of RGO/MWCNT when it was employed for the simultaneous measurement of 50 μM HQ, 30 μM DA, and 10 μM UA in 0.1 M of pH 7 phosphate buffer. Above the concentration of RGO/MWCNT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in mg mL−1 unit, there is a clear reduction in the peak current for HQ, DA, and UA, most likely caused by decreased conductivity on the electrode surface. This result indicates that a small proportion of RGO/MWCNT could give the highest conductivity of the modified electrode. Still, a higher concentration of this material composite lowered its electrocatalytic activity. This result might be due to a higher concentration of RGO/MWCNT composite, graphene as a two-dimensional material tends to stack together through noncovalent interactions which causes the blocking of catalytically active sites on the modified electrodes.88 In addition, MWCNT might be aggregated at higher concentrations due to its van der Waals forces which produce an inhomogeneous dispersion composite and thus lowered the conductivity of nanocomposite.89 Therefore, 1 mg mL−1 concentrations for RGO and MWCNT materials were employed for the subsequent experiments for simultaneous measurements of HQ, DA, and UA.
1) in mg mL−1 unit, there is a clear reduction in the peak current for HQ, DA, and UA, most likely caused by decreased conductivity on the electrode surface. This result indicates that a small proportion of RGO/MWCNT could give the highest conductivity of the modified electrode. Still, a higher concentration of this material composite lowered its electrocatalytic activity. This result might be due to a higher concentration of RGO/MWCNT composite, graphene as a two-dimensional material tends to stack together through noncovalent interactions which causes the blocking of catalytically active sites on the modified electrodes.88 In addition, MWCNT might be aggregated at higher concentrations due to its van der Waals forces which produce an inhomogeneous dispersion composite and thus lowered the conductivity of nanocomposite.89 Therefore, 1 mg mL−1 concentrations for RGO and MWCNT materials were employed for the subsequent experiments for simultaneous measurements of HQ, DA, and UA.
Fig. 5c shows the schematic illustration of a synergistic effect of the RGO/MWCNT composite on the GCE surface which is due to noncovalent interactions and van der Waals forces leading to the formation of an interconnected network structure between graphene layers and carbon nanotubes. This type of interaction could reduce the gap between RGO and MWCNT and facilitate a faster electron transport process on the electrode surface thus resulting in high electrical conductivity.90 In addition, the non-covalent interaction between sp2-hybridized regions of RGO with the sidewalls of MWCNT could promote the exfoliation of graphene layers and allow the entanglement of carbon nanotubes to expand resulting in a large surface area of RGO/MWCNT composite.91 Moreover, the porous structure of RGO/MWCNT composite on the electrode surface is beneficial in reducing the overpotential and separating the oxidation potentials of HQ, DA, and UA from each other. MWCNT could significantly enhance the oxidation currents of HQ, DA, and UA by accelerating the electron transfer rate to the electrode surface due to its high electronic conductivity and large surface area. This could be useful for the discrimination of HQ, DA, and UA during its experimental investigation using voltammetric techniques. Thus, the synergistic effects of the electrocatalytic activity between RGO and MWCNT provided the RGO/MWCNT-modified GCE with improved catalytic properties toward simultaneous detection of HQ, DA, and UA, which helped to distinguish these analytes. Fig. 5d displays the electrochemical oxidation mechanisms of HQ, DA, and UA on the surface of GCE modified with RGO/MWCNT composite to improve their anodic current responses.
As illustrated in Fig. 5c and d, the improvement in anodic current response could be attributed to the different interactions between three molecules with the composite of RGO/MWCNT-modified GCE. It is well known that RGO still bears negative charges due to a few oxygen-functional groups such as carboxyl, hydroxyl, and epoxy in its structure92 which could assist the interaction with the target molecules (HQ, DA, and UA). For HQ, forming a noncovalent interaction between hydroquinone and graphene basal plane in RGO would enhance the anodic peak current. Meanwhile, DA shows the electrostatic interaction between positively charged DA and negatively charged RGO also the noncovalent interaction between DA and the graphene layer is abundant with a hexagonal carbon structure. Nevertheless, the increased peak current of UA might be attributed to the formation of hydrogen bonds at the amide group in UA with an oxygen-rich functional group at the RGO-MWCNT composite.
3.5. Analytical performance of RGO/MWCNT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) 1)-modified GCE for simultaneous detection of HQ, DA, and UA
1)-modified GCE for simultaneous detection of HQ, DA, and UA
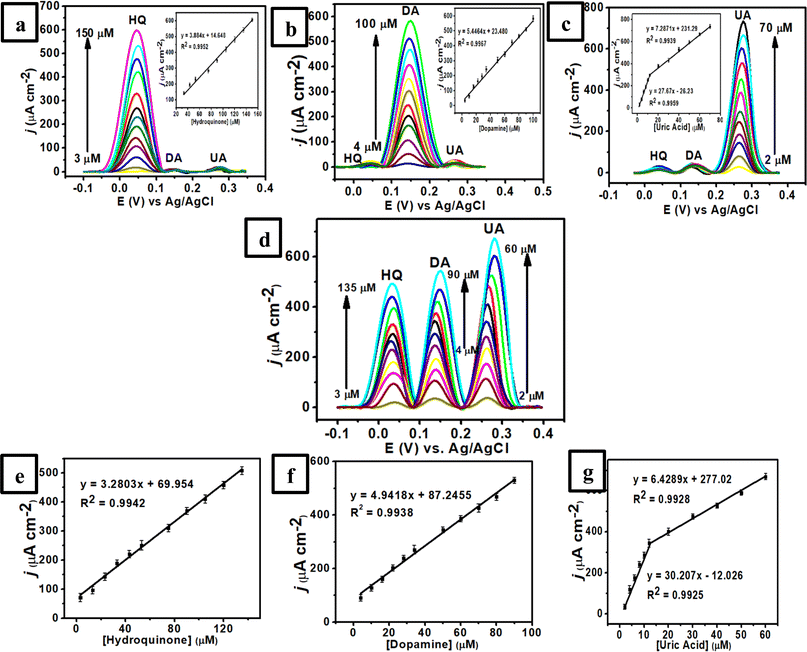
The electrooxidation of each analyte in the mixture solution was investigated by varying the concentration of a species with the other two concentrations remaining constant to further illustrate the feasibility of the proposed sensor for simultaneous detection of HQ, DA, and UA. It was revealed that the spiking of one target analyte showed a negligible effect on the peak currents and peak potentials of the other two analytes. Fig. 6a shows the DPV of RGO/MWCNT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-modified GCE at different concentrations of HQ (3–150 μM HQ) with a constant concentration of DA (4 μM) and UA (2 μM UA) in 0.1 M of pH 7 phosphate buffer. As displayed in this figure, the obtained anodic peaks for these three species were separated under similar conditions to those of DPV studies with the peak current of HQ increased linearly from 3–150 μM at fixed concentrations of DA and UA. The inset in Fig. 6a also shows the linear equation for HQ as Ipa (μA) = 3.884Chydroquinone (μM) + 14.648 (R2 = 0.9952, 3–53 μM with the sensitivity of 3.884 μA μM−1 cm−2). Meanwhile, Fig. 6b displays the anodic current of DA enhanced linearly at various concentrations from 4–100 μM while the other concentrations of HQ and UA remained constant. The corresponding linear equation of DA is defined by Ipa (μA) = 5.4464Cdopamine (μM) + 23.480 (R2 = 0.9967, 4–100 μM with the sensitivity of 5.4464 μA μM−1 cm−2) as displayed in the inset Fig. 6b. In addition, the peak current of UA increased linearly when its concentration changed from 2–12 μM (linear range 1) and 12–70 μM (linear range 2) at fixed concentrations of HQ and DA (Fig. 6c). The linear function of UA concentrations versus the peak current can be defined as Ipa (μA) = 27.67Curic acid (μM) − 26.23 (R2 = 0.9959, 2–12 μM with the sensitivity of 27.57 μA μM−1 cm−2) and Ipa (μA) = 7.2871Curic acid (μM) + 231.29 (R2 = 0.9939, 12–70 μM, with the sensitivity of 7.2871 μA μM−1 cm−2) (inset Fig. 6c).
1)-modified GCE at different concentrations of HQ (3–150 μM HQ) with a constant concentration of DA (4 μM) and UA (2 μM UA) in 0.1 M of pH 7 phosphate buffer. As displayed in this figure, the obtained anodic peaks for these three species were separated under similar conditions to those of DPV studies with the peak current of HQ increased linearly from 3–150 μM at fixed concentrations of DA and UA. The inset in Fig. 6a also shows the linear equation for HQ as Ipa (μA) = 3.884Chydroquinone (μM) + 14.648 (R2 = 0.9952, 3–53 μM with the sensitivity of 3.884 μA μM−1 cm−2). Meanwhile, Fig. 6b displays the anodic current of DA enhanced linearly at various concentrations from 4–100 μM while the other concentrations of HQ and UA remained constant. The corresponding linear equation of DA is defined by Ipa (μA) = 5.4464Cdopamine (μM) + 23.480 (R2 = 0.9967, 4–100 μM with the sensitivity of 5.4464 μA μM−1 cm−2) as displayed in the inset Fig. 6b. In addition, the peak current of UA increased linearly when its concentration changed from 2–12 μM (linear range 1) and 12–70 μM (linear range 2) at fixed concentrations of HQ and DA (Fig. 6c). The linear function of UA concentrations versus the peak current can be defined as Ipa (μA) = 27.67Curic acid (μM) − 26.23 (R2 = 0.9959, 2–12 μM with the sensitivity of 27.57 μA μM−1 cm−2) and Ipa (μA) = 7.2871Curic acid (μM) + 231.29 (R2 = 0.9939, 12–70 μM, with the sensitivity of 7.2871 μA μM−1 cm−2) (inset Fig. 6c).
In a subsequent experiment, the concentrations of HQ, DA, and UA were increased simultaneously to confirm the effect of each analyte in the presence of the other two species. Fig. 6d shows three well-separated peaks of HQ, DA, and UA with their peak currents proportional to each concentration in a linear relationship. Then, it can be obtained the linear equations for HQ (Fig. 6e) as Ipa (μA) = 3.2803Chydroquinone (μM) + 69.954 (R2 = 0.9942, 3–135 μM, with the sensitivity of 3.2803 μA μM−1 cm−2), for DA (Fig. 6f) as Ipa (μA) = 4.9418Cdopamine (μM) + 87.2455 (R2 = 0.9937, 4–90 μM, with the sensitivity of 4.9418 μA μM−1 cm−2), and for UA (Fig. 6g) as Ipa (μA) = 30.207Curic acid (μM) − 12.026 (R2 = 0.9925, 2–12 μM) and Ipa (μA) = 6.4289Curic acid (μM) + 277.02 (R2 = 0.9928, 12–60 μM) with the sensitivity of 6.429 μA μM−1 cm−2. Thus, the limit of detection (LOD) (S/N ≈ 3) for HQ, DA, and UA can be determined as 0.400 ± 0.014, 0.500 ± 0.006, and 0.300 ± 0.016 μM, respectively. In addition, the limit of quantification (LOQ) (S/N ≈ 10) for HQ, DA, and UA were 0.800 ± 0.044, 1.000 ± 0.011, and 0.600 ± 0.025 μM, respectively. Furthermore, the performance of this sensor based on RGO/MWCNT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-modified GCE can be compared to those previously reported sensors which can be summarized in Table 1.
1)-modified GCE can be compared to those previously reported sensors which can be summarized in Table 1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-modified GCE with some reported works for determination of HQ, DA, and UA
1)-modified GCE with some reported works for determination of HQ, DA, and UA
| Electrode | Analyte | Linear range (μM) | LOD (μM) | Sensitivity (μA μM−1 cm−2) | Ref. |
|---|---|---|---|---|---|
| a Covalent polyoxometalate–organic frameworks.b Co-based single atom nanozymes anchored on HOH-activated ZIF-derived porous carbon.c Carbon paste electrode.d Layered yttrium hydroxide.e Co-porphyrin-based covalent organic framework.f Graphene quantum dots.g Electrospun nanofibers.h Screen-printed carbon electrode.i Polyaniline.j Carbon quantum dots. | |||||
| PtNPs@CPOFsa-MWCNTs | HQ | 6–500 | 0.66 | 0.082 | 3 |
| Co-AcNC-3b/GCE | HQ | 4–300 | 0.034 | 21.597 | 4 |
| Bi2WO6/GCE | HQ | 20–2500 | 4.1 | 1.5 | 93 |
| Ce2(WO4)3/CPEc | HQ | 0.4–45 | 0.06 | 6.22 × 10−5 | 94 |
| AuNPs/LYH-47d/GCE | HQ | 1–100 | 0.2 | 0.8 | 95 |
| TT-COF(Co)/N-CNTse/GCE | HQ | 0.003–300 | 0.81 × 10−3 | 0.094 | 96 |
| Ni3ZnC0.7/Ni/GCE | HQ | 0.3–100 | 0.14 | 0.735 | 97 |
| N,Si-GQDsf | HQ | 5–200 | 1.35 | 0.0097 | 98 |
| Co,Mo@CNFsg/GCE | DA, UA | DA: 0.01–1000 | DA: 0.00235 | 0.86 | 5 |
| UA: 1–1000 | UA: 0.16 | 0.65 | |||
| h-WO3/MoO3/MoS2/GCE | DA, UA | DA: 1.25–495 | DA: 0.539 | 3.185 | 6 |
| UA: 10–1330 | UA: 2.402 | 0.814 | |||
| Au@Cu-MOF/SPCEh | DA, UA | DA, UA: 10–1000 | DA: 3.40 | 0.231 | 12 |
| UA: 10.36 | 0.275 | ||||
| PANIi/Cu2O-Aux/GCE | DA, UA | DA: 0.01–200 | DA: 0.0076 | 0.054 | 13 |
| UA: 0.1–1000 | UA: 0.035 | 0.026 | |||
| Au@Ni-MOF | DA, UA | DA, UA: 0.5–1000 | DA: 0.027 | 1.43 | 99 |
| UA: 0.028 | 1.35 | ||||
| ZnO/CQDsj/CPE | DA, UA | DA: 0.12–142 | DA: 0.46 | 3.375 | 100 |
| UA: 0.5–222 | UA: 0.23 | 2.411 | |||
| Ti3C2Tx/TiO2 NMs/GCE | DA, UA | DA: 2–33 | DA: 0.093 | 0.133 | 101 |
| UA: 2–33 | UA: 0.038 | 0.321 | |||
ErGO/PEDOT:PSS (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3)/GCE 3)/GCE |
DA, UA | DA: 10–110 | DA: 0.5 | 0.6365 | 102 |
| UA: 3–33 | UA: 0.4 | 0.1439 | |||
| RGO/MWCNT/GCE | HQ, DA, UA | HQ: 3–150 | HQ: 0.4 | 3.280 | This work |
| DA: 4–100 | DA: 0.5 | 4.942 | |||
| UA: 2–70 | UA: 0.3 | 6.429 | |||
3.6. Reproducibility, stability, and selectivity of RGO/MWCNT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) 1)-modified GCE
1)-modified GCE
The reproducibility of RGO/MWCNT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-modified GCE was investigated by measuring 50 μM UA, 30 μM DA, and 10 μM UA in 0.1 M of pH 7 phosphate buffer using five electrodes for each analyte. The reproducibility experiments were performed using five individual modified electrode in triplicate experiments utilizing the DPV technique at a scan rate of 25 mV s−1 and a potential range from −0.2 to 0.6 V vs. AgCl. Fig. 7a shows the values of relative standard deviation (% RSD) obtained from this work for HQ, DA, and UA were 1.53, 2.14, and 2.59%, respectively. This result demonstrated a satisfactory reproducibility of RGO/MWCNT (1
1)-modified GCE was investigated by measuring 50 μM UA, 30 μM DA, and 10 μM UA in 0.1 M of pH 7 phosphate buffer using five electrodes for each analyte. The reproducibility experiments were performed using five individual modified electrode in triplicate experiments utilizing the DPV technique at a scan rate of 25 mV s−1 and a potential range from −0.2 to 0.6 V vs. AgCl. Fig. 7a shows the values of relative standard deviation (% RSD) obtained from this work for HQ, DA, and UA were 1.53, 2.14, and 2.59%, respectively. This result demonstrated a satisfactory reproducibility of RGO/MWCNT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-modified GCE for simultaneous detection of HQ, DA, and UA. Meanwhile, the stability of RGO/MWCNT (1
1)-modified GCE for simultaneous detection of HQ, DA, and UA. Meanwhile, the stability of RGO/MWCNT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-modified GCE was also evaluated by measuring a solution containing 50 μM UA, 30 μM DA, and 10 μM UA in 0.1 M of pH 7 phosphate buffer in 7 repetitions as shown in Fig. 7b. It was obtained from this experiment that the calculated % RSD for HQ, DA, and UA were kept at 3.08, 1.08, and 0.99%, respectively. Thus, this work displayed an excellent stability of RGO/MWCNT (1
1)-modified GCE was also evaluated by measuring a solution containing 50 μM UA, 30 μM DA, and 10 μM UA in 0.1 M of pH 7 phosphate buffer in 7 repetitions as shown in Fig. 7b. It was obtained from this experiment that the calculated % RSD for HQ, DA, and UA were kept at 3.08, 1.08, and 0.99%, respectively. Thus, this work displayed an excellent stability of RGO/MWCNT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-modified GCE for simultaneous measurements of HQ, DA, and UA.
1)-modified GCE for simultaneous measurements of HQ, DA, and UA.
The investigation of selectivity studies was performed using the DPV technique by measuring a solution containing 50 μM UA, 30 μM DA, and 10 μM UA in 0.1 M of pH 7 phosphate buffer in the presence of several possible interfering species such as Mg2+, K+, Cl−, ascorbic acid, and glucose. As shown in Fig. 7c, the current response of RGO/MWCNT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-modified GCE in the presence of several interfering species displays can be maintained with a small influence indicated by its RSD value of 2.31%. Furthermore, this selectivity investigation also yielded a recovery value in the recovery range of 96–102% (Table 2) which can be assumed to be accepted in the analytical range.103 In addition, the proposed sensor based on RGO/MWCNT (1
1)-modified GCE in the presence of several interfering species displays can be maintained with a small influence indicated by its RSD value of 2.31%. Furthermore, this selectivity investigation also yielded a recovery value in the recovery range of 96–102% (Table 2) which can be assumed to be accepted in the analytical range.103 In addition, the proposed sensor based on RGO/MWCNT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-modified GCE shows a negligible change in the current response for simultaneous measurement of HQ, DA, and UA during the initial seven days as depicted in Fig. 7d. The electrode based on RGO/MWCNT (1
1)-modified GCE shows a negligible change in the current response for simultaneous measurement of HQ, DA, and UA during the initial seven days as depicted in Fig. 7d. The electrode based on RGO/MWCNT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-modified GCE can retain 80.5% of its original value of current intensity for these three analytes until seven days. Thus, the proposed sensor based on RGO/MWCNT (1
1)-modified GCE can retain 80.5% of its original value of current intensity for these three analytes until seven days. Thus, the proposed sensor based on RGO/MWCNT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-modified GCE shows a good analytical performance for simultaneous detection of HQ, DA, and UA and potential for use in real samples such as from human urine.
1)-modified GCE shows a good analytical performance for simultaneous detection of HQ, DA, and UA and potential for use in real samples such as from human urine.
| Interferences | Interferences ratio level | Recovery (%) | ||||
|---|---|---|---|---|---|---|
(Interference![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HQ) HQ) |
(Interference![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DA) DA) |
(Interference![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) UA) UA) |
HA | DA | UA | |
| K+ | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
97.4 | 97.8 | 99.5 |
| Cl− | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
102.1 | 100.1 | 98.4 |
| Ascorbic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
98.1 | 100.4 | 97.6 |
| Glucose | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
96.2 | 97.7 | 99.2 |
| Urea | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99.3 | 103.3 | 100.0 |
| Mg2+ | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
102.6 | 100.7 | 96.4 |
3.7. Simultaneous determination of HQ, DA, and UA in the samples of human urine
To assess the performance of RGO/MWCNT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-modified GCE for simultaneous detection of HQ, DA, and UA for practical applications, this proposed sensor was applied in the samples of human urine taken from 6 volunteers. Informed consent was acquired for any experiments with human subjects. Before the measurements were conducted, 6 samples of human urines were diluted 100 times using 0.1 M of pH 7 phosphate buffer to avoid any interference from the real samples. Then the urine samples were spiked with different concentrations of HQ (5, 10, 15, 20, and 25 μM), DA (3, 6, 9, 12, and 15 μM), and UA (1, 2, 3, 4, and 5 μM). Based on Table 3, the average percentage recoveries obtained from 6 samples of human urines in triplicate measurements were discovered between 98–101% for HQ, 96–99% for DA, and 97–98% for UA. Data in detail was provided in Table S1 in the ESI section.† This result indicates that this proposed sensor could be developed further as a disposable electrode due to its simplicity of electrode modifier materials which can be applied to the planar substrate for rapid measurements of biomolecules in real samples.
1)-modified GCE for simultaneous detection of HQ, DA, and UA for practical applications, this proposed sensor was applied in the samples of human urine taken from 6 volunteers. Informed consent was acquired for any experiments with human subjects. Before the measurements were conducted, 6 samples of human urines were diluted 100 times using 0.1 M of pH 7 phosphate buffer to avoid any interference from the real samples. Then the urine samples were spiked with different concentrations of HQ (5, 10, 15, 20, and 25 μM), DA (3, 6, 9, 12, and 15 μM), and UA (1, 2, 3, 4, and 5 μM). Based on Table 3, the average percentage recoveries obtained from 6 samples of human urines in triplicate measurements were discovered between 98–101% for HQ, 96–99% for DA, and 97–98% for UA. Data in detail was provided in Table S1 in the ESI section.† This result indicates that this proposed sensor could be developed further as a disposable electrode due to its simplicity of electrode modifier materials which can be applied to the planar substrate for rapid measurements of biomolecules in real samples.
| Sample | Percentage of recovery (%) | ||
|---|---|---|---|
| HQ | DA | UA | |
| Urine 1 | 98.8 ± 2.6 | 97.0 ± 2.3 | 98.0 ± 2.4 |
| Urine 2 | 103.6 ± 1.5 | 97.8 ± 2.2 | 98.0 ± 3.5 |
| Urine 3 | 103.4 ± 1.5 | 96.6 ± 0.9 | 99.2 ± 3.6 |
| Urine 4 | 101.2 ± 2.5 | 99.8 ± 2.4 | 97.2 ± 1.6 |
| Urine 5 | 101.0 ± 2.2 | 99.6 ± 1.5 | 97.4 ± 2.4 |
| Urine 6 | 100.0 ± 2.3 | 97.8 ± 1.1 | 97.2 ± 2.4 |
4 Conclusions
In this work, we have successfully developed the electrochemical sensor based on the composite of RGO and MWCNT-modified GCE for the simultaneous detection of HQ, DA, and UA in synthetic standard solutions and real samples. It was revealed that the composition of RGO with MWCNT as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in each concentration of 1 mg mL−1 exhibits the highest electrocatalytic activity towards simultaneous measurements of HQ, DA, and UA compared to other weight ratios. This is due to the synergistic effect between RGO and MWCNT related to noncovalent interactions and van der Waals forces resulting in clear improvement in the signal discrimination and response capabilities in detecting HQ, DA, and UA simultaneously. These beneficial properties of the RGO/MWCNT composite contribute to the lower limit of detection of HQ, DA, and UA as 0.8, 1, and 0.6 μM, respectively. In addition, the proposed sensor also displays several benefits for simultaneous detection of HQ, DA, and UA including wide linear range, excellent sensitivity and selectivity, outstanding repeatability, and long-term stability. This proposed sensor also demonstrated an effective detection for simultaneous measurements of HQ, DA, and UA in 6 samples of human urine with a satisfactory result. Thus, the proposed sensor based on a composite of RGO and MWCNT has the potency to be developed for in situ monitoring with minimal costs which is important for point-of-care applications. Furthermore, this proposed method offers an alternative way for the simultaneous detection of biomolecule target species which in traditional procedures require expensive equipment and a complex sample pretreatment process. These features made this proposed sensor attractive to the application of routine analysis.
1 in each concentration of 1 mg mL−1 exhibits the highest electrocatalytic activity towards simultaneous measurements of HQ, DA, and UA compared to other weight ratios. This is due to the synergistic effect between RGO and MWCNT related to noncovalent interactions and van der Waals forces resulting in clear improvement in the signal discrimination and response capabilities in detecting HQ, DA, and UA simultaneously. These beneficial properties of the RGO/MWCNT composite contribute to the lower limit of detection of HQ, DA, and UA as 0.8, 1, and 0.6 μM, respectively. In addition, the proposed sensor also displays several benefits for simultaneous detection of HQ, DA, and UA including wide linear range, excellent sensitivity and selectivity, outstanding repeatability, and long-term stability. This proposed sensor also demonstrated an effective detection for simultaneous measurements of HQ, DA, and UA in 6 samples of human urine with a satisfactory result. Thus, the proposed sensor based on a composite of RGO and MWCNT has the potency to be developed for in situ monitoring with minimal costs which is important for point-of-care applications. Furthermore, this proposed method offers an alternative way for the simultaneous detection of biomolecule target species which in traditional procedures require expensive equipment and a complex sample pretreatment process. These features made this proposed sensor attractive to the application of routine analysis.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and/or its ESI.†Informed consent
Informed consent was obtained from all volunteers who contributed human urine to this work.Author contributions
The author's contributions to this article are as follows: writing, supervision, review, and funding acquisition: Wulan Tri Wahyuni. Data acquisition: Shafa Aini Hasnawati Ta'alia, Bunga Rani Elvira, Ari Yustisia Akbar. Conceptualization and funding: Irkham, Isnaini Rahmawati, Ruri Agung Wahyuono. Writing – original draft, supervision, review, conceptualization: Budi Riza Putra.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge Riset Kolaborasi Indonesia PTNBH with contract number 13529/IT3.D10/PT.01.03/P/B/2024 fiscal year 2024 and the Rumah Program Nanoteknologi dan Material Badan Riset dan Inovasi Nasional (BRIN) 2024 with contract number 20/III.10/HK/2024.References
- R. Manikandan, T. Rajarathinam, S. Jayaraman, H.-G. Jang, J.-H. Yoon, J. Lee, H.-J. Paik and S.-C. Chang, Coord. Chem. Rev., 2024, 499, 215487, DOI:10.1016/j.ccr.2023.215487.
- M.-B. Irimes, A. Pusta, A. Cernat, B. Feier, M. Tertis, C. Cristea, A. D. Buzoianu and R. Oprean, TrAC, Trends Anal. Chem., 2024, 172, 117560, DOI:10.1016/j.trac.2024.117560.
- N. Li, J.-R. Huang, H.-Y. Zhang, M. Cui, B. Sun, C. Zhang and H.-Y. Zhao, ACS Appl. Nano Mater., 2024, 7, 1960–1969, DOI:10.1021/acsanm.3c05232.
- Y. Niu, K. Kang, B. Wang, L. Wang, C. Li, X. Gao, Z. Zhao and X. Ji, Talanta, 2024, 268, 125349, DOI:10.1016/j.talanta.2023.125349.
- Y. Xing, C. Lv, Y. Fu, L. Luo, J. Liu, X. Xie and F. Chen, Talanta, 2024, 271, 125674, DOI:10.1016/j.talanta.2024.125674.
- T. S. I. Bakavaty and K. Gurunathan, Mater. Sci. Eng., B, 2024, 299, 116967, DOI:10.1016/j.mseb.2023.116967.
- A. Valenzuela, D. Ballestero, C. Gan, G. Lorca, E. Langa and M. R. Pino-Otin, Toxics, 2024, 12, 115, DOI:10.3390/toxics12020115.
- P. Prateek, P. Kumar, R. K. Gupta, V. C. Srivastava, I. D. Mall and U. L. Stangar, J. Indian Chem. Soc., 2024, 101, 101131, DOI:10.1016/j.jics.2024.101131.
- M. M. Alam, M. Imran, T. Alshahrani, F. Khan and R. Azim, Measurement, 2024, 224, 113890, DOI:10.1016/j.measurement.2023.113890.
- X. Wang, H. Liu, Y. Xue, L. Cui, L. Chen, K.-F. Ho and Y. Huang, Environ. Chem. Lett., 2024, 22, 1327–1343, DOI:10.1007/s10311-024-01701-x.
- O. D. Bamidele, B. A. Kayode, O. I. Eniayewu, A. J. Adegbola, R. S. Olatoye, N. S. Njinga, S. T. Abdullahi and M. T. Bakare-Odunola, Sci. Rep., 2023, 13, 20992, DOI:10.1038/s41598-023-47160-2.
- J. J. Nordlund, P. E. Grimes and J. P. Ortonne, J. Eur. Acad. Dermatol. Venereol., 2006, 20, 781–787, DOI:10.1111/j.1468-3083.2006.01670.x.
- A. P. DeCaprio, Crit. Rev. Toxicol., 1999, 29, 283–330, DOI:10.1080/10408449991349221.
- F. Zhou, H. N. Lim, I. Ibrahim, N. A. Endot, E. A. Malek and N. S. K. Gowthaman, ChemPlusChem, 2024, 89, e202300686, DOI:10.1002/cplu.202300686.
- L. Lin, M. Li, P. Li, C. Ye, H. Zhuang, S. Weng and F. Chen, Microchem. J., 2024, 196, 109602, DOI:10.1016/j.microc.2023.109602.
- C. V. Raju, R. Ramya, K. Imran, C. K. Basha, J. Wilson, T. Boobalan, A. Arun, M. J. Basu and S. Saravanan, Microchem. J., 2024, 198, 110189, DOI:10.1016/j.microc.2024.110189.
- C. Liu, X. Lin, J. Liao, M. Yang, M. Jiang, Y. Huang, Z. Du, L. Chen, S. Fan and Q. Huang, Chin. Chem. Lett., 2024, 109598, DOI:10.1016/j.cclet.2024.109598.
- Y. Xue, Q. Hassan, M. Noroozifar, R. M. A. Sullan and K. Kerman, Talanta, 2024, 266, 125030, DOI:10.1016/j.talanta.2023.125030.
- F. Chen, J. Wang, L. Chen, H. Lin, D. Han, Y. Bao, W. Wang and L. Niu, Anal. Chem., 2024, 96, 3914–3924, DOI:10.1021/acs.analchem.3c05672.
- G. Deffo, C. G. Fotsop, M. C. D. Ngaha, S. G. Fogang, L. A. Vomo, B. W. Nkuigoua, C. A. Shella, A. V. Somba, T. F. N. Tene, I. K. Tchummegne, E. Njanja, I. K. Tonle, P. Puzari and E. Ngameni, Mater. Adv., 2024, 5, 3683–3695, 10.1039/D3MA01182H.
- M. A. Khairy, A. Hamad, M. Hamed, M. Locatelli and F. T. Mansour, J. Pharm. Biomed. Anal., 2024, 242, 116021, DOI:10.1016/j.jpba.2024.116021.
- J. S. Jeon, B. H. Kim, S. H. Lee, H. J. Kwon, H. J. Bae, S. K. Kim, J. A. Park, J. H. Shim, A. M. A. El-Aty and H. C. Shin, Int. J. Cosmet. Sci., 2015, 37, 567–573, DOI:10.1111/ics.12228.
- B. P. Guiard and G. Gotti, Molecule, 2024, 29, 496, DOI:10.3390/molecules29020496.
- R. Cabrera-Alonso, E. Guevara, M. G. Ramirez-Elias, B. Moncada and F. J. Gonzalez, Sking Res. Technol., 2018, 25, 20–24, DOI:10.1111/srt.12589.
- R. Cabrera-Alonso, E. Guevara, M. G. Ramirez-Elias, B. Moncada and F. J. Gonzalez, J. Nanophotonics, 2019, 13(3), 036006, DOI:10.1117/1.JNP.13.036006.
- I. B. Ansah, W.-C. Lee, C. W. Mun, J.-J. Rham, H. S. Jung, M. Kang, S.-G. Park and D.-H. Kim, Sens. Actuators, B, 2022, 353, 131196, DOI:10.1016/j.snb.2021.131196.
- A. Rybina, B. Thaler, R. Kramer and D.-P. Herten, Phys. Chem. Chem. Phys., 2014, 16, 19550–19555, 10.1039/C4CP02640C.
- H. Huang, J. Bai, L. Lei, W. Zhang, S. Yan and Y. Li, Anal. Bioanal. Chem., 2020, 412, 5291–5297, DOI:10.1007/s00216-020-02742-1.
- Y.-H. Lin, Y.-H. Yang and S.-M. Yu, J. Pharm. Biomed. Anal., 2007, 44, 279–282, DOI:10.1016/j.jpba.2007.02.004.
- X. Li, J. Pan, F. Yang, J. Feng, J. Mo and Z. Chen, Microchim. Acta, 2011, 174, 123–130, DOI:10.1007/s00604-011-0592-5.
- S. Zhao, J. Wang, F. Ye and Y.-M. Liu, Anal. Biochem., 2008, 378, 127–131, DOI:10.1016/j.ab.2008.04.014.
- C. Shao-bo, P. Yun-yue, Y. Wen-Jing and X. Zhen-dong, Anal. Test. Technol. Instrum., 2017, 23, 176–179, DOI:10.16495/j/1006-3757.2017.03.006.
- A. Chisvert, J. Sisternes, A. Balaguer and A. Salvador, Talanta, 2010, 81, 530–536, DOI:10.1016/j.talanta.2009.12.037.
- Q. Li, S. Wei, D. Wu, C. Wen and J. Zhou, BioMed Res. Int., 2018, 3461572, DOI:10.1155/2018/3461572.
- C. Xu, Q. Zheng, P. Zhao, J. Paterson, H. Chen and J. Am, Soc. Mass Spectrochem., 2019, 30, 685–693, DOI:10.1007/s13361-018-2116-6.
- G. Mo, X. He, C. Zhou, D. Ya, J. Feng, C. Yu and B. Deng, Sens. Actuators, B, 2018, 266, 784–792, DOI:10.1016/j.snb.2018.03.187.
- Z. Dong, S. Xia, A. M. A. Alboull, I. M. Mostafa, A. Abdussalam, W. Zhang, S. Han and G. Xu, ACS Appl. Nano Mater., 2024, 7, 2983–2991, DOI:10.1021/acsanm.3c05309.
- M. Kong, W. Wei, W. Wang, H. Chen and J. He, Spectrochim. Acta, Part A, 2021, 257, 119773, DOI:10.1016/j.saa.2021.119773.
- J. Qian, Z. Yang, H. Cui, K. An, C. Ren, Q. Liu and K. Wang, J. Electroanal. Chem., 2020, 868, 114177, DOI:10.1016/j.jelechem.2020.114177.
- F. A. Gorla, M. d. P. Ferreira, C. S. d. Santos, R. d. Matos, M. G. Segatelli and C. R. T. Tarley, J. Electroanal. Chem., 2024, 935, 117988, DOI:10.1016/j.jelechem.2023.117988.
- X. Ma, L. Deng, Z. Zou, Z. Pan, L. Feng, Z. Huang, Z. Liang, X. Liu, M. Li, Z. Su and H. Zheng, Talanta, 2024, 271, 125646, DOI:10.1016/j.talanta.2024.125646.
- M. P. Manikanta, R. R. Nikam, G. Tigari and N. B. Mallana, Anal. Methods, 2024, 16, 1770–1784, 10.1039/D4AY00087K.
- S. P. Jyothi, D. Vinod, D. Chandran, S. Antherjanam, B. Saraswathyamma, V. Balaraman and R. Rajamani, J. Appl. Electrochem., 2024, 54, 1819–1831, DOI:10.1007/s10800-024-02067-2.
- Z. Aryan, H. Khajehsharifi and S. Shahrokhian, Microchem. J., 2024, 198, 110087, DOI:10.1016/j.microc.2024.110087.
- R. Wang, S. Liu, X. Song, K. Jiang, Y. Hou, Q. Cheng, W. Miao, L. Tian, Y. Ren and S. Xu, Coatings, 2024, 14, 227, DOI:10.3390/coatings14020227.
- J. Suriyaprakash, I. Thangavelu, Y. Huang, Z. Hu, H. Wang, Y. Zhan, L. Wu and L. Shan, Surf. Interfaces, 2024, 46, 104021, DOI:10.1016/j.surfin.2024.104021.
- V. Mariyappan, R. Sundaresan, S.-M. Chen, R. Ramachandran, A. G. Al-Sehemi, A. Jeevika and W. Wu, Process Saf. Environ. Prot., 2024, 185, 726–738, DOI:10.1016/j.psep.2024.03.013.
- K. Prabhu, S. J. Malode, N. P. Shetti, S. Pandiaraj, A. Alodhayb and M. Muthuramamoorthy, Microchem. J., 2024, 197, 109722, DOI:10.1016/j.microc.2023.109722.
- M. H. Rahman, M. A. Rashed, N. I. Nayem, M. A. Rahaman, J. Ahmed, M. Faisal, M. Jalalah and F. A. Harraz, Mater. Chem. Phys., 2024, 314, 128915, DOI:10.1016/j.matchemphys.2024.128915.
- L. Zhang, H. Wu, Y. Chen, Y. Li, J. Xu, Y. Zhang, W. Wang, G. Zhao, C. Zhang and H. Li, ACS Appl. Electron. Mater., 2024, 6, 793–805, DOI:10.1021/acsaelm.3c01322.
- H. Wang, Y. Hao, L. Xiang, X. Qi, L. Wang, J. Ding, Y. Qu, J. Xu and W. Zhing, Mater. Res. Bull., 2024, 171, 112631, DOI:10.1016/j.materresbull.2023.112631.
- S.-H. Kim, T. H. Kim, H. K. Park, Y. C. Kang, J. S. Cho and G. D. Park, J. Energy Storage, 2024, 83, 110683, DOI:10.1016/j.est.2024.110683.
- H. Bagheri, A. Hajian, M. Rezaei and A. Shirzadmehr, J. Hazard. Mater., 2017, 324, 762–772, DOI:10.1016/j.jhazmat.2016.11.055.
- Z.-N. Huang, J. Zou, J. Teng, Q. Liu, M.-M. Yuan, F.-P. Jiao, X.-Y. Jiang and J.-G. Yu, Ecotoxicol. Environ. Saf., 2019, 172, 167–175, DOI:10.1016/j.ecoenv.2019.01.091.
- C. Ma, P. Xu, H. Chen, J. Cui, M. Guo and J. Zhao, Microchem. J., 2022, 180, 107533, DOI:10.1016/j.microc.2022.107533.
- S. Yang, M. Yang, Q. Liu, X. Wang, H. Fa, Y. Wang and C. Hou, J. Electrochem. Soc., 2019, 166, B547, DOI:10.1149/2.0011908jes.
- H. Mesker, F. Achi, A. Zouaoui, S. Ha, M. Peacock and H. Belkhalfa, Anal. Lett., 2022, 55, 1466–1481, DOI:10.1080/00032719.2021.2008951.
- S. C. Silva, R. M. Cardoso, E. M. Richter, R. A. A. Munoz and E. Nossol, Mater. Chem. Phys., 2020, 250, 123011, DOI:10.1016/j.matchemphys.2020.123011.
- P. Pasakon, J. P. Mensing, D. Phokaratkul, C. Karuwan, T. Lomas, A. Wisitsorat and A. Tuantranont, J. Appl. Electrochem., 2019, 49, 217–227, DOI:10.1007/s10800-018-1268-1.
- J.-S. Chen, Y. Li, M.-J. Yu and H.-L. Lee, J. Environ. Anal. Chem., 2020, 100, 774–788, DOI:10.1080/03067319.2019.1640874.
- X. Qiu, L. Lu, J. Leng, Y. Yu, W. Wang, M. Jiang and L. Bai, Food Chem., 2016, 190, 889–895, DOI:10.1016/j.foodchem.2015.06.045.
- B. R. Putra, U. Nisa, R. Heryanto, E. Rohaeti, M. Khalil, A. Izzataddini and W. T. Wahyuni, Anal. Sci., 2022, 38, 157–166, DOI:10.2116/analsci.21P214.
- S. Li, S. Ali, Z. Zuhra, H. Shen, J. Qiu, Y. Zeng, K. Zeng, X. Wang, G. Xie and S. Ding, Molecules, 2023, 29, 65, DOI:10.3390/molecules29010065.
- K. Hiratochi, D. Terada, H. Suga, M. Okada, K. Bandon, T. Kodaira, T. Yamada, T. Shimizu, K. Saiki and T. Kubo, Mater. Chem. Front., 2024, 8, 814–823, 10.1039/D3QM00992K.
- H. Wang, A. Wang, H. Yin, Y. Ding and C. Li, Mater. Sci. Eng., 2024, 300, 117061, DOI:10.1016/j.mseb.2023.117061.
- D. Lopez-Diaz, M. L. Holgado, J. L. Garcia-Fiero and M. M. Velazquez, J. Phys. Chem. C, 2017, 121, 20489–20497, DOI:10.1021/acs.jpcc.7b06236.
- A. Romero, M. P. Lavin-Lopez, L. Sanchez-Silva, J. L. Valverde and A. Paton-Carrero, Mater. Chem. Phys., 2018, 203, 284–292, DOI:10.1016/j.matchemphys.2017.10.013.
- M. Sieradzka, C. Slusarczyk, R. Fryczkowski and J. Janicki, J. Mater. Res. Technol., 2020, 9, 7059–7067, DOI:10.1016/j.jmrt.2020.05.026.
- L. Wang, Z. Lou, J. Deng, R. Zhang and T. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 13098–13104, DOI:10.1021/acsami.5b03978.
- P. Das, K. Ibrahim, K. Chakraborty, S. Ghosh and T. Pal, Sci. Rep., 2024, 14, 296, DOI:10.1038/s41598-023-51040-0.
- N. Sebastian, W.-C. Yu, D. Balram, A. Patel, D. Kumar and V. K. Yadav, Sens. Int., 2024, 5, 100256, DOI:10.1016/j.sintl.2023.100256.
- B. Liang, H. Hu, H. Zhu, Y. Yu, W. He and G. Li, Sci. Total Environ., 2024, 908, 168466, DOI:10.1016/j.scitotenv.2023.168466.
- M. Arslanhan and E. D. S. Parmak, Thin Solid Films, 2024, 788, 140149, DOI:10.1016/j.tsf.2023.140149.
- P. A Koyale, S. P. Kulkarni, J. L. Gunjakar, T. D. Dongale, S. S. Sutar, S. S. Soni, Y. G. Kapdi, R. Nath, S. V. Mulik and S. D. Delekar, ACS Appl. Nano Mater., 2024, 7, 2662–2674, DOI:10.1021/acsanm.3c04694.
- F. Y. Ban, S. Jayabal, A. Pandikumar, H. N. Lim and N. M. Huang, Electrocatalysis, 2015, 6, 373–381, DOI:10.1007/s12678-015-0254-1.
- A. Benchirouf, C. Muller and O. Kanoun, Nanoscale Res. Lett., 2016, 11, 4, DOI:10.1186/s11671-015-1216-5.
- Q. Kong, Z. Luo, Y. Wang and B. Wang, J. Polym. Res., 2018, 25, 231, DOI:10.1007/s10965-018-1597-0.
- Q. Liu, J. Bao, M. Yang, X. Wang, S. Lan, C. Hou, Y. Wang and H. Fa, Sens. Actuators, B, 2018, 274, 433–440, DOI:10.1016/j.snb.2018.07.146.
- B. Liu, H. Guo, L. Sun, Z. Pan, L. Peng, M. Wang, N. Wu, Y. Chen, X. Wei and W. Yang, Colloids Surf., A, 2022, 639, 128335, DOI:10.1016/j.colsurfa.2022.128335.
- A. C. Anithaa, N. Lavanya, K. Asokan and C. Sekar, Electrochim. Acta, 2015, 167, 294–302, DOI:10.1016/j.electacta.2015.03.160.
- S. A. Shahamirifard, M. Ghaedi, Z. Razmi and S. Hajati, Biosens. Bioelectron., 2018, 114, 30–36, DOI:10.1016/j.bios.2018.05.009.
- X. Yang, C. He, W. Lin, Y. Qiu, P. Li, Y. Chen, B. Huang and X. Zheng, Synth. Met., 2022, 287, 117079, DOI:10.1016/j.synthmet.2022.117079.
- U. Amara, M. T. Mehran, B. Sarfaraz, K. Mahmood, A. Hayat, M. Nasir, S. Riaz and M. H. Nawaz, Microchim. Acta, 2021, 188, 230, DOI:10.1007/s00604-021-04884-0.
- N. Vishnu, M. Gandhi, D. Rajagopal and A. S. Kumar, Anal. Methods, 2017, 9, 2265–2274, 10.1039/C7AY00445A.
- P. Karami-Kolmoti, H. Beitollahi and S. Modiri, Biomedicines, 2023, 11, 1869, DOI:10.3390/biomedicines11071869.
- C. W. Atcherley, N. D. Laude, E. B. Monroe, K. M. Wood, P. Hashemi and M. L. Heien, ACS Chem. Neurosci., 2015, 6, 1509–1516, DOI:10.1021/cn500020s.
- S. A. Shahamirifard, M. Ghaedi, Z. Razmi and S. Hajati, Biosens. Bioelectron., 2018, 114, 30–36, DOI:10.1016/j.bios.2018.05.009.
- S. Kostromin, M. Asandulesa, A. Poldshivalov and S. Bronnikov, Mater. Res. Express, 2019, 6, 115053, DOI:10.1088/2053-1591/ab46f8.
- K. A. Shiyanova, M. V. Gudkov, M. K. Torkunov, G. P. Goncharuk, A. A. Gulin, A. V. Sysa, N. G. Ryvkina, S. L. Bazhenov and V. P. Melnikov, J. Compos. Mater., 2023, 57, 111–119, DOI:10.1177/0021998322113954.
- S. Liang, H. Wang and X. Tao, J. Mater. Res. Technol., 2022, 17, 2388–2399, DOI:10.1016/j.jmrt.2022.02.004.
- O. Okhay and A. Tkach, Nanomaterials, 2021, 11, 1240, DOI:10.3390/nano11051240.
- N. S. Suhaimin, M. F. R. Hanifah, M. Azhar, J. Jaafar, M. Aziz, A. F. Ismail, M. H. D. Othman, M. A. Rahman, F. Aziz, N. Yusof and R. Mohamud, Mater. Chem. Phys., 2022, 278, 125629, DOI:10.1016/j.matchemphys.2021.125629.
- T. Thenrajan, M. M. Malar, S. Kumaravel, R. Rajaram, S. Kundu and J. Wilson, Mater. Adv., 2024, 5, 1691–1701, 10.1039/D3MA00533J.
- V. Stankovic, S. Durdic, M. Ognjanovic, G. Zlatic and D. Stankovic, Sensors, 2024, 24, 705, DOI:10.3390/s24020705.
- Y. Zhang, X. Du, J. Mao, S. He and Z. Cao, Mater. Chem. Phys., 2024, 311, 128526, DOI:10.1016/j.matchemphys.2023.128526.
- J. Li, L. Hou, Y. Jiang, M.-J. Wei, F.-Y. Kong, H.-Y. Li, Z.-X. Wang and W. Wang, Microchem. J., 2024, 199, 110007, DOI:10.1016/j.microc.2024.110007.
- Y. Liao, L. Lin, J. Liu, X. Zhang and X. Li, J. Electroanal. Chem., 2024, 954, 118028, DOI:10.1016/j.jelechem.2024.118028.
- Y.-Y. Lei, X. Zhan, Y.-W. Wu and X.-X. Yu, Talanta, 2024, 268, 125287, DOI:10.1016/j.talanta.2023.125287.
- Z. Feng, H. N. Lim, I. Ibrahim, N. A. Endot, E. A. Malek and N. S. K. Gowthaman, Appl. Organomet. Chem., 2024, 38, e7350, DOI:10.1002/aoc.7350.
- E. D. Tecuapa-Flores, C. B. Palacios-Cabrera, A. J. Santiago-Cuevas, J. G. Hernandez, J. Narayanan and P. Thangarasu, Analyst, 2024, 149, 108–124, 10.1039/D3AN01467C.
- D. Jia, T. Yang, K. Wang, L. Zhou, E. Wang, K.-C. Chou, H. Wang and X. Hou, J. Alloys Compd., 2024, 985, 173392, DOI:10.1016/j.jallcom.2023.173392.
- S. A. H. Ta'alia, E. Rohaeti, B. R. Putra and W. T. Wahyuni, Results Chem., 2023, 6, 101024, DOI:10.1016/j.rechem.2023.101024.
- S. Lu, K. Zhang, Y. Liu, X. Zhan and R. Savari, Environ. Res., 2024, 245, 117369, DOI:10.1016/j.envres.2023.117369.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra05537c |
| This journal is © The Royal Society of Chemistry 2024 |