 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
High adsorption to methylene blue based on Fe3O4–N-banana-peel biomass charcoal†
Zhu-Xiang Gonga,
Mfitumucunguzi Stevena,
Yan-Ting Chena,
Li-Zhu Huoa,
Hao Xua,
Chao-Fei Guo a,
Xue-Juan Yanga,
Yu-Xuan Wang*a and
Xi-Ping Luo
a,
Xue-Juan Yanga,
Yu-Xuan Wang*a and
Xi-Ping Luo *ab
*ab
aCollege of Chemistry and Materials Engineering, Zhejiang A&F University, Hangzhou 311300, China. E-mail: 20190050@zafu.edu.cn; luoxiping@zafu.edu.cn
bZhejiang Provincial Key Laboratory of Chemical Utilization of Forestry Biomass, Hangzhou 311300, China
First published on 15th August 2024
Abstract
This research focused on utilizing banana peel as the primary material for producing mesoporous biomass charcoal through one-step potassium hydroxide activation. Subsequently, the biomass charcoal underwent high-temperature calcination with varying impregnation ratios of KOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BC for different durations in tubular furnaces set at different temperatures. The resultant biomass charcoal was then subjected to hydrothermal treatment with FeCl3·6H2O to produce biochar/iron oxide composites. The adsorption capabilities of these composites towards methylene blue (MB) were examined under various conditions, including pH (ranging from 3 to 12), temperature variations, and initial MB concentrations (ranging from 50 to 400 mg L−1). The adsorption behavior aligned with the Langmuir model and demonstrated quasi-secondary kinetics. After five adsorption cycles, the capacity decreased from 618.64 mg g−1 to 497.18 mg g−1, indicating considerable stability. Notably, Fe3O4–N-BC exhibited exceptional MB adsorption performance.
BC for different durations in tubular furnaces set at different temperatures. The resultant biomass charcoal was then subjected to hydrothermal treatment with FeCl3·6H2O to produce biochar/iron oxide composites. The adsorption capabilities of these composites towards methylene blue (MB) were examined under various conditions, including pH (ranging from 3 to 12), temperature variations, and initial MB concentrations (ranging from 50 to 400 mg L−1). The adsorption behavior aligned with the Langmuir model and demonstrated quasi-secondary kinetics. After five adsorption cycles, the capacity decreased from 618.64 mg g−1 to 497.18 mg g−1, indicating considerable stability. Notably, Fe3O4–N-BC exhibited exceptional MB adsorption performance.
Introduction
According to statistics, more than 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 commercial dye products are introduced into the aquatic environment. The textile industry is a primary contributor to water pollution, alongside significant sectors such as paint, leather, paper, and printing industries.1,2 Methylene blue (MB) stands as a representative cationic organic dye, commonly utilized in various industries such as papermaking, silk, cotton, wool, and hair coloring. With the economy experiencing rapid growth, industrial production processes are yielding increasingly large volumes of dye effluent, predominantly composed of organic compounds like azo, amino, and phenyl compounds. The discharge of wastewater containing dyes not only pollutes the aquatic environment but also disrupts the ecological balance of aquatic life and poses risks to human health.3 Elevated levels of MB in water can lead to noticeable changes in color and turbidity, along with potential skin irritation and neurological effects. Prolonged consumption of water contaminated with dyes poses a severe risk to human health, causing significant damage to the liver and digestive system over time.4 Therefore, it is imperative to implement appropriate measures to remove MB from dye wastewater, ensuring both water quality preservation and the safeguarding of human health from contamination. Reports indicate the frequent utilization of various methods for remediating dyes in contaminated wastewater, including Fenton oxidation,5 photocatalytic oxidation,6 membrane separation,7 adsorption,8 and electrochemical approaches.9 Compared to other approaches, the adsorption method has advantages. It is one of the most extensively used wastewater treatment technologies due to its simple design, high regeneration potential, diverse material source, good performance, and cost-effectiveness.10
000 commercial dye products are introduced into the aquatic environment. The textile industry is a primary contributor to water pollution, alongside significant sectors such as paint, leather, paper, and printing industries.1,2 Methylene blue (MB) stands as a representative cationic organic dye, commonly utilized in various industries such as papermaking, silk, cotton, wool, and hair coloring. With the economy experiencing rapid growth, industrial production processes are yielding increasingly large volumes of dye effluent, predominantly composed of organic compounds like azo, amino, and phenyl compounds. The discharge of wastewater containing dyes not only pollutes the aquatic environment but also disrupts the ecological balance of aquatic life and poses risks to human health.3 Elevated levels of MB in water can lead to noticeable changes in color and turbidity, along with potential skin irritation and neurological effects. Prolonged consumption of water contaminated with dyes poses a severe risk to human health, causing significant damage to the liver and digestive system over time.4 Therefore, it is imperative to implement appropriate measures to remove MB from dye wastewater, ensuring both water quality preservation and the safeguarding of human health from contamination. Reports indicate the frequent utilization of various methods for remediating dyes in contaminated wastewater, including Fenton oxidation,5 photocatalytic oxidation,6 membrane separation,7 adsorption,8 and electrochemical approaches.9 Compared to other approaches, the adsorption method has advantages. It is one of the most extensively used wastewater treatment technologies due to its simple design, high regeneration potential, diverse material source, good performance, and cost-effectiveness.10
Activated carbon (AC) is a widely utilized adsorbent in wastewater treatment technology owing to its expansive specific surface area, well-suited pore distribution, and robust mechanical and thermal stability.11 However, most AC on the market today is made from coal, lignite, and coking coal as precursors. These resources have the disadvantage of being costly and non-renewable.12 Compared to coal, a non-renewable resource for carbon production, biomass carbon sources are both renewable and widely available. The utilization of biomass energy can reduce dependence on fossil fuels, thereby mitigating air and water pollution. Due to its larger pore structure and more active sites, biomass carbon exhibits enhanced adsorption capacity, which has garnered significant attention from researchers. Research has demonstrated that BC effectively eliminates hazardous cationic dyes from wastewater.13 Crop straws, forestry and agricultural wastes, coal, and municipal sewage can all be used to make BC.14 It can accomplish the recycling and reuse of waste resources in addition to lowering the pollution that rubbish burning and decomposition contribute to the environment. To obtain greater adsorption effects, biomass charcoal's chemical and physical characteristics must be enhanced. The original biomass charcoal's adsorption capacity to remove contaminants from wastewater is still restricted. The primary objective of modification in biomass charcoal is to enhance its specific surface area and pore structure, while concurrently enriching the material's surface functional groups. Additionally, it is crucial for biomass charcoal to facilitate easy separation into solid and liquid components. The recent surge in popularity of iron modification of biomass charcoal is attributed to its ability to introduce magnetic components, thereby facilitating efficient solid–liquid separation.15 Furthermore, adding iron to biomass charcoal can increase its surface area and create a pore structure, resulting in more active sites for adsorption.16 Nevertheless, the application of iron oxide onto biomass charcoal may potentially occupy adsorption sites or impede the pore structure, consequently diminishing its adsorption capacity.17 Nitrogen doping is a promising modifying technique. Nitrogen dopants can increase the electrical conductivity of the carbon matrix, resulting in more active catalytic sites.18–20 Nitrogen-modified biomass charcoal has been frequently employed in catalysis because of its local unpaired electrons.21 It has also been discovered that nitrogen alteration can diminish the surface area and pore volume of biomass charcoal.22 Consequently, the advantages of enhanced surface area, functional groups, and magnetic components are obtained by iron-nitrogen co-doping. The adsorption mechanisms of iron-nitrogen co-modified biomass charcoal for the removal of pollutants may involve π–π stacking, surface complexation, hydrogen bonding, and pore diffusion.23 Numerous studies have explored Fe–N modified biomass charcoal as an adsorbent. For instance, Zhou et al. pioneered the development of urea-functionalized magnetic biomass charcoal, employing it as an adsorbent for the extraction of Pb.24 Ai et al. produced NH4Cl-induced magnetic buckwheat husk powder biomass charcoal and utilized it to adsorb tetracycline and Zn(II).25 According to the literature, practically all adsorbent modification materials based on biomass charcoal are carbonized before being activated with an activator. This approach is not only energy-intensive, but it is also complex and time-consuming, and the adsorbed biomass charcoal is difficult to recover.26 Some adsorbents have low adsorption capacity due to their small specific surface area; some adsorbents have problems such as difficulty in separation and recovery; magnetic modification is an excellent solution to their separation and recovery; however, magnetic modification generally leads to a decrease in the adsorbent's adsorption capacity, so novel adsorption materials must be developed to maintain a magnetic adsorbent while also having a high adsorption capability. FeCl3 can be used as a magnetic source to improve the separation and recovery capacity of biochar due to its low cost and low pollution.
In this study, biomass charcoal was prepared with banana peels as precursors and potassium hydroxide as an activator. To investigate the impact of temperature, activator ratio, and carbonization period on adsorption performance, an orthogonal experiment was created. To make nitrogen-modified biomass charcoal (N-BC), find the ideal preparation conditions, combine it with urea in these settings, and pyrolyze. To manufacture magnetic Fe3O4, utilize a hot solvent approach. Then, dope Fe3O4 with biomass charcoal to create Fe3O4–N-BC, which is very valuable, recyclable, and makes the adsorbent magnetic to lower secondary recycling costs. The iron-nitrogen co-modification method presents several advantages, including the augmentation of surface area, enhancement of functional groups, and introduction of magnetic components, potentially rendering it a feasible alternative for biochar preparation. To explore the impacts of Fe and N modification on the morphology, chemical composition, and functional groups of biomass charcoal, the sample underwent characterization using scanning electron microscopy (SEM), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and vibrating-sample magnetometry (VSM).
Materials and methods
Materials and reagents
Banana peels were purchased from Zhejiang Province, China. Ethylene glycol, potassium hydroxide (KOH), and absolute ethanol were all of analytical grade and purchased from Macklin (Shanghai, China). Hydrochloric acid (HCl, 36–38 wt%) and sodium hydroxide (NaOH) were all of analytical grade and purchased from Sinopharm Group Chemical Reagent Co., Ltd (Shanghai, China), FeCl3·6H2O (99%), sodium acetate trihydrate (NaAc·3H2O) (99%), urea (99%), methylene blue (C16H18N3SCl) (98%) are purchased from Macklin (Shanghai, China), deionized water (18.25 MΩ cm) was prepared in the laboratory.Preparation of adsorbent
Because of the intricate interplay among reaction conditions, pinpointing suitable synthesis parameters proves challenging through single reaction conditions alone. Hence, an orthogonal design method was employed to elucidate the impact of each individual response factor.27,28 The orthogonal design table is shown in the Table S1 (ESI),† which mainly discusses the effects of biomass precursor type, carbonization time, carbonization temperature, and activator dosage on synthesis.
Adsorption kinetics. 5 mg adsorbent was mixed in methylene blue solution of 25 mL 300 mg L−1, and adsorption experiments were carried out at 25 °C and pH natural conditions at different times. Then the dynamic model is used to fit the data.
Adsorption isotherm. Dispersed 5 mg of adsorbent in 25 mL MB solutions of different concentrations, adsorb under natural pH conditions of 25 °C, 35 °C, and 45 °C, conduct isotherm research, and then used the isotherm model to fit the relevant data.
Adsorption thermodynamics. A methylene blue solution, initially concentrated at 300 mg L−1, was chosen. Successive 25 mL dye solutions were introduced to 5 mg adsorbents under ambient pH conditions. The adsorbents underwent adsorption at temperatures of 25, 35, and 45 °C. Upon reaching adsorption equilibrium, the remaining dye solution concentration was measured, followed by thermodynamic fitting.
The impact of pH on adsorption performance. 0.1 M HCl and 0.1 M NaOH were employed to modulate the pH of the dye solution within the pH range of 3–12 at 25 °C. Subsequently, 10 mg of adsorbent was dispersed in 25 mL of a 300 mg L−1 MB solution at various pH levels. Post-adsorption equilibrium, the concentration of the MB solution was ascertained.
Incorporate a specified quantity of Fe3O4–N-BC adsorbent into 25 mL of dye solutions with varying concentrations. Following adsorption at a consistent temperature for a duration, the supernatant is magnetically isolated and subjected to measurement using a UV spectrophotometer at the wavelength of maximum absorption, λ = 664 nm. According to the standard curve generated for the methylene blue dye (Fig. S2, ESI†). Measure the absorbance of the dye. The adsorption capacity qe (mg g−1) (eqn (1)) and removal rate R (%) (eqn (2)) under different conditions are calculated by the following formulas:
 | (1) |
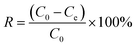 | (2) |
Characterization
The sample's morphology was analyzed using scanning electron microscopy (SEM) coupled with an energy dispersive spectrometer (EDS). After the biomass charcoal was dissolved in concentrated hydrochloric acid, the content of biomass charcoal-coated iron was determined by inductively coupled plasma mass spectrometry (ICP-MS). The absorbance of residual dye in water is measured with a UV-visible spectrophotometer (UV). An X-ray diffractometer was employed to capture the inorganic crystal morphology of the sample within the range of 10° to 70°. Raman spectra were acquired utilizing a high-resolution Raman spectrophotometer from HoribaJobin-Yvon. X-ray photoelectron spectroscopy (XPS) spectra were acquired utilizing an ESCALAB250 instrument. The magnetic properties of the samples were assessed at room temperature employing a vibrating sample magnetometer (VSM, LakeShore7404). BET specific surface area measurements were performed on a Micromeritics ASAP2020 instrument (ASAP2020, Micromeritis).Results and discussion
Modified synthesis results and characterizations
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. For KOH activation,29 first KOH interacts with carbon components and is converted into K2CO3 (eqn (3)), then K2CO3 is decomposed into K2O and CO2 (eqn (4)), and the intermediate product further reacts with carbon to form CO (eqn (5)–(7)), the gases (H2O, CO2, CO, etc.) produced by the reaction help to form a porous structure. The biomass chars utilized in subsequent characterization and adsorption experiments were synthesized at their optimal levels.
2. For KOH activation,29 first KOH interacts with carbon components and is converted into K2CO3 (eqn (3)), then K2CO3 is decomposed into K2O and CO2 (eqn (4)), and the intermediate product further reacts with carbon to form CO (eqn (5)–(7)), the gases (H2O, CO2, CO, etc.) produced by the reaction help to form a porous structure. The biomass chars utilized in subsequent characterization and adsorption experiments were synthesized at their optimal levels.| 6KOH + 2C → 2K + 3K2CO3 + 3H2 | (3) |
| K2CO3 → K2O + CO2 | (4) |
| CO2 + C → 2CO | (5) |
| K2CO3 + 2C → 2K + 3CO | (6) |
| K2O + C → 2K + CO | (7) |
Fig. 5(a) shows the XRD patterns of BC, N-BC, Fe3O4, and Fe3O4–N-BC. According to the standard PDF card (JCPDF #75-0449), the values of 2θ in the figure are at 18.4° (111), 30.3° (220), 35.7° (311), 37.4° (222), 43.4° (400), 53.9° (422), 57.5° (511), 63.1° (440), 71.6° (620), and 74.7° (533) are characteristic peaks of Fe3O4, which proves the presence of Fe3O4. Since amorphous carbon is prepared, its crystallinity is low, and broad peaks with low signal intensity appear near 28° and 40°. Because the diffraction peak intensity is weaker than that of Fe3O4, it is not obvious in the XRD pattern of Fe3O4–N-BC.23 The position of the characteristic peak of Fe3O4–N-BC is the same as that of Fe3O4, but the intensity of the peak is weakened. The XRD test results are related to the content of the sample. Compared with the pure Fe3O4 sample, the relative Fe3O4 content of Fe3O4–N-BC in the same amount of sample testing is reduced, so the XRD diffraction peak is relatively weakened.
In Fig. 5(b), the Raman spectrum of BC, N-BC, and Fe3O4–N-BC is presented. The D peak, observed around 1355 cm−1, corresponds to the disorder or defects of carbon atoms, while the G peak, positioned near 1570 cm−1, is associated with the surface stretching motion of sp2 carbon atom pairs.30 The two spectral bands of Fe3O4–N-BC illustrate the successful coating of carbon. The relative intensity ratio of peak D and peak G (ID/IG) indicates the degree of graphitization of the material.
The intensity ratios (ID/IG) of the D and G bands of BC, N-BC and Fe3O4–N-BC are 1.02, 1.00 and 0.99, respectively. The ID/IG ratio serves as an indicator of the degree of graphitization within the carbon structure. A higher ID/IG ratio suggests more pronounced defects within the carbon structure. The observed decrease in the ID/IG value of nitrogen-modified biomass charcoal signifies the emergence of more graphitic carbon. Nitrogen modification could destroy the defects of the charcoal structure in biochar due to the reaction between the N atom and the carbon structure.31 A broader half-width of the band indicates an abundance of structure within disordered carbon. Following nitrogen and iron modification, notable shifts in the positions of the D and G peaks within the biochar spectrum were observed, particularly in the G peak. Consequently, nitrogen and iron modifications exert a significant influence on the structure of graphitized charcoal. Alterations in the carbon structure of biochar will inevitably impact its adsorption efficacy.
The hysteresis loops of Fe3O4 and Fe3O4–N-BC are shown in Fig. 6(a). The saturation magnetic induction intensities of Fe3O4 and Fe3O4–N-BC decrease in sequence, and their values are 82.91 emu g−1 and 72.68 emu g−1, respectively. The decrease in saturation magnetic induction intensity is due to the carbon coating of Fe3O4. In the magnetic test, the proportion of Fe3O4 decreases and its corresponding magnetic intensity decreases. In addition, ferromagnetism is characterized by remanence and saturation magnetic susceptibility (Mr/Ms). For the composite Fe3O4–N-BC (Mr/Ms = 0.06), a Mr/Ms ratio lower than 25% indicates that the synthesized Fe3O4–N-BC materials are superparamagnetic at room temperature.32 The magnetic property facilitates the efficient separation of the dye-adsorbed composite material from water. Hence, Fe3O4–N-BC can serve as a magnetic adsorbent for the removal of pollutants from aqueous solutions.
 | ||
| Fig. 6 (a) Magnetic hysteresis loops of Fe3O4, Fe3O4–N-BC, (b) nitrogen adsorption–desorption isotherms and pore size distribution of BC, N-BC and Fe3O4–N-BC. | ||
The porous structure of magnetic biomass charcoal composites was characterized using N2 adsorption/desorption isotherms. The adsorption isotherm of the biochar exhibited a typical type-I profile, characterized by a sharp increase in adsorption at P/P0 < 0.2, followed by saturation at relatively low relative pressures. This suggests that the biochar possesses a combined microporous and mesoporous structure.33–35 As shown in Fig. 6(b), according to the pore size distribution, its pore structure is mainly distributed in the range of 2–4 nm, confirming the mesoporous structure of biomass charcoal. The specific surface areas of the prepared BC, N-BC, and Fe3O4–N-BC are shown in Table S3.† Among them, BC has the largest specific surface area of 1827.9321 m2 g−1, 3.8 times that of Fe3O4–N-BC. This indicates that KOH activation significantly enhances the specific surface area of the carbon material. In contrast to BC, nitrogen modification resulted in a decrease in the surface area and total volume of biomass char, while increasing its average pore size. Nitrogen modification appears to hinder the enhancement of biochar surface area and pore structure, a phenomenon influenced by the choice of modifying reagent (urea) and pyrolysis temperature. Additionally, some studies have suggested that nitrogen modification could potentially reduce the surface area and pore volume of biochar. Consequently, employing the iron-nitrogen co-modification technique could be deemed a favorable option for biochar production owing to its capability to introduce a substantial surface area. The decrease in biochar's specific surface area following nitrogen modification may be ascribed to (1) the narrowing of pores amidst carbon fibers within the biochar, (2) the reduction/annealing process leading to the low desorption degree of biochar,36 (3) the modification results in highly disordered and folded structures.22 The introduction of Fe3O4 leads to a smaller mass proportion of biomass charcoal in the composite material, resulting in a smaller specific surface area. Iron enters the mesoporous carbon, thereby reducing the shrinkage of the matrix carbon during carbonization.37 But compared to other magnetic biomass charcoal, its specific surface area is still larger. KOH, urea and FeCl3·6H2O react with banana peels, and the gases generated by thermal decomposition during the reaction can improve their structural characteristics and adsorption properties.
The XPS spectrum of biomass charcoal is shown in Fig. 7. C 1s and O 1s can be detected on the surface of biomass charcoal, while Fe 2p can also be detected on Fe3O4–N-BC. The high-resolution C 1s XPS (Fig. 7(b)) spectra in BC, N-BC and Fe3O4–N-BC can be convolved into three sub-peaks at 284.8/284.8/284.8, 286.3/286.1/286.3 and 287.8/287.1/288.1 eV, reflecting respectively C–C/C–H, C–O and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C.38,39 On the O 1s spectrum (Fig. 7(c)), O
C.38,39 On the O 1s spectrum (Fig. 7(c)), O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O (533.6/533.1 eV) exists in BC/N-BC, while in Fe3O4–N-BC, C
C–O (533.6/533.1 eV) exists in BC/N-BC, while in Fe3O4–N-BC, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (530.2 eV), C–O (531.1 eV) and O
O (530.2 eV), C–O (531.1 eV) and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O (533.4 eV) is convolved into three sub-peaks.40,41 After Fe modification, the peak positions of Fe 2p are 711.2, 715.7 and 724.3 eV respectively (Fig. 7(d)). 711.2 and 724.3 eV are the characteristic peaks of Fe 2p3/2 and Fe 2p1/2, respectively, and the peak at 715.7 eV is basically consistent with the Fe(III) state.39,42 This shows that there are two oxidation states of iron. Combined with XRD analysis, the Fe2+ and Fe3+ compounds in biomass charcoal are magnetite (Fe3O4) and maghemite (γ-Fe2O3).43
C–O (533.4 eV) is convolved into three sub-peaks.40,41 After Fe modification, the peak positions of Fe 2p are 711.2, 715.7 and 724.3 eV respectively (Fig. 7(d)). 711.2 and 724.3 eV are the characteristic peaks of Fe 2p3/2 and Fe 2p1/2, respectively, and the peak at 715.7 eV is basically consistent with the Fe(III) state.39,42 This shows that there are two oxidation states of iron. Combined with XRD analysis, the Fe2+ and Fe3+ compounds in biomass charcoal are magnetite (Fe3O4) and maghemite (γ-Fe2O3).43
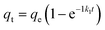 | (8) |
 | (9) |
 | (10) |
 | ||
| Fig. 9 (a) Pseudo-first-order and pseudo-second-order kinetics, fitting curves of MB on Fe3O4–N-BC, (b) intra-particle diffusion model. | ||
Fig. 9(a) illustrates the application of nonlinear regression analysis to the dataset employing dynamic equations. The adsorption dataset underwent fitting utilizing pseudo-first-order kinetics, pseudo-second-order kinetics, and intra-particle diffusion models. Detailed parameters are delineated in Table S4.† Notably, Table S4† reveals that the R2 values for the pseudo-first-order and pseudo-second-order kinetics pertaining to methylene blue adsorption by Fe3O4–N-BC stand at 0.8316 and 0.9929, respectively. Moreover, the maximum adsorption capacities are observed to be 618.15 mg g−1 and 656.08 mg g−1 correspondingly. The adsorption of methylene blue by Fe3O4–N-BC is more consistent with the pseudo-second-order kinetic model, which indicates that the adsorption of MB on this adsorbent is chemical adsorption.46 As can be seen from Fig. 9(b), there are three straight lines fitting the intra-particle diffusion to the adsorption of MB on Fe3O4–N-BC. The initial stage entails the diffusion of MB from the solution to the outer surface of Fe3O4–N-BC. Subsequently, the second stage encompasses particle diffusion, wherein MB permeates from the outer surface of Fe3O4–N-BC into the inner pores. Finally, the third stage denotes the equilibrium phase. It is noteworthy that none of these three linear progressions intersects the origin, denoting C ≠ 0, thereby suggesting that particle diffusion alone does not exclusively govern the rate-limiting step.47
 | (11) |
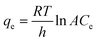 | (12) |
 | (13) |
Analysis of the fitted isotherm parameters (referenced in Table S5†) reveals that both the Temkin and Freundlich models inadequately describe the adsorption behavior of MB by Fe3O4–N-BC, as indicated by their comparatively low correlation coefficients (R2). Conversely, the Langmuir model boasts the highest correlation coefficient, nearing unity. This finding suggests that the adsorption mechanism of MB onto Fe3O4–N-BC predominantly adheres to the Langmuir adsorption isotherm. Consequently, it can be inferred that the adsorption behavior of MB on the Fe3O4–N-BC surface entails uniform monolayer adsorption, rather than heterogeneous adsorption.51 The actual adsorption capacity of MB by Fe3O4–N-BC at 298 K is 638.08 mg g−1.
In comparison to biochar investigated in prior studies, the maximum adsorption capacity of Fe3O4–N-BC for MB, as displayed in Table 1, remains notably elevated. This observation underscores the effectiveness of Fe and N modification, signifying its promising potential for practical application.
| Biochar materials | Kinetic model | Isotherm model | qmax (mg g−1) | References |
|---|---|---|---|---|
| Bamboo | Pseudo-second-order | Langmuir | 210.86 | 45 |
| Wakame | Pseudo-second-order | Langmuir | 479.49 | 52 |
| Chitosan flakes | Pseudo-second-order | Langmuir | 143.53 | 53 |
| Ashitaba biomass | — | Dubinin-Radushkevich | 438.16 | 54 |
| Langmuir | ||||
| Banana peels | Pseudo-second-order | Langmuir | 693.11 | This work |
| Fe3O4–N-BC | Pseudo-second-order | Langmuir | 651.47 | This work |
| Aminated lignin | Pseudo-second-order | Freundlich | 388.81 | 55 |
| ΔG0 = ΔH0 − TΔS0 | (14) |
 | (15) |
It can be seen from Table S6† that the positive enthalpy change (ΔH0, 11.184 kJ mol−1) indicates that MB adsorption on Fe3O4–N-BC is endothermic, and the positive entropy change (ΔS0, 44.623 J mol−1 K−1) indicates that the solid–liquid interface during the adsorption process disorder increases. The Gibbs free energy change of MB dropped from −2.1136 kJ mol−1 to −3.0061 kJ mol−1 within 298–318 K, which shows that this adsorption is a heat absorption process, and the adsorption of MB by Fe3O4–N-BC increases with the increase of temperature positive beneficial effects.
In the desorption trial, 0.1 mol L−1 HCl solution served as the eluent to extract the adsorbed MB from the biomass charcoal. After five adsorption–desorption times, the adsorption capacity of MB by Fe3O4–N-BC can still reach 497.18 mg g−1, accounting for 80.36% of the first adsorption capacity. The decrease in adsorption capacity with the number of cycles is due to the fact that MB on the surface of biomass charcoal permanently occupies some adsorption sites, resulting in some active sites being unavailable. Another reason is that part of the pore structure is blocked, resulting in fewer binding sites. The research results show that Fe3O4–N-BC has high stability and reusability, and has the potential to remove MB from wastewater.
Conclusions
In this study, N-modified banana peel biomass charcoal was generated by co-heating with urea at 700 °C and subsequently hydrothermal carbonization with Fe3O4 at 180 °C, with the calcination temperature, calcination time, and amount of activator KOH. The Fe3O4–N-BC substance was produced in 4 hours. The resulting materials were characterized using a variety of analytical techniques. The synthesized magnetic biomass charcoal material has a high specific surface area and magnetic characteristics, as well as good MB adsorption capability. At 298 K, Fe3O4–N-BC has the greatest adsorption capacity of 638.08 mg g−1 MB at adsorption equilibrium. The pseudo-second-order kinetic model and the Langmuir isotherm model are both capable of accurately describing the adsorption kinetics and isotherm. The experimental data suggest that the adsorption of MB onto Fe3O4–N-BC is characterized by homogeneous monolayer adsorption rather than heterogeneous adsorption. Furthermore, the adsorption process is dominated by chemisorption. Remarkably, the adsorbent maintained a high MB adsorption capacity of 497.18 mg g−1 even after five regeneration cycles. This work creates an MB adsorbent by recycling waste biomass resources, which not only contributes to the sustainable development of natural resources, but also broadens the potential application scope of biomass waste and aids in the resolution of dye wastewater and resource use issues. The strong adsorption performance of Fe3O4–N-BC on MB is predicted to be used for large-scale industrial wastewater treatment in the future.Data availability
The data supporting this article have been included in the main article and the ESI.†Author contributions
Conceptualization, Z.-X. G. and Y.-X. W.; methodology, Z.-X. G.; software, Z.-X. G., Y.-T. C., L.-Z. H.; writing – original draft preparation, Z.-X. G.; writing – review and editing, Z.-X. G., Y.-X. W., M. Steven., X.-J. Y., and X.-P. L.; project administration, X.-P. L.; funding acquisition, X.-P. L. All authors have read and agreed to the published version of the manuscript. All authors gave final approval for publication and agreed to be held accountable for the work performed therein. Collate acknowledgements in a separate section at the end of the article before the references and do not, therefore, individuals who provided help during the research (e.g., providing language help, writing assistance or proof reading the article, etc.).Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This research was supported by the National Fund of Forestry Research of ([2023]TS01); The key Research & Development Projects of Zhejiang Province grant number (2020C03090), Zhejiang A & F University Scientific Research and Development Fund Project grant number (2020RC032), Zhejiang Provincial Basic Public Welfare Research Program grant number (LGN22C160001).References
- V. Katheresan, J. Kansedo and S. Y. Lau, J. Environ. Chem. Eng., 2018, 6, 4676–4697 CrossRef CAS.
- P. S. Kumar, S. J. Varjani and S. Suganya, Bioresour. Technol., 2018, 250, 716–722 CrossRef PubMed.
- P. Monash and G. Pugazhenthi, Adsorption, 2009, 15, 390–405 CrossRef CAS.
- H. Ma, J.-B. Li, W.-W. Liu, M. Miao, B.-J. Cheng and S.-W. Zhu, Bioresour. Technol., 2015, 190, 13–20 CrossRef CAS PubMed.
- Y. Wei, G. Li, C. Wang and H. Guo, J. Colloid Interface Sci., 2021, 586, 233–242 CrossRef CAS PubMed.
- T. Xia, Z. Ma, M. Ai, K. Qian, S. Zhu, M. Rong, P. Zhang, Y. Ye and W. Qin, Chemosphere, 2021, 267, 128927 CrossRef CAS PubMed.
- K. Kashima, T. Inage, Y. Yamaguchi and M. Imai, J. Environ. Chem. Eng., 2021, 9, 105210 CrossRef CAS.
- L.-Z. Huo, C.-F. Guo, Z.-X. Gong, H. Xu, X.-J. Yang, Y.-X. Wang and X.-P. Luo, Materials, 2024, 17, 1046 CrossRef CAS PubMed.
- R. Salazar, M. S. Ureta-Zañartu, C. González-Vargas, C. do Nascimento Brito and C. A. Martinez-Huitle, Chemosphere, 2018, 198, 21–29 CrossRef CAS PubMed.
- B. Mu and A. Wang, J. Environ. Chem. Eng., 2016, 4, 1274–1294 CrossRef CAS.
- J. M. Illingworth, B. Rand and P. T. Williams, Process Saf. Environ., 2019, 122, 209–220 CrossRef CAS.
- A. Nasrullah, B. Saad, A. Bhat, A. S. Khan, M. Danish, M. H. Isa and A. Naeem, J. Cleaner Prod., 2019, 211, 1190–1200 CrossRef CAS.
- D. D. Sewu, P. Boakye and S. H. Woo, Bioresour. Technol., 2017, 224, 206–213 CrossRef CAS PubMed.
- Y. Zhu, J. Gao, Y. Li, F. Sun, J. Gao, S. Wu and Y. Qin, J. Taiwan Inst. Chem. Eng., 2012, 43, 112–119 CAS.
- X. Li, C. Wang, J. Zhang, J. Liu, B. Liu and G. Chen, Sci. Total Environ., 2020, 711, 134847 CrossRef CAS PubMed.
- K. Thines, E. Abdullah, N. Mubarak and M. Ruthiraan, Renew. Sustain. Energy Rev., 2017, 67, 257–276 CrossRef CAS.
- Q. Du, S. Zhang, J. Song, Y. Zhao and F. Yang, J. Hazard. Mater., 2020, 389, 122115 CrossRef CAS PubMed.
- X. Duan, Z. Ao, H. Sun, L. Zhou, G. Wang and S. Wang, Chem. Commun., 2015, 51, 15249–15252 RSC.
- X. Wang, Y. Qin, L. Zhu and H. Tang, Environ. Sci. Technol., 2015, 49, 6855–6864 CrossRef CAS PubMed.
- G. Wang, S. Chen, X. Quan, H. Yu and Y. Zhang, Carbon, 2017, 115, 730–739 CrossRef CAS.
- L. Leng, S. Xu, R. Liu, T. Yu, X. Zhuo, S. Leng, Q. Xiong and H. Huang, Bioresour. Technol., 2020, 298, 122286 CrossRef CAS PubMed.
- S. Liu, C. Zhao, Z. Wang, H. Ding, H. Deng, G. Yang, J. Li and H. Zheng, Chem. Eng. J., 2020, 386, 124015 CrossRef CAS.
- X. Li, Y. Jia, M. Zhou, X. Su and J. Sun, J. Hazard. Mater., 2020, 397, 122764 CrossRef CAS PubMed.
- X. Zhou, Y. Liu, J. Zhou, J. Guo, J. Ren and F. Zhou, J. Taiwan Inst. Chem. Eng., 2018, 91, 457–467 CrossRef CAS.
- T. Ai, X. Jiang, Q. Liu, L. Lv and S. Dai, RSC Adv., 2020, 10, 20427–20437 RSC.
- T. Chen, L. Luo, S. Deng, G. Shi, S. Zhang, Y. Zhang, O. Deng, L. Wang, J. Zhang and L. Wei, Bioresour. Technol., 2018, 267, 431–437 CrossRef CAS PubMed.
- J.-K. Kim and Y.-K. Han, Macromol. Res., 2008, 16, 734–740 CrossRef CAS.
- A. Pourjavadi, M. Ayyari and M. Amini-Fazl, Eur. Polym. J., 2008, 44, 1209–1216 CrossRef CAS.
- H. Zhao, Y. Cheng, H. Lv, G. Ji and Y. Du, Carbon, 2019, 142, 245–253 CrossRef CAS.
- P. Mohanty, S. Nanda, K. K. Pant, S. Naik, J. A. Kozinski and A. K. Dalai, J. Anal. Appl. Pyrolysis, 2013, 104, 485–493 CrossRef CAS.
- P. Zhang, X. Tan, S. Liu, Y. Liu, G. Zeng, S. Ye, Z. Yin, X. Hu and N. Liu, Chem. Eng. J., 2019, 378, 122141 CrossRef CAS.
- V. Ranjithkumar, S. Sangeetha and S. Vairam, J. Hazard. Mater., 2014, 273, 127–135 CrossRef CAS PubMed.
- P. Liu, Z. Song, L. Miao, Y. Lv, L. Gan and M. Liu, Small, 2024, 1613–6810 Search PubMed.
- Y. Qin, S. Jha, C. Hu, Z. Song, L. Miao, Y. Chen, P. Liu, Y. Lv, L. Gan and M. Liu, J. Colloid Interface Sci., 2024, 675, 1091–1099 CrossRef CAS PubMed.
- M. Mansuer, L. Miao, Y. Qin, Z. Song, D. Zhu, H. Duan, Y. Lv, L. Li, M. Liu and L. Gan, Chin. Chem. Lett., 2023, 34(3), 107304 CrossRef CAS.
- X. Duan, K. O'Donnell, H. Sun, Y. Wang and S. Wang, Small, 2015, 11, 3036–3044 CrossRef CAS PubMed.
- X. Rong, M. Xie, L. Kong, V. Natarajan, L. Ma and J. Zhan, Chem. Eng. J., 2019, 372, 294–303 CrossRef CAS.
- X. Tang, S. Ma, S. Xu, Q. Yang, Y. Huang, J. Wang and D. Hua, Chem. Eng. J., 2023, 451, 138576 CrossRef CAS.
- Y. Sun, T. Wang, C. Han, X. Lv, L. Bai, X. Sun and P. Zhang, Bioresour. Technol., 2022, 344, 126186 CrossRef CAS PubMed.
- B. Liu, T. Chen, B. Wang, S. Zhou, Z. Zhang, Y. Li, X. Pan and N. Wang, J. Hazard. Mater., 2022, 438, 129467 CrossRef CAS PubMed.
- Y. Zhou, X. Zhao, Y. Jiang, C. Ding, J. Liu and C. Zhu, Sci. Total Environ., 2023, 861, 160649 CrossRef CAS PubMed.
- K. Volgmann, F. Voigts and W. Maus-Friedrichs, Surf. Sci., 2010, 604, 906–913 CrossRef CAS.
- L. Chen, Y. Huang, M. Zhou, K. Xing, W. Lv, W. Wang, H. Chen and Y. Yao, Chemosphere, 2020, 250, 126300 CrossRef CAS PubMed.
- S. Liu, J. Li, S. Xu, M. Wang, Y. Zhang and X. Xue, Bioresour. Technol., 2019, 282, 48–55 CrossRef CAS PubMed.
- X.-N. Sun, K. Yu, J.-H. He, Y. Chen, J.-Z. Guo and B. Li, Bioresour. Technol., 2023, 373, 128715 CrossRef CAS PubMed.
- Z.-L. Chen, H. Xu, L.-Q. Bai, Y.-L. Feng and B. Li, Prog. Nat. Sci.: Mater., 2023, 33, 501–507 CrossRef CAS.
- B. Li, J. Guo, K. Lv and J. Fan, Environ. Pollut., 2019, 254, 113014 CrossRef CAS PubMed.
- P. Wang, M. Cao, C. Wang, Y. Ao, J. Hou and J. Qian, Appl. Surf. Sci., 2014, 290, 116–124 CrossRef CAS.
- K. Saini, A. Sahoo, B. Biswas, A. Kumar and T. Bhaskar, Bioresour. Technol., 2021, 342, 125924 CrossRef CAS PubMed.
- M. Zhang, B. Gao, S. Varnoosfaderani, A. Hebard, Y. Yao and M. Inyang, Bioresour. Technol., 2013, 130, 457–462 CrossRef CAS PubMed.
- M. Brdar, M. Šćiban, A. Takači and T. Došenović, Chem. Eng. J., 2012, 183, 108–111 CrossRef CAS.
- X. Yao, L. Ji, J. Guo, S. Ge, W. Lu, L. Cai, Y. Wang, W. Song and H. Zhang, Bioresour. Technol., 2020, 302, 122842 CrossRef CAS PubMed.
- F. Marrakchi, M. Ahmed, W. Khanday, M. Asif and B. Hameed, Int. J. Biol. Macromol., 2017, 98, 233–239 CrossRef CAS PubMed.
- H. Xue, X. Wang, Q. Xu, F. Dhaouadi, L. Sellaoui, M. K. Seliem, A. B. Lamine, H. Belmabrouk, A. Bajahzar and A. Bonilla-Petriciolet, Chem. Eng. J., 2022, 430, 132801 CrossRef CAS.
- C. Wang, X. Feng, S. Shang, H. Liu, Z. Song and H. Zhang, Int. J. Biol. Macromol., 2023, 237, 124200 CrossRef CAS PubMed.
- S. Fan, Y. Wang, Y. Li, Z. Wang, Z. Xie and J. Tang, Environ. Sci. Pollut. Res., 2018, 25, 29529–29540 CrossRef CAS PubMed.
- C. Peiris, S. R. Gunatilake, T. E. Mlsna, D. Mohan and M. Vithanage, Bioresour. Technol., 2017, 246, 150–159 CrossRef CAS PubMed.
- S.-Y. Yoon, C.-G. Lee, J.-A. Park, J.-H. Kim, S.-B. Kim, S.-H. Lee and J.-W. Choi, Chem. Eng. J., 2014, 236, 341–347 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra04973j |
| This journal is © The Royal Society of Chemistry 2024 |










