Open Access Article
Open Access ArticleDevelopment of N-alkylated benzimidazole based cubosome hydrogel for topical treatment of burns†
Maubashera Nawaza,
Sofia Hayata,
Umer Farooq a,
Muhammad Adnan Iqbal
a,
Muhammad Adnan Iqbal *a,
Syed Haroon Khalidbc,
Tan Wen Need,
Kooi Yeong Khawe,
Rabia Munirb and
Muhammad Umar Ijazf
*a,
Syed Haroon Khalidbc,
Tan Wen Need,
Kooi Yeong Khawe,
Rabia Munirb and
Muhammad Umar Ijazf
aDepartment of Chemistry, University of Agriculture Faisalabad, 38040, Pakistan. E-mail: adnan.iqbal@uaf.edu.pk
bDepartment of Pharmaceutics, Government College University Faisalabad, 38000, Pakistan
cDepartment of Pharmaceutical Technology, Faculty of Pharmacy, Universiti Teknologi Mara (UiTM), Puncak Alam, 42300, Selangor, Malaysia
dChemistry Section, School of Distance Education, Universiti Sains Malaysia, 11800, Malaysia
eSchool of Pharmacy, Monash University Malaysia, Jalan Lagoon Selatan, Bandar Sunway 47500, Selangor, Malaysia
fDepartment of Zoology, Wildlife and Fisheries, University of Agriculture Faisalabad, 38040, Pakistan
First published on 10th October 2024
Abstract
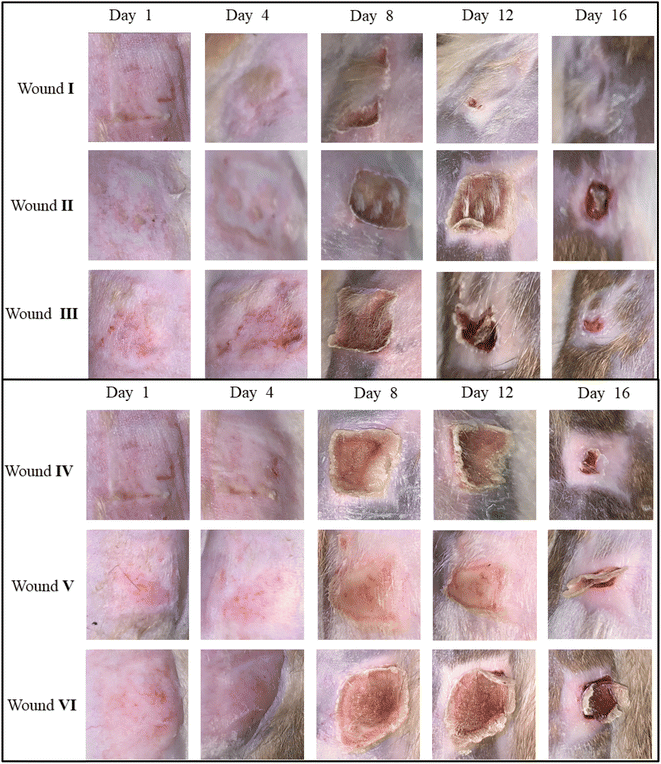
The current study focuses on assessing the activity of the N-alkylated benzimidazole based cubosomal hydrogel (cubogel) for the topical treatment of burn wounds. The study involves the synthesis of six benzimidazole derivatives (1–6) and their characterization by FT-IR and 1H and 13C NMR spectroscopy. The further study involves the design and formation of nanoparticles known as cubosomes loaded with selected 1-benzyl-1-benzimidazole (API 6) and the development of a cubogel for the topical treatment of burn wounds. Cubosomes were prepared by the homogenization method, using glyceryl monooleate (GMO) as a lipid polymer and poloxamer 407 (P407) as a surfactant. Cubosomes undergo in vitro characterizations (measurement of particle size, zeta potential, polydispersity index (PDI), % entrapment efficiency, drug release in phosphate buffer saline of pH 6.8, and surface morphology by utilizing TEM (transmission electron microscopy). Formulation D3 (2.5% of GMO, 1% of P407, and 2.5% of PVA) emerged as the optimized formulation, displaying a minimum particle size (PS) of 129.9 ± 1 nm, entrapment efficiency (%EE) of 96.67 ± 0.89%, and a drug release of 86 ± 2.7% at 24 h. Carbopol 940 hydrogel was prepared and incorporated with the optimized formulation to prepare cubogel. This optimized cubogel provided 92.56 ± 0.014% in vitro drug release within 24 h. An in vivo histopathological study was conducted on an animal model (rabbit) to assess the efficacy of cubogel in wound healing and wound contraction. Then cubogel was compared with the commercially available creams Clotrimazole® and Polyfax®. The wound treated with newly developed cubogel has maximum wound contraction (96.70%) as compared to the standard creams. The findings revealed that the newly formulated cubogel was highly effective in treating burns, showing superior performance to commercial products without inducing side effects. Additionally, benzimidazole derivative loaded cubogel caused a sustained release for treating burn wounds without any bacterial infections. The current results further suggested phase 0 clinical trials.
Introduction
Burns are the 7th universal injury with physical and financial problems for patients and their families, with an estimated 180![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 deaths occurring each year.1–5 Burns are caused by fire, flame, electric shock, or due to chemicals. The use of topical burn treatment is essential for the survival of patients with severe burns. Burn injuries account for 90% of all injuries in low and middle-income countries (LMICs).6
000 deaths occurring each year.1–5 Burns are caused by fire, flame, electric shock, or due to chemicals. The use of topical burn treatment is essential for the survival of patients with severe burns. Burn injuries account for 90% of all injuries in low and middle-income countries (LMICs).6
Topical preparations of clotrimazole are used to treat various fungal and bacterial infections. Clotrimazole is an antifungal agent with having broad spectrum of imidazole rings. Clotrimazole-loaded creams or ointments have effective penetration against stratum corneum but their efficiency is limited due to poor penetration, variable drug levels, and poor dermal bioavailability. There is also a major drawback of topical preparations because they cause infection, skin irritation, greasiness, uncontrolled evaporation, and allergic reactions at the infection site.7
Benzimidazole is a broad spectrum active pharmaceutical core that possesses pharmacological properties.8 Benzimidazoles have biological effects such as anti-inflammatory, antioxidant, anti-viral, anti-HIV, anticancer, antibacterial, and antidiabetic.9–11 Benzimidazole based derivatives (N-alkylated) bioactive agents have drawn much attention in medicinal chemistry such as albendazole, mebendazole, and astemizole.12 The benzimidazole ring has a significant heterocyclic pharmacophore.13
Scientists have been attracted to cubosomes due to the potential drug delivery system which is based on high lipid content, huge surface area, and highly organized arrangement.14 Cubosomes are lipid-based nanoparticles ranging from 100 to 500 nm, prepared by either dispersing lipid glyceryl monooleate (GMO) in water or micronizing cubic phase in water.15,16 They have well-defined bicontinuous cubic phase structures with the appropriate amount of water in them.17 They can encapsulate drugs both hydrophilic and hydrophobic, protect the drugs against physical and chemical degradation, and release the entrapped drug in a sustained manner.18 Cubosomes enhance the penetration of drugs across the skin layers with almost no observed side effects or irritation.19 They have applications in both, materials science and medicine.20
A secondary vehicle is required to put cubosome into an appropriate and practical topical formulation. This magic is possible through bioabsorbable polymers such as hydrogels. Hydrogels are hydrophilic polymer chains that can hold a large amount of water due to their hydrophilic nature. Therefore, the 3D network of the hydrogel can swell in aqueous media.21 When cubosomes are loaded into hydrogel it forms cubosomal hydrogel (cubogel). Cubogel is the cubosomal dispersion of hydrogels. The cubosomes enhance the penetration effect across the skin as the structure of cubosomes and stratum corneum are the same.22,23
The present research is based on the synthesis of N-alkylated benzimidazole (API) based cubosome hydrogel for the topical treatment of burns. API-loaded cubosomes and cubogel formulation were prepared, and characterized through in vitro (particle size, zeta potential, %EE, and drug release), and in vivo (burn wound treatment, 16 days rabbit model, histopathological evaluation).
Materials and methods
Materials
Benzimidazole, KOH, alkyl halides, chloroform, methanol, ethanol, dimethyl sulfoxide (DMSO), and n-hexane were purchased from Sigma Aldrich (St. Louis, USA). GMO was purchased from Macklin Biochemical Co. (Shanghai China). Poloxamer 407 (P407), polyvinyl alcohol (PVA), carbopol 940, and triethanolamine were purchased from Sigma Aldrich (St. Louis, USA). Distilled water (used during experiments) was obtained from the Organometallic and Coordination Chemistry Laboratory, UAF, Pakistan.Synthesis of N-alkylated benzimidazoles
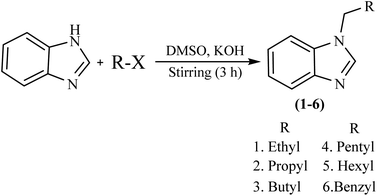
The compounds (1–6) were synthesized according to our previously reported method with minor modifications.24 Benzimidazole (1 equiv), KOH (1.5 equiv), and DMSO (40 mL) were taken in a round bottom flask and stirred at 1200 rpm for 30 minutes at RT. After that alkyl halide (1 equiv) was added dropwise and kept on stirring for 3 h. After stirring, the reaction mixture was added to 300 mL of distilled water. Suddenly turbid white solution appeared, and the reaction mixture was filtered and then washed with DMSO and n-hexane. The final product was dried at ambient temperature. The different vibrational bands and NMR signals of these compounds (1–6) have been presented in a ESI section.†Physical properties of APIs
The synthesized API 1–6 were observed physically for their appearances and melting points were measured using SANYO Gallen Kamp, JAPAN, melting point apparatus.Characterization of APIs
The bonding between benzimidazole and alkyl halide can be studied using Fourier transform infrared (FT-IR) spectroscopy initially. The sample were compressed and placed on the ATR crystal for analysis. The ATR crystal was scanned from 4000 to 650 cm−1 using an Agilent Technologies FT-IR spectrophotometer (Cary 630) at the environmental chemistry laboratory, University of Agriculture Faisalabad, Pakistan.1H and 13C-NMR spectral analyses were performed in CDCl3 solvent with a Bruker 400 MHz ultra-shield machine. δ were recorded in ppm and J values were given in Hz. The signals were labeled as s, d, t, and m, which represent singlet, doublet, triplet, and multiplet, respectively.
Preparation of cubosomes
Cubosomes were prepared according to the previously reported method with slight modifications.25 The twelve cubosomal dispersions including six blank (without drug, API) and six drug loaded formulations were prepared by homogenization of the lipid phase consisting of GMO and P407 with PVA in water.The composition of formulations is shown in Table 1. Cubosomes were prepared by melting GMO and P407 on a hot plate at 60 °C, which ensures the components were thoroughly mixed in the liquid state. Simultaneously, PVA was dissolved in distilled water and heated on the same hot plate at the same temperature for 15–20 minutes. Once GMO and P407 were completely mixed the drug was added to this mixture by increasing the temperature up to 100 °C because at this temperature drug mixed well. When PVA was completely dissolved in water then PVA containing distilled water was added dropwise to the drug loaded mixture and the stirring rate increased up to 1100 rpm for half an hour. This vigorous stirring was necessary for achieving a homogeneous mixture. After complete mixing, a yellowish color mixture was obtained. Then it was sonicated using a bath sonicator (ultrasonic cleanser) that helps in breaking down any aggregates and promotes a uniform dispersion. A well-dispersed sample had a turbid whitish consistency and no noticeable aggregates. At the end that sonicated dispersion was homogenized (Heidolph Instruments) at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min to obtain the nanosized cubosomes. The bluish-colored dispersion was obtained. The blank formulations follow the same process without the addition of API. The schematic diagram of the formation of cubosomes is given in the ESI Fig. S1.†
000 rpm for 10 min to obtain the nanosized cubosomes. The bluish-colored dispersion was obtained. The blank formulations follow the same process without the addition of API. The schematic diagram of the formation of cubosomes is given in the ESI Fig. S1.†
| Dispersions | GMO (%) | P407 (%) | PVA (%) | API (%) | Water (mL) |
|---|---|---|---|---|---|
| Blank | |||||
| B1 | 2.5 | 1 | 1.5 | — | 9.5 |
| B2 | 2.5 | 1 | 2 | — | 9.45 |
| B3 | 2.5 | 1 | 2.5 | — | 9.4 |
| B4 | 2.5 | 0.5 | 2 | — | 9.5 |
| B5 | 2.5 | 1 | 2 | — | 9.45 |
| B6 | 2.5 | 1.5 | 2 | — | 9.4 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Drug loaded | |||||
| D1 | 2.5 | 1 | 1.5 | 1 | 9.4 |
| D2 | 2.5 | 1 | 2 | 1 | 9.35 |
| D3 | 2.5 | 1 | 2.5 | 1 | 9.3 |
| D4 | 2.5 | 0.5 | 2 | 1 | 9.4 |
| D5 | 2.5 | 1 | 2 | 1 | 9.35 |
| D6 | 2.5 | 1.5 | 2 | 1 | 9.3 |
Characterization of cubosomes
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. They were then placed in clear, disposable zeta cells, with measurements taken and recorded at 25 °C.26 This procedure was replicated three times to ensure reliability, and the results are presented as mean values accompanied by their respective standard deviations.
10. They were then placed in clear, disposable zeta cells, with measurements taken and recorded at 25 °C.26 This procedure was replicated three times to ensure reliability, and the results are presented as mean values accompanied by their respective standard deviations.The spreadability of cubogel plays a pivotal role in ensuring the efficacy of topical therapy and the accurate administration of medicinal formulations. The spreadability is contingent upon the flow properties of the formulation.22 To check the spreadability, a 1 cm diameter circle (D1) was marked on a clean, dry glass slide. Subsequently, 0.5 g of cubogel was carefully applied within the circle, ensuring no spillage beyond its boundaries. Another glass slide was placed on top to cover the cubogel-loaded slide, and various weights (ranging from 100 to 500 g) were applied.31 Following this procedure, the cubogel shifted from its initial position (D1) to a new location (D2), indicative of its spreadability characteristics.
Burn wound healing of cubogel
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kg. The animals were clinically healthy with no skin wounds. The animals were housed in separate cages at room temperature with controlled humidity levels and fed with a normal diet (grass, fruits, vegetables) and water.
kg. The animals were clinically healthy with no skin wounds. The animals were housed in separate cages at room temperature with controlled humidity levels and fed with a normal diet (grass, fruits, vegetables) and water.After 16 days of study, the rabbits were slaughtered and skin samples were taken for histopathology study.
Results and discussion
Synthesis of APIs
The general procedure that involves the synthesis of APIs is shown in Scheme 1. Potassium hydroxide was added to the solution of benzimidazole in DMSO at room temperature. Respective alkyl halide was added dropwise in the same reaction medium. Since the reaction medium is exothermic a dropwise addition of alkyl halide was strictly followed and the flask was placed in a water bath to dissipate the heat. In the case of benzyl bromide (alkyl halide), the procedure was done in the fume hood to avoid the eye irritation of the benzyl bromide. The reaction mixture was stirred for 3 h at room temperature and was then poured into 300 mL of distilled water. If the oily liquid mixture was obtained after stirring then it was extracted with chloroform and the combined extract was filtered through Whatman filter paper. The process of filtration was repeated twice. The filtered compound was obtained as a thick yellowish fluid. If some solid particles are shown at the bottom of the flask after stirring. Then the product was filtered and washed with DMSO and n-hexane, and dried at room temperature. The product appeared as a white shiny powder. Both were safely stored in the Eppendorf for further characterization and activities.Characterization of APIs
| API | Name | Appearance | MP/BP (°C) |
|---|---|---|---|
| 1 | N-Ethyl benzimidazole | Colorless, thick fluid | 184–186 |
| 2 | N-Propyl benzimidazole | Colorless | 236–238 |
| 3 | N-Butyl benzimidazole | Yellow liquid | 256–258 |
| 4 | N-Pentyl benzimidazole | Thick yellowish liquid | 262–263 |
| 5 | N-Hexyl benzimidazole | Thick yellowish fluid | 270–275 |
| 6 | N-Benzyl benzimidazole | White shiny powder, solid | 110–120 |
All these APIs were prepared according to the previously reported method.38,39 According to the preliminary study, from all synthesized compounds API 6 showed the best antibacterial activity. So, in this study API 6 was incorporated into the cubosomes and cubogel, to check the antibacterial activity and burn wound healing. According to best of our knowledge, there is no commercially available topical formulation of benzimidazole that provides sustained release at this time.
1H-NMR spectrum of the selected API 6 showed the various protons in different chemical environments see Fig. 1a. 1H-NMR spectrum showed a sharp singlet proton of benzyl group CH2 resonated at 5.32 δ ppm indicating a successful N-alkylation. The chemical shifts in the range δ ppm 7.21–7.80 indicate an aromatic (CH) presence, while the signal at the downfield region, δ H 7.93 ppm, corresponds to the proton on the C7H5N2 frame NCHN. In the 13C NMR spectrum of API 6, Fig. 1b, the CH2 carbon of the benzyl group appeared at δ 48.8 and the Caliph appeared at δ 143.1 ppm. The remaining carbons appeared in the range of δ 110.0–143.9 ppm.45–48
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm49 for 10 minutes to achieve nanosized cubosomes was crucial. This high-shear process was responsible for reducing the particle size to the nanoscale, which is essential for the desired drug release profile and bioavailability.
000 rpm49 for 10 minutes to achieve nanosized cubosomes was crucial. This high-shear process was responsible for reducing the particle size to the nanoscale, which is essential for the desired drug release profile and bioavailability.Characterization of cubosomes
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N),55 1591–1522 (C
N),55 1591–1522 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C). The peaks of N-alkylated benzimidazoles were shown in the spectrum of the physical mixture indicating no interference between the drug and other bulking agents. The disappearance of the drug peaks from the cubosome spectrum indicated its solubilization within the cubosomal matrix56 are given in Table 3 and Fig. 2[a].
C). The peaks of N-alkylated benzimidazoles were shown in the spectrum of the physical mixture indicating no interference between the drug and other bulking agents. The disappearance of the drug peaks from the cubosome spectrum indicated its solubilization within the cubosomal matrix56 are given in Table 3 and Fig. 2[a].
The drug characteristic speaks are not visible in cubosomes spectrum suggesting that the drug is well integrated into the matrix, possibly to non-covalent interaction like hydrogen bonding or van der walls forces. The characteristics peak of interaction between N-alkylated benzimidazole loaded cubosomes and their bulking agent are given in Table 3 and Fig. 2[a]. Interestingly some peaks of bulking agent are still prominent in the cubosomes (Table 3).
The physicochemical properties of cubosomes were analyzed in the nano range and are shown in Table 4 the particle size of the blank and drug loaded cubo ranged from 120.8 ± 1.656 to 180.1 ± 2.645 nm and 129.9 ± 1 to 177.2 ± 1.509 nm, respectively (Fig. 2[e]). Upon loading with N-alkylated benzimidazole, it could be seen that the average particle size is decreased54 due to the amphiphilic nature of API 6.61 PDI is also decreased when the drug is loaded ranging from 0.344 ± 0.001 to 0.483 ± 0.005 and 0.309 ± 0.005 to 0.421 ± 0.001 of blank and drug loaded cubosomes, respectively. The results of PDI mono dispersity of drug loaded cubosomes approach zero. The range of zeta potential is ±30 for the physical stability of formulations.61 The blank and drug loaded cubosomes were negatively charged with a zeta potential of −10.6 ± 0.152 to −18.9 ± 0.950 and −14.3 ± 1.873 to −18.3 ± 1.442, respectively and shown in Fig. 2[g]. Therefore, the zeta potential of these cubosomes indicates their physical stability.
| Dispersions | PS (nm) | ZP | PDI | %EE | Drug release |
|---|---|---|---|---|---|
| a Effect of P407, PVA, and GMO on PS, ZP, PDI. | |||||
| B1 | 120.8 ± 1.656 | −10.6 ± 0.152 | 0.358 ± 0.006 | — | — |
| B2 | 180.1 ± 2.645 | −14.4 ± 1.250 | 0.483 ± 0.009 | — | — |
| B3 | 133.9 ± 2.645 | −18.9 ± 0.950 | 0.426 ± 0.001 | — | — |
| B4 | 154.8 ± 2.722 | −11.1 ± 1.517 | 0.344 ± 0.001 | — | — |
| B5 | 180.1 ± 2.645 | −14.4 ± 1.250 | 0.483 ± 0.005 | — | — |
| B6 | 175.1 ± 3.194 | −14.3 ± 1.582 | 0.477 ± 0.002 | — | — |
| D1 | 170.3 ± 2.475 | −17.2 ± 1.527 | 0.421 ± 0.001 | 66.59 ± 0.81 | 74 ± 1.8 |
| D2 | 167.4 ± 2.608 | −14.3 ± 1.873 | 0.378 ± 0.002 | 73.53 ± 0.83 | 66 ± 2.5 |
| D3 | 129.9 ± 1 | −16.3 ± 1.715 | 0.309 ± 0.005 | 93.67 ± 0.89 | 86 ± 2.7 |
| D4 | 177.2 ± 1.509 | −18.3 ± 1.442 | 0.398 ± 0.003 | 63.81 ± 0.84 | 72 ± 2.7 |
| D5 | 167.4 ± 2.514 | −14.3 ± 1.873 | 0.378 ± 0.003 | 74.92 ± 0.81 | 62 ± 2.5 |
| D6 | 134.8 ± 1.900 | −18.2 ± 1.562 | 0.374 ± 0.003 | 90.27 ± 0.82 | 84 ± 1.8 |
The particle size and zeta potential are crucial factors influencing the biopharmaceutical properties, drug release, entrapment efficiency, and stability of nanoparticles. These characteristics are largely determined by the concentrations of P407 and polyvinyl alcohol (PVA). The zeta potential is relatively low due to the presence of nonionic surfactant, which enhances stability by providing steric hindrance, thus preventing aggregation during storage.62,63 The data presented in Table 4 elucidates the significant impact of P407 and PVA concentrations on the particle size, zeta potential, and polydispersity index of cubosomes. In dispersions 1–3, the concentration of P407 was kept constant, while the PVA concentration was increased from 1.5% to 2.5% for both blank and drug-loaded cubosomes. This increase in PVA concentration correlated with a decrease in particle size.64 Conversely, in dispersions 4–6, the concentration of PVA was constant, and the P407 concentration was varied between 0.5% and 1.5%. This variation resulted in a notable decrease in the average particle size of the cubosomes as the P407 concentration increased, as detailed in the study.64 Throughout all these dispersions, the concentration of GMO remained unchanged, ensuring a consistent lipid content in the formulations.
Characterization of cubogel
The pH of the cubogel formulation was determined to be in the range of 5.0 to 5.3, indicating its suitability for topical application due to its compatible pH range.72,73 It aligns with the natural pH of the skin, reducing the risk of irritation and ensuring compatibility.
| Rpm | Viscosity of cubogel |
|---|---|
| 10 | 3300 ± 0.12 |
| 20 | 2593 ± 0.14 |
| 50 | 2020 ± 0.11 |
| 100 | 1265 ± 0.13 |
The study also highlights the importance of sustained drug release in wound treatment, which can enhance healing and reduce discomfort. The comparative analysis of different treatments provides valuable insights into the efficacy of various therapeutic approaches in managing burn wounds.
Conclusion
This study presents significant findings in the context of synthesis of benzimidazole derivatives and development of benzimidazole based cubosomes for the topical treatment of burns. It demonstrating the superior efficacy of API 6 loaded cubogel in promoting rapid and effective wound healing without causing irritation or other complications. In conclusion, this research successfully synthesized API 6, as benzimidazole derivative. Then benzimidazole derivative was incorporated into cubosome formulations that cause a sustained release in treating bacterial infections and burn wounds. The cubosomes, characterized by their small particle size, high entrapment efficiency, and drug release, were effectively integrated into a cubogel for topical application. This cubogel demonstrated remarkable efficacy in burn wound healing, in in vivo trials without causing adverse effects. As compared to commercial products (Clotrimazole® and Polyfax®) which cause inflammation and irritation to the burn wounds. The sustained release is characteristic of the cubosomal formulation and plays an important role in its effectiveness. Histopathological evaluations further emphasized the therapeutic potential of formulated cubogel in enhancing effective wound healing. These results have important implications for the development of more efficient and patient-friendly treatments for burn wounds. These findings highlight the potential of this innovative formulation as a superior alternative for burn wound management, showing a significant advancement in the pharmaceutical applications of benzimidazole derivatives.Abbreviation
| API | Active pharmaceutical ingredient |
| FT-IR | Fourier transform infrared spectroscopy |
| NMR | Nuclear magnetic resonance |
| GMO | Glyceryl monooleate |
| PVA | Polyvinyl alcohol |
| P407 | Poloxamer 407 |
| TEM | Transmission electron microscopy |
| D | Drug |
| B | Blank |
| UAF | University of Agriculture Faisalabad |
| EE | Entrapment efficiency |
| PS | Particle size |
| ZP | Zeta potential |
| PDI | Polydispersity index |
| RT | Room temperature |
Ethical statement
All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of National Biosafety Rules 2005, Punjab Biosafety Rules 2014, Punjab Animal Health Act 2019, and Bio-ethics protocols and approved by the Animal Ethics Committee of Chairman Institutional Biosafety and Bioethics Committee (IBC), ORIC at the University of Agriculture Faisalabad, Faisalabad-Pakistan.Data availability
All the data produced or examined during this research have been provided within the manuscript and ESI.†Conflicts of interest
All the author declares that there is no conflict of interest in publish this research.Acknowledgements
The authors are thankful to Pakistan Science Foundation (PSF) for awarding Research grant PSF/CRP/Consrm-676. The authors are thankful to University of Agriculture Faisalabad and Pakistan Science Foundation (PSF).References
- E. Siddiqui, N. Zia, A. Feroze, S. Awan, A. L. Ali, J. A. Razzak, A. A. Hyder and A. Latif, BMC Emerg. Med., 2015, 15, 1–7 CrossRef PubMed
.
- A. Wardhana, A. Basuki, A. D. H. Prameswara, D. N. Rizkita, A. A. Andarie and A. F. Canintika, Burns Open, 2017, 1, 67–73 CrossRef
.
- M. F. Sanchez, S. A. Breda, E. A. Soria, L. I. Tártara, R. H. Manzo and M. E. Olivera, Drug Delivery Transl. Res., 2018, 8, 1000–1013 CrossRef CAS PubMed
.
- L. S. Campos, M. F. Mansilla and A. M. M. de la Chica, Rev. Enfermería, 2005, 28, 67–70 Search PubMed
.
- W. W. Monafo and M. A. West, Drugs, 1990, 40, 364–373 CrossRef CAS PubMed
.
- C. Jacobs, J. Vacek, B. Many, M. Bouchard and F. Abdullah, J. Surg. Res., 2021, 257, 442–448 CrossRef
.
- P. K. Bolla, C. A. Meraz, V. A. Rodriguez, I. Deaguero, M. Singh, V. K. Yellepeddi and J. Renukuntla, Molecules, 2019, 24, 3139 CrossRef CAS PubMed
.
- Y. Li, X. Zhou, H. Wu, Z. Yu, H. Li and S. Yang, Ind. Crops Prod., 2020, 150, 112406 CrossRef CAS
.
- G. Yadav and S. Ganguly, Eur. J. Med. Chem., 2015, 97, 419–443 CrossRef CAS PubMed
.
- Y. Bansal and O. Silakari, Bioorg. Med. Chem., 2012, 20, 6208–6236 CrossRef CAS
.
- M. Gaba, S. Singh and C. Mohan, Eur. J. Med. Chem., 2014, 76, 494–505 CrossRef CAS
.
- L. Zhang, D. Addla, J. Ponmani, A. Wang, D. Xie, Y.-N. Wang, S.-L. Zhang, R.-X. Geng, G.-X. Cai and S. Li, Eur. J. Med. Chem., 2016, 111, 160–182 CrossRef CAS
.
- N. Singh, A. Pandurangan, K. Rana, P. Anand, A. Ahamad and A. K. Tiwari, Int. Curr. Pharm. J., 2012, 1, 110–118 CrossRef
.
- M. A. Ali, N. Kataoka, A.-H. Ranneh, Y. Iwao, S. Noguchi, T. Oka and S. Itai, Chem. Pharm. Bull., 2017, 65, 42–48 CrossRef CAS
.
- T. K. Kwon, S. K. Hong and J.-C. Kim, J. Ind. Eng. Chem., 2012, 18, 563–567 CrossRef CAS
.
- R. R. Bhosale, R. A. Osmani, B. R. Harkare and P. P. Ghodake, Scholars Acad. J. Pharm., 2013, 2, 481–486 Search PubMed
.
- P. T. Spicer, K. L. Hayden, M. L. Lynch, A. Ofori-Boateng and J. L. Burns, Langmuir, 2001, 17, 5748–5756 CrossRef CAS
.
- F. D. Victorelli, L. S. Manni, S. Biffi, B. Bortot, H. H. Buzzá, V. Lutz-Bueno, S. Handschin, G. Calixto, S. Murgia and M. Chorilli, J. Colloid Interface Sci., 2022, 620, 419–430 CrossRef CAS PubMed
.
- L. Boge, K. Hallstensson, L. Ringstad, J. Johansson, T. Andersson, M. Davoudi, P. T. Larsson, M. Mahlapuu, J. Håkansson and M. Andersson, Eur. J. Pharm. Biopharm., 2019, 134, 60–67 CrossRef CAS
.
- T. E. Hartnett, A. J. O'Connor and K. Ladewig, Expert Opin. Drug Delivery, 2015, 12, 1513–1526 CrossRef CAS PubMed
.
- Q. Chai, Y. Jiao and X. Yu, Gels, 2017, 3, 6 CrossRef
.
- V. Karthika, P. Sheri and M. Kuriachan, Int. J. Res. Rev., 2018, 5, 149–159 CAS
.
- A. Adak, V. Castelletto, A. de Sousa, K.-A. Karatzas, C. Wilkinson, N. Khunti, J. Seitsonen and I. W. Hamley, Biomacromolecules, 2024, 25, 1205–1213 CrossRef CAS PubMed
.
- M. A. Iqbal, R. A. Haque, S. F. Nasri, A. A. Majid, M. B. K. Ahamed, E. Farsi and T. Fatima, Chem. Cent. J., 2013, 7, 1–17 CrossRef PubMed
.
- A. E. Eldeeb, S. Salah and M. Ghorab, J. Drug Delivery Sci. Technol., 2019, 52, 236–247 CrossRef CAS
.
- L. M. Ahmed, K. M. Hassanein, F. A. Mohamed and T. H. Elfaham, Sci. Rep., 2023, 13, 17941 CrossRef CAS PubMed
.
- A. Tekade, P. Ghodke, A. Patange and P. Patil, J. Drug Delivery Sci. Technol., 2023, 87, 104797 CrossRef CAS
.
- A. Ramalheiro, J. L. Paris, B. F. Silva and L. R. Pires, Int. J. Pharm., 2020, 591, 119942 CrossRef CAS PubMed
.
- H. M. El-Laithy, A. Badawi, N. S. Abdelmalak and N. El-Sayyad, Chem. Pharm. Bull., 2018, 66, 1165–1173 CrossRef CAS PubMed
.
- K. Dua, V. R. Malipeddi, J. Madan, G. Gupta, S. Chakravarthi, R. Awasthi, I. S. Kikuchi and T. De Jesus Andreoli Pinto, Interventional Med. Appl. Sci., 2016, 8, 68–76 Search PubMed
.
- M. A. Syed, G. Aziz, M. B. Jehangir, T. A. Tabish, A. F. Zahoor, S. H. Khalid, I. U. Khan, K. M. Hosny, W. Y. Rizg and S. Hanif, Pharmaceutics, 2022, 14, 1592 CrossRef CAS PubMed
.
- S. Ahmed, M. A. Kassem and S. Sayed, Int. J. Nanomed., 2020, 9783–9798 CrossRef CAS PubMed
.
- M. Somkiat Wattanasirichaigoon, J. Med. Assoc. Thailand, 2007, 90, 724–729 Search PubMed
.
- M. I. Asad, D. Khan, A. U. Rehman, A. Elaissari and N. Ahmed, Nanomaterials, 2021, 11, 3433 CrossRef CAS PubMed
.
- N. Zeghad, A. Ejaz, K. M. Zakryya, M. Aicha and B. Abdelmalik, Curr. Issues Pharm. Med. Sci., 2023, 36, 12–17 CrossRef CAS
.
- A. Anis, A. Sharshar, S. El Hanbally and A. A. Shehata, J. Clin. Med., 2022, 11, 6417 CrossRef
.
- Q. Ou, S. Zhang, C. Fu, L. Yu, P. Xin, Z. Gu, Z. Cao, J. Wu and Y. Wang, J. Nanobiotechnol., 2021, 19, 1–12 CrossRef
.
- M. Atif, H. N. Bhatti, R. A. Haque, M. A. Iqbal, M. B. Ahamed Khadeer and A. M. S. A. Majid, Appl. Biochem. Biotechnol., 2020, 191, 1171–1189 CrossRef CAS PubMed
.
- R. A. Haque, S. F. Nasri and M. A. Iqbal, J. Coord. Chem., 2013, 66, 2679–2692 CrossRef CAS
.
- R. Y. Nadeem, M. Yaqoob, W. Yam, R. A. Haque and M. A. Iqbal, Med. Chem. Res., 2022, 31, 1783–1791 CrossRef CAS
.
- M. A. Iqbal, R. Y. Nadeem, M. Yaqoob, W. Yam and R. A. Haque, Med. Chem. Res., 2022, 31, 1783–1791 CrossRef
.
- K. Hayat, M. Shkeel, M. A. Iqbal, M. Khalid, C. K. Quah, Q. A. Wong, A. U. Rehman, M. B. K. Ahamed, U. Farooq and S. Hameed, Inorg. Chim. Acta, 2023, 557, 121694 CrossRef CAS
.
- M. A. Iqbal, SSynthesis, Structure and in Vitro Anticancer Studies of Dinuclear Silver (I)-N-heterocyclic Carbene Complexes Derived from Xylyl Linked Bis-benzimidazolium Salts, PhD thesis, Universiti Sains Malaysia, 2014
.
- A. Kamal, A. H. Ibrahim, S. S. Al-Rawi, M. A. Iqbal and H. N. Bhatti, Chem. Pap., 2023, 1–10 Search PubMed
.
- G. Lei and L. Zhou, Acta Crystallogr., Sect. E: Struct. Rep., 2009, 65, o2613 CrossRef CAS PubMed
.
- N. S. Goud, S. M. Ghouse, J. Vishnu, D. Komal, V. Talla, R. Alvala, J. Pranay, J. Kumar, I. A. Qureshi and M. Alvala, Bioorg. Chem., 2019, 89, 103016 CrossRef CAS PubMed
.
- U. B. Suleiman, U. Yunusa, A. Muhammad and M. B. Ibrahim, Port. Electrochim. Acta, 2023, 363–380, DOI:10.4152/pea.2023410504
.
- A. Chakraborty, S. Debnath, T. Ghosh, D. K. Maiti and S. Majumdar, Tetrahedron, 2018, 74, 5932–5941 CrossRef CAS
.
- N. F. Younes, S. A. Abdel-Halim and A. I. Elassasy, Int. J. Pharm., 2018, 553, 386–397 CrossRef CAS PubMed
.
- A. B. Cintra, L. A. Delboni and M. G. Lara, Braz. J. Pharm. Sci., 2023, 58, e20803 CrossRef
.
- X. Pan, K. Han, X. Peng, Z. Yang, L. Qin, C. Zhu, X. Huang, X. Shi, L. Dian and M. Lu, Curr. Pharm. Des., 2013, 19, 6290–6297 CrossRef CAS PubMed
.
- V. K. Rapalli, S. Banerjee, S. Khan, P. N. Jha, G. Gupta, K. Dua, M. S. Hasnain, A. K. Nayak, S. K. Dubey and G. Singhvi, Mater. Sci. Eng., C, 2021, 119, 111548 CrossRef CAS PubMed
.
- W. S. Alharbi and K. M. Hosny, J. Drug Delivery Sci. Technol., 2020, 57, 101710 CrossRef CAS
.
- F. Hashem, M. Nasr and M. Youssif, J. Adv. Pharm. Res., 2018, 2, 95–103 CrossRef
.
- K. C. Achar, K. M. Hosamani and H. R. Seetharamareddy, Eur. J. Med. Chem., 2010, 45, 2048–2054 CrossRef CAS PubMed
.
- H. A. Elgendy, A. M. Makky, Y. E. Elakkad, R. M. Ismail and N. F. Younes, Drug Deliv., 2023, 30, 2162159 CrossRef PubMed
.
- A. Sanjana, M. G. Ahmed and J. G. BH, Mater. Today: Proc., 2022, 50, 197–205 CAS
.
- S. S. Patil, K. Roy, B. Choudhary and K. R. Mahadik, Drug Dev. Ind. Pharm., 2016, 42, 1300–1307 CrossRef CAS PubMed
.
- A. A. El-Shenawy, M. M. Elsayed, G. M. Atwa, M. A. Abourehab, M. S. Mohamed, M. M. Ghoneim, R. A. Mahmoud, S. A. Sabry, W. Anwar and M. El-Sherbiny, Pharmaceutics, 2023, 15, 680 CrossRef CAS PubMed
.
- A. Zerriouh, A. Deghiche, W. Bououden, D. Cavallo, F. Rainone, A. Erto and N. Haddaoui, J. Mol. Liq., 2023, 382, 121914 CrossRef CAS
.
- Z. Chen, Q. Huang, Y. Song, X. Feng, L. Zeng, Z. Liu, X. Hu, C. Tao, L. Wang and Y. Qi, Biomed. Pharmacother., 2023, 166, 115316 CrossRef CAS
.
- D. C. A. Putri, R. Dwiastuti and A. K. N. Marchaban, J. Farm. Sai. Komun., 2017, 14, 79–85 Search PubMed
.
- M. Nasr, H. Younes and R. S. Abdel-Rashid, Drug Delivery Transl. Res., 2020, 10, 1302–1313 CrossRef CAS
.
- M. Sakhi, A. Khan, I. Khan, S. A. Khan, S. I. Khan, M. A. Khattak, M. N. Uddin, M. Kazi and F. Nasir, Saudi Pharm. J., 2023, 31, 101697 CrossRef CAS PubMed
.
- R. M. Zaki, A. El Sayeh Abou El Ela, A. S. Almurshedi, B. N. Aldosari, A. A. Aldossari and M. A. Ibrahim, Polymers, 2023, 15, 1774 CrossRef CAS
.
- H. A. El-Enin and A. H. Al-Shanbari, Saudi Pharm. J., 2018, 26, 790–800 CrossRef PubMed
.
- K. Prabahar, U. Uthumansha, N. Elsherbiny and M. Qushawy, Pharmaceuticals, 2023, 16, 563 CrossRef CAS PubMed
.
- N. Mukherjee, S. Ghosh, J. Sarkar, R. Roy, D. Nandi and S. Ghosh, ACS Appl. Mater. Interfaces, 2023, 15, 33457–33479 CrossRef CAS
.
- S. Sen, S. Ghosh, A. Jana, M. Jash, S. Ghosh, N. Mukherjee, D. Mukherjee, J. Sarkar and S. Ghosh, ACS Appl. Bio Mater., 2024, 7(6), 4142–4161 CrossRef CAS PubMed
.
- A. M. Al-Mahallawi, A. A. Abdelbary and S. A. El-Zahaby, Int. J. Pharm., 2021, 600, 120490 CrossRef CAS PubMed
.
- N. Mukherjee, S. Ghosh, R. Roy, D. Mukherjee, S. Sen, D. Nandi, J. Sarkar and S. Ghosh, ACS Appl. Mater. Interfaces, 2024, 30929–30957 CrossRef CAS
.
- S. M. Arman and C. Mark-Herbert, Sustainability, 2021, 13, 10242 CrossRef
.
- A. Adak, V. Castelletto, B. Mendes, G. Barrett, J. Seitsonen and I. W. Hamley, ACS Appl. Bio Mater., 2024, 7(8), 5553–5565 CrossRef CAS
.
- U. Rehman, A. Sheikh, A. Alsayari, S. Wahab and P. Kesharwani, Colloids Surf., B, 2023, 113728 Search PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra04816d |
| This journal is © The Royal Society of Chemistry 2024 |