Open Access Article
Open Access ArticleLead-free iron-doped Cs3Bi2Br9 perovskite with tunable properties†
Thiri Htuna,
Amr Elattarbc,
Hytham Elbohy d,
Kosei Tsutsumia,
Kazumasa Horiganee,
Chiyu Nakanof,
Xiaoyu Gug,
Hiroo Suzukia,
Takeshi Nishikawaa,
Aung Ko Ko Kyaw
d,
Kosei Tsutsumia,
Kazumasa Horiganee,
Chiyu Nakanof,
Xiaoyu Gug,
Hiroo Suzukia,
Takeshi Nishikawaa,
Aung Ko Ko Kyaw *g and
Yasuhiko Hayashi*a
*g and
Yasuhiko Hayashi*a
aGraduate School of Natural Science and Technology, Okayama University, Japan. E-mail: hayashi.yasuhiko@ec.okayama-u.ac.jp
bDepartment of Chemistry, Faculty of Science, Ain Shams University, Cairo, Egypt
cIndustrial & Manufacturing Engineering, FAMU-FSU College of Engineering, 2525 Pottsdamer St, Tallahassee, Florida 32310, USA
dPhysics Department, Faculty of Science, Damietta University, Egypt
eResearch Institute for Interdisciplinary Science, Okayama University, Japan
fAdvanced Science Research Center, Okayama University, Okayama, Japan
gGuangdong University Key Laboratory for Advanced Quantum Dot Displays and Lighting and Department of Electronic & Electrical Engineering, Southern University of Science and Technology, P. R. China. E-mail: aung@sustech.edu.cn
First published on 23rd July 2024
Abstract
Perovskite based on cesium bismuth bromide offers a compelling, non-toxic alternative to lead-containing counterparts in optoelectronic applications. However, its widespread usage is hindered by its wide bandgap. This study investigates a significant bandgap tunability achieved by introducing Fe doping into the inorganic, lead-free, non-toxic, and stable Cs3Bi2Br9 perovskite at varying concentrations. The materials were synthesized using a facile method, with the aim of tuning the optoelectronic properties of the perovskite materials. Characterization through techniques such as X-ray diffraction, Raman spectroscopy, X-ray photoelectron spectroscopy, energy dispersive spectroscopy (EDS), and UV-vis spectroscopy was conducted to elucidate the transformation mechanism of the doping materials. The substitution process results in a significant change in the bandgap energy, transforming from the pristine Cs3Bi2Br9 with a bandgap of 2.54 eV to 1.78 eV upon 70% Fe doping. The addition of 50% Fe in Cs3Bi2Br9 leads to the formation of the orthorhombic structure in Cs2(Bi,Fe)Br5 perovskite, while complete Fe alloying at 100% results in the phase formation of CsFeBr4 perovskite. Our findings on regulation of bandgap energy and crystal structure through B site substitution hold significant promise for applications in optoelectronics.
Introduction
In recent years, lead-based halide perovskites with the formula ABX3, where A is a cation (e.g., methylammonium, MA+, formamidinium, FA+, cesium, Cs+), B is lead (Pb2+), and X is the halogen (e.g., I−, Br−, and Cl−), has gained widespread use in ubiquitous optoelectronic devices including photovoltaics, light-emitting diode, laser and photodetector due to their excellent optical and electrical properties.1–7 These perovskites can be divided into two categories: organic–inorganic perovskites and all-inorganic perovskites, depending on the A cation.3 Organic cations are prone to decomposition compared to inorganic cations.2 Moreover, the toxicity and the moisture sensitivity of lead, a component in these perovskites, limit their stability for certain applications.8–11 Therefore, multiple attempts have been made to substitute lead ions with other metal ions exhibiting similar electronic configurations, such as Sn2+, Ge2+, Bi3+, and Sb3+, aiming for eco-friendly and stable perovskites.12,13 While tin-based perovskites present lower toxicity and a narrower bandgap than their lead-based counterparts, their susceptibility to moisture and oxygen due to rapid oxidation from Sn2+ to Sn4+ poses challenges. Germanium-based perovskites share similar stability and electronic and optical properties with lead-based perovskites, but their high cost and spontaneous oxidation during the fabrication process hinder practical applications.8,14 Alternatively, double perovskites with the general formula A2M+M3+X6 have been synthesized by changing the ABX3 structure, replacing Pb2+ with one monovalent cation, M+ (e.g., Cu+, Ag+, Au+, In+) and one trivalent cation, M3+ (e.g., Bi3+, Sb3+). This innovation results in Pb-free quaternary materials, representing a significant advancement with enhanced functionalities, particularly in solar absorbers.15–17 Another popular perovskite structure, A3B2X9, characterized by a monovalent cation A (Na+, K+, Rb+, Cs+, and CH3NH3+), a trivalent cations B (Bi3+ and Sb3+), and a halogen atom X, has recently garnered increased interest in solar cells and photonic applications.8,18–21Within the A3B2X9 perovskite structure, diverse crystal structures exist, classified as 0D, 1D, and 2D depending on the B–X sublattice. The zero-dimensional (0D) structure have a hexagonal structure, in which A3X9 units are ordered with B cations in octahedral interstitial sites, resulting in isolated [B2X9]3− dimers. In a one-dimensional structure (1D), double chains form based on corner-sharing [BX6]3− octahedra. The two-dimensional (2D) structure, where the layers are corrugated like corner-connected [BX6]3− octahedra, is the most similar to the cubic perovskite that appeared by removing the third B site cation from cubic ABX3. The 2D structure has a narrow bandgap, superior defect tolerance, higher electron and hole mobility, and excellent stability compared to the 0D and 1D structures. Therefore, it plays a crucial role in controlling the bandgap and enhancing optical absorption in lead-free perovskite materials.22,23
Bismuth-based perovskites (A3Bi2X9) emerge as attractive alternatives due to their non-toxicity, desirable moisture and air stability,24 and excelle nt water stability. Bi3+ shares a similar electronic configuration and ionic radius with Pb2+, making it a viable substitute.14,25–30 All inorganic Bi3+-based perovskites have obtained increased attention in various optoelectronic applications owing to their superior stability and low toxicity.31–33 Various Bi3+ based perovskites, including Cs3BiBr6, CsBi3I10, Cs3Bi2Br9, Cs2AgBiBr6 and Cs3Bi2I9, have been successfully synthesized.34 Among these, all-inorganic Bi-based perovskites using Cs as A cation exhibit enhanced crystallinity and superior optoelectronic properties compared to organic–inorganic counterparts.2 The choice of Cs as the cation contributes to improve structure stability and overall performance of perovskite materials.35–37 Doping strategies can further be employed on pristine perovskite material to fine-tune their optical and electronic properties. Siqi Dai et al. investigated the effect of Sb doping on pristine Cs3Bi2Br9 perovskite, finding that it reduced the bandgap from 2.59 eV to 2.22 eV. The mixed alloy occupied a smaller bandgap than both the Bi-based and Sb-based perovskites.38 Amr Elattar et al. demonstrated that doping of Cu in Cs3Bi2Br9 perovskite significantly reduces the bandgap from 2.56 eV for pristine Cs3Bi2Br9 to 1.77 eV at 100% Cu.23 Mrinmoy Roy et al. studied the effect of Pb substitution in Cs3Bi2Br9 layered perovskites, finding that the bandgap is reduced from 2.62 eV to 2.23 eV due to the emergence of defect states between the bands.39 Fuxiang et al. investigated the impact of Fe doping in the double perovskite Cs2AgInxFe1−xCl6, observing a notable reduction in bandgap from 2.8 eV in the pristine material to 1.6 eV in the Fe-doped variants. This highlights the effectiveness of Fe in substantially reducing the bandgap of perovskite materials.40 Moreover, iron, being the most abundant element on Earth's surface, offers a non-toxic, environmentally friendly, and cost-effective option.41–44
Herein, we incorporated Fe ions into the bismuth-based (Cs3Bi2Br9) perovskite, effectively modulating the bandgap of Cs3Bi2−xFexBr9 over a wide range, from 2.54 eV to 1.78 eV. Furthermore, we investigated the impact of Fe doping on the pristine Cs3Bi2Br9 perovskite. The substitution of Fe3+ for Bi3+ in the matrix, resulted in the formation of a secondary phase (Cs2(Bi,Fe)Br5) and a ternary phase (CsFeBr4) with varying proportions of alloying elements. The incorporation of Fe3+ enables the fine-tuning of the optical properties of the doped materials, paving the way for potential applications in optoelectronic devices.
Materials and methods
Materials
In our experimental procedure, we added cesium bromide (CsBr, 98%), bismuth III bromide (BiBr3, 99%), and iron III bromide (FeBr3, anhydrous) to an 8 ml volume of hydrobromic acid (HBr). It is worth noting that all chemicals were used without further purification.Synthesis of perovskite crystals
We prepared Cs3Bi2Br9 with varying Fe doping concentrations (0, 5, 20, 50, 70, and 100 at%). This was achieved by combining 3 mmol of CsBr, 2 − x mmol of BiBr3, and x mmol of FeBr3 into 8 ml of hydrobromic acid (HBr), as shown in Table 1. The mixture was then subjected to heating at 150 °C for 48 h, followed by a gradual cooling to room temperature at a rate of 10 °C day−1. This controlled thermal treatment resulted in the successful formation of Fe-doped Cs3Bi2Br9 perovskite crystals.| CsBr (mmol) | BiBr3 (mmol) | FeBr3 (mmol) | Alloying composition (%) |
|---|---|---|---|
| 3 | 2 | w/0 | 0% |
| 3 | 1.9 | 0.1 | 5% |
| 3 | 1.6 | 0.4 | 20% |
| 3 | 1 | 1 | 50% |
| 3 | 0.6 | 1.4 | 70% |
| 3 | w/0 | 2 | 100% |
Characterization
In our analysis, we employed a scanning electron microscope (SEM), specifically the JEOL JEM-2100F model, operating at 12 kV. To gain insights into the morphology and elemental composition of the samples, energy dispersive spectroscopy (EDS) was utilized. Elemental analysis was conducted using the SEM with EDS capabilities. For structural characterization, powder X-ray diffraction (XRD) data were collected at room temperature. A conventional diffractometer, specifically the Rigaku RINT-TTR III, with Bragg–Brentano geometry and a Cu-Kαradiation source (λ = 1.5418 Å), was utilized. The obtained diffraction data were analyzed using the Rietveld analysis program RIETAN2000 [A], providing detailed information about the crystal structure. Steady-state absorption spectra were acquired using a JASCO V-670 spectrophotometer. To examine lattice vibrations, Raman spectroscopy was performed with a JASCO NRS4500 NMDS instrument, employing a 532 nm excitation (green) laser. X-ray photoelectron spectroscopy (XPS) was carried out using a JEOL JPS-9030 instrument, utilizing an X-ray source of Mg K Alfa (monochromatic) with a 25 mA beam current and 12 kV. This technique provided valuable information about the elemental composition and chemical states of the samples.Results and discussion
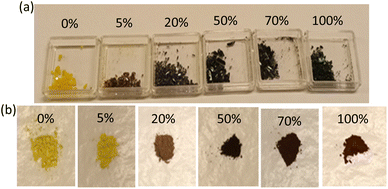
The Cs3Bi2Br9 perovskite crystals with varying Fe concentrations (0, 5, 20, 50, 70, and 100 at%) exhibit a distinct color transition from yellow to a darker shade, as depicted in Fig. 1(a). This color tunability strongly implies variations in bandgap energy and material replacement among these materials.23 Upon grinding the crystals, the materials exhibit a darker coloration at higher Fe doping concentrations, as illustrated in Fig. 1(b). It suggests that Fe3+ can be fully substituted for Bi3+ in Cs3Bi2Br9.As shown in Fig. 1(b), the color of the doped material darkens with increasing Fe concentration, suggesting a variation in bandgap energy. This visual correlation is further supported by the UV-vis absorption spectroscopy presented in Fig. 2(a). The adsorption edge of the undoped material, observed around 430 nm, aligns with previous reports.22 As the concentration of Fe increases, the optical absorbance band edge of the doping materials also increases, reaching approximately 490 nm. The UV-vis spectra exhibit three distinct types of curves corresponding to different doping concentrations, each characterized by a unique crystal structure. The optical bandgap of the materials is estimated from UV spectra using Tauc plots of (αhν)n versus hν. The Cs3Bi2Br9 perovskite possesses both direct and indirect bandgaps.39,45 The indirect bandgap values of the pristine and doped Cs3Bi2Br9 are 2.54 eV, 2.53 eV, 2.39 eV, 1.80 eV, 1.78 eV, and 1.88 eV, and the corresponding direct bandgap values are 2.69 eV, 2.67 eV, 2.61 eV, 1.99 eV, 1.97 eV, and 2.17 eV for 0%, 5%, 20%, 50%, 70%, and 100% Fe doping, respectively as shown in Fig. 2(b) and (c). The bandgap value of the pristine Cs3Bi2Br9 perovskite material is in good agreement with previous reports.40,46 These tunable optical bandgap energies align with the observed color changes in different Fe doping concentrations. Both the direct and indirect bandgaps shift towards lower values with increasing Fe content compared to the pristine Cs3Bi2Br9 perovskite. The indirect bandgap values are consistently smaller than the direct bandgap values, although the trend is the same for both methods of calculation. The PL peak does not appear when we measure the Raman spectroscopy as illustrated in ESI Fig. S1.† Therefore, we assume that both the pristine and doped Cs3Bi2Br9 perovskite have an indirect bandgap. The indirect bandgap energies exhibit a gradual decrease, ranging from 2.54 eV to 2.39 eV for the 0%, 5%, and 20% compositions of the perovskite material characterized by the Cs3Bi2Br9 perovskite structure. For the Cs2(Bi,Fe)Br5 perovskite material, the bandgap energies shift from 1.80 eV to 1.78 eV in the case of the 50% and 70% Fe doping. Notably, 70% Fe doping stands out with the lowest bandgap energy of 1.78 eV, as shown in Fig. 2(d). The bandgap energy for the CsFeBr4 structure with 100% Fe occupancy is measured at 1.88 eV. This tunability in bandgap energy holds promise for applications that require tailored optical properties, emphasizing the influence on optoelectronic devices.
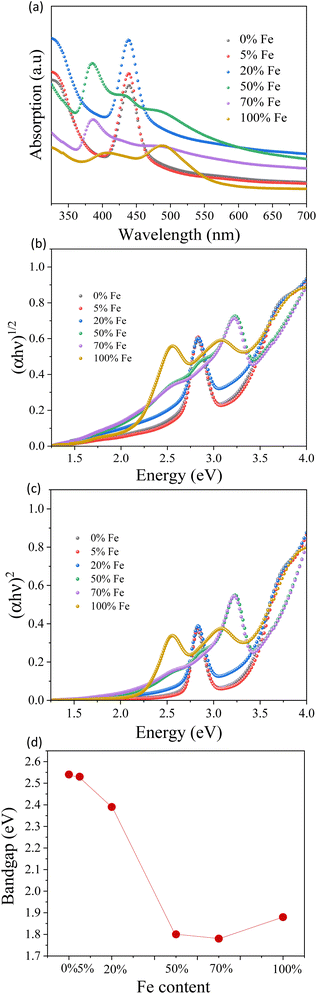 | ||
| Fig. 2 (a) UV-Vis absorption spectra, Tauc plots of (b) indirect bandgap and (c) direct bandgap, and (d) indirect bandgap energies of different Fe doping materials. | ||
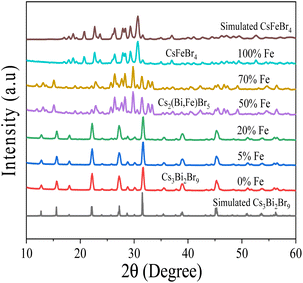
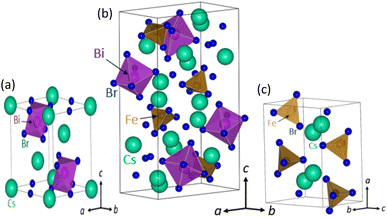
There is a possibility that variation in bandgap from 2.69 eV to 1.97 eV could be associated with crystal structure change due to Fe doping. Therefore, we further examined the crystal structure of the perovskites at various Fe doping using XRD and Rietveld analysis. Fig. 3 shows the XRD pattern of Fe-doped Cs3Bi2Br9 perovskite for concentrations ranging from 0% to 100% Fe. The powder XRD measurements of the pristine, 5% Fe-, and 20% Fe-doped perovskites are in agreement with the Cs3Bi2Br9 trigonal crystal structure illustrated in Fig. 4(a), maintaining the space group ![[P with combining macron]](https://www.rsc.org/images/entities/i_char_0050_0304.gif) 3m1 as previously reported in the literature.23 The structural parameters were refined using Rietveld analysis of XRD data, based on the structural model as shown in in ESI Fig. S2.† The XRD patterns of the pristine, 5%, and 20% Fe-doped samples were consistent with the Cs3Bi2Br9 structure as depicted in ESI Fig. S2(a)–(c).† Notably, both the a and c axes increased with higher Fe doping levels, suggesting the substitution of Fe atoms into the Bi site. Additionally, a structural transformation becomes evident at 50% Fe doping, where the diffraction patterns show an orthorhombic structure characteristic of Cs2(Bi,Fe)Br5 perovskite with a space group Pnma, as illustrated in Fig. 4(b). This crystal structure has two metal-occupied sites, namely octahedral and tetrahedral sites. Consequently, we conducted Rietveld analysis on two models: one with Bi occupying an octahedral site and Fe occupying a tetrahedral site, and the other with Bi occupying a tetrahedral site and Fe occupying an octahedral site. When the Bi atoms occupied octahedral sites, the resulting reliability factor was Rwp = 6.33% as illustrated in ESI Fig. S2(d).† On the other hand, Bi atoms occupied at tetrahedral sites, the Rwp becomes worse from 6.33% to 12.85%. This outcome indicates that Bi atoms occupied octahedral sites and Fe atoms occupy tetrahedral sites. The diffraction pattern of the 70% Fe alloy sample could be indexed by the Cs2(Bi,Fe)Br5 structure, with lattice constants increasing with Fe doping. This tendency is similar to that of Fe doping in Cs3Bi2Br9, suggesting a substitution of Fe in the pristine material via the orthorhombic structure.23 For the 100% Fe alloying material, all diffraction peaks correspond to the orthorhombic structure of CsFeBr4 perovskite, characterized by the space group Pnma, presented in Fig. 4(c). The resulting reliability factor from the Rietveld refinement was Rwp = 4.04%, as shown in ESI Fig. S2(f).† Interestingly, in CsFeBr4, Fe ions exhibit tetrahedral coordination, suggesting that the structural change induced by Fe doping is associated with the preferential tetrahedral coordination of Fe. This leads to the presence of a tertiary phase (CsFeBr4) in the Cs3Bi2Br9 perovskite at 100% Fe alloying. From the XPD analysis, we postulate that the transformation of crystal structure from Cs3Bi2Br9 to Cs2(Bi,Fe)Br5 and CsFeBr4 at higher Fe doping may also contribute to significant bandgap change in the perovskites.
3m1 as previously reported in the literature.23 The structural parameters were refined using Rietveld analysis of XRD data, based on the structural model as shown in in ESI Fig. S2.† The XRD patterns of the pristine, 5%, and 20% Fe-doped samples were consistent with the Cs3Bi2Br9 structure as depicted in ESI Fig. S2(a)–(c).† Notably, both the a and c axes increased with higher Fe doping levels, suggesting the substitution of Fe atoms into the Bi site. Additionally, a structural transformation becomes evident at 50% Fe doping, where the diffraction patterns show an orthorhombic structure characteristic of Cs2(Bi,Fe)Br5 perovskite with a space group Pnma, as illustrated in Fig. 4(b). This crystal structure has two metal-occupied sites, namely octahedral and tetrahedral sites. Consequently, we conducted Rietveld analysis on two models: one with Bi occupying an octahedral site and Fe occupying a tetrahedral site, and the other with Bi occupying a tetrahedral site and Fe occupying an octahedral site. When the Bi atoms occupied octahedral sites, the resulting reliability factor was Rwp = 6.33% as illustrated in ESI Fig. S2(d).† On the other hand, Bi atoms occupied at tetrahedral sites, the Rwp becomes worse from 6.33% to 12.85%. This outcome indicates that Bi atoms occupied octahedral sites and Fe atoms occupy tetrahedral sites. The diffraction pattern of the 70% Fe alloy sample could be indexed by the Cs2(Bi,Fe)Br5 structure, with lattice constants increasing with Fe doping. This tendency is similar to that of Fe doping in Cs3Bi2Br9, suggesting a substitution of Fe in the pristine material via the orthorhombic structure.23 For the 100% Fe alloying material, all diffraction peaks correspond to the orthorhombic structure of CsFeBr4 perovskite, characterized by the space group Pnma, presented in Fig. 4(c). The resulting reliability factor from the Rietveld refinement was Rwp = 4.04%, as shown in ESI Fig. S2(f).† Interestingly, in CsFeBr4, Fe ions exhibit tetrahedral coordination, suggesting that the structural change induced by Fe doping is associated with the preferential tetrahedral coordination of Fe. This leads to the presence of a tertiary phase (CsFeBr4) in the Cs3Bi2Br9 perovskite at 100% Fe alloying. From the XPD analysis, we postulate that the transformation of crystal structure from Cs3Bi2Br9 to Cs2(Bi,Fe)Br5 and CsFeBr4 at higher Fe doping may also contribute to significant bandgap change in the perovskites.
The elemental composition of the perovskite crystals was scrutinized using energy dispersive spectroscopy (EDS), and the alloying composition of all elements (Cs, Bi, Fe, and Br) is detailed in Table 2. Analysis reveals a correlation between the precursor and product compositions, indicating a preference for the incorporation of Fe into the pristine perovskite material. The EDS results further enable an estimation of the crystal structures. The A3B2X9 perovskite structure is observed for 0%, 5%, and 20% Fe doping, while the A2BX5 perovskite structure is identified for 50% and 70% Fe doping. Interestingly, the 100% Fe doping results in the ABX4 perovskite structure. These findings align with the XRD measurements, where the Cs3Bi2Br9 structure is retained for 0%, 5%, and 20% Fe doping, Cs2(Bi,Fe)Br5 structure emerges for 50% and 70% Fe doping, and CsFeBr4 structure is observed for 100% Fe doping. The comprehensive characterization sheds light on the structural transformations induced by varying Fe concentrations in the perovskite crystals. Energy dispersive spectroscopy (EDS) elemental mapping, shown in ESI Fig. S3,† demonstrates the same magnitude for all compositions. Despite some mappings being less clear, the overall tendency indicates a homogeneous decrease of Bi and an increase of Fe with increasing Fe content.
| Cs (at%) | Fe (at%) | Bi (at%) | Br (at%) | Estimated structure | Alloying composition (%) |
|---|---|---|---|---|---|
| 20.97 | w/0 | 12.85 | 66.17 | A3B2X9 | 0% |
| 24.85 | 1.61 | 11.91 | 61.63 | A3B2X9 | 5% |
| 23.32 | 4.88 | 7.08 | 64.71 | A3B2X9 | 20% |
| 25.41 | 6.45 | 6.38 | 61.76 | A2BX5 | 50% |
| 24.03 | 6.81 | 4.37 | 64.79 | A2BX5 | 70% |
| 15.16 | 16.99 | w/0 | 67.85 | ABX4 | 100% |
To verify the structural origin and phase purity of the pristine and alloyed perovskites, Raman spectra were obtained, as shown in Fig. 5(a). The peaks of undoped materials at 160 cm−1 and 187 cm−1 are attributed to the stretching vibrations of the Bi–Br bond with Eg and A1g symmetry in BiBr6 octahedra, respectively, which are vibrations with participation of Bi atoms. These results correspond well with prior reports.47,48 The absence of shifts in peak positions and intensity changes upon 20% or lower Fe doping suggests that Fe incorporation does not affect the crystal structure of the pristine material. New vibration mode at around 200 cm−1 appears from 50% to 100% alloyed materials, attributed to the stretching modes of Fe–Br bonds,49 absent in the parent Cs3Bi2Br9 material. The Raman peak at around 200 cm−1 broadens and shifts toward higher wavenumbers with 70% Fe and greater, as shown in Fig. 5(b), due to different ionic radius of the doping metal, Fe3+ (63 pm) and parent metal Bi3+(103 pm). This also indicates the substitution of Bi3+ with Fe3+ in the crystal structure.40
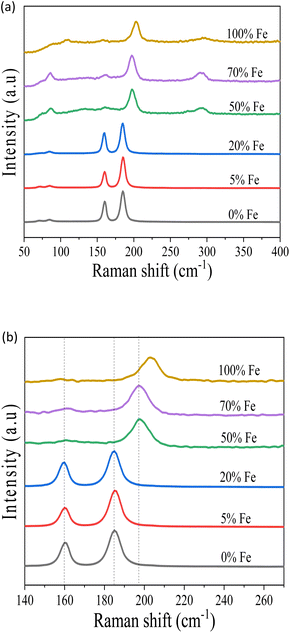 | ||
| Fig. 5 (a) Raman spectra of the pristine Cs3Bi2Br9 and Fe doped perovskite crystals. (b) Expansion of Raman spectra. | ||
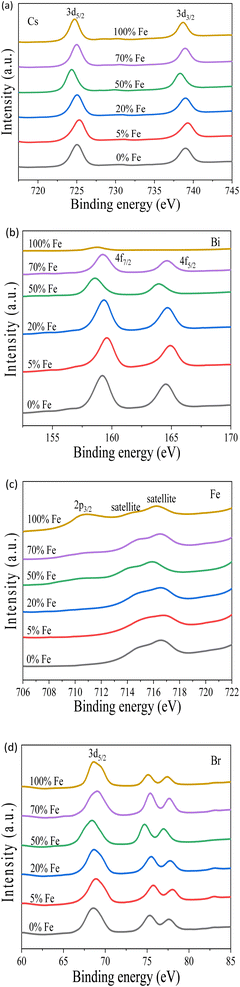
To investigate the incorporation of Fe into the pristine material, X-ray photoelectron spectroscopy (XPS) was utilized, revealing core levels of Cs 3d, Bi 4f, Br 3d, and an additional element, Fe, as depicted in Fig. 6. The Cs 3d5/2 and Cs 3d3/2 binding energies at 724 eV and 738 eV, respectively, align with previous reports.39 The Br 3d5/2 peak is located at 68 eV. Fig. 6(b) shows Bi exhibits doublet peaks corresponding to 4f7/2 at 159.3 eV and 4f5/2 at 164.6 eV, which matches with the previous results.41 At 100% Fe doping, the disappearance of these doublets is a result of the complete occupation by Fe. The binding energy of Bi 4f at 160 eV observed at 100% Fe doping is the spectral line from the 4p3/2 peak of Cs. The Fe 2p3/2 peak at 711 eV at 100% Fe doping confirms the successful doping of Fe into the pristine perovskite material as presented in Fig. 6(c). The consistency of this outcome aligns with other characterization results, including XRD and EDS analyses. Consequently, we have successfully doped Fe into the pristine Cs3Bi2Br9 perovskite material, leading to a potential reduction in its bandgap for optoelectronic applications.
Conclusions
In conclusion, the synthesis of nontoxic lead-free Cs3Bi2Br9 perovskite material with varying Fe doping concentrations has been successfully achieved through a facile method. The investigation of the structural and optical properties of Fe-doped Bi-based material has provided valuable insights. The pristine Cs3Bi2Br9 crystal structure maintains its trigonal crystal structure with space group![[P with combining macron]](https://www.rsc.org/images/entities/i_char_0050_0304.gif) 3m1. However, a secondary phase transformation to Cs2(Bi,Fe)Br5 is observed with 50% Fe doping, and successful Fe incorporation into the pristine perovskite (Cs3Bi2Br9) results in the formation of the orthorhombic crystal structure CsFeBr4 perovskite. Notably, the bandgap of the material undergoes a reduction from 2.54 eV for the pristine to 1.78 eV with 70% Fe alloying. This tunability in bandgap energy holds significant implications for potential applications in optoelectronic devices, highlighting the suitability of Fe-doped Cs3Bi2Br9 perovskite crystals for environmentally friendly, lead-free perovskite-based optoelectronic applications. This work represents a crucial step forward in the exploration and utilization of lead-free perovskite materials in the field of optoelectronics.
3m1. However, a secondary phase transformation to Cs2(Bi,Fe)Br5 is observed with 50% Fe doping, and successful Fe incorporation into the pristine perovskite (Cs3Bi2Br9) results in the formation of the orthorhombic crystal structure CsFeBr4 perovskite. Notably, the bandgap of the material undergoes a reduction from 2.54 eV for the pristine to 1.78 eV with 70% Fe alloying. This tunability in bandgap energy holds significant implications for potential applications in optoelectronic devices, highlighting the suitability of Fe-doped Cs3Bi2Br9 perovskite crystals for environmentally friendly, lead-free perovskite-based optoelectronic applications. This work represents a crucial step forward in the exploration and utilization of lead-free perovskite materials in the field of optoelectronics.
Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
The author gratefully acknowledges financial support from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan for PhD scholarship.References
- B.-W. Park, B. Philippe, X. Zhang, H. Rensmo, G. Boschloo and E. M. J. Johansson, Adv. Mater., 2015, 27, 6806–6813 CrossRef CAS.
- B. Yang, J. Chen, F. Hong, X. Mao, K. Zheng, S. Yang, Y. Li, T. Pullerits, W. Deng and K. Han, Angew. Chem., Int. Ed., 2017, 56, 12471–12475 CrossRef CAS PubMed.
- F. H. Gourji and D. Velauthapillai, Molecules, 2021, 26, 1–45 Search PubMed.
- Z.-K. Tang, Z.-F. Xu, D.-Y. Zhang, S.-X. Hu, W.-M. Lau and L.-M. Liu, Sci. Rep., 2017, 7, 1–7 CrossRef PubMed.
- J. H Heo and S. H. Im, Adv. Mater., 2016, 28, 5121–5125 CrossRef.
- H.-M. Huang, Z.-Y. Jiang, Y.-M. Lin, B. Zhou and C.-K. Zhang, Appl. Phys. Express, 2017, 10, 123002 CrossRef.
- D. Shen, X. Wang, X. Zhang, Y. Liu, Y. Shi, X. Li, X. Chen and Y. Zhang, ACS Appl. Opt. Mater., 2022, 1, 435–441 CrossRef.
- S. Ghosh, S. S. Mukhopadhyay, S. Paul, B. Pradhan and S. K. De, ACS Appl. Nano Mater., 2020, 3, 11107–11117 CrossRef CAS.
- C. Liu, L. Wang, F. Fang, Z. Zhao, J. Pan, J. Akram, S. Shafie, R. Tala-Ighil, Q. Li, Z. Zhao, J. Wu, Z. Zhu, W. Lei, X. Zhang and J. Chen, Front. Mater., 2021, 8, 682833 CrossRef.
- A. K. Baranwal, H. Masutani, H. Sugita, H. Kanda, S. Kanaya, N. Shibayama, Y. Sanehira, M. Ikegami, Y. Numata, K. Yamada, T. Miyasaka, T. Umeyama, H. Imahori and S. Ito, Nano Converg., 2017, 4, 1–14 CrossRef PubMed.
- M. Usman and Q. Yan, Crystals, 2020, 10, 62 CrossRef CAS.
- X. Li, X. Du, P. Zhang, Y. Hua, L. Liu, G. Niu, G. Zhang, J. Tang and X. Tao, Sci. China Mater., 2021, 64, 1427–1436 CrossRef CAS.
- Z. Jin, Z. Zhang, J. Xiu, H. Song, T. Gatti and Z. He, J. Mater. Chem. A, 2020, 8, 16166–16188 RSC.
- L. Romani, A. Speltini, C. N. Dibenedetto, A. Listorti, F. Ambrosio, E. Mosconi, A. Simbula, M. Saba, A. Profumo, P. Quadrelli, F. De Angelis and L. Malavasi, Adv. Funct. Mater., 2021, 31, 2104428 CrossRef CAS.
- F. Wei, F. Brivio, Y. Wu, S. Sun, P. D. Bristowe and A. K. Cheetham, J. Mater. Chem. C, 2018, 6, 3573–3577 RSC.
- S. Sun, N. T. P. Hartono, Z. Ren, F. Oviedo, A. M. Buscemi, M. Layurova, D. X. Chen, T. Ogunfunmi, J. Thapa, S. Ramasamy, C. Settens, B. L. DeCost, A. G. Kusne, Z. Liu, S. I. P. Tian, I. M. Peters, J.-P. Correa-Baena and T. Buonassisi, Joule, 2019, 3, 1437–1451 CrossRef CAS.
- M. T. Sirtl, R. Hooijer, M. Armer, F. G. Ebadi, M. Mohammadi, C. Maheu, A. Weis, B. V. van Gorkom, S. Häringer, R. A. J. Janssen, T. Mayer, V. Dyakonov, W. Tress and T. Bein, Adv. Energy Mater., 2022, 12, 2103215 CrossRef CAS.
- O. Akinbami, R. Moepya, G. N. Ngubeni, P. Tetyana, K. P. Mubiayi, M. J. Moloto and N. Moloto, J. Photochem. Photobiol. Chem., 2021, 419, 113460 CrossRef CAS.
- J.-P. Correa-Baena, L. Nienhaus, R. C. Kurchin, S. S. Shin, S. Wieghold, N. T. P. Hartono, M. Layurova, N. D. Klein, J. R. Poindexter, A. Polizzotti, S. Sun, M. G. Bawendi and T. Buonassisi, Chem. Mater., 2018, 30, 3734–3742 CrossRef CAS.
- J.-Y. Gu, G. Yan, Y. Lian, Q. Mu, H. Jin, Z. Zhang, Z. Deng and Y. Peng, RSC Adv., 2018, 8, 25802–25807 RSC.
- S.-Y. Kim, Y. Yun, S. Shin, J.-H. Lee, Y.-W. Heo and S. Lee, Scr. Mater., 2019, 166, 107–111 CrossRef CAS.
- C. J. Krajewska, S. R. Kavanagh, L. Zhang, D. J. Kubicki, K. Dey, K. Galkowski, C. P. Grey, S. D. Stranks, A. Walsh, D. O. Scanlon and R. G. Palgrave, Chem. Sci., 2021, 12, 14686–14699 RSC.
- A. Elattar, L. Kobera, J. Kangsabanik, H. Suzuki, S. Abbrent, T. Nishikawa, K. S. Thygesen, J. Brus and Y. Hayashi, J. Mater. Chem. C, 2022, 10, 12863–12872 RSC.
- N. K. Tailor, S. Mishra, T. Sharma, A. K. De and S. Satapathi, J. Phys. Chem. C, 2021, 125, 9891–9898 CrossRef CAS.
- A. J. Lehner, D. H. Fabini, H. A. Evans, C.-A. Hébert, S. R. Smock, J. Hu, H. Wang, J. W. Zwanziger, M. L. Chabinyc and R. Seshadri, Chem. Mater., 2015, 27, 7137–7148 CrossRef CAS.
- H. X. Zhu, X. H. Wang and G. C. Zhuang, Appl. Phys. A, 2019, 125, 1–10 CrossRef.
- Z. Zhang, L. Ren, H. Yan, S. Guo, S. Wang, M. Wang and K. Jin, J. Phys. Chem. C, 2017, 121, 17436–17441 CrossRef CAS.
- L. Zhang, K. Wang and B. Zou, ChemSusChem, 2019, 12, 1612–1630 CrossRef CAS PubMed.
- Z. Tan, J. Li, C. Zhang, Z. Li, Q. Hu, Z. Xiao, T. Kamiya, H. Hosono, G. Niu, E. Lifshitz, Y. Cheng and J. Tang, Adv. Funct. Mater., 2018, 28, 1801131 CrossRef.
- N. Ding, D. Zhou, G. Pan, W. Xu, X. Chen, D. Li, X. Zhang, J. Zhu, Y. Ji and H. Song, ACS Sustain. Chem. Eng., 2019, 7, 8397–8404 CrossRef CAS.
- C. Zuo and L. Ding, Angew. Chem., Int. Ed., 2017, 56, 6528–6532 CrossRef CAS.
- S. Ghosh and B. Pradhan, ChemNanoMat, 2019, 5, 300–312 CrossRef CAS.
- M. Shi, B. Yang, S. Liu, R. Zhang, K. Han, C. Li and R. Li, Energy Mater. Adv., 2022, 2022, 1–11 Search PubMed.
- Z. Ji, Y. Liu, W. Li, C. Zhao and W. Mai, Sci. Bull., 2020, 65, 1371–1379 CrossRef CAS PubMed.
- M. T. Kovsarnechan, J. Roziere, D. Mascherpa-Corral and I. inotg, Nucl. Chem., 1978, 40, 2009–2011 CrossRef CAS.
- S. Wieghold, A. S. Bieber, M. Mardani, T. Siegrist and L. Nienhaus, J. Mater. Chem. C, 2020, 8, 9714 RSC.
- S. Tang, S. Huang, G. J. Wilson and A. Ho-Baillie, Trends Chem., 2020, 2, 638–653 CrossRef CAS.
- S. Dai, X. Gan, K. Li, Q. Huang, L. Guo and H. Liu, Phys. Chem. Chem. Phys., 2023, 25, 30993 RSC.
- M. Roy, S. Ghorui, Bhawna, J. Kangsabanik, R. Yadav, A. Alam and M. Aslam, J. Phys. Chem. C, 2020, 124, 19484–19491 CrossRef CAS.
- M. N. Tran, I. J. Cleveland, G. A. Pustorino and E. S. Aydil, J. Mater. Chem. A, 2021, 9, 13026 RSC.
- X. Cheng, L. Jing, Y. Yuan, S. Du, J. Zhang, X. Zhan, J. Ding, H. Yu and G. Shi, J. Phys. Chem. C, 2019, 123, 1669–1676 CrossRef CAS.
- G. Jayanthi, S. Sumathi, K. Kannan, V. Andal and S. Murugan, Adv. Mater. Sci. Eng., 2022, 2022, 1–14 CrossRef.
- Y. Hu, X. Zhang, C. Yang, J. Li and L. Wang, RSC Adv., 2019, 9, 33017–33022 RSC.
- N. Li, Q. Zhang and W. Yang, Appl. Phys. Lett., 2020, 117, 080502 CrossRef.
- Y. Zhang, J. Yin, M. R. Parida, G. H. Ahmed, J. Pan, O. M. Bakr, J.-L. Brédas and O. F. Mohammed, J. Phys. Chem. Lett., 2017, 8, 3173–3177 CrossRef CAS PubMed.
- K. K. Bass, L. Estergreen, C. N. Savory, J. Buckeridge, D. O. Scanlon, P. I. Djurovich, S. E. Bradforth, M. E. Thompson and B. C. Melot, Inorg. Chem., 2017, 56, 42–45 CrossRef CAS PubMed.
- G. Bator, J. Baran, R. Jakubas and M. Karbowiak, Vib. Spectrosc., 1998, 16, 11–20 CrossRef CAS.
- M. Y. Valakh, M. P. Lisitsa, E. Y. Peresh, O. V. Trylis and A. M. Yaremko, J. Mol. Struct., 1997, 436–437, 309–313 CrossRef CAS.
- A. García-Saiz, I. de Pedro, P. Migowski, O. Vallcorba, J. Junquera, J. A. Blanco, O. Fabelo, D. Sheptyakov, J. C. Waerenborgh, M. T. Fernández-Díaz, J. Rius, J. Dupont, J. A. Gonzalez and J. R. Fernández, Inorg. Chem., 2014, 53, 8384–8396 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra04062g |
| This journal is © The Royal Society of Chemistry 2024 |