Open Access Article
Open Access ArticleCatalytic hydrolysis of methyl mercaptan and methyl thioether on hydroxyl-modified ZrO2: a density functional theory study†
Guihua Zhanga and
Xin Song *bc
*bc
aDepartment of Mechanical Engineering, Wuhan Vocational College of Software and Engineering, Wuhan 430205, China
bFaculty of Environmental Science and Engineering, Kunming University of Science and Technology, Kunming 650500, China. E-mail: save24176@126.com; Fax: +86-0871-65926040; Tel: +86-0871-65926040
cPingxiang Huaxing Environmental Protection Engineering Technology Co., Ltd, Pingxiang, 337022, China
First published on 9th August 2024
Abstract
The purification and removal of organic sulfur from natural gas is conducive to increasing the added value of natural gas and reducing environmental pollution. In this study, the adsorption properties of methyl mercaptan (CH3SH), dimethyl sulfide (C2H6S) and H2O on the surface of hydroxyl-modified ZrO2 were investigated using density functional theory (DFT) calculations. Additionally, a reaction mechanism was proposed for hydroxyl-modified ZrO2 catalyzing the hydrolysis of CH3SH and C2H6S. The chemisorption of H2O molecules on the catalyst surface is attributed to H–O and H–Zr bonds. The chemisorption of CH3SH and C2H6S on the catalyst surface is attributed to Zr–S bonds. Competitive adsorption between the three gases exists only between CH3SH and C2H6S. It reveals the water-resistant properties of hydroxyl-modified ZrO2 in desulfurization. The adsorption energies of the three gas molecules on the hydroxyl-modified ZrO2 surface are in the order of CH3SH − (Zr) > C2H6S − (Zr) > H2O − (OH). The natural hydrolysis of CH3SH and C2H6S is a heat-absorbing process that cannot occur spontaneously. The rate-determining step for CH3SH catalytic hydrolysis is the formation of CH3O. The fracture of CH3SHO is the rate-determining step for C2H6S catalytic hydrolysis. The depletion of the surface hydroxyl groups can be replenished by the dissociation of H2O molecules. Hydroxyl-modified ZrO2 facilitated the hydrolysis process of CH3SH to a greater extent than that of C2H6S. This study provides theoretical guidance for industrial applications and the design of hydroxyl-containing hydrolysis catalysts.
1. Introduction
In light of the imperative to sustainably develop the global climate and environment, the energy structure is undergoing continual optimization, with natural gas assuming an increasingly pivotal role in the global primary energy mix. Sulfides such as hydrogen sulfide (H2S), carbonyl sulfide (COS), methyl mercaptan (CH3SH) and dimethyl sulfide (C2H6S) are common harmful impurities in natural gas, which not only reduce the value of natural gas utilization but also cause corrosion of equipment and environmental pollution.1,2 Therefore, the purification of sulfur-containing impurities in natural gas is conducive to environmental protection and effective utilization of natural gas. The purification technologies for H2S and COS are relatively mature, so the key to natural gas desulfurization is the purification of CH3SH and C2H6S.3The removal of CH3SH and C2H6S from natural gas is more effectively and stably accomplished using dry methods such as adsorption, catalytic oxidation, catalytic decomposition, and catalytic hydrolysis.4–7 Among these methods, the catalytic hydrolysis method has been receiving increasing attention due to its low secondary pollution and high value of by-products. Zhang et al. investigated the role of H2O molecules on CH3SH removal over Cu/C-PAN catalyst.6 It was demonstrated that water promotes the adsorption of CH3SH at low temperatures and its hydrolysis at high temperatures. While H2S is the primary hydrolysis product, the reaction temperature and active components influence the type and quantity of the final product. This can diminish the desulfurization stability of the catalyst. Metal oxides, such as ZrO2, are frequently employed in the purification of gaseous pollutants, exhibiting high reaction stability and resistance to sulfur poisoning.8–10 This property is conducive to the stability of the catalytic hydrolysis process of organic sulfur and the prolongation of catalyst service life.11 Therefore, ZrO2 can be considered a potential hydrolysis catalyst for the purification of organic sulfur. Furthermore, hydroxyl groups play a pivotal role in the hydrolysis of organic sulfur. These groups are primarily derived from the dissociation of H2O molecules and the metal-hydroxyl functional groups on the surface.12 Song et al. investigated the mechanism of the COS hydrolysis reaction over a MgAlCeOx hydrotalcite-like catalyst.13 The presence of hydroxyl M–OH on the catalyst surface enhanced the hydrolysis process while providing more sites for organic sulfur adsorption. Therefore, hydroxylation modification on the ZrO2 surface can be employed to enhance the hydrolysis process of organic sulfur. However, studies on the catalytic hydrolysis of CH3SH and C2H6S using hydroxylated ZrO2 are lacking, and the reaction mechanism has not been clarified. Therefore, it is necessary to propose a catalytic hydrolysis reaction mechanism of CH3SH and C2H6S on hydroxyl ZrO2 through theoretical studies.
This work presents a systematic investigation of the competitive adsorption process of CH3SH and C2H6S, the catalytic hydrolysis reaction mechanism, and the catalytic role of surface hydroxyls. The investigation is conducted using a density-functional theory (DFT) approach. Additionally, the effects of ZrO2 and surface hydroxyl groups on the hydrolysis reaction pathway are presented.
2. Computational method
ZrO2 has been identified as a suitable catalyst for hydrolysis reactions in experimental and theoretical studies.14 Additionally, the ZrO2(100) surface exhibits suitability for surface hydroxylation modification.15 Therefore, in this study, cubic ZrO2 was employed to construct hydroxyl-modified ZrO2. The geometries and energies for the catalyst (hydroxyl-modified ZrO2), reactants (CH3SH, C2H6S, H2O), intermediates (IM), products, and transition states (TS) were calculated using Material Studio 2017 with the Dmol3 module.16,17 The GGA/PBE method employed herein provides a precise description of the interactions between electrons and encompasses a diverse range of material systems, thereby making it a highly prevalent choice in studies involving metal-containing catalytic systems.18 The calculation work employed the GGA/PBE method with a DNP 4.4 basis and spin-polarized set.19 The spin was set to unrestricted and the total charge was set to 0. In order to calibrate the energy, the basis set superposition error (BSSE) was taken into account in the calculation. To accurately describe weak interactions, dispersion-corrected density functional theory (DFT-D3 correction) was introduced.20,21 The SCF, energy, gradient, and displacement convergence tolerances were set to 1.0 × 10−6 Hartree (Ha), 1.0 × 10−5 Ha, 2.0 × 10−3 Ha Å−1 and 5.0 × 10−3 Å, respectively. The cubic ZrO2 was established according to the Crystallography Open Database. The space group of cubic ZrO2 was FM-3M, and the lattice parameters are a = b = c = 5.070 Å. In this study, a five-layer ZrO2 (100) surface with a p (3 × 3) supercell size was used. In order to construct the surface hydroxyl group, the ZrO2(100) surface layer is composed of Zr atoms. The vacuum layer is 15 Å thick and all atoms are in a relaxed state. Additionally, hydroxyl groups were introduced and immobilised on the surface of Zr atoms to simulate hydroxylated ZrO2 surfaces. Therefore, a surface hydroxyl structure of Zr–OH is constructed. The aforementioned parameters guarantee the accuracy of the resulting calculations.18 The transition states were searched, optimised and confirmed using the linear synchronous transit/quadratic synchronous transit/conjugate gradient (LST/QST/CG) calculation method.22 The adsorption energies of the binary and ternary systems were calculated using eqn (1) and (2), respectively. EA, EB and EC represented the energy of the catalysts and the gas molecules. EBSSE represented the energy of the basis set superposition error.| Eads = EAB − EA − EB + EBSSE | (1) |
| Eads = EABC − EA − EB − EC + EBSSE | (2) |
3. Results and discussion
3.1 Adsorption of CH3SH, C2H6S and H2O over hydroxyl-modified ZrO2
The adsorption properties of single and multimolecular gases on hydroxylated modified ZrO2 surfaces were investigated. To elucidate the influence of hydroxyl groups on the adsorption process, two distinct adsorption sites were selected for the study: the Zr top site distant from Zr–OH (denoted as –(Zr)) and the hydroxyl site proximal to Zr–OH (denoted as –(OH)). The optimized adsorption configurations are depicted in Fig. 1, while the corresponding adsorption energies are presented in Table 1. The adsorption energies of the two gas molecules when simultaneously adsorbed at different sites were examined, and the corresponding adsorption configurations and adsorption energies are shown in Fig. S1 and Table S1,† respectively. Following structural optimization based on the calculated adsorption energies, it was determined that adsorption configurations (i), (iv), and (vii) represent the most stable configurations for different bimolecular systems. The current adsorption site was also identified as the global minimum energy point by the sorption module. Consequently, these three conformations were employed in Fig. 1 for comparison with the unimolecular adsorption conformations. | ||
| Fig. 1 Optimised adsorption configuration for CH3SH, C2H6S and H2O over hydroxyl-modified ZrO2 (bond length in Å). | ||
| Adsorptive geometries | Adsorption energy (kJ mol−1) |
|---|---|
| H2O − (Zr) | −88.11 |
| H2O − (OH) | −123.51 |
| CH3SH − (Zr) | −192.68 |
| CH3SH − (OH) | −165.83 |
| C2H6S − (Zr) | −164.89 |
| C2H6S − (OH) | −114.63 |
| H2O + CH3SH | −219.78 |
| H2O + C2H6S | −173.37 |
| CH3SH + C2H6S | −148.04 |
The adsorption energy results indicated that the adsorption of H2O, CH3SH, and C2H6S on the top site of Zr and the hydroxyl site was generated by chemisorption. The H2O molecules exhibited a tendency to adsorb in close proximity to the Zr–OH, resulting in the formation of H–O and H–Zr bond interactions. The CH3SH and C2H6S molecules exhibited a tendency to adsorb in close proximity to the Zr top site, resulting in the Zr–S bond interactions. This suggests that there is no competitive adsorption relationship between H2O and CH3SH/C2H6S, but that there is competitive adsorption between CH3SH and C2H6S. The adsorption energies of the three gas molecules on the hydroxyl-modified ZrO2 surface are in the order of CH3SH-(Zr) > C2H6S-(Zr) > H2O-(OH). This indicates that CH3SH can preferentially adsorb on the Zr top site. When CH3SH, C2H6S and H2O are adsorbed simultaneously on the hydroxyl-modified ZrO2 surface, the adsorption energies are in the order of H2O + CH3SH > H2O + C2H6S > CH3SH + C2H6S. This indicated that H2O molecules are more capable of forming a two-gas molecularly stabilized adsorption structure with organosulfur molecules. The adsorption effects were attributed to the interaction between Zr and S/O atoms. Moreover, the adsorption energies of H2O and CH3SH/C2H6S are both greater than that of a single gas molecule when they are co-adsorbed on the hydroxyl-modified ZrO2 surface. The results indicated that the presence of the H2O molecule enhances and promotes the adsorption of CH3SH/C2H6S. Conversely, the adsorption energies are lower than those of a single gas when co-adsorption of CH3SH and C2H6S occurs. This is due to the fact that the adsorption of both CH3SH and C2H6S on the ZrO2 surface is caused by Zr–S interactions, resulting in competitive adsorption at the Zr top site. This results in a reduction in the stability of the adsorption process upon co-adsorption. This further substantiates the competitive adsorption relationship previously discussed. Consequently, the co-adsorption of H2O and CH3SH was observed to occur most stably on the catalyst surface.
3.2 Catalysis hydrolysis routes of CH3SH over hydroxyl-modified ZrO2
In order to investigate the catalytic hydrolysis of CH3SH by hydroxyl groups and ZrO2, the natural and catalytic hydrolysis routes of CH3SH were investigated. The specific reaction routes are shown in Fig. 2. Route I and Route II are natural hydrolysis reaction processes. Route III is a hydrolysis reaction process involving hydroxyl groups, and Route IV is a hydrolysis reaction process involving only ZrO2. The atomic migration process in Route IV was maintained consistent with Route II, which was employed to assess the catalytic impact of ZrO2. During natural hydrolysis, the intermediate (IM) and transition state (TS) structures in Route I and Route II were optimized and presented in Fig. 3. During catalytic hydrolysis, the optimized intermediates and transition states of Route III are shown in Fig. 4.In Route I, CH3SH and H2O first undergo physical adsorption to form IM1, releasing 38.68 kJ mol−1 of energy. After the breaking of the C–S bond in CH3SH and the O–H bond in H2O, TS1a is formed. This process requires overcoming a significant reaction energy barrier (380.69 kJ mol−1). The combination of the hydroxyl group and CH3 in TS1a, and the combination of the H atom and HS, results in the generation of Product-a (CH3OH and H2S). The relative energy of Product-a is 8.19 kJ mol−1, which suggests that Route I is a heat-absorbing process. Therefore, Route I cannot occur spontaneously.
In Route II, C–S and O–H bonds are initially broken, with H atoms from the dissociation of the H2O molecule migrating to CH3 and the hydroxyl group migrating to HS, forming TS1b. This step requires the consumption of 292.04 kJ mol−1 of energy to be realised. With the combination of C–H and O–S bonds, IM2b was generated. Subsequently, the H atom on the O–H bond in IM2b undergoes an H-transfer. This results in the detachment of the H atom from the O atom and its migration to the S atom, forming H2SO and generation of IM3b. This process necessitates the overcoming of a reaction energy barrier of 273.61 kJ mol−1 (from TS2b). Subsequently, the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond in HSHO is broken, and the O atom migrates to the vicinity of CH4, resulting in the breakage of the C–H bond and the production of TS3b. Furthermore, the O atom combines with the H atom and CH3 to form CH3OH. The process of IM3b to Product-b requires the consumption of 147.86 kJ mol−1 of energy. The reaction energy barriers of the steps in Route II indicate that the reaction rate of Route II is affected by TS1b. Therefore, the production of HSOH is the rate-determining step of Route II. Compared to Route I, Route II has a lower maximum reaction energy barrier, which suggests that Route II is more likely to occur.
O bond in HSHO is broken, and the O atom migrates to the vicinity of CH4, resulting in the breakage of the C–H bond and the production of TS3b. Furthermore, the O atom combines with the H atom and CH3 to form CH3OH. The process of IM3b to Product-b requires the consumption of 147.86 kJ mol−1 of energy. The reaction energy barriers of the steps in Route II indicate that the reaction rate of Route II is affected by TS1b. Therefore, the production of HSOH is the rate-determining step of Route II. Compared to Route I, Route II has a lower maximum reaction energy barrier, which suggests that Route II is more likely to occur.
In Route III, CH3SH and H2O first adsorb around the hydroxyl group to form the stable adsorption structure IM4, which is realized by chemisorption. Next, the C–S bond in CH3SH and the O–H bond in Zr–OH are broken to form TS4, which consumes 118.43 kJ mol−1 energy. With the formation of S–H and C–O bonds, IM5 is produced. In this step, the H2O molecule remains intact, and only CH3SH is converted to H2S through the surface hydroxyl groups. Then, the H2O molecule is dissociated into H atoms and hydroxyl groups, while the Zr–O bond is broken. Product-c (CH3OH and H2S) is generated as the hydroxyl group binds to the Zr atom and the H atom migrates to CH3O. This step necessitates overcoming the 86.28 kJ mol−1 reaction energy barrier for the generation of TS5. The maximum reaction energy barrier in Route III is the process of TS4 production, which suggests that CH3O formation is the rate-determining step. In comparison to Route II, the hydroxyl group significantly reduces the maximum reaction energy barrier during hydrolysis. Furthermore, the hydroxyl groups on the surface are capable of facilitating H-transfer, which is essential for the generation of H2S, as well as the provision of free O atoms for the formation of C–O bonds. The depletion of surface hydroxyl groups can be replenished by the dissociation of H2O molecules, which effectively enhances the role of H2O molecules in the hydrolysis reaction.
The reaction processes of Route IV and Route II are analogous. The change in reaction energy barriers indicates that ZrO2 facilitates the hydrolysis process. In contrast to Route II, the participation of ZrO2 in the hydrolysis reaction significantly reduces the reaction energy barrier of the HSOH generation step, resulting in the rate-determining step changing to HSHO generation. A comparison of Route III and Route IV revealed that ZrO2 and hydroxyl exhibited disparate catalytic effects. The introduction of hydroxyl was found to be capable of further reducing the maximum reaction energy barrier.
3.3 Catalysis hydrolysis routes of C2H6S over hydroxyl-modified ZrO2
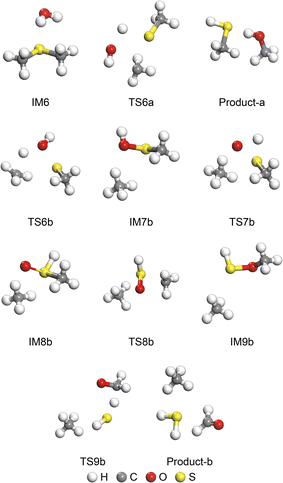
The natural and catalytic hydrolysis routes of C2H6S were investigated to ascertain the catalytic effect of hydroxyl groups and ZrO2. The four reaction routes are depicted in Fig. 5. Route V and Route VI represent natural hydrolysis reaction processes. Route VII is a hydrolysis reaction process involving hydroxyl groups, and Route VIII is a hydrolysis reaction process involving only ZrO2. The atomic migration process in Route VIII was maintained consistent with that in Route VI, which was employed to assess the catalytic impact of ZrO2. The optimized intermediate (IM) and transition state (TS) structures in Route V and Route VI are illustrated in Fig. 6, and the optimized intermediates and transition states of Route VII are depicted in Fig. 7.In Route V, C2H6S and H2O first undergo physical adsorption to form IM6, releasing 25.46 kJ mol−1 of energy. This adsorption is weaker than that between H2O and CH3SH. After the breaking of the C–S bond in C2H6S and the O–H bond in H2O, TS6a is formed. This process necessitates the overcoming of a reaction energy barrier of 66.77 kJ mol−1. The combination of the hydroxyl group and CH3 in TS6a, along with the combination of the H atom and CH3S, results in the generation of Product-a (CH3OH and CH3SH). The relative energy of Product-a is 26.68 kJ mol−1, which indicates that Route V is a heat-absorbing process. Consequently, it can be concluded that Route V is not a spontaneous process. Subsequent to this, CH3SH can undergo further hydrolysis reactions via Route II.
In Route VI, the C–S and O–H bonds are initially broken, with the H atoms from the dissociation of the H2O molecule migrating to CH3 and the hydroxyl group migrating to CH3S, forming TS6b. This step requires the consumption of 273.10 kJ mol−1 of energy to be realised. The combination of the C–H and O–S bonds resulted in the generation of IM7b (CH4 and CH3SOH). Subsequently, CH3SOH in IM7b underwent a H-transfer. The H atom on the O–H bond detached from the O atom and migrated to the S atom to form CH3SHO, leading to the generation of IM8b. This process necessitates the overcoming of a reaction energy barrier of 262.37 kJ mol−1 (from TS7b). Consequently, the C–S bond in CH3SHO is broken, and the C–O bond is generated, thus forming CH3OSH. The process of IM8b to IM9b requires the consumption of 172.62 kJ mol−1 of energy. Subsequently, TS9b is generated as the C–H and S–O bonds in CH3OSH are broken. The hydrolysis reaction is completed when the H atom completes its migration from the C–H bond to the S–H bond, and the final hydrolysis products are CH4, H2S, and HCHO. The reaction energy barriers of the steps in Route VI indicate that the reaction rate of Route VI is affected by TS6b. Therefore, the production of CH3SOH is the rate-determining step of Route VI. Compared to Route V + II, Route VI has a lower maximum reaction energy barrier, which suggests that Route VI is more likely to occur.
In Route VII, C2H6S and H2O initially adsorb around the hydroxyl group to form the stable adsorption structure IM10, which is achieved through chemisorption. Subsequently, the C–S bond in C2H6S and the O–H bond in Zr–OH are broken to form TS10, resulting in the consumption of 104.32 kJ mol−1 energy. The formation of the C–H and S–O bonds results in the production of IM11. In this step, the H2O molecule remains intact, with only C2H6S undergoing conversion to CH3SO through the surface hydroxyl groups. Subsequently, the H2O molecule is dissociated into H atoms and hydroxyl groups, while S–H is formed. The generation of IM12 occurs through TS11, with a reaction energy barrier of 94.92 kJ mol−1. The breaking of C–S and C–H bonds in CH3SO, which is accompanied by the migration of H atoms, results in the generation of IM13. This process requires overcoming a 127.21 kJ mol−1 reaction energy barrier. The hydrolysis reaction is completed when the O atom completes its migration from the Zr–O bond to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond, and the final hydrolysis products are CH4, H2S, and HCHO. The maximum reaction energy barrier in Route VII is the process of TS12 production, which suggests that the fracture of CH3SHO is the rate-determining step. Compared to Route VI, the hydroxyl group greatly reduces the maximum reaction energy barrier during hydrolysis. In addition, the hydroxyl groups on the surface are also able to provide an H-transfer for the generation of CH4 as well as free O atoms for the formation of S–O bonds. The depletion of surface hydroxyl groups can be replenished by the dissociation of H2O molecules, which effectively enhances the role of H2O molecules in the hydrolysis reaction.
O bond, and the final hydrolysis products are CH4, H2S, and HCHO. The maximum reaction energy barrier in Route VII is the process of TS12 production, which suggests that the fracture of CH3SHO is the rate-determining step. Compared to Route VI, the hydroxyl group greatly reduces the maximum reaction energy barrier during hydrolysis. In addition, the hydroxyl groups on the surface are also able to provide an H-transfer for the generation of CH4 as well as free O atoms for the formation of S–O bonds. The depletion of surface hydroxyl groups can be replenished by the dissociation of H2O molecules, which effectively enhances the role of H2O molecules in the hydrolysis reaction.
The reaction processes of Routes VIII and VI are analogous. According to the alteration of reaction energy barriers, ZrO2 facilitates the entire hydrolysis process. Nevertheless, ZrO2 did not alter the rate-determining step of the hydrolysis reaction of C2H6S. When comparing Routes VII and VIII, ZrO2 and hydroxyl exhibited disparate different catalytic effects, and the introduction of hydroxyl was capable of further reducing the maximum reaction energy barrier.
The co-promotion of ZrO2 and hydroxyl groups was found to reduce the maximum reaction energy barriers of CH3SH by 173.61 kJ mol−1 and 145.89 kJ mol−1, respectively. Furthermore, hydroxyl-modified ZrO2 was observed to facilitate the hydrolysis process of CH3SH to a greater extent than that of C2H6S.
3.4 Catalysis reaction mechanism of CH3SH and C2H6S over hydroxyl-modified ZrO2
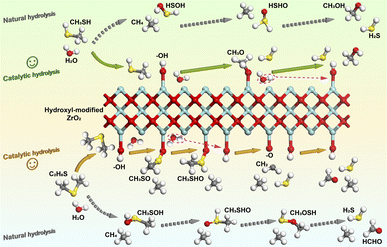
Based on the aforementioned findings, hydroxyl-modified ZrO2 has been demonstrated to enhance and modify the hydrolysis reaction process of CH3SH and C2H6S. Consequently, a reaction mechanism for the hydrolysis of CH3SH and C2H6S on the hydroxyl-modified ZrO2 surface has been proposed and depicted in Fig. 8.As illustrated in Fig. 8, the hydroxyl group on the ZrO2 surface facilitated the production of H2S and the formation of C–O bonds by providing free H atoms and free O atoms. Furthermore, the surface-free O atoms can effectively immobilize the intermediates CH3 and CH3S. The hydroxyl groups consumed on the surface can be replenished by the dissociation of H2O molecules, maintaining the continuity of the hydrolysis reaction involving the hydroxyl groups. The hydrolysis of CH3SH on the surface of hydroxyl-modified ZrO2 proceeds as follows: CH3SH (+H2O) → CH3O (+H2S) → CH3OH (+H2S). The hydrolysis process of C2H6S on the hydroxyl-modified ZrO2 surface proceeds as follows: C2H6S (+H2O) → CH3SO (+H2O + CH4) → CH3SHO (+CH4) → H2S + CH2 (+CH4) → HCHO (+CH4 + H2S).
4. Conclusions
This work investigates the competitive adsorption process of CH3SH, C2H6S, and H2O on the surface of hydroxyl-modified ZrO2 using a density functional theory approach. Additionally, a detailed catalytic hydrolysis reaction mechanism is proposed. The adsorption process of CH3SH, C2H6S, and H2O on the surface of hydroxyl-modified ZrO2 is attributed to chemisorption, with the Zr–S, H–O and H–Zr bonds playing a role. There is no competitive adsorption relationship between H2O and CH3SH/C2H6S, but there is competitive adsorption between CH3SH and C2H6S. It reveals the water-resistant properties of hydroxyl-modified ZrO2 in desulfurization. The adsorption of CH3SH on the Zr top site is favored over that of C2H6S. The hydrolysis of CH3SH and C2H6S is a heat-absorbing process that cannot occur spontaneously. The formation of CH3O and the fracture of CH3SHO are the rate-determining steps for CH3SH and C2H6S catalytic hydrolysis, respectively. The hydroxyl groups consumed on the surface can be replenished by the dissociation of H2O molecules. The hydroxyl group on the ZrO2 surface facilitated the production of H2S and the formation of C–O bonds by providing free H atoms and free O atoms, enhancing the hydrolysis of CH3SH and C2H6S. It provides theoretical guidance for industrial applications and the design of hydroxyl-containing hydrolysis catalysts.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Xin Song contributed to the study's conception and design. Calculation, data collection and analysis were performed by Xin Song and Guihua Zhang. Guihua Zhang wrote the main manuscript text. All authors read and approved the final manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (22006058).Notes and references
- P. J. Liang, D. C. Cheng, H. Y. Xu, C. Y. Xu, G. W. Chu, H. K. Zou, B. C. Sun and J. F. Chen, Sep. Purif. Technol., 2024, 346, 127374 CrossRef CAS.
- W. Jiang, Z. Li, X. Kang, L. Luo, Y. Zhou, Q. Liu, K. Liu, X. Ji and G. He, Gas Sci. Eng., 2024, 123, 205243 CrossRef CAS.
- G. Zhang, Q. Zhu, W. Zhang, Y. Zheng, Y. Cao, S. Liang, Y. Xiao, F. Liu and L. Jiang, Inorg. Chem., 2022, 61, 6083 CrossRef CAS PubMed.
- H. Zhang, X. Liu, H. Xiao, F. Shao, T. Yan, D. Cheng, L. Han and D. Zhang, Chem. Eng. J., 2024, 485, 150003 CrossRef CAS.
- X. Cao, T. Ai, Z. Xu, J. Lu, D. Chen, D. He, J. Liu, R. Tian, Y. Zhao and Y. Luo, Sep. Purif. Technol., 2023, 307, 122742 CrossRef CAS.
- Y. Zhang, K. Li, X. Sun, X. Song, F. Wang, C. Wang, P. Ning and H. He, Appl. Surf. Sci., 2021, 567, 150851 CrossRef CAS.
- T. Zhong, S. Tang, W. Huang, W. Liu, H. Zhao, L. Hu, S. Tian and C. He, Appl. Catal., B, 2024, 343, 123476 CrossRef CAS.
- R. M. Mohamed and A. A. Ismail, Ceram. Int., 2022, 48, 12592 CrossRef CAS.
- B. M. Alajmi, A. S. Basaleh, A. A. Ismail and R. M. Mohamed, Surf. Interfaces, 2023, 39, 102899 CrossRef CAS.
- A. I. Ayesh and M. D. El-Muraikhi, J. Mol. Model., 2022, 29, 15 CrossRef PubMed.
- N. Liu, P. Ning, X. Sun, C. Wang, X. Song, F. Wang and K. Li, Sep. Purif. Technol., 2021, 259, 118205 CrossRef CAS.
- X. Song, P. Ning, C. Wang, K. Li, L. Tang, X. Sun and H. Ruan, Chem. Eng. J., 2017, 314, 418 CrossRef CAS.
- X. Song, L. Sun, H. Guo, K. Li, X. Sun, C. Wang and P. Ning, ACS Omega, 2019, 4, 7122 CrossRef CAS PubMed.
- X. Sun, W. Huang, H. Xu, Z. Qu, J. Wu and N. Yan, Sep. Purif. Technol., 2023, 310, 123194 CrossRef CAS.
- E. Osei-Agyemang, A. Dasan, R. Lucas, S. Foucaud, J. P aul, S. Cristol and E. Laborde, Appl. Surf. Sci., 2022, 576, 151622 CrossRef CAS.
- B. Delley, J. Chem. Phys., 2000, 113, 7756 CrossRef CAS.
- L. Sun, P. Ning, X. Zhao, X. Song, K. Li, C. Wang, X. Sun and L. Jia, Chem. Eng. J., 2021, 412, 128752 CrossRef CAS.
- M. Ali, Z. Bibi, M. W. Younis and M. A. Iqbal, Inorg. Chem. Commun., 2024, 160, 111891 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- X. Song, X. Chen, L. Sun, K. Li, X. Sun, C. Wang and P. Ning, Chem. Eng. J., 2020, 399, 125764 CrossRef CAS.
- S. Wan, X. Song, X. Wang, C. Yuan, B. Wang, H. Chen, Y. Li, K. Ouyang and R. Chen, Sep. Purif. Technol., 2022, 302, 122158 CrossRef CAS.
- L. Jia, X. Song, L. Sun, S. Zhang, K. Li and P. Ning, Chem. Eng. J., 2022, 429, 132376 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra03801k |
| This journal is © The Royal Society of Chemistry 2024 |