Open Access Article
Open Access ArticleInteraction mechanism of Al2O3 abrasive in tantalum chemical mechanical polishing
Rui Leia,
Liang Jiang *a,
Honglin Zhanga,
Yushan Chena,
Jiaxin Zhenga,
Junhui Sun
*a,
Honglin Zhanga,
Yushan Chena,
Jiaxin Zhenga,
Junhui Sun ab,
Qijian Zhao*c and
Linmao Qian
ab,
Qijian Zhao*c and
Linmao Qian a
a
aTribology Research Institute, State Key Laboratory of Rail Transit Vehicle System, Southwest Jiaotong University, Chengdu 610031, China. E-mail: jiangliang@swjtu.edu.cn
bState Key Laboratory of Solid Lubrication, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou 730000, China
cInstitute of Machinery Manufacturing Technology, China Academy of Engineering Physics, Mianyang 621900, China. E-mail: zqj@zju.edu.cn
First published on 18th September 2024
Abstract
Al2O3 abrasive is expected to enhance chemical mechanical polishing (CMP) efficiency compared to the SiO2 abrasive. However, Al2O3 powder has dispersion issues and the material removal mechanism by Al2O3 remains unclear. This study investigated the role of Al2O3 abrasive in the tantalum CMP. It is revealed that (NaPO3)6 can effectively disperse Al2O3 powder in water. PO3− improves the stability while Na+ deteriorates it. The total Na+ concentration should be lower than the turning point to attain high stability. With stable Al2O3-containing slurries, a relatively high material removal rate of tantalum can be obtained at an alkaline pH. The characterization results indicate that the Ta element can be adsorbed on Al2O3 probably due to the chemical interaction between Al2O3 and the tantalum surface. Moreover, the Al2O3 microsphere tip starts to remove tantalum at 0.48 GPa, which is much lower than the yield strength of the tantalum surface film. For the mechanism, tantalum can be oxidized by H2O2 at alkaline pH. When Al2O3 presses and slides on the tantalum surface, tribochemical reactions occur, forming a chemical bond of Al–O–Ta at the interface. As Al2O3 moves, the bond is stretched and tantalum is detached. The findings provide mechanistic insight into Al2O3 abrasive in CMP.
1 Introduction
Tantalum/tantalum nitride has been widely used as the barrier layer for copper interconnect in integrated circuits (IC) because it can prevent copper diffusion and has good adhesion with copper and the dielectric.1 In the dual-damascene process for fabricating copper interconnect, tantalum/tantalum nitride will be polished in the P3 stage of chemical mechanical polishing (CMP) to achieve local and global planarization, enabling the subsequent photolithography.2 With the rapid development of IC, it is important to continuously improve the CMP performance of tantalum (tantalum nitride is quite like tantalum in terms of CMP) such as the CMP efficiency.Tantalum CMP can be considered a nanoscale corrosive wear process, which is mainly affected by the interaction between the tantalum surface and abrasive.3–5 Therefore, abrasive plays a critical role in the tantalum CMP. At present, SiO2 is primarily used in the tantalum CMP. Li et al.4,6 used several types of SiO2 with different specific surfaces to polish tantalum. It was revealed that the tantalum material removal rate (MRR) increases with the specific surface of SiO2 increasing. This may be because a larger specific surface possesses a higher Si–OH content on the SiO2 surface, resulting in more intensive chemical interaction between the tantalum surface and SiO2. Li et al.7 used SiO2 with different hydroxyl contents to polish tantalum. The experimental results showed that the tantalum MRR is linearly proportional to the hydroxyl content on the SiO2 surface. In addition, a large amount of tantalum is found on the SiO2 surface after polishing using atomic absorption spectrophotometry. It was concluded that a strong chemical interaction exists between tantalum and hydroxyl groups on the SiO2 surface. Vijayakumar et al.8 put forward the polishing mechanism of tantalum by SiO2. Hydroxyl groups on the SiO2 surface can react with tantalum to form Si–O–Ta bonds. As SiO2 moves, the bonds are mechanically torn, leading to the removal of tantalum.
Researchers also used Al2O3 as the abrasive in the tantalum CMP. Li et al.6 compared the polishing performance of Al2O3 and SiO2 in the tantalum CMP. The experimental results showed that Al2O3 can achieve a higher tantalum MRR than SiO2. Li et al.7 revealed that not only the hardness of the abrasive and the tantalum surface but also the hydroxyl group on the abrasive surface affects the tantalum removal. When using Al2O3 with different bulk densities to polish tantalum, Al2O3 with a higher bulk density can attain a higher tantalum MRR. This is because an increased hydroxyl content on Al2O3 results in stronger interaction between Al2O3 and the tantalum surface.
Compared to the commonly used SiO2, Al2O3 is expected to enhance the CMP efficiency further. However, the interaction mechanism between Al2O3 and the tantalum surface has not been investigated in-depth. Moreover, based on our preliminary tests, Al2O3 powder has a dispersion issue in water, hindering the development of Al2O3-containing slurries. Therefore, this study first used an effective dispersant (NaPO3)6 to disperse Al2O3 powder in ultrapure water. Then, stable Al2O3-containing slurries were prepared, and their polishing performance was studied. Afterward, transmission electron microscopy (TEM), energy dispersive spectroscopy (EDS), atomic force microscopy (AFM), and first-principles calculation were performed. Based on the experimental results, the role of Al2O3 abrasive in the tantalum CMP was revealed. The findings provide new insights into the material removal mechanism of tantalum in CMP.
2 Experimental details
Al2O3 powder (AEROXIDE Alu C, purchased from Evonik Industrial AG) was used as the abrasive. Sodium hexametaphosphate ((NaPO3)6) was used as the dispersant to disperse Al2O3 powder in ultrapure water, preparing an Al2O3 suspension.9 Specifically, (NaPO3)6 was first dissolved in ultrapure water. Then, Al2O3 powder was added under continuous stirring. Afterwards, the Al2O3 suspension was stirred for 1 h and sonicated for 15 min. Finally, if necessary, NaNO3 was added to the Al2O3 suspension to provide Na+ and control the total Na+ concentration. To characterize the long-term stability of the Al2O3 suspension, it was allowed to stand for 24 h, and then the turbidity values of the upper layer and the bottom layer of the suspension were measured with a turbidity meter (WZS-188, Shanghai Yidian Scientific Instrument Co., Ltd.). After obtaining a relatively stable Al2O3 suspension, tantalum CMP slurries were prepared by adding additional H2O2 and adjusting pH (dilute NaOH and HNO3). All the above chemical reagents were purchased from Sinopharm Chemical Reagent Co., Ltd.In the CMP experiments, a tantalum disk (50.8 mm diameter, 99.99% purity, China New Metal Materials Technology Co., Ltd.) was polished on a benchtop polisher (UNIPOL-1200S, Shenyang Kejing Auto-Instrument Co., Ltd.). Table 1 shows the CMP process conditions. During polishing, the CMP slurry was stirred continuously to maintain excellent dispersion. The weights of the tantalum disk before and after polishing were measured with a microbalance (0.01 mg readability, MSA225S, Sartorius). The MRR was calculated with the weight loss method.10 In particular, the density of tantalum is 16.68 g cm−3.11 The topographies and surface roughness Sa of the tantalum disk after polishing were measured with an optical surface profiler (SuperView W1, Chotest). The scan area was 97.9 μm × 97.9 μm. Each CMP slurry was repeated four times.
| Condition | Value |
|---|---|
| Down force | 5.0 kg set pressure displayed on the panel |
| Carrier speed | 60 rpm |
| Platen speed | 60 rpm |
| Slurry flow rate | 100 mL min−1 |
| Time of each polishing | 1 min |
| Polishing pad | Politex (Dow, conditioned for 30 s with a brush before each polishing) |
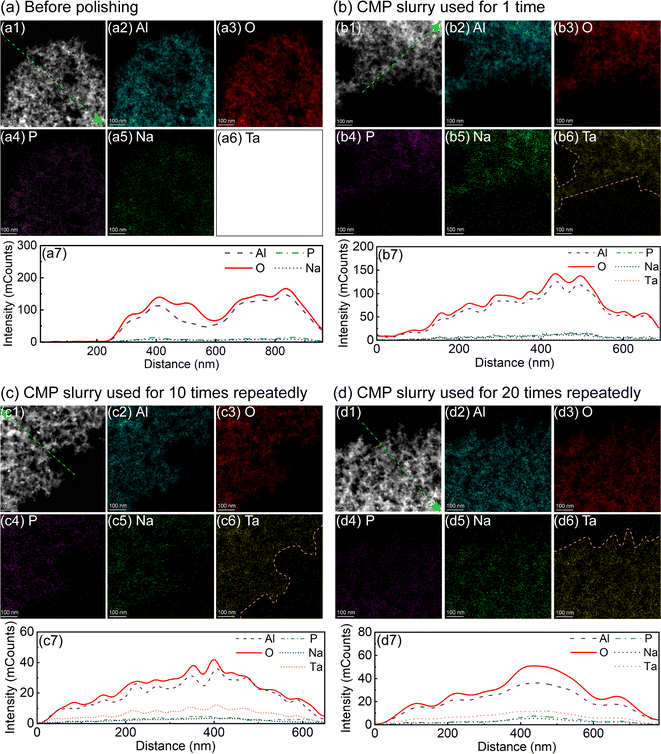
To explore the interaction between Al2O3 and tantalum, the CMP slurry was used 1 time, 10 times repeatedly and 20 times repeatedly, and then collected after polishing. The topography and compositional distribution of Al2O3 abrasive in the recycled slurry were characterized with TEM (Talos F200X, FEI) and EDS.
In order to investigate the interaction between Al2O3 and tantalum surface further, the adhesion force between an Al2O3 microsphere tip (2.5 μm nominal radius. The AFM probe was purchased from Novascan Technologies, Inc. It had a spring constant of 14.08 N m−1 calibrated by the thermal noise method12) and the tantalum surface was measured in the designated solution on an atomic force microscope (MFP-3D, Asylum Research). The dwell time was varied. The experimental conditions were as follows: 1 μm distance, 500 nm s−1 velocity, 0.5 μN applied load, and 0 s/5 s/10 s dwell time.
Moreover, AFM wear experiments were carried out to simulate the tantalum material removal process by the Al2O3 abrasive in CMP on another atomic force microscope (E-sweep, Hitachi). An Al2O3 microsphere tip was rubbed against the tantalum surface in the designated solution. The applied load was varied. The experimental conditions were as follows: 0.1 μN/0.2 μN/0.4 μN/0.6 μN/0.8 μN applied load, 1 μm s−1 relative sliding velocity, 2 μm relative sliding distance, and 100 reciprocating sliding cycles. After the AFM wear experiments, the topographies of the wear areas on the tantalum surface were scanned with a Si3N4 probe (MSCT, Bruker) in a vacuum. Moreover, the profile of the Al2O3 microsphere tip was measured by scanning a grating sample (TGT1, NT-MDT). The scanning result exhibits a reverse image of the Al2O3 microsphere tip rather than that of the grating sample because the curvature of the microsphere tip is much larger than that of the grating spikes.
To prepare a relatively smooth tantalum surface for the above AFM experiments regarding the adhesion force and wear, a small tantalum sample (10 mm × 10 mm, cut from a complete wafer purchased from SKW, Associates, Inc.) was pre-polished, then cleaned with ultrapure water and dried. The CMP slurry contained ultrapure water, 2 wt% colloidal SiO2 (YZ8040, purchased from Shanghai YZ-Lapping Material Co., Ltd.), 0.2 wt% H2O2, and at pH 10. Fig. 1 shows the topography and the average cross-sectional profile along the A–B direction. As can be seen, after polishing, the tantalum wafer surface is very smooth, meeting the requirement of AFM experiments.
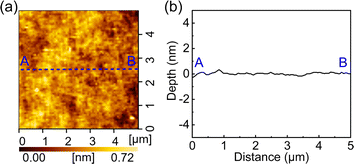 | ||
| Fig. 1 AFM measurement results of the tantalum wafer surface after polishing. (a) The topography. (b) The average cross-sectional profile along the A–B direction. | ||
To confirm the interaction between Al2O3 and tantalum, first-principles calculation based on density functional theory (DFT) was conducted using the Cambridge Sequential Total Energy Package. The exchange-correlation function was described using the generalized gradient approximation of Perdew–Burke–Ernzerhof.13 The projector-augmented wave potential was used in depicting interactions formed between electrons and ions. The Brillouin zone was sampled using Monkhorst–Pack 8 × 8 × 1 k-point grids. The calculation parameters for the plane-wave cutoff energy, total energy, and convergence tolerance force were set as 450 eV, 1 × 10−6 eV, and 0.02 eV Å−1 for accurate calculations. The spin interactions were considered, and a 15 Å vacuum layer was used to prevent periodic interactions.
3 Results and discussion
3.1 Stability of Al2O3 suspensions
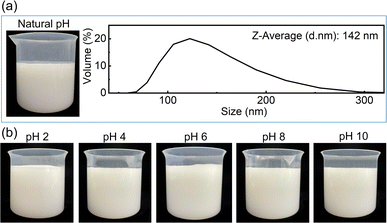
Stability is a significant indicator of the CMP slurry. Unstable CMP slurry may result in severe defects such as scratches on the workpiece surface after polishing.14 To this end, this study used (NaPO3)6 to disperse Al2O3 powder in ultrapure water. Generally, cations, such as Na+, will be introduced to the tantalum CMP slurry unavoidably, for example during adjusting the pH value. Based on the existing research, PO3− of (NaPO3)6 can improve the stability of Al2O3, whereas Na+ and other cations may deteriorate it.15 To explore the stability boundaries of Al2O3 suspensions under different concentrations of PO3−, different amounts of Na+ (mainly from NaNO3) were added on purpose. After standing for 24 h, the stability of the Al2O3 suspension was characterized by measuring the turbidity values of the bottom layer and upper layer of the suspension. If the difference between the two turbidity values (denoted as ΔTurbidity) fluctuates within a small range of 100–400 NTU and no apparent precipitate appears on the bottom, it suggests that the Al2O3 suspension is relatively stable; otherwise, it is unstable.Fig. 2 shows the stability results of the Al2O3 suspensions. As shown in Fig. 2(a), as the total Na+ concentration increases, ΔTurbidity first remains at about 100–400 NTU, then it reaches a turning point and then sharply increases until it gradually saturates. When the total Na+ concentration exceeds the turning point, the Al2O3 suspension becomes unstable, and an apparent precipitate appears on the bottom of the beaker. Fig. 2(b) summarizes the turning points of the total Na+ concentration corresponding to different concentrations of PO3−. As can be seen, the upper left unstable and bottom right stable zones are separated by the turning points. Moreover, as the PO3− concentration increases, the turning point of the total Na+ concentration gradually increases.
Fig. 2(c) shows the effects of PO3− and Na+ on the dispersion of Al2O3. For the mechanism, (NaPO3)6 can be hydrolyzed in an aqueous solution to form long chain structures in which there are a lot of negatively charged PO3−.16 PO3− can be adsorbed on the Al2O3 surface probably through hydrogen bonding.17,18 On the one hand, the zeta potential of Al2O3 becomes more negative due to the adsorbed negatively charged PO3−. On the other hand, the steric hindrance between Al2O3 increases due to the long-chain structure of the adsorption layer.16 Therefore, the stability of Al2O3 is improved. As for Na+, it can enter the electric double layer of Al2O3, neutralizing the zeta potential and compressing the electric double layer. Consequently, the stability of Al2O3 is weakened.
In summary, PO3− and Na+ play opposite roles in dispersing Al2O3. For a certain concentration of PO3−, the total Na+ concentration should be lower than the turning point to attain high stability when preparing an Al2O3-containing slurry.
3.2 CMP performance of Al2O3-containing slurries
Based on the above stability results, 5 mM (NaPO3)6 was chosen to disperse 1 wt% Al2O3 powder in ultrapure water to prepare an Al2O3 suspension. Fig. 3(a) shows the stability results. After standing for 24 h, the Al2O3 suspension at the natural pH value is quite stable without any apparent precipitate on the bottom of the beaker. The average particle size is 142 nm.On this basis, a tantalum CMP slurry containing ultrapure water, 1 wt% Al2O3 powder, 5 mM (NaPO3)6, 0.4 wt% H2O2 and 10 mM NaOH was prepared. The stability was examined at different pH values. Fig. 3(b) shows the stability results. After standing for 24 h, the Al2O3-containing slurries at pH 2 to 10 are all very stable and can be used for the following CMP.
Fig. 4 shows the MRR and surface roughness Sa results of tantalum after polishing with the above stable slurries at different pH values. With the increase in the pH value, the MRR gradually increases. The MRR reaches the maximum value at pH 10. Overall, the surface roughness Sa first decreases and then tends to be stable. The surface roughness Sa reaches 1.6 nm at pH 10.
 | ||
| Fig. 4 MRR and surface roughness Sa results after polishing with the Al2O3-containing slurries at different pH values. | ||
Fig. 5 shows the corresponding topographies of tantalum after polishing. At pH 2, the topography is quite rough with many surface defects. In comparison, at pH 10, the topography becomes much smoother, and the surface defects are largely reduced.
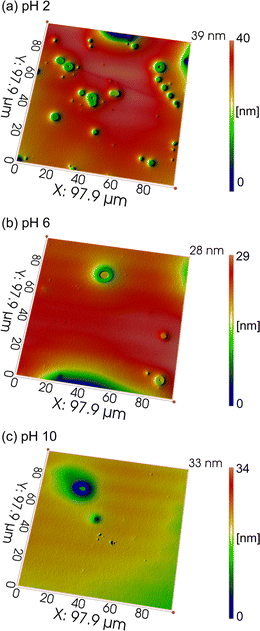 | ||
| Fig. 5 Corresponding topographies of tantalum after polishing with the Al2O3-containing slurries at different pH values. (a) pH 2. (b) pH 6. (c) pH 10. | ||
To conclude, at pH 10, a relatively high MRR and satisfactory surface quality of tantalum can be obtained. Therefore, alkaline pH is recommended for the Al2O3-containing CMP slurry for tantalum.
3.3 TEM and EDS characterization of Al2O3 before and after CMP
The above results reveal that Al2O3 can be used as an effective abrasive for the tantalum CMP. To explore the role of Al2O3 in the tantalum CMP, we used TEM and EDS to characterize Al2O3 before and after polishing, aiming to investigate the interaction between Al2O3 and the tantalum surface. Fig. 6 shows the TEM dark-field images and EDS results. As shown in Fig. 6(a), before polishing, Al2O3 in the slurry contains Al, O, P and Na elements. Al is from Al2O3, O can be from Al2O3 and (NaPO3)6, P is from (NaPO3)6, while Na can be from (NaPO3)6 and NaOH. As shown in Fig. 6(b) to (d), after polishing with the same CMP slurries 1 time, 10 times repeatedly, and 20 times repeatedly, the Ta element can be observed, and the distribution areas almost coincide with those of Al, O, P and Na. This suggests that Ta element can be adsorbed on the active sites of the Al2O3 surface, which may be the reason for the excellent CMP performance of the Al2O3-containing slurry at pH 10. In addition, the active sites may include hydroxyl group sites, Al3+ sites, and so on.19,203.4 AFM characterization of the interaction between Al2O3 and tantalum
The above TEM and EDS results indicate that Ta can be adsorbed on the Al2O3 surface. We used AFM to explore the adsorption from the microscopic perspective. The adhesion force between the Al2O3 microsphere tip and the tantalum surface in the solution was measured. The solution contained ultrapure water, 0.4 wt% H2O2, 5 mM Na+, and at pH 10. Fig. 7 shows the variation trend of the adhesion force with the dwell time. Under an applied load of 0.5 μN, the adhesion force gradually increases from 0.048 to 0.799 μN as the dwell time increases from 0 to 10 s. In principle, the adhesion force can be affected by van der Waals force, electrostatic force, capillary force, and chemical interaction.21,22 The capillary force can be neglected in the CMP slurry.23,24 Under the fixed applied load, the van der Waals force and electrostatic force remain basically unchanged, suggesting that the increased adhesion force is mainly attributed to the enhanced chemical interaction. Additionally, according to the research of Li et al.25 and Tian et al.,26 the increased dwell time will lead to an increase in the number of chemical bonds formed, resulting in an enhancement of the interfacial interaction. Therefore, it can be concluded that chemical interaction can exist between Al2O3 and the tantalum surface, which can affect the material removal in the tantalum CMP. In the following study, we performed first-principles calculation to investigate the chemical interaction between Al2O3 and the tantalum surface.AFM wear experiments were performed to examine whether the chemical interaction between Al2O3 and the tantalum surface can affect the material removal of tantalum. An Al2O3 microsphere tip was rubbed against the tantalum surface in the solution, simulating the tantalum CMP. The solution contained ultrapure water, 0.4 wt% H2O2, 5 mM Na+, and at pH 10. Fig. 8 shows the AFM wear results of tantalum. In particular, each condition was repeated twice to avoid excessive wear of the Al2O3 microsphere tip, similar to the literature.27–29 As shown in Fig. 8(a), no wear scar can be observed on the tantalum surface at 0.1 μN applied load. In comparison, a shallow wear scar appears on the tantalum surface at 0.2 μN. Moreover, as the applied load increases from 0.2 μN to 0.8 μN, the wear scar becomes deeper and clearer, and the wear depth gradually increases from about 0.1 nm to 2.3 nm (as shown in Fig. 8(b)).
Fig. 9 exhibits the Al2O3 microsphere tip after the wear experiments. No apparent wear track can be observed on the Al2O3 tip, and the profile is still spherical. The fitting radius of the Al2O3 tip is about 3.116 μm, which is close to the nominal value. In our case, Hertz contact theory was used to roughly estimate the contact pressure between the Al2O3 tip and the tantalum surface. At 0.2 μN applied load, the maximum contact pressure is calculated to be 0.48 GPa (Pmax = (6FappliedE*2/π3R2)1/3. For calculation, Fapplied is the applied load, that is 0.2 μN here; E* = [(1 − ν12)/E1 + (1 − ν22)E2]−1.30 The Young's modulus E1 and Poisson ratio ν1 of tantalum are 186 GPa and 0.35, respectively.31 The Young's modulus E2 and Poisson ratio ν2 of Al2O3 are 370 GPa and 0.22, respectively;32 R is the radius of the Al2O3 microsphere tip).
Generally, yield strength is positively correlated with hardness.33 As reported by Hariharaputhiran et al.,34 the bulk hardness of the tantalum surface film is 1100–1200 kg mm−2, which is much larger than 230 kg mm−2 of tantalum. Therefore, it can be inferred that the yield strength of the tantalum surface film should be higher than that of tantalum. As shown in Fig. 8(a), a shallow wear scar appears on the tantalum surface at 0.2 μN. Combined with the calculation result based on Hertz contact theory, the material removal of tantalum starts at a contact pressure of 0.48 GPa, which is much lower than the yield strength of tantalum (about 6–19 GPa (ref. 35 and 36)), let alone that of the tantalum surface film.
To conclude, Al2O3 can remove tantalum in the slurry under a contact pressure much lower than the yield strength of the tantalum surface film, which is quite similar to the material removal of silicon.37 A reasonable explanation can be that when Al2O3 slides on the tantalum surface, tribochemical reactions occur, lowering the energy barrier for dissociation of the interfacial bond and/or the subsurface back bond.38
3.5 First-principles calculation of interaction between Al2O3 and tantalum
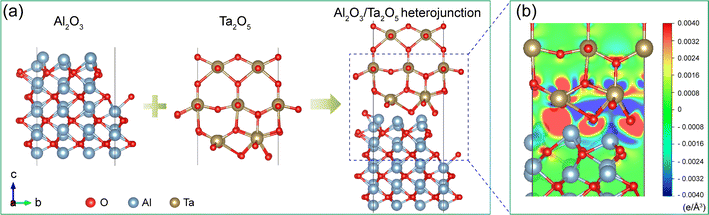
In this section, we performed first-principles calculations based on DFT to further explore the interaction between Al2O3 and the tantalum surface. As reported, tantalum can be oxidized to Ta2O5 in the H2O2-containing slurry at alkaline pH.5,39 Therefore, the interaction between Al2O3 and Ta2O5 was investigated. Fig. 10(a) exhibits the models of Al2O3, Ta2O5 and the Al2O3/Ta2O5 heterojunction. The binding energy of the Al2O3/Ta2O5 heterojunction can be calculated as follows:| E = EAl2O3+ ETa2O5− EAl2O3/Ta2O5 | (1) |
Moreover, the charge density difference of the Al2O3/Ta2O5 heterojunction is obtained by subtracting the isolated bottom (Al2O3) and upper (Ta2O5) surfaces from the total interface (the Al2O3/Ta2O5 heterojunction). As shown in Fig. 10(b), the red and blue areas indicate enrichment and depletion of the electron due to the interaction between Al2O3 and Ta2O5, respectively. The Al2O3 side is surrounded by red areas, while the Ta2O5 side is surrounded by blue areas, suggesting that electrons transfer from Ta2O5 to Al2O3, and form a chemical bond of Al–O–Ta at the Al2O3/Ta2O5 interface, which may weaken the Ta–O back bond and facilitate the removal of Ta atoms to some extent.42
3.6 Role of Al2O3 abrasive in tantalum CMP
Based on the results obtained so far and the literature, the role of Al2O3 abrasive in the tantalum CMP can be proposed. Fig. 11 demonstrates the corresponding schematic diagram. In the H2O2-containing slurry at alkaline pH, tantalum can be oxidized to form an oxide layer on the surface, which is mainly composed of Ta2O5 and Ta–OH complexes with hydroxyl groups. The chemical reactions can be depicted as follows:39,43| 6Ta + 10H2O2 + 10OH− → 3Ta2O5 + 15H2O + 10e− | (2) |
| Ta5+ + x/2H2O2 + xe− → Ta(OH)x(5−x)+ | (3) |
| Ta5+ + (x/2 + 1)H2O2 + (x + 2)e− → TaO(OH)x(3−x)+ + H2O | (4) |
When Al2O3 presses and slides on the tantalum surface, similar to silicon,38,44 tribochemical reactions can occur. Hydroxyl groups on the two contact surfaces are close to each other, and a dehydration reaction may occur to form the chemical bond of Al–O–Ta at the interface (partly confirmed by the above first-principles calculation).45,46 As Al2O3 moves, the interfacial chemical bond is stretched, and tantalum is detached from the tantalum surface. Eventually, the material removal of tantalum is achieved. In particular, as revealed by the AFM wear experimental results, the threshold contact pressure is much lower than the yield strength of the tantalum surface film when tantalum is removed, implying that the formation and stretching of the chemical bond of Al–O–Ta may lower the energy barrier for the dissociation of the Ta–Ta bond and/or Ta–O bond (weakening of the Ta–O back bond is confirmed by the above first-principles calculation).37
In addition, Li et al.6 and Hariharaputhiran et al.34 used Al2O3 (the mean aggregate size is 290–360 nm, Ferro Electronics) to polish tantalum. The MRR decreases with the increase in the hardness of the softening layer on the tantalum surface. Therefore, it can be concluded that Al2O3 may have a mechanical effect. Likewise, Al2O3 in this study may also mechanically abrade the tantalum surface besides chemical bonding. More investigation is ongoing.
This study suggests that chemical interaction exists between Al2O3 and the tantalum surface, helping achieve the material removal of tantalum. Therefore, to further enhance the CMP efficiency of tantalum, it is recommended to continuously increase the active sites that bind tantalum on the Al2O3 surface, for example hydroxyl groups.47
4 Conclusions
This study investigated the interaction mechanism of Al2O3 abrasive in the tantalum CMP using CMP and several characterization techniques including TEM, EDS, AFM, and first-principles calculation. Based on the results obtained so far, the conclusions can be drawn as follows:(1) (NaPO3)6 can be used as an effective dispersant for Al2O3 powder in water. PO3− can improve the stability of Al2O3 through electrostatic repulsion and steric hindrance, while Na+ deteriorates it. The total Na+ concentration should be lower than the turning point to attain a highly stable Al2O3 suspension. For the tantalum CMP, with stable Al2O3-containing slurries, a relatively high MRR and satisfactory surface quality of tantalum can be obtained at pH 10.
(2) TEM and EDS characterization results indicate that Ta element can be absorbed on the Al2O3 surface, which can be attributed to the chemical interaction between Al2O3 and the tantalum surface (confirmed by AFM adhesion force measurement), forming the chemical bond of Al–O–Ta (partly confirmed by first-principles calculation). Moreover, AFM wear experimental results show that the Al2O3 microsphere tip can remove tantalum under 0.48 GPa, which is much lower than the yield strength of the tantalum surface film.
(3) For the CMP mechanism, with H2O2 and at alkaline pH, tantalum can be oxidized to Ta2O5 and Ta–OH complexes, and Al2O3 can be hydrolyzed. When Al2O3 presses and slides on the tantalum surface, tribochemical reactions can occur. Hydroxyl groups on the two contact surfaces may be dehydrated to form the chemical bond of Al–O–Ta at the interface. As Al2O3 moves, the interfacial chemical bond is stretched, and tantalum is detached. Eventually, the material removal of tantalum is achieved.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work is supported by the National Natural Science Foundation of China (51991373, and 52235004), the National Key R&D Program of China (2020YFA0711001), and the Fundamental Research Funds for the Central Universities (2682024CG007).References
- M. Lane, R. H. Dauskardt, N. Krishna and I. Hashim, J. Mater. Res., 2000, 15, 203–211 CrossRef CAS.
- Y. Li, Microelectronic Applications of Chemical Mechanical Planarization, John Wiley & Sons, Inc., Hoboken, New Jersey, USA, 2007 Search PubMed.
- S. V. Babu, A. Jindal and Y. Li, JOM, 2001, 53, 50–52 CrossRef CAS.
- Y. Li, M. Hariharaputhiran and S. V. Babu, J. Mater. Res., 2001, 16, 1066–1073 CrossRef CAS.
- J. Zhang, S. Li and P. W. Carter, J. Electrochem. Soc., 2007, 154, H109 CrossRef CAS.
- Y. Li, S. Ramarajan, M. Hariharaputhiran, Y. S. Her and S. V. Babu, MRS Proc., 2000, 613, E2.4.1 CrossRef.
- Y. Li, J. Zhao, P. Wu, Y. Lin, S. V. Babu and Y. Li, Thin Solid Films, 2006, 497, 321–328 CrossRef CAS.
- A. Vijayakumar, T. Du, K. B. Sundaram and V. Desai, Microelectron. Eng., 2003, 70, 93–101 CrossRef CAS.
- M. Kitayama and J. A. Pask, J. Am. Ceram. Soc., 1996, 79, 2003–2011 CrossRef CAS.
- G. Xiao, L. Jiang, W. Peng, J. Liu, C. Deng and L. Qian, Wear, 2022, 508–509, 204466 CrossRef CAS.
- H. Yang, J. Li, Z. Zhou and J. Ruan, Mater. Lett., 2013, 100, 152–155 CrossRef CAS.
- M. L. B. Palacio and B. Bhushan, Crit. Rev. Solid State Mater. Sci., 2010, 35, 73–104 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- A. J. Khanna, S. Gupta, P. Kumar, F.-C. Chang and R. K. Singh, ECS J. Solid State Sci. Technol., 2018, 7, P423 CrossRef CAS.
- C. O. Metin, L. W. Lake, C. R. Miranda and Q. P. Nguyen, J. Nanopart. Res., 2011, 13, 839–850 CrossRef CAS.
- H. Song, Z. Cao, W. Xie, F. Cheng, K. A. M. Gasem and M. Fan, J. Cleaner Prod., 2019, 235, 259–271 CrossRef CAS.
- L. Piani and A. Papo, J. Eng., 2013, 2013, 930832 Search PubMed.
- Z.-h. Li, Y.-x. Han, Y.-j. Li and P. Gao, Trans. Nonferrous Met. Soc. China, 2017, 27, 1841–1848 CrossRef.
- H. Knözinger and P. Ratnasamy, Catal. Rev., 1978, 17, 31–70 CrossRef.
- N. Tang, Y. Cong, Q. Shang, C. Wu, G. Xu and X. Wang, ACS Catal., 2017, 7, 5987–5991 CrossRef CAS.
- M. Binggeli and C. M. Mate, Appl. Phys. Lett., 1994, 65, 415–417 CrossRef CAS.
- X. Xiao and L. Qian, Langmuir, 2000, 16, 8153–8158 CrossRef CAS.
- Q. Ouyang, K. Ishida and K. Okada, Appl. Surf. Sci., 2001, 169–170, 644–648 CrossRef CAS.
- P. Liu, S. Hong, S. Jeon, J. Lee, D. Kwak, Y. Wada, H. Hiyama, S. Hamada and T. Kim, Colloids Surf., A, 2021, 627, 127156 CrossRef CAS.
- Q. Li, T. E. Tullis, D. Goldsby and R. W. Carpick, Nature, 2011, 480, 233–236 CrossRef CAS PubMed.
- K. Tian, N. N. Gosvami, D. L. Goldsby, Y. Liu, I. Szlufarska and R. W. Carpick, Phys. Rev. Lett., 2017, 118, 076103 CrossRef PubMed.
- A. A. Tseng, Appl. Surf. Sci., 2010, 256, 4246–4252 CrossRef CAS.
- F. Ilie, Tribol.-Mater., Surf. Interfaces, 2013, 7, 211–215 CrossRef CAS.
- K.-H. Chung, Y.-H. Lee, H.-J. Kim and D.-E. Kim, Tribol. Lett., 2013, 52, 315–325 CrossRef.
- T. D. B. Jacobs, C. Mathew Mate, K. T. Turner and R. W. Carpick, in Scanning Probe Microscopy in Industrial Applications, 2013, pp. 15–48, DOI:10.1002/9781118723111.ch2.
- A. Dorogoy and D. Rittel, Mech. Mater., 2017, 112, 143–153 CrossRef.
- T.-H. Fang, T. Wang, C.-H. Liu, L.-W. Ji and S.-H. Kang, Nanoscale Res. Lett., 2007, 2, 410 CrossRef CAS.
- E. J. Pavlina and C. J. Van Tyne, J. Mater. Eng. Perform., 2008, 17, 888–893 CrossRef CAS.
- M. Hariharaputhiran, Y. Li, S. Ramarajan and S. Babu, Electrochem. Solid-State Lett., 2000, 3, 95–98 CrossRef CAS.
- M. Zhang, B. Yang, J. Chu and T. G. Nieh, Scr. Mater., 2006, 54, 1227–1230 CrossRef CAS.
- T. P. Remington, C. J. Ruestes, E. M. Bringa, B. A. Remington, C. H. Lu, B. Kad and M. A. Meyers, Acta Mater., 2014, 78, 378–393 CrossRef CAS.
- L. Chen, J. Wen, P. Zhang, B. Yu, C. Chen, T. Ma, X. Lu, S. H. Kim and L. Qian, Nat. Commun., 2018, 9, 1542 CrossRef PubMed.
- L. Chen, H. He, X. Wang, S. H. Kim and L. Qian, Langmuir, 2015, 31, 149–156 CrossRef CAS PubMed.
- T. Du, D. Tamboli, V. Desai, V. S. Chathapuram and K. B. Sundaram, J. Mater. Sci.: Mater. Electron., 2004, 15, 87–90 CrossRef CAS.
- L. Huang and X. Zhou, J. Phys.: Conf. Ser., 2023, 2639, 012013 CrossRef CAS.
- Y. Liu, G.-J. Zhao, J.-X. Zhang, F.-Q. Bai and H.-X. Zhang, Appl. Surf. Sci., 2021, 549, 149309 CrossRef CAS.
- S. FliszÁR and C. Minichino, J. Phys. Colloq., 1987, 48, 367–375 CrossRef.
- S. C. Kuiry, S. Seal, W. Fei, J. Ramsdell, V. H. Desai, Y. Li, S. V. Babu and B. Wood, J. Electrochem. Soc., 2003, 150, C36–C43 CrossRef CAS.
- C. Chen, C. Xiao, X. Wang, P. Zhang, L. Chen, Y. Qi and L. Qian, Appl. Surf. Sci., 2016, 390, 696–702 CrossRef CAS.
- J. Yu, S. H. Kim, B. Yu, L. Qian and Z. Zhou, ACS Appl. Mater. Interfaces, 2012, 4, 1585–1593 CrossRef CAS PubMed.
- Y. Li, X. Sha, W. Yue, W. Qin and C. Wang, Int. J. Refract. Met. Hard Mater., 2019, 79, 197–203 CrossRef CAS.
- V. M. Gun'ko, V. V. Turov, V. I. Zarko, E. F. Voronin, V. A. Tischenko, V. V. Dudnik, E. M. Pakhlov and A. A. Chuiko, Langmuir, 1997, 13, 1529–1544 CrossRef.
| This journal is © The Royal Society of Chemistry 2024 |