Open Access Article
Open Access ArticleGC-MS analysis, phytochemical composition of Hertia cheirifolia L. essential oil with pharmacological assessments: antioxidant, antibacterial, and antifungal activities†
Wassila Benabderrahmanea,
Hamza Fadelb,
Ines Sekharab,
Imad Mennaib,
Imed Eddine Kadic,
Mahmoud Helald,
Rokayya Sami *e,
Hala M. Abo-Dieff,
Ruqaiah I. Bedaiwi
*e,
Hala M. Abo-Dieff,
Ruqaiah I. Bedaiwi g,
Mohammad A. Alanazig,
Helal F. Al-Harthih,
Roqayah H. Kadii,
Suzan A. Abushalj,
Tasahil S. Albishik,
Alaa T. Qumsanil and
Sameer H. Qaril
g,
Mohammad A. Alanazig,
Helal F. Al-Harthih,
Roqayah H. Kadii,
Suzan A. Abushalj,
Tasahil S. Albishik,
Alaa T. Qumsanil and
Sameer H. Qaril
aDepartment of Chemistry, Faculty of Sciences, University of 20 Août 1955-Skikda, PO Box 26, El-Hadaiek Road, Algeria. E-mail: b_wassila84@yahoo.fr
bResearch Unit Valorization of Natural Resources, Bioactive Molecules, and Physicochemical and Biological Analyses, University of the Mentouri Brothers, Constantine1, Aïn El Bey Road, 25000, Constantine, Algeria. E-mail: hamzafadel64@yahoo.com; sekharaines@gmail.com; mennai_imad@umc.edu.dz
cResearch Unit in Medicinal Plants (URPM. 3000, Laghouat) Attached to the Research Centre of Biotechnology (CRBt. 25000, Constantine), Algeria. E-mail: kadi.imed.eddine@gmail.com
dDepartment of Mechanical Engineering, Faculty of Engineering, Taif University, PO 11099, Taif 21944, Saudi Arabia. E-mail: mo.helal@tu.edu.sa
eDepartment of Food Science and Nutrition, College of Sciences, Taif University, PO Box 11099, Taif 21944, Saudi Arabia. E-mail: rokayya.d@; tu.edu.sa
fDepartment of Science and Technology, University College-Ranyah, Taif University, PO Box 11099, Saudi Arabia. E-mail: h.abodeif@tu.edu.sa
gDepartment of Medical Laboratory Technology, Faculty of Applied Medical Sciences, University of Tabuk, PO Box 741, Tabuk 71491, Saudi Arabia. E-mail: rbedaiwi@ut.edu.sa; m.alenezi@ut.edu.sa
hDepartment of Biology, Turabah University College, Taif University, 21995, Saudi Arabia. E-mail: h.alharthy@tu.edu.sa
iDepartment of Biological Sciences, College of Science, University of Jeddah, Jeddah 21959, Saudi Arabia. E-mail: rhkadi@uj.edu.sa
jProgram of Food Sciences and Nutrition, Turabah University College, Taif University, PO 11099, Taif 21944, Saudi Arabia. E-mail: s.aboshal@tu.edu.sa
kBiology Department, College of Sciences, Umm Al-Qura University, Makkah, Saudi Arabia. E-mail: tsbishi@uqu.edu.sa
lDepartment of Biology, Al-Jumum University College, Umm Al-Qura University, Makkah, Saudi Arabia. E-mail: atqumsani@uqu.edu.sa; shqari@uqu.edu.sa
First published on 17th July 2024
Abstract
The genus Hertia, which belongs to the Asteraceae family, is a flowering genus with 12 species found in Africa, North and South. Among the species present in Algeria, Hertia cheirifolia L. is distributed in the eastern regions of Algeria. The aim of this study is to evaluate its phytochemical composition with following pharmacological assessments: the antioxidant, antibacterial, and antifungal activities of Hertia cheirifolia L. essential oil (EO). GC-MS analysis was used to analyze the chemical constituents of H. cheirifolia essential oil. The antioxidant capacity was assessed using DPPH, FRAP, and H2O2 tests. The EO was also tested for its ability to inhibit six strains of microorganisms, including two Gram (+) and four Gram (−) strains. The antifungal activity was tested by analyzing the effect of the EO on the mycelial growth of Fusarium oxysporum f.sp. lycopersici (FOL) fungi. Results showed that primary volatile components were α-pinene (32.59%), 2-(1-cyclopent-1-enyl-1-methylethyl) cyclopentanone (14.62%), (−)-germacrene D (11.37%), and bakkenolide A (9.57%). H. cheirifolia EO showed inhibitory effects against DPPH, H2O2, and FRAP (IC50 = 0.34 ± 0.1, 0.053 ± 0.1, and 0.047 ± 0.01 mg mL−1, respectively). The EO also exhibited moderate antibacterial effects against Staphylococcus aureus ATCC 25923 (S. aureus), Streptococcus pneumoniae ATCC 49619 (S. pneumoniae), and Enterobacter aerogenes ATCC 13048 (E. aerogenes), as well as significant antioxidant potential and varied antifungal activity based on dosage and fungal strain. To our knowledge, no previous research has examined the antifungal capacity of H. cheirifolia oil and oil-mycelial development of the FOL relationship. To fully explore the benefits of H. cheirifolia EO, more in vivo research is necessary, along with more testing on other bacterial and fungal strains.
Introduction
Around 1911 genera and 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 913 species make up the Asteraceae family.1,2 This family is among the biggest worldwide, with members across all continents, including the Mediterranean basin.3,4 Many Asteraceae species are used in various medicinal contexts and contain volatile oils that give them their distinctive scents.5–7 This family includes the genus Hertia, with 12 flowering species found in Africa, North and South and Central and Southwestern Asia.8,9 It has pharmacological properties, including antioxidant, anti-inflammatory, antibacterial, cytotoxic, spasmolytic, anthelmintic, and acaricidal effects, because of its bioactive phytochemicals.10,11 Hertia cheirifolia L., also known as “Kherchoun”12 or “Barbary ragwort”,13 is an evergreen perennial woody plant. It bears fleshy, bluish oval leaves on creeping stems, and yellow flowers in the capitulum, 2 or 3 cm in diameter, appear during the spring. This plant can withstand various environmental conditions, including wind, sea spray, cold, and drought. It is endemic to North Africa, including the Algerian Aures area and is also widely distributed in gardens.14–16 Historically, traditional healers have used it to treat hemorrhoids, spasms, inflammation, diarrhea, and a range of gastrointestinal ailments.17 In fact, literature studies indicate that H. cheirifolia possesses biological activities.18–25 Moreover, it is recommended for use as a mosquito control since it is successful against larval stages.26 To the best of our knowledge, however, no research has investigated the phytochemical constituents and pharmacological properties of the EO (essential oil) found in the aerial parts of H. cheirifolia growing in the Aures area (Algeria), and, in particular, no prior studies have looked at the antifungal potential of the EO of this species, and its association with the mycelial growth of FOL. Therefore, given the valuable chemical and biological properties of this plant, the purpose of this study was to examine the chemical makeup and biological characteristics of the EO of H. cheirifolia, which was collected for the first time from a mountainous region of Algeria. The study also aimed to compare the results with those of other studies conducted on same species to identify any similarities or differences in outcomes and factors that contributed to them.
913 species make up the Asteraceae family.1,2 This family is among the biggest worldwide, with members across all continents, including the Mediterranean basin.3,4 Many Asteraceae species are used in various medicinal contexts and contain volatile oils that give them their distinctive scents.5–7 This family includes the genus Hertia, with 12 flowering species found in Africa, North and South and Central and Southwestern Asia.8,9 It has pharmacological properties, including antioxidant, anti-inflammatory, antibacterial, cytotoxic, spasmolytic, anthelmintic, and acaricidal effects, because of its bioactive phytochemicals.10,11 Hertia cheirifolia L., also known as “Kherchoun”12 or “Barbary ragwort”,13 is an evergreen perennial woody plant. It bears fleshy, bluish oval leaves on creeping stems, and yellow flowers in the capitulum, 2 or 3 cm in diameter, appear during the spring. This plant can withstand various environmental conditions, including wind, sea spray, cold, and drought. It is endemic to North Africa, including the Algerian Aures area and is also widely distributed in gardens.14–16 Historically, traditional healers have used it to treat hemorrhoids, spasms, inflammation, diarrhea, and a range of gastrointestinal ailments.17 In fact, literature studies indicate that H. cheirifolia possesses biological activities.18–25 Moreover, it is recommended for use as a mosquito control since it is successful against larval stages.26 To the best of our knowledge, however, no research has investigated the phytochemical constituents and pharmacological properties of the EO (essential oil) found in the aerial parts of H. cheirifolia growing in the Aures area (Algeria), and, in particular, no prior studies have looked at the antifungal potential of the EO of this species, and its association with the mycelial growth of FOL. Therefore, given the valuable chemical and biological properties of this plant, the purpose of this study was to examine the chemical makeup and biological characteristics of the EO of H. cheirifolia, which was collected for the first time from a mountainous region of Algeria. The study also aimed to compare the results with those of other studies conducted on same species to identify any similarities or differences in outcomes and factors that contributed to them.
Experimental
Materials
Plant samples
Aerial portions of H. cheirifolia were gathered in March 2023 from the Ain Kercha region of Algeria's Aures zone (HC-AKA); (35° 55′ 16′′ north, 6° 41′ 58′′ east, altitude: 875 m). A voucher specimen (HC/128/VAR/04-23) has been deposited in the herbarium of the research unit VARENBIOMOL of the University of Constantine 1.| % EO yield = (weight of the extracted EO (g)/weight of the dried plant material (g)) × 100. |
The obtained essential oil was subjected to GC-MS analysis to establish its chemical makeup. Fig. 1 presents an outline of the work carried out.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80).27
80).27DPPH radical-scavenging test
The ability of H. cheirifolia essential oil to decrease DPPH was evaluated using the approach outlined by Gargouri et al.30 Aliquots of 0.1 mL of the essential oil with concentrations ranging from 31.25 to 1000 μg mL−1 were combined with 2 mL of DPPH solution (0.4%) in ethanol and vigorously shaken. After 30 minutes of incubation at 30 °C in the dark, the absorbance at 517 nm was measured, with a blank sample as reference. The following equation was used to measure the scavenging capacity of the oil:| (%) = (A0 − A1)/A0 × 100 |
A0 represents the absorbance of the control after 30 minutes, while A1 represents the absorbance by the sample after the same time period. All the samples underwent three replications.
Hydrogen peroxide scavenging testing
The EO scavenging ability against H2O2 was measured based on the technique developed by Remigante et al.31 A 40 mM solution of deionized H2O2 at pH 7.4 in a phosphate buffer was prepared. The oil was made up at different concentrations, ranging from 1 to 0.0312 mg mL−1, in H2O2 solution and allowed to react for about ten minutes at ambient temperature. The reference, α-tocopherol, was subjected to the same procedure. The absorbance of H2O2 solutions was measured at 240 nm and compared to an oil-free control. The H2O2 inhibition rate (IR%) was determined using the following formula:in which Ae is the absorbance of the tested sample, and Ac is the absorbance of the control.
Ferric reducing antioxidant power (FRAP) assay
Approximately 30 μL of diluted EO (2 mg mL−1) were mixed with 90 μL of distilled water and added to 900 μL of FRAP reagent and incubated for 30 minutes at 37 °C. The ferric reducing power was determined from the FeSO4·7H2O calibration curve and its corresponding linear regression equation.32Results are expressed as mean values ± standard errors (n = 3). Microsoft Office Excel 2007 (Microsoft, Redmond, WA, USA) was used for all statistical analyses.
Microorganism strains. The antibacterial activity of H. cheirifolia EO was tested against two Gram-positive microorganism strains (Staphylococcus aureus ATCC 25923 and Streptococcus pneumoniae ATCC 49619) and four Gram-negative microorganism strains (Klebsiella pneumoniae ATCC 700603, Escherichia coli ATCC 4330, Proteus mirabilis ATCC 7002, and Enterobacter aerogenes ATCC 13048).
Agar diffusion technique (ADT). ADT was utilized to measure the antibacterial capacity, following NCLLS recommendations. Sterile MHA was placed on Petri plates and allowed to set. Cell-culture dishes were infected with microorganisms. To create the inoculum, test microorganisms were cultured overnight and adjusted to a turbidity of 0.5 McFarland. Sterile discs (6 mm) were filled with 1/8, 1/4, 1/2, or 1 mg mL−1 of H. cheirifolia EO and later put on agar dishes harboring one of the microorganisms. These concentrations were produced by diluting the oil with DMSO (1/1, 1/2, 1/4, and 1/8 v/v), with DMSO and gentamicin serving as negative and positive controls, respectively. The plates were incubated at 37 °C for 24 hours after being kept for 30 minutes at room temperature. All disc diffusion or antibacterial assays were conducted with at least three repetitions per species. Three measurements were performed for the disc diffusion study on each bacterium to confirm the results. The antibacterial ability of H. cheirifolia essential oil was assessed by measuring the width of its inhibitory zones.19
The inhibitory activity was represented as a percentage and computed using the formula: IR = (C-T/C) × 100. In this equation, IR represents the inhibition rate as a percentage, C represents the radial growth of the phytopathogenic agent in millimeters on DMSO (control) with PDA medium, and T represents the radial growth of the phytopathogenic agent in millimeters on PDA medium with the tested complex. The EO was tested at various concentrations (1/8, 1/4, 1/2, and 1 mg mL−1 of PDA) to determine the lowest inhibitory concentration for controlling FOL growth.
Results and discussion
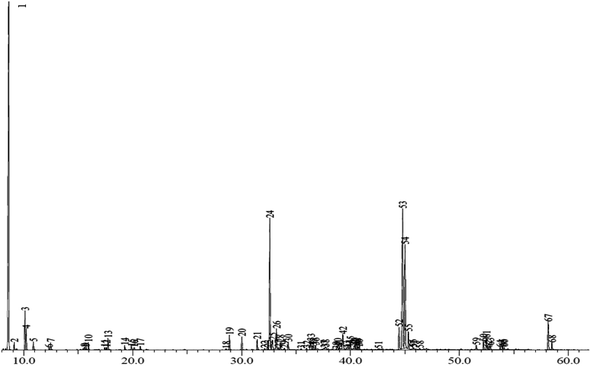
GC-MS analysis
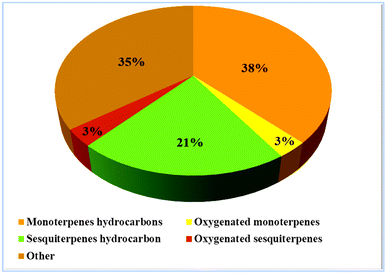
GC-MS analysis led to experimental retention indices that were compared with literature values,34 and each peak of the EI mass spectrum was compared to available mass spectra libraries (Fig. 2).In this way, sixty-eight compounds (100%) were detected by GC-MS analysis. Table S1† shows that compounds, α-pinene, 2-(1-cyclopent-1-enyl-1-methylethyl) cyclopentanone, (−)-germacrene D and bakkenolide A, are the principal volatile components of H. cheirifolia with ratios of 32.59%, 14.62%, 11.37%, and 9.57%, respectively. Oil-contained monoterpenoids (40.76%) and sesquiterpenoids (24.32%), with sesquiterpenoids (17 compounds) and oxygenated sesquiterpenoids (14 compounds) dominating their respective groups (21.06% and 3.26%, respectively), as well as 20 other substances having percentages between 0.08 and 14.62% (Fig. 3). Several investigations in Algeria found variations in the chemical makeup of H. cheirifolia essential oil. Samples of Hertia cheirifolia were collected from different regions of Algeria: Oum El Bouaghi (HC-OEB1, HC-OEB2), Batna (HC-BA), Setif (HC-SE), and Tunisia (HC-TU) and consequently coded.
As an example, sixty-two compounds were found in HC-OEB-EO,22 representing 78.3% of the total components of this EO. Monoethylhexyl phthalate, valeranone, (−)-drimenin, and tert-butylbenzene were determined to be major volatile compounds, but were not found in our sample from the Ain Kercha region of the Algeria's Aures zone, coded HC-AKA. In a previous study19 on the same species (HC-OEB2), forty-seven compounds were identified, accounting for 95.2%. Main constituents were α-pinene, β-phellandrene, and 2-(1-cyclopent-1-enyl-1-methylethyl) cyclopentanone. Ounoughi et al.20 identified fifty-seven volatile compounds in HC-BA-EO, accounting for 99.3% of the total H. cheirifolia EO. Primary components were spathulenol, α-pinene, and α-campholene aldehyde, whereas in HC-SE-EO, fifty-five components (98.57%) were identified, including α-pinene, germacrene D, caryophyllene oxide, and spathulenol.20 Notably, HC-AKA, our H. cheirifolia EO, differed from previously published investigations in terms of the presence of α-pinene. HC-SE-EO and HC-BA-EO samples had higher rates of α-pinene, while HC-AKA had a lower content (53.8, 48.5, and 32.6%, respectively). We identified fourteen common chemicals in HC-AKA compared to HC-SE and HC-BA and sixteen compounds compared to the HC-SE-EO sample. HC-AKA-EO has fifty-two components that were lacking in the HC-SE sample, and fifty components that were absent in the HC-BA-EO sample. HC-SE-EO had thirty-nine missing components, whereas HC-BA-EO had forty-three missing components. Variations might be due to harvesting time, local conditions, or a distinct chemotype.35–37 HC-TU-EO showed diversity in volatile chemical makeup across many areas. HC-TU essential oil, collected from Sousse, was found to be high in germacrene D, α-pinene, and drimane-type sesquiterpene lactones.21 In another HC-TU sample, α-pinene and α-cadinol were found to be main volatile components.16 Moreover, thymol and 2,6-dimethoxy-phenol were found to be predominant chemicals in the HC-TU-EO South-West Kef sample,24 with just nine identified compounds accounting for 93.1%. Indeed, our HC-AKA sample differed from the Tunisian sample in that it contained only thymol and 2,6-dimethoxyphenol, while HC-TU-EO contained camphor, 1,8-cineole, terpinene-4-ol, p-cymen-8-ol, myrtenol, α-terpineol, and 6-methyl-α-ionone.24 The variance in the composition of EOs from H. cheirifolia might be explained by a number of factors, including distillation technique, location, age and cultivar of the plant, environment, and equipment.21,38 Furthermore, some published research found that the essential oil content of the species may fluctuate during their growth cycle39 and may be influenced by environmental factors34,40 as well as ecological and genetic factors, such as the properties and type of soil.41 In addition, plant oil content differs substantially depending on genotype, collection season, and geographical origin.42–48 Therefore, through the results obtained and comparing them with the results of previous studies on the same plant species from different regions, it can be concluded that there are obvious differences in quantity and quality in the relative contents of the chemical components between H. cheirifolia EOs. In addition, there is a difference in its main constituents, and if there is a similarity, there is a difference in amount, even if it is a small percentage.
Antioxidant capacity
Using DPPH, H2O2, and FRAP techniques, the antioxidant effect of H. cheirifolia EO was checked (Table 1 and Fig. 4). In fact, the oil demonstrated a greater antioxidant capacity (89.55% ± 0.3) than the vitamin C standard (78.9% ± 0.1) at 1 mg mL−1 with significant DPPH activity (IC50 = 0.34% ± 0.1 mg mL−1). Regarding the H2O2 test, the results indicated a high level of antioxidant capacity with an IC50 of 0.052 ± 0.1 mg mL−1. Inhibitory rates increased progressively with the addition of concentrations of 1/32, 1/16, 1/8, 1/4, 1/2, and 1 mg mL−1. Among these concentrations, 0.25 and 0.5 mg mL−1 tested oils demonstrated the highest level of activity, exhibiting inhibition rates of 74.9% ± 0.2 and 87.9% ± 0.1, respectively. At 1 mg mL−1, the maximal activity (92.3% ± 0.3) of the essential oil was achieved, while the highest dosage of the reference compound α-tocopherol (1 mg mL−1) inhibited 90.4% ± 0.1 of the reaction. In the presence of antioxidants, the ability of plant fractions to convert ferric iron (Fe3+) to ferrous iron (Fe2+) can be measured by FRAP testing, frequently used to assess the overall antioxidant efficacy of extracts. HC-AKA-EO possessed substantial antioxidant capacity (IC50 = 0.047 ± 0.09 mg mL−1) in comparison to the ascorbic acid standard reference (IC50 = 0.09 ± 0.01 mg mL−1), at 93.4% ± 0.1. This allowed the oil to catalyze the reduction of Fe3+ to Fe2+. When compared to previously published research, H. cheirifolia essential oil (HC-AKA) showed the greatest amount of scavenging ability using the DPPH assay.22,49 The presence of hydroxylated chemicals, such as monoterpenes (α-pinene, camphene, sabinene, limonene, linalool, and verbenol) and sesquiterpenes (germacrene D, α-muurolene, and spathulenol) in the sample could account for the potent inhibitory ability of the essential oil.50–53 Compared with the results of previous studies on the same plant,11,18,21,22,25 we note that there is a difference in the results obtained. However, according to previous studies, these differences in antioxidant capacity could be due to several factors, including the molecular nature of bioactive compounds.41,54–56 In fact, the major compound (α-pinene) is known for its strong antioxidant proprieties.54,55 In contrast, minor compounds, such as limonene and β-pinene, can interact directly to create a complex of biological properties.54 In addition, antioxidant capacity was reported to be linked to the molecular structure of the EO, such as hydroxyl group substitution in phenolic aromatic rings, contributing to oxygenated monoterpenes (α-pinene oxide, linalool, and verbenol), a blend of mono and sesquiterpene hydrocarbons, and their hydrogen-donating ability.54| Concentration (mg mL−1) | DPPH test | H2O2 test | FRAP | |||
|---|---|---|---|---|---|---|
| % EO | % Vitamin C | % EO | % α-tocopherol | % EO | Ascorbic acid | |
| 1 | 89.55 ± 0.3 | 78.86 ± 0.1 | 92.34 ± 0.3 | 90.37 ± 0.1 | 94.52 ± 0.1 | 88.58 ± 0.1 |
| 0.5 | 83.04 ± 0.1 | 74.42 ± 0.2 | 87.86 ± 0.1 | 85.63 ± 0.2 | 89.12 ± 0.1 | 80.38 ± 0.1 |
| 0.25 | 39.44 ± 0.2 | 40.25 ± 0.1 | 74.89 ± 0.2 | 73.53 ± 0.0 | 79.74 ± 0.1 | 68.79 ± 0.1 |
| 0.125 | 30.93 ± 0.01 | 30.45 ± 0.0 | 53.8 ± 0.1 | 51.84 ± 0.2 | 61.56 ± 0.1 | 52.37 ± 0.1 |
| 0.0625 | 27.61 ± 0.4 | 29.24 ± 0.1 | 47.31 ± 0.0 | 40.84 ± 0.1 | 44.26 ± 0.1 | 48.65 ± 0.1 |
| 0.0312 | 24.01 ± 0.2 | 25.36 ± 0.1 | 34.34 ± 0.0 | 31.15 ± 0.1 | 28.54 ± 0.1 | 33.23 ± 0.1 |
| IC50 (mg mL−1) | 0.34 ± 0.1 | 0.39 ± 0.1 | 0.053 ± 0.1 | 0.041 ± 0.1 | 0.047 ± 0.01 | 0.09 ± 0.01 |
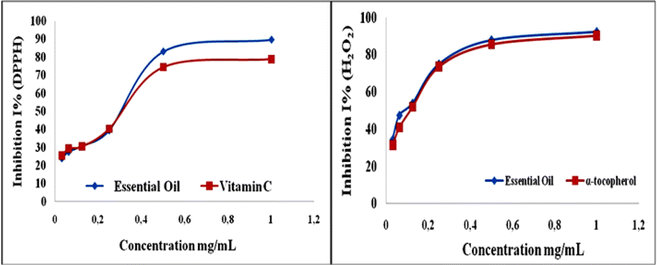 | ||
| Fig. 4 Graphic curve of the antioxidant activity of H. cheirifolia EO (DPPH and H2O2 tests) at different concentrations. | ||
According to the impressive antioxidant findings, H. cheirifolia EO showed the greatest level of antioxidant capacity in the FRAP, H2O2, and DPPH tests. Furthermore, with regard to H. cheirifolia extracts, several investigations have demonstrated that the fractions of this species contained phenolic compounds17,25,57 and demonstrated its antioxidant capacities using ABTS and DPPH assays. Additionally, the best rate of inhibition and strongest iron-reducing power were noted with the β-carotene test.18,58 Therefore, the species is regarded as a trustworthy source of natural antioxidant.
Antibacterial activity
The antibacterial effect of H. cheirifolia EO was assessed using the disc technique. After a 24 hour period of incubation at 37 °C, the inhibition zone diameter (IZD) of the disc was measured to determine the findings (Table 2). In comparison to the standard antibiotic (positive control), the findings show that the EO has significant antibacterial capacity. The findings demonstrated that the antibacterial potential of the essential oil varied depending on the type of bacteria tested. It was found to be less effective against E. coli and Proteus and be more active against Gram-(+) bacteria (IZD = 16 ± 0.01 mm to 7 ± 0.07 mm) than Gram-(−) bacteria (IZD of 13 mm to 2 mm). Additionally, IZD values showed that H. cheirifolia essential oil had the best antibacterial action in fighting S. aureus (IZD = 12 ± 0.06 mm for 0.25 mg mL−1), but its ability to combat K. pneumoniae and E. coli was lower (IZD = 1 ± 0.05 mm for 0.5 mg mL−1 and 2 mm for 0.25 mg mL−1).| Bacterial strains | IZD (mm) | ||||||
|---|---|---|---|---|---|---|---|
| Control | Concentration (mg mL−1) | ||||||
| DMSO | G | 1 | 1/2 | 1/4 | 1/8 | Sa | |
| a S = sensitivity: (+) sensitive; (++) very sensitive; and (−) not sensitive. | |||||||
| S. aureus | 0 | 21 ± 0.09 | 16 ± 0.01 | 15 ± 0.01 | 12 ± 0.20 | 8 ± 0.04 | ++ |
| S. pneumoniae | 0 | 12 ± 0.06 | 15 ± 0.01 | 13 ± 0.07 | 7 ± 0.04 | 7 ± 0.06 | ++ |
| E. coli | 0 | 16 ± 0.12 | 3 ± 0.05 | 1 ± 0.05 | 0 ± 0.01 | 0 ± 0.32 | − |
| K. pneumoniae | 0 | 14 ± 0.07 | 9 ± 0.04 | 4 ± 0.01 | 2 ± 0.06 | 0 ± 0.01 | + |
| P. mirabilis | 0 | 10 ± 0.01 | 0 ± 0.00 | 0 ± 0.00 | 0 ± 0.00 | 0 ± 0.01 | − |
| E. aerogenes | 0 | 0 ± 0.35 | 13 ± 0.00 | 10 ± 0.08 | 10 ± 0.18 | 7 ± 0.02 | + |
According to these results, H. cheirifolia EO had the same effect against Gram-(+) bacteria, especially against S. aureus, which was the bacterium that was most sensitive to H. cheirifolia EO in earlier studies on the same plant from various regions.19,20 The results of Segueni et al.22 showed considerable activity of H. cheirifolia EO against E. coli and K. pneumoniae, with the strongest inhibition zone diameter value (23–12 mm for 2000–25 μg mL−1) and (IZD = 22.33–8.6 mm for 2000–25 μg mL−1). However, our study revealed that the EO had a very weak-to-no effect on E. coli (IZD = 3–0 mm for 1–0.25 mg mL−1) and K. pneumoniae (IZD = 9–0 mm for 1–0.15 mg mL−1). Nevertheless, HC-AKA-EO was active against Gram-(−) microorganisms (E. coli and E. cloacae), in contrast to the results of Segueni et al.,22 who reported that the sample was ineffective against these bacterial species. The same observation pertains to an investigation carried out by Majouli et al.,16 which demonstrated the antibacterial ability of the Tunisian sample against Gram-positive bacteria, specifically S. aureus (MIC of 0.078 to 0.312 mg mL−1), as opposed to Gram-(−) bacterial strains. Furthermore, the H. cheirifolia extracts were ineffective against E. coli, P. aeruginosa, and S. aureus, according to the findings of Bousselsela et al.25 According to Rahali et al.,21 fluctuation in EO content may be the primary cause of these variations. Additionally, several prior studies have shown how well α-pinene works to inhibit and eradicate a broad range of microorganisms, Gram (+) and Gram (−). It has also been used to alter the resistance of bacteria to antibiotics.56,59–61 According to Rahali et al.,21 an examination of the correlation between main compounds with biological capacities, and the EO revealed that the quantities of drimenin, sesquiterpenes, lactones, and drimane-type compounds were negatively correlated with the inhibition of β-carotene bleaching, whereas a favorable correlation was observed between the contents of α-pinene and monoterpene hydrocarbons. Nevertheless, the biological activities of an essential oil rely on interactions between primary and the minor compounds, which can result in antagonistic or synergistic reactions, rather than on the concentration of the main molecule alone.62–64 As a result, the lack of a meaningful relationship between the amounts of some phytochemical components, and the degree of activity does not rule out their potential benefits because the biological capacities of these compounds have been revealed in several studies and on a large scale.
Previous studies have shown that α-pinene,54,65 β-pinene,73 sabinene,66,71 germacrene-D,74 and linalool,67,76 are volatile compounds present in many EOs extracted from several plant species with several recognized biological properties. These substances have demonstrated antioxidant, antimicrobial, anti-inflammatory, cytotoxic, and allelopathic effects.10,12,54,65,67,70
The possible mechanisms of action of these compounds are described below:
α-Pinene is a bicyclic monoterpene, whose isomer is β-pinene. It is present in EOs from plants belonging to the Asteraceae family (H. Cheirifolia,12,16,21 Matricaria recutita L.68,69 and Achillea millefolium L.70,77) This substance is known for its antimicrobial65,67,73 and antioxidant activity.54,55 Some investigations also showed that α-pinene had hypoglycemic and anti-inflammatory activities in vivo.72 Conversely, its isomer, β-pinene, is known for its good biological properties in several species of medicinal plant.73 Enaide et al.69 confirmed this, since they demonstrated that this natural compound presented hypoglycemic and hypolipidemic effects. This appears to entail, at least partially, the blockage of ATP-dependent K+ channels and activation of LPL, respectively. Additionally, the administration of β-pinene treatment demonstrated a significant anti-inflammatory effect, potentially through the inhibition of inflammatory mediators involved in the second phase of the process and/or a reduction in leukocyte migration, suggesting a connection between the action of the compound and suppression of cytokine production. It is crucial to emphasize that further research is required to fully understand the processes underlying the hypoglycemic, hypolipidemic, and anti-inflammatory benefits of β-pinene in diabetic rats. In contrast, a study by Ana Cristina et al.65 showed that only the positive enantiomers of pinene have an antimicrobial effect against MRSA, C. neoformans, R. oryzae, or C. albicans.
Germacrene-D is a hydrocarbon belonging to the class of sesquiterpenoid germacranes. This sesquiterpene has five isomers: germacrene A, B, C, D, and E. It is known for its antibacterial properties.70 According to several previous studies, this organic compound is present in the EOs of several plants (L. camara, L. alba, A. gratissima, and L. montevidensis).69 This substance can be applied as an adjuvant agent when applying azoles and aminoglycosides since it possesses antifungal and antibacterial properties.67
Sabinene is a natural organic compound classified as a monoterpene found in the EOs of various plants.67,73,77 EO antimicrobial activity is highly correlated with the composition of monoterpenes compared with other chemical families and in oxygenated molecules against hydrocarbons. Indeed, this monoterpene (sabinene) showed promising antibacterial and antifungal activities.67,73,75
Linalool is a terpene alcohol, an unsaturated tertiary alcohol, with a floral and fresh odor. It is found in a majority of essential oils, notably those of L. angustifolia, L. Officinalis, A. Millefolium and S. rosmarinus.54,66,72,76 This natural product is one of the major constituents of some of these EOs, and it has been shown in previous investigations to improve the permeability of fungal cells and negatively charged membranes.78,79 Owing to their molecular makeup, alcohols have significant affinity for attaching to various molecular structures, including proteins and glycoproteins. Because of this, they have a strong affinity for cell membranes and have a high potential to penetrate cell walls and release cytoplasm.
Antifungal activity
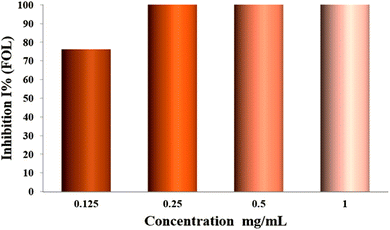
The effect of HC-AKA-EO on the mycelial growth of phytopathogenic FOL (Fusarium oxysporum f.sp. lycopersici) fungi was evaluated. FOL was 100% sensitive to oil over the six-day incubation period, as indicated by data (Table 3; Fig. 5), which also showed that the essential oil was a very efficient inhibitor. In contrast to the control (8.8 ± 0.1 cm, data is expressed as mean ± SEM, n = 3), the sample fully suppressed the development of FOL at three doses (1/4, 1/2, and 1 mg mL−1), while the maximum mycelial growth rate (2.1 ± 0.1 cm; with inhibition effect % = 76.13 ± 0.09) was recorded at a 0.125 mg mL−1 concentration. These results show that FOL responded to increasing concentrations of HC-AKA-EO, which increased the rate of inhibition and slowed mycelial development. Conceivably, these results are linked mainly to the richness of HC-AKA-EO in the bioactive compounds, especially monoterpenes and sesquiterpenes. Indeed, many studies have shown that monoterpenoids and sesquiterpenoids are considered excellent alternatives for antifungal and antibacterial agents due to their wide-reaching bioactivities and abundance of structural skeletal types.80–82 To the best of our knowledge, no prior research has looked into how the essential oil of this sap affects the mycelial growth of FOL. This work is the first to address this activity, and it is interesting to see that HC-AKA-EO has a strong antifungal effect against FOL.| Colony diameter (cm) | Concentration EO (mg mL−1) | Inhibition effect (%) | |
|---|---|---|---|
| C | T | ||
| 8.8 ± 0.1 | 2.1 ± 0.1 | 1/8 | 76.13 ± 0.09 |
| 0.0 ± 0.0 | 1/4 | 100 ± 0.14 | |
| 0.0 ± 0.0 | 1/2 | 100 ± 0.48 | |
| 0.0 ± 0.0 | 1 | 100 ± 0.07 | |
Conclusions
These findings indicate that the essential oil of H. cheirifolia may be a readily available, natural source of chemicals with antioxidant, antimicrobial, and antifungal properties. They can be applied to cosmetics, food preparation and preservation, and pharmacological treatments. Furthermore, in comparison to earlier research, it can be said that, to acquire oil of superior quality and value, the right time period for the growth of this plant as well as section from which the oil is to be harvested must be chosen. However, to fully explore the benefits of H. cheirifolia EO, more in vivo research is necessary, along with more testing on different bacterial and fungal strains, which could contribute significantly to the discovery and development of new antimicrobial and antifungal agents based on H. cheirifolia EO. In addition, we hope in future studies to examine the essential oils separately from leaves, flowers, and stems to find out which part is rich in the bioactive compounds. Additional investigations are needed to determine the compounds responsible for antioxidant, antibacterial, and antifungal activities.Abbreviations
| EO | Essential oil |
| GC-MS | Gas chromatography coupled with mass spectrometry |
| EI | Electron ionization |
| LRI | Linear retention index |
| DMSO | Dimethyl sulfoxide |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| H2O2 | Hydrogen peroxide |
| FRAP | Ferric reducing antioxidant power |
| Y | Yield |
| IC50 | Half-maximal inhibitory concentration |
| MHA | Mueller-Hinton Agar |
| ADT | Agar diffusion technique |
| IZ | Inhibition zone diameter |
| PDA | Potato, dextrose, and agar |
| FOL | Fusarium oxysporum f.sp. lycopersici |
| USA | United States of America |
| UK | United Kingdom |
| G | Gentamicin |
Data availability
The manuscript includes all the research data used in this study.Author contributions
Wassila Benabderrahmane, Hamza Fadel, Ruqaiah I. Bedaiwi, Mohammad A. Alanazi, Helal F. Al-Harthi: conceptualization, methodology, and software. Ines Sekhara, Imad Mennai, Tasahil S. Albishi, Alaa T. Qumsani, Rokayya Sami, Hamza Fadel: funding acquisition. Rokayya Sami, Hala M. Abo-Dief, Sameer H. Qari, Wassila Benabderrahmane, Mohammad A. Alanazi: validation, formal analysis, investigation, and resources. Imed Eddine Kadi, Mahmoud Helal, Tasahil S. Albishi, Alaa T. Qumsani, Mohammad A. Alanazi, Hala M. Abo-Dief, Sameer H. Qari: writing—original draft preparation. Ruqaiah I. Bedaiwi, Helal F. Al-Harthi, Wassila Benabderrahmane, Imed Eddine Kadi, Mahmoud Helal, Roqayah H. Kadi, Suzan A. Abushal: data curation, writing—review and editing, and visualization. Roqayah H. Kadi, Suzan A. Abushal, Wassila Benabderrahmane, Rokayya Sami, Ines Sekhara, Imad Mennai: software and drawings.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors extend their appreciation to Taif University, Saudi Arabia, for supporting this work through project number (TU-DSPP-2024-79). The Center for Scientific and Technical Research in Physico-Chemical Analyzes (CRAPC)-PTAPC-Laghouat, Algeria's Miss Kherifi Hadda, is much appreciated by the authors for her analysis of the essential oil sample. Further, the authors thank Marta Lores for her great efforts in editing.References
- A. Hussain, Potential Medicinal Uses of Plants From the Asteraceae (Compositae) Family in Pakistan: A Literature Review Based Meta-analysis, J. Herb. Med., 2024, 45, 100871 CrossRef.
- A. Rolnik and B. Olas, The plants of the Asteraceae family as agents in the protection of human health, Int. J. Mol. Sci., 2021, 22(6), 3009 CrossRef CAS PubMed.
- M. Denisow-Pietrzyk, L. Pietrzyk and B. Denisows, Asteraceae species as potential environmental factors of allergy, Environ. Sci. Pollut. Res., 2019, 26, 6290–6300 CrossRef.
- G. V. Ccana-Ccapatinta, M. Monge, P. L. Ferreira and F. B. Da Costa, Chemistry and medicinal uses of the subfamily Barnadesioideae (Asteraceae), Phytochem. Rev., 2018, 17, 471–489 CrossRef CAS.
- E. Piątkowska, W. Biel, R. Witkowicz and J. Kępińska-Pacelik, Chemical composition and antioxidant activity of Asteraceae family plants, Appl. Sci., 2022, 12(23), 12293 CrossRef.
- R. M. K. Yien, A. P. d. Santos Matos, A. C. C. Gomes, D. d. A. Garófalo, R. Santos-Oliveira, N. K. Simas and E. Ricci-Júnior, Nanotechnology promoting the development of products from the biodiversity of the Asteraceae family, Nutrients, 2023, 15(7), 1610 CrossRef CAS.
- M. Petrova, K. M. Georgieva and M. P. Geneva, Influence of abiotic and biotic elicitors on organogenesis, biomass accumulation, and production of key secondary metabolites in Asteraceae plants, Int. J. Mol. Sci., 2024, 25(8), 4197 CrossRef CAS.
- M. R. Akhgar, M. Shariatifar, A. R. Akhgar, M. Moradalizadeh and A. Faghihi-Zarandi, Chemical composition and antibacterial activity of the leaf essential oil from Hertia Intermedia, Chem. Nat. Compd., 2012, 48, 329–331 CrossRef CAS.
- D. Iamonico and R. El mokni, Hertia cheirifolia and H. maroccana (Asteraceae), two species endemic to North Africa: nomenclatural notes, morphology, distribution, and IUCN Red List assessments, Collect. Bot., 2021, 40, 009 Search PubMed.
- S. Soltanian, M. Sheikhbahaei, N. Mohamadi, A. Pabarja, M. F. S. Abadi and M. H. M. Tahroudi, Biosynthesis of zinc oxide nanoparticles Using Hertia intermedia and evaluation of its cytotoxic and antimicrobial activities, J. Bionanosci., 2021, 11, 245–255 CrossRef.
- K. Majouli, M. B. Hlila, A. Hamdi and A. Kenani, Phytochemical and pharmacological properties of Hertia L. genus, SOJ Pharm. Pharm. Sci., 2018, 5(2), 1–3 Search PubMed.
- M. Cherni, S. Dallali, D. Dallali, R. Mouhbi, H. Bouraoui, S. Jdidi and H. Selmi, Allelopathic activity and chemical constituents of extracts from leaves and stems of Kherchoun (Hertia cheirifolia), J. Int. Res. Med. Pharm. Sci., 2023, 18(2), 1–12 CrossRef.
- A. S. Gubb, La flore Algérienne naturelle et Acquise, Imprimerie Adolphe Jourdan, place du gouvernement, Alger, 1913 Search PubMed.
- G. Pottier-Alapetite, Flore de la Tunisie. Imprimerie officielle de la république Tunisienne, 1981, vol. 11 Search PubMed.
- N. T. Beniston and W. S. Beniston, Fleurs d'Algérie F.N.1, Alger, 1984 Search PubMed.
- K. Majouli, M. B. Hlila, G. Flamini, H. B. Jannet and A. Kenani, In vitro antibacterial activity of the Hertia cheirifolia L. essential oils, J. Coastal Life Med., 2016, 4(11), 865–867 CrossRef CAS.
- H. Bouriche, S. Kada, A. M. Assaf, A. Senator, F. Gül and I. Dimertas, Phytochemical screening and anti-inflammatory properties of Algerian Hertia cheirifolia methanol extract, Pharm. Biol., 2016, 54(11), 2584–2590 CrossRef CAS PubMed.
- K. Abdelouhab, T. Guemmaz, M. Karamać, D. E. Kati, R. Amarowicz and L. Arrar, Phenolic composition and correlation with antioxidant properties of various organic fractions from Hertia cheirifolia extracts, J. Pharm. Biomed. Anal., 2023, 235, 115673 CrossRef CAS PubMed.
- N. Bassa, S. Boulechfar, S. Boutellaa, M. M. Senoussi and A. Zellagui, Essential oils of Hertia cheirifolia Leaves in fructification stage with high anti bacterial activity, Ann. Rom. Soc. Cell Biol., 2022, 26(1), 3784–3791 Search PubMed.
- A. Ounoughi, M. Ramdani, T. Lograda, P. Chalard and G. Figueredo, Chemical composition, antimicrobial activity and chromosome number of Hertia cheirifolia L. from Algeria, Acta Sci. Nat., 2020, 7(2), 31–43 Search PubMed.
- N. Rahali, S. Mehdi, F. Younsi, M. Boussaid and C. Messaoud, Antioxidant, α-amylase, and acetylcholinesterase inhibitory activities of Hertia cheirifolia essential oils: influence of plant organs and seasonal variation, Int. J. Food Prop., 2017, 20(S2), S1637–S1651 Search PubMed.
- N. Segueni, A. Zellagui, S. Boulechfar, K. Derouiche and S. Rhouati, Essential oil of Hertia cheirifolia leaves: chemical composition, antibacterial and antioxidant activities, J. Mater. Environ. Sci., 2017, 8(2), 551–556 CAS.
- K. Majouli and A. Kenani, Bioactivity of essential oil from Hertia cheirifolia L. flowers in the control of bacteria, Trends Phytochem. Res., 2017, 1(1), 23–26 CAS.
- S. Attia, K. L. Grissa, A. C. Mailleux, S. Heuskin, G. Lognay and T. Hance, Acaricidal activities of Santolina africana and Hertia cheirifolia essential oils against the two-spotted spider mite (Tetranychusurticae), SCI Pest Manag. Sci., 2012, 68, 1069–1076 CrossRef CAS PubMed.
- H. Bousselsela, A. Benhouda, M. Yahia, S. Benbia, A. Ghecham and A. Zidani, In vitro evaluation of antioxidant and antibacterial activities of extracts of Hertia cheirifolia's leaves, Nat. Sci., 2012, 4(11), 825–831 Search PubMed.
- A. Khedidja, C. Touahria, N. E. H. Djeghader and H. Boudjelida, Laboratory study of the larvicidal efficacy of a local plant Hernia cheirifolia against the most abundant mosquito species, in Algeria, J. Entomol. Zool. Stud., 2018, 6(1), 258–262 Search PubMed.
- B. Boulanouar, K. Cheraif, A. Gherib and M. Rezzoug, GC-MS Analysis of essential oil of Lavandula officinalis (Lamiaceae) from Laghouat, Algeria, South Asian J., 2022, 12(2), 232–236 Search PubMed.
- V. I. Babushok, P. J. Linstrom and I. G. Zenkevich, Retention indices for frequently reported compounds of plant essential oils, J. Phys. Chem. Ref. Data, 2011, 40(4), 043101 CrossRef.
- S. Mahcene, F. Elhouiti, I. Mennai, D. C. G. A. Pinto, D. Tahri, M. Ouinten and M. Yousfi, A comparative study on the chemical composition, anti-microbial, antiparasitic, and cytotoxic activities of Rhanterium adpressum Coss. & Dur. and Rhanterium suaveolens Desf. essential oils from Algeria, J. Essent.Oil Bear. Plants, 2024, 27(2), 327–340 CrossRef CAS.
- O. D. Gargouri, B. Gargouri, S. K. Trabelsi, M. Bouaziz and R. Abdelhédi, Synthesis of 3-O-methylgallic acid a powerful antioxidant by electrochemical conversion of syringic acid, Biochim. Biophys. Acta, Gen. Subj., 2013, 1830(6), 3643–3649 CrossRef CAS.
- A. Remigante, S. Spinelli, E. Straface, L. Gambardella, D. Caruso, G. Falliti, S. Dossena, A. Marino and R. Morabito, Antioxidant activity of quercetin in a H2O2-induced oxidative stress model in red blood cells: functional role of band 3 protein, Int. J. Mol. Sci., 2022, 23(19), 10991 CrossRef CAS.
- M. Chokri, A. Laabidi and M. Boussaid, Myrtus communis L. Infusions: the effect of infusion time on phytochemical composition, antioxidant, and antimicrobial activities, J. Food Sci., 2012, 77(9), 941–947 Search PubMed.
- W. Song, L. Zhou, C. Yang, X. Cao, L. Zhang and X. Liu, Tomato Fusarium wilt and its chemical control strategies in a hydroponic system, Crop Prot., 2004, 23(3), 243–247 CrossRef CAS.
- R. P. Patel, R. Singh, B. R. R. Rao, R. R. Singh, A. Srivastava and R. K. Lal, Differential response of genotype×environment on phenology, essential oil yield and quality of natural aroma chemicals of five Ocimum species, Ind. Crops Prod., 2016, 87, 210–217 CrossRef CAS.
- S. F. K. Doozakhdarreh, J. Khorshidi and M. R. Morshedloo, Essential oil content and components, antioxidant activity and total phenol content of rosemary (Rosmarinus ofcinalis L.) as affected by harvesting time and drying method, Bull. Natl. Res. Cent., 2022, 46(1), 199 CrossRef.
- O. S. Tsiftsoglou, R. Stagiopoulou, N. Krigas and D. Lazari, Exploring the ecological preferences and essential oil variability in wild-growing populations of the endangered local greek endemic Thymus holosericeus (Lamiaceae), Plants, 2023, 12(2), 348 CrossRef CAS PubMed.
- F. Z. Benomari, M. Sarazin, D. Chaib, A. Pichette, H. Boumghar, Y. Boumghar and N. Djabou, Chemical variability and chemotype concept of essential oils from Algerian wild plants, Molecules, 2023, 28(11), 4439 CrossRef CAS PubMed.
- O. Izuegbuna, G. Otunola and G. Bradley, Chemical composition, antioxidant, antiinflammatory, and cytotoxic activities of Opuntia stricta cladodes, PLoS One, 2019, 14(1), e0209682 CrossRef CAS PubMed.
- Z. J. Ni, X. Wang, Y. Shen, K. Thakur, J. Han, J. G. Zhang, F. Hu and Z. J. Wei, Recent updates on the chemistry, bioactivities, mode of action, and industrial applications of plant essential oils, Trends Food Sci. Technol., 2021, 110(1), 78–89 CrossRef CAS.
- P. Ramak, S. K. Osaloo, M. Sharifi, H. Ebrahimzadeh and M. Behmanesh, Biosynthesis, regulation and properties of plant monoterpenoids, J. Med. Plants Res., 2014, 8(29), 983–991 CrossRef.
- F. Z. Kamal, G. D. Stanciu, R. Lefter, V. V. Cotea, M. Niculaua, D. C. Ababei, A. Ciobica and A. Ech-Chahad, Chemical Composition and Antioxidant Activity of Ammi visnaga L. Essential Oil, Antioxidants, 2022, 11(2), 347 CrossRef CAS.
- H. Fadel, F. Benayache, J. C. Chalchat, G. Figueredo, P. Chalard, H. Hazmoune and S. Benayache, Essential oil constituents of Juniperus oxycedrus L. and Cupressus sempervirens L. (Cupressaceae) growing in Aures region of Algeria, Nat. Prod. Res., 2019, 35(15), 2616–2620 CrossRef.
- S. Walia, S. Mukhia, V. Bhatt, R. Kumar and R. Kumar, Variability in chemical composition and antimicrobial activity of Tagetes minuta L. essential oil collected from different locations of Himalaya, Ind. Crops Prod., 2020, 150, 112449 CrossRef CAS.
- N. Farhadi, K. Babaei, S. Farsaraei, M. Moghaddam and A. G. Pirbalouti, Changes in essential oil compositions, total phenol, flavonoids and antioxidant capacity of Achillea millefolium at different growth stages, Ind. Crops Prod., 2020, 152(8), 112570 CrossRef CAS.
- S. Daghbouche, I. Ammar, D. M. Rekik, Z. E. Djazouli, B. Zebib and O. Merah, Effect of phenological stages on essential oil composition of Cytisus triflorus L'Her, J. King Saud Univ., Sci., 2020, 32(4), 2383–2387 CrossRef.
- M. Afshar, S. Najafian and M. Radi, The effect of harvest time on the natural product of Rosmarinus officinalis L. from South Iran (Fars province), Nat. Prod. Res., 2021, 36(10), 2637–2642 CrossRef.
- S. Srivastava, R. K. Lal, R. Maurya, A. Mishra, A. K. Yadav, G. Pandey, P. K. Rout and C. S. Chanotiya, Chemical diversity of essential oil among basil genotypes (Ocimum viride Willd.) across the years, Ind. Crops Prod., 2021, 173(3149362), 114153 CrossRef CAS.
- S. Rathore, S. Mukhia, S. Kapoor, V. Bhatt, R. Kumar and R. Kumar, Seasonal variability in essential oil composition and biological activity of Rosmarinus officinalis L. accessions in the western Himalaya, Sci. Rep., 2022, 12(1), 1–13 CrossRef PubMed.
- K. Majouli, M. B. Hlila, A. Hamdi, G. Flamini, H. B. Jannet and A. Kenani, Antioxidant activity and α-glucosidase inhibition by essential oils from Hertia cheirifolia (L.), Ind. Crops Prod., 2015, 82, 23–28 CrossRef.
- A. Kadri, Z. Zarai, I. B. Chobba, N. Gharsallah, M. Damak and A. Békir, Chemical composition and in vitro antioxidant activities of Thymelaea hirsute L: essential oil from Tunisia, Afr. J. Biotechnol., 2013, 10, 2930–2935 Search PubMed.
- A. Assaeed, A. Elshamy, A. E. El Gendy, B. Dar, S. Al-Rowaily and A. Abd-El Gawad, Sesquiterpenes-rich essential oil from above ground parts of Pulicaria somalensisexhibited antioxidant activity and allelopathic effect on weeds, Agronomy, 2020, 10(3), 399 CrossRef CAS.
- D. V. Sudarikov, Y. V. Krymskaya, A. K. Melekhin and O. G. Shevchenko, Synthesis and antioxidantactivity of monoterpenen itrobenzylidene sulfenimines, Chem. Pap., 2021, 75(6), 1–7 Search PubMed.
- I. Y. Chukicheva, I. V. Fedorova, O. G. Shevchenko and A. V. Kutchin, Monoterpene derivatives of sesamol. Synthesis and evaluation of antioxidant properties, Russ. Chem. Bull., 2023, 72, 2215–2223 CrossRef CAS.
- M. Chraibi, A. Farah, O. Elamin, H. M. Iraqui and K. Fikri-Benbrahim, Characterization, antioxidant, antimycobacterial, antimicrobial effects of Moroccan rosemary essential oil, and its synergistic antimicrobial potential with carvacrol, J. Adv. Pharm. Technol. Res., 2020, 11(1), 25–29 CrossRef CAS PubMed.
- B. B. Akacha, M. Michalak, I. G. Mekinić, M. Kačániová, M. Chaari, F. Brini, R. B. Saad, W. Mnif, S. Garzoli and A. B. Hsouna, Mixture design of α-pinene, α-terpineol, and 1,8-cineole: a multiobjective response followed by chemometric approaches to optimize the antibacterial effect against various bacteria and antioxidant activity, Food Sci. Nutr., 2023, 12(1), 574–589 Search PubMed.
- J. K. Nagy, Á. M. Móricz, A. Böszörményi, Á. Ambrus and I. Schwarczinger, Antibacterial effect of essential oils and their components against Xanthomonas arboricola pv. pruni revealed by microdilution and direct bioautographic assays, Front. Cell. Infect. Microbiol., 2023, 13, 2023 Search PubMed.
- A. Benslama, S. Boumerfeg, A. Beghiani, S. Aouachria, S. Khennou and L. Arrar, Total phenolic contents and antioxidant activities of Hertia cheirifolia leaves extracts, Proceeding of the 2nd African Congress on Biology and Health University Ferhat Abbas Setif1, 2012, vol. 93 Search PubMed.
- M. Menakh, D. Mahdi, S. Boutellaa, A. Zellagui, M. Lahouel and C. Bensouici, In vitro antioxidant activity and protective effect of Hertia cheirifolia L. n-butanol extract against liver and heart mitochondrial oxidative stress in rat, Acta Sci. Nat., 2020, 7(1), 33 CAS.
- A. C. R. da Silva, P. M. Lopes, M. M. B. Azevedo, D. C. M. de Costa, C. S. Alviano and D. S. Alviano, Biological activities of α-pinene and β-pinene enantiomers, Molecules, 2012, 17(6), 6305–6316 CrossRef CAS.
- B. Salehi, S. Upadhyay, I. E. Orhan, A. K. Jugran, S. L. D. Jayaweera, D. A. Dias, F. Sharopov, Y. T. N. Martins, N. Baghalpour, W. C. Cho and J. Sharifi-Rad, Therapeutic potential of α- and β-Pinene: a miracle gift of nature, Biomol, 2019, 9(11), 738 CAS.
- A. Joshi, U. Sharma, K. Samani, V. Surendra, G. Swamy and P. Manjunath, Review on therapeutic activity of pinene (C10H16): an essential oil, Int. J. Adv. Res. Pharm. Edu., 2020, 2(1), 28–33 Search PubMed.
- S. Aazza, B. Lyoussi and M. G. Miguel, Antioxidant and antiacetylcholinesterase activities of some commercial essential oils and their major compounds, Molecules, 2011, 16(9), 7672–7690 CrossRef CAS PubMed.
- J. H. Tak, E. Jovel and M. B. Isman, Comparative and synergistic activity of Rosmarinus officinalis L. essential oil constituents against the larvae and an ovarian cell line of the cabbage looper, Trichoplusiani (Lepidoptera: Noctuidae), Pest Manage. Sci., 2016, 72(3), 474–480 CrossRef CAS.
- B. Tohidi, M. Rahimmalek and A. Arzani, Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of Thymus species collected from different regions of Iran, Food Chem., 2017, 220, 153–161 CrossRef CAS PubMed.
- A. C. d. S. Rivas, P. M. Lopes, M. M. A. Barros, D. C. C. Machado, C. S. Alviano and D. S. Alviano, Biological Activities of α-Pinene and β-Pinene Enantiomers, Molecules, 2012, 17(6), 6305–6316 CrossRef.
- S. Ramos, L. B. Rojas, M. E. Lucena, G. Meccia and A. Usubillaga, Chemical Composition and Antibacterial Activity of Origanum majorana L. Essential Oil from the Venezuelan Andes, J. Essent. Oil Res., 2011, 23(5), 45–49 CrossRef CAS.
- K. Diass, M. Merzouki, K. Elfazazi, H. Azzouzi, A. Challioui, K. Azzaoui, B. Hammouti, R. Touzani, F. Depeint, A. A. Gotor and L. Rhazi, Plants, 2023, 12(7), 1571 CrossRef CAS.
- M. Kazemi, Chemical Composition and Antimicrobial Activity of Essential Oil of Matricaria recutita, Int. J. Food Prop., 2015, 18, 1784–1792 CrossRef CAS.
- I. Salamon, A. Ibraliu and M. Kryvtsova, Essential Oil Content and Composition of the Chamomile Inflorescences (Matricaria recutita L.) Belonging to Central Albania, Horticulturae, 2023, 9(1), 47 CrossRef.
- P. S. Daniel, E. L. B. Lourenço, R. M. S. Cruz, C. H. D. S. Gonçalves, L. R. M. D. Almas, J. Hoscheid, C. D. Silva, E. Jacomassi, L. B. Junior and O. Alberton, Composition and antimicrobial activity of essential oil of yarrow (Achillea Millefolium L.), AJCS, 2020, 14(3), 545–550 Search PubMed.
- W. Zaibet, H. Laouer, S. amira, G. Flamini, M. Ramdani and S. Akkal, Chemical composition and biological activities of Daucus aureus essential oils from eastern algeria, J. Chil. Chem. Soc., 2015, 60(4), 2721–2728 CAS.
- H. Özbek and B. S. Yılmaz, Anti-inflammatory and Hypoglycemic Activities of Alpha-pinene, Acta Pharm. Sci., 2017, 55(4), 7–14 Search PubMed.
- E. S. Santos, G. L. A. Coelho, Y. K. S. F. Loula, B. L. S. Landim, C. N. F. Lima, S. T. S. Machado, M. J. P. Lopes, A. D. S. Gomes, J. G. M. d. Costa, I. R. A. d. Menezes, H. D. M. Coutinho, B. Kim, C. F. B. Felipe, S. d. A. Neves and M. R. Kerntopf, Hypoglycemic, Hypolipidemic, and Anti-Inflammatory Effects of Beta-Pinene in Diabetic Rats, Evidence-Based Complementary Altern. Med., 2022, 8173307 Search PubMed.
- C. M. P. Zamora, C. A. Torres and M. B. Nuñez, Antimicrobial Activity and Chemical Composition of Essential Oils from Verbenaceae Species Growing in South America, Molecules, 2018, 23(3), 544 CrossRef.
- R. Arunkumar, S. A. Nair, K. B. Rameshkumar and A. Subramoniam, The Essential Oil Constituents of Zornia diphylla (L.) Pers, and Anti-Inflammatory and Antimicrobial Activities of the Oil, Recent Nat. Prod., 2014, 8(4), 385–393 Search PubMed.
- K. Pokajewicz, M. Białoń, L. Svydenko, R. Fedin and N. Hudz, Chemical Composition of the Essential Oil of the New Cultivars of Lavandula angustifolia Mill. Bred in Ukraine, Molecules, 2021, 26(18), 5681 CrossRef CAS PubMed.
- S. T. Chou, H. Y. Peng, J. C. Hsu, C. C. Lin and Y. Shih, Achillea millefolium L. Essential Oil Inhibits LPS-Induced Oxidative Stress and Nitric Oxide Production in RAW 264.7 Macrophages, Int. J. Mol. Sci., 2013, 14(7), 12978–12993 CrossRef.
- W. S. Alviano, R. R. Mendonça-Filho, D. S. Alviano, H. R. Bizzo, T. Souto-Padrón, M. L. Rodrigues, A. M. Bolognese, C. S. Alviano and M. M. G. Souza, Antimicrobial activity of Croton cajucara Benth linalool-rich essential oil on artificial biofilms and planktonic microorganisms, Oral Microbiol. Immunol., 2005, 20(2), 101–105 CrossRef CAS PubMed.
- F. Silva, S. Ferreira, J. A. Queiroz and F. C. Domingues, Coriander (Coriandrum sativum L.) essential oil: its antibacterial activity and mode of action evaluated by flow cytometry, J. Med. Microbiol., 2011, 60, 1479–1486 CrossRef CAS PubMed.
- F. G. Pereira, R. Marquete, L. T. Domingos, M. E. N. Rocha, A. Ferreira-Pereira, E. Mansur and D. L. Moreira, Antifungal activities of the essential oil and its fractions rich in sesquiterpenes from leaves of Casearia sylvestris Sw, An. Acad. Bras. Cienc., 2017, 89(4), 2817–2824 CrossRef CAS.
- H. Li, X. Song, H. Li, L. Zhu, S. Cao and J. Liu, Sesquiterpenes and monoterpenes from the leaves and stems of Illicium simonsii and their antibacterial activity, Molecules, 2022, 27(3), 1115 CrossRef CAS PubMed.
- H. L. Li, W. Q. Yang, X. Z. Zhou, F. Shao, T. Shen, H. Y. Guan, J. Zheng and L. M. Zhang, Antibacterial and antifungal sesquiterpenoids: chemistry, resource, and activity, Biomol., 2022, 12(9), 1271 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra03578j |
| This journal is © The Royal Society of Chemistry 2024 |