Open Access Article
Open Access ArticleExtraction and separation of anthocyanins from Kushui rose by ethanol-(NH4)2SO4 aqueous two-phase system†
Yuanyuan Li *a,
Tongyu Lia,
Hongxu Ana,
Xinyi Wanga,
Juan Hanb and
Yun Wang
*a,
Tongyu Lia,
Hongxu Ana,
Xinyi Wanga,
Juan Hanb and
Yun Wang c
c
aJingjiang College, Jiangsu University, Zhenjiang 212013, PR China. E-mail: liyuanyuan@ujs.edu.cn
bSchool of Food and Biological Engineering, Jiangsu University, Zhenjiang 212013, PR China
cSchool of Chemistry and Chemical Engineering, Jiangsu University, Zhenjiang 212013, PR China
First published on 5th July 2024
Abstract
Simultaneous extraction of anthocyanins and removal of sugars from Kushui rose was performed using an ethanol-ammonium sulphate aqueous two-phase system (ATPS). The effects of different parameters, such as type of salt, concentrations of salt and ethanol, temperature and pH on the partition coefficient and recovery of anthocyanins in the top system and sugars in the bottom system were studied. Furthermore, an experimental design of a three-level three-factor Box–Behnken design response surface methodology (RSM) was used to obtain optimal extraction conditions. The maximum partition coefficient (5.64) and recovery (78%) of anthocyanins in the top system within the investigated range were obtained at 22% (w/w) concentration of ammonium sulphate, 25% (w/w) concentration of ethanol, pH 5 and 33.5 °C. During the discussion of the main factors, the maximum recovery of sugars reached 70.09%. The HPLC profile of anthocyanins obtained from the ATPS top phase was similar to that of anthocyanins extracted by ethanol, which indicated that the ethanol-ammonium sulphate ATPS was suitable for the extraction of anthocyanins. On the basis of the anthocyanin stability experiment, anthocyanins extracted from Kushui rose should be stored at low pH and temperature.
1. Introduction
In recent years, the exploitation and application of natural pigments have caused widespread attention due to their apparent non-toxicity and safety compared with synthetic pigments.1 Anthocyanins are water-soluble natural pigments belonging to flavonoids and are responsible for pink, red, violet and blue colors of flowers, vegetables and fruits. Anthocyanins have physiological functions such as antioxidant activity and free radical scavenging, which are important in the prevention of cancer, cardiovascular disease and neurological disease.2 Therefore, finding good methods to separate anthocyanins effectively is of great significance.The organic solvent extraction is commonly used for extraction of anthocyanins in plants and the most used solvents are mixtures of ethanol and methanol/acetone because anthocyanins are soluble in polar solvents.3 However, some drawbacks of this method have emerged, such as low extraction rates, the presence of water-soluble impurities (such as sugars) in the extract, and the consumption of large amounts of organic solvents. Other extraction methods with more advanced techniques have been reported, such as pressurized solvent extraction, subcritical water extraction and supercritical fluid extraction.4–6 Limitations of these new methods are demanding more complex process conditions and higher cost in equipment.
As one of the separation methods, aqueous two-phase extraction (ATPE) has been proved to be a biocompatible and efficient technique for the downstream processing of biomolecules. Aqueous two-phase system (ATPS) not only has the function of easy processing, but also exhibits high performance in large-scale recycling of biological products. ATPS has been applied to the separation and purification of proteins,7,8 enzymes,9 antibiotics,10 genetic materials11 and low molecular weight products.12,13 The hydrophilic alcohol-salt ATPS has advantages of low viscosity and low cost14 and shows potential to achieve the desired purification and concentration of the product in a single step.15 So far, the hydrophilic alcohol-salt ATPS has been applied in the Lithospermic acid B from Salvia miltiorrhiza Bunge,16 lignans from seeds of Schisandra17 and so on. Anthocyanins have been widely found in different kinds of roses18 and Chinese Kushui rose dominates the domestic market share of more than 50% with high brand awareness. As far as we know, the paper on the hydrophilic alcohol-salt aqueous two-phase extraction of anthocyanins from rose has not been published.
This study developed a simple aqueous two-phase system for the extraction and separation of anthocyanins from Kushui rose. The anthocyanins could be enriched in the top system while the sugars were enriched in the bottom system of the ATPS. The partition coefficient and recovery of the anthocyanins in this system were optimized. In order to obtain a higher extraction rate, the influences of process parameters, including the type and concentration of salt, the concentration of ethanol, temperature and pH were studied. The concentration of salt/ethanol and temperature were optimized using response surface methodology (RSM). In addition, HPLC analysis and the stability of anthocyanins from Kushui rose were also investigated.
2. Experimental
2.1. Chemicals and materials
Kushui rose was purchased from Gansu Longcuitang Nutrition Health Priducts Co., Ltd. (Gansu, China). Methanol (HPLC grade), phenol, sulfuric acid, ethanol, ammonium sulphate, sodium dihydrogen phosphate, potassium chloride, hydrochloric acid were supplied by Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Double distilled deionized water was used throughout the experiment and all the chemicals used in the experiment were of analytical grade.2.2. Aqueous two-phase extraction
The Kushui rose petals were ground and sieved by 120 meshes, the powders were collected and subject to the experiment. A predetermined quality of ammonium sulphate solution was mixed with certain volumes of ethanol to obtain a final quality of 10.0 g. An ethanol-(NH4)2SO4 aqueous two-phase system was prepared. Then 0.1 g rose powders were added into the ATPS, the system was mixed up to form a homogeneous phase. Shaking the tube 2 min, complete phase separation was achieved by low-speed centrifugation at 3000 rpm for 10 min at 35 °C. After centrifugation the mixture was vortexed thoroughly for 2 h and held until the two phases were completely separated. The residues accumulated at the interface of two phases were discarded. The volumes of both top and bottom phases were noted. The anthocyanins and sugars concentrations in both the top and bottom phases were analyzed.2.3. Estimation of anthocyanins and total sugars
The concentration of anthocyanins was determined by a dual-wavelength pH-differential method.19 Two dilutions were prepared, one for pH 1.0 using potassium chloride buffer (0.03 M, 1.9 g KCl into 980 mL distilled water) and the other for pH 4.5 using sodium acetate buffer (0.4 M, 54.4 g CH3COONa·3H2O in 960 mL distilled water). Samples under test were diluted 5 times with the buffer solutions. A UV-visible Spectrophotometer was used for the spectral measurements. With distilled water as blank, the absorbance of each sample was measured at 520 and 700 nm, respectively. The concentrations of Kushui rose anthocyanins were expressed as cyanidin-3-glucoside (Cy-3-glc, molarextinction coefficient of 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900) equivalents.
900) equivalents.
The phenol-sulphuric acid method, based on Dubois,20 was carried out to determine the content of the total sugar in the phases. An aliquot of 1.0 mL of sample solution was mixed with 1.0 mL of phenol (5%, v/v) and 4.0 mL of sulphuric acid. The absorbance of the mixture, with the characteristic yellow–orange color, was measured at 490 nm. Dextrose was used as a standard for calibration.
The volume ratio (R) of top phase and bottom phase in the ATPS was obtained via eqn (1):
| R = VT/VB | (1) |
| K = CT/CB | (2) |
The recovery rate YT was the ratio of the anthocyanin amounts partitioned in the top phase to the total amount of anthocyanins, and it was calculated using eqn (3). The recovery rate YB was the ratio of the sugar amount partitioned in the bottom phase to the total amount of sugars, and it was calculated using eqn (4):
| YT = 100CTVT/(CTVT + CBVB) | (3) |
| YB = 100CBVB/(CTVT + CBVB) | (4) |
2.4. Experimental design of RSM
In order to obtain the optimum extraction condition, the effect of ammonium sulphate on the differential partitioning of anthocyanins and sugars in ATPS (with fixed 25% (w/w) ethanol concentration at 25 °C, no pH adjustment) was investigated with different concentrations of ammonium sulphate (from 17% to 22%, w/w). Similarly, the ATPS with varied ethanol concentrations (from 21% to 25%, w/w), under other constant conditions (with fixed 22% (w/w) ammonium sulphate concentration at 25 °C, no pH adjustment), were studied to investigate the influence of ethanol concentration on the partition behaviors. Other parameters of the ATPS were also studied: the temperature was studied in the range of 15 °C to 55 °C, and the pH was tried in the range of 1–5. All the experiments were carried out in triplicate.RSM was performed to optimize the ATPS conditions for the recovery of anthocyanins from Kushui rose. The independent variables tested were salt concentration (A), ethanol concentration (B) and temperature (C). A Box–Behnken design was selected for the optimization of the process at each of the two levels, with 17 runs including five replicates at the central point.
2.5. HPLC analysis
The HPLC system adopted in this work consisted of Agilent 1200 system (Thermo Fisher Scientific, America) equipped with a quaternary pump, surveyor plus detector. Chromatographic analysis was carried out on an Agilent TC-C18(2) column (4.6 × 250 mm, 5 μm, Shanghai, China). Sample solution was filtrated through a syringe filter (0.22 μm) and the injection volume was 10 μL. The mobile phase consisted of water (solvent A) and 100% methanol (solvent B) at a flow rate of 1.0 mL min−1. Type of isocratic elution was performed with 10% A and 90% B. The column temperature was set at 35 °C and 520 nm was selected as the detection wavelength.2.6. Stability analysis
The stability of anthocyanins from Kushui rose was evaluated at five different pHs (3.0, 4.0, 4.3, 5.0 and 6.0) by respectively fixing the temperature 70, 80 and 90 °C. Anthocyanins concentrate (0.2 g) from Kushui rose was dissolved with 50 mL deionized water (pH 4.3) and four copies of 50 mL citrate-phosphate buffers (pH 3.0, 4.0, 5.0, 6.0) in the beakers from which aliquots of 15 mL anthocyanins solution were taken out and enclosed in five glass tubes in a thermostatic water bath (preheated to a given temperature which varied ±1 °C), avoiding evaporation and shielded from light. At regular time intervals (0, 1, 3, 6, 9 and 11 h), samples were removed from the water bath and rapidly cooled by plunging into an ice water bath. The concentration of anthocyanins was measured immediately. Experiments were performed at triplicates.3. Results and discussion
3.1. Effect of salt type
The anthocyanins are known to stably exist in the form of the flavylium cation at low pH.21 The phase-forming salt selected should be acidic, ethanol-NaH2PO4 ATPS and ethanol-(NH4)2SO4 ATPS were compared to discuss the partition coefficient and recovery rate of anthocyanins and sugars from Kushui rose.In ethanol-NaH2PO4 ATPS and ethanol-(NH4)2SO4 ATPS, the differential partition of anthocyanins and sugars was studied. The recovery value of anthocyanins is close to that in ethanol-(NH4)2SO4. The difference lies in the recoveries of sugars, which are 31.14% and 58.73% in the ethanol-NaH2PO4 ATPS and ethanol-(NH4)2SO4 ATPS, respectively. Therefore, the ethanol-(NH4)2SO4 ATPS was used for the extraction of anthocyanins and sugars from Kushui rose.
3.2. Effect of salt concentration
The influence of (NH4)2SO4 concentration on the partition coefficient and recovery rate of anthocyanins and sugars in the ATPS was shown in Fig. 1. In the case of 25% (w/w) ethanol, the results indicated that partition coefficient and recovery rate of anthocyanins increased with the increase of (NH4)2SO4 concentration in ATPS. When the concentration of (NH4)2SO4 increased from 17% to 22%, the partition coefficient of anthocyanins increased from 1.06 to 5.81 and recovery rate of anthocyanins increased from 67.00% to 81.28%. It could be explained that the increase of (NH4)2SO4 concentration would lead to the increase of ethanol concentration in the upper phase and the increase of (NH4)2SO4 concentration in the bottom phase. On the one hand, the increase of ethanol concentration in the upper phase made anthocyanins favor partitioning towards the upper phase for that anthocyanins were freely soluble in ethanol. On the other hand, the increase of (NH4)2SO4 concentration in the bottom phase would enhance the salting-out effect.22 The anthocyanins were readily dissolved in the ethanol-rich phase (the upper phase) due to the driving force produced by the strengthening of salting-out effect of the salt-rich phase (the bottom phase).As the salt concentration increased, the partition coefficient of sugars in the ATPS slightly decreased from 0.85 to 0.56 while the recovery rate of sugars increased from 48.1% to 67.97%. This was because sugars were prone to be enriched in the salt-rich phase of ATPS in the presence of water-soluble small molecular organic solvents.23,24 The recovery rate of sugar reached its maximum when (NH4)2SO4 concentration was 22%, therefore this concentration of (NH4)2SO4 was chosen for further discussion.
3.3. Effect of ethanol concentration
The influence of ethanol on the partition coefficient and recovery rate of anthocyanins and sugars in the ATPS was shown in Fig. 2. In the case of 22% (w/w) (NH4)2SO4, the partition coefficient and recovery rate of anthocyanins increased with the increase of the ethanol concentration in the investigated ATPS. When the ethanol concentration increased from 21% (w/w) to 25% (w/w), the partition coefficient increased from 3.81 to 5.81 and recovery rate of anthocyanins increased from 64.91% to 81.28%.Similar to the salt effect discussed above, the ethanol concentration in the upper phase would increase when increasing the total addition amount of ethanol. Accordingly, the concentration of salt in the upper phase decreased but increased in the bottom phase.22 Due to the strengthening of salting-out effect in the bottom phase and the increase of ethanol concentration in the upper phase, the anthocyanins were readily dissolved in the ethanol-rich phase. The contaminant sugars were also prone to distribute to the bottom phase due to the incompatibility between the water-soluble small molecular organic solvents and the water-soluble monosaccharides or polysaccharides (such as glucose, glucan) as discussed above.23,24 Both the partition coefficient of sugars (0.74 to 0.56) and the recovery rate of sugars (70.09% to 67.97%) decreased slightly as the ethanol concentration increased. Thereby, 25% ethanol was finally chosen for the following discussion.
3.4. Effect of temperature
Fig. 3 showed the influence of temperature on the partition coefficient and recovery rate of anthocyanins and sugars in the ATPS. It could be observed that the partition coefficient of anthocyanins increased from 4.55 to 5.81 and the recovery rate of anthocyanins increased with the system temperature from 15 °C to 35 °C. When the temperature increased from 35 °C to 55 °C, both the partition coefficient and recovery rate of anthocyanins decreased. The recovery rate of sugars reached maximum and the partition coefficient reached minimum at 35 °C. Increasing the temperature was beneficial to cell wall-broken, which could serve to extract anthocyanins and sugars. However, the anthocyanins would be subjected to a certain damage at high temperature.25–27 This result indicated that the system temperature would influence the distribution behavior of anthocyanins and sugars in the experimental range and the best temperature was 35 °C.3.5. Effect of pH
The influence of pH on the partition coefficient and recovery rate of anthocyanins and sugars in the ATPS was investigated and shown in Fig. 4. If the pH was high, the anthocyanins would degrade rapidly. Thus, the pH of the ATPS was tested in the range of 1–5. The data indicated that the recovery rate of anthocyanins increased (72.96% to 81.28%) and the partition coefficient of anthocyanins increased (2.25 to 5.82) with increasing pH (1 to 5). The partition coefficient of sugars decreased (0.52 to 0.46) slightly while the recovery rate of sugars increased (59.31% to 68.98%). Therefore, the optimal experimental conditions were obtained with a pH value of 5 within the pH range discussed in this experiment.3.6. Modeling of the ATPS process from Kushui rose
Based on the discussion on the single factor experiment and the influence of various factors on the partition of anthocyanins, a Box–Behnken design was selected for the optimization of the process at each of the three levels, with 17 runs including five replicates at the central point. The recovery rate and partition coefficient of anthocyanins were identified as response value, and concentration of ammonium sulphate, concentration of ethanol and temperature were independent variables. And the experimental yields of three markers under various experimental conditions were presented in Table S1.†The ANONA result of response surface quadratic model for the recovery rate of anthocyanins are shown in Table S2,† the regression model established by partition coefficient is significant (P < 0.01), and R2 of the model is 0.9707 which indicates that the model shows satisfactory accuracy in fitting the experimental data. Lack of fit value P = 0.1412 which indicates the lack of fit for the equation is not significant, and the model can be used to theoretical prediction for the partition coefficient of anthocyanins. In this case A, B, C, AB, BC, B2, C2 are significant model terms. AC and A2 are not significant model terms.
The multiple regression analysis between recovery rate Y and independent variables was performed on the experimental data and the result was shown in eqn (5):
| Y = 72.84 + 4.14A + 13.74B − 5.06C − 5.44AB + 2.16AC + 4.08BC − 2.32A2 − 4.87B2 − 4.03C2 | (5) |
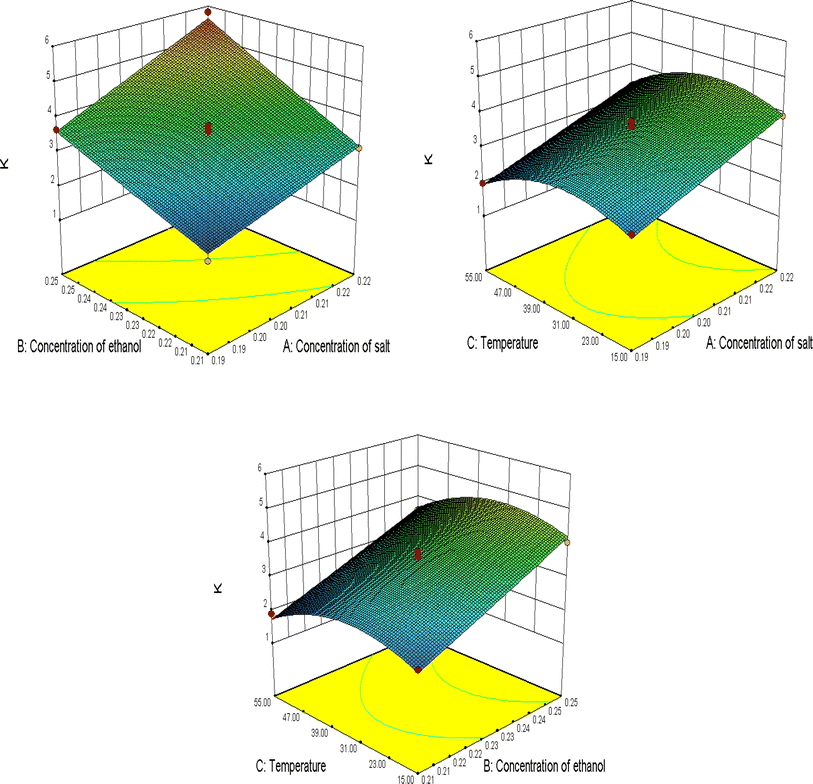
The 3D plots (Fig. 5) were constructed to predict the relationships between independent variables and dependent variables. It shows that the yield of anthocyanins increased with the increase in concentrations of salt and ethanol with the investigated range. The three factors influenced the partition coefficients are in the order: concentration of ethanol > concentration of salt > temperature.
The experimental values of the partition coefficients of anthocyanins were also presented in Table S3,† and multiple regression analysis between partition coefficient K and independent variables was performed on the experimental data and the result was shown in eqn (6):
| K = 3.59 + 0.78A + 1.00B − 0.23C + 0.22AB − 0.013AC − 0.029BC + 0.01A2 + 0.017B2 − 0.68C2 | (6) |
The ANONA result of response surface quadratic model for the partition coefficients of anthocyanins is shown in Table S3,† the regression model established by partition coefficients is significant (P < 0.01), and R2 of the model is 0.9853 which indicates that the model shows satisfactory accuracy in fitting the experimental data. Lack of fit value P = 0.1412 which indicates the lack of fit for the equation is not significant, and the model can be used to theoretical prediction for the partition coefficients of anthocyanins. In this case A, B, C, AB, C2 are significant model terms. AC, BC, A2, B2 are not significant model terms.
3D plots (Fig. 6) were constructed to predict the relationship between independent variables and dependent variables. It can be seen that the partition coefficients of anthocyanins increase with the increase in concentrations of salt and ethanol within the investigated range. The three factors influencing the partition coefficient are in the order: concentration of ethanol > concentration of salt > temperature.
Due to the partition coefficient and the yield of anthocyanins are both important to the extraction of anthocyanins from Kushui rose in order to obtain the best extraction efficiency, the partition coefficient and the yield of anthocyanins are observed corresponding to its maximum value. The results from the software indicated that optimized condition for anthocyanins extraction occurred when the concentration of ammonium sulphate, concentration of ethanol, and the temperature were set at 22% (w/w), 25% (w/w) and 33.5 °C, respectively, giving a partition coefficient of anthocyanins, 5.64 and the yield of anthocyanins, 78%. Therefore, the optimization model of the BBD is able to predict the average partition coefficient and sublation yield of anthocyanins.
3.7. HPLC analysis
The HPLC results of the Kushui rose extraction obtained by ATPE and conventional extraction were shown in Fig. 7. Two peaks were found on each chromatogram. The HPLC profiles of anthocyanins extracted by the two methods were quite similar as well as the retention time of every peak labeled in Fig. 7, which confirmed that the anthocyanins extracted by ATPE and ethanol had no obvious change.3.8. Kinetics of Kushui rose anthocyanins degradation
Previous studies have shown that thermal degradation of anthocyanins followed a first-order reaction which was expressed by the following equations:28,29| ln(Ct/C0) = −k × t | (7) |
t1/2 = −ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5/k 0.5/k
| (8) |
The temperature-dependence degradation rate constant was represented by the Arrhenius equation:
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k = ln k = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k0 − Ea/RT k0 − Ea/RT
| (9) |
It was clear from Fig. 8 that the thermal degradation of anthocyanins from Kushui rose followed first order reaction kinetics with respect to temperature. The kinetic parameters of Kushui rose anthocyanins degradation during heating (70, 80, 90 °C) at different pH levels are shown in Table 1. At the same pH levels, an increase of anthocyanins in the constant (k) and a corresponding decline in the half-life values (t1/2) with increasing heating temperature were found, which suggested that anthocyanins from Kushui rose were unstable as the temperature increased. Anthocyanins were known to be more stable at lower pH, an increase in pH may result in reduction of pigment stability. Increasing the pH from 3.0 to 6.0 hastened the degradation of Kushui rose anthocyanins, which showed pH had a strong influence on the stability of Kushui rose anthocyanins (Table 1 and Fig. 8). With the increase of pH value, the constant (k) increased, while the half-life values (t1/2) declined, in other words, the degradation of Kushui rose anthocyanins accelerated as the pH increased at the same heating temperature. When it comes to the anthocyanin stability, reactions that altered their structures may occur because of the electronic deficiency of their flavylium nuclei. Anthocyanins stability increases with the number of methoxyl in the B ring and decreases as hydroxyls increase.30 In a word, Kushui rose anthocyanins are unstable to high temperature or high pH conditions.
| pH | k (h−1) | t1/2 (h) | Ea (kJ mol−1) | ||||
|---|---|---|---|---|---|---|---|
| 70 °C | 80 °C | 90 °C | 70 °C | 80 °C | 90 °C | ||
| 3 | 0.02 | 0.040 | 0.078 | 34.6 | 17.5 | 8.9 | 70.40 |
| ±0.006 | ±0.009 | ±0.013 | ±2.5 | ±2.0 | ±1.0 | ±1.41 | |
| 4 | 0.058 | 0.088 | 0.136 | 12.0 | 7.9 | 5.1 | 44.33 |
| ±0.010 | ±0.014 | ±0.025 | ±1.3 | ±0.9 | ±0.6 | ±0.87 | |
| 4.3 | 0.069 | 0.104 | 0.152 | 10.0 | 6.7 | 4.6 | 40.78 |
| ±0.012 | ±0.019 | ±0.028 | ±1.1 | ±0.8 | ±0.5 | ±0.82 | |
| 5 | 0.119 | 0.159 | 0.208 | 5.8 | 4.4 | 3.3 | 29.10 |
| ±0.022 | ±0.031 | ±0.043 | ±0.7 | ±0.5 | ±0.4 | ±0.58 | |
| 6 | 0.251 | 0.283 | 0.314 | 2.8 | 2.4 | 2.2 | 11.73 |
| ±0.039 | ±0.044 | ±0.047 | ±0.3 | ±0.2 | ±0.1 | ±0.22 | |
The t1/2 values of Kushui rose anthocyanins extracted by the ethanol-(NH4)2SO4 ATPS were greater than those of black rice anthocyanins and blackberry anthocyanins reported at similar pH and similar temperature, which indicated that anthocyanins extracted from Kushui rose by ethanol-(NH4)2SO4 ATPS were more stable.28,29 It has been proven that the degradation rate of anthocyanins is related to the Maillard reaction of sugars to be degraded into furfural compounds.31,32 The Maillard reaction becomes strong at high temperatures, making the auxiliary color complexes of pigments unstable.32 In this work, ethanol-(NH4)2SO4 ATPS could extract and separate anthocyanins by removing the majority of sugars, which reduced the Maillard reaction and improved the stability of anthocyanins extracted from Kushui rose by ethanol-(NH4)2SO4 ATPS during heating.
The calculated Ea values for Kushui rose anthocyanins concentrate in aqueous and buffer solutions (pH 3.0–5.0) were 70.40, 44.33, 40.78, 29.10, 11.73 kJ mol−1 (Table 1). The Ea values decreased when pH changed from 3.0 to 5.0. The Ea values at pH 3.0 were highest which indicated that it was the most stable at pH 3.0. Consequently, higher Ea meant slower anthocyanins degradation, which also proved that Kushui rose anthocyanins in lower pH environment were more stable.
4. Conclusions
The present study has demonstrated the feasibility of using ATPS for the simultaneous extraction of anthocyanins and removal of sugars from Kushui rose. That the ethanol-(NH4)2SO4 ATPS has a unique advantage in terms of separation of anthocyanins and sugars needs to be particularly emphasized. The concentrations of salt and alcohol, temperature and pH discussed within investigated range produced effects on the partition behaviors of anthocyanins and sugars. Furthermore, optimal experiment was performed based on the BBD. The maximum partition coefficients (5.64) and recoveries (78%) of anthocyanins in the top system within investigated range were obtained at 22% (w/w) concentration of ammonium sulphate, 25% (w/w) concentration of ethanol, pH 5 and 33.5 °C. On the basis of HPLC analysis, the ethanol-(NH4)2SO4 ATPE was proved to be a suitable method for extraction of anthocyanins with structure unchanged. Finally, the thermal degradation of the Kushui rose anthocyanins corresponding to first-order kinetics implied that high temperature or pH should be avoided when storing or processing the anthocyanins extracted. In addition, anthocyanins extracted from Kushui rose were more stable than black rice anthocyanins and blackberry anthocyanins, so the superiority of separating anthocyanins and sugars by the ethanol-(NH4)2SO4 ATPE was further reflected through the thermal degradation experiment.Data availability
All data generated or analyzed in this study are included in this published article.Author contributions
Yuanyuan Li: conceptualization, methodology, formal analysis, data curation, writing – original draft, writing – review & editing. Tongyu Li: writing – review & editing. Hongxu An: investigation, validation, formal analysis. Xinyi Wang: formal analysis, data curation. Juan Han: investigation, resources, funding acquisition. Yun Wang: supervision, project administration, funding acquisition.Conflicts of interest
The authors declared that there are no conflicts of interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China [grant numbers 22078133, 22278191].References
- P. Chou, S. Matsui, K. Misaki and T. Matsuda, Isolation and identification of xenobiotic aryl hydrocarbon receptor ligands in dyeing wastewater, Environ. Sci. Technol., 2007, 41, 652–657 CrossRef CAS PubMed.
- I. Konczak and W. Zhang, Anthocyanins-more than nature's colours, BioMed Res. Int., 2004, 5, 239–240 CrossRef PubMed.
- W. B. Phippen and J. E. Simon, Anthocyanins in basil (Ocimum basilicum L.), J. Agric. Food Chem., 1998, 46, 1734–1738 CrossRef CAS.
- G. Fan, Y. Han, Z. Gu and D. Chen, Optimizing conditions for anthocyanins extraction from purple sweet potato using response surface methodology (RSM), LWT--Food Sci. Technol., 2008, 41, 155–160 CrossRef CAS.
- Z. Ju and L. R. Howard, Subcritical water and sulfured water extraction of anthocyanins and other phenolics from dried red grape skin, J. Food Sci., 2005, 70, 270–276 CrossRef.
- J. M. Luque-Rodríguez, M. D. Luque de Castro and P. Pérez-Juan, Dynamic superheated liquid extraction of anthocyanins and other phenolics from red grape skins of winemaking residues, Bioresour. Technol., 2007, 98, 2705–2713 CrossRef PubMed.
- S. Saravanan, J. R. Rao, B. U. Nair and T. Ramasami, Aqueous two-phase poly (ethylene glycol)–poly (acrylic acid) system for protein partitioning: influence of molecular weight, pH and temperature, Process Biochem., 2008, 43, 905–911 CrossRef CAS.
- J. C. Salgado, B. A. Andrews, M. F. Ortuzar and J. A. Asenjo, Prediction of the partitioning behaviour of proteins in aqueous two-phase systems using only their amino acid composition, J. Chromatogr. A, 2008, 1178, 134–144 CrossRef CAS PubMed.
- H. O. Johansson, F. M. Magaldi, E. Feitosa and A. P. Jr., Protein partitioning in poly (ethylene glycol)/sodium polyacrylate aqueous two-phase systems, J. Chromatogr. A, 2008, 1178, 145–153 CrossRef CAS PubMed.
- T. Furuya, Y. Iwai, Y. Tanaka, H. Uchida, S. Yamada and Y. Arai, Measurement and correlation of partition coefficients of hydrolytic enzymes for dextran + poly (ethylene glycol) + water aqueous two-phase systems at 20 oC, Fluid Phase Equilib., 1995, 110, 115–128 CrossRef CAS.
- J. Han, Y. Wang, C. Yu, Y. Yan and X. Xie, Extraction and determination of chloramphenicol in feed water, milk, and honey samples using an ionic liquid/sodium citrate aqueous two-phase system coupled with high-performance liquid chromatography, Anal. Bioanal. Chem., 2011, 399, 1295–1304 CrossRef CAS PubMed.
- S. P. Duarte, A. G. Fortes, D. M. F. Prazeres and J. C. Marcos, Preparation of plasmid DNA polyplexes from alkaline lysates by a two-step aqueous two-phase extraction process, J. Chromatogr. A, 2007, 1164, 105–112 CrossRef CAS PubMed.
- A. Salabat, M. H. Abnosi and A. R. Bahar, Amino acids partitioning in aqueous two-phase system of polypropylene glycol and magnesium sulfate, J. Chromatogr. B, 2007, 858, 234–238 CrossRef CAS PubMed.
- M. M. Bora, S. Borthakur, P. C. Rao and N. N. Dutta, Aqueous two-phase partitioning of cephalosporin antibiotics: effect of solute chemical nature, Sep. Purif. Technol., 2005, 45, 153–156 CrossRef CAS.
- Y. Wang, Y. Yan, S. Hu, J. Han and X. Xu, Phase diagrams of ammonium sulfate+ ethanol/1-propanol/2-propanol+ water aqueous two-phase systems at 298.15 K and correlation, J. Chem. Eng. Data, 2009, 55, 876–881 CrossRef.
- Y. X. Guo, J. Han, D. Y. Zhang, L. H. Wang and L. L. Zhou, An ammonium sulfate/ethanol aqueous two-phase system combined with ultrasonication for the separation and purification of lithospermic acid B from Salvia miltiorrhiza Bunge, Ultrason. Sonochem., 2012, 19, 719–724 CrossRef CAS PubMed.
- Y. X. Guo, J. Han, D. Y. Zhang, L. H. Wang and L. L. Zhou, Aqueous two-phase system coupled with ultrasound for the extraction of lignans from seeds of Schisandra chinensis (turcz.) Baill, Ultrason. Sonochem., 2013, 20, 125–132 CrossRef CAS PubMed.
- G. Dela, E. Or, R. Ovadia, A. Nissim-Levi, D. Weiss and M. Oren-Shamir, Changes in anthocyanin concentration and composition in ‘Jaguar’rose flowers due to transient high-temperature conditions, Plant Sci., 2003, 164, 333–340 CrossRef CAS.
- R. E. Wrolstad, R. W. Durst and J. Lee, Tracking color and pigment changes in anthocyanin products, Trends Food Sci. Technol., 2005, 16, 423–428 CrossRef CAS.
- M. Dubois, K. A. Gilles, J. K. Hamilton, P. A. Rebers and F. Smith, Colorimetric method for determination of sugars and related substances, Anal. Chem., 1956, 28, 350–356 CrossRef CAS.
- L. F. Reyes and L. Cisneros-Zevallos, Degradation kinetics and colour of anthocyanins in aqueous extracts of purple-and red-flesh potatoes (Solanum tuberosum L.), Food Chem., 2007, 100, 885–894 CrossRef CAS.
- Y. Wang, Y. S. Yan, S. P. Hu, J. Han and X. H. Xu, Phase Diagrams of Ammonium Sulfate + Ethanol/1-Propanol/2-Propanol + Water Aqueous Two-Phase Systems at 298.15 K and Correlation, J. Chem. Eng. Data, 2010, 55, 876–881 CrossRef CAS.
- G. B. Cardoso, T. Mourão, F. M. Pereira, M. G. Freire, A. T. Fricks, C. M. F. Soares and A. S. Lima, Aqueous two-phase systems based on acetonitrile and carbohydrates and their application to the extraction of vanillin, Sep. Purif. Technol., 2013, 104, 106–113 CrossRef CAS.
- G. B. Cardoso, I. N. Souza, T. Mourão, M. G. Freire, C. M. F. Soares and A. S. Lima, Novel Aqueous Two-Phase Systems Composed of Acetonitrile and Polyols: Phase Diagrams and Extractive Performance, Sep. Purif. Technol., 2014, 124, 54–60 CrossRef CAS.
- R. C. Khanal, L. R. Howard and R. L. Prior, Effect of heating on the stability of grape and blueberry pomace procyanidins and total anthocyanins, Food Res. Int., 2010, 43, 1464–1469 CrossRef CAS.
- N. Ghareaghajlou, S. Hallaj-Nezhadi and Z. Ghasempour, Red cabbage anthocyanins: stability, extraction, biological activities and applications in food systems, Food Chem., 2021, 365, 130482 CrossRef CAS PubMed.
- B. Enaru, G. Drețcanu, T. D. Pop, A. Stǎnilǎ and Z. Diaconeasa, Anthocyanins: factors affecting their stability and degradation, Antioxidants, 2021, 10, 1967 CrossRef CAS PubMed.
- Z. Hou, P. Qin, Y. Zhang, S. Cui and G. Ren, Identification of anthocyanins isolated from black rice (Oryza sativa L.) and their degradation kinetics, Food Res. Int., 2013, 50, 691–697 CrossRef CAS.
- W. D. Wang and S. Y. Xu, Degradation kinetics of anthocyanins in blackberry juice and concentrate, J. Food Eng., 2007, 82, 271–275 CrossRef CAS.
- M. T. Escribano-Bailón, C. Santos-Buelga and J. C. Rivas-Gonzalo, Anthocyanins in cereals, J. Chromatogr. A, 2004, 1054, 129–141 CrossRef PubMed.
- B. A. Cevallos-Casals and L. Cisneros-Zevallos, Stability of anthocyanin-based aqueous extracts of Andean purple corn and red-fleshed sweet potato compared to synthetic and natural colorants, Food Chem., 2004, 86, 69–77 CrossRef CAS.
- Z. Hou, P. Qin, Y. Zhang, S. Cui and G. Ren, Identification of anthocyanins isolated from black rice (Oryza sativa L.) and their degradation kinetics, Food Res. Int., 2013, 50, 691–697 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra03195d |
| This journal is © The Royal Society of Chemistry 2024 |