 Open Access Article
Open Access ArticleUtilizing Chamaerops humilis in removing methylene blue dye from water: an effective approach
Wassim El Malti *a,
Saja Koteichb and
Akram Hijazib
*a,
Saja Koteichb and
Akram Hijazib
aCollege of Health Sciences, American University of the Middle East, Kuwait. E-mail: Wassim.elmalti@aum.edu.kw
bResearch Platform for Environmental Science (PRASE), Doctoral School of Science and Technology, Lebanon
First published on 2nd August 2024
Abstract
Removing dyes, particularly methylene blue, from wastewater is crucial due to their detrimental effects on environmental and human health. Adsorption, recognized as a simple and efficient technique, is frequently employed to eliminate various dyes from water. Although activated carbon is a favored adsorbent for wastewater treatment, its high cost often restricts its use. As a result, there is increasing interest in utilizing inexpensive, natural materials, and waste products as alternative adsorbents. Sawdust from the European fan palm tree, specifically Chamaerops humilis, a widely available and cost-effective by-product, has demonstrated effective dye removal from wastewater. This study explored the impact of various factors such as time, agitation, adsorbent quantity, dye concentration, pH, and temperature on the adsorption of methylene blue using Chamaerops humilis sawdust. Optimal dye adsorption conditions were identified at a temperature of 25 °C, a pH of 8, an adsorbent dosage of 100 mg, a contact time of 120 min, and a dye concentration of 20 mg L−1, achieving a removal efficiency of 93.5%. Moreover, the Langmuir isotherm model described the adsorption dynamics more accurately, suggesting a maximum sorption capacity of 22.7 mg g−1 for the sawdust. Additionally, adsorption kinetics aligned better with the pseudo-second-order model than the pseudo-first-order model, underscoring the efficacy of this method in treating dye-polluted water.
Introduction
Water is essential for all forms of life, not just humans. Our planet holds about 1380 million cubic kilometers of water, consisting of salty and fresh water. The vast majority, 97.2%, is found in oceans and seas, leaving only 2.8% as freshwater. However, accessible freshwater for human use is just a fraction, about 0.07% of the total, including sources like groundwater, rivers, and lakes. Surprisingly, about 2.15% of this freshwater is locked up in ice.1With industrial growth, more pollutants enter our environment, contaminating the water with dyes, heavy metals, and other harmful substances. Dyes, in particular, are used extensively across various industries, including textiles, plastics, and paper, leading to significant environmental concerns. Even small amounts of dyes, less than 1 part per million in some cases, can be easily seen in water and are not desired due to their impact on the appearance and quality of water bodies.2 Methylene blue (MB), a common synthetic dye, is especially notorious for its persistence in aquatic environments and potential toxicity to aquatic life, affecting exposed species' reproductive and respiratory systems.3 Even low concentrations can have detrimental effects, highlighting the urgency for effective removal methods.4 Dye pollution is characterized by its large volume, intense color, high organic content, and difficulty in breaking down, posing risks to aquatic health and hindering the photosynthesis of waterborne microorganisms.3
Several treatment methods, such as adsorption, coagulation, ion exchange, membrane filtration, chemical degradation, and biodegradation, have been explored to combat dye pollution in water.5 However, many conventional techniques face high costs and operational complexities.6,7 Chemical degradation methods often involve using hazardous reagents and produce secondary pollution.7–9 Biodegradation, while environmentally friendly, typically requires long treatment times and specific conditions that can be difficult to maintain.10–12 In contrast, adsorption offers several advantages, including high efficiency, cost-effectiveness, ease of operation, and the ability to handle a wide range of dye concentrations and types.6,13–15 These attributes make adsorption a superior method for treating dye-contaminated water compared to physical, chemical, and biological methods.11 Still, the high cost and regeneration issues of activated carbon, a common adsorbent, limit its widespread use.16 Recently, research has shifted towards exploring affordable, unconventional adsorbents like clays, agricultural wastes, and industrial by-products.17–19 Many studies have examined the removal of dyes from wastewater using a range of adsorbents.20–24
Chamaerops humilis, commonly known as the European fan palm, is widely distributed across the Mediterranean region, marking its abundance in diverse environments from coastal areas to mountainous landscapes.25 Sawdust from Chamaerops humilis (SCH), an abundant and low-cost by-product of the palm industry, has emerged as a promising solution for environmental cleanup, particularly in removing heavy metals and dyes from water. Its porous structure and natural composition make it an effective adsorbent, capable of binding with and capturing a wide range of pollutants.26 Studies have shown that when treated and used in water treatment processes, SCH can significantly reduce concentrations of harmful substances, including heavy metals and synthetic dyes, from contaminated water. This makes it a sustainable option for wastewater treatment and adds value to what is otherwise considered waste material, contributing to a circular economy and reducing reliance on more expensive or non-renewable resources for water purification.27,28
This study aimed to assess the effectiveness of valorized SCH in adsorbing MB. We analyzed the chemical and physical characteristics of the adsorbent through various techniques. We conducted batch experiments to evaluate its adsorption capabilities, varying parameters such as particle size, agitation, adsorbent mass, pH, temperature, and contact time. We further examined the kinetics of the adsorption process and developed Langmuir and Freundlich isotherms under optimal conditions to explore its dynamics. The study was concluded with tests on selectivity and reusability of the adsorbent.
Materials and methods
Reagents
All reagents were sourced from commercial suppliers and used as received without additional purification. The used MB is a basic dye (C.I. 52015), presenting as a solid with a maximum absorption wavelength (λ max) of 663 nm and a molar absorptivity (ε) of 170.1 dm3 g−1 cm−1. The chemical structure of MB is depicted in Fig. 1. The study also utilized hydrochloric acid (BDH Chemicals, 36.5–38%), sodium hydroxide (Sigma-Aldrich, >97%), and methylene orange (MO), C.I.13025, with a molecular mass of 327.34 g mol−1 and a λmax of 464 nm.Adsorbent preparation
Chamaerops humilis plants were harvested from a region in Lebanon, specifically selecting the petiole part of the plant for processing into SCH (Fig. 2). Approximately 0.5 kg of this sawdust was thoroughly washed multiple times with distilled water to eliminate dust and other impurities. Subsequently, the SCH was oven-dried for 24 hours at 50 °C to achieve complete dryness.Techniques and machines
Following the preparation of the SCH, 0.5 kg of the latter was washed multiple times with distilled water, and the total dissolved solids (TDS) in the wash water were monitored to remove the residual salts and investigate the cleanliness of the sawdust.The moisture content was calculated according to eqn (1) below.
 | (1) |
Initial and final masses correspond to the SCH sample before and after drying.
The pH was measured by dispersing 0.5 g of SCH in 50 mL of distilled water, followed by stirring the mixture for 2 h at room temperature.
The pHpzc was determined by adjusting the pH of a 0.01 M NaCl solution to between 2 and 7 using 0.5 M HCl or 0.5 M NaOH. Subsequently, 0.5 g of SCH was added to 20 mL of this pH-adjusted solution and stirred at room temperature for 24 h. The final pH was measured and plotted against the initial pH, with the pHpzc identified where the initial pH (pHi) equals the final pH (pHf).
The sample's chemical composition was assessed using energy-dispersive X-ray spectroscopy on a Philips X'pert Pro MPD diffractometer. The Fourier Transform Infrared Spectroscopy (FTIR) analysis was performed using the JASCO FTIR-6300 spectrometer, covering a range from 400 to 4000 cm−1. The BET analysis was conducted on a Micromeritics ASAP 2010; the N2 adsorption isotherm was obtained by measuring the amount of N2 gas adsorbed on the surface of SCH at 77 K, while desorption isotherms were obtained by measuring the amount of gas removed from the surface of SCH as the pressure was gradually reduced. The adsorption was evaluated by ultraviolet-visible spectroscopy (UV-vis; U-2900, dual-beam spectrometer, 200 V, 664 nm wavelength).
Adsorbate preparation
MB stock solution was prepared at a concentration of 1 g L−1 by dissolving the dye in distilled water. Desired adsorbate concentrations were then achieved by diluting this stock solution accordingly.Adsorption tests
Batch mode experimental conditions were assessed using 25 mL Erlenmeyer flasks, wherein a solution of MB (20 mg L−1) was introduced along with 0.025 g of SCH adsorbent. The mixture was agitated at room temperature at medium speed for a predetermined period. Post adsorption, the MB concentration in the solution was analyzed using UV-vis spectroscopy. The study explored the influence of various parameters, including particle size, agitation, adsorbent dose, contact time, pH, and temperature, on the adsorption efficiency. The pH of the adsorptive solutions was adjusted as necessary using HCl and NaOH solutions. The findings from these experiments were utilized to determine the optimal conditions for maximizing MB removal from the aqueous solutions. The percentage of dye removal and the quantity of dye adsorbed were calculated using eqn (2) and eqn (3), respectively.
 | (2) |
 | (3) |
C0 and Ce (mg L−1) represent the initial dye concentration and equilibrium concentration, respectively. V is the volume of the solution (L), and m is the adsorbent mass (g).
Adsorption isotherm models
 | (4) |
Ce represents the MB concentration at equilibrium (mg L−1), Qe is the adsorption capacity at equilibrium (mg g−1), Qm is the maximum adsorption capacity for a monolayer (mg g−1), and KL is the Langmuir constant associated with adsorption energy (L g−1).
Subsequently, the separation factor constant (RL) derived from the Langmuir model is calculated using eqn (5):20,29,30
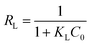 | (5) |
C0 is the initial concentration of MB (mg L−1).
 | (6) |
Qe is the equilibrium adsorption capacity (mg g−1), Ce is the equilibrium MB concentration (mg L−1), KF is the Freundlich constant related to adsorption capacity, and nF is the Freundlich exponent indicating adsorption intensity.
Reusability tests
These tests were conducted on dried samples of SCH, which had previously been loaded with MB under optimal conditions. Each sample was placed in a 150 mL Erlenmeyer flask containing 100 mL of 90% ethanol and stirred at 25 °C for 2 h. After desorption, the MB-laden solution was analyzed using UV-vis spectroscopy. This adsorption and desorption cycle was repeated four times, resulting in five uses for each adsorbent.All the experiments in this study were performed in triplicate, exhibiting a standard deviation of 1.5% and a reproducibility of 0.4%.
Results and discussion
Enhancing the adsorbent performance: the impact of washing frequency
Fig. 3 illustrates the TDS values in the wash water as the sawdust underwent successive washes. We observed a decline in water TDS from 200 to 11 ppm, indicating a reduction in residual salts. The TDS stabilized at 11 ppm after the eighth wash, suggesting no further washing was necessary as the sawdust had become sufficiently clean. Notably, a TDS level of 11 ppm is considered suitable and acceptable for adsorption.32,33Physico-chemical properties of the adsorbent
Table 1 shows that the adsorbent derived from Chamaerops humilis sawdust exhibited notable characteristics essential for its application in water purification processes. The moisture content stood at 9.80%, reflecting a good-quality adsorbent.21,34 The pH value of the adsorbent was slightly acidic at 6.5, with the point of zero charge (pHzpc) being close at 6.0, suggesting a balanced surface charge distribution under neutral conditions. The chemical analysis revealed a composition rich in carbon, highlighting its potential for adsorption, and a rich composition in oxygen, indicating various oxygen-containing functional groups. The surface properties of SCH, including specific surface area, total pore volume, and average pore size were measured using N2 adsorption–desorption isotherms (Table 1). The results revealed that the SCH has a porous structure, with a relatively small BET surface area of 12.50 m2 g−1 and an average pore diameter of 2.500 nm. According to the International Union of Pure and Applied Chemistry (IUPAC) classification on pore dimensions, adsorbent pores are categorized as micropores (d < 2 nm), mesopores (d = 2–50 nm), and macropores (d > 50 nm). Therefore, with an average pore diameter of 2.500 nm, the majority of the SCH pores fall into the mesoporous range.| Moisture content (%) | 9.8 | |
| pH | 6.5 | |
| pHzpc | 6.0 | |
| Chemical composition (wt%) | C | 58.48 |
| H | 5.580 | |
| N | 1.420 | |
| O | 34.36 | |
| Total surface area (m2 g−1) | 12.5 | |
| Total pore volume (cm3 g−1) | 0.725 | |
| Average pore size (nm) | 2.5 | |
The FTIR analysis (Fig. 4) confirmed the presence of many functional groups, with broad bands observed for O–H stretching (3220–3450 cm−1), indicative of the high presence of carboxyls, phenols, and alcohols groups; one absorption peak at about 1557.00 cm−1, characteristic of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of the carbonyl groups; the C–H stretching vibrations at 2942.30 cm−1, and C–O stretching at 1048.20 cm−1, can be attributed to the alcohol, phenol, and ether groups. These properties collectively underscore the adsorbent's potential for effectively interacting and removing MB from water.
O stretching of the carbonyl groups; the C–H stretching vibrations at 2942.30 cm−1, and C–O stretching at 1048.20 cm−1, can be attributed to the alcohol, phenol, and ether groups. These properties collectively underscore the adsorbent's potential for effectively interacting and removing MB from water.
Adsorption tests
Several variables can influence the efficiency of MB adsorption using SCH. Extensive investigation has been conducted to explore the effect of these variables on the adsorption process. For optimal adsorption results, it's crucial to fine-tune these conditions during laboratory experiments.The results, depicted in Fig. 8, show the correlation between the pH and MB adsorption on sawdust, where an increase in pH from 2 to 10 leads to an increase in adsorption percentage from 45% to 93%. Specifically, there's a sharp increase in adsorption from 45% to 90% as the pH rises from 2 to 6, followed by a more gradual increase to 93% as the pH goes from 6 to 10. At pH values below 6, the sawdust surface is positively charged, causing repulsive interactions with MB cations and a high concentration of H+ ions in the solution, which competes with MB for adsorption sites, reducing the removal rate. As the pH increases, the sawdust surface turns negatively charged, enhancing its interaction with the MB molecules.
Based on these observations, we determined that the optimal pH for our experiments is 8 to avoid conditions that are too basic and not representative of urban wastewater characteristics.
During the adsorption process, we observed three distinct phases. Initially, the removal of MB was rapid due to the abundance of free active sites on the SCH, allowing a significant amount of MB to bind quickly, which was attributed to the sample surface area and availability of active sites. Following this rapid phase, the adsorption rate decreases as the number of available active sites diminishes. Finally, the process stabilizes, reaching an equilibrium where no further MB is removed from the water, indicating the saturation phase. This slowdown is primarily due to the diminishing number of available active sites on the adsorbent as they become occupied over time.20
Adsorption kinetics
In this study, we applied both pseudo-first-order and pseudo-second-order kinetic models to pinpoint the most accurate description of the adsorption of MB onto SCH (eqn (7) and (8)).39–41 The rate constants for these models, K1 and K2, along with the calculated adsorption capacities (Qe), were derived from the plots of ln(Qe − Qt) versus time for the pseudo-first-order model, and t/Qt versus time for the pseudo-second-order model.
ln(Qe − Qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qe − K1t Qe − K1t
| (7) |
 | (8) |
Table 2 presents the values of K1 and K2, the correlation coefficients (R2), and the calculated adsorption capacities (Qe). Fig. 11a and b visually represent the kinetic plots for the pseudo-first and second-order models, respectively.
| Kinetic models | Adsorbent (SCH) |
|---|---|
| Qe.exp (mg g−1) | 4.65 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Pseudo-first order | |
| Qe.cal (mg g−1) | 2.35 |
| K1 | 0.04 |
| R2 | 0.952 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Pseudo-second order | |
| Qe.cal (mg g−1) | 4.80 |
| K2 | 0.05 |
| R2 | 0.999 |
 | ||
| Fig. 11 (a) Pseudo-first-order model; (b) pseudo-second-order models of the adsorption of MB onto SCH. | ||
The pseudo-first-order model yielded an adsorption capacity (Qe.cal) of 2.35 mg g−1 for SCH, significantly differing from the experimental data (Qe.exp). This discrepancy suggests that the pseudo-first-order model does not adequately capture the adsorption kinetics for our system. Conversely, the pseudo-second-order model's Qe.cal value of 4.80 mg g−1 aligns more closely with the experimental value of 4.65 mg g−1. Furthermore, the pseudo-second-order model's correlation coefficient (R2 = 0.999) is substantially higher than that of the pseudo-first-order model (R2 = 0.952), strongly indicating the pseudo-second-order model as the more accurate representation of the kinetics of MB adsorption onto SCH, which underscores the significance of chemisorption. This provides valuable insights into the nature of the adsorption process, suggesting that the interactions are not simply physical but involve chemical bonding between the MB molecules and the active sites on the SCH surface, leading to a more efficient adsorption system.42 In addition, the better fit of the pseudo-second-order model suggests that the adsorption capacity is closely related to the number of active sites available on the adsorbent.43 As the model assumes that the adsorption rate is proportional to the square of the number of unoccupied sites, it implies that the adsorption process becomes slower as these sites become occupied, characteristic of chemisorption processes where the active sites play a crucial role.44
Adsorption isotherm models
We conducted adsorption isothermal studies to understand how MB interacts with SCH in water and determine the maximum adsorption capacity (Qe). For this purpose, we applied both the Langmuir and Freundlich isotherm models.The summary of our isotherm analysis is shown in Fig. 12 and detailed in Table 3. The sawdust was compatible with the Langmuir and Freundlich models for removing MB, as indicated by the plots' strong correlation coefficients and linearity.
 | ||
| Fig. 12 (a) Adsorption capacity (Qe) vs. several initial concentrations of MB; (b) and (c) Langmuir and Freundlich isotherm plots for the adsorption of MB onto SCH. | ||
| Adsorption isotherm models | Parameters | Adsorbent (SCH) |
|---|---|---|
| Langmuir | Qm (mg g−1) | 22.27 |
| KL | 0.022 | |
| RL | 0.680 | |
| R2 | 0.990 | |
| Freundlich | KF | 4.620 |
| n | 3.000 | |
| R2 | 0.904 |
The dimensionless separation factor or equilibrium parameter, RL, is a crucial Langmuir model feature that predicts adsorption viability. An RL value greater than 1 indicates unfavorable adsorption, zero suggests irreversible adsorption, one denotes linear adsorption, and values between zero and one reflect favorable adsorption.29 As displayed in Table 3, our findings reveal that the RL values are within the 0 to 1 range, suggesting that the MB adsorption onto SCH is favorable.
Regarding the Freundlich model, the heterogeneity factor ‘n’ helps classify the adsorption process: 1 points to linear adsorption, less than 1 to chemical adsorption, and greater than 1 to physical adsorption (physisorption).29,45 For our study, ‘n’ values were approximately 3 for the MB adsorption onto sawdust, indicating a physical adsorption process.
Comparing the two models, the Langmuir isotherm provided higher correlation coefficients for the MB sorption, suggesting that the Langmuir model more accurately describes the sorption of MB onto SCH in our study.29
By comparing the maximum adsorption capacity of SCH with other materials (natural, synthetic, or treated) reported in the literature (Table 4), it is evident that SCH is an effective adsorbent for removing MB from water under mild conditions. This effectiveness is particularly notable given that SCH is a natural and abundant material that is used without any specific treatment.
| Adsorbent | Qm (mg g−1) | Conditions | Year |
|---|---|---|---|
| Banana peels46 | 20.80 | 30 °C, pH 6–7 | 2002 |
| Orange peels46 | 18.60 | 30 °C, pH 6–7 | 2002 |
| Neem leaf47 | 8.760 | 27 °C, pH 5–8 | 2005 |
| Fly ash48 | 13.42 | 30 °C, pH 10 | 2005 |
| Eggshell49 | 0.8000 | 25 °C, pH 7 | 2006 |
| Wheat shells50 | 16.56 | 30 °C, pH 4.5–9 | 2006 |
| Durian skin51 | 7.230 | 130 °C, pH 9 | 2015 |
| Haloxylon recurvum plant stems52 | 22.93 | 25 °C, pH 8 | 2017 |
| Ficcus palmata53 | 6.890 | 45 °C, pH 7 | 2019 |
| Modified (treated) fly ash54 | 28.65 | 25 °C, pH 7 | 2021 |
| Activated carbon from grape wood wastes55 | 5.880 | 25 °C, pH 11 | 2021 |
| Citrullus colocynthis peels | 4.480 | 19 °C, pH 8 | 2021 |
| Tumeric/polyvinyl alcohol/carboxymethyl cellulose56 | 6.270 | 25 °C, pH 6 | 2022 |
| Reused SiO2 desiccant + treated sand57 | 22.80 | 25 °C, pH 7 | 2023 |
| Cedrus atlantica sawdust58 | 7.840 | 25–60 °C, pH 10 | 2023 |
| SCH (this study) | 22.70 | 25 °C, pH 7 | 2024 |
Selectivity tests
In wastewater, dyes are never present in isolation; they coexist with other dyes. A study was conducted to evaluate the selectivity of SCH in removing cationic MB and anionic dye methyl orange (MO). For this purpose, an aqueous solution containing both dyes at equal concentrations (20 ppm) was prepared at room temperature and a pH of 8. The extent of dye removal was measured using UV-vis spectroscopy. The results, depicted in Fig. 13, indicate that the adsorption efficiency for MB (90%) surpasses that of MO. This disparity can be attributed to the strong interactions between the positively charged MB and the sawdust. Conversely, the MO molecules, being anionic, repel electrostatically against the sawdust, leading to suboptimal adsorption efficiency.Reusability tests
The employment of adsorption for wastewater treatment presents a considerable benefit over conventional methods due to the potential for adsorbent regeneration. Although SCH adsorbent is a low-cost material readily available in large quantities, particularly in the Mediterranean region, its regeneration was evaluated by exposing it to ethanol as an eluent for up to five reuse cycles at optimal conditions. As shown in Fig. 14, the SCH adsorbent maintain substantial usability for up to three cycles. However, a notable decline in adsorption capacity was observed from the fourth cycle onward, with a reduction of approximately 50% of its initial maximum capacity. This may be attributed to the partial depletion of adsorption sites after reuse or incomplete desorption of methylene blue.Conclusions
In this study, valorized sawdust from Chamaerops humilis, a low-cost precursor, has been examined and proven to be an efficient adsorbent for removing methylene blue from aqueous solutions. Given that SCH is relatively inexpensive, freely accessible, and abundant, it is a viable low-cost alternative for dye removal from aqueous solutions. This study investigated the impact of various parameters on adsorption efficiency, revealing that increasing the initial pH, adsorbent dose, and contact time enhanced the MB adsorption efficiency, whereas an increase in temperature reduced it. Agitation was shown to be crucial to improving the adsorption process. Furthermore, the pseudo-second-order model was found to more accurately describe the kinetic behavior of MB adsorption compared to the pseudo-first-order model. The values of the determined parameters (n and RL) using isothermal models indicated that the adsorption process was desirable and predominantly physical. Additionally, the correlation coefficient (R2) obtained using the Langmuir isotherm model showed a better fit than the Freundlich isotherm model.Similarly to most of the adsorption studies for wastewater treatment, the efforts exerted in this research study remain confined to the lab-scale. However, for practical industrial-scale applications, effluent treatment should be conducted in a continuous mode.
Data availability
All data generated or analyzed during this study are included in this article.Author contributions
Conceptualization, formal analysis, methodology, and supervision: WEM, AH; investigation and validation: SK and WEM; funding acquisition and project administration: AH; writing and editing: WEM.Conflicts of interest
There are no conflicts to declare.References
- V. Renaudin, Le dessalement de l’eau de mer et des eaux saumâtres, https://culturesciences.chimie.ens.fr/thematiques/chimie-physique/thermodynamique-chimique/le-dessalement-de-l-eau-de-mer-et-des-eaux, accessed 15 April 2024.
- R. Al-Tohamy, S. S. Ali, F. Li, K. M. Okasha, Y. A.-G. Mahmoud, T. Elsamahy, H. Jiao, Y. Fu and J. Sun, A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety, Ecotoxicol. Environ. Saf., 2022, 231, 113160 CrossRef CAS PubMed.
- P. O. Oladoye, T. O. Ajiboye, E. O. Omotola and O. J. Oyewola, Methylene blue dye: Toxicity and potential elimination technology from wastewater, Results Eng., 2022, 16, 100678 CrossRef CAS.
- R. Pourbaba, A. Abdulkhani, A. Rashidi and A. Ashori, Lignin nanoparticles as a highly efficient adsorbent for the removal of methylene blue from aqueous media, Sci. Rep., 2024, 14, 9039 CrossRef CAS PubMed.
- M. Shabir, M. Yasin, M. Hussain, I. Shafiq, P. Akhter, A.-S. Nizami, B.-H. Jeon and Y.-K. Park, A review on recent advances in the treatment of dye-polluted wastewater, J. Ind. Eng. Chem., 2022, 112, 1–19 CrossRef CAS.
- G. Crini, Non-conventional low-cost adsorbents for dye removal: A review, Bioresour. Technol., 2006, 97, 1061–1085 CrossRef CAS PubMed.
- V. K. Gupta, I. Ali, T. A. Saleh, A. Nayak and S. Agarwal, Chemical treatment technologies for wastewater recycling—an overview, RSC Adv., 2012, 2, 6380–6388 RSC.
- I. Sirés and E. Brillas, Remediation of water pollution caused by pharmaceutical residues based on electrochemical separation and degradation technologies: A review, Environ. Int., 2012, 40, 212–229 CrossRef PubMed.
- U. G. Akpan and B. H. Hameed, Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: A review, J. Hazard. Mater., 2009, 170, 520–529 CrossRef CAS PubMed.
- H. Ali, Biodegradation of Synthetic Dyes—A Review, Water, Air, Soil Pollut., 2010, 213, 251–273 CrossRef CAS.
- E. Forgacs, T. Cserháti and G. Oros, Removal of synthetic dyes from wastewaters: a review, Environ. Int., 2004, 30, 953–971 CrossRef CAS PubMed.
- R. G. Saratale, G. D. Saratale, D. C. Kalyani, J. S. Chang and S. P. Govindwar, Enhanced decolorization and biodegradation of textile azo dye Scarlet R by using developed microbial consortium-GR, Bioresour. Technol., 2009, 100, 2493–2500 CrossRef CAS PubMed.
- Y. C. Wong, Y. S. Szeto, W. H. Cheung and G. McKay, Adsorption of acid dyes on chitosan—equilibrium isotherm analyses, Process Biochem., 2004, 39, 695–704 CrossRef.
- V. K. Gupta, Suhas, Application of low-cost adsorbents for dye removal – A review, J. Environ. Manage., 2009, 90, 2313–2342 CrossRef CAS PubMed.
- T. Robinson, G. McMullan, R. Marchant and P. Nigam, Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative, Bioresour. Technol., 2001, 77, 247–255 CrossRef CAS PubMed.
- I. A. W. Tan, A. L. Ahmad and B. H. Hameed, Adsorption of basic dye on high-surface-area activated carbon prepared from coconut husk: Equilibrium, kinetic and thermodynamic studies, J. Hazard. Mater., 2008, 154, 337–346 CrossRef CAS PubMed.
- S. Kainth, P. Sharma and O. P. Pandey, Green sorbents from agricultural wastes: A review of sustainable adsorption materials, Appl. Surf. Sci. Adv., 2024, 19, 100562 CrossRef.
- M. M. Kwikima, S. Mateso and Y. Chebude, Potentials of agricultural wastes as the ultimate alternative adsorbent for cadmium removal from wastewater. A review, Sci. Afr., 2021, 13, e00934 CAS.
- S. Khan, S. Ajmal, T. Hussain and M. U. Rahman, Clay-based materials for enhanced water treatment: adsorption mechanisms, challenges, and future directions, J.Umm Al-Qura Univ. Appll. Sci., 2023 DOI:10.1007/s43994-023-00083-0.
- W. El Malti, A. Hijazi, Z. A. Khalil, Z. Yaghi, M. K. Medlej and M. Reda, Comparative study of the elimination of copper, cadmium, and methylene blue from water by adsorption on the citrus Sinensis peel and its activated carbon, RSC Adv., 2022, 12, 10186–10197 RSC.
- W. Al-Khatib, M. Srour, H. Bazzi, C. Haidar, A. Hijazi, A. E.-A. Al-Rekaby and W. El Malti, Test of elimination of cadmium and lead ions from water using polyurethane loaded with Cymbopogon citratus activated carbon, Biorem. J., 2023, 27, 301–310 CrossRef CAS.
- M. Rafatullah, O. Sulaiman, R. Hashim and A. Ahmad, Adsorption of methylene blue on low-cost adsorbents: A review, J. Hazard. Mater., 2010, 177, 70–80 CrossRef CAS PubMed.
- M. T. Yagub, T. K. Sen, S. Afroze and H. M. Ang, Dye and its removal from aqueous solution by adsorption: A review, Adv. Colloid Interface Sci., 2014, 209, 172–184 CrossRef CAS PubMed.
- M. A. M. Salleh, D. K. Mahmoud, W. A. W. A. Karim and A. Idris, Cationic and anionic dye adsorption by agricultural solid wastes: A comprehensive review, Desalination, 2011, 280, 1–13 CrossRef CAS.
- A. Giovino, S. Scibetta, S. Saia and C. Guarino, Genetic and morphologic diversity of European fan palm (Chamaerops humilis L.) populations from different environments from Sicily, Bot. J. Linn. Soc., 2014, 176, 66–81 CrossRef.
- R. Chikri, N. Elhadiri, M. Benchanaa and Y. El maguana, Efficiency of Sawdust as Low-Cost Adsorbent for Dyes Removal, J. Chem., 2020, 2020, 8813420 Search PubMed.
- E. Meez, A. Rahdar and G. Z. Kyzas, Sawdust for the Removal of Heavy Metals from Water: A Review, Molecules, 2021, 26(14), 4318 CrossRef CAS PubMed.
- M. N. Sahmoune and A. R. Yeddou, Potential of sawdust materials for the removal of dyes and heavy metals: examination of isotherms and kinetics, Desalination Water Treat., 2016, 57, 24019–24034 CrossRef CAS.
- T. F. Akinhanmi, E. A. Ofudje, A. I. Adeogun, P. Aina and I. M. Joseph, Orange peel as low-cost adsorbent in the elimination of Cd(II) ion: kinetics, isotherm, thermodynamic and optimization evaluations, J. Bioresour. Bioprod., 2020, 7, 34 CrossRef.
- I. Langmuir, The adsorption of gases on plane surfaces of glass, mica and platinum, J. Am. Chem. Soc., 1918, 40, 1361–1403 CrossRef CAS.
- H. Freundlich, Uber die adsorption in losungen, Int. J. Res. Phys. Chem. Chem. Phys., 1906, 57, 385–470 CAS.
- S. S. M. Hassan, H. I. Abdel-Shafy and M. S. M. Mansour, Removal of pyrene and benzo(a)pyrene micropollutant from water via adsorption by green synthesized iron oxide nanoparticles, Adv. Nat. Sci.: Nanosci. Nanotechnol., 2018, 9, 015006 Search PubMed.
- P. Beaulieu and B. Fisset, Eau du robinet : une exigence de qualité…, Cah. Nutr. Diététique, 2009, 44, 294–301 CrossRef CAS.
- M. Baudu, G. Guibaud, D. Raveau and P. Lafrance, Prévision de l’adsorption de molécules organiques en solution aqueuse en fonctions de quelques caractéristiques physico-chimiques de charbons actifs, Water Qual. Res. J., 2001, 36, 631–657 CrossRef CAS.
- N. Barka, M. Abdennouri, A. Boussaoud and M. E. Makhfouk, Biosorption characteristics of Cadmium(II) onto Scolymus hispanicus L. as low-cost natural biosorbent, Desalination, 2010, 258, 66–71 CrossRef CAS.
- K. S. Baidya and U. Kumar, Adsorption of brilliant green dye from aqueous solution onto chemically modified areca nut husk, S. Afr. J. Chem. Eng., 2021, 35, 33–43 Search PubMed.
- T. A. Saleh, in Surface Science of Adsorbents and Nanoadsorbents, ed. T. A. Saleh, Elsevier, 2022, vol. 34, pp. 39–64 Search PubMed.
- H. Esmaeili and R. Foroutan, Adsorptive Behavior of Methylene Blue onto Sawdust of Sour Lemon, Date Palm, and Eucalyptus as Agricultural Wastes, J. Dispersion Sci. Technol., 2019, 40, 990–999 CrossRef CAS.
- K. Naseem, in Sodium Alginate-Based Nanomaterials for Wastewater Treatment, ed. A. Ahmad, I. Ahmad, T. Kamal, A. M. Asiri and S. Tabassum, Elsevier, 2023, pp. 137–159 Search PubMed.
- L. Mishra, K. K. Paul and S. Jena, Adsorption Isotherm, Kinetics and Optimization Study by Box Behnken Design on Removal of Phenol from Coke Wastewater Using Banana Peel (Musa sp.) Biosorbent, Theor. Found. Chem. Eng., 2022, 56, 1189–1203 CrossRef CAS.
- H. A. Gzar, K. M. Kseer and M. J. Nasir, Kinetics and Isotherms of a Green Method for the Sorption of Metal Ions from Aqueous Solution, IOP Conf. Ser.: Mater. Sci. Eng., 2020, 888, 012077 CAS.
- Y. S. Ho and G. McKay, Pseudo-second order model for sorption processes, Process Biochem., 1999, 34, 451–465 CrossRef CAS.
- K. Vijayaraghavan and Y.-S. Yun, Bacterial biosorbents and biosorption, Biotechnol. Adv., 2008, 26, 266–291 CrossRef CAS PubMed.
- L. Wang and A. Wang, Adsorption properties of Congo Red from aqueous solution onto surfactant-modified montmorillonite, J. Hazard. Mater., 2008, 160, 173–180 CrossRef CAS PubMed.
- V. M. Bhandari and V. V. Ranade, in Industrial Wastewater Treatment, Recycling and Reuse, ed. V. V. Ranade and V. M. Bhandari, Butterworth-Heinemann, Oxford, 2014, pp. 81–140 Search PubMed.
- G. Annadurai, R.-S. Juang and D.-J. Lee, Use of cellulose-based wastes for adsorption of dyes from aqueous solutions, J. Hazard. Mater., 2002, 92, 263–274 CrossRef CAS PubMed.
- K. G. Bhattacharyya and A. Sharma, Kinetics and thermodynamics of Methylene Blue adsorption on Neem (Azadirachta indica) leaf powder, Dyes Pigm., 2005, 65, 51–59 CrossRef CAS.
- S. Wang, Y. Boyjoo and A. Choueib, A comparative study of dye removal using fly ash treated by different methods, Chemosphere, 2005, 60, 1401–1407 CrossRef CAS PubMed.
- W. T. Tsai, J. M. Yang, C. W. Lai, Y. H. Cheng, C. C. Lin and C. W. Yeh, Characterization and adsorption properties of eggshells and eggshell membrane, Bioresour. Technol., 2006, 97, 488–493 CrossRef CAS PubMed.
- Y. Bulut and H. Aydın, A kinetics and thermodynamics study of methylene blue adsorption on wheat shells, Desalination, 2006, 194, 259–267 CrossRef CAS.
- S. M. Anisuzzaman, C. G. Joseph, D. Krishnaiah, A. Bono and L. C. Ooi, Parametric and adsorption kinetic studies of methylene blue removal from simulated textile water using durian (Durio zibethinus murray) skin, Water Sci. Technol., 2015, 72, 896–907 CrossRef CAS PubMed.
- W. Hassan, U. Farooq, M. Ahmad, M. Athar and M. A. Khan, Potential biosorbent, Haloxylon recurvum plant stems, for the removal of methylene blue dye, Arab. J. Chem., 2017, 10, S1512–S1522 CrossRef CAS.
- R. Fiaz, M. Hafeez and R. Mahmood, Ficcus palmata leaves as a low-cost biosorbent for methylene blue: Thermodynamic and kinetic studies, Water Environ. Res., 2019, 91, 689–699 CrossRef CAS PubMed.
- N. T. Dinh, L. N. H. Vo, N. T. T. Tran, T. D. Phan and D. B. Nguyen, Enhancing the removal efficiency of methylene blue in water by fly ash via a modified adsorbent with alkaline thermal hydrolysis treatment, RSC Adv., 2021, 11, 20292–20302 RSC.
- S. A. Mousavi, D. Shahbazi, A. Mahmoudi and P. Darvishi, Methylene blue removal using prepared activated carbon from grape wood wastes: adsorption process analysis and modeling, Water Qual. Res. J., 2021, 57, 1–19 CrossRef.
- S. Radoor, J. Karayil, A. Jayakumar, J. Parameswaranpillai, J. Lee and S. Siengchin, Ecofriendly and low-cost bio adsorbent for efficient removal of methylene blue from aqueous solution, Sci. Rep., 2022, 12, 20580 CrossRef CAS PubMed.
- T. Juzsakova, A. D. Salman, T. A. Abdullah, R. T. Rasheed, B. Zsirka, R. R. Al-Shaikhly, B. Sluser and I. Cretescu, Removal of Methylene Blue from Aqueous Solution by Mixture of Reused Silica Gel Desiccant and Natural Sand or Eggshell Waste, Materials, 2023, 16(4), 1618 CrossRef CAS PubMed.
- C. Bouyahia, M. Rahmani, M. Bensemlali, S. E. Hajjaji, M. Slaoui, I. Bencheikh, K. Azoulay and N. Labjar, Influence of extraction techniques on the adsorption capacity of methylene blue on sawdust: Optimization by full factorial design, Mater. Sci. Energy Technol., 2023, 6, 114–123 CAS.
| This journal is © The Royal Society of Chemistry 2024 |












