Open Access Article
Open Access ArticleIdentification of acetaldehyde based on plasmonic patterns of a gold nanostructure conjugated with chromophore and H2O2: a new platform for the rapid and low-cost analysis of carcinogenic agents by colorimetric affordable test strip (CATS)†
Fatemeh Farshchia,
Arezoo Saadati‡
b,
Farnaz Bahavarniac,
Mohammad Hasanzadeh *d and
Nasrin Shadjou
*d and
Nasrin Shadjou e
e
aFundação Oswaldo Cruz, Instituto Oswaldo Cruz, Laboratório de Biologia Molecular e Doenças Endêmicas, Avenida Brasil No 4365-Manguinhos, Rio de Janeiro 21040-900, Brazil
bCentral European Institute of Technology, Brno University of Technology, Brno CZ-612 00, Czech Republic
cNutrition Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
dPharmaceutical Analysis Research Center, Tabriz University of Medical Sciences, Tabriz, Iran. E-mail: hasanzadehm@tbzmed.ac.ir
eDepartment of Nanotechnology, Faculty of Chemistry, Urmia University, Urmia, Iran
First published on 15th May 2024
Abstract
Acetaldehyde, a prevalent carbonyl compound in fermented foods, poses challenges in various applications due to its reactivity. This study addresses the need for efficient acetaldehyde detection methods across biotechnological, environmental, pharmaceutical, and food sectors. Herein, we present a novel colorimetric/UV spectrophotometric approach utilizing gold nanoparticles (AuNPs), particularly gold nano-flowers (AuNFs), for sensitive acetaldehyde identification. The method exhibits a notable sensitivity, detecting acetaldehyde at concentrations as low as 0.1 μM. The mechanism involves the interaction of acetaldehyde molecules with AuNFs, leading to a significant change in the absorbance spectrum, which serves as the basis for detection. Moreover, its applicability extends to human biofluids, notably urine samples. Integration with a cost-effective one-drop microfluidic colorimetric device (OD-μPCD) enables the development of an affordable test strip (CATS). This semi-analytical device, employing a multichannel OD-μPCD, facilitates real-time analysis of acetaldehyde in human samples. Our findings demonstrate the pioneering utilization of AuNPs for selective and sensitive acetaldehyde detection, promising advancements in environmental and occupational safety standards, and laying a foundation for enhanced detection and monitoring of related volatile organic compounds (VOCs).
1. Introduction
The presence of various toxins in the environment poses significant risks to human health and underscores the necessity for rapid and accurate detection methods.1 Among these toxins, acetaldehyde, commonly found in food and air, is particularly concerning due to its carcinogenic properties.2–4 Designated as a Group I carcinogen by the International Agency for Research on Cancer (IARC), acetaldehyde underscores the importance of monitoring food safety and air pollution.5 Consequently, there is a pressing need for simple and sensitive methods to detect acetaldehyde in specific biotechnological processes, environmental management, pharmaceuticals, and even in the analysis of drinking water and food.6Analytical techniques like HPLC and GC have been utilized for acetaldehyde detection.7 While they offer valuable insights, their intricate derivatization processes and prolonged detection times hinder immediate determination.8 In the realm of metal oxides, zinc oxide (ZnO) has shown promise in detecting volatile organic compounds (VOCs) effectively. Efforts to enhance ZnO's capabilities include structural modifications like shaping it into flower-like structures to increase surface area.9 Additionally, combining different metal oxides, such as PdO–ZnO P–N heterojunction nanostructures, has demonstrated sensitivity and selectivity in acetaldehyde detection. Doping ZnO with elements like cobalt (Co) has expanded its detection range.10 These methods are also complicated and require special laboratory equipment.
The incorporation of these technologies into microfluidic platforms holds potential for new systems that can be marketed as diagnostic tools.11,12 This type of sensor enables simultaneous detection of diverse parameters.13,14 A widely recognized approach is to employ paraffin wax and metal molds, known for their flexibility and ease of fabrication. These materials remain in demand and are commercially viable due to their cost-effectiveness.15
One of the most widely adopted methods for colorimetric analysis involves leveraging gold nanoparticles (AuNPs) as indicators, effectively enhancing system precision and sensitivity. The unique properties of AuNPs, including their localization of plasmonic bands and ease of synthesis, render them extensively used in biomedical applications.16 Moreover, nanoparticle characteristics make them an excellent platform for biological analysis, impacting optical properties based on size and shape variations, thereby enhancing diagnostic sensitivity.17,18 Advancements in spectroscopic technology have facilitated the development of optical sensors for acetaldehyde detection, including microfluidic sensors based on colorimetry.19 Despite the utility of colorimetric-based sensors, challenges such as the need for trained personnel, limited resolution, and complex procedures persist.19 Consequently, researchers are exploring modifications to existing technologies or investigating novel diagnostic methods to design cost-effective, dependable, disposable laboratories that operate independently of electrical connections.20
To execute chemical reactions using simple and cost-effective methods, microfluidic systems have been suggested.21 Paper-based microfluidic designs, utilizing hydrophobic barriers to form hydrophilic channels, offer portability and versatility, suitable for various settings.22,23 This study presents a novel diagnostic platform for rapid and accurate detection of acetaldehyde using UV-visible spectroscopy and colorimetric analysis.24 Various types of AuNPs with different shapes and pH values were initially employed as analytes, establishing a reliable colorimetric method in combination with UV-visible spectroscopy for identifying acetaldehyde in real samples. Integrated with an optimized one-droplet microfluidic glass fiber-based device (OD-μPCD), the system offers semi-quantitative results. The study utilized glass fiber paper to manufacture microfluidic wafers, demonstrating their effectiveness in detecting acetaldehyde using analytical techniques. The developed colorimetric microfluidic sensor presents a novel and viable approach for acetaldehyde detection in human urine samples, offering insights into advanced analytical tools for monitoring environmental health and safety.25,26
In this investigation, a novel paper-based microfluidic matrix was created using fiber glass paper, offering a unique platform for acetaldehyde analysis. These microfluidic devices operate via a color change mechanism, enabling the naked-eye detection of target molecules, while UV spectrophotometers provide quantitative analysis. The utilization of fiber glass paper in manufacturing microfluidic wafers facilitates the detection of acetaldehyde using analytical techniques. The findings from this study demonstrate that the application-based colorimetric microfluidic sensor presents a novel and viable approach for detecting acetaldehyde in human urine samples. The alterations in color and absorbance spectrum resulting from the interaction between the marker and acetaldehyde underscore the capability of the developed method to quantify acetaldehyde in authentic specimens. Moreover, this research marks the initial utilization of AuNPs for the precise and sensitive detection of acetaldehyde in natural samples, offering insights into advanced analytical tools and measurement platforms for acetaldehyde and other volatile organic compounds (VOCs). The simplicity, affordability, and portability of data-oriented microfluidic devices make them a promising option for tracking and monitoring acetaldehyde levels, contributing to enhanced environmental health and safety measures. The success of this colorimetric technique, combined with the simplicity and reliability of microfluidic devices, positions it as a potential off-the-shelf apparatus for widespread application in monitoring acetaldehyde.
It sets the stage for experimentation and observation. To enhance clarity, the process of synthesizing and applying an optical probe for the identification of acetaldehyde is illustrated in Scheme 1.
 | ||
| Scheme 1 Synthesis and application of optical probe conjugated with TMB + H2O2 to identification of acetaldehyde by μPCD. | ||
2. Experiment
2.1 Chemicals and materials
Acetaldehyde, formaldehyde, TMB (3,3′,5,5′-tetramethylbenzidine), NaOH (sodium hydroxide), HCl (hydrogen chloride), AgNO3 (silver nitrate), HAu chloroauric acid, cysteamine (CysA), TSC (trisodium citrate, Na3C6H5O7), PVP K-30 (polyvinylpyrrolidone), DDT (dichlorodiphenyltrichloroethane), acetone, ethanol, hexane, NaBH4 (sodium boro2tide (0), H%), HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), K2CO3 (potassium carbonate), CTAB (cetyltrimethylammonium bromide) were obtained from Sigma-Aldrich (Ontario, Canada). Glass fiber was purchased from Whatman Company (Maid Stone, England).2.2 Instrumentation
Examined the size of nanoparticles using atomic force microscopy (AFM) in tapping mode and Nanosurf (AG Gräubernstrasse 124![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 410 Liestal Switzerland). Surface area and size were also evaluated by zeta measurement, Zetasizer Ver.7.11 and, dynamic light analysis (DLS) (Malvern Instruments Ltd, MAL1032660, UK). Energy dispersive spectroscopy (EDS) was utilized to measure the composition of the nanoparticles, and the best resolution of scanning electron microscopy (FE-SEM, Hitachi-Su8020, Czech Republic-operating voltage 3 kV) was used to measure the source. The size and morphology of the synthesized nanoparticles were analyzed by transmission electron microscopy (TEM) (Adelaide, Australia, operating voltage 200 kV). EDS is used to analyze the content of nanoparticles. Optical analysis was examined using a UV-visible spectrophotometer Shimadzu UV-1800 with 1 nm resolution.27
410 Liestal Switzerland). Surface area and size were also evaluated by zeta measurement, Zetasizer Ver.7.11 and, dynamic light analysis (DLS) (Malvern Instruments Ltd, MAL1032660, UK). Energy dispersive spectroscopy (EDS) was utilized to measure the composition of the nanoparticles, and the best resolution of scanning electron microscopy (FE-SEM, Hitachi-Su8020, Czech Republic-operating voltage 3 kV) was used to measure the source. The size and morphology of the synthesized nanoparticles were analyzed by transmission electron microscopy (TEM) (Adelaide, Australia, operating voltage 200 kV). EDS is used to analyze the content of nanoparticles. Optical analysis was examined using a UV-visible spectrophotometer Shimadzu UV-1800 with 1 nm resolution.27
2.3 Synthesis of AuNPs with different sizes and morphologies
Gold nanoflowers (AuNFs), gold nanoparticles coated with CysA and DDT (AuNPs-CysA/AuNPs-DDT), and gold nanostars (GNSs) were synthesized according to our previous reports.28–312.4 Identification of acetaldehyde by proposed optical chemosensor
For this purpose, using optical methods, various AuNPs with different morphologies and sizes were used as probes and mixed with a combination of bluish green TMB and H2O2 solution (volume ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 V/V). The TMB solution is oxidized in the presence of H2O2, and with the addition of AuNPs, the NPs act as peroxide and change the color of the solution. Finally, with the addition of the test chemical (acetaldehyde) at room temperature, the color of the chemical changes due to oxidation. The color change was confirmed by UV-visible spectrophotometry as an identification system in the wavelength range of 200–800 nm. For colorimetric analysis, the color change in the solution is monitored with a mobile phone camera and, identification paper is used in the lighting environment.
1 V/V). The TMB solution is oxidized in the presence of H2O2, and with the addition of AuNPs, the NPs act as peroxide and change the color of the solution. Finally, with the addition of the test chemical (acetaldehyde) at room temperature, the color of the chemical changes due to oxidation. The color change was confirmed by UV-visible spectrophotometry as an identification system in the wavelength range of 200–800 nm. For colorimetric analysis, the color change in the solution is monitored with a mobile phone camera and, identification paper is used in the lighting environment.
2.5 Construction of one-droplet microfluidic glass fiber-based device (OD-μPCD)
The OD-μPCD was constructed using a novel method aimed at generating a useable color response from samples in a cost-effective and user-friendly manner. According to our previous publications,27,29,32 fiberglass papers were immersed in molten paraffin for 30 seconds, followed by removal and allowing the paraffin to dry on the paper for 1 minute. Subsequently, the paraffin-free paper was placed beneath the paraffin-coated paper on the surface of a robust magnet. An 8-pronged iron pattern, heated to 150 °C for 5 minutes and completely hot, was then utilized as a stamp on the papers. The interaction between the magnet and the iron pattern facilitated the creation of hydrophilic channels and hydrophobic areas on the paraffin-free paper.3. Results and discussion
3.1 Characterization of NPs
In this study, we have included the nanoparticles' characterization information, which were originally presented in our previous publication.29,31,33–413.2 Qualitative study (naked-eye and spectrophotometry analysis)
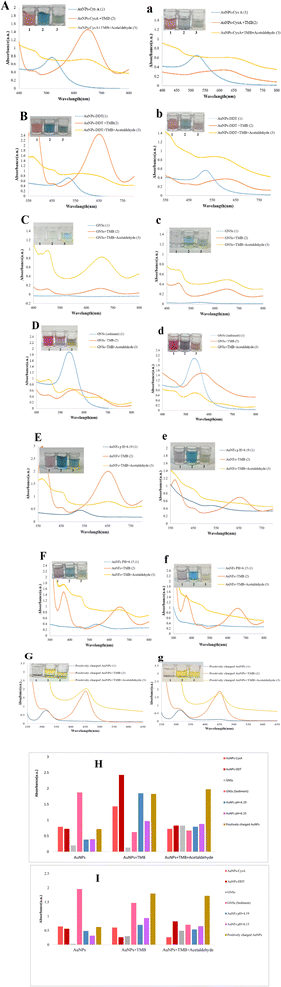
AuNPs demonstrate remarkable structure, surface, and photonic characteristics, which have generated significant interest for their utilization in optical chemical sensors. Nanomaterials are widely acknowledged for their physical and chemical attributes, particularly their surface area/volume ratio.34 The interaction between AuNPs and light gives rise to a phenomenon termed localized surface plasmon resonance, which is distinctive to metal nanoparticles, notably AuNPs.35 The exceptional optical properties of AuNPs make them well-suited for quick and uncomplicated measurements.36 Given their unique properties, AuNPs are ideal for integration into optical devices to enable the detection of specific analytes. The findings indicated a decrease in the absorbance intensity of AuNPs-CysA after incubation with TMB + H2O2 and TMB + H2O2 + acetaldehyde.In contrast, AuNPs-CysA alone demonstrates negligible exchange rate, highlighting the effectiveness of the nanoparticles on the monitoring of candidate target. It is paved that metal nanoparticles and other nanomaterial-driven chemical sensors exhibit diverse physical and chemical characteristics.37 The specific product's functionality is closely linked to the surface area-to-volume ratio.38 When nanoscale systems interact with light, various processes such as transmission, absorbance, reflection, light scattering, and fluorescence take place.39 AuNPs boast exceptional structural, surface, and photonic properties, capturing attention as optical devices. Nanomaterials are widely acknowledged for their physical and chemical properties, directly influenced by their environment.40 The interaction between light and AuNPs leads to the emergence of localized surface plasmon resonance,35 a phenomenon distinctive to metal nanoparticles, particularly AuNPs. This phenomenon is contingent on the dielectric constant of the surrounding medium.36 The luminescence effect known as a surface plasmon absorbance band arises from the presence of free electrons in AuNPs. This phenomenon occurs when the accumulation of electron nanoparticles resonates with light. AuNPs exhibit remarkable optical properties, making them ideal for quick and uncomplicated measurements.38
The enlargement of AuNP size leads to a shift of the plasmonic absorbance towards longer wavelengths, producing a red light. Moreover, the widening of the absorbance peak indicates the expansion of the suspension and the formation of aggregates.41 AuNPs play a significant role in numerous drug reactions due to their high surface area-to-surface ratio.42 The optical characteristics of AuNPs with different morphologies have been extensively examined to enable the identification of analytes through UV-visible spectroscopy.43 Capping agents or stabilizers are employed to avert the binding or aggregation of AuNPs with other compounds. The application of coating nanoparticles with additives induces electrostatic repulsion, which contributes to their stability.44 The distinct attributes of AuNPs make them suitable for integration into optical devices for the purpose of detecting specific analytes.45 Explore of chemosensory behavior in AuNPs-CysA: Here, we utilized cysteamine-modified gold nanoparticles (AuNPs-CysA) as a probe for the detection of acetaldehyde (0.1 μM). The catalytic activity of AuNPs-CysA increased the oxidation rate of TMB. The mobility of small colloidal particles enhances the absorbance of visible light.46 Stabilizers are utilized to prevent nanoparticles from binding or forming compounds with other materials. As previously mentioned, the application of additives to coat nanoparticles enhances their stability by generating electrostatic repulsion. The negative charge present on the surface of nanoparticles contributes to their unique attributes. The interaction between acetaldehyde and the modified probe causes the accumulation of AuNPs-CysA in the solution, resulting in a change in color and UV-visible spectral wavelengths.
AuNPs can be easily tailored on their surface by attaching functional molecules like cysteamine (CysA) to establish specific binding sites for target molecules. This research investigates the interplay of AuNPs-CysA with acetaldehyde in the presence of TMB + H2O2. As depicted in Fig. 1A, the presence of acetaldehyde in the solution induces a shift in the resonance of AuNPs-CysA, leading to a change in the absorbance peak from 520 nm to 655 nm on the UV-visible spectrum. The interaction between acetaldehyde and AuNPs-CysA is predicated on the generation of a Schiff base between the amino group of acetaldehydes and the thiol group of CysA, resulting in the accumulation of AuNPs-CysA in the solution. The binding of AuNPs-CysA to acetaldehyde was ascertained by measuring the absorbance at 550 nm, which increased proportionately with the acetaldehyde concentration. To enhance the detection sensitivity of the AuNPs-CysA-based sensor, TMB was employed as a redox reagent. In the presence of acetaldehyde, AuNPs-CysA facilitates the oxidation of TMB by H2O2, causing the color to transition from colorless to green. This green shade is attributed to the formation of the oxidized form of TMB, exhibiting a characteristic peak at 650 nm in the UV-visible spectrum. The intensity of this peak is directly proportional to the concentration of acetaldehyde in the solution. Overall, the interplay among AuNPs-CysA, acetaldehyde, and TMB furnishes a precise and selective technique for the detection of acetaldehyde in a diverse array of samples, encompassing indoor air, food, and biological fluids. In addition, the stability and performance of the chemical sensor are evaluated after one hour. Fig. 1A shows that the absorption density of AuNPs-CysA in the presence of TMB containing and acetaldehyde decreased. In comparison, the exchange rate of AuNPs-CysA alone is negligible, indicating the effectiveness of the nanoparticles.
The colorimetric response of the AuNPs to acetaldehyde was based on the aggregation and oxidation of the nanoparticles, respectively. According to Fig. 1B, even if the nanoparticles are estimated to have good stability, the system does not have good stability after one hour.
Further studies are needed to elucidate the nature of the interaction between acetaldehyde and GNS and improve detection sensitivity. No significant change in UV was observed after one hour for GNSs, GNSs/TMB + H2O2, or GNSs/TMB + H2O2/acetaldehyde solutions (Fig. 1C); shows that the interaction between acetaldehyde and GNSs does not cause obvious morphological or chemical changes. Although no change was observed, it was determined that interactions occurred on the GNS surface, and as a result, acetaldehyde absorbance happened on the surface.
As shown in Fig. 1E and F, the reaction of TMB + H2O2 with AuNFs leading to the formation of the Au(I)–TMB complex. The construction of this complex changes the color of the solution to blue-green and increases the absorbance of wavelengths from 521 to 660 nm. In the presence of acetaldehyde, the H2O2 produced by the reaction of TMB with HRP is consumed by the acetaldehyde, resulting in less use of H2O2 to oxidize AuNFs. For this reason, the color of the drug turns light green. After one hour, no absorbance was visible in the UV-visible spectrum, making the solution colorless (Fig. 1E and F).
3.3 Analytical study
In this study, we utilized the peroxidative activity of AuNPs to detect acetaldehyde through a colorimetric method. We employed the UV-vis technique to investigate the distribution of acetaldehyde (0.1–10−7 M) using the optical probe in the presence of AuNPs and TMB solutions. The absorbance spectra were recorded within the range of 200–800 nm, as shown in Fig. S1(A–G),† and the absorbance/concentration ratio is presented in Fig. S1(a–g).† Critical analytical parameters, such as the linear band characteristic and lower limit of quantitation (LLOQ), were determined by constructing a standard curve based on the absorbance change in the 400–700 nm band at different acetaldehyde concentrations. We observed a central dipole resonance peak at 455, 542, and 655 nm and a color change at high acetaldehyde levels when exposed to UV-visible light. We attributed this phenomenon to the change in AuNPs morphology and the preference for gold atoms deposited on its surface.In the presence of certain nanoparticles such as AuNPs-CysA and AuNFs, the absorption increases as the concentration decreases, whereas other nanoparticles like AuNPs-DDT and GNSs exhibit a decrease in absorption with decreasing concentration. This phenomenon can be attributed to the interaction of acetaldehyde molecules with these nanoparticles in solution, leading to a change in the local refractive index and a subsequent modulation of the absorbance peak intensity. The surface area-to-volume ratio of smaller nanoparticles is higher compared to larger nanoparticles, resulting in a more pronounced decrease in absorbance efficiency at lower acetaldehyde concentrations on the larger surface area of small AuNPs. Consequently, the decrease in absorbance efficiency of larger AuNPs may be less significant at lower acetaldehyde concentrations when compared to smaller nanoparticles. The size-dependent properties of AuNPs influence the interaction between nanoparticles and acetaldehyde molecules, thereby affecting the observed absorbance energy in the UV-visible light spectrum. Additionally, these interactions often manifest as discernible color changes that can be observed with the naked eye.
For a comprehensive comparison, the relationships and corresponding equations for each nanoparticle are detailed in Table 1.
| Type of optical prob | Linear range | LLOQ |
|---|---|---|
| AuNPs-CysA | 0.1 μM–10 M | 0.1 μM |
| AuNPs-DDT | ||
| AuNFs pH = 4.19 | ||
| GNSs | 0.1 μM–0.1 M | 0.1 μM |
| GNSs (sediment) | ||
| AuNFs pH = 6.15 | 1 μM–1 M | 1 μM |
| PCAuNPs | 10 μM–1 M | 10 μM |
As shown in obtained results and comparison of data that indicated in Table 1, AuNFs demonstrated the most pronounced color change at pH = 6.15. Also, as shown in Table 2, this design process offers numerous advantages, including portability, disposability, speed, accuracy, convenience, and affordability. One of its primary strengths is the ability to conduct straightforward field tests through direct testing, eliminating the need for pre-testing or measurement tools and manual dexterity. In contrast, other methods discussed in the study are limited by reliance on expertise, laboratory equipment, preclinical samples, repeated trial time, and require more effort to compensate for human error. Thus, the developed system overcomes these limitations and makes research more accessible and valuable for users.
| Method | Reaction system | Linear range | LOD/LLOQ | Ref. |
|---|---|---|---|---|
| Electrochemical | MgO-templated carbon (GMgoc) | 0.02–0.1 ppm | 0.02 ppm | 47 |
| CeO2–MWCNT nanocomposite | 10−8 to 10−5 M | 7.4 × 10−9 M | 48 | |
| Spectrophotometry | Coenzyme NAD+ | 0.33 μM | 0.33 μM | 49 |
| Spectro electrochemical enzymatic sensor | K3[Fe (CN)6]/K4[Fe (CN)6] | 2.13 ± 0.05 mM (white wine) | 1.99 mM (white wine) | 50 |
| 5.41 ± 0.16 mM (red wine) | 5.51 mM (red wine) | |||
| Amperometric biosensor | Two platinum thin-film electrodes and a hydrophilic polytetrafluoroethylene membrane | 1.00–200 μmol l−1 | 1.00–200 μmol l−1 | 51 |
| Fluorescence | CPDs-Tb3+ | 0.04–42.48 | 0.02 mM | 52 |
| Colorimetric/UV-vis | AuNPs-CysA | 0.1 μM–10 M | 0.1 μM | This work |
| AuNPs-DDT | ||||
| AuNFs pH = 4.19 | ||||
| GNSs | 0.1 μM–0.1 M | 0.1 μM | ||
| GNSs (sediment) | ||||
| AuNFs pH = 6.15 | 1 μM–1 M | 1 μM | ||
| PCAuNPs | 10 μM–1 mM | 1 μM |
3.4 Analytical evaluation in real sample
Human urine samples were collected from healthy individuals without prior treatment to evaluate the effectiveness of acetaldehyde detection in real samples. These urine samples were mixed with different concentrations of acetaldehyde (ranging from 17.35 to 10−7 M) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Our study results showed a positive relationship between the concentration difference and the corresponding colorimetric measurements in human urine samples. This finding demonstrates the success of our research in accurately detecting and measuring acetaldehyde levels in real samples using colorimetric methods. The regression equations illustrating the relationship between the measured optical response and the concentration of acetaldehyde enable precise quantification of acetaldehyde levels in urine samples, enhancing the reliability and accuracy of our detection method.
1 ratio. Our study results showed a positive relationship between the concentration difference and the corresponding colorimetric measurements in human urine samples. This finding demonstrates the success of our research in accurately detecting and measuring acetaldehyde levels in real samples using colorimetric methods. The regression equations illustrating the relationship between the measured optical response and the concentration of acetaldehyde enable precise quantification of acetaldehyde levels in urine samples, enhancing the reliability and accuracy of our detection method.
This way, urine is first tested for its shape and UV-visible spectrum. The color change of the urine sample is shown in Fig. S2.† UV-visible spectrophotometry was used to study absorbance. UV-visible light analysis showed that the urine samples exhibited small peaks at wavelengths between 400 and 600 nm. In the case of GNSs, AuNPs-CysA, AuNFs and PCAuNPs as the concentration decreases, the absorbance intensity increases, but the opposite is true for other AuNPs. There is no color change visible in GNS currently. The different behaviors can be attributed to many factors, including the size, and shape of AuNPs and their chemical composition. The surface properties of AuNPs can vary depending on the synthesis method and the functionalization or modification steps involved. These surface features, such as surface charges and functional groups, can affect the absorbance behavior of acetaldehyde. The complexity of the real matrix structure may affect the absorbance behavior and suggest other factors to consider. Colorimetric and UV-visible results demonstrated the reliability and validity of the chemically produced product in detecting acetaldehyde in real urine. Therefore, this optical device could be an efficient and effective measurement platform for detecting acetaldehyde in urine samples.
3.5 Analytical validity
The change in the bipolar resonance peak of AuNPs can be attributed to the change in AuNP morphology and the release of new AuNP atoms on the AuNP surface and is utilized in the wavelength shift model. In the presence of TMB and interference (GLU/ethanol/acetone and acetaldehyde), the absorbance peak at 450 nm increased and the 650 nm band showed an increase in absorbance. The PC-AuNPs showed an absorbance peak at 450 nm, which was significantly affected by interfering substances, especially acetaldehyde. Furthermore, the presence of acetaldehyde caused a visible color change and absorbance peak in the 450 nm range, whereas no noticeable color change was observed in the presence of glucose, ethanol, and acetone. In this case, the appearance of the peak was solely due to the staining effect. Similar patterns were observed for other types of AuNPs, such as AuNF and eluted AuNPs, which performed well. When tested with all types of AuNPs, acetaldehyde outperformed other analytes in terms of selectivity in real urine samples. Obtained graphs in Fig. S3(H and I)† describe the changes in peak observed in the presence of the target drug, indicating a successful oxidation reaction.
Overall, our research demonstrates that different preparations exhibit varying responses to different factors, with acetaldehyde exhibiting the most significant difference.
Interestingly, small changes in the absorbance spectrum are observed due to interfering modes, indicating that the drug sensor selectively controls acetaldehyde even in the presence of interference properties unrelated to the detection mechanism (Fig. S4†). Human urine samples were used to conduct experiments to test the effectiveness of the drug sensor. The results demonstrate the drug's potential for detecting and quantifying acetaldehyde levels in biological processes (Fig. S5†). Additionally, the chemical sensor accurately detects and measures acetaldehyde levels even in real-world conditions (Fig. S6†).
In conclusion, the study showed that the interaction between AuNPs and related products is a powerful choice for chemical sensors to detect acetaldehyde. The sensor selectively controls acetaldehyde even in the presence of interference properties unrelated to the detection mechanism. Human urine testing confirmed the effectiveness and efficiency of the chemical sensor for detecting acetaldehyde. Additionally, we used a paper-based microfluidic system as a powerful diagnostic tool for accurately and rapidly detecting acetaldehyde. This new system combines simplicity and efficiency, monitoring various assays, including chemicals, viruses, and markers. Paper sensors have attracted attention due to their low cost and ease of use, which has led to progress in this research. It is hoped that these sensors will eventually replace laboratory procedures and enable cost-effective measurements.
3.6 Colorimetric determination of acetaldehyde using OD-μPCD
In our study, we utilized fiberglass paper as the substrate for the chemical sensor due to its superior flow characteristics and lower friction compared to other paper substrates. By incorporating microchannels into the design, we were able to facilitate capillary action, promoting efficient liquid flow and ease of measurement. To detect acetaldehyde, we utilized co-embedded AuNPs in the sensor and added TMB + H2O2 and acetaldehyde analyte. As depicted in Fig. 2–4, the presence of acetaldehyde was clearly indicated by a noticeable color change. Moreover, after an hour of incubation, the successful fabrication of hydrophilic channels in the paper substrate was confirmed, further attesting to the reliability and robustness of our system.Based on the UV visibility results, AuNFs at pH 6.15 demonstrated good detection ability and performance across different acetaldehyde concentrations. As a result, we selected AuNFs (pH 6.15) as the most promising material for further investigation with OD-μPAD. To assess the effect of acetaldehyde concentration in human urine samples, we introduced synthetic AuNFs into the sensing area and observed a noticeable color change upon addition of the test sample (Fig. S7†). This system shows potential as a diagnostic tool for clinical research. Another aspect of our investigation involved measuring the capillary energy of microchannels. By placing AuNFs (probes) and TMB + H2O2 in the detection zones and 50 μl of acetaldehyde (analyte) in the sensor area, we observed a lack of color due to redox reactions over time, affirming successful construction of μPADs and drug-sensing substrates with high efficiency, friction-free, and flow rates. We conducted meticulous evaluations of several relevant operators to ensure selection of appropriate chemical sensors. This involved combining relevant products such as acetone, ethanol, glucose, and acetaldehyde with dyes and AuNPs and careful analysis of the results.
The stability of microfluidic preparations was also investigated in this study. The results demonstrated that the system effectively analyzed the acetaldehyde over a period of three days, marking a notable achievement for microfluidic biosensors, particularly considering their early developmental stage. To achieve this, we utilized OD-μPADs for colorimetric analysis of target ions via immersion optical probes. After drying at room temperature, the AuNPs combine and turn the color of the paper substrate light purple, with the drying and mixing process taking approximately 10 minutes. This marked the beginning of the development of a nanoparticle-modified OD-μPADs for rapid and accurate identification of acetaldehyde.
The concentration (index) of nanoparticles in the cellulose fibers of the paper, the shape of the paper, and the location of the nanoparticles affect the optical and physical interaction between nanoparticles and the paper substrate. The penetration and accumulation of nanoparticles into the porous structure of the paper cause a change in light compared to the non-porous structure. To preserve their optical properties, it is essential to maintain the solid state of nanoparticles during drying and aggregation.
Our study is the first to successfully utilize OD-μPAD for accurate and powerful identification of acetaldehyde by using AuNPs. To the best of our knowledge, no previous research has employed this method for such purposes. Furthermore, this model exhibits potential for identification of other analytes, including amino acids. The OD-μPAD's low cost and convenience, coupled with its small size, make it a highly portable alternative to larger, more expensive laboratories with specialized operator.
In summary, our study demonstrates significant scientific implications for utilizing AuNPs in microfluidic systems to sensitive detection of acetaldehyde in real samples. The use of microfluidic devices allows for efficient and affordable maintenance analysis. By thoroughly examining specific chemical properties, we showcase the impact of interfering properties on the absorbance spectra of AuNPs. It's important to highlight that the selection and effectiveness of AuNPs are contingent on the type of AuNPs and the interfering agent used in this study.
4. Conclusion
In this study, we developed an enhanced colorimetric method combined with UV-visible spectroscopy for the detection of acetaldehyde in real samples. By employing gold nanoparticles (AuNPs) with varied sizes and morphology, we achieved real-time and selective detection of acetaldehyde amidst diverse interferents. The interaction between acetaldehyde and AuNPs induced significant changes in the absorbance spectrum, enabling rapid and reliable measurement of acetaldehyde concentration. This innovative approach represents a novel application of AuNPs for specific and sensitive acetaldehyde detection in human samples. The microfluidic device utilized in our study offers simplicity, affordability, and portability, rendering it suitable for widespread in situ and ex situ acetaldehyde monitoring in environmental, industrial, and biomedical settings. Moreover, engineered one-drop microfluidic colorimetric devices (OD-μPCDs) demonstrate excellent stability for long-term storage and analysis, making them indispensable for field applications and remote sensing. The advanced colorimetric method, coupled with the simplicity and reliability of the microfluidic format, can be readily commercialized as a user-friendly device. By replacing conventional laboratory techniques with the innovative approach proposed in this study, we anticipate enhanced accessibility to acetaldehyde analysis for environmental organizations, manufacturers, and medical facilities. Continued refinement and exploration of this approach holds the potential to enhance its performance, broaden its applicability to other compounds, and address new challenges in chemical analysis. We envision that our work will inspire further research and contribute to the development of superior technology for detecting and monitoring acetaldehyde on a global scale.In summary, our study represents a significant breakthrough in colorimetric detection, highlighting the promise of AuNPs and paper-based microfluidics for sensitive and selective acetaldehyde analysis. This work lays the foundation for future advancements in analytical chemistry, facilitating rapid and accurate measurement of acetaldehyde exposure to bolster environmental, health, and safety standards. Further exploration and refinement may enhance the effectiveness of this approach, extend its suitability for other substances, and tackle emerging challenges in chemical analysis. Overall, our study marks a noteworthy progression in color detection, showcasing the potential of AuNPs and paper-based microfluidics for precise and selective detection of acetaldehyde, while serving as a platform for advancing the detection of acetaldehyde and other volatile organic compounds (VOCs) across diverse regions.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the pharmaceutical analysis Research Center, Tabriz University of Medical Sciences for instrumental supporting of this research (Grant No. 73702).References
- F. O. Adeola, Handbook of Global Health, 2020, pp. 1–30 Search PubMed.
- J. J. Baert, J. De Clippeleer, L. De Cooman and G. Aerts, J. Am. Soc. Brew. Chem., 2015, 73, 100–108 CrossRef CAS.
- J. I. Garaycoechea, G. P. Crossan, F. Langevin, L. Mulderrig, S. Louzada, F. Yang, G. Guilbaud, N. Park, S. Roerink and S. Nik-Zainal, Nature, 2018, 553, 171–177 CrossRef CAS PubMed.
- K. C. Zenki, B. H. M. Mussulini, E. P. Rico, D. L. de Oliveira and D. B. Rosemberg, Toxicol. in Vitro, 2014, 28, 822–828 CrossRef CAS PubMed.
- H. Lin, H. Jiang, S. Y.-S. S. Adade, W. Kang, Z. Xue, M. Zareef and Q. Chen, Crit. Rev. Food Sci. Nutr., 2023, 63, 8226–8248 CrossRef PubMed.
- Y. Liu, C. Liu, X. Xu, C. Niu, J. Wang, F. Zheng and Q. Li, Foods, 2022, 11, 3450 CrossRef CAS PubMed.
- S. S. H. Ho and J. Z. Yu, Environ. Sci. Technol., 2004, 38, 862–870 CrossRef CAS PubMed.
- R. P. Dator, M. J. Solivio, P. W. Villalta and S. Balbo, Toxics, 2019, 7, 32 CrossRef CAS PubMed.
- Q. A. Drmosh, I. Olanrewaju Alade, M. Qamar and S. Akbar, Chem.–Asian J., 2021, 16, 1519–1538 CrossRef CAS PubMed.
- M. M. Rahman, S. B. Khan, M. Faisal, A. M. Asiri and K. A. Alamry, Sens. Actuators, B, 2012, 171, 932–937 CrossRef.
- B. L. Gray, IEEE Nanotechnol. Mag., 2014, 8, 6–16 Search PubMed.
- N. Maluf and K. Williams, An Introduction to Microelectromechanical Systems Engineering, Artech House, 2004 Search PubMed.
- M. Razmkhah, S. Abtahi and A. Ghaderi, Curr. Stem Cell Res. Ther., 2019, 14, 43–51 CrossRef CAS PubMed.
- V. Rincón Montes, PhD dissertation, RWTH Aachen University, 2021.
- V. N. Ataide, L. F. Mendes, L. I. Gama, W. R. de Araujo and T. R. Paixão, Anal. Methods, 2020, 12, 1030–1054 RSC.
- K. Nejati, M. Dadashpour, T. Gharibi, H. Mellatyar and A. Akbarzadeh, J. Cluster Sci., 2021, 1–16 Search PubMed.
- H. Jans and Q. Huo, Chem. Soc. Rev., 2012, 41, 2849–2866 RSC.
- K. Kelly, E. Coronado and L. L. Zhao, J. Phys. Chem. B, 2003, 107, 668–677 CrossRef CAS.
- X. Bai, Y. Wang, Z. Song, Y. Feng, Y. Chen, D. Zhang and L. Feng, Int. J. Mol. Sci., 2020, 21, 2480 CrossRef CAS PubMed.
- P. Nosovitskiy, G. Nosovitskiy, K. Nandigam, R. Abozaid and S. Karan, in Breath Analysis: an Approach for Smart Diagnostics, Springer, 2022, pp. 161–200 Search PubMed.
- C.-W. Tsao, Micromachines, 2016, 7, 225 CrossRef PubMed.
- X. Qin, J. Liu, Z. Zhang, J. Li, L. Yuan, Z. Zhang and L. Chen, TrAC, Trends Anal. Chem., 2021, 143, 116371 CrossRef CAS.
- H. Canbolat, IEEE Trans. Instrum. Meas., 2009, 58, 3762–3768 Search PubMed.
- J. B. Nielsen, R. L. Hanson, H. M. Almughamsi, C. Pang, T. R. Fish and A. T. Woolley, Anal. Chem., 2019, 92, 150–168 CrossRef PubMed.
- K. Mahato, A. Srivastava and P. Chandra, Biosens. Bioelectron., 2017, 96, 246–259 CrossRef CAS PubMed.
- E. Noviana, T. Ozer, C. S. Carrell, J. S. Link, C. McMahon, I. Jang and C. S. Henry, Chem. Rev., 2021, 121, 11835–11885 CrossRef CAS PubMed.
- F. Farshchi, A. Saadati, M. Hasanzadeh, Y. Liu and F. Seidi, RSC Adv., 2023, 13, 6225–6238 RSC.
- A. Mobed, F. Kohansal, A. Ahmadalipour, M. Hasanzadeh and F. Zargari, Anal. Methods, 2021, 13, 311–321 RSC.
- F. Farshchi, A. Saadati, M. Hasanzadeh and F. Seidi, RSC Adv., 2021, 11, 27298–27308 RSC.
- N. Razmi, M. Hasanzadeh, M. Willander and O. Nur, Anal. Methods, 2022, 14, 1562–1570 RSC.
- S. Ahmadi, Z. Ghasempour and M. Hasanzadeh, Food Chem., 2023, 423, 136307 CrossRef CAS PubMed.
- A. Saadati, F. Farshchi, M. Jafari, H. Kholafazad, M. Hasanzadeh and N. Shadjou, RSC Adv., 2024, 14, 8602–8614 RSC.
- A. Saadati, F. Farshchi, M. Hasanzadeh and F. Seidi, Anal. Methods, 2021, 13, 3909–3921 RSC.
- H. Yin, H. Too and G. Chow, Biomaterials, 2005, 26, 5818–5826 CrossRef CAS PubMed.
- J. A. Delaire and K. Nakatani, Chem. Rev., 2000, 100, 1817–1846 CrossRef CAS PubMed.
- A. E. Miroshnichenko, S. Flach and Y. S. Kivshar, Rev. Mod. Phys., 2010, 82, 2257 CrossRef CAS.
- A. Sharma, Y. Zhu, E. J. Spangler and M. Laradji, J. Chem. Phys., 2022, 156, 4689–4698 Search PubMed.
- V. Amendola, R. Pilot, M. Frasconi, O. M. Maragò and M. A. Iatì, J. Phys.: Condens. Matter, 2017, 29, 203002 CrossRef PubMed.
- H. E. Toma, V. M. Zamarion, S. H. Toma and K. Araki, J. Braz. Chem. Soc., 2010, 21, 1158–1176 CrossRef CAS.
- F. Flory, L. Escoubas and G. Berginc, J. Nanophotonics, 2011, 5, 052502 CrossRef.
- N. E. Larm, J. B. Essner, K. Pokpas, J. A. Canon, N. Jahed, E. I. Iwuoha and G. A. Baker, J. Phys. Chem. C, 2018, 122, 5105–5118 CrossRef CAS.
- P. Boomi, R. Ganesan, G. Prabu Poorani, S. Jegatheeswaran, C. Balakumar, H. Gurumallesh Prabu, K. Anand, N. Marimuthu Prabhu, J. Jeyakanthan and M. Saravanan, Int. J. Nanomed., 2020, 7553–7568 CrossRef CAS PubMed.
- E. C. Dreaden, A. M. Alkilany, X. Huang, C. J. Murphy and M. A. El-Sayed, Chem. Soc. Rev., 2012, 41, 2740–2779 RSC.
- S. Zeng, D. Baillargeat, H.-P. Ho and K.-T. Yong, Chem. Soc. Rev., 2014, 43, 3426–3452 RSC.
- E. Borgarello, J. Kiwi, M. Graetzel, E. Pelizzetti and M. Visca, J. Am. Chem. Soc., 1982, 104, 2996–3002 CrossRef CAS.
- B. Metz, G. F. Kersten, P. Hoogerhout, H. F. Brugghe, H. A. Timmermans, A. De Jong, H. Meiring, J. ten Hove, W. E. Hennink and D. J. Crommelin, J. Biol. Chem., 2004, 279, 6235–6243 CrossRef CAS PubMed.
- I. Shitanda, T. Oshimoto, N. Loew, M. Motosuke, H. Watanabe, T. Mikawa and M. Itagaki, Biosens. Bioelectron., 2023, 238, 115555 CrossRef CAS PubMed.
- R. M. Shereema, S. R. Nambiar, S. S. Shankar and T. P. Rao, Anal. Methods, 2015, 7, 4912–4918 RSC.
- H. Zhu, R. Gonzalez and T. A. Bobik, Appl. Environ. Microbiol., 2011, 77, 6441–6450 CrossRef CAS PubMed.
- D. Ibáñez, M. B. González-García, D. Hernández-Santos and P. Fanjul-Bolado, Biosensors, 2022, 12, 1032 CrossRef PubMed.
- T. Gessei, T. Arakawa, H. Kudo, H. Saito and K. Mitsubayashi, Anal. Lett., 2014, 47, 1361–1374 CrossRef CAS.
- R. Guan, S. Zhang, X. Fan, X. Shao, Y. Hu, T. Liu, S. Wang and Q. Yue, J. Fluoresc., 2022, 32, 759–770 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra02814g |
| ‡ Co-first author. |
| This journal is © The Royal Society of Chemistry 2024 |