 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Polyacetylenes with (hetero)aryl-, styryl-, and amino-phenothiazinyl sidechains: synthesis and photophysics†
Wladislaw Pisetsky and
Thomas J. J. Müller *
*
Heinrich Heine University Düsseldorf, Faculty of Mathematics and Natural Sciences, Institute of Organic Chemistry and Macromolecular Chemistry, Universitätsstrasse 1, D-40225 Düsseldorf, Germany. E-mail: ThomasJJ.Mueller@uni-duesseldorf.de
First published on 2nd April 2024
Abstract
A novel generation of 7-aryl phenothiazinyl substituted polyacetylenes is readily accessible via controlled rhodium-catalyzed polymerization of the corresponding 3-ethynyl 7-aryl phenothiazines. The monomers are synthesized by Suzuki coupling, Heck coupling, or Buchwald–Hartwig amination, and Bestmann–Ohira reaction. This allows for the introduction of electron donating and releasing substituents with different ligation patterns. The obtained polymers display narrow molecular weight distributions, with very few exceptions, and are soluble in many organic solvents. The photophysical properties of novel monosubstituted polyacetylenes and corresponding monomers were compared. While the monomers exhibit strong emission in solution with quantum yields of up to 0.84 only selected polymers are luminescent (Φf = 0.06) and display moderate Stokes shifts and positive emission solvatochromism.
Introduction
In recent years the venerable electron-rich phenothiazine heterocycles have stimulated increasing scientific interest and have been successfully established in organic electronics, such as organic light-emitting diodes (OLEDs), organic field-effect transistors (OFETs), dye-sensitized solar cells (DSSCs), molecular wires and organic hole-transport layers.1–3 Due to their low reversible oxidation potential they qualify as good donor molecules, and butterfly shaped phenothiazines can be easily oxidized to give stable planar colorful radical cations.4 The optical and electrochemical properties of phenothiazines can be tuned by introducing substituents in 3- and 7-position using cross-coupling reactions, such as Suzuki and Sonogashira coupling.5–7 As previously shown by our group, the first oxidation potentials of phenothiazines can be lowered using monodisperse oligophenothiazines that are accessible via one-pot bromine–lithium exchange-borylation–Suzuki (BLEBS) coupling.8 Cyclic voltammetry revealed an electronic intramolecular communication between the phenothiazine units, which can be affected by integration of covalently bound organometallic communicators, such as ferrocene.9 The self-assembly characteristics of oligophenothiazines bearing thioacetate groups were successfully achieved by chemisorbing the moieties on gold surfaces.10,11 However, solubility issues impeded the applicability of early generations of oligophenothiazines.12,13Therefore, we became mesmerized to generate a novel conjugated organic polymer, uniting the interesting semiconductivity of polyacetylene with the light emission of the electron-rich heterocycle of phenothiazine. Among the conjugated polymers especially the archetypal polyacetylene and novel generations of mono- and disubstituted polyacetylenes received scientific interest, showing broad applications as devices in, for instance, xerography and photovoltaic cells.14–17
Very recently, we synthesized and characterized a series of novel emissive phenothiazinyl merocyanine substituted polyacetylenes accessible via rhodium catalysis following Taniguchi's protocol.18,19 Indeed, the photonic properties of phenothiazinyl merocyanines are transferable into the corresponding polymers. As monosubstituted polyacetylenes are typically regarded to be non-emissive due to unfavorable energetics of ground and excited states these results were quite surprising.20 In addition, well-known solubility issues of polyacetylenes could be overcome by introducing a branched aliphatic chain.
Here, we focus on the synthesis of (oligo)phenothiazinyl substituted polyacetylenes bearing electron withdrawing and donating groups with different ligation patterns for investigating the structure–property relationships.
Results and discussion
Monomer synthesis and polymerization
The synthesis of an asymmetric alkynylated phenothiazine dimer and trimer was started with a one-pot BLEBS sequence of the mono brominated 1a and dibrominated phenothiazine 1b moieties according to our established protocol.8 In order to overcome well-known solubility issues, a branched alkyl substituent was introduced by N-alkylation of the phenothiazine moiety.12 The dibrominated phenothiazine 1b was used in excess to minimize the formation of unfavorable symmetrical oligophenothiazines via double BLEBS sequence (Scheme 1).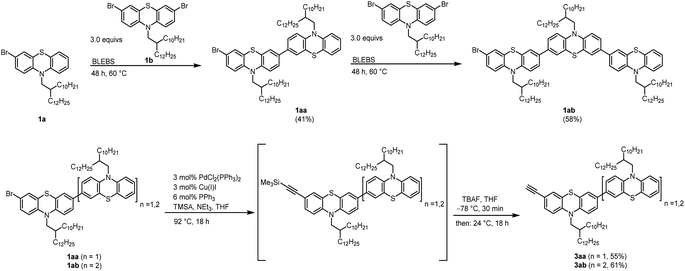 | ||
| Scheme 1 Synthesis of unsymmetrical oligophenothiazines 1aa–ab via BLEBS sequence and following synthesis of alkynylated oligophenothiazines 3aa–3ab by Sonogashira-coupling-desilylation-sequence. | ||
The unsymmetrical brominated phenothiazines 1aa and 1ab were transferred into the corresponding alkynylated moieties 3aa and 3ab in a one-pot fashion by Sonogashira-coupling-desilylation-sequence using (trimethylsilyl)acetylene (TMSA) as a coupling reagent and tetrabutylammonium fluoride (TBAF) to deprotect the TMS group.19
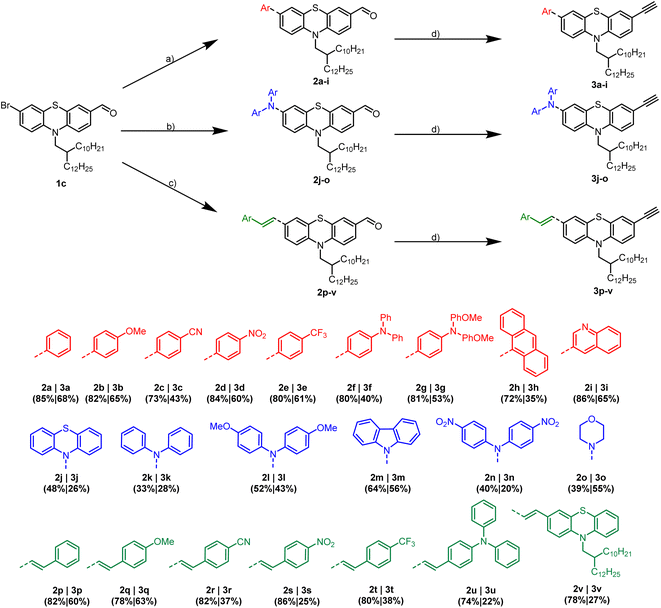
The preparation of 3-substituted alkynylated phenothiazines 3 commences from bifunctional 3-bromo-7-formyl phenothiazine 1c as starting material (Scheme 2). As recently published, 1c is readily accessible by N-alkylation of 10H-phenothiazine with a branched swallowtail, followed by two-step Vilsmeier formylation–bromination.20 Since cross-coupling reactions are a convenient method to generate libraries of substituted phenothiazines with various ligation patterns, we exploit the reactivity of phenothiazines and introduce substituents in 3- and 7-positions of the phenothiazine unit.
In the first step, electron donating and withdrawing aryl-substituents are introduced via Suzuki coupling using Pd(PPh3)4 and K2CO3 following our protocol5 to give arylated products 2a–i in good yields (71–82%).
The donor strength of the substituents can be increased by coupling of secondary amines via Buchwald–Hartwig arylamination of 1c.21 The corresponding phenothiazines 2j–o are obtained in moderate yields (33–64%). Selective secondary amines are synthesized via Ullmann coupling since Buchwald–Hartwig arylamination is unsuccessful. Especially, electron-poor secondary amines are quite unreactive (see ESI†). Regarding the morpholino substituted derivative 2o, an L-proline catalyzed variation of Ullmann coupling is required to obtain the desired product.
Furthermore, the π-system of the phenothiazine moieties can be extended by Heck coupling of substituted styrenes with 1c in the presence of Pd2dba3 and cataCXium® PtB as a ligand.7 The vinylated compounds 2p–v are isolated in good yields (74–82%). Finally, Bestmann–Ohira reaction of 2a–v furnishes alkynylated phenothiazines 3a–v in moderate yields, following mild conditions using a methanol–water mixture as a solvent at room temperature.6
The structures of the novel aldehydes 2a–v and monomers 3a–v are unambiguously characterized by 1H and 13C NMR, IR spectroscopy and mass spectrometry. The purity of the bulk material is supported by combustion analysis.
The polymerization of the substituted alkynylated phenothiazines 3a–v and 3aa–3ab is accomplished following the recently optimized protocol by Taniguchi via rhodium catalysis (Scheme 3).18 In contrast to monomers, which are isolated as colorful oils, the corresponding polymers are precipitated in methanol furnishing orange solids. Interestingly, the obtained polymers 4 show different morphologies, including rubbers, powders, and brittle glasses.
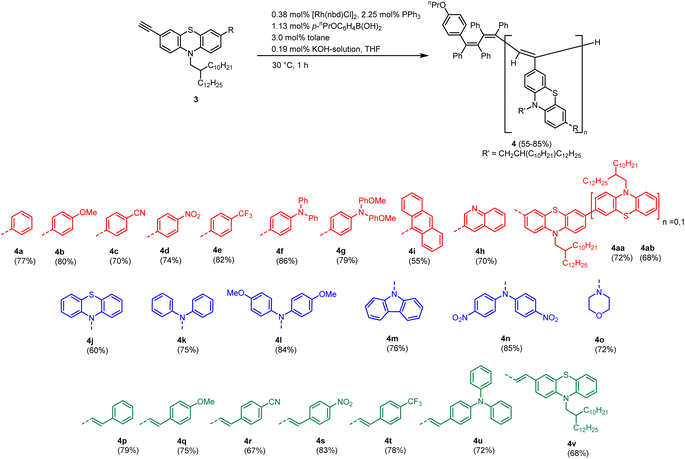 | ||
| Scheme 3 Synthesis of phenothiazinyl-substituted polymers 4 by rhodium-catalyzed polymerization of 3-alkynyl phenothiazines. | ||
The novel polymers 4 are characterized using gel permeation chromatography (GPC), IR spectroscopy, UV-vis absorption and emission spectroscopy. As already observed for parent polyacetylenes, NMR spectra reveal considerably broadened signals hampering a proper structural characterization.19
The solubility-promoting 2-decyl-tetradecyl N-substituent on the phenothiazine allows for characterization of all polymers except for anthracenyl substituted polymer 4i, which is completely insoluble. The polymerization data are investigated using GPC measurements.
As already observed for parent polymers, the polydispersities of phenothiazinyl substituted polyacetylenes are strongly dependent on the substitution pattern at position 7 of the phenothiazine moiety. Additionally, GPC data reveal an additional dependence on the ligation pattern. In contrast to recently published phenothiazinyl merocyanine substituted polyacetylenes,19 the obtained polymers generally show narrow weight distributions, despite applying same reaction conditions. On the one hand, aryl-substituted polymers 4a–h and amino-substituted polymers 4j–o show low dispersities (1.04–1.13) and high molecular weights, hinting at the living nature of this rhodium catalyzed polymerization (Table 1). On the other hand, vinyl substituted polymers 4p–4v show wider spread of molecular weights and broad dispersities. Noteworthy, nitro substituted derivatives 4d, 4n and 4s display significantly broader molecular weight distributions and lower molecular weights, which might be rationalized by interchain charge-transfer interactions of the electron-rich phenothiazinyl moieties with electron-deficient nitrostyryl parts.
| Polymer | Mw [kDa] | Mn [kDa] | ĐM | Polymer | Mw [kDa] | Mn [kDa] | ĐM |
|---|---|---|---|---|---|---|---|
| 4a | 205 | 188 | 1.11 | 4l | 25.9 | 23.8 | 1.09 |
| 4b | 106 | 103 | 1.06 | 4m | 9.82 | 9.28 | 1.08 |
| 4c | 138 | 133 | 1.04 | 4n | 92.3 | 70.2 | 1.31 |
| 4d | 1227 | 859 | 1.43 | 4o | 34.9 | 32.7 | 1.06 |
| 4e | 140 | 135 | 1.04 | 4p | 68.2 | 66.2 | 1.03 |
| 4f | 311 | 294 | 1.05 | 4q | 300 | 156 | 1.92 |
| 4g | 107 | 103 | 1.06 | 4r | 392 | 201 | 1.95 |
| 4h | 285 | 263 | 1.08 | 4s | 187 | 11.8 | 15.8 |
| 4i | — | — | — | 4t | 93.2 | 90.0 | 1.03 |
| 4aa | 107 | 97.1 | 1.11 | 4u | 109 | 85.5 | 1.28 |
| 4ab | 76.2 | 67.1 | 1.19 | 4v | 57.7 | 57.8 | 1.01 |
| 4j | 18.8 | 17.6 | 1.06 | 4w19 | 60.0 | 55.8 | 1.05 |
| 4k | 12.3 | 11.2 | 1.13 |
An additional trend can be observed regarding the oligophenothiazine polymers 4aa–4ab. In this case, longer sidechains clearly lead to broader molecular weight distributions. Comparing triad 4ab to unsubstituted pendant 4w (R = H),19 significantly higher polydispersities become evident indicating a low control of the polymerization (see Table 1).
Photophysical properties
The photophysical properties of monomers 3 and the corresponding polymers 4 were studied via UV-vis and fluorescence spectroscopy. All spectra were recorded in dichloromethane since significant emission was observed in polar solvents.Expectedly, alkynylated phenothiazines 3 show typical photonic properties for phenothiazines. Longest wavelength absorption maxima λmax,abs are between 334–431 nm including additional absorption bands in UV region caused by π–π* transition. Obviously, the absorption behavior is influenced by the electronic properties of the aryl substituents. A redshift of the longest wavelength absorption maxima within each series of monomers was observed for derivatives carrying electron withdrawing substituents. Therefore, the absorption maximum of nitro-substituted derivative 3n lies at 406 nm in contrast to donor substituted phenothiazine 3j bearing an electron-donating phenothiazine group (328 nm). Further regularities were observed comparing aryl substituted moieties 3a–i and corresponding vinyl substituted derivatives 3p–v. The extension of the π-system causes a redshifted absorption from 334 to 374 nm. The same trend can be seen with phenothiazine dyad 3aa and triad 3ab.
Except for nitro-substituted derivatives, monomers 3 display a pronounced emission in dichloromethane. The emission maxima lie between 466 and 570 nm. Again, the strongest redshift in emission is recorded for the vinylated acceptor substituted derivative 3r. Within the series, highest fluorescence quantum yields are detected for 3c (0.76), 3e (0.69), 3r (0.87) and 3t (0.84), bearing electron withdrawing cyano and trifluoromethyl substituents.
The optical properties of monomers 3 can be compared to the corresponding polymers 4. Generally, the absorption and emission spectra of all monomers and polymers show a similar shape. However, for the polymers 4 additional bands are observed at 500 nm, caused by the absorption of the polyene backbone. Noteworthy, the band appears as a broad shoulder. The remaining absorption maxima of polymers 4 coincide with the corresponding monomers since optical properties are determined by the phenothiazine moiety. A juxtaposition of monomer 3t and polymer 4t shows a redshifted absorption and emission. In contrast to alkynylated phenothiazines 3 and phenothiazinyl merocyanine substituted polyacetylenes (Fig. 1 and Table 2),19 polymers 4 are mostly weakly emissive (see ESI, Table S9†). Emission spectra are recorded, but the quantum yields are not determined since emission is very weak. Noteworthy, to measure the emission spectra of the polymers 4, excitation is not achieved at the longest wavelength absorption band (shoulder at around 500 nm), as usually, but at the next hypsochromically shifted maximum.
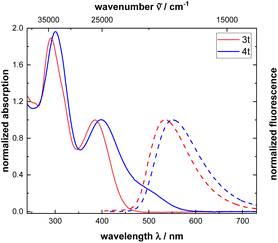 | ||
| Fig. 1 UV-vis absorption and emission spectra of monomer 3t and polymer 4t recorded in dichloromethane (c(3t,4t) = 10−5 M, T = 293 K). | ||
Exceptionally polymers 4r and 4t show a moderate emission in dichloromethane with fluorescence quantum yields of 0.06, however quantum yields are significantly lower than corresponding monomers. Moreover, both polymers exhibit pronounced polarity effects including positive emission solvatochromism (see Fig. 2 and Table 3). The lower fluorescence quantum yield in comparison to phenothiazinyl merocyanine substituted polyacetylenes19 and the lower energy of the longest wavelength absorption maxima of these series indicate that the sidechain chromophore only contributes to an emission of the polymer, if the excitation energy stems from the appended fluorophore moieties.
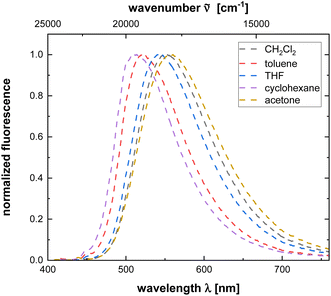 | ||
| Fig. 2 Emission spectra of polymer 4t recorded in solvents of different polarity (c(4t) = 10−5 M, T = 293 K). | ||
| Solvent | λmax,em [nm] | Stokes shift Δ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) [cm−1] [cm−1] |
|---|---|---|
| Cyclohexane | 514 | 5800 |
| Toluene | 522 | 6000 |
| THF | 543 | 6700 |
| Dichloromethane | 551 | 6900 |
| Acetone | 558 | 7700 |
Conclusion
In summary, we have successfully extended the recently published library of novel soluble phenothiazinyl substituted polyacetylenes obtained via rhodium catalysis and analyzed their structure–property relationship. Three series of substituted alkynyl phenothiazines bearing electron withdrawing and donating substituents with different ligation patterns were synthesized applying Suzuki coupling, Buchwald–Hartwig arylamination, Heck coupling and Bestmann–Ohira reaction. In contrast to parent polyacetylenes, most obtained polymers display narrow molecular weight distributions and low dispersities supporting living nature of polymerization. The photophysical properties of obtained alkynylated phenothiazines were compared to corresponding polymers. Contrary to highly emissive monomers, especially donor substituted polyacetylenes can be regarded as non-emissive. Among the series, only two polymers show moderate emission in dichloromethane (0.06), including emission solvatochromism.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors cordially thank the Fonds der Chemischen Industrie and the Deutsche Forschungsgemeinschaft (Mu1088/9-1) for financial support. We also thank the CeMSA @ HHU (Center for Molecular and Structural Analytics @ Heinrich Heine University) for recording the mass spectrometric and the NMR spectroscopic data.References
- T. Meyer, D. Ogermann, A. Pankrath, K. Kleinermanns and T. J. J. Müller, J. Phys. Chem., 2012, 77, 3704–3715 CAS.
- P. S. Gangadhar, G. Reddy, S. Prasanthkumar and L. Giribabu, Phys. Chem. Chem. Phys., 2021, 23, 14969–14996 RSC.
- J. J. Lade, S.-S. Chiou, Y.-H. Tseng, M.-H. Chiu, Y.-F. Lin, C.-H. Chang, P. Chetti and A. C. Chaskar, ChemistrySelect, 2021, 6, 11029–11038 CrossRef CAS.
- D. Pan and D. L. Phillips, J. Phys. Chem. B, 1999, 103, 4737–4743 CrossRef CAS.
- M. Hauck, M. Stolte, J. Schönhaber, H.-G. Kuball and T. J. J. Müller, Chem.–Eur. J., 2011, 17, 9984–9998 CrossRef CAS PubMed.
- C. S. Krämer and T. J. J. Müller, Eur. J. Org Chem., 2003, 2003, 3534–3548 CrossRef.
- M. Stephan, J. Panther, F. Wilbert, P. Ozog and T. J. J. Müller, Eur. J. Org Chem., 2020, 2020, 2086–2092 CrossRef CAS.
- A. W. Franz and T. J. J. Müller, Synthesis, 2008, 2008, 1121–1125 CrossRef.
- M. Sailer, F. Rominger and T. J. J. Müller, J. Organomet. Chem., 2006, 691, 299–308 CrossRef CAS.
- M. Sailer, A. W. Franz and T. J. J. Müller, Chem.–Eur. J., 2008, 14, 2602–2614 CrossRef CAS PubMed.
- A. Franz, S. Stoycheva, M. Himmelhaus and T. J. J. Müller, Beilstein J. Org. Chem., 2010, 6, 72 Search PubMed.
- T. J. J. Müller, A. W. Franz, C. S. Barkschat, M. Sailer, K. Meerholz, D. Müller, A. Colsmann and U. Lemmer, Macromol. Symp., 2010, 287, 1–7 CrossRef.
- H. Khelwati, A. W. Franz, Z. Zhou, W. R. Thiel and T. J. J. Müller, Molecules, 2021, 26, 2950 CrossRef CAS PubMed.
- H. Z. Chen, R. S. Xu, Q. Sun, J. W. Y. Lam, M. Wang and B. Z. Tang, Polym. Adv. Technol., 2000, 11, 442–449 CrossRef CAS.
- A. R. Murad, A. Iraqi, S. B. Aziz, S. N. Abdullah and M. A. Brza, Polymers, 2020, 12, 2627 CrossRef CAS PubMed.
- H. S. Kang, D. H. Kim and T. W. Kim, Sci. Rep., 2021, 11, 3885 CrossRef CAS PubMed.
- J. Yang, Z. Zhao, S. Wang, Y. Guo and Y. Liu, Chem, 2018, 4, 2748–2785 CAS.
- T. Taniguchi, T. Yoshida, K. Echizen, K. Takayama, T. Nishimura and K. Maeda, Angew. Chem., Int. Ed., 2020, 59, 8670–8680 CrossRef CAS PubMed.
- W. Pisetsky, P. Budny and T. J. J. Müller, Angew. Chem., Int. Ed., 2024, 63, e202316246 CrossRef CAS PubMed.
- S. Wang, D. Hu, X. Guan, S. Cai, G. Shi, Z. Shuai, J. Zhang, Q. Peng and X. Wan, Angew. Chem., Int. Ed., 2021, 60, 21918–21926 CrossRef CAS PubMed; B. K. Börüsah and T. J. J. Müller, Organics, 2022, 3, 502–506 CrossRef.
- L. Mayer, R. Kohlbecher and T. J. J. Müller, Chem.–Eur. J., 2020, 26, 15130–15134 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra01912a |
| This journal is © The Royal Society of Chemistry 2024 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600), 292 (35
600), 292 (35