Open Access Article
Open Access ArticleHighly homogeneous and stable single-walled carbon nanotubes dispersion modified by polyvinylpyrrolidone and alkanolamine in water
Minghua Li *ab,
Shujun Yuab,
Xiting Fangc,
Zhiqiang Duab and
Xiaojin Geab
*ab,
Shujun Yuab,
Xiting Fangc,
Zhiqiang Duab and
Xiaojin Geab
aSchool of Energy Materials and Chemical Engineering, Hefei University, Hefei, Anhui 230601, China. E-mail: liminghua@hfuu.edu.cn
bAnhui Provincial Engineering Research Center for Green Coatings High-performance Additives, Hefei, Anhui 230601, China
cAnhui Research Institute of Chemical Industry, Hefei, Anhui 230041, China
First published on 22nd April 2024
Abstract
A novel noncovalent surface modification of commercial single-walled carbon nanotubes (SWCNTs) was successfully carried out by using ball grinding technology between SWCNTs and mixed dispersants (polyvinylpyrrolidone (PVP) and alkanolamine), affording a highly homogeneous and stable PA-SWCNTs dispersion in water. The homogeneous dispersibility and long storage stability were systematically investigated by transmittance spectroscopy, absorption spectroscopy, zeta potential analyzer, sedimentation photo and transmittance electron microscopy. Under the optimized conditions, the PA-SWCNTs dispersion modified with 0.7 wt% PVP and 0.25 wt% alkanolamine under the condition of total 6 h ball grinding time using paint shaker can be easily well-dispersed in water and has good storage stability, and no sedimentation is observed more than one month. From an industrial perspective, this method is green and easy to operate in industry.
1. Introduction
Single-walled carbon nanotubes (SWCNTs) have aroused great interest among researchers owing to their extraordinary mechanical, electronic, and optical properties with practicable roles in low-weight conductive reinforcements in polymers,1,2 conductive inks for flexible displays,3 transparent electrodes,4 lithium ion battery,5 and more. Compared with spherical nanoparticles, the high aspect ratio and high conductivity of commercial SWCNTs provide an outstanding advantage in constructing a conductive network in the polymer matrix at a much lower content.6 However, the bundled aggregates of commercial SWCNTs induced by the particularly high aspect ratio and high interaction energy block their homogeneous dispersion. To utilize these excellent properties, commercial SWCNTs should be dispersed into a medium individually. Presently, dispersion with good dispersibility and long storage stability still presents a really tough challenge because a tube–tube contact produces high van der Waals interaction energy.In recent years, various methods have been proposed to decrease the CNTs agglomerations by using chemical and physical strategies.7–16 Chemical strategies based on surface modification of CNTs with strong acids such as HNO3 and H2SO4 create defects on the surface of CNTs, and then to graft soluble functional groups or hydrophilic chains.13,14 Although this has been recognized as an effective strategy for obtaining stable dispersion of CNTs in a medium, it usually results in the destruction of the intrinsic sp2 hybridized network and degrades the mechanical and electrical properties of the commercial SWCNTs.15,16 Besides, many researchers have vigorously adopted non-covalent strategies to disperse carbon nanotubes into the aqueous system, generally with the assistance of surfactants, biomolecules, aromatic compounds, natural macromolecules, and polymers onto the CNTs surface by using van der Waals forces or π–π stacking interactions.17–23 Because this strategy avoids complicated chemical reactions under strongly acidic conditions and preserves the conjugated structure and inherent properties of commercial SWCNTs, it has been considered to be an easy, green modification method. However, the long-term stability of carbon nanotubes dispersion with highly homogeneous dispersibility is generally worse than that of chemical modified dispersion.
Presently, the need to use environmentally friendly materials has accelerated increased researches on water-based formulations for water-borne coatings. In an effort to realize this goal, different water-borne coatings were developed, which during the coating deposition release water to the atmosphere instead of volatile organic compounds (VOCs). However, compared to that of solvent-borne coatings, the performance of water-borne polymer is not good enough.24 Song et al. reported that addition of multi-walled carbon nanotubes (MWCNTs) to the water-borne acrylic coatings resulted in an up to ∼50% increase in the adhesion strength. The nanocomposite coating with 1 wt% MWCNTs showed the highest efficiency in water retardation.25 Huang et al. made use of SWCNTs dispersion modified by sodium dodecylbenzenesulfonate (SDBS) and Triton X-100 to make water-borne polyurethanes coating for poly (ethylene terephthalate) (PET) film. The antistatic films obtained had special characteristics, such as high transparency, low sheet resistance and excellent resistance to water and heat.26
In this study, we report an easy, green modification of SWCNTs by using non-covalent strategy between commercial SWCNTs and mixed dispersants (polyvinylpyrrolidone (PVP) and alkanolamine), as shown in Fig. 1. PVP polymer with macromolecular chains provided greater steric hindrance and alkanolamine formed electrostatic repulsion, which facilitated the formation of well-dispersed SWCNTs. The optimum ratio of PVP and alkanolamine was explored for preparing highly homogeneous and stable SWCNTs dispersion in water.
Further, this water-based dispersion of commercial SWCNTs can be easily used to prepare SWCNTs/water-borne coatings nanocomposites under the condition of simple mixing. Importantly, this preparation route above is easy and economical due to easy operation and cheap raw materials in industry. We are profoundly convinced that this green experimental work will promote the application of SWCNTs in water-borne coatings.
2. Experimental
2.1 Materials
Commercial SWCNTs (black powder: SWCNT length >5 μm, BET: 300 m2 g−1, SWCNTs outer mean diameter: 1.6 ± 0.4 nm) was obtained from OCSiAl (Novosibirsk, Russia). Polyvinylpyrrolidone (PVP) and alkanolamine (2-amino-2-methyl-1-propanol) bought from market were used without purification. Deionized water was prepared using corresponding equipment.2.2 Modification of SWCNTs
2.3 Characterization
The transmittance and absorption of the SWCNTs dispersion were systematically determined with a quartz cell using an UV2600 ultraviolet spectrophotometer (Shimadzu Instruments).The zeta potential of the SWCNTs dispersion was investigated by a 90Plus PALS Zeta Potential (Brookhaven Instruments). Thermogravimetry analysis of commercial SWCNTs and modified SWCNTs was performed under nitrogen on a 209F1 thermogravimetric analyzer (Netzsch Instruments) with a temperature range of 25–600 °C at a heating rate of 10 °C min−1. Morphology of the diluted SWCNTs dispersion was characterized by transmittance electron microscopy (TEM, FEI F20). For TEM, the diluted dispersion (in water) was placed onto a carbon-coated copper grid.3. Results and discussion
3.1 Transmittance spectroscopy
From an industrial perspective, it is fast and easy to measure the optical transmittance of carbon materials while giving a repetitive and quick indication of dispersion quality.23,27 Fig. 2a shows the transmittance spectroscopy of diluted commercial SWCNTs, modified P-SWCNTs-0.5%, 0.6%, 0.7% and PA-SWCNTs with incident light wavelength ranging from 200 nm to 800 nm. We compared the experimental data at 550 nm from commercial SWCNTs, P-SWCNTs modified with different PVP content in Fig. 2b. From this it is can be seen that the modified P-SWCNTs dispersions have the different optical transmittance in water. Compared to 93.5% at 550 nm of commercial SWCNTs dispersion, the optical transmittance of the modified P-SWCNTs dispersion is lower, especially the P-SWCNT-0.7% (36.1%), indicating that commercial SWCNTs were poorly dispersed in water due to their high specific surface area, strong van der Waals forces and inherent hydrophobicity, resulting in serious agglomeration. Because of the new non-covalent interaction between commercial SWCNTs and hydrophilic PVP macromolecular chains, the agglomeration was controlled effectively. With the increasing PVP content, the optical transmittance of P-SWCNTs decreased gradually. This may because when the PVP content is increased, the tight agglomeration was controlled effectively.However, it is hard to improve SWCNTs dispersion further by using steric hindrance of PVP polymer separately. Considering the good dispersibility and long storage stability simultaneously, P-SWCNTs dispersion has been modified by using alkanolamine with electrostatic repulsion. Compared to 36.1% at 550 nm of optimal P-SWCNT-0.7% dispersion, the optical transmittance of water-based PA-SWCNTs dispersion is 32.7% in Fig. 2a, indicating that the dispersibility of PA-SWCNTs has been improved by alkanolamine owing to electrostatic repulsion interaction between SWCNTs and alkanolamine displayed in Fig. 1.
3.2 Absorption spectroscopy
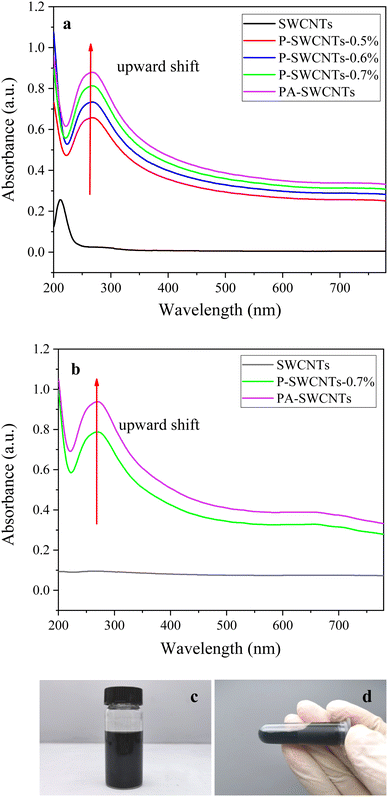
To utilize the physical properties of SWCNTs, the entangled bundles of the commercialized SWCNTs need to be modified before using as functional additives in the various materials.2,8,11 It is a good way to prove the surface modification effect of SWCNTs by comparing the absorbance intensity of carbon nanotubes remaining after a centrifugation.28,29 Therefore, the dispersing ability at varied dispersant content was characterized using UV-vis spectroscopy. As reported in previous studies, the greater absorbance proved the better dispersibility because the intensity of absorbance is related to the number of nanoparticles contained in the unit volume of the dispersion.23Fig. 3a shows the absorbance spectra of diluted dispersion of commercial SWCNTs and modified P-SWCNTs and PA-SWCNTs taken after centrifugation for 15 min. It is seen that the dispersion of the modified SWCNTs shows an upward shift of the baseline. The shorter the wavelength is, the larger the baseline shift is. The phenomenon is due to wavelength dependence of light scattering by SWCNTs in water.19 In addition, the absorbance of SWCNTs dispersion decreases from visible to near infra-red region, which is partly due to scattering in lower wavelength region. From the figure, it is clearly seen that the absorbance of SWCNTs dispersion taken after centrifugation is the lowest due to heavy sedimentation. Compared to that of SWCNTs dispersion, the absorbance intensity of diluted P-SWCNTs dispersion modified by different hydrophilic PVP content is enhanced owing to a little sedimentation and that of diluted PA-SWCNTs dispersion without sedimentation (see Fig. 3d) is the highest, proving that non-covalent steric hindrance interaction and electrostatic repulsion interaction have happened simultaneously between SWCNTs and mixed dispersants. Fig. 3b shows the absorbance spectra of diluted dispersion of commercial SWCNTs and diluted P-SWCNTs-0.7% and PA-SWCNTs after one month. Compared to that of Fig. 3a, the absorbance intensity of diluted PA-SWCNTs dispersion after one month is still high, indicating that it has good storage stability. This also shows that a stable PA-SWCNTs dispersion without sedimentation has been prepared successfully.
3.3 Dispersibility and stability
The zeta potential is an important parameter that characterizes the surface properties of electrostatically stabilized nanomaterials in aqueous solution.23 Fig. 4 shows the relation between zeta potential and SWCNTs dispersion modified by different dispersant. This plot shows that the zeta potential of P-SWCNTs dispersion decreases with increasing hydrophilic PVP content; then the zeta potential of P-SWCNTs-0.7% reaches the absolute value at 24.40 mV. However, it is hard to obtain the highly homogeneous and stable P-SWCNTs dispersion modified by different PVP content after a centrifugation under the condition of 900 rpm for 15 min, indicating that non-covalent steric hindrance interaction of PVP macromolecular chain is still insufficient. | ||
| Fig. 4 Relation between zeta potential and water-based SWCNTs dispersion modified by different dispersant. a: Commercial SWCNTs, b: P-SWCNTs-0.5%, c: P-SWCNTs-0.6%, d: P-SWCNTs-0.7%, e:PA-SWCNTs. | ||
To solve this problem, mixed dispersants including PVP polymer and alkanolamine are used to modify the commercial SWCNTs. Under the optimized conditions, the zeta potential of the modified PA-SWCNTs dispersion mixed with 0.7 wt% PVP and 0.25 wt% alkanolamine correspondingly under the condition of total 6 h ball grinding time using paint shaker reaches the absolute value at 25.30 mV and has good storage stability after a centrifugation. Therefore, it can be presumed that the non-covalent steric hindrance interaction and electrostatic repulsion interaction are important for preparing the highly homogeneous and stable PA-SWCNTs dispersion.
Sedimentation analysis is often used to estimate the dispersibility and storage stability of SWCNTs in water.30 Fig. 5 shows comparison photographs of water-based modified SWCNTs dispersions stored for one month. The dispersibility of the PVP modified SWCNTs is much better than that of their totally physical mixture without dispersant in water. It is clear that the commercial SWCNTs dispersion without dispersant has obvious black sediment in water. In contrast to water-based P-SWCNTs dispersion modified by different PVP content, the commercial SWCNTs is insoluble, while three kinds of P-SWCNTs dispersion in Fig. 5A can be easily well-dispersed in water, and no sedimentation is observed more than one month. This suggests that P-SWCNTs dispersions have good solubility in water. However, the P-SWCNTs dispersions diluted 100 times have a little sediment in Fig. 5B and the amount of sediment decreases in sequence, indicating that PVP dispersant is not enough to bring mutual exclusion and steric hindrance effect. Therefore, this indicates that P-SWCNT is not suitable for water-borne coatings.
Furthermore, the dispersibility of PA-SWCNT is also much better than that of their totally physical mixture. Compared with commercial SWCNTs and the best P-SWCNT-0.7% modified by hydrophilic PVP polymer in Fig. 5A, the PVP and alkanolamine modified PA-SWCNTs dispersion diluted 100 times can be easily well-dispersed in water, and no sedimentation is observed in Fig. 5B. The above results demonstrate that the PA-SWCNTs dispersion is a physically stable system and can be considered as an ideal functional additive for water-borne coatings.
3.4 TGA measurements
Supporting evidence for the non-covalent modification of commercial SWCNTs comes from the thermogravimetry analysis.27,28 Actual SWCNTs compositions of commercial SWCNTs, P-SWCNT and PA-SWCNTs at a temperature rate of 10 °C min−1 are shown in Fig. 6. The TGA plot of commercial SWCNTs indicates a gradual mass loss of around 1.8% as the temperature reaches 600 °C. However, there is a distinct mass loss region between 300 and 600 °C for modified P-SWCNT and PA-SWCNTs. The results show that the mass loss of P-SWCNTs increases with increasing hydrophilic PVP content. Compared with commercial SWCNTs, the mass loss around 59.1% of P-SWCNTs-0.7% terminates at approximately 600 °C, which was due to the pyrolysis of hydrophilic PVP polymer. Furthermore, we noted that PA-SWCNTs showed a significant weight loss around 62.8% corresponding to the pyrolysis of PVP and alkanolamine dispersant.3.5 Dispersion morphology
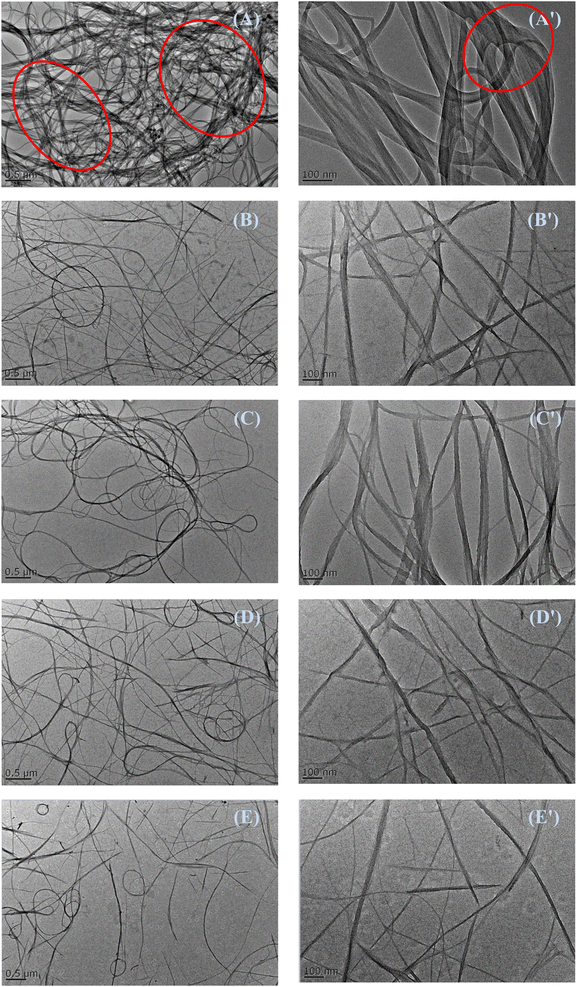
It is essential to investigate the influence of different dispersants on the dispersion of carbon nanotubes in water because physical properties of nanocomposites are directly dependent on their dispersion morphology.31–33 The dispersion morphologies of commercial SWCNTs, P-SWCNTs and PA-SWCNTs in water are checked by TEM, as shown in Fig. 7. The commercial SWCNTs dispersed in water at the carbon membrane show tight agglomeration and exist large carbon bundles about 50 nm,26 which is attributed to the poor dispersion of carbon nanotubes (marked as red circles in Fig. 7 A/A′). In comparison, we find small carbon bundles in the images of PVP-modified P-SWCNTs, which is due to the surface modification of hydrophilic PVP. It is obvious to find that the diameter of commercial SWCNTs is reduced.This phenomenon is consistent with SWCNTs functionalization with noncovalent interactions, where the organic chain may bleach the strong interactions between individual SWCNTs.Furthermore, after the modification with hydrophilic PVP and alkanolamine dispersant, the PA-SWCNTs nanotubes are also well dispersed in water, and show good dispersibility and exist small bundles in Fig. 7 (D/D′). Compared to that of commercial SWCNTs, the diameter of PA-SWCNTs is between 5 and 20 nm. The above results can demonstrate that the PA-SWCNTs dispersion is a physically stable system. Thus, it can be inferred from the dispersion morphology that PA-SWCNTs with good dispersibility and long storage stability is easy to disperse in water-borne coatings and build good physical properties.
4. Conclusions
In summary, a highly homogeneous and stable PA-SWCNTs dispersion was successfully prepared in water by non-covalent surface functionalization technique between SWCNT and mixed dispersants. The results show that the best modified PA-SWCNTs with the lowest optical transmittance (32.7%) at 550 nm and the largest zeta potential (25.30 mV) can be easily well-dispersed in water, and the aggregates of SWCNTs are successfully debundled into highly dispersed nanotubes by using paint shaker. The diluted PA-SWCNTs dispersion with no sedimentation more than one month has the potential application as a functional conductive or performance additive for water-borne coatings, which gives a new strategy for the high value-added utilization of SWCNTs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Hefei municipal natural science foundation (No. 202337) and Anhui provincial key research and development program (No. 2023z04020004).References
- B. Krause, P. Pötschke, E. Ilin and M. Predtechenskiy, Polymer, 2016, 98, 45 CrossRef CAS.
- A. A. Green and M. C. Hersam, Nano Lett., 2016, 8, 1417 CrossRef.
- T. J. Simmons, D. Hashim, R. Vajtai and P. M. Ajayan, J. Am. Chem. Soc., 2007, 129, 10088 CrossRef CAS.
- S. Li, K. Wang, M. Feng, H. Yang, X. Liu, Y. He, C. Zhang, J. Wang and J. Fu, Carbon Lett., 2020, 30, 651 CrossRef.
- Z. Y. He, Z. X. Xiao, H. J. Yue, Y. X. Jiang, M. Y. Zhao, Y. K. Zhu, C. H. Yu, Z. X. Zhu, F. Lu, H. R. Jiang, C. X. Zhang and F. Wei, Adv. Funct. Mater., 2023, 33, 2300094 CrossRef CAS.
- M. B. Basat and N. Lachman, Nanomaterials, 2021, 11, 2618 CrossRef.
- J. Zou, S. Khondaker, Q. Huo and L. Zhai, Adv. Funct. Mater., 2009, 19, 479 CrossRef CAS.
- M. M. Hamedi, A. Hajian, A. B. Fall, K. Hakansson, M. Salajkova, F. Lundell, L. Wagberg and L. A. Berglund, ACS Nano, 2014, 8, 2467–2476 CrossRef CAS.
- M. Liebscher, A. Lange, C. Schrofl, R. Fuge, V. Mechtcherine, J. Plank and A. Leonhardt, J. Mater. Sci., 2017, 52, 2296 CrossRef CAS.
- Y. Han, C. Cai, J. Lin, S. Gong, W. Xu and R. Hu, Rapid Commun., 2018, 39, 1880 Search PubMed.
- T. Wang, L. Jing, Q. Zhu, A. Ethiraj and X. Fan, J. Colloid Interface Sci., 2020, 577, 300 CrossRef CAS.
- X. Li, W. Chen and C. Zou, Powder Technol., 2020, 361, 957 CrossRef CAS.
- S. Fernando, Y. Lin and Y. P. Sun, Langmuir, 2004, 20, 4777 CrossRef PubMed.
- M. H. Li, Z. Y. Xu, J. Y. Chen and S. E. Zhu, J. Polym. Eng., 2018, 38, 537 CrossRef CAS.
- S. Peng and K. Cho, Nanotechnology, 2000, 11, 57–60 CrossRef CAS.
- B. Sohrabi, N. Poorgholami-Bejarpasi and N. Nayeri, J. Phys. Chem. B, 2014, 118, 3094 CrossRef CAS PubMed.
- M. H. Li, P. Xu, J. G. Yang, H. Ying, K. Haubner, L. Dunsch and S. F. Yang, J. Phys. Chem. C, 2011, 115, 4584 CrossRef CAS.
- E. Zelikman, D. Alperstein, G. Mechrez, R. Suckeveriene and M. Narkis, Polym. Bull., 2013, 70, 1195 CrossRef CAS.
- G. Liu, N. Liu, A. López-Moreno, P. Zhao and W. B. Dai, ChemistrySelect, 2018, 3, 6081 CrossRef CAS.
- B. Sharma, S. K. Sharma, S. M. Gupta and A. Kumar, Arabian J. Sci. Eng., 2018, 43, 6155 CrossRef CAS.
- W. M. Peter, W. Pawel, L. O. David and M. G. Dirk, J. Porphyrins Phthalocyanines, 2018, 22, 1 CrossRef.
- Y. Kim, J. S. Hong, S. Y. Moon, J. Y. Hong and J. U. Lee, Carbon Lett., 2021, 31, 1327 CrossRef.
- M. H. Li, D. M. Jiang, Z. Q. Du, S. J. Yu, X. J. Ge and Y. He, J. Nanopart. Res., 2023, 25, 85 CrossRef CAS.
- S. Huo, H. Y. Zhou and J. X. Wang, React. Funct. Polym., 2021, 163, 104892 CrossRef CAS.
- D. D. Song, Z. W. Yin, F. J. Liu, H. X. Wan, J. Gao, D. W. Zhang and X. G. Li, Prog. Org. Coat., 2017, 110, 182–186 CrossRef CAS.
- Y. Tian, X. C. Zhang, H. Z. Geng and H. J. Yang, RSC Adv., 2017, 7, 53018 RSC.
- M. H. Li, X. Y. Lu, J. J. Jiang, L. Gao, J. Gao and D. M. Jiang, RSC Adv., 2022, 12, 6037 RSC.
- L. Liu, P. X. Yu, M. Y. Wu, Q. Y. Wu, J. Y. Liu, J. J. Yang and J. A. Zhang, Ind. Eng. Chem. Res., 2021, 60, 12353 CrossRef CAS.
- A. U. Rehman, S. M. Abbas, H. M. Ammad, A. Badshah, Z. Ali and D. H. Anjum, Mater. Lett., 2013, 108(1), 253 CrossRef CAS.
- B. Sharma, S. K. Sharma and S. M. Gupta, Mater. Res. Express, 2018, 5, 055511 CrossRef.
- L. Bai, Y. Bai and J. Zheng, J. Mater. Sci., 2017, 52, 7516 CrossRef CAS.
- T. Hussain, F. Bashir and A. Mujahid, Silicon, 2022, 14, 10807 CrossRef CAS.
- B. Sharma, S. K. Sharma, S. M. Gupta and A. Kumar, Arabian J. Sci. Eng., 2018, 43, 6155–6163 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |