 Open Access Article
Open Access ArticleAdvancements in application of chitosan and cyclodextrins in biomedicine and pharmaceutics: recent progress and future trends
Farnaz Bahavarniaa,
Mohammad Hasanzadeh *b,
Parinaz Bahavarniac and
Nasrin Shadjou
*b,
Parinaz Bahavarniac and
Nasrin Shadjou d
d
aNutrition Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
bPharmaceutical Analysis Research Center, Tabriz University of Medical Sciences, Tabriz, Iran. E-mail: hasanzadehm@tbzmed.ac.ir
cFood and Drug Safety Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
dDepartment of Nanotechnology, Faculty of Chemistry, Urmia University, Urmia, Iran
First published on 24th April 2024
Abstract
The global community is faced with numerous health concerns such as cancer, cardiovascular and neurological diseases, diabetes, joint pain, osteoporosis, among others. With the advancement of research in the fields of materials chemistry and medicine, pharmaceutical technology and biomedical analysis have entered a new stage of development. The utilization of natural oligosaccharides and polysaccharides in pharmaceutical/biomedical studies has gained significant attention. Over the past decade, several studies have shown that chitosan and cyclodextrin have promising biomedical implications in background analysis, ongoing development, and critical applications in biomedical and pharmaceutical research fields. This review introduces different types of saccharides/natural biopolymers such as chitosan and cyclodextrin and discusses their wide-ranging applications in the biomedical/pharmaceutical research area. Recent research advances in pharmaceutics and drug delivery based on cyclodextrin, and their response to smart stimuli, as well as the biological functions of cyclodextrin and chitosan, such as the immunomodulatory effects, antioxidant, and antibacterial properties, have also been discussed, along with their applications in tissue engineering, wound dressing, and drug delivery systems. Finally, the innovative applications of chitosan and cyclodextrin in the pharmaceutical/biomedicine were reviewed, and current challenges, research/technological gaps, and future development opportunities were surveyed.
1. Introduction
Oligosaccharides and polysaccharides are a diverse group of natural polymers with important biological functions.1 The highest class of functional oligosaccharides are considered carbohydrates, whose monosaccharide units are fructose, galactose, glucose and/or xylose.2 These molecules are called prebiotics because they promote the growth of beneficial bacteria, especially Bifidobacterium species. These beneficial oligosaccharides have physical and physiological benefits that help improve consumers' health. In this way, the application of oligosaccharides as additives in beneficial nutraceuticals has great potential to make major advances in the quality of nutraceuticals affecting the user's health. Functional oligosaccharides offer various health benefits and can be used as nutrients, medicine, animal feed, beauty products, immune-stimulating, and prebiotics.3,4 In expansion, known functional oligosaccharides incorporate arabinose–oligosaccharides, arabinogalactan–oligosaccharides, arabinoxylo–oligosaccharides, galacturonic–oligosaccharides and human draining oligosaccharides (HMOs). In particular, cyclodextrins, made from starch with modifications in the product, are a class of macrocyclic oligosaccharides.5,6 Cyclodextrins are cyclic α-(1/4)-glucans that polymerize levels of 6, 7, and 8 monosaccharide units, respectively. Macrocyclic carbohydrates are widely associated with supramolecular chemistry products, drug carriers, atomic reactors, and artificial devices.7 Since the 1950s, cyclodextrins have been recognized for their physicochemical properties and potential to enhance the solubility, stability, and bioavailability of molecular therapies. They can serve as multi-functional drug carriers through the formation of inclusion complexes or CyD/drug conjugates, making them attractive for drug delivery applications. The unique biocompatibility and functional capabilities of cyclodextrins and their derivatives have led to their development for biomedical materials. Beneficial oligosaccharides have been shown to promote intestinal regeneration and reduce8 the risk of intestinal diseases, obesity, cancer, body weight, and type 2 diabetes. As a result, they are recommended as important nutrients for metabolic diseases.9Polysaccharides have various functional groups including hydroxyl, amino, carboxylate, sulfate and ester groups. Certain polysaccharides and their derivatives, such as alginate, starch, and particularly chitosan, exhibit mucosal adhesion properties due to modifications in hydrogen bonding, electrostatic attraction, and hydrophobic interactions.10
Polysaccharides like cellulose, xylan, and chitosan are structural components of plant cell walls and the shells of fish and reptiles. Other polysaccharides like glycogen, amylose, and amylopectin are important for sugar storage in bacteria and plants.11 Chitosan has attracted major scientific and industrial interests since the late 1970s. Because of its particular macromolecular structure, biocompatibility, biodegradability and other intrinsic functional properties, chitosan and its derivatives have practical applications in pharmacy, medicine, and chemistry.25 There has been notable advancement in understanding how certain compounds are synthesized in living organisms.12 This understanding has helped in discovering new types of sugars by exploring genetic information and has opened up possibilities to modify these sugars in a way that improves their medicinal properties.13 Many polysaccharides have anticancer activity.14 Usually, the way it works is by making the macrophages in the host become active.15 Sugar-based carbohydrates, which are used in biomedical applications to improve the ratio, are also used in unconventional applications.16 In biomedical applications, PEG (polyethylene glycol) is known as a safe and consistent material that can enhance the relaxation of some paramagnetic substances such as gadolinium diethylenetriaminepentacetate (Gd-DTPA) or ferrite.17 However, researchers have studied how saccharides affect T1 and T2 relativities as possible options.18 Polysaccharides like heparin, pullulan, and chitosan are used to target tumor cells.19 Small sugars can be used as a source of energy in living organisms. These small sugars can be broken down easily and quickly to provide fuel for cells. Additionally, glycan is now being used in nanotechnology to prepare materials for purposes such as tissue engineering, drug delivery, inhibiting enzymes, and creating biosensors.16,20–22 The one important use the carbohydrates in medical science is their ability to be recognized and taken into cells by lectins on the surface of mammalian cells. Also having multiple valence or charge states is a common characteristic of transition metal elements.23 Scientists discovered that when multiple patterns of a substance join together on a specific target, it can increase the attachment strength between carbohydrates and protein receptors, this is called a multivalent ligand.24
This study aimed to explore the use of oligosaccharides derived from natural sources and bioactive polysaccharides. The review introduced various types of saccharides, defined natural biopolymers such as cyclodextrin and chitosan, and discussed their wide applications in biomedical fields that have received attention from researchers. Recent research advances in pharmaceutics, especially in drug delivery based on the response of cyclodextrin to smart stimuli, were also discussed. The review covered the biological functions of cyclodextrin and chitosan, including their antimicrobial, anti-oxidative, and immunomodulatory effects, as well as their applications in tissue engineering, wound dressing, and drug delivery systems. Innovative applications of saccharides were investigated. Finally, current challenges and future development opportunities were discussed. To aid in understanding the procedure, a summary is provided in Fig. 1.
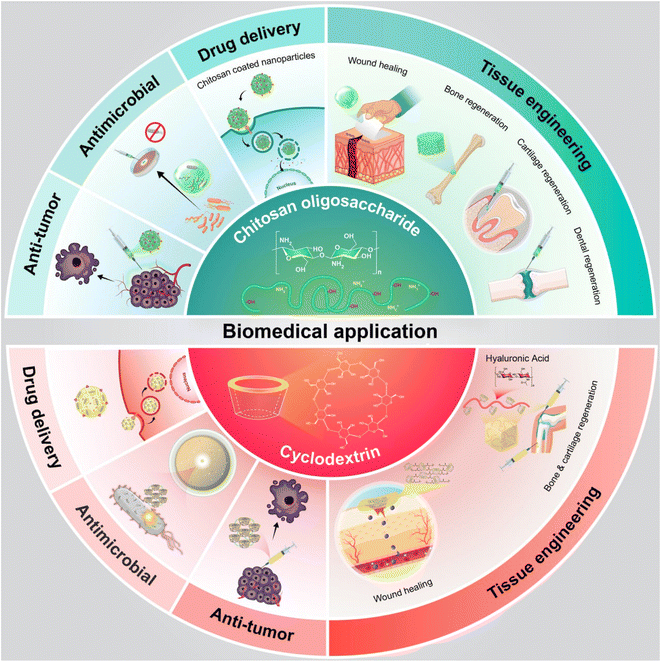 | ||
| Fig. 1 Schematic representation of pharmaceutical/biomedical application of natural chitosan and cyclodextrin. | ||
2. Chitosan oligosaccharide (COS)
Chitosan is a heteropolysaccharide composed of 2-acetamido-2-deoxy-D-glucopyranose and 2-amino-2-deoxy-D-glucopyranose that are distributed linearly and randomly. Chitosan is a biopolymer obtained from chitin, one of the most abundant and renewable materials on Earth. Chitin is a primary component of cell walls in fungi, the exoskeletons of arthropods, such as crustaceans, e.g. crabs, lobsters and shrimps, and insects, the radulae of molluscs, cephalopods, and the scales of fish and lissamphibians. The arthropod's exoskeleton is first smashed and cleaned, then soaked in hot sodium hydroxide (NaOH) to dissolve the proteins and sugars, leaving behind only basic chitin. The remaining chitin is washed with deionized water, which is then drained off. The chitin is converted into chitosan by the acetylation process.26 Chitosan is widely used in various fields such as biomaterials, cosmetics, pharmaceuticals, environmental science, agriculture, and food processing due to its ability to interact with other polyelectrolytes and its effectiveness in tasks such as flocculation and clarification. Chemical studies of chitosan (CS) have mainly focused on its use in drug delivery systems for biomedical applications.27,28Chitosan, unlike its chitin precursor, is mostly insoluble in solvents and can be rapidly converted into filaments, cast into films, or various microscopic morphologies from its acidic aqueous solution.29 Electrospinning from vinegar solutions has been recorded to produce tiny fibers. As a natural biopolymer, chitosan has the ability to create porous structures when its solutions freeze and lyophilize (freeze-drying), or by using simple methods such as the “internal bubble process”. This makes chitosan very useful for tissue engineering, particularly in orthopedics for bone regeneration and cartilage. There are several methods for preparing chitosan material for laboratory-cultured cells. These materials can be sponges, gels, fibers, or poriferous materials such as chitosan and ceramics, as well as other materials such as collagen or gelatin.11,30,31
Tissues were formed from cells by mixing chitosan alginate with various substances like calcium phosphate, polymethyl methacrylate (PMMA), poly-L-lactic acid (PLLA), and hydroxyapatite, hyaluronic acid. Alginate is a suitable material for cartilage engineering, but it does not adhere to cells. Researchers have shown that alginate-based chitosan hybrid polymer fibers have better cell adhesion and growth ability in the laboratory than alginate fibers alone. Additionally, when mixed with alginate, it acts like a body, controlling the release of important substances in the body called bioactive macromolecules such as hirudin. Researchers have mixed chitosan with different materials such as alginate, hydroxyapatite, hyaluronic acid, calcium phosphate, PMMA, poly-L-lactic acid (PLLA), and the growth materials that could be used for lab-grown tissue.32–37
3. Use of chitosan-based biomaterials in tissue engineering
Skin is the largest organ and the first line of defense against external agents such as viruses and mutagens.37. Skin injuries caused by heat, chemicals, or electricity can be detrimental, causing long-lasting injuries that are difficult to heal. Traditional skin graft treatments and techniques are not very effective, and they can cause a delayed or incorrect healing process, as well as a negative immune response. In this field, engineering provides effective solutions by morphologically mimicking natural systems,38 allowing cells to grow and multiply in three dimensions39 and helping skin cells move and change properly. Chitosan is an ideal material for tissue engineering because it is non-toxic, biodegradable and compatible with living cells. It can also be modified to create structures that resemble the body's natural framework.393.1. Capability of chitosan in tissue building
The use of chitosan-based membrane structures for tissue engineering has been widely studied. In vitro studies using L929 mouse fibroblasts demonstrated accelerated growth, reduced apoptosis, and oxidative stress when compared to control plastic. Furthermore, protein analysis confirmed the presence of fibroblast markers for cell survival and membrane development.40 In vitro studies using dermal fibroblast cultures showed that the designed membrane promoted fibroblast growth. These results suggest that the chitosan-based membrane has the potential to be used as an antibacterial agent in the treatment of skin burns.41 Furthermore, a different study examined the potential of a chitosan-aloe-curcumin layer in facilitating tissue regeneration in large wounds. The study also found that incorporating curcumin into the membrane composites may aid in wound healing and controlling infections.5 The study investigated the wound-healing effect of a polyvinyl alcohol chitosan layer in combination with ibuprofen. In vitro studies of the drug release membrane showed that the layer promoted drug release. Furthermore, biocompatibility testing confirmed that the membrane did not affect human dermal fibroblasts (NHDF) and also increased the adhesion and growth of the culture. Microscopic images of cells showed skin healing and reduced inflammation in samples treated with ibuprofen membranes.42 Similarly, chitosan-silk microfiber membranes with suitable properties were prepared, which were biocompatible and did not harm L929 cells and helped them grow.42 Furthermore, in vivo experiments conducted on mice with burns demonstrated that applying the chitosan-based membrane resulted in a reduction of the inflammatory response, even distribution of fibroblast cells, and deposition of sufficient collagen.43 The study also involved seeding the membrane with mesenchymal stem cells (MSCs) due to their good adhesion properties. Results from in vivo experiments showed that chitosan films mixed with MSCs enhanced wound healing outcomes.44 Similarly, another study examined the anti-inflammatory effect of a chitosan–hyaluronic acid–edaravone membrane in wound healing. Additionally, in vivo analysis of mouse skin showed that the membranes caused less swelling and facilitated the movement of fibroblasts, keratinocytes, and endothelial cells, leading to wound healing.45 They used microwave drying to fabricate a strong and elastic composite membrane with effective antimicrobial properties against both Gram positive and negative bacteria. The chitosan-based composite membrane also showed significant wound healing properties in shallow burn wounds in rats, as indicated by Masson staining revealing the presence of vascular endothelial growth factor.46 MTT analysis revealed that the membrane formation did not harm fibroblasts, and fluorescent dye tests showed that chitosan-based membranes actively promoted fibroblast growth and maturation. The fibroblast cells had a usual shape and a strong network structure, showing that they stuck to the membrane, the experiments found that chitosan-based membrane material can help heal cells and prevent infections. This makes it a good option for making membranes for skin tissue engineering.47 In conclusion, chitosan-based layers have had a major impact on the development of chitosan science and biomedical fields including tissue engineering. Most researchers only use simulated fluids, animals (mice, pigs, etc.) or cells to test the biomedical properties of chitosan-based biomaterials, to bridge the gap in biotechnology.48 So, further research and technology are needed to develop chitosan-based biomaterials for tissue engineering. Scientists have done a lot of research on how chitosan-based hydrogels can be used in treating skin issues.49 In a research experiment, scientists made a substance called chitosan hydrogel by mixing glutaraldehyde and genipin. The test in the lab showed that cell growth increased on the chitosan hydrogel surface compared to the control. A research study combined chitosan and alkaline lignin to produce a biocompatible hydrogel.50 The chitosan–lignin hydrogel promoted rat migration in scratch wound healing, with the 3T3 fibroblast cell line dispersed throughout the area, and long-term incubation showing fibroblasts in all scratch areas. It enhanced collagen strand formation and the number of fibroblast cells at the wound site in skin injuries. The researchers observed that the chitosan hydrogel with the small carrier system is adhesive and reduces the chances of phenytoin absorption through the skin. Additionally, a unique gel composed of chitosan and fluorinated methacrylamide was tested on rats with diabetes, showing increased collagen fibers, improved skin healing, and formation of new blood vessels.51–53 In another research, scientists looked at a special gel made with alginate and chitosan that included hesperidin, and they tested it on rats with wounds. Furthermore, the hydrogel also exhibited powerful antioxidant properties because it contains chitosan amino groups and silver nanoparticles.54 Enhancing chitosan's properties through modifications and combination with other substances, such as complex formation, holds promising potential. The porous structure of sponges follows the structure of the body, promoting integration, interaction, growth and development.55 Cell-derived sponges can maintain their porous structure and are suitable for skin engineering applications.56 A study documented the tissue engineering of an innovative chitosan–gelatin sponge-like skin treatment. In another study, biocompatible chitosan gelatin sponges containing curcumin were investigated for tissue building. It contains less gelatin than sponge chitosan due to its wound-healing properties. Additionally, in another study, researchers developed and tested sponges containing dermal fibroblasts and chitosan. Sponges aid the body's healing process by accelerating cell growth and increasing collagen production. Scientists combined chitosan sponge with basic fibroblast growth factor to create a novel tissue engineering material with a highly porous structure and increased elasticity as chitosan content was raised. This chitosan-based sponge releases growth factors, aiding in wound healing, and has shown impressive physical properties. Another study explored a collagen and chitosan sponge infused with MSCs, demonstrating effective reduction of inflammation and promotion of blood vessel growth in diabetic rats, accelerating wound healing. mRNA expression study showed sustained presence of β-catenin in treated tissues, highlighting its crucial role in wound healing and dermis formation.59 This sponge shows effective water retention, expansion, support, and protection for damaged tissue. Chitosan, derived from shellfish shells, possesses properties that reduce inflammation, fight infections, and promote cell growth. Applying these sponges to wounds can create a favorable environment for tissue repair, promoting cell growth and aiding in the healing process. Chitosan sponges have the potential as supportive structures for damaged skin. In summary, chitosan-based sponge materials are notable for their compact size, impressive biocompatibility, and capacity for self-repair in the body's natural environment. Fiber-based biomaterials can support the 3D growth and development of cells as well as biomimicry. Experimental results in wound healing show that fibers promote cell adhesion and expansion towards their surface. The resulting composite nanofibers displayed a smooth, uniform structure with hydrophobic properties.57–68 Additionally, the presence of starch and chitosan promoted L929 cell migration, aiding in wound healing. Overall, these nanofibers have the potential to eliminate pathogens and enhance wound healing. Scientists combined chitosan and collagen microfibrils and tested the mixture on 3T3 fibroblasts and HaCa T keratinocytes, facilitating healing. Another study connected silk and chitosan fibers using genipin and glutaraldehyde, with genipin showing low toxicity and promoting rapid fibroblast cell growth.65 In the identical experiment, researchers fabricated a scaffold utilizing chitosan-polyvinyl alcohol nanofibers and investigated the adhesion of skin fibroblasts, a specific type of cell, to the material. In summary, researchers have developed and assessed wound dressings made by blending chitosan and polyvinyl alcohol nanofibers with carboxymethyl chitosan nanoparticles. These nanofibers enhance bioactive substance availability, regulate degradation, and effectively combat Staphylococcus aureus and Escherichia coli. Histopathological examinations have shown that chitosan-based nanofibers promote collagen expression and epithelial cell regeneration, demonstrating their dual capabilities in antibacterial properties and wound recovery. The resulting nanofiber filament demonstrated enhanced stability and effectiveness in reducing colonies of Staphylococcus aureus and E. Cytotoxicity assessments showed that the composite nanofiber structure was biocompatible with keratinocytes and human dermal fibroblast cell lines. Studies indicate that chitosan composite biomaterials exhibit favorable properties and biocompatibility, making them suitable for various applications.69In concluding this section, it's evident that the impact of chitosan on the development of biomedical fields, such as tissue engineering, pharmaceutics, drug delivery, and bacteria eradication, is significant. Research has shown that chitosan-based membrane materials contribute to cell healing and disease protection, making them ideal for constructing tissue engineering membranes. These membranes have been effective in eradicating Gram-positive and Gram-negative bacteria and have also demonstrated the promotion of healing without causing severe burns in experiments involving mice. The membrane preparation process displays properties such as good crystallinity, flexibility, and antibacterial effectiveness against Staphylococcus aureus. Significant advancements have been made, yet a notable disparity remains between the research efforts focused on chitosan-based biomaterials and the actual number of approved products available in the market. Furthermore, the expedient development of chitosan-based coatings poses a persistent challenge in areas where the attainment of precise geometries and high performance presents difficulties. To bridge this gap in biotechnology, a majority of researchers opt to explore the biomedical properties of chitosan-based biomaterials using simulated fluids, animal models (mice, pigs, etc.), or cellular tests. Therefore, advancing research and technology is imperative to propel the progress of chitosan-based biomaterials within the domain of tissue engineering. Notably, hydrogel offers the benefit of being soft, flexible, non-toxic, and conducive to stable pore size control, and poses disadvantages in terms of mechanical properties for particular pore sizes and the potential for toxic responses. Moreover, there could be variations based on chitosan's activity and its combination with other substances (complex formation) can enhance its characteristics. The sponge's porous structure mimics the human body's structure, promoting integration, interaction, growth, and development. Sponges derived from cells can preserve their porous structure, making them suitable for skin engineering applications. These sponges are biocompatible with human keratinocytes (HaCa T), aiding the body's healing process by stimulating cell growth and increasing collagen production. Chitosan sponges are a favorable option for modeling damaged tissue. Chitosan-based sponges are widely preferred due to their small size, good biocompatibility, and potential in physiological settings. Despite this potential, the development of chitosan-based self-healing hydrogels (e.g., PEG) remains challenging.
3.2. Other applications of chitosan
Unrestrained hemorrhaging from an injury can result in various complications, including hypothermia, low blood pressure, infection, and even shock. The risk is heightened when there is minimal obstruction between the key components of innovation or discovery, such as the idea and the experiment.70If not addressed, it can result in severe health complications and mortality.73 Surgical interventions such as those performed on the heart, liver, and bones can lead to bleeding and adverse impacts on the body, such as low blood volume shock and hypothermia, which can affect blood coagulation.74 As a result, researching the cessation of blood flow and discovering methods to swiftly halt bleeding are crucial for emergency lifesaving measures.71 Moreover, when in contact with blood or fluids, hemostats may become contaminated by bacteria. A torn dressing can cause prolonged pain and delay the healing of a wound, thus limiting the possibility of their reuse.76 Furthermore, they are unable to be adapted for irregular, deep, or minor wounds.77 Over the recent years, scientists have created specific medications that aid in halting bleeding during surgical procedures and emergency scenarios. These medications, known as topical hemostatic adhesives and sealants, work by speeding up the blood clotting process.78–80 However, these products have their limitations. While cyanoacrylates serve as potent hemostatic adhesives, they have the potential to trigger allergic reactions. Additionally, when these adhesives solidify, they generate heat rapidly and form substances that may be harmful to the human body. On the other hand, hydrogels, which comprise natural or synthetic components, can be used in the production of hemostatic materials. Traditional hydrogels can be easily inserted and applied to specific areas, including uniquely shaped wounds, with minimal adverse effects. Natural polysaccharides, often used as raw elements for hydrogel formulations, possess properties like biodegradability, good biocompatibility, and the capacity to enhance hemostatic effects after wound healing.72,73 An appropriate hemostatic hydrogel should possess the following characteristics (I) undergo rapid conversion into a gel to halt bleeding and promote wound healing (II) exhibit good viscosity and durability in wet environments to ensure effective wound coverage. With the advancement of biomaterial science, chitosan-based hemostatic hydrogels have demonstrated effective hemostatic effects, biodegradability, anti-inflammatory capabilities, and healing properties.74
Adequate control of bleeding is necessary to diminish patient suffering and mortality, and a thorough understanding of hemostasis is crucial for achieving effective hemostasis. Chitosan (CS) possesses favorable qualities such as biodegradability, biocompatibility, and non-toxicity, making it extensively applicable in fields like biomedicine, chemicals, food industry, cosmetics, and other industries. Optimization of chitosan-based composite material structures can facilitate rapid hemostasis. Moreover, CS can serve as antibiotic, anti-inflammatory, wound healing agents, and other applications. It can also be utilized to develop diverse hemostatic components leveraging its properties.77 Mixing chitosan and succinic anhydride in pyridine yields a product known as CS-succinic acid (CSS). Research has demonstrated the efficacy and safety of a hemostatic made with CSS for managing hemostasis in rats with liver disease. This formulation has potential for treating severe bleeding via intravenous injection.78
In a separate study, Prof. Xu's research team examined a unique gel type created by combining silk fibroin with CS using N-hydroxy succinimide. This gel boasts a range of functionalities, such as antibacterial, hemostatic, and slow drug release properties. The CS dressing, incorporating fibroin fibers, demonstrates improved water absorption, hemostasis, and enhanced air permeability. Results indicated that the CS/SF hydrogel exhibits potent bactericidal and hemostatic capabilities without causing harm to human skin cells, showing promising applications as a wound dressing.64
Chitosan combined with CSS presents an eco-friendly alternative to traditional plastic packaging derived from non-renewable resources, as it is composed of biodegradable polymers. The quality of the cast chitosan membrane is influenced by the choice and application method of the plasticizer used in the product. Research indicates that the proper grafting of succinic acid occurred without altering the structure of the chitosan sample. The inclusion of succinic acid as a plasticizer offers various advantages, including its ability to function as a biodegradable material for bacterial eradication and hemorrhage control, in addition to its potential usage as a wound dressing.80
In summary, hemostatic, adhesives, and sealants play a crucial role in controlling bleeding during surgeries and in emergency scenarios. While cyanoacrylates in hemostatic adhesives are powerful, their potential to trigger allergic reactions and release harmful substances upon hardening should be noted. Nonetheless, the benefits of these medical products in reducing patient morbidity and mortality cannot be overlooked. The combination of silk fibroin with CS using N-hydroxy succinimide yields a versatile product with antibacterial, hemostatic, and anti-secretory properties. The CS dressing, enriched with fibroin fibers, exhibits improved water absorption, effective hemostasis, and permeability to excess air. Studies show that the CS/SF hydrogel displays significant bactericidal and hemostatic capabilities without causing harm to human skin, making it suitable as a wound dressing. The combination of chitosan and succinic anhydride results in CSS, which has been proven to effectively control bleeding in mice with liver disease. In cases of severe bleeding, injections may be employed for treatment. The use of succinic acid stands out as it serves as a biodegradable, sterilizing substance that aids in preventing bleeding and acts as a wound dressing, albeit with potential toxicity in certain instances. Despite ongoing advancements in hemostatic agents in modern medicine, challenges persist, hindering their successful application in treating uncontrolled bleeding. Therefore, there is a pressing need for the rapid development of efficient, user-friendly, and versatile hemostatic products, with CS-based hydrogels positioned to replace existing options, thereby enhancing the quality of such medical interventions in surgical and emergency settings.
4. Cyclodextrin and types of cyclodextrins
Cyclodextrins (CDs) are short-chain oligosaccharide molecules that result from the degradation of starch. Comprising D-glucopyranose units joined by a glycosidic bond between carbon 1 and carbon 4, there are three types of cyclodextrins consisting of 6 units (α), 7 units (β), and 8 units (γ). Notably, cyclodextrin offers various advantages, such as being non-toxic, readily soluble in water, easily modifiable, and possessing high bioavailability. Furthermore, it is widely accessible and brings significant benefits across diverse research domains.81,82Cyclodextrins stand out due to their unique characteristics – a hydrophilic surface combined with a hydrophobic cavity inside, forming clathrates through weak interactions without undergoing chemical reactions.83 The cone-shaped cavity of cyclodextrins allows them to encapsulate other molecules, providing chemical and physical stability to the encapsulated molecule.84 This ability enables them to protect light or oxygen-sensitive substances, alter the chemical reactivity of the host molecules, reduce the evaporation of volatile compounds, enhance the dissolution of substances, and safeguard against breakdown by microorganisms.85,86 Among the native CDs, α-CD is noted for its robust and stable structure, attributed to its comparatively smaller internal space.87 From a chemical perspective, β-CD exhibits optimal clathrate-forming ability, thanks to its structural features. Nonetheless, native CDs, with their hydroxyl groups that can be functionalized in different ways, offer a wide range of functionalities.88 Modified cyclodextrins can effectively incorporate specific host molecules and enhance solubility, but they often come with a higher price tag than native CDs.89 They are also utilized for controlled drug release, capitalizing on the empty spaces inside the cyclodextrins and their ability to bind to drugs in a way that allows for eventual release.90
Therefore, the host molecules are gradually and controllably released without modifying the drug's properties, encompassing physical, chemical, and biological attributes. Moreover, cyclodextrins (CDs) facilitate the sustained retention of molecules within the emitting substance, ultimately amplifying their effectiveness and accuracy in targeting specific tissues for an extended period.7,91
CDs are generally inactive within the body, yet they are utilized as ingredients in numerous medications. They have found widespread use in pharmaceutical science and technologies for several purposes, including enhancing the dissolution and duration of drugs in liquids, improving the efficacy of drug absorption into the body, masking odors and tastes, regulating the release rate of drugs in the body, reducing local and systemic toxicity, and facilitating the passage of drugs through biological barriers.92,93
Medications containing cyclodextrins can be administered orally, intranasally, ocularly, or transdermally.94 In order to enhance the pharmaceutical characteristics of natural cyclodextrins for medical applications, researchers have subjected them to chemical modifications.95 These modifications improve their solubility, capacity to encapsulate other substances, controlled drug release, and reduce potential harm to the body.96 Examples of these modifications include increasing solubility and hydrophilic or hydrophobic properties.97 Cyclodextrins are utilized for drug delivery and gene therapy, with modifications tailored to specific properties and enhanced molecular recognition abilities.98
4.1. Amphiphilic CD
CD amphiphiles have been created with the aim of enhancing CD's interaction with biological organisms and boosting its binding capacity in water. Depending on the specific groups linked to CDs, the derivative substances can be classified into three groups: neutral, positive, and negative CD amphiphilic.994.2. Neutral CD amphiphilic
It is possible to generate CD molecules with dual hydrophilic and hydrophobic characteristics by linking certain molecules to the secondary hydroxyl group at a specific position.100 This leads to the formation of CD molecules containing two fatty acid chains with lengths varying from 2 to 14 carbons.101–103Amphiphilic CDs have the capability to bind with cholesterol-derived drugs. A novel drug called amphiphilic hepta (6-alkylthio-6-deoxy)-β-CD 2-oligo (ethylene glycol)118 was recently developed by scientists. This was achieved through the modification of perbrominated CDs using a process known as nucleophilic displacement, along with the addition of another chemical, ethylene carbonate.119 Besides the multi-part amphiphilic CDs, single-part amphiphilic CDs have also been successfully produced.120–122 To further enhance the characteristics of CD amphiphiles, different responsive connections or targeting entities have been incorporated.123 CD amphiphiles have been formed by linking hydrophobic elements to CDs through disulfide bonds that respond to redox reactions. Additional special molecules such as galactosyl and mannosyl can be attached to the chains of oligo(ethylene glycol) to enable recognition and targeting of specific cells.124
4.3. Cationic CD amphiphilic
Cationic CD amphiphiles are synthesized for the supramolecular assembly of soft materials with specific surface chemistry and charge.104,105 Recent attention has focused on their ability to provide genetic information.106 A simple way to synthesize cationic β-CD amphipathic compounds is to incorporate the amide group in the core of CD with alkyl chains at the 2- and 3-position, O-alkyl ethers are readily available.104 Cationic amphiphiles can also be formed by replacing the amphiphilic-containing oligo (ethylene oxide) oligomer chain with the ω-amino group.107 Recently, a series of amphiphilic β-CD polycations were produced by replacing the primary hydroxyl group with various amino-containing moieties.100 By changing the alkyl chains at positions 2 and 3, the density and hydrophobic–hydrophilic balance of the resulting product can be adjusted.4.4. Anionic CD amphiphilic
Amphiphilic CDs with carboxyl or sulfate groups have been produced, with the possibility of loading on either the first or second side. To make the carboxyl-containing CD amphiphilic, alkyl groups were introduced at the 6-position, while carboxyl-methyl groups were added at the 2- and 3-positions.108 Sulfated amphiphilic cyclodextrins can be synthesized by introducing sulfate groups to the 6-hydroxyls of cyclodextrins that have been esterified at the 2nd and 3rd positions.1094.5. Hydrophobic CD
Modifying CDs with hydrophobic groups can create carriers for controlled release. To achieve this, it is necessary to synthesize alkylated or acylated derivatives like hepta (2,3,6-tri-O-ethyl) and hepta (2,3,6-tri-O-butyryl)-CD.131 Hydrophobic derivatives of cyclodextrin (CD) have been created by carefully acetylating CD using 2-methoxypropene as the acetylation agent.87,110,111 The acetylated materials can decompose effectively in solvents like acetone, ethanol, methylene chloride, or THF. The hydrolysis rate, which is dependent on pH and molecular structure, was assessed with acetylated CD materials. The degradation rate can be readily adjusted by controlling the acetal type and level of modification through the manipulation of reaction kinetics. Importantly, both in vitro and in vivo toxicological tests have demonstrated the excellent biocompatibility of acetylated CD, with its nanoparticles planned for secure local or systemic administration. Furthermore, when combined with adamantyl-terminated polyethylene glycol (Ada-PEG), acetylated CD can develop either parallel or cylindrical particles according to their weight ratio, indicating the well-preserved complex capabilities of acetylated CDs. Later on, we examined the growing popularity of the use of plant-based slimy substance in medicine, particularly in tissue engineering. This substance is utilized to facilitate the growth of new tissue, aid in wound healing, administer medication, and support pharmaceutical applications.112Covering with mucilage reduces moisture loss during the storage period. Humidity decreases due to the continuous flow of water into the environment, so it is classified as hydrophobic. Mucilage is utilized to facilitate the growth of new tissue, and aid in wound healing.
The structure of oligosaccharides undergoes significant changes throughout the developmental process, with specific groups of oligosaccharides emerging at different stages of development. Moreover, alterations in the sugar molecules found on the outer surface of cells are associated with various diseases, including cancer development. Oligosaccharides, as large molecules, play a crucial role in safeguarding integral parts of proteins, regulating the interaction of sugar compounds with other molecules, and influencing the pace of processes involving changes in shape. The process of glycosylation within cells is susceptible to changes in cellular functionality. Abnormal glycosylation may serve as an indicator for the presence of certain diseases such as rheumatoid arthritis and cancer.113
4.6. Application of CD-based biomaterials in tissue engineering
Cyclodextrin (CD) is composed of both linear and curved chain polymers along with minerals such as hyaluronic acid, chitosan, alginate, hydroxyapatite, and calcium phosphate. This process facilitates the transformation of cells into specific types, enhances the compatibility of natural materials with the body, strengthens connections through robust bonds, and improves the properties necessary for cell and tissue regeneration. CD is considered a safe substance for use in both animals and humans, according to several reports and the FDA, as it does not elicit an immune response and exhibits minimal toxicity. The solubility of CD in water varies, with αCD at 13%, βCD at 2%, and γCD at 26%. This essentially means “each corresponds in sequence.” The limited solubility of βCD in water is attributed to the strong internal bonds that it forms. However, this bond can be disrupted by modifying βCD with secondary hydroxyl groups, resulting in a modified version known as hydroxypropyl-βCD, which exhibits much higher solubility in water compared to βCD.114In the continuation of this topic, the use of biometrics modified with cyclodextrins in tissue regeneration and tissue engineering was investigated.
For instance, researchers led by Rod-ell produced a soft gel that exhibits decreased thickness under compression. They achieved this by using CD-HA to engineer the gel. The study states that the CD-CD-HA transforms into a substance known as tosylated β-CD, which is then joined with an intermediate material called HA, or hyaluronic acid–tetrabutylammonium hydroxide, through the amidation process. These structures have the ability to engage with adamantane–HA conjugates, leading to the formation of hydrogel materials through the combination of adamantane and CD.116
The research indicates that blending HA with β-CDs creates a potential material for achieving drug delivery objectives. HA has the capability to regulate the discharge of the material from the gels. The HA grafted with β-CDs hindered crystallization by preserving a unified dispersion of the drug, thus expediting the diffusion of the material out of the gel network. Upon application of force, the developed hydrogel can transition to a more fluid state and be utilized as an injectable substance within the body.114
Glucuronylglucosyl-cyclodextrin (GUG-CD) conjugates can be used as gene transfer vectors. Studies have shown that GUG-β-CD reduces amyloid fibril formation in vitro and in vivo without side effects. Therefore, GUG-β-CD has the potential to be a promising drug for the treatment of FAP.125
Additionally, hydroxyapatite nanoparticles (HA-Ca5(PO4)·3(OH)) were used as additives in CD culture medium to evaluate the biological properties of new CD scaffolds for bone regeneration.133 To evaluate the effectiveness of the composite in promoting bone regeneration, a CD scaffold was implanted into the tibia defect of rats. The results showed that there was no anti-inflammatory reaction around the implant and the intact bone was replaced by new bone 4 weeks after the implant.133 In a study by Barreiro et al., the fabrication of composite materials using porous cyclodextrins (CDs) in combination with sand scaffolds was reported. It was found that the sand scaffold, primarily composed of CaMg(CO3)2 from Clypaster subdepressus, did not provide adequate support for the CD composites, leading to the accumulation of apatite particles on the CD surface during cultivation in Gluconobacter hansenii cultures. The study recommended that the scaffold's compressive strength should be tailored to facilitate bone regeneration, particularly in the tibial bone. An ideal wound dressing should exhibit moisture at the wound site, offer protection with oxygen permeability, and effectively absorb excess fluids. Essential characteristics of such a dressing include being composed of biomaterials, minimally invasive, incorporating antibiotics, facilitating wound healing, and allowing painless removal.149,156 Efforts have been made to develop minimally invasive biomaterials with antimicrobial properties that promote wound healing while minimizing discomfort. Various external electrical devices aimed at accomplishing this have also been developed. Recently, attention has been directed toward the investigation of bacterial cellulose (BC) composites for potential wound dressing applications. For example, composite materials like bacterial cellulose/glucose and bacterial cellulose/dextrin, produced through in situ fermentation, showed enhanced porosity and promising properties for this purpose.134
Moreover, this study also showed that β-CD based modifies the composite material led to increases in its ability to retain water which is important in wound dressings.134 It should be noted that the change in position only changes the morphology and physical properties of the bacterial cellulose membrane. However, the chemical composition of the bacterial cellulose membrane does not change in space. Combination therapy has recently been investigated as a potential factor in wound healing. Dressings such as bacterial cellulose/glucose and bacterial cellulose/dextrin composites are prepared by in situ fermentation.
The MTS (multi-task semantic segmentation) method shows that composite materials have greater porosity and better biocompatibility ((3-(4,5)-dimethylthiazol-2-yl)-5-(3-carboxymethoxy phenyl)-2-(4-sulfophenyl)-2H-tetrazol) and bacteria compared to the control of cellulose group.134
In conclusion, the enhanced products are manufactured from biomaterials requiring minimal processing, possessing antibacterial properties, and promoting wound healing without causing harm or requiring extraction. As a result, numerous bacterial cellulose composites have been under recent investigation as potential materials for medical use. For instance, bacterial cellulose/glucose and bacterial cellulose/dextrin composites, with increased porosity and enhanced biocompatibility, are being widely utilized in various tissue engineering applications.157 Furthermore, other aspects such as in situ modification and the interaction between external materials and bacterial cellulose fibril growth, as well as the process control of bacterial cellulose nanofibers, remain important. Cellulose hydrogels have garnered attention in the medical field due to their biodegradability, biocompatibility, and their ability to create a moist environment conducive to healing. Utilizing various hydrogels modified by CD for wound healing composites enables the controlled release of additional drugs, leading to enhanced clinical outcomes.135
4.7. Other innovative applications of CDs
Industrialization have caused the pollution of air, water, soil and many foods. At the same time, people's lifestyles have changed dramatically, and malnutrition has become a fast-food culture media.136–138 Many challenges are faced, such as determining the health benefits of certain foods, improving immunity, preventing certain diseases, and reducing side effects and medical costs. Promote safe and sound clinical research to evaluate the mechanisms of action and effectiveness of nutrients in response to nutritional challenges.139–142 Safety and health evaluations are encouraged to evaluate the processing and effectiveness of nutrients in response to nutritional challenges.143In conclusion, the requirements to achieve therapeutic goals are include bioavailability, biocompatibility, solubility, loading efficiency, and toxicity of the drug, as well as pharmacokinetic, including release, long-term effect, and development.144 In this regard, we can talk about the use of dextrin in the pharmaceutical industry, as they are non-toxic, biodegradable and biocompatible, easily swelled by drugs with varying water solubility or high pressure.145 Dextrin is starch derivative known for its great potential on the development of hydrogels due to their excellent absorbability and proven clinical tolerance associated with amylase degradation.146 CD and linear dextrin have the same physicochemical and biological properties, but CD is more resistant to non-enzymatic hydrolysis due to its structure.95 Maltodextrin is a linear dextrin composed of linear (amylase) and branched (amylopectin) carbohydrates.147,148 However, some limitations of dextrins are loss of viscosity during storage, poor solubility, uncontrolled hydration rate, and microbial contamination.149
In the continuation of this topic, the applications of (1) Cyclodextrin nano sponge, (2) carbonated NS of CD, (3) ether-NS of CD, (4) ester-NS of CD, (5) maltodextrins are mentioned as innovative applications of cyclodextrin.
In alternative studies, cyclodextrin-based polymers are created by dissolving cyclodextrin in dimethyl sulfoxide and introducing hexamethylene diisocyanate (HDI) as a cross-linking agent. Subsequently, the antibiotic Mitomycin C (MMC) is incorporated into the polymers to establish a beneficial interaction between MMC and the polymer. As demonstrated in this study, the release of MMC is fine-tuned to be more gradual and consistent. In comparison to traditional treatments, this approach presents reduced risk to patients and surgical staff due to the lower level of MM.151
In summary, the defining characteristic of cyclodextrin nanosponges today lies in their interconnected three-dimensional structure comprised of cross-linked CD units. The effectiveness of CD nanosponges can be attributed to their capacity to evolve over time while preserving their inherent attributes, including cost-efficiency, eco-friendliness, non-toxicity, and the ability to encapsulate diverse molecules. Conversely, researchers attach significance to the potential benefits that can be derived from their utilization. Consequently, further exploration of their present applications, such as serving as a carrier, signifies their capability to open new prospects in the realms of biomedicine and human health.152
Additionally, Dhakar et al. found through their studies that resveratrol exhibited significant effectiveness, and oxyresveratrol proved to be even more potent when encapsulated in β-CD: CDI NSs. The NSs loaded with resveratrol and oxyresveratrol demonstrated superior solubility compared to the individual drug molecules, indicative of improved antioxidant activity. Matencio and his research team delved into the interaction of oxyresveratrol with two varying quantities of β-CD: CDI NSs using an innovative technique. Employing UV-Vis measurements, the scientists determined the inclusion constant (KFapp) between β-CD: CDI NSs and oxyresveratrol, making developments to the Benesi–Hildebrand method to achieve this. As per the findings of this study, complexes of CD modified NSs hold potential for utilization in nutritional products.154
In conclusion, the development of novel nanocarriers for anti-cancer medications offers the potential to overcome some of the current treatment limitations. Another innovative synthetic approach involves incorporating an active molecule, like carbonate, during the cross-linking process to create molecularly imprinted nanosponges. Active molecules enable very slow-release kinetics. Researchers are exploring targeting nanosponges for tumor-specific drug accumulation in the future by modifying the structure and combining cationic nanosponges with high cross-links to enhance loading capacity.155
Researchers have formulated a innovative biocompatible hydrogel using hydroxypropyl cyclodextrin as the base, with ethylene glycol diglycidyl ether (EGDE) serving as the cross linker, along with hydroxypropyl methylcellulose (HPMC) and other polymers. Diclofenac, a non-steroidal anti-inflammatory drug, was chosen as an appropriate candidate for incorporation into synthetic materials. The hydrogel possesses the capability to encapsulate diclofenac and steadily release it over an extended period.158
Ether NS based CD are the product of the coupling reaction of CD and epoxy reagents. Most research still focuses on epichlorohydrin. In this case, ether NS based on CD exhibit high anti-inflammatory properties. CDs and their chemical modifiers and also, tailor-made components, including polymeric nanomaterials, facilitate delivery of species and improve clinical outcomes. Also, CD-related polymeric nanostructures are widely used in the selection of drug molecules for tissue repair. CD pendant polymers can serve as a host to encapsulate many drug molecules that cannot be easily encapsulated by free CD.61
The synthesis of Ester-NS of CD involves subjecting CD to a reaction with dianhydride or di/polycarboxylic acid. This allows Ester-NS of CD to absorb significant amounts of water and create hydrogels, with the degree of crosslinking directly impacting its water absorption and swelling properties. Limited crosslinking results in greater water uptake, but also reduces its chemical stability, rendering it more susceptible to hydrolysis in aqueous environments compared to polyurethane or carbonate species.61
Maltodextrins have many uses in the food, medical, beverage, and pharmaceutical industries because of their properties and qualities. Maltodextrin is considered the foremost utilized starch hydrolysate by the nourishment industry.165 A single study demonstrated that the in vitro release test revealed the strong protective effects of casein-maltodextrin-PAs nanoparticles under storage and heat treatments, while also affecting the bioavailability of PAs. As a result, casein-maltodextrin-PA nanoparticles are being recognized as innovative antioxidants suitable for use in pharmaceutical and nutraceutical products.166 In addition, Gurturk and colleagues discovered that the use of maltodextrin-modified liposomes containing levodopa proved to be the most potent treatment for relieving Parkinson's disease symptoms. This discovery ultimately paved the way for a successful method of targeting the blood–brain barrier (BBB) and minimizing cytotoxic effects.167
In a study, maltodextrins were utilized to produce a film, with the addition of glycerin to enhance flexibility and improve the absorption of orally administered quercetin. Maltodextrin films have the capability to maintain freshness for extended periods while rapidly dissolving.168 As per the research carried out by Helal and her team, maltodextrin forms an ester bond with vitamin E succinate through a chemical reaction. Bio-conjugated compounds, as a result, exhibit increased water solubility, reduced impact on major organs within the body, and may offer protection against damage caused by less effective molecules compared to vitamin E succinate.164
In a particular research, scientists incorporated ciprofloxacin (CFX) into synthetic polypropylene (PP) gastric inserts that had been treated with citric acid acidifier and hydroxypropyl-β-CD (HPϒCD) or maltodextrin for functionalization. The employment of HPϒCD and maltodextrin as two types of carbohydrate oligomers derived from starch promotes a sustainable and environmentally friendly approach by sustaining diverse methods for activities such as blood or electric current. The subsequent CFX group and maltodextrin report during the HPϒCD introduction period. The HPϒCD-terminated networks exhibit chemical resistance due to the inner cavity determination, independent of the presence of ions and hydrogen, between CFX and CD-terminated materials.163 Maltodextrin has also been utilized in the construction of proteasomes, identifying potential materials for hydrophobic or amphipathic drug delivery.169
In a study, it combines maltodextrin, lactose monohydrate and rubulan to create a unique product containing resveratrol. Blocked resveratrol has a stable release and is more stable in the stomach and intestines.170 Similar to CD, maltodextrin can be modified for better performance. Compared to CD, maltodextrin is cheaper and better soluble in water.171 In medicine, modified CD has been studied more than modified maltodextrin. Therefore, scientists have developed special CDs that can be used for many purposes. These modified CDs can be modified to work better and help deliver nutrients. Castro-Carbado and others explained this in their studies. Researchers combined maltodextrin with citric acid and tartaric acid to create a new product.172
Cecone and colleagues not only developed environmentally friendly polymers using corn maltodextrin as the main material, but also other chemicals such as 1,4-butanediol diglycidyl ether, 1,4-diazabicyclo-[2,2,2] octane (DABCO), imidazole and etc. This method can be used on a larger scale.173 These materials are seen as eco-friendly alternatives and have the potential to be explored as sustainable adsorbents for a range of uses, including in the environmental, medical, and pharmaceutical fields, owing to their high adsorption capabilities. Notably, Melendez-Ortiz and colleagues developed hydrogels based on maltodextrin with antibacterial qualities. These were modified using glycidyl methacrylate (GMA) and copolymerized with acrylic acid (AAc) or acrylamide (Aam) to enhance their mechanical and chemical properties. This process led to the creation of bioactive nanoparticles, such as zinc oxide nanoparticles (ZnONPs).174
Yan et al. conducted another study that presented a novel approach to hinder the lipid-lowering drug simvastatin (SIM) within an injectable maltodextrin-based micelle/hydrogel composite for enhancing bone regeneration. Aldehyde-modified micelles loaded with SIM were linked to the hydrogel through Schiff base bonds. This method represents a significant enhancement in the mechanical properties of the hydrogel, its favorable biocompatibility, controlled release in the body, and its capability to promote bone formation.175 Maltodextrin appreciates the helical structure of amylose chains and can act as a complexing agent; proposes strategies for incorporating drug carriers into hydrogels for drug delivery and tissue engineering applications.176
The results show that supercritical fluid extracts of maltodextrin have potential as ingredients in the cosmetic and pharmaceutical industries due to their properties and properties.
In addition to examining oligosaccharides and polysaccharides in biomedical research, in this review article, we also surveyed their applications in biomedicine and drug delivery.
5. Applications of CS oligosaccharide and CD in biomedicine and pharmaceutical studies
Most of the researches on chitosan has focused on its ability to deliver of drugs, genes along with usage in medical and food production,177 However, there are some important studies on the abilities of chitosan as an antioxidant,178 anti-inflammatory,179 immunostimulatory,180 wound healing/hemostatic,25 and antibacterial effect.181 CD biopolymers are commonly utilized as the structural basis for a wide range of potential medical and drug delivery devices. The molecule is loaded by placing the drug in the center of the CD. Furthermore, various interactions between chemicals and polymer materials support the loading process. These interactions are primarily associated with the carboxyl group of the polymer network, enabling binding to the drug through hydrogen bonding. The utility of CD inclusion complexes as therapeutic agents is particularly notable in the context of antibiotics, anti-inflammatory drugs, and antioxidant drugs.89 This review continues the latest research on biomedical and pharmaceutical application of above-mentioned saccharides. So, in the first part, we discuss different kind of application of CS in biomedicine/pharmaceutical studied such as antimicrobial, tissue engineering, drug delivery, gene delivery and so on. Also, at the next subsection the usage of CD on the biomedicine and pharmaceutical studies will be discussed. In addition, recent progress and challenges on the biomedical and pharmaceutical application of oligosaccharide/polysaccharide like CD/CS based composite will be surveyed focusing on their biological activities towards medical approaches.5.1. Chitosan oligosaccharide's antimicrobial activity
The ability of chitosan or its derivatives to kill germs depends on different things such as their size, degree of modification, and other physico–chemical properties. It also depends on the type of germs that they are targeting, specifically if they are Gram-negative or Gram-positive. There are substantial efforts invested in studying how COS (chitosan oligosaccharide's) could be used to treat serious infections caused by bacteria and fungi. They are looking into the potential benefits and other ways it could be used. For many clinically important bacteria, COS has been shown to be effective in killing harmful bacteria and fungi, including, E. coli, Vibrio parahaemolyticus, Salmonella typhimurium, P. aeruginosa, Mycobacterium luteus, Streptococcus mutans, Streptococcus faecalis, Streptococcus epidermidis, Staphylococcus aureus, Bacillus subtilis, Bacillus cereus, Lactobacillus plantarum, Bifidobacterium.182 Interestingly, chitosan oligosaccharide (COS) has been found to not target specific pathogenic bacteria, therefore affecting both pathogenic microbes and the normal flora in the gastrointestinal tract. Significantly, COS inhibited the typical growth of seven particular non-pathogenic anaerobic colonic bacteria, including species of Clostridium and Bacteroides.183In a study measuring the highest inhibitory concentration of COS, it was found that COS can effectively exert its antibacterial properties to impact the balance of the colon, which has been shown to be a crucial polysaccharide in the development of both intestinal and non-intestinal infections and inflammations.184
In a different investigation, it was observed that chitosan oligosaccharide (COS) derivatives possess anti-virulence properties against infections caused by Shiga toxin-producing E. coli, which often lead to severe cases of hemolytic uremic syndrome. One notable development was the successful conjugation of COS with a Shiga toxin ligand, effectively countering the cytotoxic effects of the toxin. Moreover, COS has been found to exhibit non-specific antibacterial activity, thereby inhibiting the growth of pathogenic microbes as well as indigenous microorganisms in the gastrointestinal tract (GIT). Furthermore, COS hindered the normal proliferation of seven specific bacteria present in the colon, including species of Clostridium and Bacteroides. This illustrates the ability of COS to eradicate bacteria and influence colon function. Recent research has also indicated that COS plays a significant role in the development of both intestinal and non-intestinal infections and inflammations.185 Additionally, the specific ingredient of COS successfully killed a Gram-negative bacterium of Vibrio vulnificus.186 COS derivatives have been demonstrated to possess the ability to inhibit the activity of Shiga toxin-producing E. coli bacteria, which can be life-threatening and result in hemolytic uremic syndrome by damaging red blood cells. COS primarily binds to a component known as the Shiga toxin ligand, specifically trisaccharide globulotriose, effectively counteracting the harmful effects of the Shiga toxin. The antibacterial properties of COS can be attributed to its relationship with chitin and chitosan. According to this report, COS initiates its antimicrobial action by targeting the receptors of microbial cell-dividing bacteria and viruses. This initiation leads to the movement of potassium granules (K+) across the cell membrane, resulting in (K+) efflux and the stimulation of extracellular fermentation.186 So, it causes an increase in cell differentiation and an increase in Ca2+ uptake. This limits the abilities of microbial cells and causes them to die. Studies have shown that COS-N-chlorogenic acid manganese (COS-N-MB),187 exerts an antibacterial effect by being well scavenged and promotes cell wall adsorption through electrostatic interaction and internal chelation ions, thus promoting bacterial growth permeability, cell membrane, which causing cell leakage and membrane disruption. This process leads to the complete breakdown of the cell membrane, distortion of the membrane and target, and eventual migration of the cell. The positive charge signal at the C-2 position of the glucosamine unit helps regulate cell division throughout the bacterial cell.188
In a different analysis, it was found that the carboxylic acid group of macromolecules present on the surface of bacteria interacts negatively with the positively charged glucosamine, impacting the cellular biological processes. An investigation was carried out to assess the influence of chitosan oligosaccharide supplementation in post-weaning feed on E. coli and Lactobacilli proliferation, fecal excretion, complement assimilation, and structural integrity of the frozen region of the small intestine. Chitosan has been observed to offer positive alterations to the gut microbiota and its appendage.189
5.2. Chitosan oligosaccharide's anti-fungi
COS significantly impacts by eliminating a wide range of bacteria and fungi due to its antibacterial properties.190 Based on previous reports, COS could increase cell membrane permeability, thus causing the destruction of fungal cells.191 COS appears to have a better antifungal effect due to its lower MW (molecular weight) and DA (degree of acetylation). However, some studies have shown that COS with moderate polymerization has the strongest inhibitory activity against yeast.192 Briefly, as indicated by past research, chitosan and COS share similar mechanisms of action. They both interact with receptors within microbial cell structures, resulting in the displacement of potassium and efflux of K+ from the cell layer. Consequently, this alteration causes a delay in cellular fermentation and enhances the uptake of calcium ions, eventually culminating in cell death and the proliferation of calcium particle intake, ultimately resulting in cellular destruction.193 Another study indicated that positively charged glucosamine units also provide energy for different carboxyl strands of bacterial cell division and inhibit bacterial cell growth.1945.3. Chitosan oligosaccharide's anti-tumor activity and anti-cancer
Cancer is characterized by the rapid growth of irregular cells, which possess the capacity to invade and destroy healthy cells, as well as spread extensively throughout the body's organs and tissues. Research has indicated that COS expression can impact the progression of cancer during various stages, including growth, invasion, and metastasis.195 Earlier research has indicated that chitosan oligosaccharide (COS) is capable of stimulating the release of clinically significant cells such as ascites. Several instances have demonstrated COS's potential to diminish inflammation in cancer cells and to facilitate the formation of self-assembled micelles for delivering nano-biomaterials within the body. Moreover, COS has exhibited promise in inhibiting the proliferation of cancer cells in both laboratory settings and live organisms by impeding specific enzymes known as matrix metallopeptidase (MMP).196 In simple words, COS was found to stop the movement and invasion of stomach cancer cells in a test tube.197 It was also discovered that when these cells were treated with COS, a protein called MMP-2, which can damage a specific type of collagen, was not produced. Mainly a special concentration of COS prevents inflammation of colon cancer cells of HT-29. This is achieved by reducing the expression of nitric oxide synthase (iNOS) and MMP-2.198 Scientists also investigated the potential of COS and chitin oligomers in combating cancer and alleviating inflammation. They delved into the usage of these oligomers as a component of a balanced diet to potentially prevent cancer and inflammatory diseases. Apart from their tumor-fighting efficacy, these oligomers possess a high affinity for tumor cells, enabling them to deliver potent doses for cancer treatment.179COS can slow tumor growth by producing lymphocyte cytokines to increase T-cell proliferation. However, there have been no further studies to confirm this idea. The ability of COS to stop MMP-9 from being produced in fibrosarcoma cells is very important for the spread and growth of cancer.199 COS has been shown to non-competitively bind and neutralize MMP-2 in melanoma cells, preventing the viability of these cells.200 This prevents the spread of cells and reduces CD147 levels. COS helps prevent cancer by reducing inflammation and increasing antioxidant activity in the body.198
COS has the ability to hinder the growth of tumors by disrupting critical cancer pathways. Furthermore, it impedes the formation of blood vessels in tumor vasculature cells. COS also facilitates the formation of tight junctions in cells through its dependence on the protein AMPK. These discoveries imply that COS can stimulate AMPK via an inflammatory response and calcium release, offering potential benefits for diarrhea treatment and colorectal cancer prevention. Moreover, COS contributes to the establishment of tight junctions reliant on AMPK, presenting a novel means through which COS triggers AMPK activation involving an inflammatory response and calcium release. Understanding this process could be instrumental in the management of diarrhea and the prevention of colorectal cancer.201 In another study, treatment of human colon cancer cells (HT-29) with COS (molecular weight 3–5 kDa) resulted in increased production of antioxidants, including glutathione, reduced glutathione in –S-transferase, and kinin reductase.202
A research study has shown that COS has a positive impact on the immune system of piglets weaned at an early age. COS regulates the production of antibodies and cytokines, thereby boosting the activity of superoxide dismutase and catalase enzymes and enhancing their ability to combat harmful substances when administered through their diet at a specific dosage.203 An interesting new research work was conducted by Lee and his team in 2017, It was found that giving piglets chitosan in their food in different amounts increased the levels of prostaglandin E2(PGE2), leukotriene B4(LTB4). The increase in levels was either straight or curved depending on the dosage. The activity of certain chemicals in the blood was increased in either a straight line or curved line pattern. These chemicals are serum cytosolic-phospholipase A2, COX-2, and 5-lipoxygenase. These findings show that chitosan can affect how the body processes arachidonic acid.204,205
The effect depends on the amount of chitosan taken. In most studies that have been published, the results show that chitosan can help with growth just like antibiotics in food. So, chitosan is a good and useful option instead of antibiotics.206
5.4. Chitosan oligosaccharide's: biomaterials for drug and DNA delivery activities
Redaction of multidrug resistance (MDR) in infectious and proliferative diseases, in the era of the evolution of one of the main problems in drug therapy and gene therapy, is to increase the effectiveness of drugs and/or DNA delivered to the cell, and tissue target via drug administration and/or DNA.207 Interestingly, COS has shown potential in biomedical applications as a drug and DNA delivery agent for the biomolecule chitosan.208,209 The excellent gelling properties, of chitosan, can provide morphogenic and pharmaceutical properties in the formulation. To overcome MDR, it is important to develop chemotherapy drugs that deliver antibodies to cancer/tumor sites.210Also, in another study, stearic acid self-assembled COS (COS-SA) nanoparticle conjugates were used to deliver DOX for target delivery, demonstrating pH-dependent sustained release of DOX with less cytotoxicity. The cell turnover rate of DOX was higher in nanoparticle-treated cells compared with cells without DOX. According to this report, it can improve the response and penetration of nanoparticles into tissues while reducing drug delivery to other organs such as the heart. While the level of nanoparticles increases due to the reduction of nanoparticles by macrophages and the reduced adsorption of nanoparticles to proteins in the blood, the increase of amount of nanoparticles by tumor cells increases the number, growth and cell shrinkage of cancer cells. COS-based nanocarriers were used in cross-linking systems with glutaraldehyde and genipin b, revealing the potent cytotoxicity of COS micelles containing antibodies against various types of cancer.211 COS has also been shown to enhance DNA delivery and drug absorption across epithelial cells. Polymer micelles made from COS and stearic acid have been tested and proven to be an effective method of DNA delivery in the laboratory and in living organisms. The sticky property of COS is very important for the ability of nanocarriers to stick to cells, which helps them interact with the walls of the cells. This data shows that COS and its derivatives are safe and can be used for delivering drugs or genes effectively.212
5.5. Chitosan oligosaccharide's anti-inflammatory activity
Inflammation occurs when the body responds to harmful agents like bacteria, damaged cells, or allergens, initiating a defensive response using immune cells and chemicals that cause swelling and attract more immune cells to the affected area. Inflammatory processes are linked to various diseases such as cancer, heart disease, diabetes, and other health issues. The cell wall of Gram-negative bacteria contains lipopolysaccharide (LPS), which contributes to the pathogenesis of inflammatory diseases. When LPS binds to the TLR4 receptor, it triggers an inflammatory response in the body by hindering the degradation of IκB or blocking specific pathways involving MAPK, a signal-transmitting complex consisting of three subunits (p38, MAPK, ERK1/2, JNK1/2).213 The main component of MAPK helps translocate a protein activating into the nucleus, which then activates inflammatory genes.214 COS has been shown to block LPS-induced inflammation. Similar effects were observed in cells and animals tested using different concentrations of COS.215One study showed protection against ovalbumin (OVA)-induced lung inflammation in a mouse model of induced asthma expressing COS, IL-4, and IL-5 at the mRNA and protein levels. It can reduce inflammation and the level of certain proteins in the lungs and fluid. This is achieved by reducing the expression of iNOS, a protein activated by LPS, in L9 microglia.71
In another study, the inhibitory effect of COS on nitric oxide (NO) production was determined, and COS decreased the expression of iNOS in L9 microglia activated by LPS.52
Glc-containing low molecular weight COS (b1 kDa) inhibits antigen-stimulated degranulation and cytokine production in RBL-2H3 cells.51 High molecular weight COS (b1 kDa) containing Glc prevents antigen-induced degranulation and cytokine production in RBL-2H3 cells.50 These data indicate that oral administration of low molecular weight COS is effective in reducing allergic reactions and is therefore a potential product for the treatment of mastcell-mediated allergic asthma.216 Another study found that porcine epithelial cells were effective in suppressing intestinal inflammation and the immune response of low-molecular-weight COS. p38, a COS-related factor that inhibits LPS-induced MAPK activation, including JNK1/2 and ERK1/2, thereby inhibiting AP-1 nuclear translocation.75
5.6. Chitosan oligosaccharide's immuno-stimulating activity
With the increasing prevalence of diseases like AIDS, cancer, and diabetes, there is a growing need to develop therapies and vaccines that boost the immune system. Recent research suggests using substances to strengthen the immune system, especially in supporting cancer treatment with chemotherapy. Chitosan oligosaccharides (COS) have shown to alleviate inflammation, combat harmful molecules, and enhance the immune system's response. COS enhances the growth and abilities of specific cells to fight harmful substances and promotes the proliferation and activity of certain immune cells. Studies revealed that COS treatment increased TLR4 and iNOS mRNA levels in macrophages, highlighting its potent anti-inflammatory effects through TLR4 activation.217The research utilized subtilis Rec to assess its effects at varying dosages. This study suggests that increased COS levels have a greater potential to prevent mutations, dependent on the quantity rather than the size of COS molecules. COS activates macrophages, located on cell membranes, which are essential for pattern recognition, mediated by TLR4 to trigger intracellular immune responses.72 A study conducted by Zhang and colleagues show that COS helps RAW 264.7 cells, a type of immune system, grow and consume harmful substances. So, increased levels of NO and TNF-α are important components of the immune system. When COS is taken orally, macrophages take up FITC-COS and TITC-COS. However, the increase in FITC-COS was stopped when anti-mouse TLR4 was used.111
5.7. Chitosan oligosaccharide's anti-AIDS
HIV is the most common clinical virus found worldwide. Interestingly, some studies have shown that certain types of chitosan, called oligomeric derivatives, can block the effects of HIV on human T lymphoblastoid CEM-SS cells.73Interestingly, AE-chitosan was found to be effective against HIV-1 in a three-days test at a concentration of a specified amount. Researchers created a new compound by combining chitosan oligomers with CS-O-isopropyl-5′-O-d4T monophosphate. This compound was found to be effective against HIV and harmful to certain cells. This research led to some of strategies on the treatment of HIV using chitosan and nucleoside reverse transcriptase inhibitor (NRTI). The treatment was designed to be more effective in fighting HIV when used with antiretroviral therapy.74 Recently, it's found that CS and its oligomers have shown effectiveness against HIV. This is about a very interesting discovery, where they made a new composite by combining different small parts of proteins, namely tryptophan, methionine, and glutamine combined, with COS. The evaluation of the results of this report showed that QMW-COS and WMQCOS defended C8166 cells from the harmful effects of the HIV-1RF strain, as well as stopping the formation of HIV-induced syncytia. Different combinations of tripeptides had various effects that changed gradually and repeatedly.194
5.8. Chitosan oligosaccharide's antioxidant
Reactive oxygen species (ROS) increase the risk of diseases such as diabetes, heart disease and cancer. Injury and infection cause some immune systems to produce harmful chemicals called free radicals. These substances can damage cells and cause ongoing inflammation. Lately, researchers have been studying COS and similar substances because they have been shown to have powerful benefits for fighting damage caused by harmful molecules and reducing swelling, which could potentially be helpful in treating or preventing certain severe conditions. Various studies have obtained similar findings. According to the report of Sun et al., COS, with molecular weight of 3–5 kDa, was combined with L-ascorbic acid and used as antioxidant. This combination showed the ability to remove of harmful molecules (free radicals) and protect against damage caused by them.76According to another report, COS stops the activity of myeloperoxidase enzyme, which helps reduce damage to proteins and genetic material in mouse macrophage cells. Researchers found that a treatment called COS was able to prevent damage to the tissues caused by lipopolysaccharide injection. COS helps prevent cell damage and ageing by acting as an antioxidant in different ways. For instance, it increases the level of GSH reduced glutathione, which helps in capturing and removing harmful substances inside cells. However, COS also makes AMPK adenosine monophosphate-activated kinase more active, and this helps control of other processes in the living organs of body. These include regulating genes like NF-kB, limiting the effect of β-catenin, and activating caspase-3. These responses are triggered when the body has too many harmful molecules called ROS and is under a lot of oxidative stress.216
However, it might be possible in future to deliver insulin directly to the right place in the body by using chitosan that sticks well to the intended area of absorption. Chitosan dissolves in water and has a low weight helping insulin to be processed easily. Sulfated chitosan greatly opens up the channels for insulin to pass through. For this purpose, chitosan and its derivatives can be use as carriers for insulin. In conclusion chitosan is the second most used natural polymer and among many biopolymers. It has important properties such as mucoadhesion, improved permeability, biocompatibility and biodegradability. Chitosan and its derivatives play an important role in the safety and treatment of various diseases. The activity of chitosan makes it suitable for biomedical and pharmaceutical applications. Chitosan-based nanocarriers have emerged as promising drug delivery methods. Chitosan nanoparticles (CSNP) are safe and effective nanocarrier systems with controlled drug release and release. CSNPs have broad biomedical applications in medicine and drug delivery. Since chitosan and its derivatives have many physical and chemical properties, they can be used effectively in the pharmaceutical and biomedical industries.218
Table 1 indicates the analysis results from different literature summarizing the ingredients and applications of chitosan used in biomedical research. It can be seen that there are many treatments such as antibiotics, anti-inflammatory drugs, anti-inflammatory drugs, anti-epithelial cells, anti-transplantation, immune stimulation, anti-HIV, antioxidant activity, and various biomedicine/pharmaceutical applications. It is often used in research as the basis for various medical conventions and drug-delivery devices.246–252
| Saccharides | Natural source | Applications | Advantage | Disadvantage | Ref. |
|---|---|---|---|---|---|
| Chitosan oligosaccharides | Depolymerised of chitosan or chitin | Antimicrobial, to determine the pathogenesis of enteric infections and inflammation | The chitosan backbone can provide good safety and performance as a carrier for biological agents, as demonstrated by the transport of DNA or drugs such as insulin and famotidine | None of the treatments affected the survival of the disease-free mice | 184 |
| Prepared from 90–95% deacetylated chitosan | Anti-inflammatory effects, it blocks the cytotoxic effect of (Vibrio vulnificus) on intestinal epithelial cells | COS has potent antibacterial activity against Vibrio vulnificus both in vitro and in vivo. Its molecular weight. It can be used as a therapeutic agent to treat Vibrio vulnificus infection | Low concentrations of COS have a weak inhibitory effect on the growth of Vibrio vulnificus and have no effect within 5 hours after application | 219 | |
| Chitosan conjugates bearing trisaccharide globotriose | Antimicrobial, antiviral immunity against Shiga toxin-producing E. coli | Chitosan oligosaccharide (COS) derivatives have anti-inflammatory properties against Shiga toxin-producing Escherichia coli infection, which often causes severe hemolytic uremic disease | Its antibacterial effect is not as good as LMWC | 185 | |
| Depolymerised products of chitosan or chitin | Antimicrobial, leads to the relocation of potassium particles (K+) from the cell film, causing an efflux of K+ and incitement of extracellular fermentation | COS derivatives have been demonstrated to possess the ability to inhibit the activity of Shiga toxin-producing E. coli bacteria | The antibacterial properties of COS can be attributed to its relationship with chitin and its molecular weight | 186 | |
| Chitosan and its derivatives(the negatively charged carboxylic acid groups and positively charged glucosamine units) | Assess the impacts of the distinctive effect of dietary chitosan oligosaccharide level on the growth efficiency of Escherichia coli and lactic acid bacteria in faecal excretion | Chitosan and its derivatives as a pig-feed additive provides positive antimicrobial, anti-oxidative, immunoregulatory, and blood cholesterol-limiting effects | The molecular mechanisms of these bioactivities and the precise influences of the physicochemical properties of these substances on their various bioactivities remain to be understood | 189 | |
| Depolymerised of chitosan | Anti-tumor, inhibitory effect of COS on orthotopic liver cancer | Molecular weight and DDA of chitosan oligosaccharides are important factors for suppressing cancer cell growth | Antitumor activity of chitosan seems to depend not only on molecular size but also on their chemical structure | 220 | |
| Nanoparticles called Galactosylated COS and (Gal-COS/ATP) | Anti-tumor, COS prevent the spread of cancer cells in both lab dishes and live animals by blocking certain enzymes called matrix metallopeptidase (MMP) | COS significantly inhibited SGC-7901 cell proliferation and metastasis in a dose-dependent manner | The underlying mechanisms and the direct influence of COS on gastric cancer cells have not been fully tested in detail | 197 | |
| In simple words, to stop the movement and invasion of stomach cancer cells in a test tube | |||||
| COS oligomer and chitin oligomer | Anti-tumor, these oligomers can be used as part of a healthy diet to help prevent cancer and inflammatory diseases | COS derivatives are effective in targeted drug therapy/gene therapy | The exact mechanisms behind the actions of NACOS and COS are not yet fully dissected, and further mechanistic studies will be required to harness the benefits of NACOS and COS in therapeutics | 179 | |
| Depolymerised products of chitosan or chitin | Anti-cancer, it decreased NF-κB activity and COX-2 expression while increasing AMPK activity and antioxidant activity | COS is an antitumor metastatic agent for the treatment of colon cancer | Without COS pretreatment, NO production by cytokines is impossible | 198 | |
| Depolymerised products of chitosan | Anti-cancer, β-catenin caused the arrest of mTOR, pyruvate kinase, and ornithine decarboxylase. Additionally, COS inhibits the growth of blood vessels in tumors by reducing VEGF and urokinase-type plasminogen activators in blood vessel cells | COS inhibits the growth of blood vessels in tumor activators in blood vessel cells | The amount of chitosan used should be much higher to accumulate strongly in the tumor tissue after intravenous injection, leading to almost complete inhibition of growth and metastasis | 221 | |
| Chitosan | Anti-cancer, the production of several antioxidants, including glutathione, glutathione-S-transferase, and kinin reductase, as well as ornithine decarboxylase and cyclin reduction in oxygenase (COX-2) | Due to the cationic nature of chitosan, it is very useful in cell absorption and transport in the correction of physiological and degenerative disorders such as diabetes, obesity and AD | Due to its composition of multiple NGlc units and its vulnerability to degradation, its safety is of concern | 202 | |
| Depolymerised products of chitosan or chitin | Anti-cancer, COS promotes cell-mediated immune response by regulating the production of antibodies and cytokines in early-weaned piglets | COS promotes cell-mediated immune response by regulating the production of antibodies, and passive tumor targeting can be a promising anticancer drug delivery system for tumor-targeted therapy | Cytotoxicity in vitro should be investigated | 203 | |
| Depolymerised products of chitosan or chitin | Anti-cancer, increase in the activity of COX-2, and an increase in 5-lipoxygenase activity | Chitosan is a well-known elicitor that strongly affects both secondary metabolites and biomass production by plants | The effect of chitosan on S. marianum cell suspension is not known yet | 204 | |
| Depolymerised products chitosan or chitin | Anti-inflammatory, protection against ovalbumin (OVA) -induced lung inflammation and reduced inflammation and levels of certain proteins in the lung tissue and fluids | COS effectively reduced the expression of inflammatory mediators (TNF-α, iNOS, MCP-1, RANTES, fractalkine, and ICAM-1), COS could also abate the ability of the recruited lymphocytes to secrete chemokines and cytokines, further blocking the attraction of leukocytes to the inflammatory sites | COS reduces inflammatory damage by affecting different components of the inflammatory response, but it has not been reported which components it is effective on | 71 | |
| Depolymerised products of chitosan or chitin | Anti-inflammatory, COS inhibit NO production by inhibiting the expression of inducible nitric oxide synthase (iNOS) in activated microglia | COS could suppress NO production in LPS-induced N9 microglial cells, mediated by p38 MAPK and ERK1/2 pathways | It is not possible without chitosan pretreatment | 222 | |
| Depolymerised products of chitosan | Anti-inflammatory epithelial cells, LPS-induced activation of MAPKs, thus inhibits AP-1 nuclear translocation. In addition, COS inhibits NF-κB activation, resulting in decreased NF-κB nuclear translocation | LCOS significantly attenuated mRNA expression of IL-8 and MCP-1 induced by TNF-α in the cells | PKA (protein kinase A)-specific inhibitor, reversed the mRNA expression of IL-8 when co-cultured with LCOS | 75 | |
| Depolymerised products of chitosan | Prevent mutations, COS's ability to indirectly reduce mutations, the COS mechanism for boosting the immune system starts by activating certain cells called macrophages | COS's ability to indirectly reduce mutations | Mutagenic activity of indirect-acting mutagen was inhibited by ∼50% in the gene expression system | 217 | |
| Depolymerised products chitosan | Immuno-stimulating | COS possesses potent immune-stimulating properties by activating TLR4 on macrophages | Chitosan has limitations such as poor solubility in physiological conditions | 111 | |
| It causes an increase in nitric oxide, an important molecule in the immune system, and tumor necrosis factor | |||||
| Chitosan oligomers | Anti-HIV activity the creation of a new treatment using chitosan and nucleoside reverse transcriptase inhibitor (NRTI) | Chitosan-nucleoside reverse transcriptase inhibitor (NRTI) conjugate with a phosphoramide linkage an efficient approach for improving NRTI therapy efficacy in antiretroviral treatment | More attention has been paid to partially hydrolyzed chitosan oligosaccharides | 74 | |
| Chitosan and itsoligomers | Anti-HIV activity combining different small parts of proteins, namely tryptophan, methionine, and glutamine, with COS. QMW-COS and WMQCOS defended C8166 cells from the harmful effects of the HIV-1RF strain, as well as stopping the formation of HIV-induced syncytia | Chitosan prevents the formation of syncytium caused by HIV | Only three proteins(Tryptophan, methionine, and glutamine have been investigated | 76 | |
| Depolymerised products chitosan | Antioxidants activity, COS was combined with L-ascorbic acid, showed the ability to remove harmful molecules called free radicals and protect against damage caused by them | The antioxidant assays demonstrated that conjugation significantly improved the antioxidant activities, being dramatically higher than that of free chitosan | The antioxidant activity of conjugated chitosan is higher than its free state | 194 | |
| Antioxidants COS stops the myeloperoxidase enzyme from working in cells, which helps reduce damage to proteins and genetic material in mouse macrophage cells | chitosan oligosaccharides attenuated organ dysfunction and improved survival rates after LPS injection | Need for more studies on chitosan oligosaccharides with different molecular weights and which specific monomer has the best anti-inflammatory effect | 216 |
5.9. CDs anti-inflammatory and antimicrobial therapy
In a study conducted by Kfoury, it was shown that Anatole and eugenol can help decrease levels of inflammatory chemicals called interleukin-6 (IL-6) and IL-8 in lung and liver cells exposed to air pollution.78 It was tested on mice to see if it could help wounds heal to done faster. They looked at how the wound area changed and how much inflammation there was by checking for a type of white blood cell that gets into the skin. The results of this report showed that when using tobramycin and inclusion complexes together in the hydrogel made of dialdehyde carboxymethyl cellulose, there was a greater decrease in these two aspects compared to when they were used separately.79Also, Xu and colleagues made a bandage for wounds using a type of gel that included eugenol and β-CD. This made the bandage more stable and able to dissolve in water easily. It also helped the bandage release of VOC (Volatile organic compounds) slowly, which stayed at a higher concentration in the bandage for a longer time. Based on the results of this report, under in vitro conditions the number of cells involved in blood vessel growth has been increased and the activation of inflammatory substances was decreased. Experiments conducted on living organisms showed that the wound heals done faster after a few days. In addition, the growth of bacteria was stopped.223 In various studies, caryophyllene was encapsulated in M-β-CD, which made it easier to dissolve in water. The inclusion complex reduced swelling, while free caryophyllene reduced swelling to a lesser extent than the inclusion complex.152 According to this report, if mice were pretreated with a caryophyllene/M-β-CD inclusion complex, the injury was reduced better than when they were given caryophyllene or free omeprazole. In addition to reducing inflammation, caryophyllene also acts as an antioxidant to protect the lining of the stomach.224
Interestingly, certain substances were successfully trapped inside a material called randomly methylated-β-cyclodextrin, which made them easier to dissolve in water and protected them from outside conditions. These trapped substances helped fight bacteria better than when they were free, and they also had antioxidant properties in lower amounts. For example, in a research work, cinnamaldehyde was trapped in CDs and used to make wound dressings with antibacterial properties.225
In another experiment, cinnamaldehyde mixed with different CDs and used to make nanofibers with antibacterial properties and tested. The very thin fibers were placed on agar plates with the bacteria Escherichia coli. The study lasted for 24 hours and showed that the nanofibres containing only CDs did not have any effect in stopping the growth of Escherichia coli bacteria. The authors of this report believed that the antibacterial activity is caused by the cinnamaldehyde/CD inclusion complexes, Nanofibers containing β-CD did not kill bacteria, but nanofibers containing cinnamaldehyde and β-CD did it. Additionally, when the cinnamaldehyde/β-CD complex is included in the nanofibers, it does not cause brain damage, unlike free cinnamaldehyde.226
Also, linalool was encapsulated in three different types of modified CDs to produce antibacterial nanofibres. Prepared nanocomposite, showed that nanofibers with linalool/M-β-CD inclusion complexes were the most stable and effective in slowly releasing the substance. Because thymol has antibacterial abilities, researchers looked into using it in fibrous membranes made by electrospinning. The findings showed that the combination of thymol and β-CD had a stronger ability to kill bacteria because it dissolved better in water and released volatile organic compounds slowly over time.227
Interestingly, a study conducted by Paiva in 2022 show that thymol was enclosed inside β-CDs. They added the material Eudragit®EPO to the mixture of thymol and β-CD to hide the strong bad taste and smell of thymol. This substance did not change how thymol was enclosed. So, this mixture was selected for the laboratory and live tests. In studies on how medicine moves through the body, the inclusion complex was better than free thymol. It got into the body faster and stayed there longer, so people didn't need to take it as often during the day. Furthermore, the findings showed that thymol is mostly absorbed by the stomach and is not absorbed well by the intestinal lining. Based on the results of this report the inclusion complex enhances thymol's movement through the digestive system, causing more thymol to build up on the surface of the intestine. This could potentially help thymol fight against bacterial intestinal problems.228
In another research, thymol was put inside different kinds of CDs and it was found that HP-β-CD) 2-hydroxypropyl-β-cyclodextrin (was the best choice. The antifungal effect of thymol was greatly enhanced when it was encapsulated in HP-β-CD. This is likely because its ability to dissolve in a liquid. Additionally, the encapsulation increased the amount of time of product incubation compared to regular thymol. It also made the product more stable when exposed to high temperatures. The tests conducted on free limonene showed that it is effective against Staphylococcus aureus and Pseudomonas aeruginosa. When Pseudomonas aeruginosa is combined with gentamicin, there is a beneficial interaction. Simply put, limonene did not alter the antibacterial effect of gentamicin when incorporated into β-CD. Inclusion complexes also did not show any improvement, probably due to β-CD interfering with the interaction of organic compounds (VOCs) and bacteria. Also, Piletti and coworkers proposed cugenel/BCD incorporation lead to a substance with excellent antibacterial activity.229
5.10. CDs pain control activity
The scientists examined the combination of carvacrol and β-CD to see if it could help lessen the pain that comes with muscle inflammation. In a study conducted by Souza, rats were given different treatments before muscle inflammation was caused. The treatments done by carvacrol/β-CD and dexamethasone. Based on the results of this report, the control group showed high levels of markers that cause inflammation and pain, while the groups that were given carvacrol/β-CD inclusion complex or dexamethasone before showed similar ability to reduce inflammation in most cases.230Interestingly, Silva conducted a study in which they injected formalin into mice to induce pain and then tested whether complexes containing carvacrol/β-CD reduced that pain. Free carvacrol in the first (neurogenic) phase of the response is ineffective, causing side effects in the second (inflammatory) phase. The carvacrol/β-CD combination reduced pain in two phases. Based on this report, the combination of carvacrol and β-CD can reduce pain more effectively and longer than carvacrol alone. This is an appropriate treatment good because it reduces the problems associated with the toxicity of carvacrol. Also, combination of caryophyllene and β-CD also helps reduce pain by activating the body's natural pain relief system,231 Santos created a mixture of citronellal and β-CD to make citronellal more effective in reducing pain. Studies of these research group shown that the combination of citronellal/beta-CD and tramadol (painkiller), just as well as each other in reducing pain. The way it might be by citronellal interacting with glutamate receptors, which then activates a pathway that stops signals from going up to the brain. Normally, these medicines make your muscles stronger, but the citronellal/β-CD mixture did not have the same effect. Similarly, the treatment that was received every day did not show any signs of needing it more.232
In another study, researchers found that limonene became more water soluble and remained stable longer when combined with β-CD. Additionally, the combination of limonene and β-CD has no effect on muscle tone. Another study found that linalool and the β-CD compound could relieve pain without affecting muscle tone. This may be because they interfere with the body's pain relief and reduce a protein called Fos in the spinal cord. But the key difference between free linalool and the compound is how long the effect lasts. Similarly, the combination of α-terpineol with β-CD increases its water solubility, increases its use in the body and remains stable. Inclusion complexes provide longer-lasting pain relief than α-terpineol without binding to other drugs.233
5.11. CDs anti-hypertensive treatment
The anti-inflammatory activity of linalool was examined in spontaneously hypertensive rats and compared with the effects provided by linalool/β-CD containing complexes and specific products in its composition, compared with other samples.234 This means that the free linalool had a positive impact on lowering blood pressure, but the effects were even better when it was pronounced or emphasized. Also, the addition complex made the substance more stable and improved its ability to be used by the body. Recently, researchers tested both carvacrol and the carvacrol enclosed in β-CD to see how they affected blood pressure in rats that are prone to high blood pressure. Carvacrol given for free did not lower blood pressure compared to the control group. However, when carvacrol was combined with β-CD, a decrease in blood pressure began on the 15th day of treatment. Furthermore, a combination of carvacrol and β-CD can reduce inflammation and lower blood pressure.2355.12. CDs antioxidant behavior
To evaluate the behaviour of antioxidants effect of β-CD, eugenol was packaged into CDs and incorporated into nanofibers by electrospinning. This led to water solubility inurement and thermal stability. In a time-dependent antioxidant test, the incorporated eugenol/M-β-CD inclusion complex showed a faster antioxidant response and a better overall antioxidant performance than free eugenol.236 Xiao prepared sulfobutyl ether β-cyclodextrin (SBE-β-CD) encapsulated borneol to improve the apparent solubility and stability of VOCs and used this incorporated compound together with tetramethylpyrazine in transdermal drug delivery systems to protect middle cerebral artery occlusion. Application of borneol/SBE-β-CD inclusion complex promotes the opening of the blood–brain barrier. The absorption of this drug in the brain is increased when administered with tetramethylpyrazine, enhancing the protective effect of this drug against middle cerebral artery occlusion. HP-β-CD is a CD widely used in drug formulations to encapsulate caryophyllene to improve its solubility and bioavailability.237 In addition, caryophyllene/HP-β-CD plays a role in improving cerebral blood flow, inhibiting neuronal apoptosis and reducing brain degeneration in vascular dementia model mice, thus improving memory and spatial learning. Complexes containing caryophyllene/HP-β-CD also activate type 2 cannabinoid receptors located in the brain's immune system.2285.13. CDs anti-tumor therapy
Cancer is a disease that often leads to death, so scientists are working on ways to make the treatment better with fewer side effects. They want it to be more effective in fighting of cancer. These cancer drugs are harmful to normal cells and can make cancer resistant to treatment.238 So, the important things to do are to make the drugs better and reduce any bad effects they may have.239 The carrier for anticancer drugs is a way to treat cancer. In this situation, The Scientists focus on methods that actively target using DDS. So far, scientists have found different things that can be used for targeting, like folate, transferrin, EGF, Her2, and RGD peptide. Folic acid is a commonly used ligand because it targets cells with high levels of folate receptors, which is often found in more severe forms of cancer.240 Moreover, FA also has advantages such as low cost, low molecular weight, and high safety immunity. However, there have been only a few reports where anticancer drugs have been successfully targeted in living organisms using FA-conjugated CDs.210Another study made two types of CDs with folate attached to them. The use of caproic acid as a spacer between the folate and CD molecules tested how well these CDs can form complexes with DOX (Fig. 1). According to the obtained results of this report, the DOX/Fol-c1-CD complex is safer than the DOX/Fol-c2-CD complex because it has no effect on blood chemistry once injected into the muscle of the tumor.241,242
5.14. Other pharmaceutical applications
Carvacrol is a substance that is good for fighting cancer. Scientists have put carvacrol into β-CDs to make them even better at treating diseases by making them easier to dissolve, more stable, and easier for the body to absorb when taken orally. To test if the combination of carvacrol and β-CD can fight cancer, based on the results of this report, carvacrol alone caused the death of 35% of cells, while carvacrol combined with β-CD caused 95% of cell death, indicating that the combination was more toxic.243 Carvacrol can reduce inflammation in the body230,193. To understand this characteristic and see if the carvacrol/β-CD inclusion complex can help with Parkinson's disease, researchers caused inflammation in the brains of rats. Some rats were given carvacrol/β-CD inclusion complexes for 15 days before triggering inflammation. The rats that did not receive any treatment showed high levels of molecules that cause inflammation and a loss of special cells in the brain. However, the rats that were given a special substance called carvacrol/β-CD did not show any differences compared to the rats that did not have inflammation in their brains. Therefore, the inclusion complex showed strong anti-inflammatory effects and could protect against the loss of a certain type of brain cells called dopaminergic neurons, which is a characteristic symptom of Parkinson's disease. In another study, researchers examined how β-CD can make linalool more effective in protecting the stomach.244 Because linalool is more easily dissolved in water and remains stable when encapsulated, so, it shown an improved ability to protect the stomach lining from called free radicals.244Celebioglu made nanofibers without using polymers. with the use of HP-β-CD) hydroxypropyl-beta-cyclodextrin (, HP-γ-CD) hydroxypropyl-gamma-cyclodextrin (, and M-β-CD)methyl-beta-cyclodextrin(, which contain complexes that have the ability to join together and form structures in concentrated solutions. Thymol incorporated to β-CD helps create a material for electrospinning that has a big surface area. This allows thymol to dissolve better in water and withstand higher temperatures. It also makes the thymol very good at stopping damage from oxidation, achieving a near-perfect antioxidant activity of 100.0% so, tiny fibers with a mixture of linalool and M-β-CD can help fight off damaging substances in the body, even in small amounts. This is because the M-β-CD can hold onto the linalool better and the fibers provide a lot of surface area for the mixture. Authors of this report indicate that the CDs make the healing properties of the substances stronger and more reliable.245 Although most of these studies were performed in vitro and in vivo condition, they demonstrated that CDs can improve the therapeutic properties and stability of VOCs, leading to the future use of VOC/CD in medicine, particularly involving complexes in oral and cosmetics.
Interestingly CD is a type of carrier used to improve the pharmacokinetics of drug molecules.
These cyclic oligosaccharides have medical and pharmaceutical applications because they can form bonds with poorly water-soluble molecules. The advantage of complexes in improving the chemical and biological properties of drug molecules; solubility, bioavailability, stability, non-toxicity and shelf life. The first compounds used in the treatment are α-, β- and α-, β-, γ-CD has been shown to be effective but has some specific nephrotoxicity. Currently, to solve these problems, sulfobutyl ether-β-CD, hydroxypropyl-β-CD, and etc., can be used.
The data in Table 2 presents an analysis of different studies on the utilization and benefits of Cyclodextrins in biomedical research. The results reveal the diverse applications of CD biopolymers, including their role in anti-inflammatory, antimicrobial, pain control, anti-hypertensive, antioxidant, and anti-tumor activities. These findings underscore the frequent use of CD biopolymers as a basis for numerous potential medical and drug delivery devices.
| Saccharides | Natural source | Applications | Advantage | Disadvantage | Ref. |
|---|---|---|---|---|---|
| CD | β-Cyclo-dextrin | Anti-inflammatory, without the use of surfactants and solvents to produce VOCs, HP-β-CD may be a better environmental choice | The addition of either free or encapsulated essential oil components to particulate matter exposed cells decreased up to 96% of the cytokine IL-6 level and by up to 87% of the cytokine IL-8 leve | It should be investigated on other essential oils with more careful measurement | 78 |
| β-Cyclo-dextrin | Anti-inflammatory, the gel is formulated with tobramycin and borneol/mono-6-(2-hydroxy-3(trimethylammonium)propyl)-β-cyclodextrin (BN/EPTAC-β-CD) to promote rapid wound healing | The positively charged cyclodextrin derivatives containing the auxiliary drug borneol are dispersed inside the hydrogel by electrostatic attraction with carboxyl groups, imine bonds can break in response thereby drugs and moisture inside the hydrogel are released to promote wound healing | It has only been investigated in weakly acidic environments | 79 | |
| β-Cyclo-dextrin | Anti-inflammatory, made a bandage for wounds using a type of gel that included eugenol and β-CD, the bandage was more stable and able to dissolve in water easily | β-Cyclodextrin accelerated diabetic wound healing by reducing the lectin-like oxidized low-density lipoprotein receptor-1/nuclear factor kappa-B-induced dysfunction in endothelial cells and promoting angiogenesis | Eugenol is poorly soluble, pungent smelling, and volatile, thus preventing its clinical use as a potential therapeutic agent | 223 | |
| Caryophyllene enclosed in M-β-CD | Anti-inflammatory, apart from reducing inflammation, caryophyllene also works as an antioxidant to protect the stomach lining | Caryophyllene also acts as an antioxidant to protect the lining of the stomach | Antiproliferative, anti-migratory, cytotoxic, and pro-apoptotic effects of OEO-PbH may lead to the drying and falling off of the skin tags | 223 and 247 | |
| β-Cyclo-dextrin | Anti-inflammatory and antibacterial properties of cinnamaldehyde are found in β-CD. The findings show that β-CD helps cinnamaldehyde remain stable even after 3 weeks, similar to the effect of free cinnamaldehyde | β-Cyclo-dextrin has strong anti-inflammatory and antioxidant effects, similar to those of tCIN when used alone | tCIN self-inclusion in the β-CD polymer did not elevate the toxicity to more than that of tCIN alone | 248 | |
| Methylated-β-cyclodextrin | Antimicrobial, a chemical called cinnamaldehyde was trapped in cyclodextrins and is used as a wound dressing along with antibiotics | Methylated/β-CD improved the antibacterial activities of the mixture against Escherichia coli and Staphylococcus aureus | Highest effective antibacterial activities of PLA/β-CD/CA-3 for E. coli and S. aureus were preserved for 60 h | 225 | |
| Combination of cinnamaldehyde with different CDs | Antimicrobial, a combination of cinnamaldehyde with different CDs was used to produce nanofibers with antibacterial properties. The complex containing cinnamaldehyde/HP-β-CD was inhibited at 4.83 cm | Cinnamaldehyde has kept its antibacterial activity in cinnamaldehyde/CD-IC NF samples when tested against Escherichia coli | Pure cinnamaldehyde is insoluble in water in nature, cinnamaldehyde is a highly volatile compound | 226 | |
| Limonene/M-β-CD | Antimicrobial, nanofibers with linalool/M-β-CD inclusion complex are the most stable and effective in the slow release of drugs, showing that it is better at killing bacteria. E. coli and S. aureus | CD/limonene-IC-NFs exhibited high antibacterial activity against E. coli and S. aureus | It can be used for a short time period | 249 | |
| Three different types of modified CDs | Antimicrobial, strong immunity against Escherichia coli and Staphylococcus aureus | Characteristics of liquid linalool have been preserved in a solid nanofiber form and designed CD/linalool-IC-NFs confer high loading capacity, enhanced shelf life and strong antibacterial activity of linalool | The limitation of this test is related to the fact that it has been evaluated only for Gram-positive bacteria | 227 | |
| Thymol/β-CDs | Antimicrobial, research results show that thymol is mainly absorbed from the stomach and poorly absorbed from the small intestine. Encapsulation compounds support the movement of thymol throughout the digestive tract, causing more thymol to build up on the surface of the intestine. This could potentially help thymol fight against bacterial intestinal problems | The ingredients in the capsule support the movement of thymol throughout the digestive tract and cause more thymol to accumulate on the surface of the gut, which can help thymol fight against bacterial problems in the gut | Thymol is mainly absorbed from the stomach and poorly absorbed from the small intestine | 228 | |
| Thymol withβ-CDs | Antimicrobial, the tests conducted on free limonene showed that it is effective against S. Bacteria Aureus and P are two things. When Pseudomonas aeruginosa is combined with gentamicin, there is a beneficial interaction | The tests conducted on free limonene showed that it is effective against S. Bacteria aureus and P are two things | When Pseudomonas aeruginosa is combined with gentamicin, there is a beneficial interaction | 229 | |
| Carvacrol/β-CD | Pain control activity, while the control group showed high signs of pain and inflammation, when the group was given carvacrol/β-CD with complex or dexamethasone. There was a similar ability to reduce pain in most cases | βCD-carvacrol reduces inflammation and nociception in a model of acute injury to skeletal muscles | Not reported | 230 | |
| Carvacrol/β-CD | Pain control activity, administration of free carvacrol was ineffective in the first phase of the response (neurogenic) but the carvacrol/β-CD combination reduced pain | The encapsulation of carvacrol in β-cyclodextrin can act as a considerable therapeutic agent for orofacial pain management | The low polarity and water solubility limit their pharmacological uses | 231 | |
| Mixture of citronellal and β-CD | Pain control activity, by citronellal interacting with glutamate receptors, activates a pathway that stops signals from going up to the brain. Normally, these medicines make your muscles stronger | By interacting with glutamate receptors, citronella activates a pathway that stops signals from going up to the brain, so these drugs make your muscles stronger | β-CD poor solubility and their applications are limited | 232 | |
| Limonene combined with β-CD | Pain control activity, the combination of these drugs reduces the severity of spinal symptoms by reducing the amount of Fos protein. Its pain-relieving effect is related to its interaction with certain receptors in the brain | βCP reduced hyperalgesia produced by a chronic muscle pain model. The inhibition of the superficial dorsal horn of the spinal cord lamina I is involved in this process, possibly evoked by CNS activation, specifically the descending inhibitory pain system | The βCP curve showed an endothermic event in the range of 165–47 °C | 250 | |
| Linalool and β-CD combination | Pain control activity, linalool/β-CD inclusion complex helps reduce stomach damage using small doses of linalool, and the analgesic effect of the inclusion complex lasts for 24 hours after administration, while the effects of the free linalool didn't last as long because it was more easily absorbed and stable in the inclusion complex | LIN-CD improved the analgesic profile of LIN with the possible involvement of descending pain pathways and the analgesic effect of linalool in an animal model of chronic non-inflammatory muscle pain | So far, only the investigations in animal models of inflammatory pain and supraspinatus have been published | 251 | |
| Carvacrol enclosed in β-CD | Anti-hypertensive therapy when carvacrol was combined with β-CD, a decrease in blood pressure. Additionally, the combination of carvacrol and β-CD may reduce inflammation, lower blood pressure, and treat high blood pressure | Encapsulation of CARV in β-CD can improve cardiovascular activity, showing potential anti-inflammatory and antihypertensive effects | low polarity and water solubility, which restricts its pharmacological | 235 | |
| Borneol encapsulated with SBE-β-CDs | Antioxidant activity, borneol/SBE-β-CD leads to the opening of the blood–brain barrier. The absorption of this drug in the brain is increased when combined with tetramethylpyrazine, contributing to the protection of this drug against middle cerebral artery occlusion | The solubility of DMY was significantly improved in the presence of natural (α-, β-, γ-) CDs and their derivatives, namely hydroxypropyl-β-cyclodextrin (HP-β-CD) | Poor water solubility and low chemical stability, its applications in food and pharmaceutical fields remain limited | 237 | |
| FA is combined with b-CD | Anti-tumor, when FA is combined with b-CyD using a PEG spacer, it helps rhodamine-B enter KB cells, which are a type of human squamous carcinoma cell (FR-a (+)). However, this combination does not have the same effect on MCF7 cells, which are human breast cancer cells (FR-a (−)). When b-CyD is combined with FA using a click chemistry strategy, it forms a small particle in water with 5-fluorouracil. This particle can enter cells that have a lot of FR-a proteins without needing clathrin or GPI-anchored proteins | CD/FA nanoparticles with excellent long-term optical properties have great prospects for the development of targeting tracers and anti-tumor biomedical research | This combination does not have the same effect on MCF7 cells, which are human breast cancer cells (FR-a (−)) | 243 | |
| CDs with folate attached to them | Anti-tumor, DOX complex had similar abilities to fight tumors both in lab dishes and in living organisms | These findings suggest that DOX-β-CyD could be useful as a tumor-selective carrier for anticancer drugs | β-Cyclodextrin poor solubility, volatility and sensitivity to environmental factors pose challenges for formulation scientists | 244 and 252 |
6. Conclusions, challenges, gap discovery, and future perspectives
Naturally occurring oligosaccharides and polysaccharides show promise for biomedical and drug delivery applications due to their cost-effectiveness and compatibility with biological systems. However, their structures and physical properties often need to be tailored through chemical or physical methods to meet specific application requirements. Chondroitin sulfate, an amino polysaccharide, has potential in the biomedical industry, but its limited water solubility hinders widespread use, requiring the functionalization of polymers with water-soluble molecules. Diverse research approaches are essential for the development of novel polymers with medical applications. Cyclodextrins are valuable additives in the medical field and can be used in creating supramolecular assemblies for biomedical purposes. Various methods, including mixing, solubilization, encapsulation, emulsification, as well as processes such as lyophilization, compression, casting, and absorption, are employed to formulate these carriers.Cyclodextrins offer multiple benefits for improving the performance and containment of anti-inflammatory drugs, enhancing administration, release, and delivery. Further investigation in this area is essential for advancing nanomedicine solutions. They have proven to be optimal for manufacturing antibiotics and bioactive drugs and new forms have been developed to accommodate larger molecules like proteins. Research interest in this field remains high.
Cyclodextrins (CDs) have proven clinical effectiveness in cancer treatment and diagnosing cardiovascular diseases. They offer advantages such as longer duration, increased payload, tailored size and properties for tissue penetration, passive targeting, cellular/subcellular traffic, and adaptability for customization to meet different objectives. Integrating CDs into supramolecular platforms improves biocompatibility, simplifies functionalization, and enhances drug payload and therapeutic target identification. However, the use of CDs, specifically β-CD, is limited to oral and topical applications due to low water solubility and potential nephrotoxicity. Despite their advantages, CD-based formulations have limitations, particularly related to spatio-temporal controlled charge distribution tailored to individual body characteristics and disease progression across different conditions.
Ongoing concerns remain about the potential harm of certain chitosan derivatives used to transport drugs from the nasal cavity to the brain. More research is essential to comprehend how polysaccharides interact with proteins and the properties of chitosan–protein complexes. Protonated chitosan plays a key role in stabilizing these complexes under acidic conditions. Furthermore, there is a need to enhance chitosan-based wound dressings by integrating sensors and therapeutic agents for simultaneous release to address microbial growth. Taking a proactive approach, wounds could potentially release bioactive compounds in response to environmental changes linked to primary infection, such as pH, temperature, or UV light chitosan and its derivatives can be used as carriers for insulin. Thousands of studies have been submitted over the years, but they have still been unsuccessful and the applications of chitosan in biomedical fields are still limited. There are still many unanswered questions and problems that need to be resolved; many composite materials have been developed for human clinical use. However, the processes related to drug delivery selection, in vitro and in vivo toxicity and safety issues of chitosan-based biomaterials, and their synthesis process need to be thoroughly examined.
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
We would like to thank Tabriz University of Medical Sciences for their support with this research under grant Number (74103).References
- D. R. G. Fouad, Chitosan as an antimicrobial compound: modes of action and resistance mechanisms, Univ., Diss., Bonn, 2008.
- S. I. Mussatto and I. M. Mancilha, Carbohydrate Polym., 2007, 68, 587–597 CrossRef CAS.
- S. Patel and A. Goyal, World J. Microbiol. Biotechnol., 2011, 27, 1119–1128 CrossRef CAS.
- T. Sako, K. Matsumoto and R. Tanaka, Int. Dairy J., 1999, 9, 69–80 CrossRef CAS.
- S. Li, C. Li, Y. Zhang, X. He, X. Chen, X. Zeng, F. Liu, Y. Chen and J. Chen, Theranostics, 2019, 9, 4993 CrossRef CAS PubMed.
- C.-D. Radu, O. Parteni and L. Ochiuz, J. Controlled Release, 2016, 224, 146–157 CrossRef CAS PubMed.
- S. Muthana, H. Yu, H. Cao, J. Cheng and X. Chen, J. Org. Chem., 2009, 74, 2928–2936 CrossRef CAS PubMed.
- Z. Yang, D. J. McClements, Z. Xu, M. Meng, C. Li, L. Chen, C. Qiu, J. Long and Z. Jin, Food Hydrocolloids, 2022, 131, 107729 CrossRef CAS.
- P. Stribling and F. Ibrahim, Clin. Nutr., 2023, 55, 340–356 Search PubMed.
- X. Li, H. Wang, Y. Li, R. Chen, P. Zhang, X. Wang, Z. Zou, X. Shen, A. Roy and W. Luo, Nanomaterials, 2023, 55, 126 Search PubMed.
- B. Lepenies, J. Yin and P. H. Seeberger, Curr. Opin. Chem. Biol., 2010, 14, 404–411 CrossRef CAS PubMed.
- M. Kussmann, D. H. A. Cunha and S. Berciano, Front. Nutr., 2023, 10, 1193848 CrossRef PubMed.
- J. Berdy, J. Antibiot., 2005, 58, 1–26 CrossRef CAS PubMed.
- N. M. Krishnakumar, B. T. Ramesh and S. A. Ceasar, Stud. Nat. Prod. Chem., 2023, 76, 113–148 CrossRef CAS PubMed.
- W. Li, K. Song, S. Wang, C. Zhang, M. Zhuang, Y. Wang and T. Liu, Mater. Sci. Eng. C, 2019, 98, 685–695 CrossRef CAS PubMed.
- A. Khan and K. A. Alamry, Carbohydr. Res., 2021, 506, 108368 CrossRef CAS PubMed.
- R. Sheervalilou, M. Shirvaliloo, S. Sargazi and H. Ghaznavi, Expet Opin. Drug Deliv., 2021, 18, 949–977 CrossRef CAS PubMed.
- A. Polo, M. A. Albiac, A. Da Ros, V. N. Ardèvol, O. Nikoloudaki, F. Verté, R. Di Cagno and M. Gobbetti, Nutrients, 2023, 15, 590 CrossRef CAS PubMed.
- F. Seidi, R. Jenjob, T. Phakkeeree and D. Crespy, J. Contr. Release, 2018, 284, 188–212 CrossRef CAS PubMed.
- K. E. Sapsford, W. R. Algar, L. Berti, K. B. Gemmill, B. J. Casey, E. Oh, M. H. Stewart and I. L. Medintz, Chem. Rev., 2013, 113, 1904–2074 CrossRef CAS PubMed.
- V. Prajapati, J. Ferreir, R. Patel, S. Patel and P. Joshi, Biosens. Nanotechnol., 2023, 419–442 Search PubMed.
- A. Karimian, H. Parsian, M. Majidinia, M. Rahimi, S. M. Mir, H. S. Kafil, V. Shafiei-Irannejad, M. Kheyrollah, H. Ostadi and B. Yousefi, Int. J. Biol. Macromol., 2019, 133, 850–859 CrossRef CAS PubMed.
- R. Clément, Z. Lun and G. Ceder, Energy Environ. Sci., 2020, 13, 345–373 RSC.
- V. Georgakilas, J. A. Perman, J. Tucek and R. Zboril, Chem. Rev., 2015, 115, 4744–4822 CrossRef CAS PubMed.
- N. Morin-Crini, E. Lichtfouse, G. Torri and G. Crini, Sustainable Agriculture Reviews 35: Chitin and Chitosan: History, Fundamentals and Innovations, 2019, pp. 49–123 Search PubMed.
- T. S. Trung, W. W. Thein-Han, N. T. Qui, C.-H. Ng and W. F. Stevens, Bioresour. Technol., 2006, 97, 659–663 CrossRef CAS PubMed.
- J. Aggarwal, S. Sharma, H. Kamyab and A. Kumar, J. Environ. Treat. Tech., 2020, 8, 1005–1016 Search PubMed.
- B. Tian, Y. Liu and J. Liu, Carbohydrate Polym., 2021, 251, 116871 CrossRef CAS PubMed.
- S. Petroni, I. Tagliaro, C. Antonini, M. D'Arienzo, S. F. Orsini, J. F. Mano, V. Brancato, J. Borges and L. Cipolla, Mar. Drugs, 2023, 21, 147 CrossRef CAS PubMed.
- P. Laurienzo, Mar. Drugs, 2010, 8, 2435–2465 CrossRef CAS PubMed.
- M. M. Islam, M. Shahruzzaman, S. Biswas, M. N. Sakib and T. U. Rashid, Bioact. Mater., 2020, 5, 164–183 Search PubMed.
- Q. Hu, B. Li, M. Wang and J. Shen, Biomaterials, 2004, 25, 779–785 CrossRef CAS PubMed.
- J. Saremi, N. Mahmoodi, M. Rasouli, F. E. Ranjbar, E. L. Mazaheri, M. Akbari, E. Hasanzadeh and M. Azami, Biomed. Pharmacother., 2022, 146, 112529 CrossRef CAS PubMed.
- J. Girones Molera, J. Alberto Mendez and J. San Roman, Curr. Pharmaceut. Des., 2012, 18, 2536–2557 CrossRef PubMed.
- J. Bizeau and D. Mertz, Adv. Colloid Interface Sci., 2021, 287, 102334 CrossRef CAS PubMed.
- B. Seal, T. Otero and A. Panitch, Mater. Sci. Eng. R Rep., 2001, 34, 147–230 CrossRef.
- J. Jagur-Grodzinski, E-Polymers, 2003, 3, 012 Search PubMed.
- E. V. Campos, J. L. Oliveira and L. F. Fraceto, Front. Chem., 2017, 5, 93 CrossRef PubMed.
- N. H. Syed, H. Rajaratinam and A. A. Nurul, in Sustainable Material for Biomedical Engineering Application, Springer, 2023, pp. 87–106 Search PubMed.
- S. S. Behera, U. Das, A. Kumar, A. Bissoyi and A. K. Singh, Int. J. Biol. Macromol., 2017, 98, 329–340 CrossRef CAS PubMed.
- L. Sanhueza, R. Melo, R. Montero, K. Maisey, L. Mendoza and M. Wilkens, PLoS One, 2017, 12, e0172273 CrossRef PubMed.
- P. I. Morgado, S. P. Miguel, I. J. Correia and A. Aguiar-Ricardo, Carbohydrate Polym., 2017, 159, 136–145 CrossRef CAS PubMed.
- M. Wang, X. Xu, X. Lei, J. Tan and H. Xie, Burns Trauma, 2021, 9, tkab002 CrossRef PubMed.
- D. Bellini, C. Cencetti, A. C. Sacchetta, A. M. Battista, A. Martinelli, L. Mazzucco, A. S. D'Abusco and P. Matricardi, J. Mech. Behav. Biomed. Mater., 2016, 64, 151–160 CrossRef CAS PubMed.
- T. M. Tamer, K. Valachová, M. A. Hassan, A. M. Omer, M. El-Shafeey, M. S. M. Eldin and L. Šoltés, Mater. Sci. Eng. C, 2018, 90, 227–235 CrossRef CAS PubMed.
- H. Merzendorfer and E. Cohen, Extracellular Sugar-Based Biopolymers Matrices, 2019, pp. 541–624 Search PubMed.
- V. Vivcharenko, M. Wojcik and A. Przekora, Cells, 2020, 9, 1185 CrossRef CAS PubMed.
- S. Zhang, F. Xia, S. Demoustier-Champagne and A. M. Jonas, Nanoscale, 2021, 13, 7471–7497 RSC.
- T.-C. Ho, C.-C. Chang, H.-P. Chan, T.-W. Chung, C.-W. Shu, K.-P. Chuang, T.-H. Duh, M.-H. Yang and Y.-C. Tyan, Molecules, 2022, 27, 2902 CrossRef CAS PubMed.
- J. Fu, F. Yang and Z. Guo, New J. Chem., 2018, 42, 17162–17180 RSC.
- C. Ji, A. Khademhosseini and F. Dehghani, Biomaterials, 2011, 32, 9719–9729 CrossRef CAS PubMed.
- A. M. Cardoso, E. G. de Oliveira, K. Coradini, F. A. Bruinsmann, T. Aguirre, R. Lorenzoni, R. C. S. Barcelos, K. Roversi, D. R. Rossato and A. R. Pohlmann, Mater. Sci. Eng. C, 2019, 96, 205–217 CrossRef CAS PubMed.
- T. Murati, M. Miletić, J. Pleadin, B. Šimić and I. Kmetič, J. Appl. Toxicol., 2020, 40, 1592–1601 CrossRef CAS PubMed.
- F. Khan, D. T. N. Pham, S. F. Oloketuyi, P. Manivasagan, J. Oh and Y.-M. Kim, Colloids Surf., B, 2020, 185, 110627 CrossRef CAS PubMed.
- M. Lazaridou, D. N. Bikiaris and D. A. Lamprou, Pharmaceutics, 2022, 14, 1978 CrossRef CAS PubMed.
- F. Han, Y. Dong, Z. Su, R. Yin, A. Song and S. Li, Int. J. Pharm., 2014, 476, 124–133 CrossRef CAS PubMed.
- T. H. M. Nguyen, C. Abueva, H. Van Ho, S.-Y. Lee and B.-T. Lee, Carbohydrate Polym., 2018, 180, 246–255 CrossRef CAS PubMed.
- R. Deepa, W. Paul, T. Anilkumar and C. P. Sharma, J. Biomim. Biomater. Tissue Eng., 2013, 3, 261–272 CrossRef CAS.
- M. M. Farag, J. Mater. Sci., 2023, 58, 527–558 CrossRef CAS.
- A. Rónavári, N. Igaz, D. I. Adamecz, B. Szerencsés, C. Molnar, Z. Kónya, I. Pfeiffer and M. Kiricsi, Molecules, 2021, 26, 844 CrossRef PubMed.
- Á. Sarabia-Vallejo, M. d. M. Caja, A. I. Olives, M. A. Martín and J. C. Menéndez, Pharmaceutics, 2023, 15, 2345 CrossRef PubMed.
- M. E. Hoque, T. Nuge, T. K. Yeow, N. Nordin and R. Prasad, J. Polym. Res., 2015, 9, 15 Search PubMed.
- A. Madni, R. Kousar, N. Naeem and F. Wahid, J. Bioresour. Bioprod., 2021, 6, 11–25 CrossRef CAS.
- Z. Xu, T. Chen, K. Q. Zhang, K. Meng and H. Zhao, Polym. Int., 2021, 70, 1741–1751 CrossRef CAS.
- A. Moeini, P. Pedram, P. Makvandi, M. Malinconico and G. G. d'Ayala, Carbohydrate Polym., 2020, 233, 115839 CrossRef CAS PubMed.
- A. Partovinia and M. Koosha, Express Polym. Lett., 2019, 13, 484–499 CrossRef CAS.
- R. Rodríguez-Rodríguez, H. Espinosa-Andrews, C. Velasquillo-Martínez and Z. Y. García-Carvajal, Int. J. Polym. Mater. Polym. Biomater., 2020, 69, 1–20 CrossRef.
- S. Abid, T. Hussain, A. Nazir, A. Zahir, S. Ramakrishna, M. Hameed and N. Khenoussi, Int. J. Biol. Macromol., 2019, 135, 1222–1236 CrossRef CAS PubMed.
- S. B. Qasim, M. S. Zafar, S. Najeeb, Z. Khurshid, A. H. Shah, S. Husain and I. U. Rehman, Int. J. Mol. Sci., 2018, 19, 407 CrossRef PubMed.
- M. Paul-Clark, P. George, T. Gatheral, K. Parzych, W. Wright, D. Crawford, L. Bailey, D. Reed and J. Mitchell, Pharmacol. Therapeut., 2012, 135, 200–215 CrossRef CAS PubMed.
- I.-M. Fang, C.-H. Yang and C.-M. Yang, Mediat. Inflamm., 2014, 2014, 827847 Search PubMed.
- Y. Bulut, K. S. Michelsen, L. Hayrapetian, Y. Naiki, R. Spallek, M. Singh and M. Arditi, J. Biol. Chem., 2005, 280, 20961–20967 CrossRef CAS PubMed.
- M. Artan, F. Karadeniz, M.-M. Kim and S.-K. Kim, J. Biotechnol., 2008, 136, S539 CrossRef.
- R. Zeng, Z. Wang, H. Wang, L. Chen, L. Yang, R. Qiao, L. Hu and Z. Li, Macromol. Res., 2012, 20, 358–365 CrossRef CAS.
- J. Yang, G. Tian, D. Chen, Y. Yao, J. He, P. Zheng, X. Mao, J. Yu, Z. Huang and B. Yu, J. Anim. Physiol. Anim. Nutr., 2018, 102, 252–259 CrossRef CAS PubMed.
- Y. Zou, C. Xiang, L.-X. Sun and F. Xu, Biosens. Bioelectron., 2008, 23, 1010–1016 CrossRef CAS PubMed.
- Z. Hu, D.-Y. Zhang, S.-T. Lu, P.-W. Li and S.-D. Li, Mar. Drugs, 2018, 16, 273 CrossRef PubMed.
- M. Kfoury, M. Borgie, A. Verdin, F. Ledoux, D. Courcot, L. Auezova and S. Fourmentin, Environ. Chem. Lett., 2016, 14, 345–351 CrossRef CAS.
- X. Fan, L. Yang, T. Wang, T. Sun and S. Lu, Eur. Polym. J., 2019, 121, 109290 CrossRef CAS.
- P. Fan, Y. Zeng, D. Zaldivar-Silva, L. Agüero and S. Wang, Molecules, 2023, 28, 1473 CrossRef CAS PubMed.
- Y. Chen, Y. Ye, R. Li, Y. Guo and H. Tan, Fibers Polym., 2013, 14, 1058–1065 CrossRef CAS.
- X. Huang, X. Huang, X.-H. Jiang, F.-Q. Hu, Y.-Z. Du, Q.-F. Zhu and C.-S. Jin, J. Microencapsul., 2012, 29, 1–8 CrossRef CAS PubMed.
- R. J. Dilley and W. A. Morrison, Int. J. Biochem. Cell Biol., 2014, 56, 38–46 CrossRef CAS PubMed.
- M. Ciaccia and S. Di Stefano, Org. Biomol. Chem., 2015, 13, 646–654 RSC.
- G. Ioele, M. De Luca, A. Garofalo and G. Ragno, Drug Delivery, 2017, 24, 33–44 CrossRef CAS PubMed.
- Y. M. Zhang, Y. H. Liu and Y. Liu, Adv. Mater., 2020, 32, 1806158 CrossRef CAS PubMed.
- H. Chen, X. Liu, Y. Dou, B. He, L. Liu, Z. Wei, J. Li, C. Wang, C. Mao and J. Zhang, Biomaterials, 2013, 34, 4159–4172 CrossRef CAS PubMed.
- G. Jakab, D. Bogdán, K. Mazák, R. Deme, Z. Mucsi, I. M. Mándity, B. Noszál, N. Kállai-Szabó and I. Antal, AAPS PharmSciTech, 2019, 20, 1–12 CrossRef CAS PubMed.
- K. Escobar, K. A. Garrido-Miranda, R. Pulido, N. Naveas, M. Manso-Silván and J. Hernandez-Montelongo, Pharmaceutics, 2023, 15, 296 CrossRef CAS PubMed.
- Y. Hua, L. Chen, C. Hou, S. Liu, Z. Pei and Y. Lu, Int. J. Nanomed., 2020, 5873–5899 CrossRef CAS PubMed.
- E. Beňová, D. Bergé-Lefranc, V. Zeleňák, M. Almáši, V. Huntošová and V. Hornebecq, Appl. Surf. Sci., 2020, 504, 144028 CrossRef.
- Z. Liu, L. Ye, J. Xi, J. Wang and Z.-g. Feng, Prog. Polym. Sci., 2021, 118, 101408 CrossRef CAS.
- H. Xie, X. Ma, W. Lin, S. Dong, Q. Liu, Y. Chen and Q. Gao, Polymers, 2021, 13, 4187 CrossRef CAS PubMed.
- A. Laza-Knoerr, R. Gref and P. Couvreur, J. Drug Target., 2010, 18, 645–656 CrossRef CAS PubMed.
- S. V. Kurkov and T. Loftsson, Int. J. Pharm., 2013, 453, 167–180 CrossRef CAS PubMed.
- G. Tiwari, R. Tiwari and A. K. Rai, J. Pharm. BioAllied Sci., 2010, 2, 72 CrossRef CAS PubMed.
- V. Rizzi, J. Gubitosa, R. Signorile, P. Fini, C. Cecone, A. Matencio, F. Trotta and P. Cosma, Chem. Eng. J., 2021, 411, 128514 CrossRef CAS.
- H. Mousazadeh, Y. Pilehvar-Soltanahmadi, M. Dadashpour and N. Zarghami, J. Controlled Release, 2021, 330, 1046–1070 CrossRef CAS PubMed.
- F. Sallas and R. Darcy, Eur. J. Org Chem., 2008, 2008, 957–969 CrossRef.
- A. Díaz-Moscoso, L. Le Gourriérec, M. Gómez-García, J. M. Benito, P. Balbuena, F. Ortega-Caballero, N. Guilloteau, C. Di Giorgio, P. Vierling and J. Defaye, Chem.–Eur. J., 2009, 15, 12871–12888 CrossRef PubMed.
- P. Zhang, L. Chang-Chun, A. W. Coleman, H. Parrot-Lopez and H. Galons, Tetrahedron Lett., 1991, 32, 2769–2770 CrossRef CAS.
- P. Zhang, H. Parrot-lopez, P. Tchoreloff, A. Baszkin, C. c. Ling, C. de Rango and A. W. Coleman, J. Phys. Org. Chem., 1992, 5, 518–528 CrossRef CAS.
- P. Tchoreloff, M. M. Boissonnade, A. W. Coleman and A. Baszkin, Langmuir, 1995, 11, 191–196 CrossRef CAS.
- H. Parrot-Lopez, C. C. Ling, P. Zhang, A. Baszkin, G. Albrecht, C. De Rango and A. W. Coleman, J. Am. Chem. Soc., 1992, 114, 5479–5480 CrossRef CAS.
- R. Dong, Y. Zhou, X. Huang, X. Zhu, Y. Lu and J. Shen, Adv. Mater., 2015, 27, 498–526 CrossRef CAS PubMed.
- C. Ortiz Mellet, J. M. Benito and J. M. Garcia Fernandez, Chem.–Eur. J., 2010, 16, 6728–6742 CrossRef CAS PubMed.
- R. Donohue, A. Mazzaglia, B. J. Ravoo and R. Darcy, Chem. Commun., 2002, 2864–2865 RSC.
- T. Kraus, M. Buděšínský and J. Závada, J. Org. Chem., 2001, 66, 4595–4600 CrossRef CAS PubMed.
- T. Sukegawa, T. Furuike, K. Niikura, A. Yamagishi, K. Monde and S.-I. Nishimura, Chem. Commun., 2002, 430–431 RSC.
- J. Zhang, Y. Jia, X. Li, Y. Hu and X. Li, Adv. Mater., 2011, 23, 3035–3040 CrossRef CAS PubMed.
- P. Zhang, W. Liu, Y. Peng, B. Han and Y. Yang, Int. Immunopharm., 2014, 23, 254–261 CrossRef CAS PubMed.
- G. Goksen, D. Demir, K. Dhama, M. Kumar, P. Shao, F. Xie, N. Echegaray and J. M. Lorenzo, Int. J. Biol. Macromol., 2023, 123146 CrossRef CAS PubMed.
- K. Chen, Y. Qian, C. Wang, D. Yang, X. Qiu and B. P. Binks, J. Colloid Interface Sci., 2021, 591, 352–362 CrossRef CAS PubMed.
- J. Wankar, N. G. Kotla, S. Gera, S. Rasala, A. Pandit and Y. A. Rochev, Adv. Funct. Mater., 2020, 30, 1909049 CrossRef CAS.
- G. Mattheolabakis, L. Milane, A. Singh and M. M. Amiji, J. Drug Target., 2015, 23, 605–618 CrossRef CAS PubMed.
- J. E. Mealy, C. B. Rodell and J. A. Burdick, J. Mater. Chem. B, 2015, 3, 8010–8019 RSC.
- M. E. Lamm, L. Song, Z. Wang, M. A. Rahman, B. Lamm, L. Fu and C. Tang, Macromolecules, 2019, 52, 8967–8975 CrossRef CAS.
- A. Celebioglu and T. Uyar, Chem. Commun., 2010, 46, 6903–6905 RSC.
- E. Hsiung, A. Celebioglu, R. Chowdhury, M. E. Kilic, E. Durgun, C. Altier and T. Uyar, J. Colloid Interface Sci., 2022, 610, 321–333 CrossRef CAS PubMed.
- O. Donoso-González, L. Lodeiro, Á. E. Aliaga, M. A. Laguna-Bercero, S. Bollo, M. J. Kogan, N. Yutronic and R. Sierpe, Pharmaceutics, 2021, 13, 261 CrossRef PubMed.
- A. C. Fonseca, M. H. Gil and P. N. Simoes, Prog. Polym. Sci., 2014, 39, 1291–1311 CrossRef CAS.
- M. Deng, J. Wu, C. A. Reinhart-King and C.-C. Chu, Acta Biomater., 2011, 7, 1504–1515 CrossRef CAS PubMed.
- M. A. Lysik and S. Wu-Pong, J. Pharmaceut. Sci., 2003, 92, 1559–1573 CrossRef CAS PubMed.
- R. Rial, M. González-Durruthy, M. Somoza, Z. Liu and J. M. Ruso, Molecules, 2021, 26, 5855 CrossRef CAS PubMed.
- T. Anno, T. Higashi, Y. Hayashi, K. Motoyama, H. Jono, Y. Ando and H. Arima, J. Drug Targeting, 2014, 22, 883–890 CrossRef CAS PubMed.
- D. Romanov, A. Khripunov, Y. G. Baklagina, A. Severin, N. Lukasheva, D. Tolmachev, V. Lavrent’Ev, A. Tkachenko, N. Arkharova and V. Klechkovskaya, Glass Phys. Chem., 2014, 40, 367–374 CrossRef CAS.
- A. Barreiro, D. Recouvreux, D. Hotza, L. Porto and C. Rambo, J. Mater. Sci., 2010, 45, 5252–5256 CrossRef CAS.
- C. Brackmann, M. Zaborowska, J. Sundberg, P. Gatenholm and A. Enejder, Tissue Eng. C Methods, 2012, 18, 227–234 CrossRef CAS PubMed.
- M. Zaborowska, A. Bodin, H. Bäckdahl, J. Popp, A. Goldstein and P. Gatenholm, Acta Biomater., 2010, 6, 2540–2547 CrossRef CAS PubMed.
- A. Bodin, S. Bharadwaj, S. Wu, P. Gatenholm, A. Atala and Y. Zhang, Biomaterials, 2010, 31, 8889–8901 CrossRef CAS PubMed.
- E.-M. Feldmann, J. F. Sundberg, B. Bobbili, S. Schwarz, P. Gatenholm and N. Rotter, J. Biomater. Appl., 2013, 28, 626–640 CrossRef CAS PubMed.
- A. J. Engler, S. Sen, H. L. Sweeney and D. E. Discher, Cell, 2006, 126, 677–689 CrossRef CAS PubMed.
- S. Saska, H. Barud, A. Gaspar, R. Marchetto, S. J. L. Ribeiro and Y. Messaddeq, Int. J. Biomater., 2011, 2011, 175362 CAS.
- T. R. Stumpf, R. A. Pértile, C. R. Rambo and L. M. Porto, Mater. Sci. Eng. C, 2013, 33, 4739–4745 CrossRef CAS PubMed.
- Y. Zhang, X. Gao, X. Tang, L. Peng, H. Zhang, S. Zhang, Q. Hu and J. Li, Int. J. Biol. Macromol., 2023, 126693 CrossRef CAS PubMed.
- C. Kumar, S. Kumar, S. Prabu and T. Suriyaprakash, Int. J. Health Allied Sci., 2012, 1, 47 CrossRef.
- L. Das, E. Bhaumik, U. Raychaudhuri and R. Chakraborty, J. Food Sci. Technol., 2012, 49, 173–183 CrossRef CAS PubMed.
- S. Agarwal, S. Hordvik and S. Morar, Toxicology, 2006, 221, 44–49 CrossRef CAS PubMed.
- S. L. Prabu, T. SuriyaPrakash, C. D. Kumar, S. SureshKumar and T. Ragavendran, Elixir Pharm, 2012, 46, 8372–8377 Search PubMed.
- J. Zhao, Recent Pat. Biotechnol., 2007, 1, 75–97 CrossRef CAS PubMed.
- F. Shahidi, Trends Food Sci. Technol., 2009, 20, 376–387 CrossRef CAS.
- R. C. Gupta, R. Lall and A. Srivastava, Nutraceuticals: Efficacy, Safety and Toxicity, Academic Press, 2021 Search PubMed.
- D. Paolino, A. Mancuso, M. Cristiano, F. Froiio, N. Lammari, C. Celia and M. Fresta, Nanomaterials, 2021, 11(3), 792 CrossRef CAS PubMed.
- A. Utrata-Wesołek, B. Trzebicka, J. Polaczek, I. Radecka and M. Kowalczuk, Polymers, 2023, 15, 1810 CrossRef PubMed.
- G. Hoti, A. Matencio, A. Rubin Pedrazzo, C. Cecone, S. L. Appleton, Y. Khazaei Monfared, F. Caldera and F. Trotta, Int. J. Mol. Sci., 2022, 23, 4102 CrossRef CAS PubMed.
- A. López Ruiz, A. Ramirez and K. McEnnis, Pharmaceutics, 2022, 14, 421 CrossRef PubMed.
- C. Takeiti, T. Kieckbusch and F. Collares-Queiroz, Int. J. Food Prop., 2010, 13, 411–425 CrossRef CAS.
- J. Sun, R. Zhao, J. Zeng, G. Li and X. Li, Molecules, 2010, 15, 5162–5173 CrossRef CAS PubMed.
- A. Shukla, A. P. Singh and P. Maiti, Signal Transduct. Targeted Ther., 2021, 6, 63 CrossRef CAS PubMed.
- T. R. Thatiparti and H. A. von Recum, Macromol. Biosci., 2010, 10, 82–90 CrossRef CAS PubMed.
- S. R. Merritt, G. Velasquez and H. A. von Recum, Exp. Eye Res., 2013, 116, 9–16 CrossRef CAS PubMed.
- I. Krabicová, S. L. Appleton, M. Tannous, G. Hoti, F. Caldera, A. Rubin Pedrazzo, C. Cecone, R. Cavalli and F. Trotta, Polymers, 2020, 12, 1122 CrossRef PubMed.
- C. Jullian, L. Moyano, C. Yanez and C. Olea-Azar, Spectrochim. Acta Mol. Biomol. Spectrosc., 2007, 67, 230–234 CrossRef PubMed.
- N. K. Dhakar, A. Matencio, F. Caldera, M. Argenziano, R. Cavalli, C. Dianzani, M. Zanetti, J. M. López-Nicolás and F. Trotta, Pharmaceutics, 2019, 11, 545 CrossRef CAS PubMed.
- M. F. Aldawsari, A. H. Alhowail, M. K. Anwer and M. M. Ahmed, Int. J. Nanomed., 2023, 2239–2251 CrossRef CAS PubMed.
- F. Caldera, M. Tannous, R. Cavalli, M. Zanetti and F. Trotta, Int. J. Pharm., 2017, 531, 470–479 CrossRef CAS PubMed.
- R. Machín, J. R. Isasi and I. Vélaz, Carbohydrate Polym., 2012, 87, 2024–2030 CrossRef.
- C. Rodriguez-Tenreiro, C. Alvarez-Lorenzo, A. Rodriguez-Perez, A. Concheiro and J. J. Torres-Labandeira, Pharm. Res., 2006, 23, 121–130 CrossRef CAS PubMed.
- P. K. Shende, R. Gaud, R. Bakal and D. Patil, Colloids Surf., B, 2015, 136, 105–110 CrossRef CAS PubMed.
- Y. J. Wang and L. Wang, Starch-Stärke, 2000, 52, 296–304 CrossRef CAS.
- V. A. Guntero, M. Peralta, P. Noriega, M. N. Kneeteman and C. A. Ferretti, Chem. Proc., 2020, 3, 74 Search PubMed.
- I. Siemons, R. Politiek, R. Boom, R. Van der Sman and M. Schutyser, Food Res. Int., 2020, 131, 108988 CrossRef CAS PubMed.
- S. Barthold, M. Hittinger, D. Primavessy, A. Zapp, H. Groß and M. Schneider, Eur. J. Pharm. Biopharm., 2019, 142, 405–410 CrossRef CAS PubMed.
- H. M. Helal, W. M. Samy, E. M. El-Fakharany, E. A. Kamoun, S. M. Mortada and M. A. Sallam, J. Drug Delivery Sci. Technol., 2020, 60, 102097 CrossRef CAS.
- G. Rezende and L. N. Hashizume, RGO, Rev. Gaucha Odontol., 2018, 66, 257–262 CrossRef CAS.
- X. Sun, X. Wu, X. Chen, R. Guo, Y. Kou, X. Li, Y. Sheng and Y. Wu, Food Chem., 2021, 346, 128952 CrossRef CAS PubMed.
- Z. Gurturk, A. Tezcaner, A. D. Dalgic, S. Korkmaz and D. Keskin, MedChemComm, 2017, 8, 1337–1345 RSC.
- F. Lai, I. Franceschini, F. Corrias, M. C. Sala, F. Cilurzo, C. Sinico and E. Pini, Carbohydrate Polym., 2015, 121, 217–223 CrossRef CAS PubMed.
- A. I. Blazek-Welsh and D. G. Rhodes, Pharm. Res., 2001, 18, 656–661 CrossRef CAS PubMed.
- P. Shruthi, H. A. Pushpadass, M. E. E. Franklin, S. N. Battula and N. L. Naik, LWT--Food Sci. Technol., 2020, 134, 110127 CrossRef CAS.
- T. Loftsson and D. Duchene, Int. J. Pharm., 2007, 329, 1–11 CrossRef CAS PubMed.
- M. Castro-Cabado, A. Casado and J. San Román, Eur. Polym. J., 2016, 78, 91–105 CrossRef CAS.
- C. Cecone, G. Costamagna, M. Ginepro and F. Trotta, RSC Adv., 2021, 11, 7653–7662 RSC.
- H. I. Meléndez-Ortiz, R. Betancourt-Galindo, B. Puente-Urbina, A. Ledezma and O. Rodríguez-Fernández, Int. J. Polym. Mater. Polym. Biomater., 2022, 71, 959–968 CrossRef.
- S. Yan, J. Ren, Y. Jian, W. Wang, W. Yun and J. Yin, Biomacromolecules, 2018, 19, 4554–4564 CrossRef CAS PubMed.
- S. Demasi, M. Caser, F. Caldera, N. K. Dhakar, F. Vidotto, F. Trotta and V. Scariot, Ind. Crops Prod., 2021, 164, 113346 CrossRef CAS.
- J. Sharifi-Rad, C. Quispe, M. Butnariu, L. S. Rotariu, O. Sytar, S. Sestito, S. Rapposelli, M. Akram, M. Iqbal and A. Krishna, Cancer Cell Int., 2021, 21, 1–21 CrossRef PubMed.
- W. Xia, P. Liu, J. Zhang and J. Chen, Food Hydrocolloids, 2011, 25, 170–179 CrossRef CAS.
- K. Azuma, T. Osaki, S. Minami and Y. Okamoto, J. Funct. Biomater., 2015, 6, 33–49 CrossRef PubMed.
- Y. Zeng, Y. Xiang, R. Sheng, H. Tomás, J. Rodrigues, Z. Gu, H. Zhang, Q. Gong and K. Luo, Bioact. Mater., 2021, 6, 3358–3382 CAS.
- S. Qie, Y. Hao, Z. Liu, J. Wang and J. Xi, Acta Chim. Sin., 2020, 78, 232 CrossRef CAS.
- O. M. Sharaf, M. S. Al-Gamal, G. A. Ibrahim, N. M. Dabiza, S. S. Salem, M. F. El-Ssayad and A. M. Youssef, Carbohydrate Polym., 2019, 223, 115094 CrossRef CAS PubMed.
- J. Šimůnek, V. Brandysová, I. Koppová and J. Šimůnek, Folia Microbiol., 2012, 57, 341–345 CrossRef PubMed.
- S. Ghaisas, J. Maher and A. Kanthasamy, Pharmacol. Therapeut., 2016, 158, 52–62 CrossRef CAS PubMed.
- X. Li, P. Wu, S. Cheng and X. Lv, J. Med. Chem., 2012, 55, 2702–2710 CrossRef CAS PubMed.
- R. Krishnaveni and K. Senthilkannan, Preamble to Biomaterials and its Applications in Science and Technology, Lulu. com, 2019 Search PubMed.
- A. Peña, N. S. Sánchez and M. Calahorra, BioMed Res. Int., 2013, 2013, 527549 Search PubMed.
- Q. A. Acton, Glycoside Hydrolases—Advances in Research and Application, 2012th edn, 2012 Search PubMed.
- G. Guan, M. A. K. Azad, Y. Lin, S. W. Kim, Y. Tian, G. Liu and H. Wang, Front. Physiol., 2019, 10, 516 CrossRef PubMed.
- F. Seyfarth, S. Schliemann, P. Elsner and U.-C. Hipler, Int. J. Pharm., 2008, 353, 139–148 CAS.
- J. H. Sandoval-Sánchez, R. Ramos-Zúñiga, S. L. de Anda, F. López-Dellamary, R. Gonzalez-Castañeda, J. De la Cruz Ramírez-Jaimes and G. Jorge-Espinoza, World Neurosurg., 2012, 77, 577–582 CrossRef PubMed.
- G. Benchamas, G. Huang, S. Huang and H. Huang, Trends Food Sci. Technol., 2021, 107, 38–44 CrossRef CAS.
- N. A. S. Rozman, W. Y. Tong, C. R. Leong, W. N. Tan, M. A. Hasanolbasori and S. Z. Abdullah, J. Microbiol. Biotechnol., 2019, 29(7), 1009–1013 CrossRef CAS PubMed.
- M. Z. Karagozlu, F. Karadeniz and S.-K. Kim, Int. J. Biol. Macromol., 2014, 66, 260–266 CrossRef CAS PubMed.
- L. Liu, M. Li, M. Yu, M. Shen, Q. Wang, Y. Yu and J. Xie, Int. J. Biol. Macromol., 2019, 121, 743–751 CrossRef CAS PubMed.
- X. L. Zhu, Y. Z. Du, R. S. Yu, P. Liu, D. Shi, Y. Chen, Y. Wang and F. F. Huang, Int. J. Mol. Sci., 2013, 14, 15755–15766 CrossRef PubMed.
- Z. Luo, X. Dong, Q. Ke, Q. Duan and L. Shen, Oncol. Lett., 2014, 8, 361–366 CrossRef CAS PubMed.
- K.-S. Nam, M.-K. Kim and Y.-H. Shon, J. Microbiol. Biotechnol., 2007, 17, 2042–2045 CAS.
- A. V. Kumar and R. Tharanathan, Carbohydr. Polym., 2004, 58, 275–283 CrossRef CAS.
- C. Gorzelanny, B. Poeppelmann, E. Strozyk, B. M. Moerschbacher and S. W. Schneider, Biomacromolecules, 2007, 8, 3035–3040 CrossRef CAS PubMed.
- C. Muanprasat, P. Wongkrasant, S. Satitsri, A. Moonwiriyakit, P. Pongkorpsakol, T. Mattaveewong, R. Pichyangkura and V. Chatsudthipong, Biochem. Pharmacol., 2015, 96, 225–236 CrossRef CAS PubMed.
- M. Naveed, L. Phil, M. Sohail, M. Hasnat, M. M. F. A. Baig, A. U. Ihsan, M. Shumzaid, M. U. Kakar, T. M. Khan and M. Akabar, Int. J. Biol. Macromol., 2019, 129, 827–843 CrossRef CAS PubMed.
- J. Y. Lee, M. Y. Lee, D. H. Kim, S. Y. Lee, J. S. Kim, H. J. Cho and D. D. Kim, Chemosensitizing indomethacin-conjugated chitosan oligosaccharide nanoparticles for tumor-targeted drug delivery, Acta Biomater., 2017, 57, 262–273 CrossRef CAS PubMed.
- J.-Y. Lee, U. Termsarasab, M. Y. Lee, D.-H. Kim, S. Y. Lee, J. S. Kim, H.-J. Cho and D.-D. Kim, Acta Biomater., 2017, 57, 262–273 CrossRef CAS PubMed.
- H. Zong, S. Liu, R. Xing, X. Chen and P. Li, Ecotoxicol. Environ. Saf., 2017, 138, 271–278 CrossRef CAS PubMed.
- J. Liang, H. Yan, P. Puligundla, X. Gao, Y. Zhou and X. Wan, Food Hydrocolloids, 2017, 69, 286–292 CrossRef CAS.
- A. Aied, U. Greiser, A. Pandit and W. Wang, Drug discovery today, 2013, 18, 1090–1098 CrossRef CAS PubMed.
- J. J. Wang, Z. W. Zeng, R. Z. Xiao, T. Xie, G. L. Zhou, X. R. Zhan and S. L. Wang, Int. J. Nanomed., 2011, 765–774 Search PubMed.
- H. Fonouni, A. Kashfi, A. Majlesara, O. Stahlheber, L. Konstantinidis, N. Gharabaghi, T. W. Kraus, A. Mehrabi and H. Oweira, J. Biomed. Mater. Res. B Appl. Biomater., 2018, 106, 1307–1316 CrossRef CAS PubMed.
- Y. Guo, M. Chu, S. Tan, S. Zhao, H. Liu, B. O. Otieno, X. Yang, C. Xu and Z. Zhang, Mol. Pharm., 2014, 11, 59–70 CrossRef CAS PubMed.
- Y.-Z. Du, P. Lu, J.-P. Zhou, H. Yuan and F.-Q. Hu, Int. J. Pharm., 2010, 391, 260–266 CrossRef CAS PubMed.
- Q. Luo, J. Zhao, X. Zhang and W. Pan, Int. J. Pharm., 2011, 403, 185–191 CrossRef CAS PubMed.
- M. Molteni, S. Gemma and C. Rossetti, Mediat. Inflamm., 2016, 2016, 6978936 Search PubMed.
- M. Yousef, R. Pichyangkura, S. Soodvilai, V. Chatsudthipong and C. Muanprasat, Pharmacol. Res., 2012, 66, 66–79 CrossRef CAS PubMed.
- M. J. Chung, J. K. Park and Y. I. Park, Int. Immunopharm., 2012, 12, 453–459 CrossRef CAS PubMed.
- Y. Qiao, X.-F. Bai and Y.-G. Du, Int. Immunopharm., 2011, 11, 121–127 CrossRef CAS PubMed.
- K.-S. Nam, Y.-R. Choi and Y.-H. Shon, Biotechnol. Lett., 2001, 23, 971–975 CrossRef CAS.
- A. Harugade, A. P. Sherje and A. Pethe, React. Funct. Polym., 2023, 105634 CrossRef CAS.
- B. C. Lee, M. S. Kim, S. H. Choi, K. Y. Kim and T. S. Kim, Int. J. Mol. Med., 2009, 24, 327–333 CAS.
- J. K. Park, M. J. Chung, H. N. Choi and Y. I. Park, Int. J. Mol. Sci., 2011, 12, 266–277 CrossRef CAS PubMed.
- J. Xiao, X. Duan, Q. Yin, Z. Miao, H. Yu, C. Chen, Z. Zhang, J. Wang and Y. Li, Biomaterials, 2013, 34, 5381–5390 CrossRef CAS PubMed.
- P. Wei, P. Ma, Q.-S. Xu, Q.-H. Bai, J.-G. Gu, H. Xi, Y.-G. Du and C. Yu, Glycoconjugate J., 2012, 29, 285–295 CrossRef CAS PubMed.
- W. Xu, Y. Chen and Y. Liu, ACS Appl. Mater. Interfaces, 2021, 13, 3109–3118 CrossRef CAS PubMed.
- P. S. Santos, L. K. Souza, T. S. Araujo, J. V. R. Medeiros, S. C. Nunes, R. A. Carvalho, A. C. Pais, F. J. Veiga, L. C. Nunes and A. Figueiras, ACS Omega, 2017, 2, 9080–9094 CrossRef CAS PubMed.
- Y. Liu, X. Liang, R. Zhang, W. Lan and W. Qin, Polymers, 2017, 9, 464 CrossRef PubMed.
- Z. I. Yildiz, M. E. Kilic, E. Durgun and T. Uyar, J. Agric. Food Chem., 2019, 67, 11066–11076 CrossRef CAS PubMed.
- Z. Aytac, Z. I. Yildiz, F. Kayaci-Senirmak, T. Tekinay and T. Uyar, Food Chem., 2017, 231, 192–201 CrossRef CAS PubMed.
- A. C. Paiva-Santos, L. Ferreira, D. Peixoto, F. Silva, M. J. Soares, M. Zeinali, H. Zafar, F. Mascarenhas-Melo, F. Raza and P. G. Mazzola, Colloids Surf., B, 2022, 112758 CrossRef CAS PubMed.
- R. Piletti, A. Bugiereck, A. Pereira, E. Gussati, J. Dal Magro, J. Mello, F. Dalcanton, R. Ternus, C. Soares and H. Riella, Mater. Sci. Eng. C, 2017, 75, 259–271 CrossRef CAS PubMed.
- A. C. A. Souza, F. F. Abreu, L. R. Diniz, R. Grespan, J. M. DeSantana, L. J. Quintans-Júnior, P. P. Menezes, A. A. Araújo, C. B. Correa and S. A. Teixeira, Pharmacol. Rep., 2018, 70, 1139–1145 CrossRef CAS PubMed.
- J. C. Silva, J. R. Almeida, J. S. Quintans, R. G. Gopalsamy, S. Shanmugam, M. R. Serafini, M. R. Oliveira, B. A. Silva, A. O. Martins and F. F. Castro, Biomed. Pharmacother., 2016, 84, 454–461 CrossRef CAS PubMed.
- V. Suvarna and S. Chippa, Curr. Drug Delivery, 2023, 20, 770–791 CrossRef CAS PubMed.
- M. G. Oliveira, R. G. Brito, P. L. Santos, H. G. Araujo-Filho, J. S. Quintans, P. P. Menezes, M. R. Serafini, Y. M. Carvalho, J. C. Silva and J. R. Almeida, Chem.-Biol. Interact., 2016, 254, 54–62 CrossRef CAS PubMed.
- D. Lecca and S. Ceruti, Biochem. Pharmacol., 2008, 75, 1869–1881 CrossRef CAS PubMed.
- L. Barreto da Silva, S. B. Camargo, R. d. A. Moraes, C. F. Medeiros, A. d. M. Jesus, A. Evangelista, C. F. Villarreal, L. J. Quintans-Júnior and D. F. Silva, Clin. Exp. Pharmacol. Physiol., 2020, 47, 1798–1807 CrossRef CAS PubMed.
- A. Celebioglu, Z. I. Yildiz and T. Uyar, J. Agric. Food Chem., 2018, 66, 457–466 CrossRef CAS PubMed.
- Y. Wu, Y. Xiao, Y. Yue, K. Zhong, Y. Zhao and H. Gao, Food Hydrocolloids, 2020, 103, 105718 CrossRef CAS.
- S. Gandhi and P. Shende, J. Controlled Release, 2021, 339, 41–50 CrossRef CAS PubMed.
- M. Aquib, M. A. Farooq, P. Banerjee, F. Akhtar, M. S. Filli, K. O. Boakye-Yiadom, S. Kesse, F. Raza, M. B. Maviah and R. Mavlyanova, J. Biomed. Mater. Res., Part A, 2019, 107, 2643–2666 CrossRef CAS PubMed.
- T. Loftsson, J. Pharm. Sci., 2021, 110, 654–664 CrossRef CAS PubMed.
- U. Termsarasab, H.-J. Cho, D. H. Kim, S. Chong, S.-J. Chung, C.-K. Shim, H. T. Moon and D.-D. Kim, Int. J. Pharm., 2013, 441, 373–380 CrossRef CAS PubMed.
- H. Arima, Y. Hayashi, T. Higashi and K. Motoyama, Expet Opin. Drug Deliv., 2015, 12, 1425–1441 CrossRef CAS PubMed.
- C. T. Ribeiro, J. Gasparotto, L. L. Petiz, P. O. Brum, D. O. Peixoto, A. Kunzler, H. T. da Rosa Silva, R. C. Bortolin, R. F. Almeida and L. J. Quintans-Junior, Neurochem. Int., 2019, 126, 27–35 CrossRef PubMed.
- G. Wadhwa, S. Kumar, L. Chhabra, S. Mahant and R. Rao, J. Inclusion Phenom. Macrocycl. Chem., 2017, 89, 39–58 CrossRef CAS.
- M. S. J. Manzoni, E. A. Rossi, N. D. Pauly-Silveira, R. A. Pinto, M. N. Roselino, I. Z. Carlos, M. B. Quilles, M. B. de Abreu Glória and D. C. U. Cavallini, Food Res. Int., 2017, 99, 495–500 CrossRef CAS PubMed.
- B. Almeida, C. Domingues, F. Mascarenhas-Melo, I. Silva, I. Jarak, F. Veiga and A. Figueiras, Int. J. Mol. Sci., 2023, 24, 2974 CrossRef CAS PubMed.
- L. Bora, T. Burkard, M. H. S. Juan, H. H. Radeke, A. M. Mut, L. L. Vlaia, I. Z. Magyari-Pavel, Z. Diaconeasa, S. Socaci and F. Borcan, Pharmaceutics, 2022, 14, 2413 CrossRef CAS PubMed.
- M. Davaatseren, Y.-J. Jo, G.-P. Hong, H. J. Hur, S. Park and M.-J. Choi, Molecules, 2017, 22, 1868 CrossRef PubMed.
- Z. Aytac, Z. I. Yildiz, F. Kayaci-Senirmak, N. O. San Keskin, S. I. Kusku, E. Durgun, T. Tekinay and T. Uyar, J. Agric. Food Chem., 2016, 64, 7325–7334 CrossRef CAS PubMed.
- L. J. Quintans-Júnior, A. A. Araújo, R. G. Brito, P. L. Santos, J. S. Quintans, P. P. Menezes, M. R. Serafini, G. F. Silva, F. M. Carvalho and N. K. Brogden, Life Sci., 2016, 149, 34–41 CrossRef PubMed.
- S. S. Nascimento, E. A. Camargo, J. M. DeSantana, A. A. Araújo, P. P. Menezes, W. Lucca-Júnior, R. L. Albuquerque-Júnior, L. R. Bonjardim and L. J. Quintans-Júnior, N. Schmied. Arch. Pharmacol., 2014, 387, 935–942 CrossRef CAS PubMed.
- K. Motoyama, R. Onodera, A. Okamatsu, T. Higashi, R. Kariya, S. Okada and H. Arima, J. Drug Target., 2014, 22, 211–219 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |
