DOI:
10.1039/D4RA01271B
(Paper)
RSC Adv., 2024,
14, 7601-7608
A mild protocol for efficient preparation of functional molecules containing triazole†
Received
19th February 2024
, Accepted 27th February 2024
First published on 4th March 2024
Abstract
The construction of a class of novel triazole molecules containing sulfonyl fluoride functionalities was achieved through Cu-catalyzed click chemistry in good to excellent yields. The sulfonyl fluoride moieties were cleaved completely under base conditions to produce N-unsubstituted triazoles quantitatively, which provides a strategy to combine SuFEx click chemistry with Cu-catalyzed click chemistry ingeniously.
Functionalized triazoles as core motifs are present in numerous biologically active molecules with wide applications in pharmaceuticals, agrochemicals, and other functional materials.1–4 Many triazole derivatives have been successfully developed as drugs or drug candidates for the treatment of a variety of diseases (Fig. 1). Especially, the discovery and development of CuAAC Click Chemistry in 2001![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 by Professor K. B. Sharpless. Based on the significance of both sulfonyl fluorides and triazoles, finding a portal to the assembly of molecules bearing both sulfonyl fluoride and triazole moieties would unquestionably increase the chance of identifying drug candidates and significantly contribute to lead compounds optimization.
5 by Professor K. B. Sharpless. Based on the significance of both sulfonyl fluorides and triazoles, finding a portal to the assembly of molecules bearing both sulfonyl fluoride and triazole moieties would unquestionably increase the chance of identifying drug candidates and significantly contribute to lead compounds optimization.
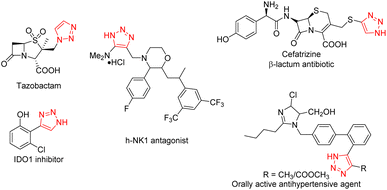 |
| | Fig. 1 Representative active molecules or drugs containing triazole. | |
On the other hand, sulfur(VI) fluoride exchange (SuFEx) has turned into a powerful chemical strategy, attracting increasing attention with wide applications in organic chemistry,6–8 medicinal chemistry8–11 and material science12–14 since the great discovery developed by Professor K. B. Sharpless and coworkers in 2014.15 The sulfonyl fluoride moiety has been the core of SuFEx methodology, which has drawn wide notice for its preparation. Aliphatic sulfonyl fluorides were a representative class of SuFEx family. The most famous methods for achieving aliphatic sulfonyl fluorides were mainly divided into two categories. The first involves ethenesulfonyl fluoride (ESF) and its olefin derivatives participated addition reactions, such as michael addition,16–18 cycloaddition reaction,19–21 radical addition (Fig. 2a).22–25 The second category includes transformations of aliphatic sulfonyl fluoride reagents, which also yields aliphatic sulfonyl fluorides (Fig. 2b).19,26 Because of the great importance of sulfonyl fluorides moieties, the development of efficient and reliable methods to synthesize sulfonyl fluoride containing scaffolds continues to be of great significance, and therefore, extensive investigations have been performed.
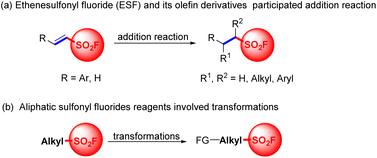 |
| | Fig. 2 General synthesis of aliphatic sulfonyl fluorides. (a) Ethenesulfonyl fluoride (ESF) and its olefin derivatives participated addition reaction; (b) aliphatic sulfonyl fluoride reagents involved transformations. | |
Under the inspiration of classic CuAAC5 strategy and previous work of our group,27 a series of different substituted 1-bromo-2-(4-phenyl-1H-1,2,3-triazol-1-yl)ethane-1-sulfonyl fluorides (BTESFs) 3 were synthesized by different terminal alkynes and aliphatic sulfonyl fluoride reagent 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 (more details see ESI†). As illustrated in Table 1, twenty-six alkynes 2 were smoothly converted to their corresponding BTESFs 3 in good to excellent yields after Cu-catalyzed cycloaddition with azide 1. For aromatic alkynes, no matter electron-donating groups 2b, 2c or electron-withdrawing groups 2f, 2g were all compatible. Sterically hindered substrates 2l, 2m did not affect the efficiency of cycloaddition. It was worth noting that heterocyclic products 3n–p were also generated in good yields with either a nitrogen or sulfur atom. In addition, aliphatic alkynes 2q–w were smoothly transformed into their corresponding triazoles in satisfactory yields. Especially, the alkynes derived from phenols, such as 2t–w all gave corresponding triazoles in nearly quantitative yields. Natural products ethynyl estradiol 2x and norethindrone 2y were applicable to be modified by the cycloaddition with 1 and furnished corresponding BTESFs 3x and 3y in 91% and 82% yields respectively. Interestingly, when 2.0 equivalents of azide 1 was used, ditriazole substituted BTESF 3z was generated in 88% yield after the reaction with the derivative of diethylstilbestrol 2z.
27 (more details see ESI†). As illustrated in Table 1, twenty-six alkynes 2 were smoothly converted to their corresponding BTESFs 3 in good to excellent yields after Cu-catalyzed cycloaddition with azide 1. For aromatic alkynes, no matter electron-donating groups 2b, 2c or electron-withdrawing groups 2f, 2g were all compatible. Sterically hindered substrates 2l, 2m did not affect the efficiency of cycloaddition. It was worth noting that heterocyclic products 3n–p were also generated in good yields with either a nitrogen or sulfur atom. In addition, aliphatic alkynes 2q–w were smoothly transformed into their corresponding triazoles in satisfactory yields. Especially, the alkynes derived from phenols, such as 2t–w all gave corresponding triazoles in nearly quantitative yields. Natural products ethynyl estradiol 2x and norethindrone 2y were applicable to be modified by the cycloaddition with 1 and furnished corresponding BTESFs 3x and 3y in 91% and 82% yields respectively. Interestingly, when 2.0 equivalents of azide 1 was used, ditriazole substituted BTESF 3z was generated in 88% yield after the reaction with the derivative of diethylstilbestrol 2z.
Table 1 Cu(I)-catalyzed cycloaddition for the synthesis of BTESFs 3a
| Reaction conditions: CuSO4·5H2O (5 mol%, 12.5 mg), sodium ascorbate (10 mol%, 19.8 mg), 1 (1 mmol, 232 mg) and 2 (2.0 equiv., 2.0 mmol) were dissolved in MeOH (5 mL) and reacted at room temperature for 12–24 h. 1 (2 mmol, 464 mg) was used. |
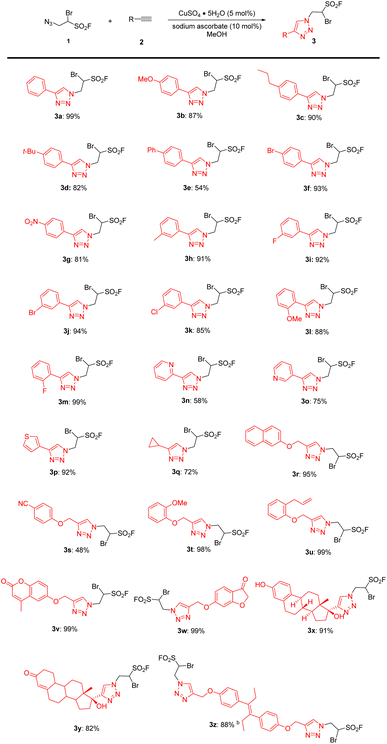 |
Meaningfully, NH-1,2,3-triazoles will be obtained by the reaction of BTESFs 3 and base through the elimination step. These N-unsubstituted 1,2,3-triazole motifs are ubiquitous in pharmaceutical, agrochemistry and material fields (Fig. 1). And the increasing importance of NH-1,2,3-triazoles has highlighted the development of new synthetic methods for these kinds of compounds. However, most of the reports resulted in N-substituted 1,2,3-triazoles,26 with only a few generating NH-triazoles,28 which mainly involved sodium azide29–34 and trimethylsilyl azide (TMSN3)35–38 participated cycloaddition of alkynes or alkyne precursors39–43 (Fig. 3). With our designed BTESFs 3 in hand, NH-1,2,3-triazoles were formed facilely with excellent yields.
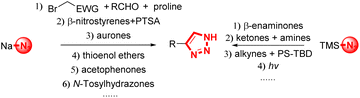 |
| | Fig. 3 Synthesis of NH-1,2,3-triazoles. | |
Afterwards, a simple condition screening was carried out using BTESF 3a as model starting material to test the formation of 4-phenyl-1H-1,2,3-triazole 4a (Table 2). The desired product 4a was furnished with a yield of 97% when the reaction was proceeded in the presence of 2 equivalents of pyrrolidine at room temperature for 12 hours in 1,4-dioxane (entry 1). Encouraged by the result, we further examined other organic and inorganic bases for the promotion of this reaction. The results indicated that secondary amines (entries 2 and 3) were beneficial for the generation of 4a compared with other tertiary amines (entries 4–8). While inorganic bases such as Na2CO3, NaOH, Cs2CO3 and K2CO3 only provided the desired product in less than 30% yields (entries 9–12). Considering that pyrrolidine is more commercially available than piperidine (entry 2), finally, pyrrolidine was chosen as the suitable base for the synthesis of other NH-1,2,3-triazoles 4.
Table 2 Optimization for the synthesis of NH-1,2,3-triazoles 4a
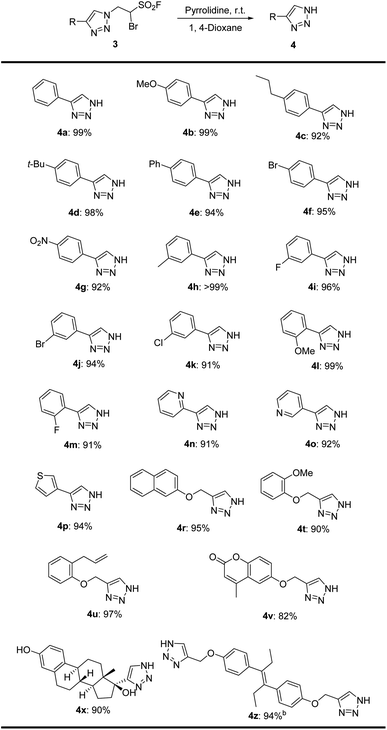
With the optimized conditions in hand, we explored the substrate scope and functional-group tolerance for the formation of NH-1,2,3-triazoles from their corresponding BTESFs 3 (Table 3). To our delight, most BTESFs 3 were converted to their desired NH-triazoles in excellent to quantitative yields under the condition of pyrrolidine. 4-Aromatic substituted BTESFs bearing electron-donating (3b–e, 3h, 3l) or electron-withdrawing (3f, 3g, 3i–k, 3m) groups all generated their corresponding triazoles efficiently in excellent yields. 4-Heterocyclic substituted BTESFs (3n–p) tolerated well and provided corresponding triazoles 4n, 4o and 4p in 91%, 92% and 94% yields respectively. 4-Phenol derivatives substituted BTESFs were also amenable for the process. 2-Naphthol 3r, guaiacol 3t, 2-allylphenol 3u and coumarin phenol 3v were converted smoothly to their desired products in good to excellent yields. Especially, the derivative of diethylstilbestrol 3z generated disubstituted triazole 4z in 94% yield when treated with 4.0 equivalents of pyrrolidine. Ethynyl estradiol substituted triazole 4x was also generated with yields of 90%.
Table 3 Scope of NH-1,2,3-triazoles 4a
| Reaction conditions: 3 (0.3 mmol) and pyrrolidine (0.6 mmol, 42.7 mg) were dissolved in 1,4-dioxane (2 mL) and reacted at room temperature for 12 h. Pyrrolidine (1.2 mmol, 85.4 mg) was used. |
 |
A more detailed mechanism was proposed and illustrated in Fig. 4.44 Initially, the terminal alkyne 2 coordinates with the copper(I) to give the copper(I)-alkyne intermediate I; then, the nitrogen atom connected with carbon atom in 1 displaces one of the ligands on the copper(I) alkyne I and forms a linkage with copper to form the intermediate II; subsequently, the terminal nitrogen atom in II attacks the C-2 carbon atom of the alkyne to form an unstable six-membered copper ring III. It has been shown that this process is an endothermic process that can form a stable five-membered triazole intermediate IV by releasing heat through ring contraction; finally, the intermediate IV undergoes hydrolysis and releases triazole product 3 to complete the catalytic cycle. Under the condition of nucleophilic base pyrrolidine,45 the highly electrophilic sulfonyl fluoride moiety of 3 cleaved subsequently with the generation of NH-1,2,3-triazoles together with byproduct enaminyl sulfonyl fluoride.27
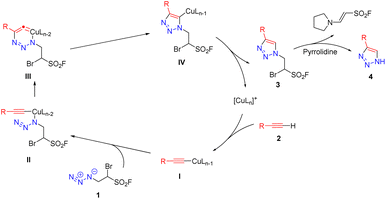 |
| | Fig. 4 Proposed mechanism. | |
Conclusions
In conclusion, a mild method has been developed for the construction of a novel series of triazoles containing sulfonyl fluoride moiety using the sulfonyl fluoride reagent we developed. All compounds are obtained in good to excellent yields mildly. Significantly, the aliphatic sulfonyl fluoride moiety proved sensitive to bases, allowing for smooth transformation into N-unsubstituted triazoles under mild conditions. This offers a convenient and efficient protocol for synthesizing sulfonyl fluorides and triazoles, both of which are versatile functionalities in medicinal discovery. Further studies of these scaffolds in organic chemistry and medicinal chemistry are ongoing in our laboratory.
Experimental section
General procedure for synthesis of 3
An oven-dried round-bottle flask (20 mL) was charged with CuSO4·5H2O (5 mol%, 12.5 mg), sodium ascorbate (10 mol%, 19.8 mg), alkyne 2 (2 mmol), 2-azido-1-bromoethane-1-sulfonyl fluoride 1 (1 mmol, 232 mg) and 5 mL MeOH. The mixture was stirred at room temperature for 12–24 h with monitoring by TLC. After the reaction was completed, the solution was concentrated to dryness and the residue was purified through silica gel chromatography using ethyl acetate/petroleum ether = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to afford desired product 3.
2 to afford desired product 3.
General procedure for synthesis of 4
An oven-dried round-bottle flask (10 mL) was charged with 3 (0.3 mmol), pyrrolidine (0.6 mmol, 42.7 mg) and 1,4-dioxane (2 mL). The mixture was stirred at room temperature for 12 h with monitoring by TLC. After the reaction was completed, the solution was concentrated to dryness and the residue was purified through silica gel chromatography using a mixture of ethyl acetate and petroleum ether from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to pure dichloromethane to afford desired product 4.
2 to pure dichloromethane to afford desired product 4.
1-Bromo-2-(4-phenyl-1H-1,2,3-triazol-1-yl)ethane-1-sulfonyl fluoride (3a). White solid, 331 mg, 99%. M.p. 162–163 °C. 1H NMR (500 MHz, DMSO) δ 8.66 (s, 1H), 7.85 (d, J = 7.4 Hz, 2H), 7.47 (t, J = 7.7 Hz, 2H), 7.37 (t, J = 7.4 Hz, 1H), 7.00–6.97 (m, 1H), 5.47 (dd, J = 15.1, 5.2 Hz, 1H), 5.30 (dd, J = 15.1, 7.6 Hz, 1H). 13C NMR (126 MHz, DMSO) δ 146.5 (s), 130.2 (s), 129.1 (s), 128.2 (s), 125.3 (s), 122.6 (s), 55.4 (d, J = 18.9 Hz), 50.3 (s). 19F NMR (471 MHz, DMSO) δ 47.1 (s, 1F). ESI-MS HRMS calculated for C10H10BrFN3O2S [M + H]+ 333.9656, found 333.9655.
1-Bromo-2-(4-(4-methoxyphenyl)-1H-1,2,3-triazol-1-yl)ethane-1-sulfonyl fluoride (3b). 315 mg, 87%. M.p. 131–132 °C. 1H NMR (500 MHz, CDCl3) δ 7.83 (s, 1H), 7.76 (d, J = 8.9 Hz, 2H), 6.97 (d, J = 8.9 Hz, 2H), 5.70–5.67 (m, 1H), 5.34 (dd, J = 14.8, 5.1 Hz, 1H), 4.96 (dd, J = 14.7, 8.4 Hz, 1H), 3.84 (s, 3H). 13C NMR (126 MHz, DMSO) δ 159.2 (s), 146.4 (s), 126.6 (s), 122.8 (s), 121.6 (s), 114.4 (s), 55.4 (d, J = 18.7 Hz), 55.2 (s), 50.2 (s). 19F NMR (471 MHz, CDCl3) δ 46.8 (s, 1F). ESI-MS HRMS calculated for C11H12BrFN3O2S [M + H]+ 363.9761, found 363.9758.
1-Bromo-2-(4-(4-propylphenyl)-1H-1,2,3-triazol-1-yl)ethane-1-sulfonyl fluoride (3c). White solid, 338 mg, 90%. M.p. 128–130 °C. 1H NMR (500 MHz, DMSO) δ 8.60 (s, 1H), 7.76 (d, J = 8.1 Hz, 2H), 7.28 (d, J = 8.1 Hz, 2H), 6.99–6.97 (m, 1H), 5.46 (dd, J = 15.1, 5.2 Hz, 1H), 5.29 (dd, J = 15.1, 7.6 Hz, 1H), 2.58 (t, J = 7.5 Hz, 2H), 1.65–1.57 (m, 2H), 0.90 (t, J = 7.3 Hz, 3H). 13C NMR (126 MHz, DMSO) δ 146.5 (s), 142.3 (s), 129.0 (s), 127.7 (s), 125.2 (s), 122.2 (s), 55.4 (d, J = 18.8 Hz), 50.3 (s), 37.0 (s), 23.9 (s), 13.6 (s). 19F NMR (471 MHz, CDCl3) δ 47.1 (s, 1F). ESI-MS HRMS calculated for C13H16BrFN3O2S [M + H]+ 376.0125, found 376.0123.
1-Bromo-2-(4-(4-(tert-butyl)phenyl)-1H-1,2,3-triazol-1-yl)ethane-1-sulfonyl fluoride (3d). White solid, 318 mg, 82%. M.p. 168–170 °C. 1H NMR (500 MHz, DMSO) δ 8.62 (s, 1H), 7.78 (d, J = 7.1 Hz, 2H), 7.49 (d, J = 8.3 Hz, 2H), 6.99–6.97 (m, 1H), 5.47 (dd, J = 15.1, 5.1 Hz, 1H), 5.30 (dd, J = 15.1, 7.6 Hz, 1H), 1.31 (s, 9H). 13C NMR (126 MHz, DMSO) δ 150.7 (s), 146.5 (s), 127.4 (s), 125.7 (s), 125.1 (s), 122.2 (s), 55.4 (d, J = 18.8 Hz), 50.3 (s), 34.4 (s), 31.0 (s). 19F NMR (471 MHz, DMSO) δ 47.1 (s, 1F). ESI-MS HRMS calculated for C14H18BrFN3O2S [M + H]+ 390.0282, found 390.0280.
2-(4-([1,1′-Biphenyl]-4-yl)-1H-1,2,3-triazol-1-yl)-1-bromoethane-1-sulfonyl fluoride (3e). White solid, 224 mg, 55%. M.p. 195–197 °C. 1H NMR (500 MHz, DMSO) δ 8.72 (s, 1H), 7.95 (d, J = 8.4 Hz, 2H), 7.79 (d, J = 8.4 Hz, 2H), 7.72 (d, J = 7.3 Hz, 2H), 7.48 (t, J = 7.7 Hz, 2H), 7.38 (t, J = 7.4 Hz, 1H), 7.01–6.99 (m, 1H), 5.49 (dd, J = 15.1, 5.2 Hz, 1H), 5.32 (dd, J = 15.1, 7.5 Hz, 1H). 13C NMR (126 MHz, DMSO) δ 146.1 (s), 139.8 (s), 139.5 (s), 129.3 (s), 129.0 (s), 127.6 (s), 127.3 (s), 126.6 (s), 125.8 (s), 122.7 (s), 55.4 (d, J = 18.9 Hz), 50.3 (s). 19F NMR (471 MHz, DMSO) δ 47.1 (s, 1F). ESI-MS HRMS calculated for C16H14BrFN3O2S [M + H]+ 409.9969, found 409.9968.
1-Bromo-2-(4-(4-bromophenyl)-1H-1,2,3-triazol-1-yl)ethane-1-sulfonyl fluoride (3f). White solid, 380 mg, 93%. M.p. 132–134 °C. 1H NMR (500 MHz, DMSO) δ 8.71 (s, 1H), 7.82 (d, J = 8.6 Hz, 2H), 7.67 (d, J = 8.5 Hz, 2H), 6.98–6.96 (m, 1H), 5.46 (dd, J = 15.2, 5.2 Hz, 1H), 5.31 (dd, J = 15.2, 7.5 Hz, 1H). 13C NMR (126 MHz, DMSO) δ 145.4 (s), 132.0 (s), 129.4 (s), 127.3 (s), 123.0 (s), 121.2 (s), 55.3 (d, J = 18.9 Hz), 50.4 (s). 19F NMR (471 MHz, DMSO) δ 47.1 (s, 1F). ESI-MS HRMS calculated for C10H9Br2FN3O2S [M + H]+ 411.8761, found 411.8760.
1-Bromo-2-(4-(4-nitrophenyl)-1H-1,2,3-triazol-1-yl)ethane-1-sulfonyl fluoride (3g). White solid, 306 mg, 81%. M.p. 156–157 °C. 1H NMR (500 MHz, DMSO) δ 8.93 (s, 1H), 8.34 (d, J = 8.9 Hz, 2H), 8.14 (t, J = 5.7 Hz, 2H), 7.01–6.98 (m, 1H), 5.51 (dd, J = 15.2, 5.2 Hz, 1H), 5.37 (dd, J = 15.2, 7.4 Hz, 1H). 13C NMR (126 MHz, DMSO) δ 146.9 (s), 144.5 (s), 136.5 (s), 126.1 (s), 124.8 (s), 124.5 (s), 55.2 (d, J = 19.1 Hz), 50.4 (s). 19F NMR (471 MHz, DMSO) δ 47.2 (s, 1F). ESI-MS HRMS calculated for C10H9BrFN4O4S [M + H]+ 378.9506, found 378.9504.
1-Bromo-2-(4-(m-tolyl)-1H-1,2,3-triazol-1-yl)ethane-1-sulfonyl fluoride (3h). White solid, 318 mg, 91%. M.p. 127–129 °C. 1H NMR (500 MHz, DMSO) δ 8.63 (s, 1H), 7.68 (s, 1H), 7.63 (d, J = 7.7 Hz, 1H), 7.35 (t, J = 7.6 Hz, 1H), 7.18 (d, J = 7.5 Hz, 1H), 6.99–6.96 (m, 1H), 5.46 (dd, J = 15.1, 5.2 Hz, 1H), 5.30 (dd, J = 15.1, 7.5 Hz, 1H), 2.37 (s, 3H). 13C NMR (126 MHz, DMSO) δ 146.5 (s), 138.2 (s), 130.1 (s), 128.9 (s), 128.8 (s), 125.8 (s), 122.6 (s), 122.4 (s), 55.4 (d, J = 18.9 Hz), 50.3 (s), 21.0 (s). 19F NMR (471 MHz, DMSO) δ 47.1 (s, 1F). ESI-MS HRMS calculated for C11H12BrFN3O2S [M + H]+ 347.9812, found 347.9811.
1-Bromo-2-(4-(3-fluorophenyl)-1H-1,2,3-triazol-1-yl)ethane-1-sulfonyl fluoride (3i). White solid, 323 mg, 92%. M.p. 116–117 °C. 1H NMR (500 MHz, DMSO) δ 8.74 (s, 1H), 7.71–7.65 (m, 2H), 7.54–7.50 (m, 1H), 7.20 (td, J = 8.6, 2.4 Hz, 1H), 6.98–6.95 (m, 1H), 5.46 (dd, J = 15.2, 5.2 Hz, 1H), 5.31 (dd, J = 15.2, 7.4 Hz, 1H). 13C NMR (126 MHz, DMSO) δ 162.7 (d, J = 242.2 Hz) 145.4 (d, J = 2.7 Hz), 132.6 (d, J = 8.6 Hz), 131.3(d, J = 8.6 Hz), 123.5 (s), 121.4 (d, J = 2.7 Hz), 115.0 (d, J = 21.1 Hz), 111.9 (d, J = 22.9 Hz), 55.4 (d, J = 18.8 Hz), 50.4 (s). 19F NMR (471 MHz, DMSO) δ 47.2 (s, 1F), −112.0 to −112.1 (m, 1F). ESI-MS HRMS calculated for C10H9BrF2N3O2S [M + H]+ 351.9561, found 351.9560.
1-Bromo-2-(4-(3-bromophenyl)-1H-1,2,3-triazol-1-yl)ethane-1-sulfonyl fluoride (3j). White solid, 384 mg, 94%. M.p. 101–102 °C. 1H NMR (500 MHz, DMSO) δ 8.77 (s, 1H), 8.06 (t, J = 1.6 Hz, 1H), 7.87 (d, J = 7.8 Hz, 1H), 7.58–7.55 (m, 1H), 7.44 (t, J = 7.9 Hz, 1H), 6.99–6.96 (m, 1H), 5.47 (dd, J = 15.2, 5.2 Hz, 1H), 5.31 (dd, J = 15.2, 7.4 Hz, 1H). 13C NMR (126 MHz, DMSO) δ 145.0 (s), 132.5 (s), 131.3 (s), 130.9 (s), 127.7 (s), 124.2 (s), 123.5 (s), 122.3 (s), 55.3 (d, J = 18.9 Hz), 50.4 (s). 19F NMR (471 MHz, DMSO) δ 47.2 (s, 1F). ESI-MS HRMS calculated for C10H9Br2FN3O2S [M + H]+ 411.8761, found 411.8760.
1-Bromo-2-(4-(3-chlorophenyl)-1H-1,2,3-triazol-1-yl)ethane-1-sulfonyl fluoride (3k). White solid, 314 mg, 85%. M.p. 117–119 °C. 1H NMR (500 MHz, DMSO) δ 8.77 (s, 1H), 7.91 (t, J = 1.7 Hz, 1H), 7.83 (d, J = 7.7 Hz, 1H), 7.51 (t, J = 7.9 Hz, 1H), 7.44–7.42 (m, 1H), 6.98–6.95 (m, 1H), 5.47 (dd, J = 15.2, 5.2 Hz, 1H), 5.32 (dd, J = 15.2, 7.4 Hz, 1H). 13C NMR (126 MHz, DMSO) δ 145.1 (s), 133.8 (s), 132.3 (s), 131.0 (s), 128.0 (s), 124.8 (s), 123.8 (s), 123.5 (s), 55.3 (d, J = 19.0 Hz), 50.4 (s). 19F NMR (471 MHz, DMSO) δ 47.2 (s, 1F). ESI-MS HRMS calculated for C10H9BrClFN3O2S [M + H]+ 367.9266, found 367.9265.
1-Bromo-2-(4-(2-methoxyphenyl)-1H-1,2,3-triazol-1-yl)ethane-1-sulfonyl fluoride (3l). White solid, 322 mg, 88%. M.p. 152–154 °C. 1H NMR (500 MHz, DMSO) δ 8.57 (s, 1H), 8.16 (dd, J = 7.7, 1.7 Hz, 1H), 7.36 (td, J = 7.8, 1.8 Hz, 1H), 7.15 (d, J = 7.8 Hz, 1H), 7.07 (td, J = 7.6, 1.0 Hz, 1H), 6.98–6.95 (m, 1H), 5.47 (dd, J = 15.0, 5.3 Hz, 1H), 5.31 (dd, J = 15.0, 7.8 Hz, 1H), 3.92 (s, 3H). 13C NMR (126 MHz, DMSO) δ 155.5 (s), 142.2 (s), 129.4 (s), 126.7 (s), 125.2 (s), 120.9 (s), 118.7 (s), 111.8 (s), 55.7 (s), 55.6 (d, J = 18.9 Hz), 50.1 (s). 19F NMR (471 MHz, DMSO) δ 47.1 (s, 1F). ESI-MS HRMS calculated for C11H12BrFN3O3S [M + H]+ 363.9761, found 363.9769.
1-Bromo-2-(4-(2-fluorophenyl)-1H-1,2,3-triazol-1-yl)ethane-1-sulfonyl fluoride (3m). White solid, 350 mg, 99%. M.p. 132–133 °C. 1H NMR (500 MHz, DMSO) δ 8.61 (d, J = 3.7 Hz, 1H), 8.17 (td, J = 7.6, 1.6 Hz, 1H), 7.46–7.41 (m, 1H), 7.38–7.33 (m, 2H), 7.03–7.00 (m, 1H), 5.53 (dd, J = 15.0, 5.3 Hz, 1H), 5.37 (dd, J = 15.0, 7.7 Hz, 1H). 13C NMR (126 MHz, DMSO) δ 158.5 (d, J = 247.2 Hz), 139.8 (d, J = 2.4 Hz), 130.0 (d, J = 8.4 Hz), 127.3 (d, J = 3.4 Hz), 125.1 (s), 125.0 (d, J = 9.6 Hz), 117.9 (d, J = 13.0 Hz), 116.1 (d, J = 21.3 Hz), 55.3 (d, J = 18.9 Hz), 50.1 (s). 19F NMR (471 MHz, DMSO) δ 47.1 (s, 1F), −114.2 to −114.3 (m, 1F). ESI-MS HRMS calculated for C10H9BrF2N3O2S [M + H]+ 351.9561, found 351.9560.
1-Bromo-2-(4-(pyridin-2-yl)-1H-1,2,3-triazol-1-yl)ethane-1-sulfonyl fluoride (3n). White solid, 194 mg, 58%. M.p. 123–124 °C. 1H NMR (500 MHz, DMSO) δ 8.72 (s, 1H), 8.62 (d, J = 4.8 Hz, 1H), 8.06 (d, J = 7.9 Hz, 1H), 7.91 (td, J = 7.8, 1.7 Hz, 1H), 7.38–7.35 (m, 1H), 7.02–6.99 (m, 1H), 5.52 (dd, J = 15.0, 5.3 Hz, 1H), 5.37 (dd, J = 15.0, 7.6 Hz, 1H). 13C NMR (126 MHz, DMSO) δ 149.3 (s), 149.0 (s), 146.9 (s), 137.9 (s), 124.7 (s), 123.4 (s), 119.8 (s), 55.3 (d, J = 19.0 Hz), 50.2 (s). 19F NMR (471 MHz, DMSO) δ 47.1 (s, 1F). ESI-MS HRMS calculated for C9H9BrFN4O2S [M + H]+ 334.9608, found 334.9605.
1-Bromo-2-(4-(pyridin-3-yl)-1H-1,2,3-triazol-1-yl)ethane-1-sulfonyl fluoride (3o). White solid, 250 mg, 75%. M.p. 133–134 °C. 1H NMR (500 MHz, DMSO) δ 9.08 (d, J = 1.6 Hz, 1H), 8.82 (s, 1H), 8.58 (dd, J = 4.8, 1.5 Hz, 1H), 8.26 (dt, J = 4.8, 1.5 Hz, 1H), 7.53 (dd, J = 8.3, 5.1 Hz, 1H), 7.01–6.98 (m, 1H), 5.50 (dd, J = 15.2, 5.2 Hz, 1H), 5.36 (dd, J = 15.2, 7.4 Hz, 1H). 13C NMR (126 MHz, DMSO) δ 145.8 (s), 143.1 (s), 142.5 (s), 136.4 (d, J = 3.4 Hz), 127.7 (s), 125.7 (s), 124.3 (s), 55.3 (d, J = 18.9 Hz), 50.5 (s). 19F NMR (471 MHz, DMSO) δ 47.2 (s, 1F). ESI-MS HRMS calculated for C9H9BrFN4O2S [M + H]+ 334.9608, found 334.9605.
1-Bromo-2-(4-(thiophen-3-yl)-1H-1,2,3-triazol-1-yl)ethane-1-sulfonyl fluoride (3p). White solid, 312 mg, 92%. M.p. 133–134 °C. 1H NMR (500 MHz, DMSO) δ 8.51 (s, 1H), 7.89 (dd, J = 2.9, 1.2 Hz, 1H), 7.66 (dd, J = 5.0, 2.9 Hz, 1H), 7.52 (dd, J = 5.0, 1.2 Hz, 1H), 6.96–6.93 (m, 1H), 5.44 (dd, J = 15.2, 5.2 Hz, 1H), 5.29 (dd, J = 15.2, 7.4 Hz, 1H). 13C NMR (126 MHz, DMSO) δ 143.1 (s), 131.5 (s), 127.5 (s), 125.8 (s), 122.4 (s), 121.5 (s), 55.5 (d, J = 18.8 Hz), 50.3 (s). 19F NMR (471 MHz, DMSO) δ 46.7 (s, 1F). ESI-MS HRMS calculated for C8H8BrFN3O2S2 [M + H]+ 339.9220, found 339.9219.
1-Bromo-2-(4-cyclopropyl-1H-1,2,3-triazol-1-yl)ethane-1-sulfonyl fluoride (3q). White solid, 213 mg, 72%. M.p. 100–101 °C. 1H NMR (500 MHz, DMSO) δ 7.92 (s, 1H), 6.88–6.84 (t, J = 6.4 Hz, 1H), 5.31 (dd, J = 15.1, 5.2 Hz, 1H), 5.14 (dd, J = 15.1, 7.7 Hz, 1H), 2.00–1.94 (m, 1H), 0.94–0.90 (m, 2H), 0.73–0.69 (m, 2H). 13C NMR (126 MHz, DMSO) δ 149.2 (s), 122.1 (s), 55.5 (d, J = 18.6 Hz), 50.0 (s), 7.7 (s), 6.4 (s). 19F NMR (471 MHz, DMSO) δ 47.0 (s, 1F). ESI-MS HRMS calculated for C7H10BrFN3O2S [M + H]+ 297.9656, found 297.7654.
1-Bromo-2-(4-((naphthalen-2-yloxy)methyl)-1H-1,2,3-triazol-1-yl)ethane-1-sulfonyl fluoride (3r). White solid, 394 mg, 95%. M.p. 110–111 °C. 1H NMR (500 MHz, DMSO) δ 8.39 (s, 1H), 7.85–7.81 (m, 3H), 7.50 (d, J = 2.5 Hz, 1H), 7.47 (t, J = 7.0 Hz, 1H), 7.36 (t, J = 7.0 Hz, 1H), 7.20 (dd, J = 8.9, 2.5 Hz, 1H), 6.97–6.94 (m, 1H), 5.45 (dd, J = 15.1, 5.2 Hz, 1H), 5.32–5.27 (m, 3H). 13C NMR (126 MHz, DMSO) δ 155.9 (s), 142.9 (s), 134.2 (s), 129.4 (s), 128.7 (s), 127.5 (s), 126.8 (s), 126.5 (s), 125.9 (s), 123.8 (s), 118.7 (s), 107.4 (s), 61.1 (s), 55.4 (d, J = 18.9 Hz), 50.1(s). 19F NMR (471 MHz, DMSO) δ 47.0 (s, 1F). ESI-MS HRMS calculated for C14H14BrFN3O3S [M + H]+ 413.9918, found 413.9915.
1-Bromo-2-(4-((4-cyanophenoxy)methyl)-1H-1,2,3-triazol-1-yl)ethane-1-sulfonyl fluoride (3s). White solid, 186 mg, 48%. M.p. 102–104 °C. 1H NMR (500 MHz, DMSO) δ 8.36 (s, 1H), 7.78 (d, J = 8.9 Hz, 2H), 7.22 (d, J = 8.9 Hz, 2H), 6.95–6.92 (m, 1H), 5.44 (dd, J = 15.1, 5.2 Hz, 1H), 5.31–5.27 (m, 3H). 13C NMR (126 MHz, DMSO) δ 161.5 (s), 142.3 (s), 134.3 (s), 126.3 (s), 119.2 (s), 116.0 (s), 103.4 (s), 61.4 (s), 55.4 (d, J = 18.9 Hz), 50.2 (s). 19F NMR (471 MHz, DMSO) δ 47.1 (s, 1F). ESI-MS HRMS calculated for C12H11BrFN4O3S [M + H]+ 388.9714, found 388.9714.
1-Bromo-2-(4-((2-methoxyphenoxy)methyl)-1H-1,2,3-triazol-1-yl)ethane-1-sulfonyl fluoride (3t). White solid, 385 mg, 98%. M.p. 109–110 °C. 1H NMR (500 MHz, DMSO) δ 8.30 (s, 1H), 7.11 (dd, J = 7.9, 1.6 Hz, 1H), 6.98–6.86 (m, 4H), 5.43 (dd, J = 15.1, 5.2 Hz, 1H), 5.28 (dd, J = 15.1, 7.7 Hz, 1H), 5.15 (s, 2H), 3.74 (s, 3H). 13C NMR (126 MHz, DMSO) δ 149.3 (s), 147.5 (s), 143.2 (s), 125.9 (s), 121.7 (s), 120.7 (s), 114.3 (s), 112.4 (s), 61.8 (s), 55.5 (s), 55.4 (d, J = 16.3 Hz), 50.1 (s). 19F NMR (471 MHz, DMSO) δ 47.0 (s, 1F). ESI-MS HRMS calculated for C12H14BrFN3O4S [M + H]+ 393.9876, found 393.9876.
2-(4-((2-Allylphenoxy)methyl)-1H-1,2,3-triazol-1-yl)-1-bromoethane-1-sulfonyl fluoride (3u). White solid, 400 mg, 99%. M.p. 60–61 °C. 1H NMR (500 MHz, CDCl3) δ 7.78 (s, 1H), 7.19–7.14 (m, 2H), 6.95–6.91 (m, 2H), 6.00–5.92 (m, 1H), 5.70 (dd, J = 8.1, 5.2 Hz, 1H), 5.30 (dd, J = 14.8, 5.1 Hz, 1H), 5.24 (s, 2H), 5.03–5.02 (m, 1H), 5.00 (s, 1H), 4.95 (dd, J = 14.8, 8.2 Hz, 1H), 3.38 (d, J = 6.6 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 155.8 (s), 145.3 (s), 136.9 (s), 130.2 (s), 129.0 (s), 127.5 (s), 124.3 (s), 121.5 (s), 115.6 (s), 112.0 (s), 62.2 (s), 55.1 (d, J = 22.4 Hz), 51.4 (s), 34.4 (s). 19F NMR (471 MHz, CDCl3) δ 46.8 (s, 1F). ESI-MS HRMS calculated for C14H16BrFN3O3S [M + H]+ 404.0074, found 404.0073.
1-Bromo-2-(4-(((4-methyl-2-oxo-2H-chromen-6-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)ethane-1-sulfonyl fluoride (3v). White solid, 443 mg, 99%. M.p. 178–180 °C. 1H NMR (500 MHz, DMSO) δ 8.35 (s, 1H), 7.36–7.29 (m, 3H), 6.95–6.93 (m, 1H), 6.39 (s, 1H), 5.44 (dd, J = 15.1, 5.2 Hz, 1H), 5.32–5.27 (m, 3H), 2.43 (s, 3H). 13C NMR (126 MHz, DMSO) δ 156.0 (s), 154.3 (s), 153.1 (s), 147.5 (s), 142.7 (s), 126.0 (s), 120.2 (s), 119.9 (s), 117.6 (s), 114.8 (s), 109.6 (s), 61.6 (s), 55.4 (d, J = 18.8 Hz), 50.1 (s), 18.2 (s). 19F NMR (471 MHz, DMSO) δ 47.1 (s, 1F). ESI-MS HRMS calculated for C15H14BrFN3O5S [M + H]+ 445.9816, found 445.9816.
1-Bromo-2-(4-(((3-oxo-2,3-dihydrobenzofuran-6-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)ethane-1-sulfonyl fluoride (3w). White solid, 416 mg, 99%. M.p. 117–118 °C. 1H NMR (500 MHz, DMSO) δ 8.39 (s, 1H), 7.54 (d, J = 8.6 Hz, 1H), 6.99 (d, J = 2.1 Hz, 1H), 6.95–6.92 (m, 1H), 6.76 (dd, J = 8.6, 2.1 Hz, 1H), 5.45 (dd, J = 15.1, 5.2 Hz, 1H), 5.33–5.28 (m, 3H), 4.77 (s, 2H). 13C NMR (126 MHz, DMSO) δ 197.3 (s), 175.7 (s), 166.3 (s), 142.1 (s), 126.3 (s), 124.8 (s), 114.4 (s), 112.0 (s), 97.8 (s), 75.6 (s), 61.7 (s), 55.4 (d, J = 18.9 Hz), 50.2 (s). 19F NMR (471 MHz, DMSO) δ 47.1 (s, 1F). ESI-MS HRMS calculated for C13H12BrFN3O5S [M + H]+ 419.9660, found 419.9660.
1-Bromo-2-(4-((8R,9S,13S,14S,17S)-3,17-dihydroxy-13-methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthren-17-yl)-1H-1,2,3-triazol-1-yl)ethane-1-sulfonyl fluoride (3x). White solid, 478 mg, 91%. M.p. 205–206 °C. 1H NMR (500 MHz, DMSO) δ 9.01 (brs, 1H), 7.97 (s, 1H), 6.95 (d, J = 8.4 Hz, 1H), 6.92–6.89 (m, 1H), 6.46 (dd, J = 8.3, 2.1 Hz, 1H), 6.42 (s, 1H), 5.38 (dt, J = 14.9, 4.7 Hz, 1H), 5.23 (dd, J = 15.0, 7.5 Hz, 1H), 2.72–2.65 (m, 2H), 2.35–2.30 (m, 1H), 2.08–2.06 (m, 1H), 1.99–1.94 (m, 1H), 1.83–1.82 (m, 2H), 1.77–1.72 (m, 1H), 1.65–1.58 (m, 1H), 1.49–1.15 (m, 6H), 0.92 (s, 3H), 0.63–0.59 (m, 1H). 13C NMR (126 MHz, DMSO) δ 154.9 (s), 154.5 (d, J = 4.4 Hz), 137.3 (s), 130.5 (s), 126.1 (s), 124.0 (d, J = 7.8 Hz), 115.0 (s), 112.8 (s), 81.2 (s), 55.7 (d, J = 18.4 Hz), 55.6 (d, J = 18.4 Hz), 50.0 (d, J = 3.4 Hz), 47.7 (s), 46.9 (s), 43.3 (s), 37.3 (s), 32.5 (s), 29.3 (s), 27.3 (s), 26.2 (s), 23.6 (s), 14.4 (s). 19F NMR (471 MHz, DMSO) δ 47.2 (d, J = 22.8 Hz, 1F). ESI-MS HRMS calculated for C22H28BrFN3O4S [M + H]+ 528.0962, found 528.0962.
1-Bromo-2-(4-((13S,17R)-13-methyl-3-oxo-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)-1H-1,2,3-triazol-1-yl)ethane-1-sulfonyl fluoride (3y). White solid, 414 mg, 82%. M.p. 97–98 °C. 1H NMR (500 MHz, CDCl3) δ 7.61 (d, J = 6.3 Hz, 1H), 5.79–5.78 (m, 1H), 5.70–5.67 (m, 1H), 5.30–5.23 (m, 1H), 5.00–4.93 (m, 1H), 2.47–2.02 (m, 9H), 1.88–1.86 (m, 2H), 1.71–1.69 (m, 1H), 1.52–1.41 (m, 5H), 1.25–1.16 (m, 2H), 1.06 (s, 3H), 0.64–0.60 (m, 1H), 0.48–0.42 (m, 1H). 13C NMR (126 MHz, CDCl3) δ 200.0 (s), 166.8 (s), 154.4 (s), 124.7 (s), 123.2 (s), 82.3 (d, J = 11.5 Hz), 55.2 (dd, J = 22.3, 15.8 Hz), 51.5 (s), 49.1 (d, J = 13.2 Hz), 48.3 (s), 47.3 (d, J = 3.5 Hz), 42.6 (s), 41.2 (s), 38.1 (s), 36.6 (s), 35.6 (s), 32.7 (s), 30.9 (s), 26.6 (s), 26.2 (s), 23.7 (d, J = 6.4 Hz), 14.3 (s). 19F NMR (471 MHz, DMSO) δ 46.8 (d, J = 7.5 Hz, 1F). ESI-MS HRMS calculated for C22H30BrFN3O3S [M + H]+ 514.1170, found 514.1170.
(E)-2,2′-((((Hex-3-ene-3,4-diylbis(4,1-phenylene))bis(oxy))bis(methylene))bis(1H-1,2,3-triazole-4,1-diyl))bis(1-bromoethane-1-sulfonyl fluoride) (3z). White solid, 706 mg, 88% (when corresponding alkyne 1 mmol and 1 2 mmol were used, 3z was obtained in 88% yield, no 3z′ was obtained). M.p. 206–208 °C. 1H NMR (500 MHz, DMSO) δ 8.34 (s, 2H), 7.13 (d, J = 8.5 Hz, 4H), 7.05 (d, J = 8.6 Hz, 4H), 6.96–6.94 (t, J = 5.3 Hz, 2H), 5.45 (dd, J = 15.1, 5.2 Hz, 2H), 5.30 (dd, J = 15.0, 7.6 Hz, 2H), 5.20 (s, 4H), 2.09 (q, J = 7.4 Hz, 4H), 0.72 (t, J = 7.4 Hz, 6H). 13C NMR (126 MHz, DMSO) δ 156.6 (s), 143.2 (s), 138.1 (s), 134.6 (s), 129.6 (s), 125.9 (s), 114.5 (s), 61.0 (s), 55.5 (d, J = 18.7 Hz), 50.1 (s), 28.1 (s), 13.3 (s). 19F NMR (471 MHz, DMSO) δ 47.1 (s, 2F). ESI-MS HRMS calculated for C28H31Br2F2N6O6S2 [M + H]+ 809.0055, found 809.0055.
(E)-1-Bromo-2-(4-((4-(4-(4-(prop-2-yn-1-yloxy)phenyl)hex-3-en-3-yl)phenoxy)methyl)-1H-1,2,3-triazol-1-yl)ethane-1-sulfonyl fluoride (3z′). White solid, 140 mg, 24% (when corresponding alkyne 1 mmol and 1 1 mmol were used, corresponding 3z was obtained in 75% yield (300 mg), and 3z′ was obtained in 24% yield). M.p. 101–102 °C. 1H NMR (500 MHz, DMSO) δ 8.35 (s, 1H), 7.15–7.13 (m, 4H), 7.05 (d, J = 8.5 Hz, 2H), 7.00 (d, J = 8.5 Hz, 2H), 6.96–6.94 (t, J = 5.4 Hz, 1H), 5.45 (dd, J = 15.1, 5.2 Hz, 1H), 5.30 (dd, J = 15.1, 7.6 Hz, 1H), 5.20 (s, 2H), 4.80 (d, J = 2.1 Hz, 2H), 3.55 (s, 1H), 2.08 (q, J = 6.9 Hz, 4H), 0.71 (t, J = 7.4 Hz, 6H). 13C NMR (126 MHz, DMSO) δ 156.6 (s), 155.8 (s), 143.1 (s), 138.1 (s), 138.0 (s), 134.9 (s), 134.5 (s), 129.52 (s), 129.47 (s), 125.9 (s), 114.5 (s), 114.4 (s), 79.5 (s), 78.2 (s), 61.0 (s), 55.4 (d, J = 16.6 Hz), 55.4 (s), 50.1 (s), 28.1 (s), 13.29(s), 13.27 (s). 19F NMR (471 MHz, DMSO) δ 47.1 (s, 1F). ESI-MS HRMS calculated for C26H28BrFN3O4S [M + H]+ 576.0962, found 576.0962.Note: In the 13C NMR spectrum of 3z′, theoretically, there should be twenty-six peaks. Due to the compact overlaying, it is difficult to specify the overlaying peaks.
4-Phenyl-1H-1,2,3-triazole (4a)46. White solid, 43 mg, 99%. 1H NMR (500 MHz, DMSO) δ 14.98 (s, 1H), 8.24 (s, 1H), 7.86 (d, J = 7.3 Hz, 2H), 7.45 (t, J = 7.6 Hz, 2H), 7.35 (t, J = 7.0 Hz, 1H).
4-(4-Methoxyphenyl)-1H-1,2,3-triazole (4b)46. White solid, 52 mg, 99%. 1H NMR (500 MHz, DMSO) δ 14.83 (s, 1H), 8.14 (s, 1H), 7.79 (d, J = 7.9 Hz, 2H), 7.01 (d, J = 8.2 Hz, 2H), 3.79 (s, 3H).
4-(4-Propylphenyl)-1H-1,2,3-triazole (4c)47. White solid, 52 mg, 92%. 1H NMR (500 MHz, DMSO) δ 14.90 (s, 1H), 8.20 (s, 1H), 7.76 (d, J = 7.1 Hz, 2H), 7.26 (d, J = 7.3 Hz, 2H), 2.57 (t, J = 7.5 Hz, 2H), 1.64–1.56 (m, 2H), 0.89 (t, J = 7.3 Hz, 3H).
4-(4-(tert-Butyl)phenyl)-1H-1,2,3-triazole (4d)48. White solid, 59 mg, 98%. 1H NMR (500 MHz, DMSO) δ 14.90 (s, 1H), 8.19 (s, 1H), 7.78 (d, J = 7.4 Hz, 2H), 7.47 (d, J = 8.0 Hz, 2H), 1.30 (s, 9H).
4-([1,1′-Biphenyl]-4-yl)-1H-1,2,3-triazole (4e)49. White solid, 62 mg, 94%. 1H NMR (500 MHz, DMSO) δ 15.00 (s, 1H), 8.30 (s, 1H), 7.96 (d, J = 7.7 Hz, 2H), 7.76 (d, J = 7.8 Hz, 2H), 7.72 (d, J = 7.5 Hz, 2H), 7.48 (t, J = 7.6 Hz, 2H), 7.38 (t, J = 7.1 Hz, 1H).
4-(4-Bromophenyl)-1H-1,2,3-triazole (4f)50. White solid, 64 mg, 95%. 1H NMR (500 MHz, DMSO) δ 15.07 (s, 1H), 8.30 (s, 1H), 7.83 (d, J = 7.9 Hz, 2H), 7.65 (d, J = 8.1 Hz, 2H).
4-(4-Nitrophenyl)-1H-1,2,3-triazole (4g)46. Yellow solid, 52 mg, 92%. 1H NMR (500 MHz, DMSO) δ 15.30 (s, 1H), 8.45 (s, 1H), 8.30 (d, J = 8.6 Hz, 2H), 8.13 (d, J = 8.2 Hz, 2H).
4-(m-Tolyl)-1H-1,2,3-triazole (4h)50. White solid, 47 mg, 99%. 1H NMR (500 MHz, DMSO) δ 14.92 (s, 1H), 8.22 (s, 1H), 7.69 (s, 1H), 7.65 (d, J = 7.4 Hz, 1H), 7.33 (d, J = 7.5 Hz, 1H), 7.17 (d, J = 7.3 Hz, 1H), 2.36 (s, 3H).
4-(3-Fluorophenyl)-1H-1,2,3-triazole (4i)51. White solid, 47 mg, 96%. 1H NMR (500 MHz, DMSO) δ 15.08 (s, 1H), 8.34 (s, 1H), 7.93 (s, 1H), 7.85 (d, J = 7.2 Hz, 1H), 7.49 (t, J = 7.8 Hz, 1H), 7.42–7.41 (m, 1H).
4-(3-Bromophenyl)-1H-1,2,3-triazole (4j)46. White solid, 63 mg, 94%. 1H NMR (500 MHz, DMSO) δ 15.12 (s, 1H), 8.34 (s, 1H), 8.07 (s, 1H), 7.89 (d, J = 7.3 Hz, 1H), 7.54–7.53 (m, 1H), 7.42 (t, J = 7.8 Hz, 1H).
4-(3-Chlorophenyl)-1H-1,2,3-triazole (4k)48. White solid, 49 mg, 91%. 1H NMR (500 MHz, DMSO) δ 15.11 (s, 1H), 8.34 (s, 1H), 7.93 (s, 1H), 7.85 (d, J = 7.0 Hz, 1H), 7.48 (t, J = 7.8 Hz, 1H), 7.41–7.40 (m, 1H).
4-(2-Methoxyphenyl)-1H-1,2,3-triazole (4l)50. White solid, 52 mg, 99%. 1H NMR (500 MHz, DMSO) δ 15.14 (s, 1H), 8.18–8.00 (m, 2H), 7.34 (t, J = 7.2 Hz, 1H), 7.13 (d, J = 8.3 Hz, 1H), 7.05 (t, J = 7.5 Hz, 1H), 3.90 (s, 3H).
4-(2-Fluorophenyl)-1H-1,2,3-triazole (4m)52. White solid, 44 mg, 91%. 1H NMR (500 MHz, DMSO) δ 15.34 (s, 1H), 8.20 (s, 1H), 8.05–8.03 (m, 1H), 7.41–7.39 (m, 1H), 7.35–7.29 (m, 2H).
2-(1H-1,2,3-Triazol-4-yl)pyridine (4n)53. White solid, 40 mg, 91%. 1H NMR (500 MHz, DMSO) δ 15.13 (s, 1H), 8.62–8.62 (m, 1H), 8.25 (s, 1H), 8.05–7.88 (m, 2H), 7.38–7.36 (m, 1H).
3-(1H-1,2,3-Triazol-4-yl)pyridine (4o)46. White solid, 40 mg, 92%. 1H NMR (500 MHz, DMSO) δ 15.16 (s, 1H), 9.09 (s, 1H), 8.69–8.37 (m, 2H), 8.23 (d, J = 7.6 Hz, 1H), 7.50–7.47 (m, 1H).
4-(Thiophen-3-yl)-1H-1,2,3-triazole (4p)48. White solid, 43 mg, 94%. 1H NMR (500 MHz, DMSO) δ 14.88 (s, 1H), 8.12 (s, 1H), 7.89 (s, 1H), 7.64 (s, 1H), 7.54 (d, J = 5.0 Hz, 1H).
4-((Naphthalen-2-yloxy)methyl)-1H-1,2,3-triazole (4r). White solid, 64 mg, 95%. M.p. 122–123 °C. 1H NMR (500 MHz, DMSO) δ 14.98 (s, 1H), 7.93 (s, 1H), 7.84–7.81 (m, 3H), 7.50–7.45 (m, 2H), 7.36 (t, J = 7.4 Hz, 1H), 7.20 (d, J = 7.5 Hz, 1H), 5.30 (s, 2H). 13C NMR (126 MHz, DMSO) δ 155.9 (s), 143.0 (s), 134.2 (s), 133.5 (s), 129.4 (s), 128.6 (s), 127.5 (s), 126.7 (s), 126.4 (s), 123.7 (s), 118.7 (s), 107.2 (s), 61.0 (s). ESI-MS HRMS calculated for C13H12N3O [M + H]+ 226.0975, found 226.0974.
4-((2-Methoxyphenoxy)methyl)-1H-1,2,3-triazole (4t). White solid, 55 mg, 90%. M.p. 121–123 °C. 1H NMR (500 MHz, DMSO) δ 14.94 (s, 1H), 7.84 (s, 1H), 7.11–7.09 (m, 1H), 6.96 (d, J = 7.6 Hz, 1H), 6.92 (t, J = 7.3 Hz, 1H), 6.89–6.86 (m, 1H), 5.14 (s, 2H), 3.73 (s, 3H). 13C NMR (126 MHz, DMSO) δ 149.3 (s), 147.5 (s), 143.2 (s), 133.6 (s), 121.5 (s), 120.6 (s), 114.1 (s), 112.3 (s), 61.6 (s), 55.4 (s). ESI-MS HRMS calculated for C10H12N3O2 [M + H]+ 206.0924, found 206.0922.
4-((2-Allylphenoxy)methyl)-1H-1,2,3-triazole (4u). White solid, 62 mg, 97%. M.p. 80–82 °C. 1H NMR (500 MHz, DMSO) δ 14.93 (s, 1H), 7.84 (s, 1H), 7.21–7.18 (m, 1H), 7.12–7.11 (m, 2H), 6.90 (t, J = 7.3 Hz, 1H), 5.96–5.88 (m, 1H), 5.19 (s, 2H), 5.01–4.97 (m, 2H), 3.29 (d, J = 6.4 Hz, 2H). 13C NMR (126 MHz, DMSO) δ 155.7 (s), 143.4 (s), 136.8 (s), 133.1 (s), 129.6 (s), 128.2 (s), 127.4 (s), 120.8 (s), 115.5 (s), 112.2 (s), 61.4 (s), 33.9 (s). ESI-MS HRMS calculated for C12H14N3O [M + H]+ 216.1131, found 216.1131.
6-((1H-1,2,3-Triazol-4-yl)methoxy)-4-methyl-2H-chromen-2-one (4v). White solid, 63 mg, 82%. M.p. 172–173 °C. 1H NMR (500 MHz, DMSO) δ 14.98 (s, 1H), 7.89 (s, 1H), 7.36–7.29 (m, 3H), 6.39 (s, 1H), 5.28 (s, 2H), 2.43 (s, 3H). 13C NMR (126 MHz, DMSO) δ 159.9 (s), 154.3 (s), 153.0 (s), 147.4 (s), 142.8 (s), 133.5 (s), 120.2 (s), 119.8 (s), 117.5 (s), 114.7 (s), 109.5 (s), 61.7 (s), 18.2 (s). ESI-MS HRMS calculated for C13H12N3O3 [M + H]+ 258.0873, found 258.0872.
(8R,9S,13S,14S,17S)-13-Methyl-17-(1H-1,2,3-triazol-4-yl)-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol (4x). White solid, 91 mg, 90%. M.p. 233–235 °C. 1H NMR (500 MHz, DMSO) δ 14.45 (s, 1H), 8.94 (s, 1H), 7.60 (s, 1H), 6.94 (d, J = 8.4 Hz, 1H), 6.46 (d, J = 8.3 Hz, 1H), 6.41 (s, 1H), 5.15 (s, 1H), 2.72–2.64 (m, 2H), 2.09–1.75 (m, 6H), 1.51–1.17 (m, 6H), 0.93 (s, 3H), 0.53–0.41 (m, 1H). 13C NMR (126 MHz, DMSO) δ 154.9 (s), 137.1 (s), 132.5 (s), 130.4 (s), 126.0 (s), 114.9 (s), 112.7(s), 81.1 (s), 50.5 (s), 47.6 (s), 46.7 (s), 43.2 (s), 37.4 (s), 32.7 (s), 29.2 (s), 27.2 (s), 26.0 (s), 23.4 (s), 14.3 (s). ESI-MS HRMS calculated for C20H26N3O2 [M + H]+ 340.2020, found 340.2020.
(E)-4,4′-(((Hex-3-ene-3,4-diylbis(4,1-phenylene))bis(oxy))bis(methylene))bis(1H-1,2,3-triazole) (4z). White solid, 121 mg, 94%. M.p. 154–156 °C. 1H NMR (500 MHz, DMSO) δ 14.96 (s, 2H), 7.88 (s, 2H), 7.13 (d, J = 8.6 Hz, 4H), 7.05 (d, J = 8.6 Hz, 4H), 5.19 (s, 4H), 2.09 (q, J = 7.3 Hz, 4H), 0.72 (t, J = 7.4 Hz, 6H). 13C NMR (126 MHz, DMSO) δ 156.6 (s), 143.2 (s), 138.0 (s), 134.5 (s), 133.4 (s), 129.4 (s), 114.3 (s), 61.0 (s), 28.0 (s), 13.2 (s). ESI-MS HRMS calculated for C24H27N6O2 [M + H]+ 431.2190, found 431.2189.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
We are grateful to the Natural Science Fund for Colleges and Universities in Jiangsu Province (Grant No. 21KJB150017), Natural Science Foundation of Yangzhou Polytechnic Institute (Grant No. 2021xjzk006), and the project of Lvyang JinFeng from Yangzhou government for their continuous encouragement towards the research and financial support.
Notes and references
- L. S. Kallander, Q. Lu, W. Chen, T. Tomaszek, G. Yang, D. Tew, T. D. Meek, G. A. Hofmann, C. K. Schulz-Pritchard, W. W. Smith, C. A. Janson, M. D. Ryan, G.-F. Zhang, X. K. O. Johanson, R. B. Kirkpatrick, T. F. Ho, P. W. Fisher, M. R. Mattern, R. K. Johnson, M. J. Hansbury, J. D. Winkler, K. W. Ward, D. F. Veber and S. K. Thompson, J. Med. Chem., 2005, 48, 5644 CrossRef CAS PubMed.
- S. A. Bakunov, S. M. Bakunova, T. Wenzler, M. Ghebru, K. A. Werbovetz, R. Brun and R. R. Tidwell, J. Med. Chem., 2010, 53, 254 CrossRef CAS PubMed.
- R. Sood, A. Donnadio, S. Giancola, A. Kreisz, D. J. Jones and S. Cavaliere, ACS Appl. Mater. Interfaces, 2016, 8, 16897 CrossRef CAS PubMed.
- D. A. Reed, D. J. Xiao, M. I. Gonzalez, L. E. Darago, Z. R. Herm, F. Grandjean and J. R. Long, J. Am. Chem. Soc., 2016, 138, 5594 CrossRef CAS PubMed.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004 CrossRef CAS PubMed.
- Q. Zheng, J. Dong and K. B. Sharpless, J. Org. Chem., 2016, 81, 11360 CrossRef CAS PubMed.
- H. L. Qin, Q. Zheng, G. A. L. Bare, P. Wu and K. B. Sharpless, Angew. Chem., Int. Ed., 2016, 55, 14155 CrossRef CAS PubMed.
- T. S.-B. Lou and M. C. Willis, Nat. Rev. Chem., 2022, 6, 146 CrossRef CAS PubMed.
- Q. Zhao, X. Ouyang, X. Wan, K. S. Gajiwala, J. C. Kath, L. H. Jones, A. L. Burlingame and J. Taunton, J. Am. Chem. Soc., 2017, 139, 680 CrossRef CAS PubMed.
- Q. Zheng, J. L. Woehl, S. Kitamura and K. B. Sharpless, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 18808 CrossRef CAS PubMed.
- M. Gehringer and S. A. Laufer, J. Med. Chem., 2018, 62, 5673 CrossRef PubMed.
- H. Wang, F. Zhou, G. Ren, Q. Zheng, H. Chen, B. Gao, L. Klivansky, Y. Liu, B. Wu, Q. Xu, J. Lu, K. B. Sharpless and P. Wu, Angew. Chem., Int. Ed., 2017, 56, 11203 CrossRef CAS PubMed.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed.
- B. Gao, L. Zhang, Q. Zheng, F. Zhou, L. M. Klivansky, J. Lu, Y. Liu, J. Dong, P. Wu and K. B. Sharpless, Nat. Chem., 2017, 9, 1083 CrossRef CAS PubMed.
- J. Dong, L. Krasnova, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2014, 53, 9466 CrossRef CAS PubMed.
- B. Moku, W.-Y. Fang, J. Leng, E. A. B. Kantchev and H.-L. Qin, ACS Catal., 2019, 9, 10477 CrossRef CAS.
- J. J. Krutak, R. D. Burpitt, W. H. Moore and J. A. Hyatt, J. Org. Chem., 1979, 44, 3847 CrossRef CAS.
- Q. Chen, P. Mayer and H. Mayr, Angew. Chem., Int. Ed., 2016, 55, 12664 CrossRef CAS PubMed.
- C. Li, Y. Zheng, K. P. Rakesh and H.-L. Qin, Chem. Commun., 2020, 56, 8075 RSC.
- H. Jangra, Q. Chen, E. Fuks, I. Zenz, P. Mayer, A. R. Ofial, H. Zipse and H. Mayr, J. Am. Chem. Soc., 2018, 140, 16758 CrossRef CAS PubMed.
- Y. A. Skalenko, T. V. Druzhenko, A. V. Denisenko, M. V. Samoilenko, O. P. Dacenko, S. A. Trofymchuk, O. O. Grygorenko, A. A. Tolmachev and P. K. Mykhailiuk, J. Org. Chem., 2018, 83, 6275 CrossRef CAS PubMed.
- R. Xu, T. Xu, M. Yang, T. Cao and S. Liao, Nat. Commun., 2019, 10, 3752 CrossRef PubMed.
- X. Zhang, W. Fang, L. Ravindar, W. Tang and H. Qin, Adv. Synth. Catal., 2020, 362, 3358 CrossRef CAS.
- N. Yang, C. Mao, H. Zhang, P. Wang, S. Li, L. Xie and S. Liao, Org. Lett., 2023, 25, 4478 CrossRef CAS PubMed.
- P. Wang, H. Zhang, M. Zhao, S. Ji, L. Lin, N. Yang, X. Nie, J. Song and S. Liao, Angew. Chem., Int. Ed., 2022, 134, e202207684 CrossRef.
- X. Zhang, B. Moku, J. Leng, K. P. Rakesh and H.-L. Qin, Eur. J. Org Chem., 2019, 1763 CrossRef CAS.
- J. Leng, W. Tang, W.-Y. Fang, C. Zhao and H.-L. Qin, Org. Lett., 2020, 22, 4316 CrossRef CAS PubMed.
- I. V. Efimov, Chem. Heterocycl. Compd., 2019, 55, 28 CrossRef CAS.
- X.-J. Quan, Z.-H. Ren, Y.-Y. Wang and Z.-H. Guan, Org. Lett., 2014, 16, 5728–5731 CrossRef CAS PubMed.
- G.-L. Wu and Q.-P. Wu, Adv. Synth. Catal., 2018, 360, 1949–1953 CrossRef CAS.
- G.-L. Wu and Q.-P. Wu, Synthesis, 2018, 50, 2768–2774 CrossRef CAS.
- H. A. Swarup, Kemparajegowda, K. Mantelingu and K. S. Rangappa, ChemistrySelect, 2018, 3, 703–708 CrossRef CAS.
- A. Kafle, S. Bhattarai and S. T. Handy, Synthesis, 2020, 52, 2337–2346 CrossRef CAS.
- W.-M. Shu, X.-F. Zhang, X.-X. Zhang, M. Li, A.-J. Wang and A.-X. Wu, J. Org. Chem., 2019, 84, 14919–14925 CrossRef CAS PubMed.
- L. Yang, Y. Wu, Y. Yang, C. Wen and J.-P. Wan, Beilstein J. Org. Chem., 2018, 14, 2348–2353 CrossRef CAS PubMed.
- P. R. Clark, G. D. Williams, J. F. Hayes and N. C. O. Tomkinson, Angew. Chem., Int. Ed., 2020, 59, 6740–6744 CrossRef CAS PubMed.
- I. V. Efimov, Chem. Heterocycl. Compd., 2019, 55, 28–30 CrossRef CAS.
- K. Qvortrup and T. E. Nielsen, Chem. Commun., 2011, 47, 3278–3280 RSC.
- J. C. Loren and K. B. Sharpless, Synthesis, 2005, 9, 1514 Search PubMed.
- Q. Hu, Y. Liu, X. Deng, Y. Li and Y. Chen, Adv. Synth. Catal., 2016, 358, 1689 CrossRef CAS.
- L. Hu, C. Mìck-Lichtenfeld, T. Wang, G. He, M. Gao and J. Zhao, Chem.–Eur. J., 2016, 22, 911 CrossRef CAS PubMed.
- X. Wang, C. Kuang and Q. Yang, Eur. J. Org Chem., 2012, 424 CrossRef.
- T. Jin, S. Kamijo and Y. Yamamoto, Eur. J. Org Chem., 2004, 3789 CrossRef CAS.
- F. Himo, T. Lovell and R. Hilgraf, et al., J. Am. Chem. Soc., 2005, 127, 210–216 CrossRef CAS PubMed.
- Y. M. Shafran, A. A. Hussein, N. A. Beliaev, V. A. Shevyrin, S. Shityakov, T. V. Beryozkina and V. A. Bakulev, ACS Omega, 2022, 7, 5008–5031 CrossRef CAS PubMed.
- W.-M. Shu, X.-F. Zhang, X.-X. Zhang, M. Li, A.-J. Wang and A.-X. Wu, J. Org. Chem., 2019, 84, 14919–14925 CrossRef CAS PubMed.
- L. S. Kallander, Q. Lu, W. Chen, T. Tomaszek, G. Yang, D. Tew, T. D. Meek, G. A. Hofmann, C. K. Schulz-Pritchard, W. W. Smith, C. A. Janson, M. D. Ryan, G.-F. Zhang, K. O. Johanson, R. B. Kirkpatrick, T. F. Ho, P. W. Fisher, M. R. Mattern, R. K. Johnson, M. J. Hansbury, J. D. Winkler, K. W. Ward, D. F. Veber and S. K. Thompson, J. Med. Chem., 2005, 48, 5644–5647 CrossRef CAS PubMed.
- J. Kalisiak, K. B. Sharpless and V. V. Fokin, Org. Lett., 2018, 10, 3171–3174 CrossRef PubMed.
- H. Cha, K. Lee and D. Y. Chi, Tetrahedron, 2017, 73, 2878–2885 CrossRef CAS.
- X.-J. Quan, Z.-H. Ren, Y.-Y. Wang and Z.-H. Guan, Org. Lett., 2014, 16, 5728–5731 CrossRef CAS PubMed.
- A. Coelho, P. Diz, O. Caamano and E. Sotelo, Adv. Synth. Catal., 2010, 352, 1179–1192 CrossRef CAS.
- Y. He, E. Sun, Y. Zhao, L. Hai and Y. Wu, Tetrahedron Lett., 2014, 55, 111–115 CrossRef CAS.
- J. Thomas, S. Jana, S. Liekensb and W. Dehaen, Chem. Commun., 2016, 52, 9236–9239 RSC.
|
| This journal is © The Royal Society of Chemistry 2024 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article a,
Jie Xua,
Yanan Lia,
Shi-Meng Wangb and
Hua-Li Qin
a,
Jie Xua,
Yanan Lia,
Shi-Meng Wangb and
Hua-Li Qin *c
*c
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 by Professor K. B. Sharpless. Based on the significance of both sulfonyl fluorides and triazoles, finding a portal to the assembly of molecules bearing both sulfonyl fluoride and triazole moieties would unquestionably increase the chance of identifying drug candidates and significantly contribute to lead compounds optimization.
5 by Professor K. B. Sharpless. Based on the significance of both sulfonyl fluorides and triazoles, finding a portal to the assembly of molecules bearing both sulfonyl fluoride and triazole moieties would unquestionably increase the chance of identifying drug candidates and significantly contribute to lead compounds optimization.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 (more details see ESI†). As illustrated in Table 1, twenty-six alkynes 2 were smoothly converted to their corresponding BTESFs 3 in good to excellent yields after Cu-catalyzed cycloaddition with azide 1. For aromatic alkynes, no matter electron-donating groups 2b, 2c or electron-withdrawing groups 2f, 2g were all compatible. Sterically hindered substrates 2l, 2m did not affect the efficiency of cycloaddition. It was worth noting that heterocyclic products 3n–p were also generated in good yields with either a nitrogen or sulfur atom. In addition, aliphatic alkynes 2q–w were smoothly transformed into their corresponding triazoles in satisfactory yields. Especially, the alkynes derived from phenols, such as 2t–w all gave corresponding triazoles in nearly quantitative yields. Natural products ethynyl estradiol 2x and norethindrone 2y were applicable to be modified by the cycloaddition with 1 and furnished corresponding BTESFs 3x and 3y in 91% and 82% yields respectively. Interestingly, when 2.0 equivalents of azide 1 was used, ditriazole substituted BTESF 3z was generated in 88% yield after the reaction with the derivative of diethylstilbestrol 2z.
27 (more details see ESI†). As illustrated in Table 1, twenty-six alkynes 2 were smoothly converted to their corresponding BTESFs 3 in good to excellent yields after Cu-catalyzed cycloaddition with azide 1. For aromatic alkynes, no matter electron-donating groups 2b, 2c or electron-withdrawing groups 2f, 2g were all compatible. Sterically hindered substrates 2l, 2m did not affect the efficiency of cycloaddition. It was worth noting that heterocyclic products 3n–p were also generated in good yields with either a nitrogen or sulfur atom. In addition, aliphatic alkynes 2q–w were smoothly transformed into their corresponding triazoles in satisfactory yields. Especially, the alkynes derived from phenols, such as 2t–w all gave corresponding triazoles in nearly quantitative yields. Natural products ethynyl estradiol 2x and norethindrone 2y were applicable to be modified by the cycloaddition with 1 and furnished corresponding BTESFs 3x and 3y in 91% and 82% yields respectively. Interestingly, when 2.0 equivalents of azide 1 was used, ditriazole substituted BTESF 3z was generated in 88% yield after the reaction with the derivative of diethylstilbestrol 2z.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (v/v)].
50 (v/v)].![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to afford desired product 3.
2 to afford desired product 3.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to pure dichloromethane to afford desired product 4.
2 to pure dichloromethane to afford desired product 4.







