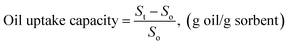Open Access Article
Open Access ArticlePreparation of bio-based porous material with high oil adsorption capacity from bio-polyurethane and sugarcane bagasse
Thai Dinh Cuong,
Le Quang Dien and
Phan Huy Hoang *
*
School of Chemistry & Life Sciences, Hanoi University of Science & Technology, No.1, Dai Co Viet Street, Hanoi, Vietnam. E-mail: hoang.phanhuy@hust.edu.vn
First published on 26th February 2024
Abstract
This work presents the fabrication of bio-based porous material for highly efficient removing of oil from oil/water system. The sunflower oil-based polyol was synthesized and then used to replace the petro-polyol in the simultaneous preparation of a sugarcane bagasse-polyurethane composite (SC-PU composite) by inserting sugarcane fiber filler into the PU matrix. The bio-polyol was obtained from sunflower oil with a hydroxyl number of 182 mg KOH g−1, and functionality of 3.5 OH groups per mol. The bio-polyol and the newly designed bio-based SC-PU composite were characterized by NMR, FT-IR and SEM analysis. The effect of several parameters such as bio-polyol/petro-polyol ratio, dosage of adding sugarcane fiber and size of filler particles on oil adsorption capacity of a new sorbent material were also investigated. Oil sorption capacity of the newly designed sorbent was relatively high, up to 15.2 g g−1 when 20% sugarcane bagasse with a particle size of 1 mm was added into the bio-polyurethane matrix. This is nearly four times higher than that of neat PU foam without the biomass filler and lignocellulosic materials. This finding demonstrated the importance of selecting the right components to fabricate a cost-effective, highly renewable and biodegradable sorbent with high oil–water separation efficiency, reducing the use of chemicals from fossil sources.
Introduction
Annually, there occurs many accidents in transportation, storage, and utilization of oil. The transportation and utilization of oil could increase the risk of oil spill that would cause environmental damage. Moreover, the pollution by petro-oils seriously affects sea life, water environments, and the economy because of the properties of oils.1,2 The adsorbents have been proved to have a high capacity for oil uptake to reduce the costs for oil spill treatment and minimize dangerous effects on ecosystems.3–6 Suitable absorbent materials for largescale oil spill handling should present a high oil absorption capacity and selectivity, and at the same time, should be cost-effective. According to these requirements, among many materials that have been developed to effectively absorb oil, polyurethane (PU) foams are the most promising adsorbent materials.6–8 Many works have reported that the oil absorption capacity and oil-water selectivity of PU foams are increased by using chemical methods to change their surface and/or structure properties. The parameters of the morphological structure of the foam are evaluated as an important intrinsic factor that can affect the oil absorption efficiency, which has important implications for the optimal design of the efficient absorbent.4,9,10 The structure of the PU foam could be adjusted by using different amount of bio-polyol for replacing petrochemical oil. There have been some literature that focus on the bio-polyurethane with tuneable structure by partially replacing petroleum-based polyols with plant-based bio-polyols.10,11However, a major disadvantage of synthetic sorbents is non-biodegradable, they degrade very slowly in comparison with natural adsorbents. Thus using these synthetic materials could induce the secondary pollution for environment. The natural oil-sorption materials have several advantages of biodegradability, economy, technical feasibility, and environmental acceptability for cleanup of oil spill.5,12–14 However, such materials have been observed to show the disadvantages of poor buoyancy properties, low hydrophobicity and relatively low oil sorption capacity compare to synthetic sorbents.5 Therefore, it is very important to develop a newly designed sorbent that possesses good properties such as high oil sorption capacity, biodegradability, environmentally friendly and economy. There have been several studies focusing on partial replacement of synthetic PU with natural adsorbent to create a new adsorbent that combines the advantages of both.1,15–17 The new potential adsorption materials can be beneficial for oil spill cleanup by improving the efficiency of oil sorption and by incorporating the advantages of the two materials including biodegradability, economy of natural sorbents and hydrophobicity, oleophilicity of synthetic materials.
Nevertheless, there have been no studies that combine the use of bio-polyurethane and natural adsorbents from lignocellulosic biomass sources to fabricate bio-based adsorbents to take advantage of the superior properties of these adsorption materials. Furthermore, polyols have a profound influence on the processing and characterization properties of the finished PU and the vegetable oil polyols confer the good hydrophobicity and lipophilicity of PU foam.10,18,19 Hence, applying vegetable oil polyol for preparation of bio-based PU foaming, and simultaneously combining with sugarcane bagasse filler to fabricate new designed oil-absorbing porous materials is a new and very potential research direction. Beside, the use of bagasse fiber from plant sources and use as filler in bio-based fiber-polyurethane porous adsorbent will make the overall composite more biodegradable and renewable. Moreover, the utilization of sugarcane bagasse for fabrication of oil sorbent is an effective solution to achieve two main goals: treatment of huge amount of agricultural residue in Vietnam and creation of an efficient adsorption biomaterial for oil spill treatment.
Therefore, this current work presents the fabrication of bio-based porous sorbent by combination of bio-based polyurethane and a natural sorbent-sugarcane bagasse (SC), which is the abundant agriculture residue in Vietnam. Bio-based PU was prepared from sunflower oil-based polyol that was synthesized from sunflower oil via controlled hydroxylation of vegetable oils in one conversion step using a mixture of hydrogen peroxide and formic acid. The as-synthesized bio-polyol was then used to replace fossil-based polyol in synthesis of bio-based polyurethane. Bagasse fiber was added simultaneously in to the bio-polyol mixture during preparation process to obtain porous SC fiber-polyurethane composite material with high oil adsorption capacity. The new designed bio-sorbent showed high oil uptake capacity when adsorbing oil from the water surface.
Materials and methods
Materials
Hydrogen peroxide (H2O2, 30%), acid formic (HCOOH, 99%), sodium carbonate (Na2CO3, ≥99.5%), acetone (99.5%), Wijs solution, potassium iodide (KI, 99.0%), cyclohexan (99.5%), glacial acetic acid (99.5%), 0.1 N sodium thiosulfate (Na2S2O3) standard solution, diphenylmethane diisocyanate (p-MDI), silicone L-580 and triethylamin N(C2H5)3 (99.5%) were purchased from Sigma and all chemicals were used as received. Starch (99.9%) and sunflower oil were supplied from Vietnam.Synthesis of bio-polyol from sunflower oil
The bio-polyol was synthesized by hydroxylation of sunflower oil in one conversion step using a mixture of hydrogen peroxide and formic acid followed the method reported in literature.20 Sunflower oil sample, HCOOH acid catalyst with specified ratio was added to the conical flask, and mixed well by magnetic stirrer. H2O2 solution with certain volume was slowly added to the above conical flask, stirred until obtaining homogeneous solution. Next, closed the conical flask was heated to the required temperature by heated magnetic stirrer. After the reaction time, the separating funnel was used to obtain the bio-polyol product. In particular, two layer of reaction mixture was separated by funnel and the upper layer was washed 2–3 times with a solvent consisting of 10% Na2CO3 solution and acetone (ratio 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 in volume). The liquid layer that contained bio-polyol product was then washed with a washing solution of water and acetone until the pH of 7. At the end of the washing stage, most of the acetone and water were separated by a separating funnel. Then, rotary distillation apparatus was used to remove the remaining acetone and water in the product. The final product was completely dried with anhydrous Na2SO4 and anhydrous silica in a desiccator for 24 h. The hydroxylation process was repeated twice, and the variance was recorded.
40 in volume). The liquid layer that contained bio-polyol product was then washed with a washing solution of water and acetone until the pH of 7. At the end of the washing stage, most of the acetone and water were separated by a separating funnel. Then, rotary distillation apparatus was used to remove the remaining acetone and water in the product. The final product was completely dried with anhydrous Na2SO4 and anhydrous silica in a desiccator for 24 h. The hydroxylation process was repeated twice, and the variance was recorded.
Preparation of bio-based porous sorbent from polyurethane and sugarcane bagasse fiber
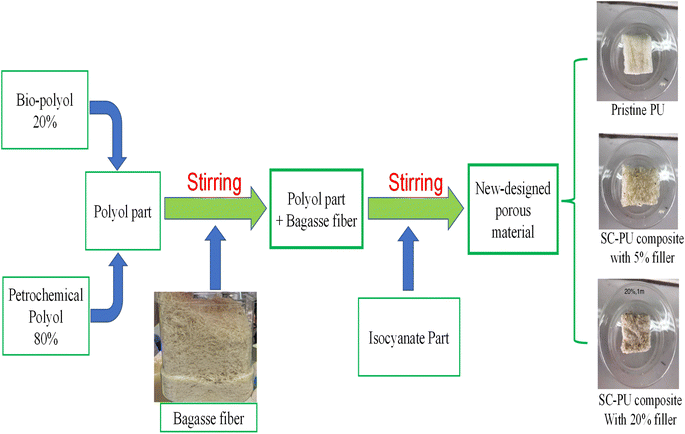
The sample of sugarcane bagasse (SC) was collected from Thanh Hoa province (in the North of Vietnam). The bagasse was cut, ground and sieved to select samples in different sieve sizes: 1 mm; 2 mm; 4 mm. Then, these sugarcane bagasse samples were treated with hot water at 170 °C in 2 h (pressure of approximate 7.9 bar) before using as filler for preparation of SC-PU composite.Process diagram for fabrication of bagasse fiber-PU composite was shown in Fig. 1. Bio-polyols from sunflower oil (20 wt%) was mixed with petrochemical polyol (80 wt%). This mixture was added with sugarcane bagasse fiber and stirred. The bio-based composite was prepared with adding of bagasse fiber (5, 10, 15, 20 and 25 wt%) and three different particle sizes (1, 2 and 4 mm). To this mixture isocyanate part was added and stirred well for about 10–20 seconds. Next this mixture was poured into a mold cup for foam formation. At the end of the curing reaction, the composite was removed from the mold and washed with water and cut into small piece for further experiments. Fig. 1 showed the components and the expanded PU foams, indicating the visible difference between pristine PU and the SC-PU composites with 5 and 20% filler.
Characterizations
Iodine index was determined according to ISO 3961:2013 by Wijs method with 0.1 N Na2S2O3 standard solution, KI and starch as indicator. The content of hydroxyl groups was determined according to AOAC 965:32 standard based on the acetylation reaction of free hydroxyl radicals of polyol and acetic anhydride in pyridine solvent. After the reaction, the remaining unreacted acetic anhydride was converted to acetic acid and titrated with KOH. Nuclear magnetic resonance (NMR) analysis was performed on a 600 MHZ Bruker Avance 500 high-resolution magnetic resonance spectrometer (USA). Fourier transform infrared spectroscopy (FT-IR, Alpha, 200619) was carried out for sunflower oil, oil-based polyol and SC-PU porous material. Morphology studies were carried out using Scanning electron microscopy (SEM) was performed using a JSM-7000F, JEOL, Japan.The method to determine the oil adsorption capacity of the SC-PU composite was based on the ASTM F726-99 Standard Test Method for Sorbent Performance of Adsorbents for use on Crude Oil and Related Spills.6 To determine the oil adsorption coefficient, the adsorbent was placed on the Diesel oil (DO)/water surface. In particular, 80 ml of water was poured into a beaker, then added 20 ml of Diesel oil. Immerse the adsorbent, which has been weighed, in the beaker containing the oil/water for different periods of time. Next, the adsorbent was removed from the liquid surface and vacuum filtrated to remove free water on the surface for about 2 minutes. Then the adsorbent was weighed to calculate the adsorption capacity as following equation:
The oil adsorption test was repeated twice, and the variance was recorded.
Results & discussion
Synthesis of bio-polyol from sunflower oil
Based on the results reported in the literature,20,21 the suitable reaction conditions for the synthesis of polyols from sunflower oil by oxidizing the double bond in the oil using hydroperoxide and formic acid are chosen as following: reaction temperature: 50 °C; reaction time: 5 hours; molar ratio of H2O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oil of 5
oil of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; molar ratio of acid
1; molar ratio of acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oil of 16
oil of 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. At the above conditions, the efficiency of bio-polyol synthesis reached about 90%, with a hydroxyl number of product OH# ≈ 182 mgKOH g−1 and function f ≈ 3.5 OH groups per mol. The 1H-NMR spectrum of sunflower oil and as-obtained polyol was shown in Fig. 1.
1. At the above conditions, the efficiency of bio-polyol synthesis reached about 90%, with a hydroxyl number of product OH# ≈ 182 mgKOH g−1 and function f ≈ 3.5 OH groups per mol. The 1H-NMR spectrum of sunflower oil and as-obtained polyol was shown in Fig. 1.
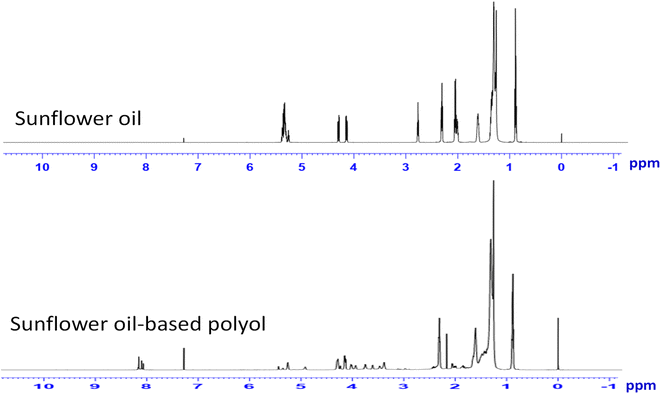
NMR spectrum of sunflower oil (Fig. 2A) showed the characteristic of oliphinic proton in the vinyl group (–HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–) at δ 5.2–5.4 ppm; divinyl methylene proton (
CH–) at δ 5.2–5.4 ppm; divinyl methylene proton (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH2–CH
CH–CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ) at δ 2.7–2.8 ppm, and allyl methylene proton (–CH2–CH
) at δ 2.7–2.8 ppm, and allyl methylene proton (–CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH2–) at δ 2 ppm.20,22 Other peaks were also observed such as methinic proton (in the glyceryl group) at the region δ 4.9–5.2 ppm; methylene protons (in the glyceryl group) at the δ 4.1–4.3 ppm region, α-methylene protons attached to the carbonyl group at the δ 2.3 ppm region; β-methylene protons calculated from the carbonyl group at δ ∼1.6 ppm.
CH–CH2–) at δ 2 ppm.20,22 Other peaks were also observed such as methinic proton (in the glyceryl group) at the region δ 4.9–5.2 ppm; methylene protons (in the glyceryl group) at the δ 4.1–4.3 ppm region, α-methylene protons attached to the carbonyl group at the δ 2.3 ppm region; β-methylene protons calculated from the carbonyl group at δ ∼1.6 ppm.
The as-synthesized bio-polyol obtained from vegetable oil involves the formation of a hydroxyl group attached to the C of original double bond in oil. In this study, the presence of hydroxyl group in the structure of the as-synthesized polyol was confirmed by nuclear magnetic resonance spectroscopy analysis of the product samples compared with the original sunflower oil control sample. In can be seen from the 1H-NMR spectrum of as-synthesized polyols that the conversion of double bonds into epoxy groups and then into hydroxyl groups can be confirmed by Fig. 2B where the presence of new peaks δ 3.4–4.2 ppm, corresponding to the signature of methinic protons bound to hydroxyl and formiate (HC–OH) groups and epoxy group (–CH(O)CH) at δ 2.8–3.2 ppm were observed. The characteristic unsaturated binding regions correspond to the proton oliphinic peaks of the double bond at δ 5.2–5.4 ppm, the proton divinyl methylene proton at δ 2.7–2, 8 ppm and allyl methylene protons (δ 2 ppm) were observed to decline sharply in intensity or almost disappear.
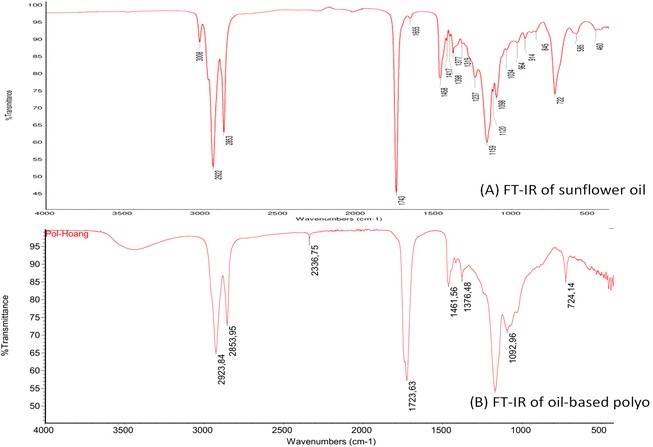
In the spectra of sunflower oil (Fig. 3A), the bands for triglycerides such as –CH2 stretching of aliphatic hydrocarbons at 2923, 2853 cm−1 and carbonyl ester groups at 1723 cm−1 were observed.23,24 The peak at 3008 cm−1 corresponding to ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH group of double bonds of sunflower oil was also observed.25 In the FTIR spectra of the sunflower oil-based bio-polyol (Fig. 3B), it can be seen that the bands for triglycerides still remain while the new band at 3496 cm−1 corresponding to –OH (broad stretching) which indicates the formation of polyol from the vegetable oil was observed. Moreover, the disappearance of band at 3008 cm−1 corresponding to
CH group of double bonds of sunflower oil was also observed.25 In the FTIR spectra of the sunflower oil-based bio-polyol (Fig. 3B), it can be seen that the bands for triglycerides still remain while the new band at 3496 cm−1 corresponding to –OH (broad stretching) which indicates the formation of polyol from the vegetable oil was observed. Moreover, the disappearance of band at 3008 cm−1 corresponding to ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH group of double bonds of oil indicates the conversion of double bond into hydroxyl groups.
CH group of double bonds of oil indicates the conversion of double bond into hydroxyl groups.
It is known that the difference in the double bonds in the fatty acid chains resulting in the different possible of –OH groups which will be introduced into the oils after its conversion to polyol. In this work, the original sunflower oil was an unsaturated oil with a high iodine value (IV) about 120.6 gI2/100 g. Meanwhile, the iodine value of the bio-polyol from sunflower oil determined by chemical titration according to the Wijs method was almost zero. The hydroxyl number of starting sunflower oil was about 8 mg KOH g−1, after the reaction hydroxyl number of oil-based polyol increased sharply to about 182.6 mg KOH g−1. The significantly increase in the hydroxyl number and the sharply decrease in the iodine value of the oil-based polyol product compared with the original oil sample confirmed again the conversion of the double bond and the formation of the hydroxyl group in bio-polyol. Thus, bio-polyol was successfully synthesized by hydroxylation of sunflower oil in a conversion step using a mixture of hydrogen peroxide (H2O2) and formic acid (HCOOH).
Under the identified reaction conditions, the hydroxyl number of oil-based bio-polyol was lower than theoretical result, about ≈182 mg KOH g−1. The hydroxyl number of bio-polyol in this work was not really high due to incomplete epoxidation and hydroxylation.26 It can be explained that the epoxy ring opening reaction needs to be catalyzed by strong acids (e.g., sulfuric acid or p-toluene sulfonic acid). When weakly organic acids such as HCOOH are used, the carboxylic acid addition reaction that opens the epoxy ring to form hydroxyalkyl structures preferentially takes place.20 Moreover, besides the ring-opening reaction with formic acid, a number of other side reactions can also occur such as: esterification of newly formed hydroxyls with excess formic acid leads to the loss of hydroxyl groups and/or the reaction of the hydroxyl group in the product with the epoxy ring under the H+ catalyst to form the dimer or trimer.
Fabrication of fiber-polyurethane bio-composite for oils adsorption
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 2500–2270 cm−1 disappeared confirming that the complete consumption of isocyanate compound and formation of polyurethane.25,27
O at 2500–2270 cm−1 disappeared confirming that the complete consumption of isocyanate compound and formation of polyurethane.25,27
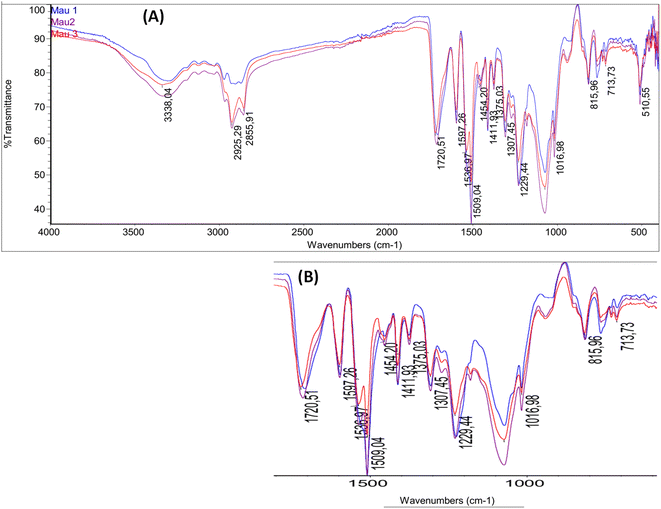 | ||
| Fig. 4 (A) FT-IR spectrum of neat PU (blue line) and SC-PU composite with 10% sugarcane bagasse (red line) and 20% sugarcane bagasse (purple line); (B) magnification of the band from 1720–700 cm−1. | ||
The important characteristic features of polyurethane were observed from FT-IR spectrum of all samples in Fig. 4. The presence of bands of –NH at 3340 cm−1 can be attributed to the urethane group stretching, the band of 1720 cm−1 can be attributed to the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations from urethane groups, 1536 cm−1 can be attributed to the –NH bending, 1230 cm−1 can be attributed to the –C–N stretching and 1105 cm−1 for –C–O stretching in the urethane group (–NHCOO–). Thus, the formation of urethane linkage in the polyurethane is confirmed. Since some characteristic features of lignocellulose biomass are same as polyurethane, it was not possible to separate specific peaks of sugarcane lignocellulose.25,28 Moreover, in the FT-IR spectrum of SC-PU composite the band at 2850–2937 cm−1 for –CH2 stretching vibrations of lignocellulose material or vegetable oil-based polyol was also observed to be stronger than that in neat PU (only come from –CH2 vibration of vegetable oil-based polyol).
O stretching vibrations from urethane groups, 1536 cm−1 can be attributed to the –NH bending, 1230 cm−1 can be attributed to the –C–N stretching and 1105 cm−1 for –C–O stretching in the urethane group (–NHCOO–). Thus, the formation of urethane linkage in the polyurethane is confirmed. Since some characteristic features of lignocellulose biomass are same as polyurethane, it was not possible to separate specific peaks of sugarcane lignocellulose.25,28 Moreover, in the FT-IR spectrum of SC-PU composite the band at 2850–2937 cm−1 for –CH2 stretching vibrations of lignocellulose material or vegetable oil-based polyol was also observed to be stronger than that in neat PU (only come from –CH2 vibration of vegetable oil-based polyol).
SEM images of neat PU and its composites were shown in Fig. 5. SEM image of the virgin PU showed the existence of mixture of elongated and spherical shaped pores. The morphologies of treated sugarcane bagasse filled PU showed the increase in the porosity, which was composed of many pores and fissures.
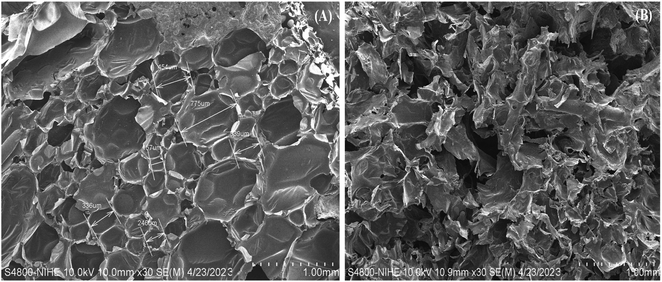 | ||
| Fig. 5 SEM image of virgin polyurethane (A) and biomass-PU composite with 20% sugarcane bagasse filler (B). | ||
It is obviously seen that biomass-PU composite had an half-open cell structure compare to closed-cell structure of virgin PU foam. The addition of fillers to polyurethane foam formulations has a direct influence on the size and shape of their cells of the resulting composites and the appearance of pores in their three-dimensional structure during the in situ polymerization.6 All those properties, in turn, can also alter the sorption capacity of the composite foam in relation to the blank foam (without filler). The pores and capillaries of composite were formed uniformly with pore size of a few hundred micrometers composed the hierarchical roughness required for a high oil sorption capacity. This result can be related to the further expansion of the polymer matrix mass due to the addition of the filler, which changed the structure of the system. These increased open structures are expected to increase the adsorption capacity of the PU composites.23
Oil adsorption studies
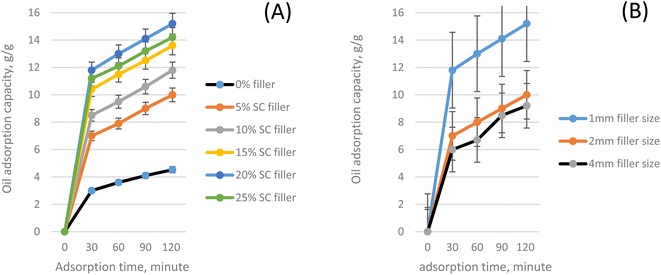
In previous studies, biomass was added to the polyurethane foam network to enhance the oil separation ability of the composite.6,15,29 In this current work, sugarcane bagasse was used as filler to fabricate SC-PU composite with the aim to improve biodegradability and oil adsorption capabilities of sorbent. The chemical composition of sugarcane bagasse fiber was found to be high cellulose content of about 43.2%, 27.8% pentozan and 18.4% lignin. The oil adsorption capacity of sugarcane bagasse (SC) fiber is not high, about 3.1 g g−1 due to the high hydrophilicity and low hydrophobicity of fiber. After treatment with hot water at 170 °C in 2 h, oil adsorption capacity of SC fiber increased to 4.2 g g−1. In the process of hydrothermal treatment, extractives and hemicelluloses were hydrolyzed and depolymerized at elevated temperature, and the hydroxyl groups in the lignocellulose material decreased leading to the deconstruction of lignocellulosic structure.30,31 Thus, the Lignin-Hemi-Cellulose network structure of the sugarcane bagasse was partially deconstructed after treatment by hot water, leading to the increase of the surface area and pore volume of the material, the adsorption ability thus would increase.29 The oil adsorption ability of SC-PU foam were then investigated to find out the optimum conditions.Fig. 6 showed the adsorption results of samples after 120 minutes of submerging the samples in oil/water mixture. It can be seen that adding sugarcane fiber to the bio-based polyurethance would increase the oil adsorption capacity of the SC-PU porous material significantly. It can be explained that: by adding biomass fibers such as sugarcane bagasse to the PU foam during formation process, the cell wall of foam become less stable, the membrane is broken easily leading to the “open cell” structure of the SC-PU composite. The highly open cell content helps the oil, when adsorbed, move easily between the cells into the foam instead of being hindered by cell wall as before. Furthermore, sugarcane bagasse itself is also a lignocellulose fiber with a light porous structure, which has been proven to have a relatively high oil adsorption capacity. When forming foam, lignocellulose fiber can adsorb the oil as well as act as a bridge and/or conduit to allow oil to penetrate deeper into the structure of the foam, leading to the increase of oil absorption capacity of SC-PU foam.20
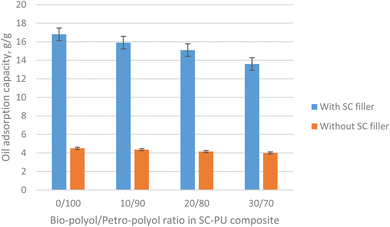 | ||
| Fig. 6 Oil adsorption capacity of SC-PU composite with different bio-polyol/petro-polyol ratio - with 20% SC filler with size of 1 mm (blue column) and without SC filler (orange column). | ||
A 120 minute oil adsorption test of the SC-PU porous samples also showed that as the amount of bio-polyol (that used to replace petrochemical polyol component) increased, the oil adsorption capacity of the SC-PU foam decreased in the both cases of with or without SC filler. It can be attributed to the increase of density of the PU foam (thus decrease of porosity) when bio-polyol/petro-polyol ratio increased (data not shown), leading to lower oil adsorption capacity. Moreover, high dosage of bio-polyol resulted in the high density of the PU material leading to a heavier and low buoyancy material, which can be a hindering factor when dealing with oil spills. Hence, to ensure porous formation to obtain porous materials with high oil adsorption capacity and to ensure high renewable bio-based content in material, the dosage of bio-polyol used to replace petro-polyol in forming PU foam was selected at 20% (bio-polyol/petro-polyol ratio of 20/80). This result is consistent with the conclusions in previous report.19
The influence of SC filler dosage on oil adsorption capacity of SC-PU composite was estimated and shown in Fig. 7A. It is seen that the amount of filler affects significantly on the oil adsorption ability of the new designed adsorbent. The oil adsorption capacity increased with the increase of filler dosage (from 5 to 20% filler). However, when the amount of sugarcane fiber increased to a 25%, the oil absorption capacity of SC-PU material decreased slightly. The adsorption capacity of the SC-PU sorbent reached its highest values of 15.2 g g−1 after 120 min. contact time when 20% of SC filler was used. This phenomenon can be attributed to by the fact that the more rice straw used, the higher open-cell structure of sugarcane bagasse-polyurethane foam with higher porosity, thus more possibilities for oil to be adsorbed into the sorbent. The open pores are essential to provide oil storage space that enhances the oil absorption, and the small pore sizes can reduce the foams' mass transfer resistance, which accelerates the oil absorption rate.4,6 It is known that increased filling of the porous structure can be achieved when two main conditions are simultaneously accomplished: the porous structure should be interconnected (because unconnected voids are inaccessible for the oil storage), and there should be a factor which drives the oil into the porous structure.4,15 The oil is driven through the pores, and subsequently retained in the void, in the presence of a capillary pressure. Thus, surface properties and structural parameters such as the pore size and their connectivity are significant parameters that influence the ability of a porous sorbent to adsorb the oil. J. Pinto et al.4 has found that there is a clear effect of the pore size on the oil adsorption efficiency. The average oil adsorption efficiency increases with the decrease of the pores size, reaching maximum and reproducible values for pore sizes less than several of μm, where the capillary pressure in each pore is strong enough to completely fill the porous structure of the sorbent with oil and retain it after extraction. Moreover, the highly porous materials with lower pore sizes increase the surface area, promote more interactions, leading to high oil adsorption efficiency.32 Furthermore, the buoyancy of the new porous composite increased as the increase of the content of filler providing a higher ability to contact oil for easy adsorption. However, at a filler ratio of 25%, the oil adsorption capacity of the material began to decrease. Because using higher amount of filler leading to reduction of polyurethane matrix resulted in a decrease of adsorption capacity of the sorbent. At high filler loading, the structure of SC-PU composite was broken easily leading to the lost of the pore and channel inside sorbent and then leading to lower adsorption efficiency. Therefore, 20% of sugarcane bagasse filler was selected for further investigations.
SC filler was set at 20% as mentioned earlier, different filler sizes of 1, 2, and 4 mm were used to investigate the effect of sugarcane fiber size on adsorption capacity of the SC-PU composite sorbent. Influence of fiber size on oil adsorption capacity was shown in Fig. 7B. Similar to filler dosage, sugarcane fiber size was also an important factor influencing the adsorption capacity of the SC-PU composite. As seen from Fig. 7B, increasing the sugarcane fiber size resulted in decreasing adsorption capacity of SC-PU composite. The oil adsorption capacity of the sorbent reached to highest value at the SC filler size of 1 mm and decreased when increasing the size of filler. This phenomenon may be associated with the molecular restructuring in the interface foam-oil for the reinstatement of equilibrium, leading to increased oil adsorption.6 The decrease in sugar fiber size would increase its surface area and thus induced the increase of total surface area and pore volume of the SC-PU composite.
Moreover, smaller sizes provided higher number of SC filler particles, which led to higher number of channel/pores and thus higher porosity of the SC-PU sorbent material. It is known that the surface area and porosity show considerable effects on oil adsorption capacity of sorbent materials. A material with higher porosity presents more sites for oil interaction, increasing the oil removal efficiency of material. Therefore, by inserting SC fiber filler the filling of all the voids by oil and the retaining of the oil inside the porous sorbent was then enhanced. This explains why when the fiber was filled with the right size, the porous material has a higher oil absorption than the original PU material. Based on these results, SC filler with size of 1 mm was, hence, selected as the suitable size of the filler for fabrication of the SC-PU porous composite.
From aforementioned results, the suitable condition for fabrication of SC-PU sorbent was chosen as bio-polyol/petro-polyol ratio of 20/80; 20% sugarcane bagasse filler with size of 1 mm. At these conditions, oil adsorption capacity of the as-prepared SC-PU sorbent was relatively high, up to 15.2 g g−1, and was comparable and/or higher than that of Azolla plant,3 several vegetable fibers,14 polypropylene,2 and porous polystyrene.33 This adsorption capacity even was improved over three times compared with treated sugarcane fiber or neat polyurethane without being incorporated in polyurethane matrix.
Conclusion
In conclusion, an efficient sugarcane bagasse-polyurethane sorbent with high oil adsorption capacity has been fabricated from bio-polyurethane foam and sugarcane bagasse fiber. The bio-based SC-PU porous composite was fabricated by adding sugarcane fiber filler in to bio-PU matrix, that was obtained by replacing the 20% petro-polyol with as-synthesized bio-polyol. The bio-polyol was synthesized by controlled hydroxylation in one conversion step of sunflower oil using a mixture of hydrogen peroxide and formic acid. The use of the as-obtained sorbent for treatment oil was investigated in various conditions. Oil sorption capacity of the newly designed sorbent was relatively high, up to 15.2 g g−1 when 20% sugarcane bagasse with particle size of 1 mm was added into bio-polyurethane matrix. This is nearly four times higher than that of neat PU foam without biomass filler and lignocellulosic materials. Several advantages of the new sorbent obtained from polyurethane and bagasse such as low cost, eco-friendly, highly biodegradability and ready availability in Vietnam made it an efficient sorbent material for oil removal. This opens a new route for oil treatment in large quantities of pilot scale that required to studied in next experiment.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research is funded by the Hanoi University of Science and Technology (HUST) under project number T2023-PC-105.References
- P. H. Hoang, A. T. Hoang, N. H. Chung, L. Q. Dien, X. P. Nguyen and X. D. Pham, The Efficient Lignocellulose-Based Sorbent for Oil Spill Treatment from Polyurethane and Agricultural Residue of Vietnam, Energy Sources, Part A, 2018, 40, 312–319, DOI:10.1080/15567036.2017.1415397.
- Q. F. Wei, R. R. Mather, A. F. Fotheringham and R. D. Yang, Evaluation of Nonwoven Polypropylene Oil Sorbents in Marine Oil-Spill Recovery, Mar. Pollut. Bull., 2003, 46, 780–783, DOI:10.1016/S0025-326X(03)00042-0.
- J. Sayyad Amin, M. Vared Abkenar and S. Zendehboudi, Natural Sorbent for Oil Spill Cleanup from Water Surface: Environmental Implication, Ind. Eng. Chem. Res., 2015, 54, 10615–10621, DOI:10.1021/acs.iecr.5b01715.
- J. Pinto, A. Athanassiou and D. Fragouli, Effect of the Porous Structure of Polymer Foams on the Remediation of Oil Spills, J. Phys. D: Appl. Phys., 2016, 49, 145601, DOI:10.1088/0022-3727/49/14/145601.
- M. O. Adebajo, R. L. Frost, J. T. Kloprogge, O. Carmody and S. Kokot, Porous Materials for Oil Spill Cleanup: A Review of Synthesis and Absorbing Properties, J. Porous Mater., 2003, 10, 159–170, DOI:10.1023/A:1027484117065.
- O. S. H. Santos, M. Coelho da Silva, V. R. Silva, W. N. Mussel and M. I. Yoshida, Polyurethane Foam Impregnated with Lignin as a Filler for the Removal of Crude Oil from Contaminated Water, J. Hazard. Mater., 2017, 324, 406–413, DOI:10.1016/j.jhazmat.2016.11.004.
- Q. Zhu, Y. Chu, Z. Wang, N. Chen, L. Lin, F. Liu and Q. Pan, Robust Superhydrophobic Polyurethane Sponge as a Highly Reusable Oil-Absorption Material, J. Mater. Chem. A, 2013, 1, 5386–5393, 10.1039/c3ta00125c.
- B. Li, X. Liu, X. Zhang, J. Zou, W. Chai and J. Xu, Oil-Absorbent Polyurethane Sponge Coated with KH-570-Modified Graphene, J. Appl. Polym. Sci., 2015, 132, 1–7, DOI:10.1002/app.41821.
- N. E. Marcovich, M. Kurańska, A. Prociak, E. Malewska and K. Kulpa, Open Cell Semi-Rigid Polyurethane Foams Synthesized Using Palm Oil-Based Bio-Polyol, Ind. Crops Prod., 2017, 102, 88–96, DOI:10.1016/j.indcrop.2017.03.025.
- Z. S. Petrović, W. Zhang and I. Javni, Structure and Properties of Polyurethanes Prepared from Triglyceride Polyols by Ozonolysis, Biomacromolecules, 2005, 6, 713–719, DOI:10.1021/bm049451s.
- P. Alagi, Y. J. Choi and S. C. Hong, Preparation of Vegetable Oil-Based Polyols with Controlled Hydroxyl Functionalities for Thermoplastic Polyurethane, Eur. Polym. J., 2016, 78, 46–60, DOI:10.1016/j.eurpolymj.2016.03.003.
- D. Angelova, I. Uzunov, S. Uzunova, A. Gigova and L. Minchev, Kinetics of Oil and Oil Products Adsorption by Carbonized Rice Husks, Chem. Eng. J., 2011, 172, 306–311, DOI:10.1016/j.cej.2011.05.114.
- V. Singh, R. J. Kendall, K. Hake and S. Ramkumar, Crude Oil Sorption by Raw Cotton, Ind. Eng. Chem. Res., 2013, 52, 6277–6281, DOI:10.1021/ie4005942.
- T. R. Annunciado, T. H. D. Sydenstricker and S. C. Amico, Experimental Investigation of Various Vegetable Fibers as Sorbent Materials for Oil Spills, Mar. Pollut. Bull., 2005, 50, 1340–1346, DOI:10.1016/j.marpolbul.2005.04.043.
- L. S. Martins, N. C. Zanini, L. S. Maia, A. G. Souza, R. F. S. Barbosa, D. S. Rosa and D. R. Mulinari, Crude Oil and S500 Diesel Removal from Seawater by Polyurethane Composites Reinforced with Palm Fiber Residues, Chemosphere, 2021, 267, 129288, DOI:10.1016/j.chemosphere.2020.129288.
- L. S. Maia, N. C. Zanini, A. M. Claro, N. C. do Amaral, H. S. Barud and D. R. Mulinari, Eco-Friendly Foams of Castor Oil Based-Polyurethane with Artemisia Residue Fillers for Discarded Vegetable Oil Sorption, J. Appl. Polym. Sci., 2021, 138, 1–16, DOI:10.1002/app.51259.
- M. Gandara, D. R. Mulinari, F. M. Monticeli and M. R. Capri, Sugarcane Bagasse Fibers Reinforced in Polyurethane for Sorption of Vegetal Oil, J. Nat. Fibers, 2021, 18, 1983–1994, DOI:10.1080/15440478.2019.1710653.
- L. Maisonneuve, G. Chollet, E. Grau and H. Cramail, Vegetable Oils: A Source of Polyols for Polyurethane Materials, OCL: Oilseeds Fats, Crops Lipids, 2016, 23, D508, DOI:10.1051/ocl/2016031.
- A. P. Piotr Rojek, Effect of Different Rapeseed-Oil-Based Polyols on Mechanical Properties of Flexible Polyurethane Foams Piotr, J. Appl. Polym. Sci., 2012, 125, 2936–2945, DOI:10.1002/app.
- P. H. Hoang, N. M. Dat and B. Q. Hung, One-Pot Synthesis of Bio-Polyol from Vegetable Oil for Preparation of Bio-Polyurethane Based Porous Sorbent with High Oil Adsorption Capacity, J. Appl. Polym. Sci., 2023, 1–9, DOI:10.1002/app.54332.
- R. De Vasconcelos Vieira Lopes, N. P. D. Loureiro, A. P. T. Pezzin, A. C. M. Gomes, I. S. Resck and M. J. A. Sales, Synthesis of Polyols and Polyurethanes from Vegetable Oils-Kinetic and Characterization, J. Polym. Res., 2013, 20, 238, DOI:10.1007/s10965-013-0238-x.
- M. Borowicz, J. Paciorek-Sadowska and M. Isbrandt, Synthesis and Application of New Bio-Polyols Based on Mustard Oil for the Production of Selected Polyurethane Materials, Ind. Crops Prod., 2020, 155, 112831, DOI:10.1016/j.indcrop.2020.112831.
- A. Chandrashekar, S. Vargheese, J. A. Jyothy G Vijayan and T. N. P. Gopi, Highly e Cient Removal of Rhodamine B Dye Using Nanocomposites Made from Cotton Seed Oil-Based Polyurethane and Silylated Nanocellulose, J. Polym. Environ., 2022, 30, 4999–5011 CrossRef CAS.
- S. N. Shah, FTIR Characterization and Physicochemical Evaluation of Cottonseed Oil, Pak. J. Anal. Environ. Chem., 2017, 18, 46–53, DOI:10.21743/pjaec/2017.06.04.
- J. G. Vijayan, A. Chandrashekar, J. Ag and T. N. Prabhu, Polyurethane and Its Composites Derived from Bio-Sources : Synthesis , Characterization and Adsorption Studies, Polym. Polym. Compos., 2022, 30, 1–18, DOI:10.1177/09673911221110347.
- A. S. A. Hazmi, M. M. Aung, L. C. Abdullah, M. Z. Salleh and M. H. Mahmood, Producing Jatropha Oil-Based Polyol via Epoxidation and Ring Opening, Ind. Crops Prod., 2013, 50, 563–567, DOI:10.1016/j.indcrop.2013.08.003.
- S. Matsumura, A. R. Hlil, C. Lepiller, J. Gaudet, D. Guay, Z. Shi, S. Holdcroft and A. S. Hay, A Novel Vegetable Oil–Lactate Hybrid Monomer for Synthesis of High-Tg Polyurethanes, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 243–250, DOI:10.1002/pola.
- Z. Fang, C. Qiu, D. Ji, Z. Yang, N. Zhu, J. Meng, X. Hu and K. Guo, Development of High-Performance Biodegradable Rigid Polyurethane Foams Using Full Modified Soy-Based Polyols, J. Agric. Food Chem., 2019, 67, 2220–2226, DOI:10.1021/acs.jafc.8b05342.
- P. H. Hoang, H. T. Dat, T. D. Cuong and L. Q. Dien, Pretreatment of Coir Lignocellulose for Preparation of a Porous Coir-Polyurethane Composite with High Oil Adsorption Capacity, RSC Adv., 2022, 12, 14976–14985, 10.1039/d2ra01349e.
- C. Wan, Y. Zhou and Y. Li, Liquid Hot Water and Alkaline Pretreatment of Soybean Straw for Improving Cellulose Digestibility, Bioresour. Technol., 2011, 102, 6254–6259, DOI:10.1016/j.biortech.2011.02.075.
- Y. Zhang, S. Yang, J. Q. Wu, T. Q. Yuan and R. C. Sun, Preparation and Characterization of Lignocellulosic Oil Sorbent by Hydrothermal Treatment of Populus Fiber, Materials, 2014, 6, 6733–6747, DOI:10.3390/ma7096733.
- T. Zhang, L. Kong, M. Zhang, F. Qiu, J. Rong and J. Pan, Synthesis and Characterization of Porous Fibers/Polyurethane Foam Composites for Selective Removal of Oils and Organic Solvents from Water, RSC Adv., 2016, 6, 86510–86519, 10.1039/c6ra10916k.
- N. Zhang, S. Zhong, T. Chen, Y. Zhou and W. Jiang, Emulsion-Derived Hierarchically Porous Polystyrene Solid Foam for Oil Removal from Aqueous Environment, RSC Adv., 2017, 7, 22946–22953, 10.1039/c7ra02953e.
| This journal is © The Royal Society of Chemistry 2024 |