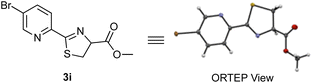Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
2-cyanopyridine derivatives enable N-terminal cysteine bioconjugation and peptide bond cleavage of glutathione under aqueous and mild conditions†
Tetsuya Yano,
Takahiro Yamada *,
Hiroaki Isida
*,
Hiroaki Isida ,
Nami Ohashi
,
Nami Ohashi and
Toshimasa Itoh
and
Toshimasa Itoh *
*
Showa Pharmaceutical University, Machida, Tokyo 194-8543, Japan. E-mail: t-yamada@ac.shoyaku.ac.jp; titoh@ac.shoyaku.ac.jp
First published on 22nd February 2024
Abstract
Inspired by the chemical reactivity of apalutamide, we have developed an efficient method for N-terminal cysteine bioconjugation with 2-cyanopyridine derivatives. Systematic investigations of various 2-cyanopyridines revealed that 2-cyanopyridines with electron-withdrawing groups react efficiently with cysteine under aqueous and mild conditions. Moreover, the highly reactive 2-cyanopyridines enable the peptide bond cleavage of glutathione. The utility of our method is demonstrated by its application to the cysteine-selective chemical modification of bioactive peptides.
Introduction
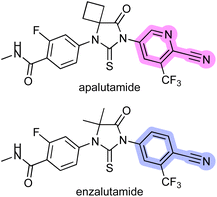
Apalutamide is a potent, specific, and orally administered inhibitor of the androgen receptor (AR) and an attractive drug for the treatment of patients with non-metastatic castration-resistant prostate cancer (nmCRPC).1 However, apalutamide showed a higher rate of skin rash as a side effect compared with placebo in the phase 3 SPARTAN trial.2 In contrast, the rate of skin rash across all clinical trials for enzalutamide,3 an antiandrogen drug structurally similar to apalutamide, is comparable to that of the placebo (Fig. 1).4 Subsequent studies suggested that the increased skin rash associated with apalutamide might be linked to a structural difference between the two drugs, and that the skin rash could be the result of drug-induced, immune-mediated hypersensitivity.5 The chemical structures of the two compounds show that the most important structural difference between the two drugs is that apalutamide contains a 2-cyanopyridine moiety, whereas enzalutamide possesses 2-cyanophenyl (Fig. 1). 2-Cyanopyridines have been experimentally and computationally confirmed to be more reactive than 2-cyanophenyls,6 and previous studies revealed that the 2-cyanopyridine moiety of apalutamide could chemically react with a thiol nucleophile such as glutathione, resulting in thiazoline ring formation (Scheme 1).5 In general, non-specific covalent binding of small organic molecules to proteins can lead to immune responses and induce adverse events.7 These findings indicate that the 2-cyanopyridine moiety in apalutamide acts as a hapten that reacts with cysteine residues in proteins, which could trigger an immune response and resulting in increased incidence of skin rash in patients.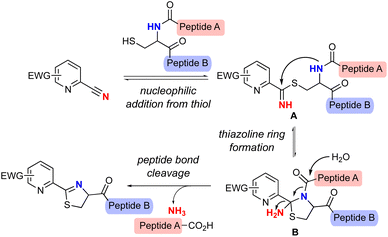 | ||
| Scheme 1 Proposed reaction mechanism of Cys-selective peptide bond cleavage by 2-cyanopyridine derivatives. | ||
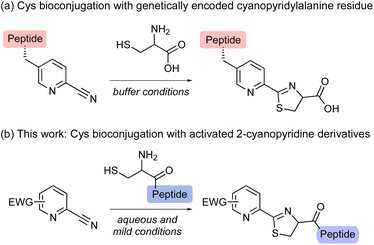
Two reaction mechanisms for thiazoline ring formation have been proposed to date,5,8 one of which is described in Scheme 1.8 This proposed reaction mechanism suggests that cysteine-containing peptide chains could be cleaved by a reaction between 2-cyanopyridine derivatives and thiols. The nucleophilic addition of the thiol group of cysteine to 2-cyanopyridine leads to the reversible formation of thioimidate A, which is then cyclized by intramolecular nucleophilic addition of the amide nitrogen in cysteine to give 2-amidethiazolidine intermediate B. Subsequent irreversible hydrolysis of the amide bond and release of ammonia from B produces an N-terminal peptide fragment and the cyclic thiazoline-modified C-terminal fragment. By further optimizing the reaction system and simplifying the chemical structure of apalutamide, we envisioned that 2-cyanopyridine derivatives might allow cysteine-selective peptide bond cleavage.
Activated heteroaromatic nitriles have recently been attracting increasing attention because of their rapid formation of a thiazoline ring with an N-terminal cysteine, as in click reactions.9 In particular, we were drawn to the reaction between 2-cyanopyridine derivatives and 1,2-aminothiols, which proceeds under biological conditions and can be applied to in-cell protein modifications.10 For example, Huber and co-workers demonstrated that the genetic encoding of 2-cyanopyridylalanine enables the site-specific attachment of a wide range of functionalities in cells (Scheme 2a).10a However, most studies have been limited to the use of 2-cyanopyridylalanine residues, and the scope of cyanopyridine substrates that have been experimentally explored to date remains very narrow.11 Although electron-withdrawing substituents might increase the rate of reaction with N-terminal cysteine,6,9a there have been no systematic investigations of the substituent effects on cyanopyridines in the reaction.
Herein, we investigated the reaction efficiency of 2-cyanopyridine derivatives bearing electron-withdrawing substituents in the reaction with cysteine (Scheme 2b). The study was inspired by the chemical reactivity of the 2-cyanopyridine moiety of apalutamide. Cysteine-selective peptide bond cleavage of glutathione was realized by using structurally optimized 2-cyanopyridine derivatives. The key features of our method are its mild reaction conditions in aqueous media and a substrate scope applicable to the bioconjugation of cysteine-containing bioactive peptides. In addition, 15N-labeling experiments allowed us to determine the reaction mechanism for the formation of thiazoline product from 2-cyanopyridine derivatives and cysteine.
Results and discussion
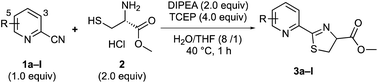
First, we investigated the reaction between 2-cyanopyridines 1 and cysteine methyl ester 2 in an aqueous medium with THF to solubilize the substrate (Table 1). To prevent disulfide bond formation and keep Cys thiols reduced, the reactions were conducted in the presence of 4.0 equiv. of tris(2-carboxyethyl)phosphine (TCEP).12 As expected, the reaction of 2-cyanopyridine 1a with cysteine 2 afforded the thiazoline product 3a, in 67% yield (Table 1). It should be noted that the obtained product 3a did not show optical activity and was racemic,13 indicating that thiazoline products 3 racemize very rapidly.| 2-Cyano pyridines | R | Products | Yield (%) |
|---|---|---|---|
| a Reaction conditions: 1a–l (0.3 mmol, 1.0 equiv), L-cysteine methyl ester hydrochloride 2 (2.0 equiv), DIPEA (2.0 equiv), and TCEP (4.0 equiv) in H2O/THF (8/1, 2.0 mL) were stirred at 40 °C for 1 h. All yields are isolated yield. | |||
| 1a | None | 3a | 67 |
| 1b | 3-CF3 | 3b | 29 |
| 1c | 5-CF3 | 3c | 73 |
| 1d | 3-F | 3d | 97 |
| 1e | 5-F | 3e | 94 |
| 1f | 5-NO2 | 3f | 0 |
| 1g | 5-SO2NH2 | 3g | 53 |
| 1h | 5-SO2NHCH2CH2Ph | 3h | 55 |
| 1i | 5-Br | 3i | 79 |
| 1j | 5-OMe | 3j | 53 |
| 1k | 5-NH2 | 3k | 41 |
| 1l | 5-NHAc | 3l | 62 |
To improve the reaction efficiency, we screened several 2-cyanopyridines with various substituents on the pyridine ring (Table 1). The reaction employing 3-trifluoromethyl-2-cyanopyridine (1b), a substructure of apalutamide, decreased the reaction efficiency and the corresponding product 3b was obtained in 29% yield. Interestingly, 2-cyanopyridine 1c with a trifluoromethyl group at the 5-position slightly improved reactivity, giving the desired thiazoline 3c in 73% yield. These results indicate that the reactivity of 2-cyanopyridines with cysteine depend not only on the electronic nature of the nitrile group but also on steric hindrance around the cyanocarbon. Next, we explored the reactions with 2-cyanopyridines bearing various electron-withdrawing groups (1d–i). To our delight, the reaction with 2-cyanopyridines 1d and 1e bearing a fluoro group proceeded efficiently, affording the desired products 3d and 3e in 97% and 94% yields, respectively. In general, fluorine is highly electronegative and has a strong tendency to act as an electron-withdrawing group, which presumably contributes to the increased reactivity of the cyanocarbons. In contrast, nitro-substituted 2-cyanopyridine 1f did not provide the desired product, probably because the nitro group could be reduced to an amino group in the presence of TCEP. The reaction with 5-sulfonamide-2-cyanopyridines 1g and 1h gave the corresponding products in 53% and 55% yields, respectively. 5-Bromo-2-cyanopyridine 1i also reacted with cysteine 2 and the structure of thiazoline product 3i was confirmed to be racemic by X-ray crystal structure analysis (Scheme 3).14 2-Cyanopyridines 1j–l with an electron-donating group (OMe, NH2, NHAc) clearly reduced reaction efficiency.
We investigated various amino acids with 2-cyanopyridine 1d to evaluate the Cys-selectivity of this ring formation reaction (see ESI†). Fortunately, 1d did not react with Ser, Thr, Lys, His, Tyr, Trp, Arg, Asp, and Glu, and the starting pyridine 1d was completely recovered. These results indicated that the developed bioconjugation reaction with 2-cyanopyridine derivatives is highly selective for cysteine.
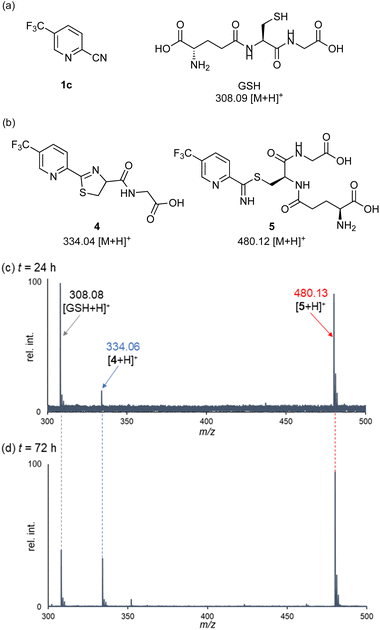
Having identified highly reactive 2-cyanopyridines, we next examined the peptide bond cleavage of glutathione (GSH), a tripeptide of γ-Glu-Cys-Gly (Fig. 2). Previous studies reported that apalutamide reacts with GSH under buffer conditions to form several adducts, with an elimination half-life with GSH of over 50 hours.5 Based on this report, we monitored the progress of the reaction between 2-cyanopyridine 1c and GSH under ammonium acetate buffer (pH 7.0) conditions using ESI-MS (Fig. 2). Stirring at 40 °C for 24 h provided two products: the thiazoline product 4 with an m/z of 334.06 (calcd 334.04 [M + H]+) and a thioimidate intermediate 5 with an m/z of 480.13 (calcd 480.12 [M + H]+). After 72 h, the concentration of GSH decreased and the relative intensity of the MS peak for the thiazoline 4 increased over time. The thioimidate 5 was formed by nucleophilic addition of the thiol group of GSH to the cyanocarbon of 1c, indicating that peptide bond cleavage proceeds via intramolecular cyclization from an in situ generated thioimidate intermediate. Monitoring the reactions between various 2-cyanopyridines with GSH showed that activated 2-cyanopyridines 1b, 1d, and 1e, bearing electron-withdrawing substituents, also cleaved the peptide bond of glutathione (see ESI†).
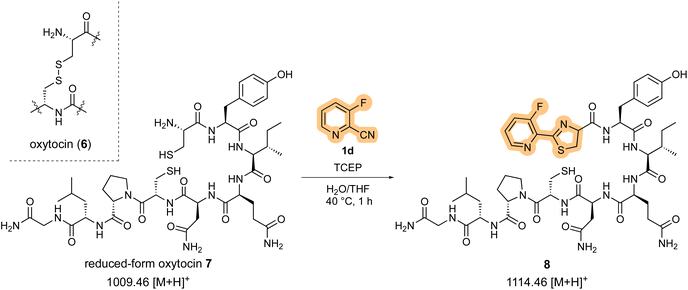
To further investigate the peptide cleavage reaction and N-terminal cysteine bioconjugation, we next focused on the reaction between 2-cyanopyridine derivatives and cysteine-containing bioactive peptides. For oxytocin (6), a bioactive peptide with an intramolecular disulfide bond,15 1d reacted only with the N-terminal cysteine residue of the in situ generated reduced form oxytocin 7, leaving the internal cysteine residue intact (Scheme 4).
After stirring at 40 °C for 1 h, HPLC analysis showed that the starting peptide 7 with an m/z = 1009.41 (calcd 1009.46 [M + H]+; Fig. 3a) was completely consumed and the N-terminal thiazoline product 8 with an m/z = 1114.44 (calcd 1114.46 [M + H]+; Fig. 3b) appeared as a major peak. However, cysteine-selective cleavage of the peptide bond was not observed. The same adduct formations were observed in the reaction between 1d and vasopressin (arginine vasopressin, argipressin) or lypressin (lysine vasopressin),16 derivatives of oxytocin bearing a similar disulfide bridge (see ESI†). These results indicate that the developed method successfully supported the N-terminal cysteine bioconjugation of bioactive oligopeptides, but cysteine-selective peptide cleavage by 2-cyanopyridine 1d may be less applicable to internal cysteine residues.
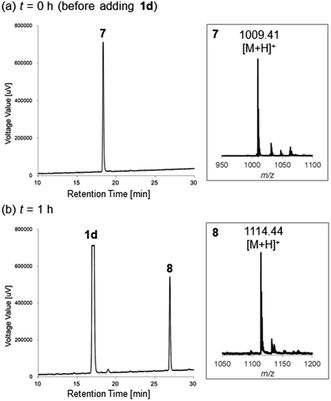 | ||
| Fig. 3 HPLC charts of the reaction between oxytocin and 1d described in Scheme 4 at (a) t = 0 h (before adding 1d) and (b) t = 1 h. The reaction was monitored by RP-HPLC (gradient: 10–40% of acetonitrile/0.1% TFA against H2O/0.1% TFA with a flow rate of 1.0 mL min−1 over 30 min). Insets show the MS corresponding peaks of reduced-form oxytocin 7 and the N-terminal thiazoline product 8. | ||
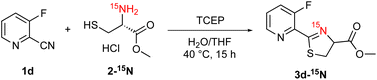
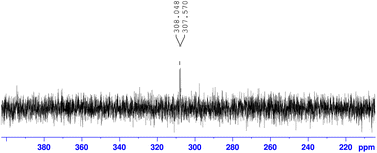
Additionally, we gained insight into the reaction mechanism by conducting 15N-labeling experiments (Scheme 5). After treating 15N-labeled L-cysteine methyl ester 2-15N with 2-cyanopyridine 1d under the optimized reaction conditions, 15N-NMR analysis of the obtained product revealed that the thiazoline product 3d contained 15N-labeled nitrogen (Fig. 4). HRMS analysis confirmed the formation of a15N-labeled thiazoline product,17 indicating that the nitrogen atom of the thiazoline ring derives from the cysteine residue. These results strongly support the reaction mechanism described in Scheme 1, including the formation of 2-amidethiazolidine intermediate B and subsequent irreversible thiazoline formation with the release of ammonia.8,11c
Conclusion
In conclusion, we have developed an efficient method for N-terminal cysteine bioconjugation using 2-cyanopyridine derivatives. Exploration of substituent effects on the 2-cyanopyridine structure revealed that the nitrile group can be made more reactive by the installation of fluoro and trifluoromethyl groups, and the highly reactive 2-cyanopyridines allow efficient thiazoline ring formation with cysteine in aqueous media. The highlights of this reaction are its wide scope and mild reaction conditions that allow rapid bioconjugation with bioactive peptides. Moreover, it is noteworthy that the highly reactive 2-cyanopyridines enable the peptide bond cleavage of glutathione. The developed peptide cleavage reaction using 2-cyanopyridine derivatives holds promise as a valuable chemical tool for site-selective peptide modifications. Further applications to realize the cysteine-selective bond cleavage of oligopeptides and protein chains are currently underway in our laboratory.Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) N. J. Clegg, J. Wongvipat, J. D. Joseph, C. Tran, S. Ouk, A. Dilhas, Y. Chen, K. Grillot, E. D. Bischoff, L. Cai, A. Aparicio, S. Dorow, V. Arora, G. Shao, J. Qian, H. Zhao, G. Yang, C. Cao, J. Sensintaffar, T. Wasielewska, M. R. Herbert, C. Bonnefous, B. Darimont, H. I. Scher, P. Smith-Jones, M. Klang, N. D. Smith, E. D. Stanchina, N. Wu, O. Ouerfelli, P. J. Rix, R. A. Heyman, M. E. Jung, C. L. Sawyers and J. H. Hager, Cancer Res., 2012, 72, 1494–1503 CrossRef CAS PubMed; (b) R. de Vries, F. Jacobs, G. Mannens, J. Snoeys, F. Cuyckens, C. Chien and P. Ward, Drug Metab. Dispos., 2019, 47, dmd.118.084517 CrossRef PubMed.
- M. R. Smith, F. Saad, S. Chowdhury, S. Oudard, B. A. Hadaschik, J. N. Graff, D. Olmos, P. N. Mainwaring, J. Y. Lee, H. Uemura, A. Lopez-Gitlitz, G. C. Trudel, B. M. Espina, Y. Shu, Y. C. Park, W. R. Rackoff, M. K. Yu, E. J. Small and S. Investigators, N. Engl. J. Med., 2018, 378, 1408–1418 CrossRef CAS PubMed.
- (a) C. Tran, S. Ouk, N. J. Clegg, Y. Chen, P. A. Watson, V. Arora, J. Wongvipat, P. M. Smith-Jones, D. Yoo, A. Kwon, T. Wasielewska, D. Welsbie, C. D. Chen, C. S. Higano, T. M. Beer, D. T. Hung, H. I. Scher, M. E. Jung and C. L. Sawyers, Science, 2009, 324, 787–790 CrossRef CAS PubMed; (b) J. A. Gibbons, T. Ouatas, W. Krauwinkel, Y. Ohtsu, J.-S. van der Walt, V. Beddo, M. de Vries and J. Mordenti, Clin. Pharmacokinet., 2015, 54, 1043–1055 CrossRef CAS PubMed.
- (a) H. I. Scher, K. Fizazi, F. Saad, M.-E. Taplin, C. N. Sternberg, K. Miller, R. de Wit, P. Mulders, K. N. Chi, N. D. Shore, A. J. Armstrong, T. W. Flaig, A. Fléchon, P. Mainwaring, M. Fleming, J. D. Hainsworth, M. Hirmand, B. Selby, L. Seely, J. S. de Bono and A. Investigators, N. Engl. J. Med., 2012, 367, 1187–1197 CrossRef CAS PubMed; (b) T. M. Beer, A. J. Armstrong, D. E. Rathkopf, Y. Loriot, C. N. Sternberg, C. S. Higano, P. Iversen, S. Bhattacharya, J. Carles, S. Chowdhury, I. D. Davis, J. S. de Bono, C. P. Evans, K. Fizazi, A. M. Joshua, C.-S. Kim, G. Kimura, P. Mainwaring, H. Mansbach, K. Miller, S. B. Noonberg, F. Perabo, D. Phung, F. Saad, H. I. Scher, M.-E. Taplin, P. M. Venner, B. Tombal and P. Investigators, N. Engl. J. Med., 2014, 371, 424–433 CrossRef PubMed; (c) M. Hussain, K. Fizazi, F. Saad, P. Rathenborg, N. Shore, U. Ferreira, P. Ivashchenko, E. Demirhan, K. Modelska, D. Phung, A. Krivoshik and C. N. Sternberg, N. Engl. J. Med., 2018, 378, 2465–2474 CrossRef CAS PubMed.
- C. Ji, M. Guha, X. Zhu, J. Whritenour, M. Hemkens, S. Tse, G. S. Walker, E. Evans, N. K. Khan, M. B. Finkelstein, E. Callegari and R. S. Obach, Chem. Res. Toxicol., 2020, 33, 211–222 Search PubMed.
- (a) R. M. Oballa, J.-F. Truchon, C. I. Bayly, N. Chauret, S. Day, S. Crane and C. Berthelette, Bioorg. Med. Chem. Lett., 2007, 17, 998–1002 CrossRef CAS PubMed; (b) A. Berteotti, F. Vacondio, A. Lodola, M. Bassi, C. Silva, M. Mor and A. Cavalli, ACS Med. Chem. Lett., 2014, 5, 501–505 CrossRef CAS PubMed.
- For selected reviews, see: (a) J. Uetrecht, Chem. Res. Toxicol., 2008, 21, 84–92 Search PubMed; (b) A. S. Kalgutkar and D. Dalvie, Annu. Rev. Pharmacol. Toxicol., 2015, 55, 1–20 CrossRef PubMed.
- K. Mizuno, K. Takeuchi, K. Umehara and M. Nakajima, Drug Metab. Dispos., 2019, 47, 809–817 CrossRef CAS PubMed.
- For recent reviews, see: (a) M. Proj, N. Strašek, S. Pajk, D. Knez and I. Sosič, Bioconjugate Chem., 2023, 34, 1271–1281 CrossRef CAS PubMed; (b) Y. Zhu, X. Zhang, Q. You and Z. Jiang, Bioorg. Med. Chem., 2022, 68, 116881 CrossRef CAS PubMed; (c) F. Chen and J. Gao, Chem.–Euro. J., 2022, 28, e202201843 CrossRef CAS PubMed; (d) Y. Wang, R. An, Z. Luo and D. Ye, Chem.–Euro. J., 2018, 24, 5707–5722 CrossRef CAS PubMed; (e) Y. Yuan and G. Liang, Org. Biomol. Chem., 2013, 12, 865–871 RSC; (f) For selected examples, see: H. Ren, F. Xiao, K. Zhan, Y. Kim, H. Xie, Z. Xia and J. Rao, Angew. Chem., Int. Ed., 2009, 48, 9658–9662 CrossRef CAS PubMed; (g) M. Liu, R. Yoshisada, A. Amedi, A. J. P. Hopstaken, M. N. Pascha, C. A. M. Haan, D. P. Geerke, D. A. Poole and S. A. K. Jongkees, Chem.–Euro. J., 2023, 29, e202203923 CrossRef CAS PubMed; (h) R. Padanha, R. A. N. Cavadas, P. Merino, J. P. M. António and P. M. P. Gois, Org. Lett., 2023, 25, 5476–5480 CrossRef CAS PubMed.
- For selected examples of bioconjugation reactions between cysteine and cyanopyridylalanine, see: (a) E. H. Abdelkader, H. Qianzhu, J. George, R. L. Frkic, C. J. Jackson, C. Nitsche, G. Otting and T. Huber, Angew. Chem., Int. Ed., 2022, 61, e202114154 CrossRef CAS PubMed; (b) C. Nitsche, H. Onagi, J.-P. Quek, G. Otting, D. Luo and T. Huber, Org. Lett., 2019, 21, 4709–4712 CrossRef CAS PubMed; (c) N. A. Patil, J.-P. Quek, B. Schroeder, R. Morewood, J. Rademann, D. Luo and C. Nitsche, ACS Med. Chem. Lett., 2021, 12, 732–737 CrossRef CAS PubMed; (d) M. Liu, R. Morewood, R. Yoshisada, M. N. Pascha, A. J. P. Hopstaken, E. Tarcoveanu, D. A. Poole, C. A. M. de Haan, C. Nitsche and S. A. K. Jongkees, Chem. Sci., 2023, 14, 10561–10569 RSC.
- For selected examples of thiazoline formation reactions between cysteine and cyanopyridines, see: (a) O. Maltsev, V. Walter, M. Brandl and L. Hintermann, Synthesis, 2013, 45, 2763–2767 CrossRef CAS; (b) F. de J. Cortez, P. Nguyen, C. Truillet, B. Tian, K. M. Kuchenbecker, M. J. Evans, P. Webb, M. P. Jacobson, R. J. Fletterick and P. M. England, ACS Chem. Biol., 2017, 12, 2934–2939 CrossRef PubMed; (c) T. Toyama, T. Saitoh, Y. Takahashi, K. Oka, D. Citterio, K. Suzuki and S. Nishiyama, Chem. Lett., 2017, 46, 753–755 CrossRef CAS; (d) R. Morewood and C. Nitsche, Chem. Sci., 2020, 12, 669–674 RSC.
- (a) C. Walling and R. Rabinowitz, J. Am. Chem. Soc., 1957, 79, 5326 CrossRef CAS; (b) R. E. Humphrey and J. L. Potter, Anal. Chem., 1965, 37, 164–165 CrossRef CAS.
- The thiazoline products did not show optical activity and single-crystal X-ray diffraction analysis of thiazoline 3i confirmed that the product was obtained as a racemate. See Supporting Information for details. For selected examples of racemization of 2-thiazoline-4-esters, see: (a) P. Raman, H. Razavi and J. W. Kelly, Org. Lett., 2000, 2, 3289–3292 CrossRef CAS PubMed; (b) H. Emtenäs, M. Carlsson, J. S. Pinkner, S. J. Hultgren and F. Almqvist, Org. Biomol. Chem., 2003, 1, 1308–1314 RSC . For a selected review on the chemistry of 2-thiazolines, see:; (c) A.-C. Gaumont, M. Gulea and J. Levillain, Chem. Rev., 2009, 109, 1371–1401 CrossRef CAS PubMed.
- Deposition number CCDC-2310511 for 3i contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- For selected reviews, see: (a) A. Argiolas and G. L. Gessa, Neurosci. Biobehav. Rev., 1991, 15, 217–231 CrossRef CAS PubMed; (b) P. Richard, F. Moos and M. J. Freund-Mercier, Physiol. Rev., 1991, 71, 331–370 CrossRef CAS PubMed; (c) C. S. Carter, W. M. Kenkel, E. L. MacLean, S. R. Wilson, A. M. Perkeybile, J. R. Yee, C. F. Ferris, H. P. Nazarloo, S. W. Porges, J. M. Davis, J. J. Connelly and M. A. Kingsbury, Pharmacol. Rev., 2020, 72, 829–861 CrossRef PubMed.
- For selected reviews, see: (a) L. Bankir, D. G. Bichet and N. G. Morgenthaler, J. Intern. Med., 2017, 282, 284–297 CrossRef CAS PubMed; (b) S. Sparapani, C. Millet-Boureima, J. Oliver, K. Mu, P. Hadavi, T. Kalostian, N. Ali, C. M. Avelar, M. Bardies, B. Barrow, M. Benedikt, G. Biancardi, R. Bindra, L. Bui, Z. Chihab, A. Cossitt, J. Costa, T. Daigneault, J. Dault, I. Davidson, J. Dias, E. Dufour, S. El-Khoury, N. Farhangdoost, A. Forget, A. Fox, M. Gebrael, M. C. Gentile, O. Geraci, A. Gnanapragasam, E. Gomah, E. Haber, C. Hamel, T. Iyanker, C. Kalantzis, S. Kamali, E. Kassardjian, H. K. Kontos, T. B. U. Le, D. LoScerbo, Y. F. Low, D. M. Rae, F. Maurer, S. Mazhar, A. Nguyen, K. Nguyen-Duong, C. Osborne-Laroche, H. W. Park, E. Parolin, K. Paul-Cole, L. S. Peer, M. Philippon, C.-A. Plaisir, J. P. Marroquin, S. Prasad, R. Ramsarun, S. Razzaq, S. Rhainds, D. Robin, R. Scartozzi, D. Singh, S. S. Fard, M. Soroko, N. S. Motlagh, K. Stern, L. Toro, M. W. Toure, S. Tran-Huynh, S. Trépanier-Chicoine, C. Waddingham, A. J. Weekes, A. Wisniewski and C. Gamberi, Biomedicines, 2021, 9, 89 CrossRef CAS PubMed; (c) L. Bankir, D. G. Bichet and N. G. Morgenthaler, J. Intern. Med., 2017, 282, 284–297 CrossRef CAS PubMed.
- HRMS analysis showed the formation of 15N-labeled thiazoline product 3d-15N; HRMS (ESI) m/z calcd for C10H7F15N14NO2S+Na+: 264.0241 [M+Na]+; found: 264.0237.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2310511. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4ra00437j |
| This journal is © The Royal Society of Chemistry 2024 |