Open Access Article
Open Access ArticleGreening up organic reactions with caffeine: applications, recent developments, and future directions
Ankita Chaudhary
 *a,
Divya Mathur
*b,
Ritu Gaba
a,
Raaina Pasricha
a and
Khyati Sharma
a
*a,
Divya Mathur
*b,
Ritu Gaba
a,
Raaina Pasricha
a and
Khyati Sharma
a
aDepartment of Chemistry, Maitreyi College, University of Delhi, Delhi – 110 021, India. E-mail: achaudhary@maitreyi.du.ac.in
bDepartment of Chemistry, Daulat Ram College, University of Delhi, Delhi – 110 007, India
First published on 18th March 2024
Abstract
Numerous studies in the field of alkaloid chemistry have provided researchers with valuable insights into their unique properties as catalysts. Among the diverse natural catalysts, caffeine has emerged as a green, expedient, and biodegradable catalyst with high efficiency and applicability. Interest in using caffeine as a catalyst has burgeoned over the past few years with its role in diverse multicomponent reactions. Preparation of its imidazolium salts and further conversion to Nitrogen Heterocyclic Carbene (NHC) ligands and ionic liquids offers new paradigms. Caffeine has also played a multifaceted role as a support material in influencing the structural properties of nanoparticles. We hope that the chemistry of caffeine and its applications for sustainable organic transformations discussed in this review will stimulate new thinking and open new avenues in this field.
1 Introduction
Nature is an irrefutable provider of innumerable chemicals that not only provide a novel template for drug discovery of lead compounds but also provide frontier opportunities to explore new sources of material, polymer, and energy. Hence, when it comes to looking for opportunities to improve chemical reactions by making them more sustainable, the researchers will look toward the large pool of yet unexploited natural compounds that can act as green, bio-renewable, and non-toxic catalysts. Methylxanthine alkaloids, such as caffeine, theobromine, and theophylline are examples of the less explored and utilized class of bio-sourced catalysts. Recently, the application of caffeine as a catalyst made headlines as it was reported to create flexible biocompatible polymer gels that can be used in drug delivery and other medical applications.1 Caffeine also finds applications in the treatment of Parkinson's disease, Alzheimer's disease, and cancer immunotherapy, lowers the risk of cardiovascular diseases, and behaves as a weak bronchodilator as well as an analgesic synergist for severe pain.2 Numerous reviews have addressed caffeine's effects on either physical or cognitive performance.3–6Leveraging natural and benign resources in catalysis and organometallic chemistry is one of the major missions of green and sustainable chemistry. There are various advantages of using caffeine because it is readily available, cheap, biodegradable, and non-toxic. The easy functionalization of caffeine along with its eco-friendly features makes it an excellent candidate as a catalyst for the construction of chemically and biologically important organic frameworks. Owing to the presence of the imidazole ring, caffeine has been used as an N-heterocyclic carbene precursor for the preparation of gold, silver, palladium, and platinum complexes, and was used for various coupling reactions. Caffeine has also been reportedly used as support in the design of heterogeneous catalysts and ionic liquids. The role of caffeine as a stabilizing agent in the synthesis of nanoparticles as well as an organic modifier in the preparation of hydroxyapatite (HA) nanorods has also been described in the literature.
However, a review that can offer a full-scale lens to discuss the function of caffeine as a catalyst in various organic reactions has not been adumbrated so far. The main objective of this review is to present the existing knowledge pertaining to the exploitation of caffeine in various organic transformations. The selection of varied examples is intended to demonstrate the advancement in this field. The effect of functionalization on scope, selectivity/yields of the product, and recyclability (wherever applicable) along with an illustration of the catalyst role through mechanisms is attempted through this review.
1.1 Caffeine biosynthesis and catabolism
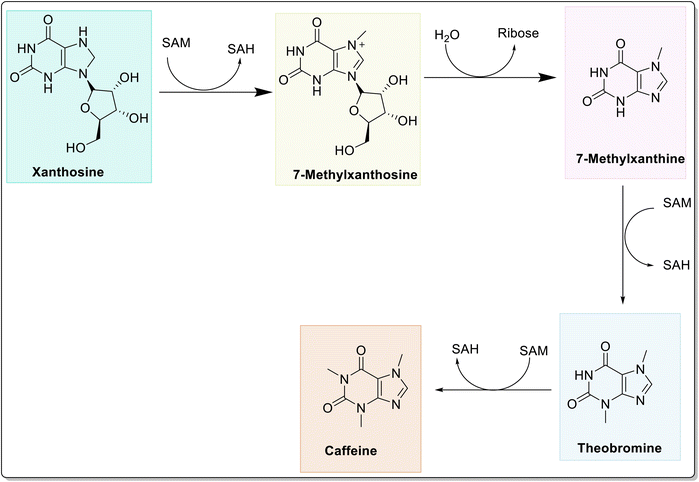
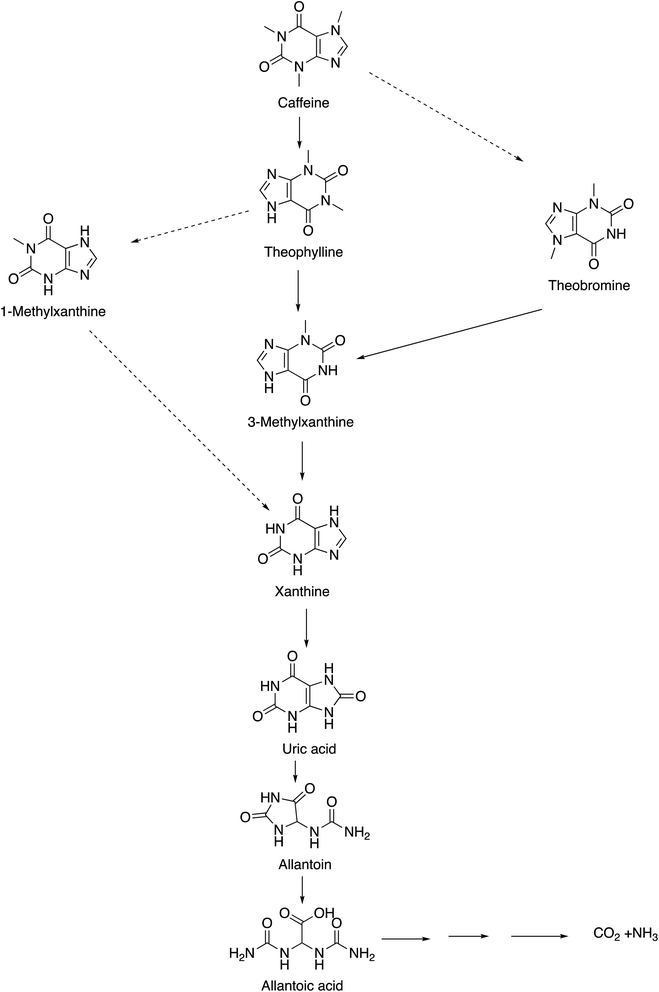
Caffeine (1,3,7-trimethylxanthine or 3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione) exists as a white, odorless solid with a slightly bitter taste and is found in over 60 plant species, of which cocoa pods, tea leaves, kola nuts, and coffee beans are the most well-known. It is one of the most widely consumed psychostimulant drugs worldwide and exhibits diverse pharmacological activities. As one of the most predominantly consumed foods and supplements in the world, caffeine is not only consumed as coffee7 but is also present in various other foods, drugs, and beverages8,9 Caffeine was first isolated in 1819, by a German chemist named Friedlieb Ferdinand Runge and was termed “Kaffebase”, while the name caffeine was coined by Pierre-Joseph Pelletier.10–12 It is produced either by extraction from natural sources or by synthetic procedures (e.g., methylation of various xanthines and theophylline). Although there are numerous potential biosynthetic routes to caffeine in the plants, the major biosynthetic pathway of caffeine from xanthosine is depicted in Fig. 1.13,14 Herein, initially, the xanthosine undergoes N7-methylation with consequent deribosylation to afford 7-methylxanthine. Thereafter, methylation at N3 produces theobromine, which results in the formation of caffeine through enzymatic N1-methylation.The key route of caffeine catabolism involves the conversion of caffeine to theophylline, 3-methylxanthine, xanthine, uric acid, allantoin, allantoic acid (Fig. 2) and eventually to CO2 and NH3.15
1.2 Extraction and synthesis of caffeine
Apart from the conventional extraction techniques like the Soxhlet method, Maceration, Reflux extraction, Decoction, etc. employed for the extraction of caffeine from natural sources,16 many other environment benevolent extraction techniques have been recently developed such as ultrasound-assisted extraction (UAE),17 supercritical fluid extraction (SFE),18 microwave-assisted extraction (MAE),19 pressurized liquid extraction,20 high-pressure processing (HPP),21 extraction involving deep eutectic solvents22 etc. (Table 1). As compared to conventional approaches, green extraction techniques offers numerous advantages like reduction in the extraction solvent usage, employment of non-hazardous substances, short extraction time, and energy efficient.23,24| Techniques | Key features of the technique |
|---|---|
| Supercritical fluid extraction (SFE) | • CO2, under pressure at 7.39 MPa and at 31.3 °C as critical temperature, is the most used solvent for the Super critical extraction method |
| • Enhances extraction yields, shorter production cycles, reusability of revered CO2 are some of the merits of the protocol | |
| Microwave-assisted extraction (MAE) | • The microwave assisted extraction involves the application of microwave irradiation which serves as a non-contact heat source, which makes heating more efficient and selective and helps to accelerate energy and mass transfer |
| • High reproducibility, less solvent as well as power consumption and greater purity of the final product are the merits of this method | |
| Ultrasound-assisted extraction (UAE) | • Ultrasonication is utilised in this extraction process |
| • A frequency range between 20 to 40 kHz; and intensity range varying from 10–1000 W cm−2 is employed | |
| • The cavitation phenomenon involved in the process increases the efficiency of extraction | |
| • Involves use of less energy as well as solvents | |
| High pressure processing (HPP) | • High pressure processing (HPP) is non-thermal process that results in removal of active components from the natural biomaterial under high pressure conditions |
| • Compared with conventional extraction processes, HPP extraction has excellent advantages, such as shorter extraction time, higher yield and lower energy consumption | |
| Pressurized liquid extraction (PLL) | • High temperature under reduced pressure conditions are used in this protocol, wherein the temperature range is dependent on the solvent used |
| Extraction using deep eutectic solvent (DES) | • It utilizes green and biodegradable solvent i.e. deep eutectic solvent |
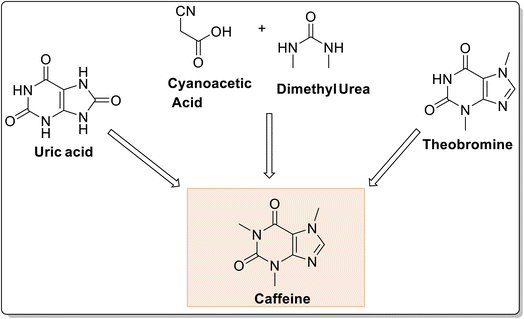
The first synthesis of caffeine from uric acid was reported in 1895 by Hermann Emil Fisher (Fischer & Ach, 1895),25 and later on in 1902, was awarded with the Nobel Prize for the same. Later on, its synthesis was reported by cyanoacetic acid and urea or dimethyl urea by Traube26 (Fig. 2). Thereafter, a number of procedures have been developed for caffeine synthesis by N-methylation of theobromine under different conditions (Fig. 3) namely methyl iodide in the presence of sodium hydroxide as a base;27 N dimethyl sulfate,28 dimethyl sulfate in the presence of alumina impregnated with KF in acetonitrile,29 methyl iodide in methanolic sodium methoxide solution.26 Recently, green synthesis of caffeine has been reported from sodium theophylline using DMC as the methylating, Turkish red oil as a solvent, and DBU as the catalyst.30
2 Application of caffeine as a catalyst
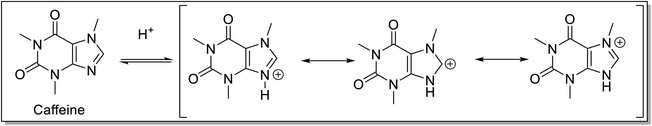
Various authors have explored the weak basic property of caffeine (Fig. 4) for catalyzing various organic transformations. It behaves as a very feeble base with pKa 14.2 and reacts with acids. Caffeine is often combined with a wide variety of compounds, such as sodium benzoate, H2SO4, H3PO4, HClO4, and HNO3 to increase the solubility and its efficacy and the salt produced can be easily hydrolyzed. The scope of using caffeine in a variety of solvent systems is immense as it is soluble in almost all solvents. The caffeine's solubility decreases in the order of chloroform, acetone, ethyl acetate, water, methanol, and ethanol.31 There are recent reports on the application of caffeine and conjugated caffeine as a catalyst in green, convenient, and high-yielding multicomponent reactions.Mohebat and co-workers32 reported the synthesis of benzo[a]pyrano[2,3-c]phenazine derivatives 5 by domino reaction of 2-hydroxy-1,4-naphthoquinone 1, 1,2-diaminobenzenes 2, aldehydes 3 and malononitrile 4 (Scheme 1). The initial condensation of 2-hydroxy-1,4-naphthoquinone and 2-phenylenediamine proceeded under solvent-free conditions at 75 °C to yield benzo[a]phenazin-5-ol, which subsequently underwent reaction with aldehydes and malononitrile in presence of caffeine under conventional heating or microwave irradiation to afford the benzo[a]pyrano[2,3-c]phenazines in high yield. Notably, the solubility of caffeine in water offers the possibility to be recovered from the reaction mixture by simple filtration and reused for the next run. The application of microwave irradiation resulted in a shorter reaction time in comparison to conventional heating. The developed protocol offers several advantages such as the use of inexpensive, non-toxic, highly reactive, reusable solid green catalysts, short reaction times, and operational simplicity. The mechanism for the caffeine-catalyzed synthesis of benzo[a]pyrano[2,3-c]phenazines has been depicted in Scheme 1. The formation of 5 proceeded via the formation of benzo[a]phenazin-5-ol by initial condensation of 2-hydroxy-1,4-naphthoquinone and diamine. Simultaneously, caffeine promoted Knoevenagel condensation of aldehyde with malononitrile afforded arylidenemalononitrile, which underwent Michael addition with benzo[a]phenazin-5-ol, followed by cyclization and tautomerisation to form 5.
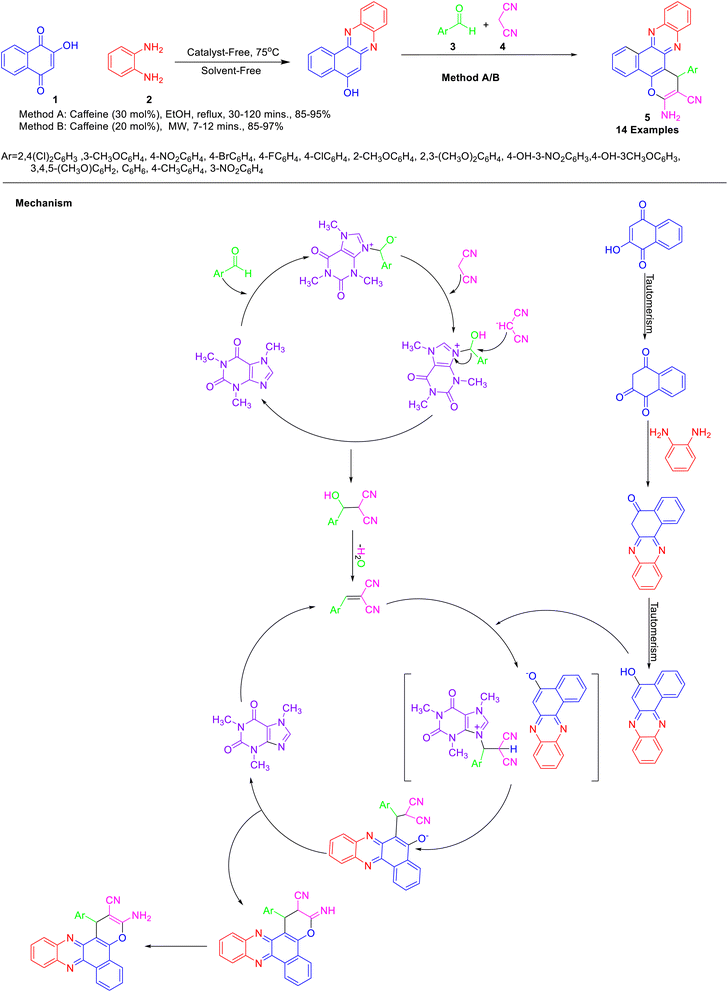 | ||
| Scheme 1 Synthesis of benzo[a]pyrano[2,3-c]phenazines 5 in the presence of caffeine as a catalyst and its mechanism. | ||
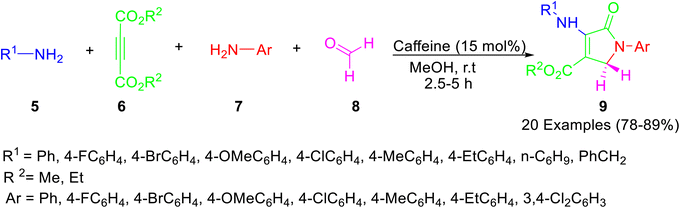
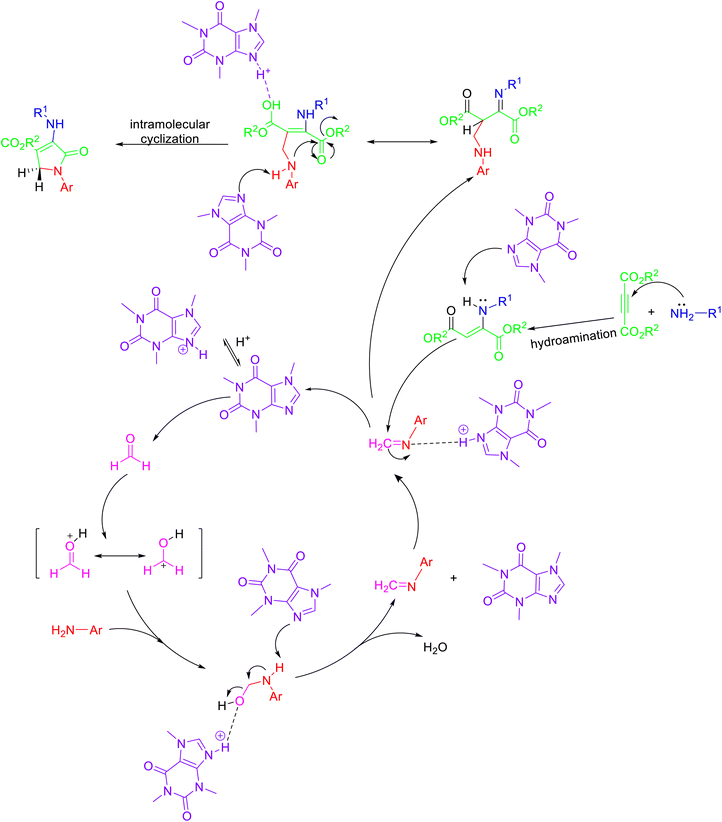
Mohamadpour33 exploited caffeine as a catalyst for the synthesis of polysubstituted dihydro-2-oxypyrroles 9 via multicomponent domino reaction between aromatic amines 7, aliphatic amines 5 dialkyl acetylenedicarboxylate 6 and formaldehyde 8 in methanol as solvent at ambient temperature (Scheme 2). The probable mechanism for the caffeine-catalyzed formation of oxypyrroles 9 is shown in Scheme 3. The use of natural, biodegradable, and inexpensive catalysts, simple workup, less reaction times, and good to high yields of the product are some of the advantages of the above protocol.
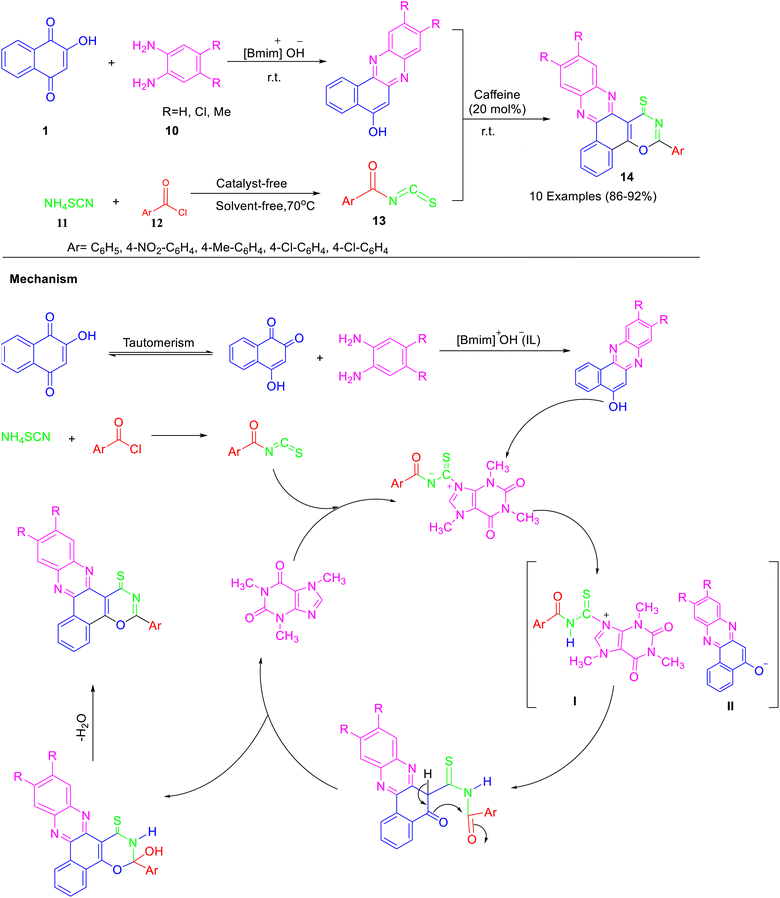
Various benzo[a][1,3]oxazino[6,5-c]phenazine-1-thiones were prepared by one pot, three component sequential condensation reaction of 2-hydroxy-1,4-naphthoquinone 1, 1,2-diaminobenzenes 2, and aroyl isothiocyanate 13 (formed from reaction of ammonium thiocyanate 11 and acid chloride 12) using caffeine as catalyst in [Bmim]OH at room temperature (Scheme 4).34 The reaction carried out with 4,5-dichlorobenzene-1,2-diamine as well as 4,5-dimethylbenzene-1,2-diamine resulted in a longer reaction time as compared to 1,2-diaminobenzene. The rationale mechanism for the formation of the desired oxazine is depicted in Scheme 4. The reaction proceeds via [Bmim]OH promoted formation of benzo[a]phenazin-5-ol by the reaction of 2-hydroxy-1,4-naphthoquinone with benzene-1,2-diamine. Benzo[a]phenazin-5-ol so formed, reacts with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adduct of aroyl isothiocyanate with caffeine and affords anion of benzo[a]phenazin-5-ol (II) and intermediate I. Finally, intermediate I undergo a cyclization reaction and dehydration to produce the desired product.
1 adduct of aroyl isothiocyanate with caffeine and affords anion of benzo[a]phenazin-5-ol (II) and intermediate I. Finally, intermediate I undergo a cyclization reaction and dehydration to produce the desired product.
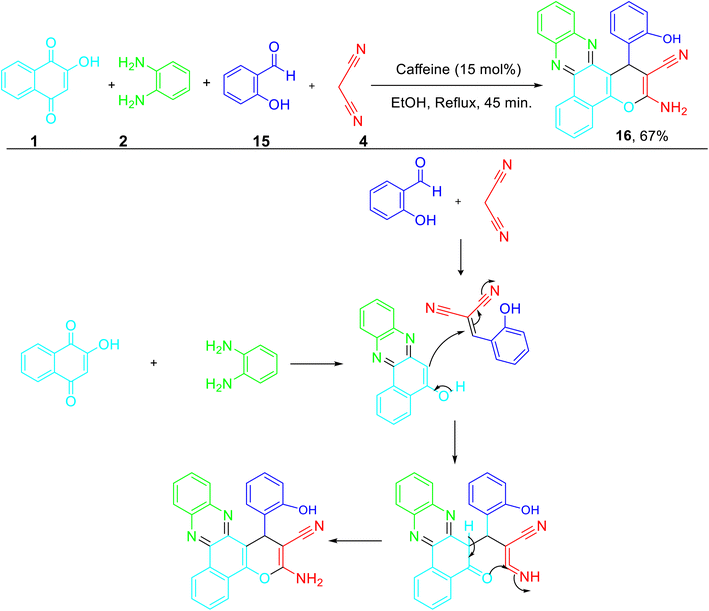
Caffeine promoted one-pot synthesis of benzo[a]pyrano[2,3-c]phenazines 16 from the multicomponent reaction of 2-hydroxy-1,4-naphthoquinone 1, o-phenylenediamine 2, malononitrile 4, and 2-hydroxybenzaldehyde 15 in ethanol under reflux conditions (Scheme 5) was reported by Liandi and co-workers.35 DPPH assay was employed to evaluate the antioxidant property of synthesized phenazine and was found to be a good antioxidant with an IC50 value of 14.26 ppm.
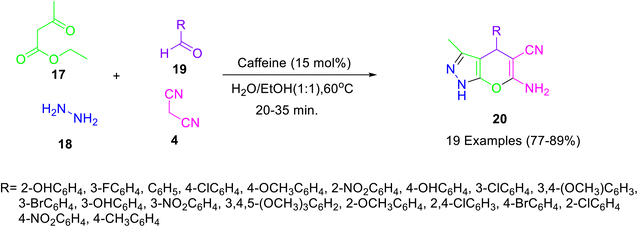
Mohamadpour and co-workers36 have reported caffeine-catalysed multicomponent synthesis of dihydropyrano[2,3-c]pyrazoles 20 via the domino Knoevenagel–Michael cyclocondensation reaction of malononitrile 4, ethyl acetoacetate 17, hydrazine hydrate 18, and aryl aldehydes 19 in aqueous ethanol (Scheme 6). Various dihydropyrano[2,3-c]pyrazoles were prepared in good yields and short reaction times under the developed reaction conditions.
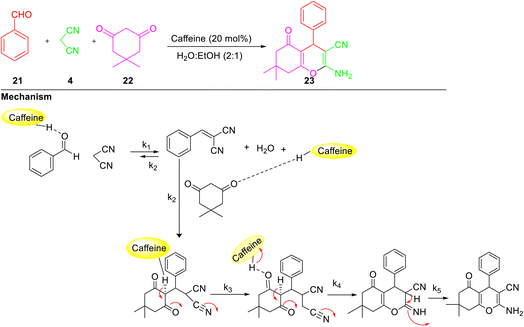
A mechanistic insight for the synthesis of 4H-tetrahydrobenzo[b]pyrans 23 via reaction of benzaldehyde 21, malononitrile 4, and dimedone 22 in a mixture of (ethanol/water, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as solvent utilising caffeine as a biodegradable catalyst has been reported by carrying out UV-vis spectroscopy (Scheme 7) by Habibi-Khorassani and co-workers.37 They have reported that the above reaction followed second-order kinetics and the partial orders with respect to benzaldehyde, malononitrile, and dimedone were one, one, and zero, respectively. Based on the steady-state approximation, the first step of the probable pathway was identified as a rate-determining step (k1). The various reaction parameters like activation energy (Ea), activation enthalpy (DHÂ), free energy of activation (DGÂ), and entropy of activation (DSÂ) were also calculated.
2) as solvent utilising caffeine as a biodegradable catalyst has been reported by carrying out UV-vis spectroscopy (Scheme 7) by Habibi-Khorassani and co-workers.37 They have reported that the above reaction followed second-order kinetics and the partial orders with respect to benzaldehyde, malononitrile, and dimedone were one, one, and zero, respectively. Based on the steady-state approximation, the first step of the probable pathway was identified as a rate-determining step (k1). The various reaction parameters like activation energy (Ea), activation enthalpy (DHÂ), free energy of activation (DGÂ), and entropy of activation (DSÂ) were also calculated.
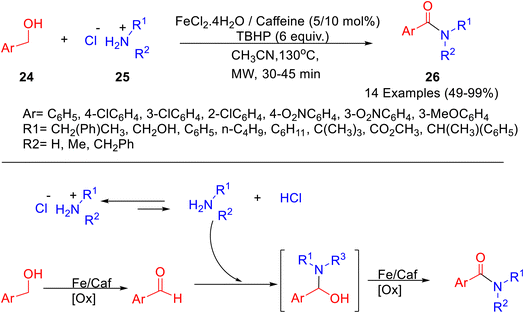
Several benzamides 26 were formed from the reaction of benzyl alcohols 24 and amine hydrochloride salts 25 under microwave irradiation, using a catalytic system that involves Fe(II) and caffeine as stabilizing ligands (Scheme 8).38 The role of microwave irradiation was established by the fact that a low yield of product was observed by traditional heating. Furthermore, the replacement of caffeine with using natural sources of caffeine like coffee beans, or tea leaves, resulted in a much lower yield of product.
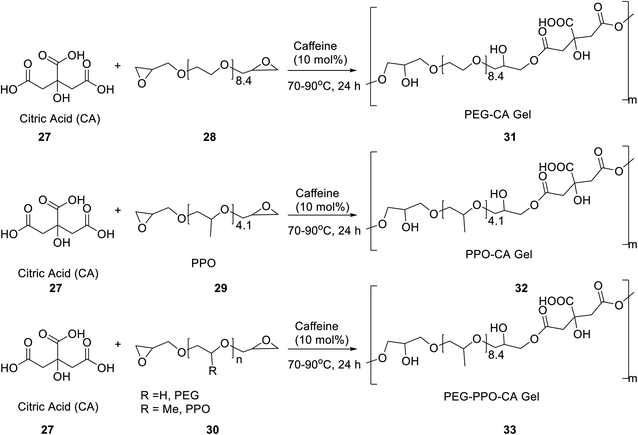
Caffeine has been employed as a catalyst for the formation of 3D-covalently crosslinked gels 31–33 via deprotonation of citric acid 27 and epoxide ring opening of diglycidyl ether functionalized oligomers of poly(ethylene glycol) (PEG) and poly(propyleneoxide) (PPO) 28–30 (Scheme 9).39 The gels so formed, demonstrated dynamic physical, chemical, and mechanical properties, which can be tailored in shape, surface texture, and tensile strength, among other potential attributes. The low cost, versatile, and facile synthesis of these gels, makes them suitable candidates for a broad range of customized engineering applications viz. drug delivery constructs, tissue engineering scaffolds, etc.
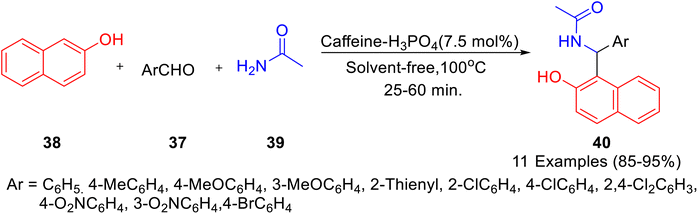
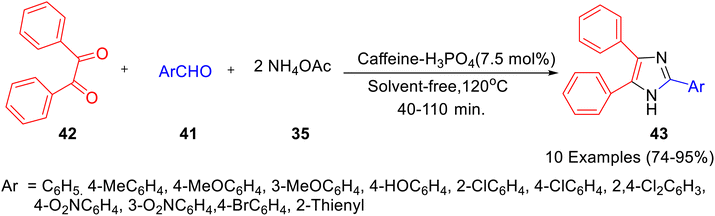
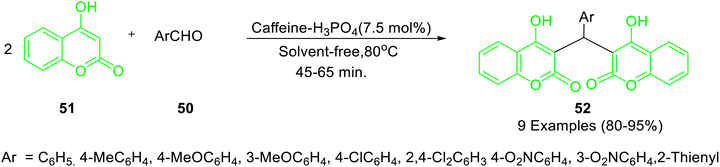
Caffeine-based catalysts i.e. caffeine-H3PO4, caffeine-HClO4 and caffeine-HNO3 were prepared and employed for one-pot preparation of polyhydroquinolines 36 through reaction of dimedone 22, ethylacetoacetate 17, aldehydes 34 and ammonium acetate 35 by Saghanezhad et al.40 Among the tested catalysts, caffeine-H3PO4 demonstrated excellent catalytic potential and afforded polyhydroquinolines in high yields under solvent-free conditions (Scheme 10). The developed protocol was further exploited for the preparation of 1-amidoalkyl-2-naphthols 40 via a reaction of aromatic aldehydes 37, 2-naphthol 38, and acetamide 39 (Scheme 11); 4,5-trisubstituted imidazoles 43 via reaction of aromatic aldehydes 41, benzil 42 and ammonium acetate 35 (Scheme 12); bis(indolyl) methanes 46 through reaction of aromatic aldehydes 45 and indole 44 (Scheme 13); 4,4′-(arylmethylene) bis(1H-pyrazol-5-ols) 49 via reaction of aldehydes 48 and 3-methyl-5-pyrazolone 47 (Scheme 14); 3,3′-(arylmethylene) bis(4-hydroxycoumarins) 52 via reaction of aromatic aldehydes 50 and 4-hydroxycoumarin 51 (Scheme 15).
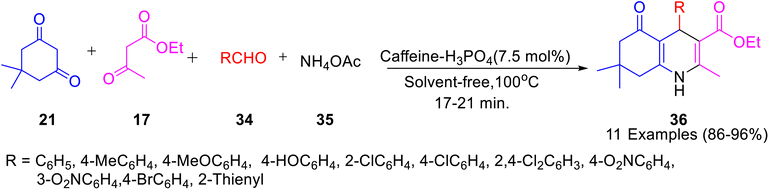 | ||
| Scheme 10 One-pot preparation of polyhydroquinolines 36 in the presence of caffeine based catalyst i.e. caffeine-H3PO4. | ||
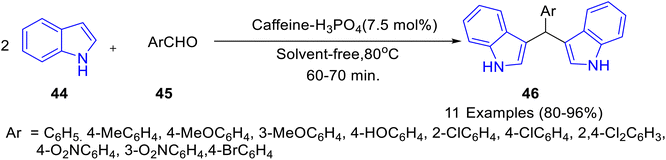 | ||
| Scheme 13 Synthesis of bis(indolyl) methanes 46 in the presence of catalytic amount of caffeine-H3PO4. | ||
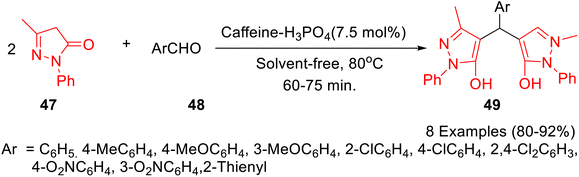 | ||
| Scheme 14 Synthesis of 4,4′-(arylmethylene) bis(1H-pyrazol-5-ols) 49 in the presence of catalytic amount of caffeine-H3PO4. | ||
One-pot domino Knoevenagel–Michael synthesis of bis-cyclohexenones 54 and 1,8-dioxooctahydroxanthenes 55 was demonstrated by caffenium hydrogen sulphate catalyzed reaction of dimedone 22 with various aldehydes 53 in aqueous ethanol (Scheme 16).41 The reusability of the catalyst was also evaluated for synthesis of both bis-cyclohexanones and 1,8-dioxooctahydroxanthenes for up to 4 consecutive runs without any prominent loss in catalytic potential. Saghanezhad42 has also reported the synthesis of 1,8-dioxo-octahydroxanthene derivatives 55 by reaction of dimedone with various aldehydes in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio utilizing caffeine hydrogen sulphate as a catalyst.
2 molar ratio utilizing caffeine hydrogen sulphate as a catalyst.
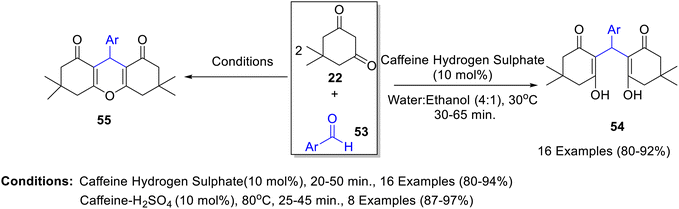 | ||
| Scheme 16 Synthesis of bis-cyclohexanones 54 and 1,8-dioxooctahydroxanthenes 55 using caffeine hydrogen sulphate as a catalyst. | ||
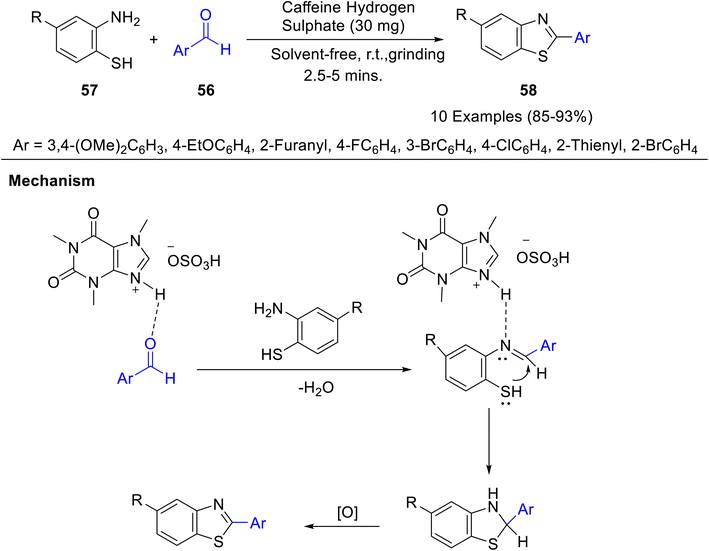
A mechanochemical synthesis of 2-arylbenzothiazoles 58 has been developed by Kalal and co-workers43 by the reaction of substituted aromatic aldehydes 56 and 2-aminothiophenol 57 using caffeine hydrogen sulphate as an eco-friendly catalyst at room temperature (Scheme 17). The catalyst was reusable up to 5 times without any noticeable loss of activity. The probable reaction mechanism for the synthesis of 2-arylbenzothiazoles is shown in Scheme 17. The reaction starts with the nucleophilic addition of the amine group of 2-aminobenzenethiole to the activated carbonyl carbon of aldehyde to afford imine. The attack of the SH group to imine (activated by caffeine hydrogen sulphate), followed by oxidation, results in the formation of 2-arylbenzothiazole.
Various α-amino acid derivatives 62 were synthesized via Kabachnik–Fields reaction of 2-aminothiazole 59 with several aldehydes 60 and dialkyl phosphites 61 using caffeine hydrogen sulfate as catalyst under both microwave irradiation and solvent-free conditions (Scheme 18).44 The microwave irradiation-assisted synthesis resulted in a higher yield of product and shorter reaction times as compared to conventional heating. The recovery and recyclability of the catalyst have also been demonstrated by the authors. The recycled catalyst was reportedly reused for six subsequent runs, without any substantial loss in catalytic potential. The proposed mechanism for the synthesis of α-substituted thiazolyamine methyl phosphonates has been shown in Scheme 18. Initially, the nucleophilic addition of amine to the carbonyl group of aldehydes (activated by CHS) leads to form an imine. Thereafter, aminophosphonates are formed by the nucleophilic attack of phosphate to imine with the simultaneous release of CHS.
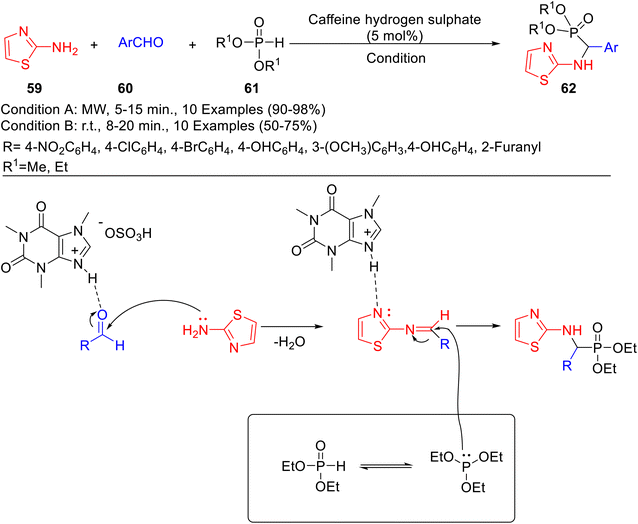 | ||
| Scheme 18 Caffeine hydrogen sulphate promoted synthesis of α-amino acid derivatives 62 and its mechanism. | ||
3 Caffeine based ionic liquids
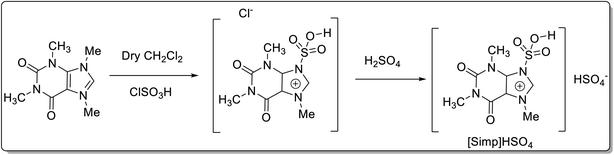
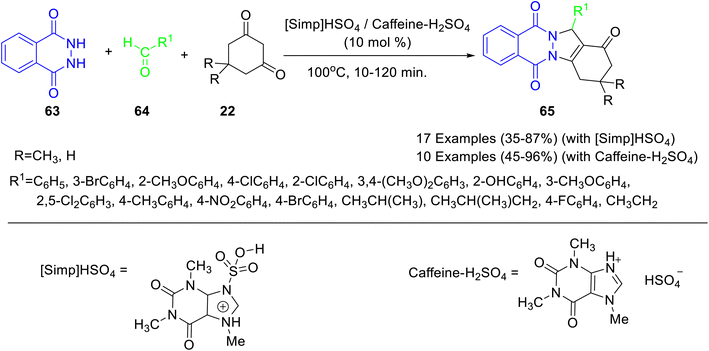
Ionic liquids (ILs) are quaternary salts based on organic–inorganic anions and bulky organic cations.45 ILs are considered environment friendly due to their nonflammable, non-volatile, low vapor pressure, high thermal stability, tunable polarity, and broad electrochemical stability. Caffeine poses as an inexpensive and easily accessible feedstock for acquiring ionic liquids that can be utilized in catalytic reactions. Caffeine-based ionic liquids have an easy preparation technique, and their use as a catalyst involves a simple work-up procedure, purification, easy recovery, and reusability.Tayebee and co-workers46 reported the synthesis of a caffeine-based Brønsted acid ionic liquid, viz. 3-sulfonic acid-1-imidazolopyridinium hydrogen sulfate ([Simp]HSO4) (Scheme 19), and employed it for the synthesis of 2H-indazolo[2,1-b]phthalazine-1,6,11(13H)-triones 65 through one pot, three-component condensation reaction of phthalhydrazide 63, aromatic aldehydes 64, and dimedone 22 under solvent-free conditions (Scheme 20). The authors have demonstrated the reusability of the recovered catalyst up to 6 runs without any appreciable loss in catalytic potential. The plausible mechanism for the formation of 2H-indazolo[2,1-b]phthalazine-1,6,11(13H)-triones has been shown in Scheme 21. Initially, the formation of heterodyne by condensation of aldehyde and dimedone takes place. Thereafter, the Michael-type addition of phthalhydrazide with heterodiene, followed by cyclization and dehydration affords the corresponding product. The authors have reported the probable role of the catalyst in not only activating the carbonyl, amine, and enamine groups towards the nucleophilic attack but also arranging the starting materials in closer proximity due to the dual hydrogen-bonding properties.
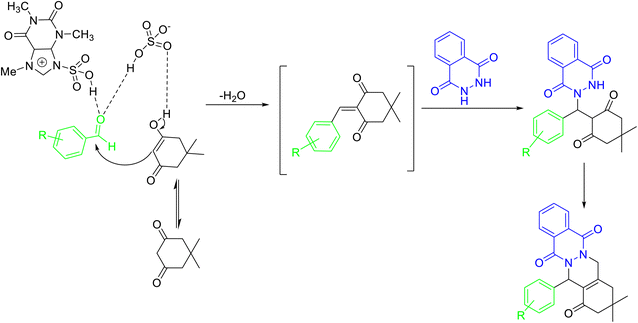 | ||
| Scheme 21 Probable mechanism for [Simp]HSO4 promoted synthesis of 2H-indazolo[2,1-b]phthalazinetriones 65. | ||
Later on in 2017, the synthesis of 2H-indazolo[2,1-b]phthalazinetriones was also reported utilizing catalytic amount of caffeine-H2SO4 under solvent-free conditions from the reaction of aromatic aldehydes, phthalhydrazide, and dimedone.47 Various aldehydes bearing both electron-withdrawing as well as electron-donating groups underwent a reaction smoothly with phthalhydrazide and dimedone, under the developed reaction conditions. The recovery and reusability of the catalyst was studied by the authors who reported a slight decrease in the catalytic activity of the catalyst after the fifth cycle.
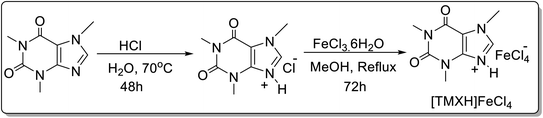
A novel nano-sized cube-shaped magnetic ionic liquid derived from caffeine i.e. [TMXH]FeCl4 was prepared (Scheme 22)48 and used for the synthesis of 12-aryl-8,9,10,12-tetrahydrobenzo[a]xanthene-11-ones 67 in good yield by three-component reaction of dimedone 22, aldehyde 66 and 2-naphthol 38 (Scheme 23). The catalyst could be easily recovered and reused for up to 4 runs without appreciable loss in efficacy and stability. The plausible mechanism for the [TMXH]FeCl4 catalyzed synthesis of 12-aryl-8,9,10,12-tetrahydrobenzo[a]xanthene-11-ones has been highlighted in Scheme 23.
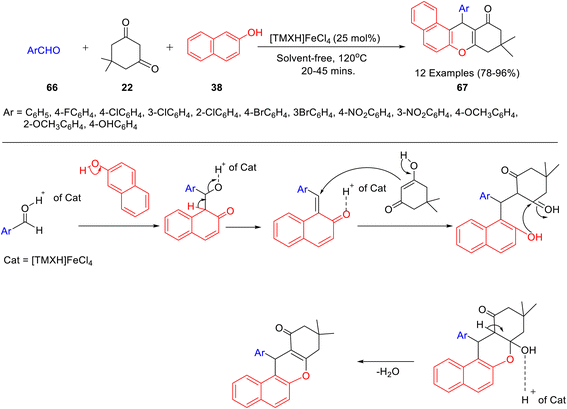 | ||
| Scheme 23 Synthesis of synthesis of 12-aryl-8,9,10,12-tetrahydrobenzo[a]xanthene-11-ones 67 catalyzed by [TMXH]FeCl4 and its mechanism. | ||
A new caffeine-based acidic ionic liquid i.e. [TMXH][TSA], was prepared by Salami and co-workers49 by the reaction of caffeine with p-toluenesulfonic acid, and its catalytic activity was evaluated for the synthesis of 1,8-dioxooctahydroxanthenes (Scheme 24). Various aldehydes underwent a reaction smoothly with dimedone in the presence of [TMXH][TSA] under solvent-free conditions to afford 1,8-dioxooctahydroxanthenes in high yields. Furthermore, it was observed that the recovered catalyst can be used for 3 consecutive cycles without notable loss of activity. The mechanistic pathway leading to the formation of the desired product using a catalytic amount of [TMXH][TSA] has been shown in Scheme 25. Other caffeine-derived-ionic liquid i.e. CaffBAIL was also employed for the synthesis of 1,8-dioxooctahydroxanthene derivatives 69 by reaction of dimedone 22 with various aldehydes 68 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio by Hataminejad and co-workers.50
2 molar ratio by Hataminejad and co-workers.50
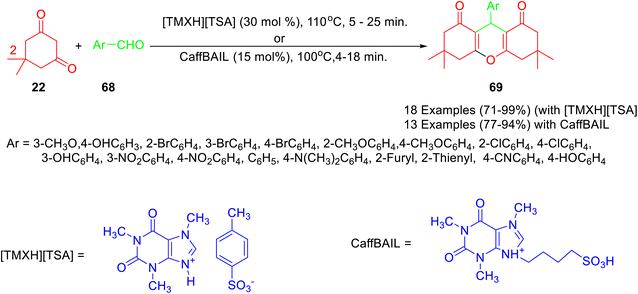 | ||
| Scheme 24 Synthesis of 1,8-dioxo-octahydroxanthene derivatives 69 in the presence of caffeine based ionic liquids. | ||
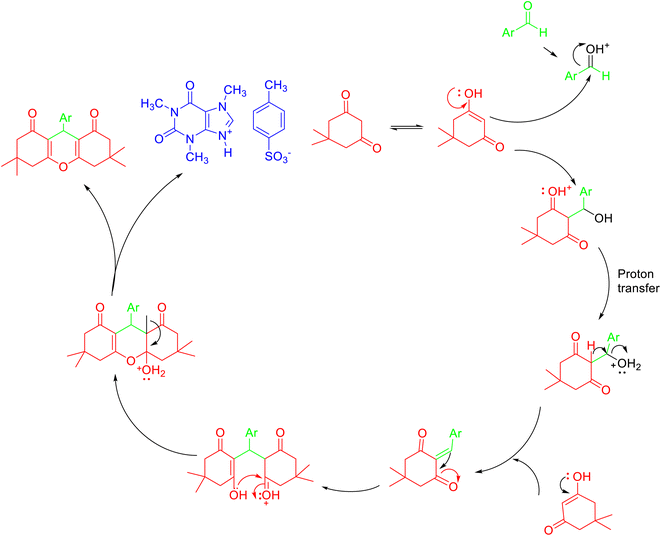 | ||
| Scheme 25 Plausible mechansim for the synthesis of 1,8-dioxo-octahydroxanthenes 69 in the presence of [TMXH][TSA]. | ||
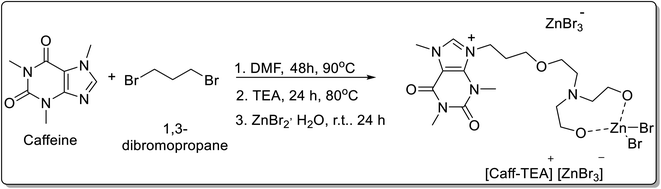
A natural-based ionic liquid ([Caff-TEA]+[ZnBr3]−) was reportedly synthesized using caffeine, triethanolamine, and zinc bromide by Ghafuri and co-workers51 (Scheme 26). The use of caffeine-based ionic liquid in the absence of ZnBr2 is limited owing to its high melting point of about 176 °C, but the addition of ZnBr2 to it, results in the decrease of melting point to 76 °C, thereby allowing its use for reaction under 100 °C in milder reaction conditions possible. The synthesized [Caff-TEA]+[ZnBr3]− demonstrated high catalytic activity towards condensation reactions of various aldehydes 70 and dimedone 22/cyclohexane-1,3-dione 71 for the synthesis of benzylidenes 72, bis-hydroxyenones 73 and xanthenes 74 under ultrasonic irradiation (Scheme 27).
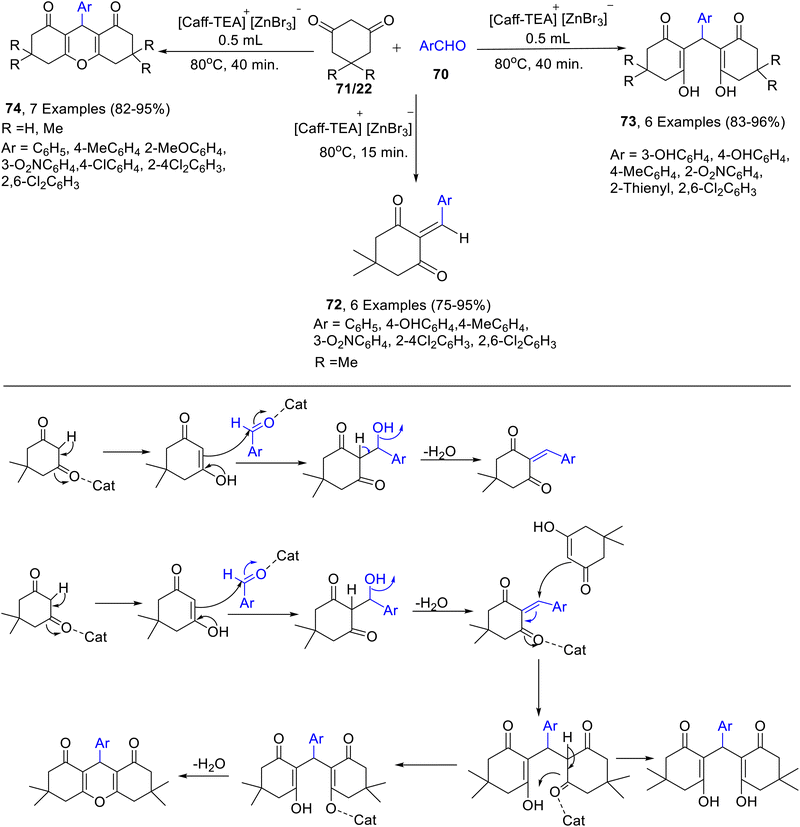 | ||
| Scheme 27 [Caff-TEA]+[ZnBr3]− catalyzed synthesis of benzylidenes 72, bis-hydroxyenones 73, and xanthenes 74 under ultrasonic irradiation and its mechanism. | ||
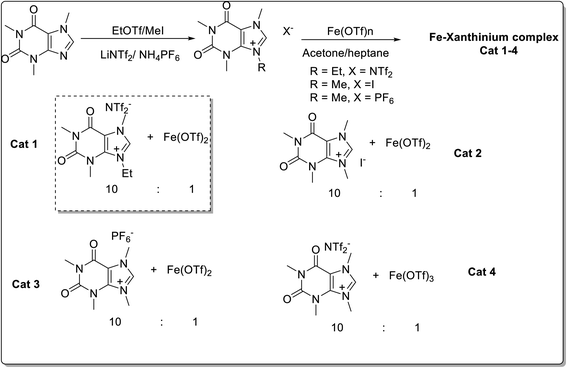
Four caffeine-derived methylxanthinium salts were prepared (Scheme 28) and their catalytic potential was evaluated for the Diels–Alder reaction of cyclopentadiene and 3-acryloyl-1,4-oxazolidin-2-one (Scheme 29).52 The recyclable ionic salt/iron triflate catalyst (Cat 1), prepared from xanthinium salt and iron(II) triflate gave the best results. Various dienophiles i.e. α,β-unsaturated carbonyl 76a and N-acyloxazolidinone derivatives 76b underwent a Diels–Alder reaction with cyclopentadiene 75 in presence of caffeine-derived xanthinium–Fe(OTf)2 complex (1 mol%), in dimethyl carbonate (DMC) as green solvent (Scheme 29). The recovered catalyst (Cat 1) could be recycled for up to five runs without any significant decrease in the yield of the product.
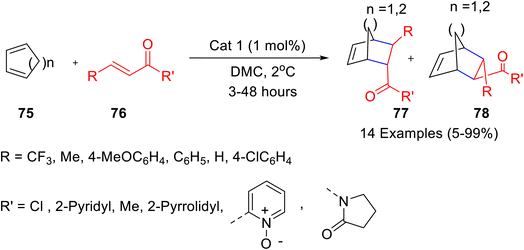 | ||
| Scheme 29 Diels–Alder reaction of cyclopentadiene and 3-acryloyl-1,4-oxazolidin-2-one in the presence of caffeine-derived xanthinium–Fe(OTf)2 complex. | ||
4 Caffeine-based N-heterocyclic carbene ligands
The caffeine structure has two substructures, a pyrimidinedione and an imidazole ring, fused together. In particular, in the imidazole ring, the deprotonation of the C–H bond between the two nitrogen atoms can be achieved to form useful ligands for the coordination of transition metal ions i.e. N-heterocyclic carbene (NHC) precursor. The quintessential use of caffeine in NHCs by modification of its imidazole ring forming caffeine-8-ylidene was started way back in the 1970s by Taube53 caffeine-based NHC metal complexes can be procured through direct metalation at the C8 position.54 It has been demonstrated that the reactivity of caffeine-based NHC as a nucleophile or base is strongly influenced by the substituents of the imidazole ring. Alkylation of the xanthine ring confines the number of metal binding sites and also increases the solubility of the complex.55 Alkylation at N7 and N9 positions followed by subsequent deprotonation at the C8 position is another way to generate the NHC.56 Regioselective C8-metalation of N7- or N9-blocked caffeine by oxidative addition of the C-X in 8-halocaffeine to Pt complexes also represents a facile and straightforward method for building NHCs.57The bioactive components of caffeine can be effectively incorporated with metal NHCs to form pharmacologically active motifs with antimicrobial and antiproliferative activity. Caffeine NHC–silver acetate complex demonstrated antimicrobial activity against numerous resistant respiratory pathogens including members of the Burkholderia cepacia complex.58 Also, Casini and coworkers designed caffeine–gold(I) NHC complexes and found them effective with antiproliferative activities in different cancerous and nontumorigenic cell lines.59
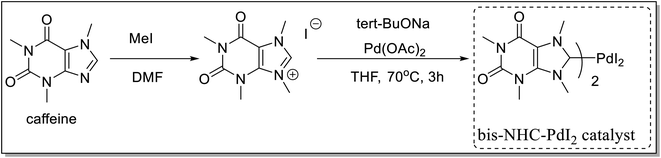
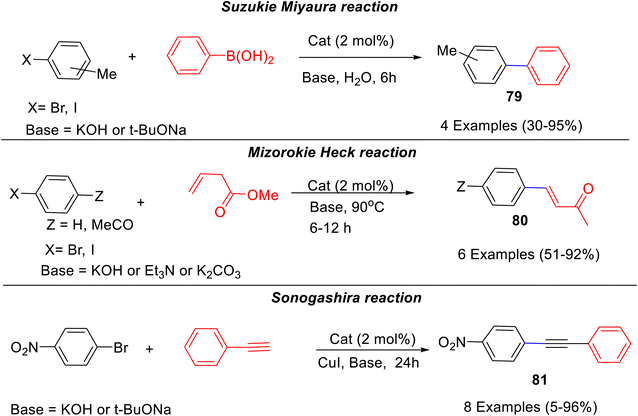
A bis-NHC-Pd catalyst derived from caffeine was synthesized by Luo et al.60 in a two-step process. 1,3,7,9-Tetramethylxanthinium obtained by reaction of caffeine with excess of methyl iodide in dry dimethyl formamide, DMF, was reacted with sodium tert-butoxide in the presence of Pd(OAc)2 in dry THF to afford the desired bis-NHC-palladium catalyst i.e. bis(1,3,7,9-tetramethylxanthine8-ylidene)palladium diiodide (Scheme 30). The authors have further demonstrated the role of this catalyst for various C–C bond-forming reactions like Suzuki–Miyaura, Mizoroki–Heck, and Sonogashira reactions in water with or without additives resulting into corresponding products 79–81 (Scheme 31).
In a pursuit to overcome the drawbacks associated with above mentioned, caffeine-based homogeneous bis NHC-Pd catalyst60 two polystyrene-supported palladium(II)–N-heterocyclic carbene complex PS-NHC-Pd(II) derived from theophylline and caffeine were designed by Mohammadi and co-workers61 and their catalytic potential towards Heck–Matsuda reaction was evaluated (Scheme 32). PS-NHC-Pd(II) (Caff) was successfully utilized as catalyst for the cross-coupling reaction of arenediazonium tetrafluoroborate salts 82 with ethyl acrylate 83 resulting in the desired coupled product 84 but demonstrated lower catalytic efficiency as compared to theophylline based PS-NHC-Pd(II) catalyst. Furthermore, the authors have studied the reaction of different olefins and arenediazonium tetrafluoroborate salts in the presence of a heterogeneous PS-NHC-Pd(II) catalyst based on theophylline. The remarkable acidity of the alkylated caffeine-based catalyst allowed the isolation of zwitterionic caffeine monocarbene palladium complexes.
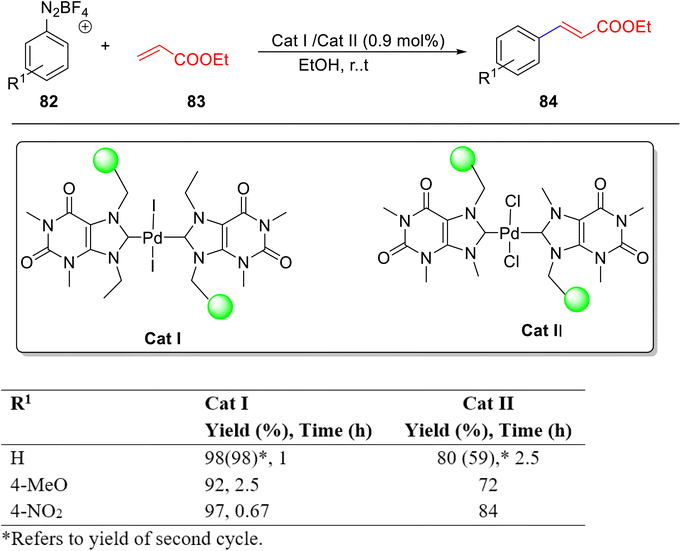 | ||
| Scheme 32 Heck–Matsuda reaction in the presence of heterogeneous PS-NHC-Pd(II) catalyst based on theophylline and caffeine. | ||
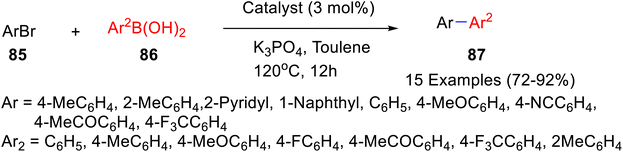
Later on, in 2022, Zhang and co-workers62 demonstrated the synthesis of air and moisture-stable cyclopentadienyl nickel–NHC complexes from caffeine as well as theophylline (Scheme 33) and checked their efficacy as catalyst for the Suzuki–Miyaura cross-coupling reaction. These catalysts were made by the reaction of NiCp2 with the different imidazolium salt obtained from the methylation of caffeine and theophylline. Out of the three synthesized catalyst used, the cyclopentadienyl nickel–NHC complexes from caffeine gave the best results probably owing to aryl boronic acid's higher reactivity of [Ni–NHC] catalyst due to its less sterically demanding structure. [Ni–NHC] was investigated further for other Suzuki–Miyaura cross-coupling of various aryl bromides 85 with aryl boronic acids 86 and gave the desired coupled product 87 (Scheme 34). Several aryl bromides as well as aryl boronic acids containing both electron-rich and electron-deficient groups underwent Suzuki cross-coupling smoothly in the presence of Ni–NHC.
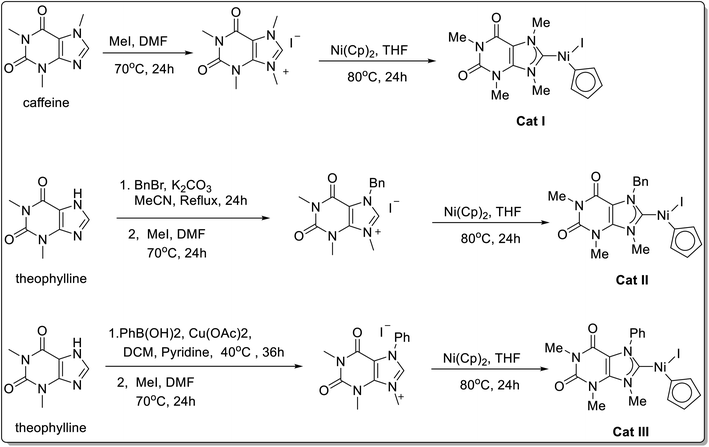 | ||
| Scheme 33 Synthesis of cyclopentadienyl nickel–NHC complexes derived from caffeine and theophylline. | ||
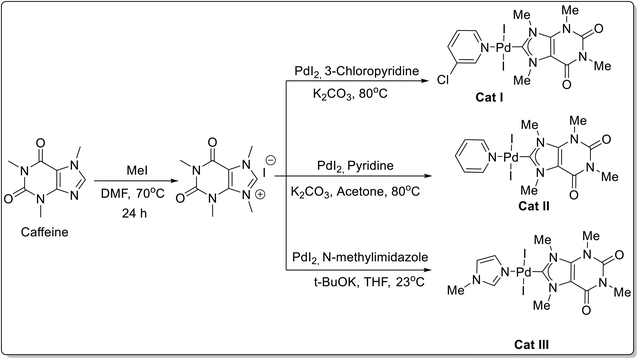
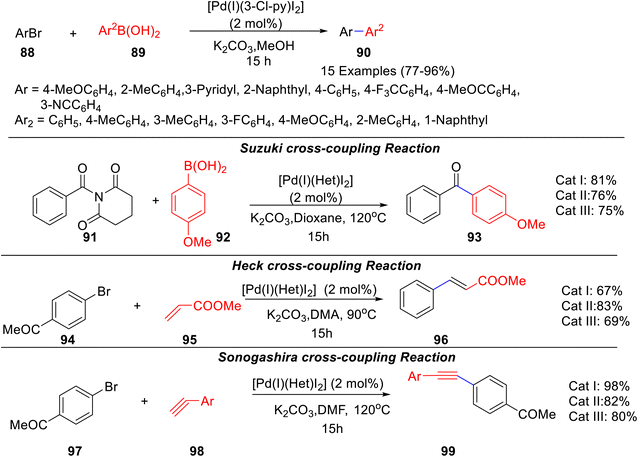
Three caffeine-derived Pd–PEPPSI complexes viz. [Pd(I)-(Het)I2] (Het = 3-chloro-pyridine, pyridine, 1-methylimidazole) were synthesized and their efficacy as a catalyst for Suzuki cross-coupling of aryl bromides, Suzuki cross-coupling of amides, Heck cross-coupling, and Sonogashira cross-coupling was evaluated.63 The catalysts bearing 3-chloro-pyridine, pyridine, and N-methylimidazole ancillary ligands were synthesized from the corresponding N9-Me caffeine imidazolium salt (prepared by methylation at N-9 position of caffeine by methyl iodide in DMF) by direct deprotonation, followed by coordination to PdX2 in the presence of N-heterocycles (Scheme 35). All complexes used demonstrated stability to air and moisture. The reactivity of all three synthesized Pd–PEPPSI catalysts was evaluated towards the Suzuki cross-coupling of inactivated aryl bromides and was found to promote the coupling reaction with high efficiency using a combination of K2CO3 and MeOH at 80 °C. The catalytic potential of [Pd(I)(3-Cl-py)I2] was further explored for Suzuki–Miyaura cross-coupling reactions of numerous aryl bromides 88 and aryl boronic acids 89 to give diaryls 90 (Scheme 36).
All three catalysts were effective in promoting Suzuki cross-coupling of amide, Heck cross-coupling, and Sonogashira cross-coupling (Scheme 36) and gave the targeted products 93, 96, 99. Out of the three catalysts used, the Cat III, Cat was found to be most efficient towards Suzuki–Miyaura cross-coupling of N-benzoyl-glutarimide 91 with aryl boronic acid 92 under mild carbonate base conditions as well as Sonogashira cross-coupling of 4-acetyl-1-bromobenzene 94 with phenylacetylene 95; whereas Cat II gave the best result in Heck cross-coupling of 4-acetyl-1-bromobenzene 97 and methyl acrylate 98, using K2CO3 in DMA at 90 °C.
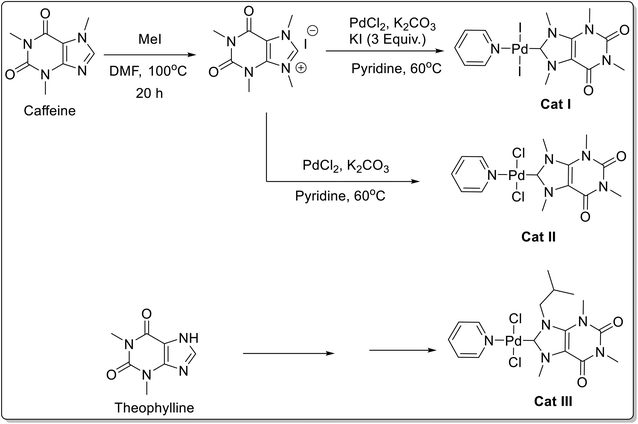
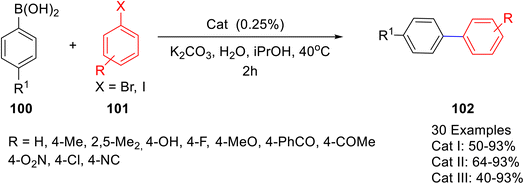
Mazars and co-workers64 synthesized three Pd–PEPPSI catalysts derived from xanthine alkaloids, such as caffeine and theophylline (Scheme 37). These complexes were reported to exhibit high catalytic activity in the Suzuki–Miyaura cross-coupling of aryl halides 100 with phenylboronic acids 101 (Scheme 38). Several aryl bromides and iodides with both electron-donating and electron-withdrawing substituents afforded the corresponding biaryl 102 derivatives in high yields. Low catalyst loading (0.25 mol%), use of water-based reaction media, and natural, non-toxic, widely available, cheap xanthine derivative-based catalysts are some of the prominent features of the protocol developed.
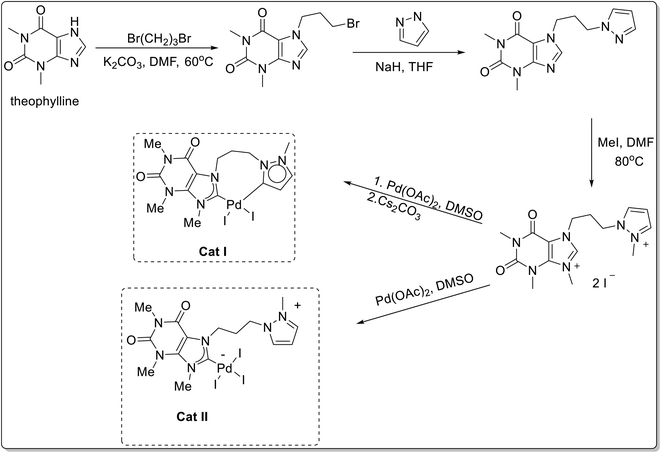
The [PdI2(NHC)(Py)] (Cat I) and [PdCl2(NHC)(Py)] (Cat II) were two-step protocol involving the initial reaction of caffeine, with methyl iodide to afford 1,3,7,9-tetrametylxanthinium iodide, followed by treatment with palladium(II) chloride and in presence of KI and absence of KI respectively.
Several homo- and heterodicarbene palladium complexes bearing caffeine-derived N-heterocyclic carbene ligands were synthesized by Teng et al.65 Amongst all the synthesized complexes, caffeine-pyrazole-derived complexes i.e. cis-[PdI2(Caf-Py)] (Cat I) and [PdI3(Caf-Py)] (Cat II) (Scheme 39) demonstrated highest catalytic potential for the cyanation of aryl halides 103 with K4[Fe(CN)6]·3H2O 104 to afford 105 (Scheme 40).
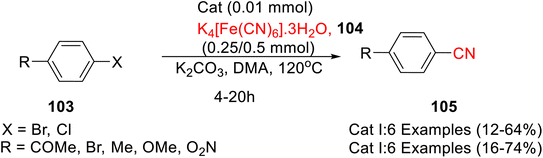 | ||
| Scheme 40 Caffeine-derived N-heterocyclic carbene palladium complexes catalysed cyanation of aryl halides. | ||
A Pd(II) complex containing a tridentate bis-NHC caffeine-derived ligand was synthesized by Bysewski66 and co-workers (Scheme 41) and its catalytic potential towards various coupling reactions was evaluated. The complex exhibited high catalytic activity in the Suzuki–Miyaura between aryl halides 106 and phenylboronic acid 107 to afford 108; Mizoroki–Heck cross-coupling reactions between aryl halides 109 and ethyl acrylate to give 110; Sonogashira cross-coupling of aryl halides 111 with phenylacetylene 112 to afford 113 (Scheme 41). Though the Suzuki reaction involving aryl chlorides did not result in the formation of the product, the analogous bromides as well as iodides yielded the desired products under mild conditions.
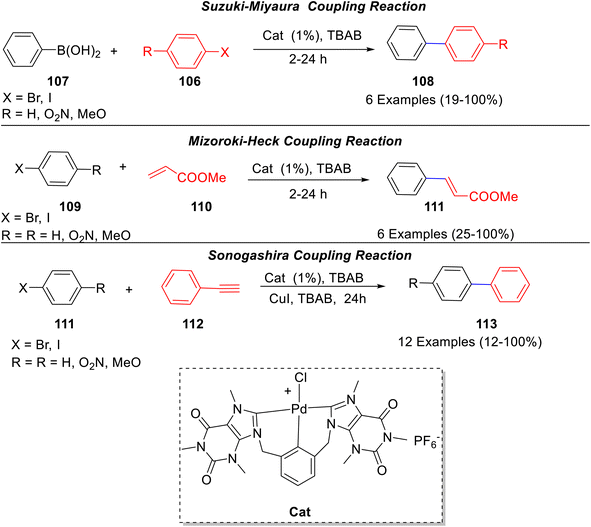 | ||
| Scheme 41 Cross coupling reactions catalyzed by Pd(II) complex containing a tridentate bis-NHC caffeine-derived ligand. | ||
5 Caffeine-based nanoparticles
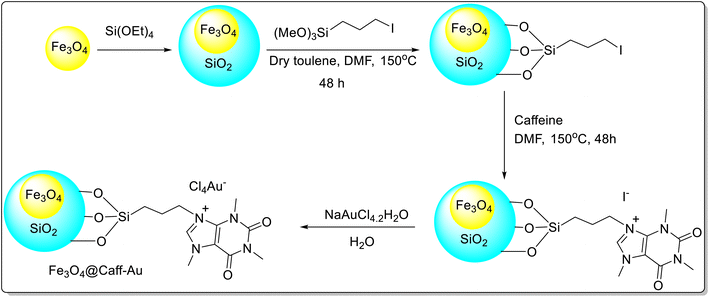
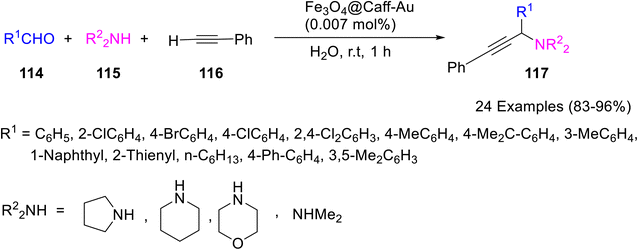
Caffeine has been employed as a stabilizing, capping, and structure-directing agent and as a support material enhancing the stability of nanoparticles via anchoring. In addition to exhibiting outstanding biological activity, these caffeine nanoparticles also serve as an effective catalyst in organic reactions. They can be easily synthesized and recycled many times with little loss in activity. Caffeine support and immobilization can significantly affect the catalytic reactions. Metal nanoparticles can be anchored and stabilized onto caffeine and as a support can transfer electrons from and to the metal providing acidity or basicity to caffeine-supported metal catalysts for organic transformations.Caffeine gold complex supported on magnetic nanoparticles viz. Fe3O4@Caff-Au was prepared (Scheme 42)67 and its catalytic potential towards A3 coupling reactions of terminal alkynes (phenylacetylene) 116, aldehydes 114, and secondary amines 115 was examined. Various amines (piperidine, morpholine, pyrrolidine, and dimethylamine) underwent an A3 coupling reaction successfully affording the targeted products 117 in excellent yields (Scheme 43). The catalyst demonstrated excellent recyclability for at least nine consecutive runs without substantial loss of activity and with a slight accumulation of Au species. The high TOF, low catalyst loading, less reaction time, and use of green recyclable catalysts are some of the prominent features of the protocol.
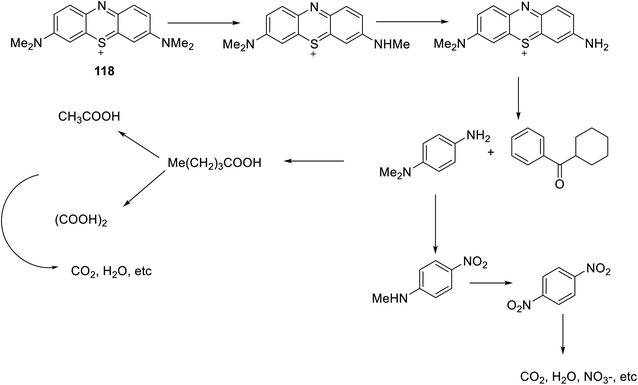
A mitigated orthorhombic structured CeTiO4 NPs immobilized with caffeine and alginate biopolymer blend i.e. Caf–Alg@CeTiO4 was synthesized through chemical coprecipitation reaction by Hasan and co-workers68 The resultant bionanocomposite was probed as a photocatalyst to degrade methylene blue (MB) 118 under visible solar radiation (Scheme 44). Through a combination of statistical model response surface methodology (RSM) and Box–Behnken design (BBD), the optimized values of reaction parameters were computed such as 50 min for solar irradiation, 6.57 pH value, 98.6 mg L−1 MB concentration, and 0.57 g L−1 catalyst dose which favored maximum degradation of MB (98%).
The formation of electrons in the conduction band and holes in the valence band were observed during the reaction process of photoabsorption by Caf–Alg@CeTiO4 material. Initially, water molecules from the solvent media interact with holes and get converted into the reactive ˙OH radicals whereas the O2 molecules entrap electrons and forms O2−˙ radical. The collision of MB molecule with photon, results in the transfer of MB to an excited state, wherein it reacts with O2−˙ radical thereby leading to the formation of various non-toxic degraded products like CO2 and H2O reaction. The plausible fragmentation mechanism of MB by Caf–Alg@CeTiO4 BNC under optimized reaction conditions by O2−˙ radicals has been depicted in Scheme 44.
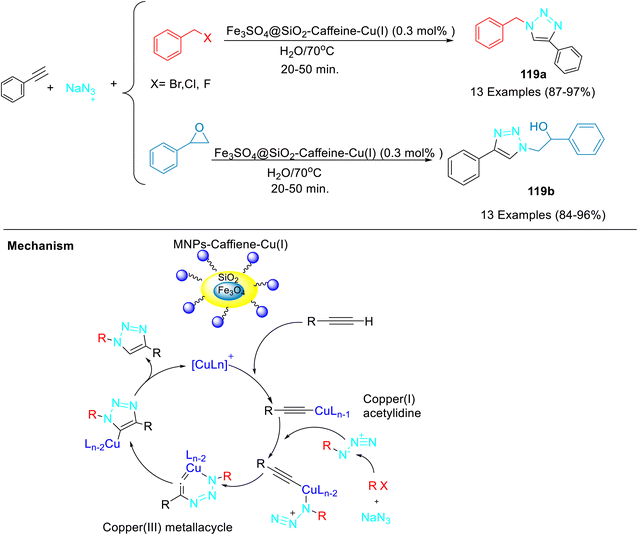
The silica-coated magnetite nanoparticles of Fe3O4 were synthesized and caffeine was immobilized on its surface (Scheme 45).69 The synthesized copper(I)–caffeine complex immobilized on silica-coated magnetite nanoparticles was utilised for the synthesis of 1,2,3-triazoles 119a–b through a reaction between several terminal alkynes and in situ generated organic azides from organic halides as well as epoxides in an aqueous medium (Scheme 46). The authors further studied the reusability of the catalyst for this three-component click reaction. Notably, the catalyst was recycled for at least 5 runs without any significant loss of activity.
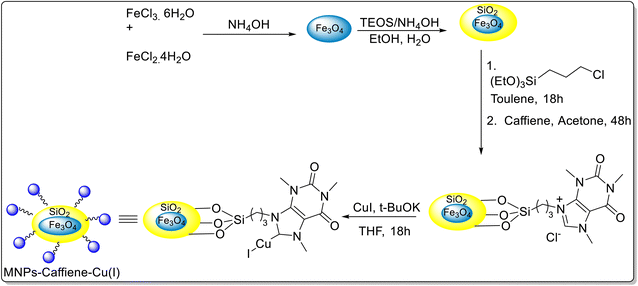 | ||
| Scheme 45 Synthesis of copper(I)–caffeine complex immobilized on silica-coated magnetite nanoparticles (MNPs-caffeine–Cu(I)). | ||
A probable pathway for the synthesis of triazoles has been shown in Scheme 46. Initially, the Cu(I)–acetylidine complex was formed from the reaction between Cu(I) and aryl acetylene. Thereafter, the azide group adds to the copper acetylidine complex to give π-complex as an intermediate. In the next step, terminal nitrogen of azide attacks at the carbon-2 of the Cu–acetylidine to afford a six-membered metallacycle. Subsequent ring contraction and protonolysis give the desired product with the regeneration of the Cu(I) catalyst.
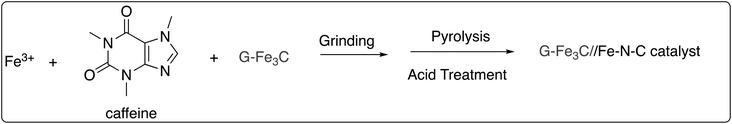
Wang and co-workers reported the synthesis of caffeine-derived graphene-wrapped Fe3C nanoparticles entrapped in hierarchically porous Fe–N–C nanosheets (G-Fe3C/Fe–N–C catalyst) (Scheme 47) and investigated its catalytic properties by employing cathodic oxidation–reduction reaction (ORR) as a model in a 0.1 M KOH solution.70 The obtained catalyst showed electrocatalytic activity better than Pt/C in a similar environment (Eonset = 1.09 V, E1/2 = 0.88 V), coupled with remarkable stability (only 0.18 mV negative shift in E1/2 after 2000 cycles). The remarkable ORR performance of the G-Fe3C/Fe–N–C in the alkaline electrolyte can be accounted for by following facts: (i) the co-doped N and Fe promotes the generation of several active sites for the ORR, owing to strong interaction between Fe3C center and pyridinic N; (ii) the interfacial mass and electron transport for nanosheets is accelerated by large amount contact area and abundant channels of G-Fe3C/Fe–N–C, (iii) graphene-wrapped Fe3C entrapped on graphitic carbon improves the catalytic activity as well as durability. Moreover, the synergistic effects of Fe3C and Fe–N–C increase the ORR performance.
6 Miscellaneous
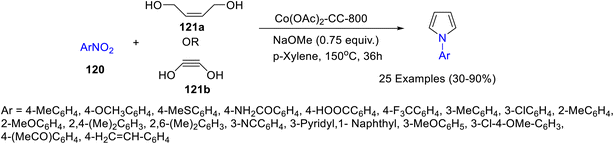
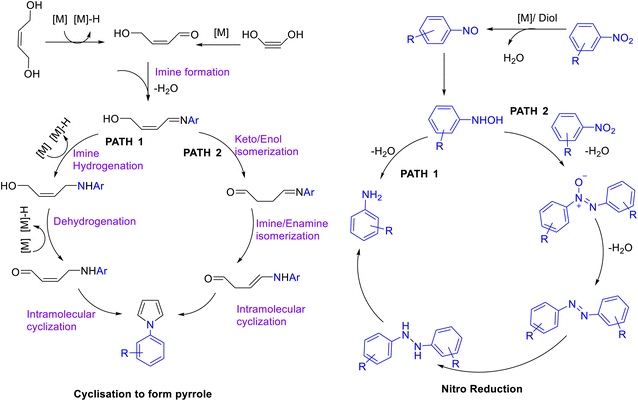
Several caffeine carbon-supported cobalt-based heterogeneous catalysts viz. Co(OAc)2-CC-T, (T = 700 °C, 800 °C, 900 °C) were prepared by Panja and co-workers71 (Scheme 48) and their catalytic potential was studied for the synthesis of pyrroles 123 by coupling between nitroarenes 120 and diols 121a–b. Among all the catalysts Co(OAc)2-CC-800 exhibited the highest catalytic activity for this transformation (Scheme 49). Nitroarenes with electron-donating substituents resulted in a comparatively higher yield of the desired pyrroles in comparison with those containing electron-withdrawing groups. This could be probably due to the higher nucleophilicity of amines (generated in situ), with electron-donating groups which facilitate the cyclization process. The authors have calculated the green chemistry metrics for the protocol involved in synthesis of 1-(4-(methylthio)phenyl)-1H-pyrrole and reported E-factor of 6.19, 73.54% atom economy, 53.97% atom efficiency, 100% carbon efficiency, and 46.06% of reaction mass efficiency, thereby demonstrating the remarkable sustainability of the protocol. Furthermore, the recyclability of the catalyst up to 6 cycles without an appreciable decline in the catalytic activity has been reported by the authors. The probable mechanism for the formation of N-phenylpyrrole consists of two parts: (i) reduction of nitrobenzene to aniline and (ii) C–N coupling followed by cyclization (Scheme 50). The acid leaching experiment suggested an important role of Co–Nx and CoOx in the catalytic activity of Co(OAc)2-CC-800.Subramanian and co-workers72 have explored the potential of caffeine as an organic modifier in the synthesis of hydroxyapatite (HA) nanorods. HA nanorods with high crystallinity with well-defined shapes were formed as conformed by FT-IR, X-ray diffraction studies, and TEM image analysis. The effect of different concentrations of caffeine on the size, shape, and morphology of HA nanorods was also undertaken. The increased concentrations of the caffeine content resulted in an increase in the crystallinity of HA nanorods. As an organic modifier, caffeine produces new surfaces by breaking the bond between the neighbouring atoms thereby resulting in the formation of smaller particles by controlling the driving force between the nanoparticle.
Mary et al.73 have utilized caffeine as stabilizing agent in the synthesis of CuO nanoparticles by precipitation method. The effect of caffeine on the morphology, size and shape of copper oxide nanoparticles was examined by varying the concentrations of caffeine. The synthesized CuO NPs were characterized using Fourier-transform infrared spectroscopy (FTIR), X-ray diffraction study (XRD) scanning electron microscopy diffraction (SEM), and transmission electron microscope (TEM). The morphological study of the nanoparticles revealed that at higher concentrations of caffeine, the leafy particles transformed into spherical nanoparticles.
7 Conclusions
Catalysts are pervasive in synthetic organic chemistry and medicinal chemistry because they enable reactions to be carried out selectively, faster, and under milder experimental conditions. In line with the twelve principles of “Green Chemistry,” caffeine and its derived biobased catalysts adds value as it is environment-friendly, available at low cost, and caffeine extraction and separation from renewable plant sources requires only water and supercritical CO2. Caffeine is also a vital raw material in medicine and food additives because of its biological effects and role in gastric acid secretion, central nervous system, and cardiovascular system. Attempts are also being made to develop caffeine-based gels and drug delivery systems since they will be water-compatible, bio-compatible, and clinically competent.Further, caffeine is also an appealing catalyst. The use of caffeine in multicomponent reactions specifically involving Knoevenagel condensation has been explored with assorted substrates and experimental conditions. Another quality of caffeine that tempts chemists is that in sufficiently acid solutions it converts into conjugate acid. Caffeine being basic, a lone pair of electrons on one of its nitrogen atoms forms the conjugate acid salt under acidic conditions. This also results in an increased water solubility as a cation. The classic example is the caffeine hydrogen sulphate which is an efficient heterogeneous catalyst together with the less explored caffeine-HClO4, caffeine-H3PO4, and caffeine-HNO3 being used in one-pot reactions with water as solvent. Here, the use of these heterogeneous catalysts needs to be further analyzed in reactions.
The imidazole ring of caffeine serves as a common scaffold for the preparation of ionic liquids and its properties can be fine-tuned via altering the nature of the anion and the length or nature of the alkyl chain. The prepared reagent can serve the purpose of Brønsted acidic catalyst and a green solvent. However, the development of caffeine-based ionic liquids is only in its infancy and remains unexplored. The imidazole unit of caffeine has also been exploited for the synthesis of carbene-type ligands and metal complexes with abundantly available earth transition metals. Caffeine NHC complexes have shown antimicrobial, anticancer, and photoluminescent properties alongside their catalytic properties in complex organic reactions. Furthermore, the proper choice of metal and alkyl groups can widen the applicability of the caffeine–NHC complexes. Also, outstanding in vitro results of caffeine–NHC complexes offer promising perspectives in their use as metallodrugs. Thus, the discovery of metal complexes of NHC ligands derived from caffeine contributes to developing a more sustainable and ecological organometallic chemistry.
Caffeine with a high dipole moment, and positive charge on the nitrogen atom, interacts electrostatically with negatively charged groups. The adsorption of caffeine onto inorganic surfaces occurs because caffeine is positively ionized below its pKa. The use of caffeine support in nanoparticles can be attributed to dipole–dipole interactions, where nitrogen atoms, two carbonyl groups, and π-aromatic electrons act as a stabilizing factor in nanoparticles. Herein, the use of caffeine as nanoparticle support opens the door to its catalytic applications which also need thoughtful investigations.
Based on these developments, it is anticipated that the reactions involving many other diverse substrates will be investigated by employing caffeine as a catalyst. Furthermore, there is scope for the development of new classes of caffeine-based catalysts.
Conflicts of interest
There are no conflicts to declare.References
- https://news.mit.edu/2018/polymer-synthesis-gets-jolt-caffeine-0413.
- G. Faudone, S. Arifi and D. Merk, J. Med. Chem., 2021, 64, 7156–7178 CrossRef CAS PubMed.
- T. M. McLellan, J. A. Caldwell and H. R. Lieberman, Neurosci. Biobehav. Rev., 2016, 71, 294–312 CrossRef CAS PubMed.
- J. M. Davis, Z. Zhao, H. S. Stock, K. A. Mehl, J. Buggy and G. A. Hand, Am. J. Physiol.: Regul., Integr. Comp. Physiol., 2003, 284, R399–R404 CrossRef CAS.
- J. Shearer and T. E. Graham, Nutr. Rev., 2014, 72, 121–136 CrossRef PubMed.
- L. L. Spriet, Sports Med., 2014, 44, 175–184 CrossRef.
- J. J. Barone and H. R. Roberts, Food Chem. Toxicol., 1996, 34, 119–129 CrossRef CAS PubMed.
- S. Li, J. Berger and S. Hartland, Anal. Chim. Acta, 1990, 232, 409–412 CrossRef CAS.
- Encyclopedia of Dietary Supplements, CRC Press, 2010 Search PubMed.
- B. A. Weinberg and B. K. Bealer, The World of Caffeine, Routledge, 2004 Search PubMed.
- F. F. Runge, Neueste phytochemische Entdeckungen zur Begründung einer wissenschaftlichen Phytochemie, 1820, vol. 1 Search PubMed.
- N. P. Adelon, Dictionnaire de médecine, 1822, vol. 4 Search PubMed.
- H. Ashihara, H. Sano and A. Crozier, Phytochemistry, 2008, 69, 841–856 CrossRef CAS PubMed.
- R. Huang, A. J. O'Donnell, J. J. Barboline and T. J. Barkman, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 10613–10618 CrossRef CAS PubMed.
- H. Ashihara, T. Yokota and A. Crozier, in Advances in Botanical Research, Academic Press Inc., 2013, vol. 68, pp. 111–138 Search PubMed.
- A. F. M. Cláudio, A. M. Ferreira, M. G. Freire and J. A. P. Coutinho, Green Chem., 2013, 15, 2002 RSC.
- L. Wen, Z. Zhang, D. Rai, D. W. Sun and B. K. Tiwari, J. Food Process Eng., 2019, 42(6), e13191 CrossRef.
- M. Sökmen, E. Demir and S. Y. Alomar, J. Supercrit. Fluids, 2018, 133, 171–176 CrossRef.
- G. R. Lopes, C. P. Passos, C. Rodrigues, J. A. Teixeira and M. A. Coimbra, Food Res. Int., 2020, 129, 108864 CrossRef CAS PubMed.
- D. V. Bermejo, J. A. Mendiola, E. Ibáñez, G. Reglero and T. Fornari, Food Bioprod. Process., 2015, 96, 106–112 CrossRef CAS.
- X. Jun, J. Food Eng., 2009, 94, 105–109 CrossRef CAS.
- A. Loukri, C. Sarafera, A. M. Goula, K. Gardikis and I. Mourtzinos, Appl. Food Res., 2022, 2, 100176 CrossRef CAS.
- I. M. Koina, Y. Sarigiannis and E. Hapeshi, Separations, 2023, 10, 121 CrossRef CAS.
- I. Usman, M. Hussain, A. Imran, M. Afzaal, F. Saeed, M. Javed, A. Afzal, I. Ashfaq, E. Al Jbawi and S. A. Saewan, Int. J. Food Prop., 2022, 25, 1215–1233 CrossRef CAS.
- E. Fischer and L. Ach, Ber. Dtsch. Chem. Ges., 1895, 28, 3135–3143 CrossRef CAS.
- W. Traube, Ber. Dtsch. Chem. Ges., 1900, 33, 3035–3056 CrossRef CAS.
- E. Fischer, Ber. Dtsch. Chem. Ges., 1898, 31, 3266–3277 CrossRef CAS.
- H. Biltz and P. Damm, Justus Liebigs Ann. Chem., 1917, 413, 186–197 CrossRef.
- J. Yamawaki, T. Ando and T. Hanafusa, Chem. Lett., 1981, 10, 1143–1146 CrossRef.
- S. Yang, Z. Dong, C. Yin, H. Yue, W. Gao and F. Yang, J. Chin. Chem. Soc., 2020, 67, 1715–1720 CrossRef CAS.
- A. Shalmashi and F. Golmohammad, Lat. Am. Appl. Res., 2010, 40, 283–285 CAS.
- A. Y. E. Abadi, M. T. Maghsoodlou, R. Heydari and R. Mohebat, Res. Chem. Intermed., 2016, 42, 1227–1235 CrossRef CAS.
- F. Mohamadpour, Bull. Chem. Soc. Ethiop., 2019, 33, 149–158 CrossRef CAS.
- R. Mohebat and A. Yazdani-Elah-Abadi, Chin. Chem. Lett., 2017, 28, 1340–1344 CrossRef CAS.
- A. R. Liandi and A. H. Cahyana, J. Pharm. Res., 2022, 26, 954–961 CAS.
- F. Mohamadpour, Org. Prep. Proced. Int., 2020, 52, 453–457 CrossRef CAS.
- S. M. Habibi-Khorassani, M. Shahraki and S. S. Pourpanah, Orient. J. Chem., 2016, 32, 1559–1570 CrossRef CAS.
- X. Bantreil, P. Navals, J. Martinez and F. Lamaty, Eur. J. Org. Chem., 2015, 2015, 417–422 CrossRef CAS.
- A. M. DiCiccio, Y. A. L. Lee, D. L. Glettig, E. S. E. Walton, E. L. de la Serna, V. A. Montgomery, T. M. Grant, R. Langer and G. Traverso, Biomaterials, 2018, 170, 127–135 CrossRef CAS PubMed.
- S. J. Saghanezhad, M. H. Sayahi, I. Imanifar, M. Mombeni and S. Deris Hamood, Res. Chem. Intermed., 2017, 43, 6521–6536 CrossRef CAS.
- S. Agarwal, R. Poddar, M. Kidwai and M. Nath, ChemistrySelect, 2018, 3, 10909–10914 CrossRef CAS.
- S. J. Saghanezhad, Rev. Roum. Chim., 2018, 63(1), 67–72 Search PubMed.
- P. Kalal, A. Sethiya, J. Soni, I. Patel, D. Gandhi and S. Agarwal, J. Sulfur Chem., 2022, 43, 233–241 CrossRef CAS.
- M. Sudileti, V. Chintha, S. Nagaripati, M. Gundluru, S. H. Yasmin, R. Wudayagiri and S. R. Cirandur, Med. Chem. Res., 2019, 28, 1740–1754 CrossRef CAS.
- Z. Lei, B. Chen, Y.-M. Koo and D. R. MacFarlane, Chem. Rev., 2017, 117, 6633–6635 CrossRef PubMed.
- R. Tayebee, M. Jomei, B. Maleki, M. K. Razi, H. Veisi and M. Bakherad, J. Mol. Liq., 2015, 206, 119–128 CrossRef CAS.
- S. J. Saghanezhad and S. Sayyahi, Res. Chem. Intermed., 2017, 43, 2491–2500 CrossRef CAS.
- M. Salami and A. Ezabadi, Res. Chem. Intermed., 2020, 46, 4611–4626 CrossRef CAS.
- M. Salami and A. Ezabadi, Res. Chem. Intermed., 2019, 45, 3673–3686 CrossRef CAS.
- E. Hataminejad and A. Ezabadi, Res. Chem. Intermed., 2022, 48, 2535–2556 CrossRef CAS.
- H. Ghafuri, S. Yaghoubi and H. R. E. Zand, Appl. Organomet. Chem., 2019, 33(10), e5149 CrossRef.
- D. Meng, D. Li and T. Ollevier, RSC Adv., 2019, 9, 21956–21963 RSC.
- M. J. Clarke and H. Taube, J. Am. Chem. Soc., 1975, 97, 1397–1403 CrossRef CAS.
- H. J. Krentzien, M. J. Clarke and H. Taube, Bioinorg. Chem., 1975, 4, 143–151 CrossRef CAS PubMed.
- V. Stoppa, T. Scattolin, M. Bevilacqua, M. Baron, C. Graiff, L. Orian, A. Biffis, I. Menegazzo, M. Roverso, S. Bogialli, F. Visentin and C. Tubaro, New J. Chem., 2021, 45, 961–971 RSC.
- W. Beck and N. Kottmair, Chem. Ber., 1976, 109, 970–993 CrossRef CAS.
- D. Brackemeyer, A. Hervé, C. Schulte to Brinke, M. C. Jahnke and F. E. Hahn, J. Am. Chem. Soc., 2014, 136, 7841–7844 CrossRef CAS.
- A. Kascatan-Nebioglu, A. Melaiye, K. Hindi, S. Durmus, M. J. Panzner, L. A. Hogue, R. J. Mallett, C. E. Hovis, M. Coughenour, S. D. Crosby, A. Milsted, D. L. Ely, C. A. Tessier, C. L. Cannon and W. J. Youngs, J. Med. Chem., 2006, 49, 6811–6818 CrossRef CAS PubMed.
- B. Bertrand, L. Stefan, M. Pirrotta, D. Monchaud, E. Bodio, P. Richard, P. Le Gendre, E. Warmerdam, M. H. de Jager, G. M. M. Groothuis, M. Picquet and A. Casini, Inorg. Chem., 2014, 53, 2296–2303 CrossRef CAS PubMed.
- F. T. Luo and H. K. Lo, J. Organomet. Chem., 2011, 1262–1265 CrossRef CAS.
- E. Mohammadi and B. Movassagh, J. Mol. Catal. A: Chem., 2016, 418–419, 158–167 CrossRef CAS.
- J. Zhang, M. M. Rahman, Q. Zhao, J. Feliciano, E. Bisz, B. Dziuk, R. Lalancette, R. Szostak and M. Szostak, Organometallics, 2022, 41, 1806–1815 CrossRef CAS PubMed.
- M. M. Rahman, J. Zhang, Q. Zhao, J. Feliciano, E. Bisz, B. Dziuk, R. Lalancette, R. Szostak and M. Szostak, Organometallics, 2022, 41, 2281–2290 CrossRef CAS PubMed.
- F. Mazars, G. Zaragoza and L. Delaude, J. Organomet. Chem., 2022, 978, 122489 CrossRef CAS.
- Q. Teng, Y. Zhao, Y. Lu, Z. Liu, H. Chen, D. Yuan, H. V. Huynh and Q. Meng, Organometallics, 2022, 41, 161–168 CrossRef CAS.
- O. Bysewski, A. Winter and U. S. Schubert, Inorganics, 2023, 11, 164 CrossRef CAS.
- M. Gholinejad, M. Afrasi and C. Najera, Appl. Organomet. Chem., 2019, 33(4), e4760 CrossRef.
- I. Hasan and F. A. Alharthi, J. Photochem. Photobiol., A, 2022, 433, 114126 CrossRef CAS.
- A. Salamatmanesh, M. Kazemi Miraki, E. Yazdani and A. Heydari, Catal. Lett., 2018, 148, 3257–3268 CrossRef CAS.
- M.-T. Chen, Z.-X. Huang, X. Ye, L. Zhang, J.-J. Feng and A.-J. Wang, J. Colloid Interface Sci., 2023, 637, 216–224 CrossRef CAS PubMed.
- D. Panja, A. Sau, B. Balasubramaniam, P. Dhara, R. K. Gupta and S. Kundu, J. Catal., 2021, 402, 244–254 CrossRef CAS.
- R. Subramanian, P. Murugan, G. Chinnadurai, K. Ponmurugan and N. A. Al-Dhabi, Mater. Res. Express, 2020, 7, 015022, DOI:10.1088/2053-1591/ab619a.
- A. P. A. Mary, A. T. Ansari and R. Subramanian, Inorg. Nano-Met. Chem., 2021, 51, 174–181 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |