Open Access Article
Open Access ArticlePulsed electric field technology as a promising pre-treatment for enhancing orange agro-industrial waste biorefinery
Ramon Bocker and
Eric Keven Silva *
*
Faculdade de Engenharia de Alimentos (FEA), Universidade Estadual de Campinas (UNICAMP), Rua Monteiro Lobato, 80, Campinas-SP CEP:13083-862, Brazil. E-mail: ekeven@unicamp.br
First published on 9th January 2024
Abstract
In the processing of orange juice, 50–70% of the fresh fruit weight is converted into organic waste. Orange processing waste (OPW) primarily consists of peels, seeds, and pulp. Improper disposal of this residue can lead to greenhouse gas emissions, environmental pollution, and the wastage of natural resources. To address this ecological issues, recent research has focused on developing innovative process designs to maximize the valorization of OPW through biorefinery strategies. However, the current challenge in implementing these methods for industrial waste management is their significant energy consumption. In response to these challenges, recent studies have explored the potential of employing pulsed electric field (PEF) technology as a pre-treatment to improve energy efficiency in biorefinery processes. This non-thermal and emerging technology can enhance the mass transfer of intracellular components via electroporation of cell walls, thereby resulting in shorter processing times, lower energy inputs, greater retention of thermosensitive components, and higher extraction yields. In this regard, this review offers a comprehensive discussion on the innovative biorefinery strategies to the valorization of OPW, with a specific focus on recent studies assessing the technical feasibility of methodologies for the extraction of phytochemical compounds, dehydration processes, and bioconversion methods. Recent studies that discussed the potential of PEF technology to reduce energy demand by increasing the mass transfer of biological tissues were emphasized.
1 Introduction
Orange production is one of the most relevant contributors to the economic output of the agro-industrial sector. Globally, oranges rank as the most produced and consumed fruit. This prominent position can be primarily attributed to the exceptional nutritional profile and widespread sensory appeal that oranges offer.1,2 These attributes have led to an increasing demand for oranges, resulting in a significant share of total citrus fruit production. Orange production accounts for a noteworthy 82% of total citrus fruit production, 47% of global imports, and 44% of global exports of citrus fruits.3 Nonetheless, these statistics underscore the profound global significance of the orange supply chain.The large-scale processing chain of orange fruit results in a proportional generation of waste. In the orange juice processing 50–70% of the fresh fruit processed is converted into organic waste, consisting of peels, internal tissues, and seeds.4 This orange processing waste (OPW) produced raises ecological concerns due to the high volume produced globally. Currently, the disposal methods used for managing these residues include ensiling, landfilling, and incineration.5 Nevertheless, these practices have some drawbacks, including low energy-efficiency, production of leachate, emission of greenhouse gasses, water and soil pollution, disruption to wildlife, and resource wastage.4,6 Limitations that underscore the pressing need for effective OPW management strategies.
OPW has a high availability of components recognized as potential bioresources for several industrial segments, such as food production, pharmaceuticals, and cosmetics.7,8 The main methods for OPW valorization include phytochemical extraction, dehydration, and bioconversions.9–11 Resistance of plant cells to the mass transfer of their intracellular components limits the energy efficiency of these biorefinery methods. This barrier promoted by cell walls restrict the overall process energy-efficiency, as more energy is required when there is greater resistance to mass transfer.12 Therefore, to address these challenges, recent studies have explored the use of innovative technologies to integrate the biorefinery of OPW through improving the accessibility of their intracellular compounds.
Recent studies have explored the integration of pulsed electric field (PEF) technology into biorefinery processes. This emerging technology applies electric fields to the food matrix, promoting the formation of pores in biological cells. This phenomenon, well-known as electroporation, destabilizes the lipid molecules that primarily constitute the cell membrane, thereby enhancing the mass transfer of intracellular components into the different solvents. Indeed, depending on electric field intensity, temporary or permanent pores or channels are created in the cell membrane, allowing the passage of molecules, ions, and other particles through the membrane that would normally be blocked. By making cell walls more permeable, PEF technology can integrate extraction, dehydration, and bioprocessing, reducing the energy demand and time of the processes. These aspects contribute to the economic feasibility of an industrial process for the valorization of OPW. Nevertheless, further research is essential to assess economic feasibility and enhance the process conditions and parameters for the large-scale industrial integration of PEF.12–14
Currently, some studies present PEF technology as an environmentally friendly and promising method for improving the extraction of phytochemical compounds from biological tissues. As demonstrated in recent studies, pre-treatment with PEF technology is a suitable approach to enhance the extraction of soluble dietary fiber, limonene, naringin, phenolic compounds, polyphenols, hesperidin, narirutin, and ascorbic acid from OPW.15–19 The use of PEF as a pre-treatment in extraction processes can enhance higher yields, shorter processing times, and reduced reliance on organic solvents. Attributes that make PEF a suitable technology for reducing overall extraction costs.
On the other hand, OPW biorefinery, such as dehydration and bioconversion methods, have scarce literature regarding the technical feasibility of PEF application and represent a potential for future research.20,21 In the context of dehydration, as presented in study,21 the utilization of PEF reduces the energy required for water evaporation, thereby reducing the energy demand of the process. Additionally, PEF shows promise in enhancing access to sugars in plant tissue, leading to higher concentrations available for fermentation. This may result in a reduction in the overall energy expenditure of the fermentation process.
Fig. 1 presents a significant increase in research output between 2013 and 2023 concerning PEF technology within the Scopus database, using “pulsed electric field technology” as the keyword. This underscores the growing interest and relevance of PEF, positioning it as an essential subject for future studies. It is important to note that further studies are necessary to evaluate the techno-economic feasibility and optimize the process parameters for the integration of PEF technology into OPW biorefinery processes.
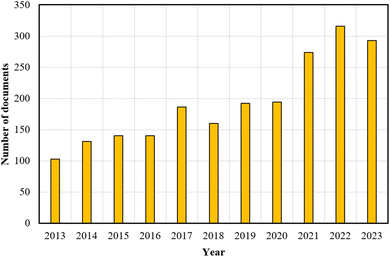 | ||
| Fig. 1 The number of documents returned from a Scopus database search using the keyword “pulsed electric field technology”. | ||
Addressing the increasing global demand for efficient strategies in biorefinery processes for OPW, this review conducted a comprehensive discussion on the potential of PEF treatment to enhance mass transfer in biological tissues reducing the energy demand. Recent advances in pathways for OPW valorization were emphasized. Furthermore, valuable insights into the integration of biorefinery methods with PEF technology to enhance energy-efficiency were provided.
2 Orange processing chain
2.1 Global economic landscape
Agro-industry is a significant contributor to the global economy output, representing 20% of the gross domestic product in emerging countries.1,22 Among fruit crops, citrus fruits account for 98% of industrialized crops.1 These commodities exhibit a substantial import and export values, estimated at 14.57 million metric tons and 15.62 million metric tons, respectively.22 As a result, oranges production stands up as a relevant contributor to the global agricultural output, generating both employment opportunities and revenue for local economies.23Nowadays, the estimated orange production for the 2022–2023 period is to be 5% lower than 47.5 Mt. This decrease is primarily attributed to reduced production in the European Union and the United States but is partially compensated by an increase in Egypt production. Therefore, both consumption and the amount of fruit used for processing have declined.22 However, the worldwide demand for oranges has historically increased due to the dissemination of information regarding their high technological and nutritional quality. This has elevated orange crops to become one of the most important fruits for the agro-economy globally, leading both in terms of production and total acreage.
Global citrus fruit production in 2020 reached approximately 158.49 Mt. Asia emerged as the leading global contributor to orange production, followed by Africa, the Americas, Europe, and Oceania. Within citrus-producers, China led with a production of 44.63 Mt of citrus fruits, corresponding to 28.16% of the over-all production of citrus fruit in 2020. Brazil, Mexico, and India are other important countries, each manufacturing over 5% of the total global citrus fruit production in 2020.22 Worldwide, about 10.07 million hectares of land are dedicated to citrus fruit production. China, Brazil, Nigeria, India, and Mexico are the main worldwide citrus-production.22 This extensive use of land on a worldwide scale has led to increased research efforts aimed at mitigating citrus production chain ecological footprint.
Sweet oranges account for approximately 60% of the entire production of fresh fruit and processed juice consumption. From 2021 to 2022, there was an observed increase of 1.4 Mt in the total orange production destined for the citrus processing industry.22 Out of the total citrus fruit production, more than 20% is allocated for processing, mainly in the orange juice industry.1 From this point of view, oranges play a significant role on the global economic landscape, being commercialized as a fresh fruit, or processed for juice production, besides other by-products.
2.2 Orange juice processing
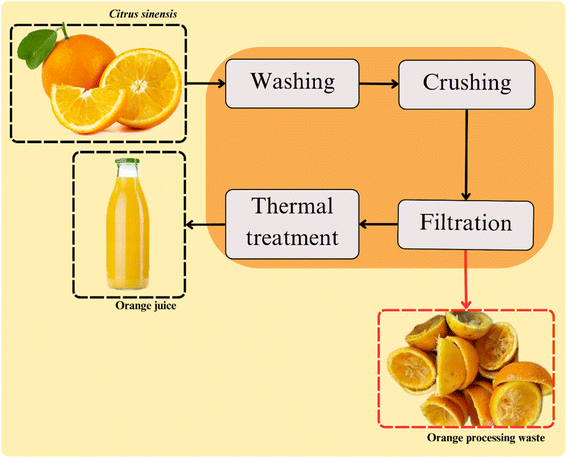
The conventional method used for orange juice extraction is based on four steps. These include washing, crushing, filtration, and thermal treatment (Fig. 2).Oranges processing first step is the fruit selection and washing. This step is conducted to ensure the beverage quality and safety by removing the remaining dirt, chemical residues, and other contaminants from the postharvest. Washing typically involves adding organic acids and chlorine-based compounds into the washing water to reduce microbial activity. The generated waste of this step includes residual washing water and oranges that do not meet the industry quality standards.24,25
Crushing is the next stage of orange processing, which is conducted through mechanical methods, such as centrifugation and pressing. At this step, the fruit is crushed or cut and squeezed for the juice extraction. The waste generated in this stage includes pulp, seeds, peel, and fruit membranes. Enzymatic methods are considered an effective pre-treatment for mechanically crushed oranges. Despite their higher cost and slower processing speed, they can significantly enhance the release of biocomponents from plants tissues. This higher extraction yield is due to the enzyme activities on the cell walls of the fruits. The main enzymes used in the process are pectinases and cellulases. These catalysts act on the food matrix promoting the release of more components that contribute to the beverage's sensory profile.26
Performed after juice extraction, the filtration step is carried out for ensuring the beverage quality. At this stage the peel fragments, seeds, and pulp that are remaining on the beverage are removed. Subsequently, the clarification is usually performed to adjust the juice turbidity, ensuring its suitable sensory aspect. In this step, enzymes are typically added to break down suspended particles in the beverage, including pectin, proteins, and other components in suspension. Pectinases are catalysts widely used to pectin degradation, contributing to the viscosity and clarity of the beverage.27,28
Afterward, the juice conventionally undergoes a heat treatment to ensure food quality and safety by reducing the microbial and enzyme activities. For the orange juice, the time/temperature binomial typically employed are sufficient for the inactivation of pectinolytic enzymes in the beverage.29 This is crucial for ensuring the kinetic stability of the beverage. Fact that is related with the property of pectin (targeted by these enzymes) on maintaining solid compounds suspended.30,31
3 Orange agro-industrial waste from sweet orange (Citrus sinensis)
3.1 Characterization of OPW
Orange (Citrus sinensis) has a high amount of micro and macronutrients and bioactive compounds recognized as promising biological resources. The main components of oranges composition are water, carbohydrates, fibers, vitamins (vitamin C, vitamin E, B-complex vitamins and, and vitamin A), minerals (potassium, calcium, and magnesium), flavonoids, organic acids, carotenoids, and essential oils.8 These components composition and distribution in the fruit vary according to some factors, such as species, cultivation conditions, and degree of ripeness.7 This nutritional profile underscore orange as a suitable and health product for consumers.Depending on the composition and processing technology, citrus fruits processing for juice extraction generates an organic waste ranging from 50–70% (w/w) of the fresh fruit weight. This OPW is composed of peels, internal tissues, and seed. Material that has different fractions of fiber, saccharides, protein, minerals, terpenes, fat and volatile compounds.4 These components are concentrated in the mesocarp and epicarp of the fruit. In the epicarp, there are present significant amounts of terpenes, fat, and volatile compounds. On other hand, the fibers, saccharides, minerals and proteins are available in the mesocarp.4
OPW is composed of peel, seed, pulp, and water. Residue that has a high a availability of components recognized as potential bioresources primarily destined for industrial segments, such as food production, pharmaceuticals, and cosmetics.32 These organic materials present a series of utilization opportunities, such as the production of biofuel, packaging material, bio-adsorbent, bio-fertilizer, and activated carbon.4,10,32,33
3.2 Nutraceutical properties
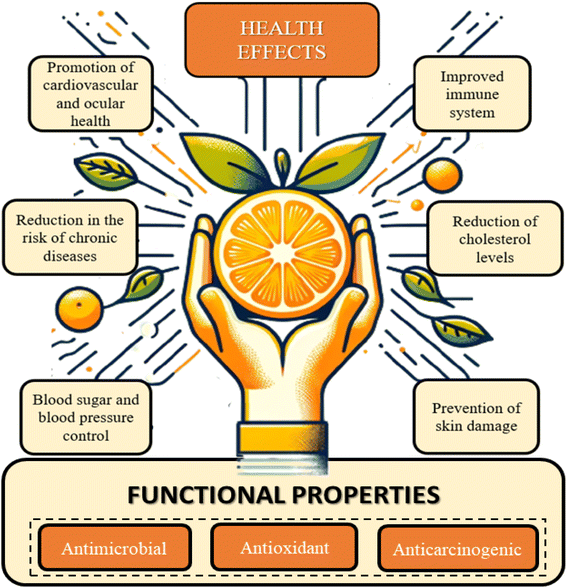
Oranges has a great nutritional profile due to the nutraceutical properties of several micro- and macronutrients present in their composition. The principal phytochemical and bioactive components in the fruit are flavonoids, terpenes, vitamin C, carotenoids, fibers, and others. Constituents that have their regular intake consistently related with health and wellness promotion, such as antiviral, antioxidant, antifungal, and anticarcinogenic activity.7,8,34–36Fig. 3 provides an overview of the primary functional properties and health-promoting effects associated with the adequate intake of orange components. These encompass a spectrum of benefits, including immune system enhancement, promotion of cardiovascular and ocular health, improved digestion, reduced risk of non-communicable chronic diseases, regulation of blood sugar and blood pressure levels, enhanced hydration, lowered cholesterol levels, and prevention of skin damage.7,37
Orange nutritional profile exhibits an excellent dietary fiber content. The regular intake of this carbohydrate is consistently associated with effects that promote longevity and quality of life. These include the contribution of soluble fiber (SF) to lowering blood glucose and lipid levels, mitigating the chances of cardiovascular diseases and colorectal cancer, boosting gastrointestinal immunity, and promoting satiety.7 As for insoluble fibers (IF), they play a key role in water absorption in the digestive tract and the prevention and relief of constipation.36
Also related with health promotion, flavonoids are components with relevant functional properties.35 Ciumărnean et al. (2020) elucidate in their study that the intake of flavonoids reduces the risk of non-communicable chronic and cardiovascular diseases, as well as the prevention of carcinogenic processes. These beneficial effects are a consequence of antioxidant and anti-inflammatory properties provided by flavonoids, which makes oranges regular consumption a health-promoting factor.37,38
Terpenes, such as limonene, are another class of nutraceutical compounds present. These components exhibit therapeutic effects attributed to their ability to protect cellular components from oxidative stress.39 This property is related to a reduced risk of cancerous processes and heart diseases.40,41 Vitamin C also represents a health-promoting component present in oranges, whose antioxidant action has consistently been related with a decrease in the risk of chronic diseases.42 Furthermore, studies also indicate the role of ascorbic acid in act on the immune system health and preventing diseases, such as scurvy.43
Regarding the mineral composition of the fruit, the most abundant are potassium, magnesium, and calcium.44 The first one has important effects on the control of muscle function, blood pressure, and fluid balance in cellular components. Meanwhile, magnesium and calcium are also nutritionally relevant as they prevent muscle weakness and osteoporosis. Additionally, other micronutrients available in smaller proportions are thiamine and folate, which are important components for the nervous system proper functioning.45
3.3 Technological properties
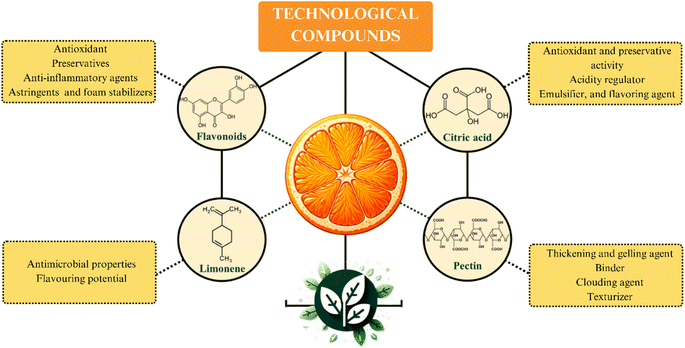
Fig. 4 presents a schematic representation of the primary components of oranges with significant technological applications and their respective utilizations. As depicted, these orange components demonstrate noteworthy technological applications, contributing economic value to their by-products.32 These compounds find extensive usage, particularly within the realms of the food, pharmaceutical, and cosmetic industries.Pectin is one of the most relevant technological components of the orange. This SF has a widespread range of applications in the food industry, such as a thickening and gelling agent, binder, clouding agent, and texturizer. Pectin is usually added in formulations aiming to improve texture and consistency of food products, such as pie fillings, sauces, preserves, and jellies. Additionally, the technological activity of this SF is also explored in fruit juice processing as a stabilizing agent. In the juice formulation step, pectin promotes kinetic stability by slowing up the sedimentation process.9
Citric acid also has relevant technological properties, such as antioxidant and preservative activity, acidity regulator, emulsifier, and flavoring agent.46 These characteristics add economic value to this organic acid, which has an extensive range of applications in food, cosmetics, and pharmaceuticals sectors.46 Another technological component in oranges composition is limonene. This lipophilic terpene exhibits antimicrobial properties and aromatic potential that contribute to a diverse array of applications, which include volatile organic compounds; fragrance in cosmetics and perfumes; flavoring in ice creams; solubilizing agent in medications; insecticide and pesticide.7,47
Flavonoids are another class of compounds with relevant technological applications present in significant amounts in citrus fruit. These phenolic compounds are used in sectors of the pharmaceutical, food, and oral hygiene product industries, among others. These molecules exhibit important activities as antioxidants (hesperidin and naringin), preservatives (hesperidin), anti-inflammatory agents (hesperidin and diosmin), astringents (hesperetin and naringenin), and foam stabilizers (naringin).48
4 Circular economy and key challenges
One of the central pillars of the circular economy is developing renewable and sustainable production chains. This economic model, aligned with the principles of sustainability, focuses on promoting a sustainable cycle of production, consumption, and disposal. This economical approach contributes to the reduction of the environmental impact generated by the growing global demand for natural resources. To achieve this goal, the circular economy explores process design that mitigates the wastage of bioresources. One of the fronts of this economic model is based on the reuse or conversion of agro-industrial waste into new products and ingredients.1,49Ensuring the feasibility of an industrial process while preserving natural resources stand as one of the primary challenges in its design. The method adopted for the disposal of residues significantly impacts the guarantee of a lower environmental impact. This is because of a high volume of waste generated in the beverage processing, which corresponds to approximately 50% of the mass of fresh fruit.32 These organic materials, when not properly disposed of, can lead to water and soil pollution, greenhouse gas emissions, harm to fauna, and waste of natural resources. Therefore, the treatment of this high annual volume of waste generated globally is essential for an eco-friendly processing chain.1
Nowadays, the conventional methods for the disposal of organic waste generated in orange processing are ensiling, landfilling, and incineration.5 These activities present many disadvantages, such as high energy demand, leachate production, greenhouse gas generation, and waste of natural resources.4,6 Therefore, aiming to overcome these issues, the valorization of orange components through the comprehensive use of OPW is investigated through biorefinery methods. However, some limitations are remaining for the implementation of these opportunities on an industrial scale, such as the high volume produced, seasonality, and limonene content.4,50 In addition, industrial-scale suitability also requires further studies on economic feasibility, aiming for higher energy efficiency and shorter processing time.12,13
Recent studies have explored the energy-efficiency of process integration strategies. This integration of different technologies for biomass valorization allows optimization of natural resource waste and promotes cost reduction and environmental impact mitigation in orange processing. Consequently, these integrated technologies facilitates the bioconversion of these residues into many by-products with health and sustainability appeal.12
The Clevenger hydrodistillation is a well-established method for the recovery of essential oil from OPW.51–53 This process generates wastewater rich in phytochemicals, which presents challenges for responsible waste management. Citrus wastewaters have a highly variable composition due to variations in raw material composition and industrial conditions. These limitations pose a challenge in establishing cost-effective biorefinery methods for waste management. Therefore, the typical fate of this material is treatment and subsequent disposal in the environment.54 However, several studies shown many opportunities being investigated to valorize citrus wastewater. This allows for the consideration of reusing the waste components to obtain dyes, antioxidants, nutraceuticals, antimicrobials, and flavorings.55
Beyond the environmental impact, the current management methods of these residues also lead to significant economic loss. This is because citrus waste, composed of peel, seed, pulp, and wastewater, has a high availability of components with high nutritional and technological value. These components enable the potential use of these residues as bioresources for industrial sectors such as food, pharmaceutical, and cosmetic production. These materials exhibit components with high nutritional and technological attributes, allowing their conversion into by-products with a significant market price. Therefore, biorefinery strategies to valorize orange processing waste has been increasingly explored in recent research.12,54–56
The effective management of orange waste is crucial for the feasibility of orange processing. Recent studies have extensively investigated the integration of innovative technologies into the valorization of agro-industrial waste. These environmentally friendly technologies, including PEF, supercritical carbon dioxide, and high-intensity ultrasound, offer sustainable solutions for valorization of biocomponents.52,57–59 By utilizing non-thermal processes, these unconventional technologies reduce the energy demand and aid in the preservation of heat-sensitive components.54–56
Although the search for opportunities to valorize citrus waste is environmentally positive for implementation in industrial systems, some challenges need to be overcome. One of the primary limitations is the difficulty and high investment needed for residue collection and transportation. Furthermore, the quality of the waste is also a limiting factor, as any physicochemical alterations or contamination directly affect the product quality.1 Another important aspect in the conversion of these residues into by-products is the need for suitable processing technologies, which may require a significant initial investment. Considering all these factors, the biorefinery of orange processing waste demands economically sustainable processes that demonstrate technical feasibility and comply with current waste treatment regulations.33
5 Recent pathways for OPW valorization
OPW have many components with uses in the food, pharmaceutical, and cosmetic industries. The non-soluble carbohydrates present in this residue composition can be applied as gelling agents, structures for solid–liquid fermentation, and stabilizing. Additionally, the soluble carbohydrates, such as sucrose, fructose and galactose can contribute to the fermentation process as a carbon feedstock. Thus, the content of lipids present in OPW composition can be applied to produce biofuels. Polyphenols and flavonoids, owing to their antioxidant properties, find application in the pharmaceutical, food, and cosmetic sectors. The essential oils, limonoids, pigments and carotenoids have several uses in the cosmetic and pharmaceutical industries as flavoring, natural antioxidants, and flavorings.Many studies focus on exploring new ways to utilize OPW, aiming to valorize this residue, which is rich in micro and macronutrients. Table 1 highlights OPW versatility, yielding valuable components such as soluble dietary fiber, limonene, naringin, phenolic compounds, polyphenols, hesperidin, sugar-rich concentrate, narirutin, ascorbic acid, biobutanol, ethanol, biogas, methane, silver nanoparticles, silver oxide nanoparticles, short-chain fatty acids, α-amylase, ellagic acid, mucic acid, endo-polygalacturonase, and citric acid.4,9–11,46,47,58,60–70 Utilizing these residues for new products supports sustainable practices by reducing food waste and aligning with eco-friendly methods.32
| Raw material | Process | By-product | Ref. |
|---|---|---|---|
| Peel and pulp of sweet orange | Acid hydrolysis | Pectin | Vaez, et al. (2021)9 |
| Sweet orange peel | Solid-liquid extraction with hexane and other acids | Limonene | Ozturk, et al. (2019)47 |
| Fruit peel residues (Citrus limetta) | High intensity ultrasound | Limonene | Khandare, Tomke, and Rathod (2021)58 |
| Sweet orange pulp | Hot extraction | Concentrated dietary fibers | Perez-Pirotto, et al. (2022)60 |
| Rice residues (Oryza sativa) and orange peel | Clostridium sp. in a fermentation bioreactor with a volume of 1 L at 500 rpm and 37 °C | Biobutanol | Su, et al. (2022)10 |
| Orange peel and activated sludge from waste | Fermentation bioreactor with orange and iodine in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio, pH 9, 200 rpm, 35 °C for 10 days 2 ratio, pH 9, 200 rpm, 35 °C for 10 days |
Short-chain fatty acids | Shao, et al. (2023)11 |
| Peel and pulp of sweet orange | S. cerevisiae in a fermentation bioreactor at 38 °C for 3 days | Ethanol | Vaez, et al. (2021)9 |
| Residues of orange pulp peel | Mesophilic anaerobic digestion at 37 °C in 118 mL dark glass bottles | Biogas | Vaez, et al. (2021)9 |
| Orange peel waste and organic fraction of municipal solid waste | Sewage sludge degasified at an operating temperature of 37 °C | Methane | Bouaita, et al. (2022)61 |
| Orange peel waste | Streptomyces sp. in a fermentation bioreactor at 30 °C, with orbital agitation at 140 rpm, for 5 days | α-amylase | Ousaadi, et al. (2021)62 |
| Polyphenols from orange peel waste | Aspergillus fumigatus MUM 1603 in a fermentation bioreactor at 30 °C, with orbital agitation at 200 rpm for 78 h | Ellagic acid | Sepúlveda, et al. (2020)63 |
| Residue of fiber and proteins from pectin extract of orange peel waste | Fermentation bioreactor at 35 °C and pH 4, with co-substrates (D-lactose and yeast extract) | Mucic acid | Ortiz-Sanchez, et al. (2020)71 |
| Orange peel powder and beet pulp powder | Aspergillus niger AUMC 4156, Penicillium oxalicum AUMC 4153, and P. variotii AUMC 4149 in a fermentation bioreactor at 30 °C and 150 rpm for 5 d | Endo-polygalacturonate | Almowallad, et al. (2022)64 |
| Waste from orange pulp peel | Aspergillus niger CECT-2090 in solid-state fermentation with inoculum concentration ranging from 0.5 × 103 to 0.7 × 108 spores per g of dried orange peel, bed loading between 1.0 and 4.8 g of dried orange peel, and moisture content kept between 50 and 100% of the material's maximum water-holding capacity | Citric acid | Torrado, et al. (2011)46 |
| Residual water from orange processing | 50 mL of a NiCl2 solution (0.1 mM) was mixed with 60 mL of aqueous extract from orange residue. The process was conducted at a temperature of 70 °C for 20 min | Nickel oxide nanoparticles (NiONPs) | Narayanan, et al. (2023)65 |
| Orange peel | 40 mL of AgNO3 (0.25–6.0 mmol L−1) was added to 40 mL of orange peel extract for 5–10 min at 75 °C (until the mixture changed color to brown) | Silver nanoparticles | Skiba and Vorobyova (2019)66 |
| Orange peel | Self-hydrolysis at a temperature of 150–180 °C and pH of 3.73 | Pectooligosaccharides | Morales, García-Martín, and Ladero (2023)67 |
| Orange peel | KOH concentration: 0–4%, reaction time: 0–40 min, and solids loading: 40–120 g L−1 | Sugar-rich concentrate | Jang, Lee, and Yoo (2021)68 |
| Oil (37% by weight) extracted from sweet orange seeds residue | Transesterification at 60 °C of orange seeds (200 g; 0.232 mol) and methanol (56.7 mL; 1.392 mol; molar ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) using sodium methoxide catalyst. Reaction time of 1 h 1) using sodium methoxide catalyst. Reaction time of 1 h |
Biodiesel | Moser, Dorado, Bantchev, Winkler-Moser, and Doll (2023)69 |
| Orange peel | Treatment with 0.05 M KO2 for 1 h | Sugar-rich concentrate | Davaritouchaee, Mosleh, Dadmohammadi, and Abbaspourrad (2023)70 |
Table 1 outlines the primary by-products derived from OPW. Scaling up the repurposing of these by-products into marketable goods on an industrial scale significantly amplifies their value, potentially creating economic opportunities from biomaterials that might otherwise be discarded.32 OPW offer versatile products applicable across diverse industries, including food, pharmaceuticals, and cosmetics, due to their inherent nutritional and technological qualities.4,7 Therefore, harnessing OPW holds significant promise in fostering sustainable innovation and ensuring economic viability within the realm of bioproduct development.54 However, the production of these by-products from OPW encounters several challenges. The high production costs require significant investments in research, development, and production, potentially impacting the final product cost.1 Additionally, navigating regulatory frameworks, which depend on specific product features, demands compliance with complex food safety standards, adding complexity and time to the process.4 Achieving commercial viability, especially in the initial phase, proves challenging, needing both scaling up and gaining market acceptance. The variability in waste composition further complicates establishing efficient process parameters due to the fluctuating composition of these residues.32,33
To scale up the production of by-products at industrial levels from OPW, certain challenges must be overcome. The recovery of components from residues faces several limitations, such as residue complexity, economic viability, production of toxic waste, and energy efficiency.50,72 To address these issues, a promising strategy is applying pre-treatments to improve mass transfer and reduce energy requirements for processes such as extraction, dehydration, and bioconversion.61 Table 2 summarizes the technologies that can be employed to enhance the efficiency of these biorefinery processes, outlining their advantages and disadvantages.
| Technology | Mechanism | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| Mechanical pressing | The application of pressure to the plant material induces cell rupture, facilitating the release of phytochemicals | - Simple process | - Limited efficiency for some hard-to-extract compounds | Occhiuto et al. (2022);73 Prestes et al. (2023).74 |
| - Conservation of nutritional value | - Lower yields compared to other high-pressure methods | |||
| - Lower energy input compared to thermal treatments (cold pressing) | - Possible degradation of thermosensitive compounds (hot pressing) | |||
| - Minimal equipment requirements | ||||
| Steam pretreatment (explosion) | The sudden release of high-pressure steam within the cell structure disrupts the biomass matrix, increasing porosity and facilitating mass transfer by breaking down the cellulosic structure | - Enhanced porosity | - Energy-intensive process | Jedvert et al. (2012);75 DeMartini (2015).76 |
| - Uniform treatment | - Degradation of thermosensitive components | |||
| - Process flexibility | - Limited efficiency for some biomass | |||
| Hydrothermal methods | Applying high pressure and temperature to biomass in liquid water induces the breakdown of components like acetal in hemicelluloses into acetic acid, promoting mass transfer of intracellular components | - Lignin solubilization | - Energy-intensive process | Borrega, Nieminen, and Sixta (2011);77 Manzanares et al. (2020);78 Prado et al. (2016).79 |
| - Hemicellulose dissolution | - Degradation of thermosensitive components | |||
| - Temperature versatility | - Equipment requirements | |||
| - Fractionation capability | ||||
| Alkaline and acid methods | Low pH hydrolysis specifically targets the breakdown of the hemicellulose fraction, capitalizing on its susceptibility at lower pH levels. Conversely, high pH hydrolysis focuses on the breakdown of lignin | - Selectivity towards hemicelluloses (low pH) or lignin (high pH) | - Risk of undesired compounds formation | Huang et al. (2019);80 Yuan et al. (2018);81 Qian et al. (2019).82 |
| - High efficiency in sugar conversion | - Less effective with high lignin content | |||
| Organic solvents/organosolv/green solvents | Organic solvents dissolve lignin and hemicellulose present in the biomass, breaking β-aryl-ether bonds and degrading hemicelluloses, yielding valuable sugars and phytochemicals | - Selective solvent properties | - Complex solvent recovery process | Bouxin, Jackson, and Jarvis (2014);83 Zhang et al. (2016);84 Chin et al. (2019);85 Lê et al. (2016).86 |
| - Potential for solvent recovery | - Solvents cost | |||
| - Potential toxicity of organic solvents | ||||
| - Flammability of solvents | ||||
| - Environmental concerns | ||||
| Enzymes | Enzymatic methods involve the targeted action of enzymes on biomass components, catalyzing the breakdown of complex structures | - Mild and specific treatment | - Costly enzymes | Studer et al. (2010);87 Chandra (2016).88 |
| - Environmentally friendly | - Process optimization challenges | |||
| - Product specificity | - Enzyme stability and recovery | |||
| High intensity ultrasound | Ultrasound induces acoustic cavitation through the creation of alternating high-pressure and low-pressure cycles in the system, leading to the formation and subsequent collapse of microbubbles at precise micropoints. The collapse of these microbubbles generates shear stresses, effectively disrupting the cellular structure | - Increased mass transfer of intracellular components | - Equipment complexity and cost | Prestes et al. (2023);74 Heydari (2021);52 Mello (2021).21 |
| - Reduced processing time | - Possible generation of reactive compounds | |||
| - Minimal heat generation | - Challenges associated with scaling up to industrial levels | |||
| - Lower energy input | ||||
| - Improved retention of thermosensitive compounds compared to thermal technologies | ||||
| Pulsed electric field | Pulsed electric field technology applies short pulses of high-voltage electricity to biomass, inducing the formation of pores in cell membranes at precise micropoints. These pores compromise cellular integrity, facilitating the extraction of intracellular compounds | - Enhanced mass transfer | - Complexity and cost of equipment | Prestes et al. (2023);74 Bocker, and Silva (2022);89 Arshad et al. (2020).90 |
| - Reduced energy consumption | - Challenges associated with scaling up to industrial levels | |||
| - Preservation of thermosensitive compounds | - Potential generation of toxic by-products | |||
| - Shortened processing time | ||||
| - Lower energy input | ||||
| - Enables continuous operation |
The comprehensive overview provided in Table 2 outlines various conventional and emerging pre-treatment methodologies utilized in biorefinery processes. These methodologies play a crucial role in enhancing mass transfer efficiency. Some of the highlighted methodologies prominently utilize heat to facilitate mass transfer, such as the steam pretreatment (explosion) and hydrothermal methods.45,91 However, the efficacy of these methods is tempered by their tendency to degrade heat-sensitive compounds, thereby requiring a significant input of energy.5,12,77,79 Conversely, enzymatic methods demonstrate a remarkable level of selectivity, resulting in reduced energy requirements and improved retention of heat-sensitive compounds.32 However, their widespread adoption faces challenges due to the high costs associated with enzymes and their specialized operational requirements.92 Similarly, solvent-based approaches offer advantages but encounter constraints due to their costs and potential environmental impacts linked to solvent use during extraction processes.84,85 Emerging technologies, notably PEF and high-intensity ultrasound, depart from conventional heat-centric methods, relying on phenomena such as electroporation and sonoporation to facilitate mass transfer.90,93 These alternatives exhibit promising attributes, including enhanced mass transfer, decreased energy consumption, preservation of thermosensitive compounds, and accelerated processing times.59,90 Nonetheless, their implementation on an industrial scale poses challenges related to equipment complexity, cost, and the potential generation of toxic by-products. This underscores the necessity for further development and refinement in these domains.59
6 PEF technology for valorization of agro-industrial waste
6.1 PEF fundamentals
PEF is an innovative technology recognized for its non-thermal and minimally invasive action mechanism. This innovative pre-treatment stands up as a sustainable solution for treating biological tissues and biomaterials. PEF applications extend across many industry sectors, such as food, biotechnology, and medicine.94 In food systems, PEF technology is recognized for its exceptional performance in assisting phytochemical compounds extraction from plant matrices, facilitating dehydration processes, inactivating microorganisms, and enzymes, and concentrating bioactive compounds. PEF pre-treatment is also related to enhancing the physicochemical, rheological, and structural properties of food products.14,57,95–97PEF pre-treatment generates a potential difference through a conductive biological material. This innovative technology operates based on the principle of cell membrane electroporation; a phenomenon that occurs through the application of repetitive energy pulses discharged onto the cell's membrane surface. Accumulating electric charge on the cell membrane facilitates the reorientation of electrical charges within lipid molecules. This phenomenon induces reduction on the membrane selectivity to extracellular components and an increase on the membrane potential. The intensity of the cell's membrane potential reached depends on the food conductivity, electric field amplitude, cell radius, and shape.96,97
In critical values of electric pulses, electric charge accumulation induces the formation of pores in cells membranes. The intensity of these pulses determines whether electroporation is reversible or permanent. Depending on the strength of the electric field, this phenomenon can have adverse effects on the cell integrity. These effects may include protein aggregation on the plasma membrane, cell fusion, the incorporation of micro and macromolecules, and damage to the cell structure. Therefore, the applied field strength is crucial for achieving the intended applications.13,14,57,96
The electroporation induced by PEF technology encompasses a dynamic and gradual process, described by some phases. The initial phase, developing around 10 ns, involves the cell's membrane charging and polarization. This induces the preliminary creation of pores and temporary disruption of the membrane integrity. The subsequent stage encompasses the entire duration of the sample exposure to electrical pulses, which lasts less than 1 s. During this period, the pores expand and aggregate together. This second phase is determinant to the electroporation phenomenon reversibility. During the final phase of electroporation, there is an attempt to restore the cell membrane to the former semi-permeable structure.57,95
PEF technology induces the formation of pores in the food sample, which allows for an increased mass transfer ratio of the cell intracellular components. This increase is a direct consequence of the membrane permeability alteration promoted by the formation of pores within the food sample, facilitating an enhanced mass transfer ratio of the cell intracellular components. This effect contributes significantly to the overall energy efficiency of the extraction process. Consequently, PEF technology emerges as an efficacious method for facilitating the extraction of micro and macronutrients from biological tissues. This non-thermal technology enhances the extraction of carbohydrates, proteins, lipids, pigments, phenolics, and others.15–19
PEF treatment has been associated with a decrease in the lignin content of biomass structures. This reduction in lignin can facilitate fermentation by reducing the complexity of cell walls composed of lignocellulose. However, the precise mechanism behind the reduction of lignin through PEF treatment is not yet fully understood, necessitating further research.96
During PEF pre-treatment, besides electroporation, various electrochemical non-desirable reactions occur at the interfaces between the electrodes and the cell walls. These reactions can lead to the formation of gases (H2 and O2), dissolution of the electrode material, electrolysis of water, the generation of toxic chemicals (HCl, H2O2, and HClO), and changes in pH and electrical conductivity near the electrode surfaces. These electrochemical reactions can also cause fouling, leading to the distortion of the local electric field, arcing, contamination of the treated material, and interruption of fluid flow.72
Additionally, corrosion can damage the electrodes significantly, increasing surface roughness due to the release of metal, which further contributes to electric field arcing and distortion. Consequently, the electrodes have a limited lifespan of only a few hours of operation. The extent of undesired effects from electrode reactions depends on chamber design, electrode material, electrical parameters (including pulse shape, peak voltage, energy input, polarity, and pulse duration), and the properties of treated products.57,95
6.2 Large-scale processing
PEF treatment starts with inserting the organic material between two electrodes within a treatment chamber. The conductive properties of the material create an electric potential difference, facilitated by the electrodes.96 The treatment intensity is determined by both the electrical flow applied and the electrodes configuration.98 Several operating parameters are considered for PEF treatments, including holding time, electric field strength, polarity, pulse specific energy, pulse repetition frequency, pulse number, pulse shape, and pulse width.99Industrial-scale uses of PEF technology requires the full comprehension of the electric engineering, biology and fluid mechanics related with the mechanism of induced transmembrane potential. This innovative electromagnetic technology induces alterations in cell permeability through the accumulation of electric charge on the plant cell membrane. This electromagnetic method comprises a pulse generator and a treatment chamber. To achieve process standards in large-scale PEF, treatment chambers and high pulse frequencies are required to provide the necessary processing energy.100 Accommodating large treatment volumes necessitates wider electrode gaps, which, in turn, require higher pulse voltage amplitudes and larger electrode areas.72
To meet current needs, large PEF treatment devices use one or more pulse generators coupled to an electrode for continuous processing. When transitioning from laboratory to industrial scale, the power output must be scaled up, ranging from a few kilowatts to systems exceeding 100 kW. The transfer of electrical energy to the treated media occurs within the treatment chamber, with significant design parameters including electrode configuration, electrode area, electrode gap, and flow pattern.96,97
PEF batch processing and laboratory settings utilize static chambers, while continuous chambers are suitable for industrial scale uses. Batch chambers on a laboratory scale can treat small-volume samples, efficient temperature control through electrode cooling, and the ability to adjust repetition rates. On the other hand, continuous chambers are necessary for handling large volumes and can be effortlessly integrated into existent food processing systems. Currently, there are three main treatment chamber types: parallel plates, coaxial plates, and collinear chambers. Parallel and coaxial plates are used for batch processing, while collinear chambers are for continuous processing.101
The scale-up of PEF-assisted extraction for industrial applications requires the optimization of process parameters. The key process parameters that significantly affect the performance of PEF in the extraction process comprise pulse number, electric field strength, treatment duration, specific energy input, and temperature.90 These parameters regulate the formation of pores in cell walls. Increasing of PEF process parameters, intensifies electroporation, leading to a higher release of intracellular compounds. However, the effectiveness of these parameters may vary depending on the characteristics of the organic material being processed, such as the size distribution of cells between the peel and pulp, the structure, chemical and physical stability of the target compound, as well as its location within the plant cell and its ability to bind to the surrounding matrix.102
Industrial-scale standards requires electrical discharges or pulses generated with power enough to allow continuous and large-scale treatment. PEF integration into the extraction process operating within a flow rate of 1000 to 2000 kg h−1 requires a 3 kW power input to generate pulses within the 20 to 30 kV range, with a frequency of 200–300 Hz. Additionally, PEF technology should feature a spacious treatment chamber and one or more pulse generators to meet the substantial processing volume demanded by industrial standards.14 Intricate design and features contribute to the high cost of PEF technology machinery.103 Therefore, deploying this technology on an industrial scale necessitates a substantial initial investment, ranging from €75,000 to €400,000, contingent upon the energy requirements of the process and the scale of production.14
Recent studies have shown PEF technology as a suitable pre-treatment for integrating industrial processes. This emerging and non-thermal technology is provided by several companies, including, Pulsemaster (Germany), PurePulse (Netherlands), Scandinova (Sweden), DTI/Elea (Germany), and Steribeam (Germany).103 However, further research is needed to implement PEF in large-scale industrial processes worldwide. Additional studies are necessary to optimize processes parameters.90
6.3 Benefits and main limitations
Effective implementation of emerging technologies in industrial scale necessitates consideration of economic, social, and environmental factors associated with system manufacturing. The current requirements of the food market emphasize the adoption of green technologies. Contemporary demands within the food market underscore the importance of embracing environmentally friendly technologies, a trend driven by the commitment to the Agenda 2030 for sustainability and development of the United Nations. Among the innovative and promising technologies in the food market, PEF technology stand out. Numerous studies have identified PEF technology as a noteworthy pre-treatment for developing processes that can mitigate the formation and usage of harmful substances.96,97,104This non-thermal and low-energy technology can assist quantitative and qualitative recovery of compounds from biosources. PEF-assisted by the phenomenon of electropermeabilization reduces extraction time, diminishes the necessity for intense heating processes, and minimizes the reliance on solvents.16,19,105
PEF-assisted extraction processes support the conceptions of green chemistry and sustainability. PEF technology by increasing the mass transfer of intracellular components allow the use of eco-friendly solvents for extracting natural colorants and bioactive compounds, preventing the contamination of food and beverages with harmful chemicals.15,60 In terms of sustainability, PEF-assisted extraction processes require less energy consumption than isolated extraction methods. The short treatment time characteristic of PEF-based processes contributes to energy conservation.72 However, there is a lack of comprehensive life cycle assessment research exploring the environmental effects of PEF technology in the extraction of phytochemical compounds. Therefore, there is a need for further discussions on sustainability, green chemistry principles, and life cycle assessment of this emerging technologies.89
As discussed for Ahmed Taha, et al. (2022),101 the main drawback of PEF pre-treatment is the presence of bubbles, which can create operational difficulties and inconsistent results. Factors like dissolved gasses or gas release from the product contribute to bubble formation, disrupting the electrical field distribution and treatment efficiency. Limited availability of commercial PEF units in certain regions due to high costs and specialized expertise required hinders widespread implementation. This innovative technology also entails high energy consumption, leading to increased operating costs and potential environmental concerns. However, ongoing research aims to address these challenges by minimizing bubble formation, reducing costs, optimizing energy consumption, and enhancing accessibility. With these advancements, PEF technology could revolutionize sectors such as food processing and wastewater treatment, offering efficient and sustainable solutions for preservation and purification.
7 OPW biorefinery assisted by PEF technology
7.1 Phytochemical extractions
Conventional phytochemical extractions are carried out through methods such as hydro-distillation, Soxhlet extraction, percolation, grinding, maceration, soaking, and others. These methods are applied to obtaining valuable compounds from plant matrices.59,106 Nevertheless, these methods have relevant drawbacks that limit their applicability, such as low extraction yields, high solvent consumption, potential degradation of thermosensitive compounds, the need for filtration and centrifugation, adverse impacts on the environment, and long processing time.58 Hence, to enhance energy-efficiency and environmental friendliness, technologies such PEF have been investigated to integrate the extraction of biocomponents from biological tissues.9–11PEF technology affects the permeability of cell walls, enhancing a non-selective mass transfer of intracellular components.21 Thus, this phenomenon leads to a decrease in the resistance of the plant cell to mass transfer, resulting in higher extraction yields. By combining an emerging technology with traditional extraction methods, energy efficiency increases, easing enhanced phytochemical extraction. Thus, this contribution leads to enhancement of natural resource utilization while diminishing the requirement for excessive organic solvents and elevated temperatures.14,57,95–97
Fig. 5 provides an overview of the primary beneficial effects associated with electropermeabilization phenomena, including shorter processing times, lower energy input, greater retention of thermosensitive components, and higher extraction yield. Parameters modulating PEF application – such as electric field strength, pulse width, pulse number, frequency, and specific energy input – are depicted. Notably, low energy input and shorter treatment times emphasize the PEF potential to enhance phytochemical extraction yields. By utilizing electric pulses, this non-thermal technology not only increases extraction yields but also aids in preserving thermosensitive components.56,104 These attributes position PEF as an appealing technology for industrial applications, particularly concerning thermosensitive compounds, thus making it a potential point for research and development.
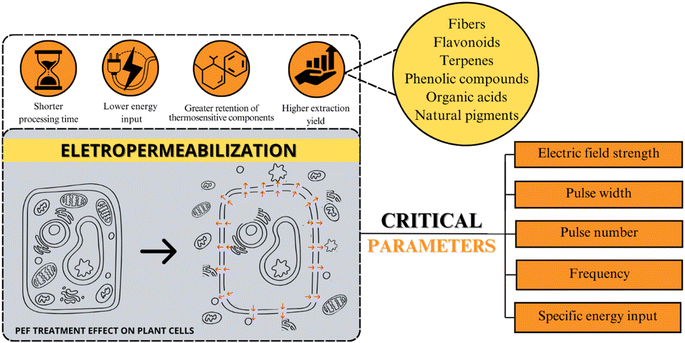 | ||
| Fig. 5 Overview of PEF effects and modulating parameters for phytochemical extraction on plant cells. | ||
As illustrated in Fig. 5, the main factor that affects PEF efficiency is the equipment parameters (electric field strength, pulse number, frequency, pulse width, and specific energy inputs) and the sample composition (homogeneity, pH, and conductivity).21
Luengo, et al. (2013)17 conducted an evaluation of the potential of PEF pretreatment in assist in the extraction process of polyphenols from OPW. Their findings showed that PEF treatment promoted an increase of 159% in polyphenol yield. Furthermore, compared to the non-treated samples, antioxidant activity of treated samples significantly increased by 192%.102
Fan, et al. (2022)105 assessed the potential of PEF technology for the extraction of soluble dietary fiber from OPW. Their results demonstrated a significant enhancement in the physicochemical properties of the treated samples. Notably, the PEF-treated samples showed substantial improvements in water solubility, as well as heightened water and oil holding capacities, emulsifying activity, swelling capacity, emulsion stability, and foam stability. These outcomes underscore the potential of PEF technology on improving the physicochemical attributes of OPW components.
Carpentieri, et al. (2021)15 further investigated the potential of PEF-assisted extraction for obtaining limonene from OPW. The strategic application of PEF pre-treatment to orange peels led to a noteworthy 33% increase in the limonene content within ethanolic extracts, compared to conventional extraction methods. This compelling result underscores the promising prospects of PEF technology in easing the extraction of terpenes from botanical sources.
There is still limited information on the potential of PEF technology assistance extraction of pigments from OPW. As outlined in a review conducted by Bocker and Silva (2022),89 PEF is a promising and innovative method for enhancing the extraction of food pigments from plant matrices. The research highlights this non-thermal technology potential to enhance mass transference while preserving thermosensitive components. Consequently, the electroporation enhances the yield and energy-efficiency of pigment extraction. Therefore, integration of PEF technology in the extraction of pigment from OPW requires further research.
PEF pre-treatment has demonstrated potential in assisting extraction processes by enhancing mass transfer in biological tissues. However, there are still few studies presenting PEF as an innovative approach to assistance the extraction of compounds from OPW. Table 3 presents a systematic analysis of the literature conducted to find current articles about PEF assisting the extraction of phytochemical compounds from OPW. The studies demonstrated that PEF technology is suitable for facilitating the extraction of various compounds, including soluble dietary fiber, limonene, naringin, phenolic compounds, polyphenols, hesperidin, narirutin, and ascorbic acid from OPW. Across all studies, the integration of PEF consistently resulted in improved mass transfer, evidenced by increased extraction yields.
| Raw material | Process conditions | By-product | Major findings | Ref. |
|---|---|---|---|---|
| Orange [C. sinensis (L.) Osbeck] peel | Electric field strength: 2–10 kV cm−1 | Soluble dietary fiber | Samples subjected to PEF treatment exhibited increased water solubility, water and oil holding capabilities, emulsifying activity, swelling capacity, emulsion stability, and foam stability | Fan, et al. (2022)105 |
| Pulse width: 20 μs | ||||
| Pulse number: 10–50 | ||||
| Frequency: 1 Hz | ||||
| Temperature: 25–75 °C | ||||
| Orange [C. sinensis (L.) Osbeck] peel | Electric field strength: 1–5 kV cm−1 | Limonene | The application of PEF pre-treatment to orange peels resulted in a significant 33% increase in the limonene content in ethanolic extracts compared to the control extraction | Carpentieri, et al. (2021)15 |
| Specific energy input: 1–40 kJ kg−1 | ||||
| Pulse width: 20 μs | ||||
| Frequency: 5 Hz | ||||
| Temperature: 20 °C | ||||
| Pomelo fruits (shantian variety) | Electric field strength: 2–10 kV cm−1 | Naringin | The extraction yield showed a 20% increase when exposed to pulses of 30 at an electric field intensity of 4 kV cm−1, as compared to the non-PEF treatment | Niu, et al. (2021)16 |
| Pulse number: 10–50 | ||||
| Temperature: 40 °C | ||||
| Orange [C. sinensis (L.) Osbeck] peel | Electric field strength: 1–7 kV cm−1 | Phenolic compounds | In comparison to the nontreated sample, PEF treatments of 1, 3, 5 e 7 kV cm−1 increased the antioxidant activity up to 51%, 94%, 148%, and 192%, respectively | Luengo, et al. (2013)17 |
| Specific energy input: 0,06–3.77 kJ kg−1 | ||||
| Pulse width: 3 μs (15 a 150 μs) | ||||
| Pulse number: 5–50 | ||||
| Frequency: 1 Hz | ||||
| Orange (Citrus sinensis), pomelo (Citrus maxima); lemon (Citrus limon) | Electric field strength: 3–10 kV cm−1 | Polyphenols | The application of PEF treatment resulted in significant improvements in juice yield from different citrus fruits. Specifically, orange juice yield increased by 25%, pomelo juice yield by 37%, and lemon juice yield by 59%. Moreover, when high electric field strength was applied to orange peels, the extraction of polyphenols was enhanced, reaching up to 22 mg GAE (Gallic Acid Equivalents) per gram of dry matter | El Kantar, et al. (2018)18 |
| Pulse width: 70 μs | ||||
| Pulse number: 5–50 | ||||
| Frequency: 12–100 kHz | ||||
| Orange (Citrus unshiu) peel | Electric field strength: 3 kV cm−1 | Hesperidin and narirutin | With increasing PEF treatment time, the concentrations of both hesperidin and narirutin showed a noticeable rise. The application of PEF resulted in a significant increase in the extracted amounts of hesperidin and narirutin, with increments of 22.1% and 33.6%, respectively | Hwang, et al. (2021)19 |
| Specific energy input: 72–152 kJ kg−1 | ||||
| Pulse width: 70 μs | ||||
| Pulse number: 0–225 | ||||
| Frequency: 50 Hz |
7.2 Dehydration process
Dehydration is a preservation method conventionally performed through hot-air convective drying. This method promotes the establishment of a vapor pressure gradient among the heated drying air and the products, leading to a reduction in the volume and weight of the samples. However, there are several drawbacks that limit the use of this conventional dehydration method. Some of these drawbacks include changes in sensory characteristics, low energy efficiency, and the degradation of thermosensitive components due to prolonged exposure to elevated temperatures. To address these limitations, the integration of emerging technologies such PEF into this dehydration process can enhance energy efficiency.21PEF-assisted dehydration is a smart biorefinery strategy that reduces the energy required for the biomass drying, which lowers the evaporation energy required during subsequent stages. This effect promotes the formation of pores on the cells membrane structure, resulting in an improved water and vapor diffusion. The change in the cell permeability leads to higher yields and greater overall efficiency in biomass processing. Aspects that make PEF technology a suitable pre-treatment to reduce processes costs.72
Sack, et al. (2008)20 reported that PEF technology can improve energy efficiency in drying processes. These findings indicated a reduction in the drying time for biomass to 2-3 times less than that of non-PEF-treated materials. The utilization of this innovative technology has improved the reduction in processing time, which in turn has contributed to the mitigation of sensory and nutritional changes in the food matrix. Additionally, Mello, et al. (2021)21 evaluated the integration of PEF and high-intensity ultrasound into the drying process of orange peel. The findings demonstrated that integrating these two emerging technologies assisted preserve the color, and they also led to higher phenolic content and increased antioxidant activity.
7.3 Bioconversions
Bioconversion technologies, such as digestion and fermentation, offer a sustainable method for the valorization of OPW. These processes are carried out by microorganisms that utilize agro-industrial residue as a substrate to produce valuable bioactive compounds and phytochemicals. The current literature reports several environmentally friendly products resulting from the bioconversion of OPW, including biobutanol, ethanol, biogas, methane, short-chain fatty acids, α-amylase, ellagic acid, mucic acid, endo-polygalacturonase, and citric acid. Since these products originate from sustainable sources, they align with the current demand in the modern consumer market for eco-friendly products.33,63OPW exhibits a composition rich in polysaccharides, phenolic compounds, and dietary fibers.48 These components, with an excellent nutritional profile, make this biomass a suitable alternative substrate for fermentative processes.7 In fact, OPW is an energetic, renewable, and sustainable resource that can be an alternative to the use of lignocellulosic biomass. This organic residue has a higher level of polysaccharides and a lowest lignin content when compared to lignocellulosic biomass.10 The low concentration of lignin in the orange waste may eliminates the need of enzymatic hydrolysis pre-treatments. Nevertheless, OPW has a high pectin content compared to lignocellulosic biomass, which poses challenges during the fermentation process. This necessitates specific saccharification processes due to the high content of non-metabolizable sugars in pectin, such as L-rhamnose, L-arabinose, galacturonic acid, and galactose.32,33
A limiting factor in scaling saccharification process to an industrial level is their extensive reaction time. To address this issue, thermal methods in conjunction with chemical treatment are commonly employed. However, the use of heat not only promotes high energy consumption but also leads to the loss of OPW thermosensitive components. Therefore, these limitations underscore the pressing need for non-thermal routes to enhance the enzymatic digestibility of orange residues.
Although there are no studies that elucidate the potential of PEF for improving sugar accessibility in OPW, this emerging technology has been associated with enhancing sugar extraction yield of other biological tissues. Dastangoo, Hamed Mosavian, and Yeganehzad (2020)107 evaluated the effect of PEF technology on the extraction of sugars from carrots (Daucus carota). The study exhibited a significant increase in the accessibility of sugar due to the permeabilization of the cell tissue. This highlights the potential of PEF pre-treatment to increase the enzymatic accessibility of OPW. Nevertheless, the evaluation of the main challenges and benefits that come with the application of PEF technology requires further research and investigation.
A critical factor that impacts bioconversions is the kinetics of releasing digestible material from cell structures. This factor makes PEF technology a suitable method to enhance fermentation processes. By altering the cell permeability, PEF technology increases the bioavailability of intracellular components. This effect promotes a shorter retention time and faster digestion. However, further research is necessary to fully assess the potential of PEF technology in biofuel production.
Biofuels are a significant by-product derived from the processing of OPW. The demand for these alternative fuels is driven by many ecological concerns related with the use of fossil fuels. These concerns are associated with a wide range of observed climate changes worldwide, as well as the wastage of natural resources and environmental pollution.92 However, the high energy density and existing infrastructure of fossil fuels production give them a competitive advantage in the market over biofuels. To tackle this challenge, agro-industrial residues can play a vital role in making these processes more feasible. This is primarily because OPW offers the advantages of low raw material cost, high availability, and alignment with sustainable strategies.10,12
Compared to fossil fuel sources, biofuels currently lack efficiency regarding outputs and energy consumption. These alternative fuels have low energy-efficiency that limits their application on a global scale. However, the use of biomass for biofuel production has a low-carbon, low-water footprint, versatility in product offerings, and widespread availability, both locally and globally. This serves as strong motivation for the development of novel processes and technologies aimed at optimizing the energy efficiency of biofuels. For instance, PEF treatment stands out as a potential non-thermal approach for assisting the bioconversion process and increasing the energy efficiency of biorefineries.72
8 Critical observations and final considerations
The rich nutritional and technological profile of OPW makes this organic waste a potential bioresource for obtaining products and ingredients with sustainability and health appeal. Therefore, the appropriated disposal of these residues is not only an environmental need but also an economic opportunity.4,10,32,33Due to the high content of compounds considered valuable bioresources, current studies have been exploring methods for the valorization of OPW through processes such as phytochemical extraction, dehydration, and bioconversion.9–11 As mentioned earlier in this review, the resistance of plant cells to mass transfer limits the energy efficiency of biorefinery methods. The intracellular components of biological tissue are protected by cell walls, requiring more energy for the extraction of valued components from biological tissues.12 This resistance to mass transfer of intracellular components limits extraction yield. Therefore, to address these challenges, recent studies have explored PEF as an innovative technology to integrate the biorefinery of OPW.15–19
As discussed throughout this review, PEF technology has shown potential of improving the nutritional and sensory profile, reducing energy consumption, and increasing extraction yield of OPW. Although this technology has a high initial investment, integrating PEF in waste treatment is a strategy to optimize energy consumption during processing. However, as they are still new in the industrialization of products, these technologies still require further studies to assess their economic viability on an industrial scale for processing agro-industrial waste.93
Regarding technical feasibility, there are only a few studies that focus on the use of PEF technology to assist in the dehydration and bioconversion of OPW. In the context of dehydration,20 the utilization of PEF for electroporation of biological tissue has the potential to significantly reduce the energy required for water evaporation, thereby enhancing the energy-efficiency of the process. Moreover, Furthermore, PEF holds promise in enhancing the accessibility of sugars within plant tissue, resulting in higher substrate concentrations available for microorganisms to convert. This, in turn, leads to a more energy-efficient overall process.
Further studies are essential for the evaluation of the optimum parameters for PEF applications. These enhancements have the potential to reduce energy demand, particularly for future utilization within industrial scale. Furthermore, techno-economic viability studies of these procedures are required. On the other hand, exploring potential synergies between PEF and other technologies is needed. Such investigations will provide valuable insights into whether the substantial initial investment required for widespread implementation of this groundbreaking technology can yield significant economic benefits.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by the São Paulo Research Foundation - FAPESP (grant number 2020/11255-3). Ramon Bocker thanks FAPESP for his doctoral scholarship (grant number 2023/09158-8). Eric Keven Silva thanks FAPESP (grant number 2023/01876-9) for the Young Investigator Fellowship.References
- S. Suri, A. Singh and P. K. Nema, Appl. Food Res., 2022, 2, 100050 CrossRef CAS.
- M. Buniowska, C. J. Carbonell, A. Zulueta, A. Frigola and M. J. Esteve, Food Process. Technol., 2015, 1(3), 77–83 Search PubMed.
- K. Aparna, M. Sowmya, M. R. Sahoo, P. D. Mayengbam, M. Dasgupta and M. Sreedhar, J. Pharmacogn. Phytochem., 2018, 7, 3032–3036 Search PubMed.
- M. Ortiz-Sanchez, A. B. Omarini, J.-A. González-Aguirre, M. Baglioni, J. A. Zygadlo, J. Breccia, R. D'Souza, L. Lemesoff, M. Bodeain, C. A. Cardona-Alzate, I. Pejchinovski and M. H. Fernandez-Lahore, Chem. Eng. Process., 2023, 189, 109407 CrossRef CAS.
- B. Santiago, M. T. Moreira, G. Feijoo and S. González-García, Biomass Bioenergy, 2020, 143, 105844 CrossRef CAS.
- N. Ferronato and V. Torretta, Int. J. Environ. Res. Public Health, 2019, 16, 1060 CrossRef CAS PubMed.
- R. Richa, D. Kohli, D. Vishwakarma, A. Mishra, B. Kabdal, A. Kothakota, S. Richa, R. Sirohi, R. Kumar and B. Naik, J. Agric. Food Res., 2023, 14, 100718 CAS.
- E. Tütem, K. Sözgen Başkan, Ş. K. Ersoy and R. Apak, in Nutritional Composition and Antioxidant Properties of Fruits and Vegetables, ed. A. K. Jaiswal, Academic Press, 2020, pp. 353–376, DOI:10.1016/B978-0-12-812780-3.00022-2.
- S. Vaez, K. Karimi, S. Mirmohamadsadeghi and A. Jeihanipour, Process Saf. Environ. Prot., 2021, 152, 513–526 CrossRef CAS.
- G. Su, C. Chan and J. He, Renewable Energy, 2022, 193, 576–583 CrossRef CAS.
- Q. Shao, S. Fang, X. Fang, M. Zhang, W. Huang, F. Wang, X. Duan, Y. Wu and J. Luo, Bioresour. Technol., 2023, 380, 129128 CrossRef CAS PubMed.
- J. A. Okolie, E. I. Epelle, M. E. Tabat, U. Orivri, A. N. Amenaghawon, P. U. Okoye and B. Gunes, Process Saf. Environ. Prot., 2022, 159, 323–344 CrossRef CAS.
- P. Raffaini and L. Manfredi, in Endorobotics, ed. L. Manfredi, Academic Press, 2022, pp. 337–358, DOI:10.1016/B978-0-12-821750-4.00015-3.
- E. Puértolas and F. J. Barba, Food Bioprod. Process., 2016, 100, 172–184 CrossRef.
- S. Carpentieri, A. Režek Jambrak, G. Ferrari and G. Pataro, Front. Nutr., 2021, 8, 792203 CrossRef PubMed.
- D. Niu, E.-F. Ren, J. Li, X.-A. Zeng and S.-L. Li, Sep. Purif. Technol., 2021, 265, 118480 CrossRef CAS.
- E. Luengo, I. Álvarez and J. Raso, Innovative Food Sci. Emerging Technol., 2013, 17, 79–84 CrossRef CAS.
- S. El Kantar, N. Boussetta, N. Lebovka, F. Foucart, H. N. Rajha, R. G. Maroun, N. Louka and E. Vorobiev, Innovative Food Sci. Emerging Technol., 2018, 46, 153–161 CrossRef CAS.
- H. J. Hwang, H. J. Kim, M. J. Ko and M. S. Chung, Food Sci. Biotechnol., 2021, 30, 217–226 CrossRef CAS PubMed.
- M. Sack, C. Eing, T. Berghofe, L. Buth, R. Stangle, W. Frey and H. Bluhm, IEEE Trans. Plasma Sci., 2008, 36, 2577–2585 CAS.
- R. E. Mello, A. Fontana, A. Mulet, J. L. G. Corrêa and J. A. Cárcel, Innovative Food Sci. Emerging Technol., 2021, 72, 102753 CrossRef CAS.
- FAOSTAT, Crops and Livestock Products, 2023, https://www.fao.org/faostat/en/#data/QCL Search PubMed.
- J. Wang, D. Chen, Y. Lei, J.-W. Chang, B.-H. Hao, F. Xing, S. Li, Q. Xu, X.-X. Deng and L.-L. Chen, PLoS One, 2014, 9, e87723 CrossRef PubMed.
- B. Acoglu and P. Y. Omeroglu, LWT, 2021, 147, 111690 CrossRef CAS.
- N. E. Martínez-gonzáles, L. Martínez-Chávez, C. Martínez-Cárdenas and A. Castillo, J. Food Prot., 2011, 74, 1684–1691 CrossRef PubMed.
- K.-S. Shin and J.-H. Lee, Food Sci. Biotechnol., 2021, 30, 663–673 CrossRef CAS PubMed.
- A. Ahmed and M. Sohail, J. King Saud Univ., Sci., 2020, 32, 955–961 CrossRef.
- L. Dal Magro, J. P. S. Pessoa, M. P. Klein, R. Fernandez-Lafuente and R. C. Rodrigues, Catal. Today, 2021, 362, 184–191 CrossRef CAS.
- H. M. Shahbaz, J. U. Kim, S.-H. Kim and J. Park, in Fruit Juices, ed. G. Rajauria and B. K. Tiwari, Academic Press, San Diego, 2018, pp. 341–361, DOI: DOI:10.1016/B978-0-12-802230-6.00018-7.
- N. Grigelmo-Miguel and O. Martı́n-Belloso, Food Res. Int., 1998, 31, 355–361 CrossRef.
- A. Gomes, A. L. R. Costa, P. D. Rodrigues, R. J. S. d. Castro and E. K. Silva, Food Control, 2022, 131, 108391 CrossRef CAS.
- D. Z. Cypriano, L. L. da Silva and L. Tasic, Waste Manage., 2018, 79, 71–78 CrossRef CAS PubMed.
- D. Jeong, H. Park, B.-K. Jang, Y. Ju, M. H. Shin, E. J. Oh, E. J. Lee and S. R. Kim, Bioresour. Technol., 2021, 323, 124603 CrossRef CAS PubMed.
- D. U. D. Carvalho, M. A. D. Cruz, R. C. Colombo, L. S. Watanabe, Z. H. Tazima and C. S. V. J. Neves, Braz. J. Food Technol., 2020, 23 Search PubMed.
- O. S. Omoba, R. O. Obafaye, S. O. Salawu, A. A. Boligon and M. L. Athayde, Antioxidants, 2015, 4, 498–512 CrossRef CAS PubMed.
- L. Wang, H. Xu, F. Yuan, Q. Pan, R. Fan and Y. Gao, Biocatal. Agric. Biotechnol., 2015, 4, 250–258 CrossRef.
- Z. Berk, in Citrus Fruit Processing, ed. Z. Berk, Academic Press, San Diego, 2016, pp. 261–279, DOI:10.1016/B978-0-12-803133-9.00013-8.
- M. V. Vavoura, I. K. Karabagias, I. S. Kosma, A. V. Badeka and M. G. Kontominas, Molecules, 2022, 27, 6166 CrossRef CAS PubMed.
- H. Falleh, M. Ben Jemaa, M. Saada and R. Ksouri, Food Chem., 2020, 330, 127268 CrossRef CAS PubMed.
- A. Masyita, R. Mustika Sari, A. Dwi Astuti, B. Yasir, N. Rahma Rumata, T. B. Emran, F. Nainu and J. Simal-Gandara, Food Chem.: X, 2022, 13, 100217 CAS.
- J. K. R. Silva, P. L. Figueiredo, K. G. Byler and W. N. Setzer, Int. J. Mol. Sci., 2020, 21, 3426 CrossRef PubMed.
- T. Kietzmann, Redox Biol., 2023, 63, 102753 CrossRef CAS PubMed.
- M. Hofmann, M. Hofmann, J. Louis, P. Smith and E. Nwabuogu, J Am Med Dir Assoc, 2021, 22, B3–B4 CrossRef PubMed.
- B. Sallato, C. Bonomelli and J. Mártiz, J. Plant Nutr., 2017, 40, 954–963 CrossRef CAS.
- M. Ishfaq, M. Aadil, S. R. Ejaz, W. Hassan, N. M. Panduro-Tenazoa, M. E. El Sayed, M. N. Murshed and Z. M. El-Bahy, J. Alloys Compd., 2023, 960, 170661 CrossRef CAS.
- A. M. Torrado, S. Cortés, J. M. Salgado, B. Max, N. Rodríguez, B. P. Bibbins, A. Converti and J. M. Domínguez, Braz. J. Microbiol., 2011, 42, 394–409 CrossRef CAS PubMed.
- B. Ozturk, J. Winterburn and M. Gonzalez-Miquel, Biochem. Eng. J., 2019, 151, 107298 CrossRef CAS.
- T. A. Sial, Z. Lan, M. N. Khan, Y. Zhao, F. Kumbhar, J. Liu, A. Zhang, R. L. Hill, A. H. Lahori and M. Memon, Waste Manage., 2019, 87, 125–134 CrossRef CAS PubMed.
- D. Panwar, A. Saini, P. S. Panesar and H. K. Chopra, Trends Food Sci. Technol., 2021, 111, 549–562 CrossRef CAS.
- F. Fazzino, F. Mauriello, E. Paone, R. Sidari and P. S. Calabrò, Chemosphere, 2021, 271, 129602 CrossRef CAS PubMed.
- A. Mohsin, M. H. Hussain, W. Q. Zaman, M. Z. Mohsin, J. Zhang, Z. Liu, X. Tian, S. Rehman, I. M. Khan, S. Niazi, Y. Zhuang and M. Guo, Crit. Rev. Biotechnol., 2022, 42, 1284–1303 CrossRef CAS PubMed.
- M. Heydari, O. Rostami, R. Mohammadi, P. Banavi, M. Farhoodi, Z. Sarlak and M. Rouhi, J. Food Process. Preserv., 2021, 45, e15585 CAS.
- D. Thuy, K. Thang, M. C. Lưu, T. Giang, N. Ngọc Thạnh, L. Bui and P. Huynh, Cienc. Rural, 2023, 54, e20230240 Search PubMed.
- S. Consoli, C. Caggia, N. Russo, C. L. Randazzo, A. Continella, G. Modica, S. O. Cacciola, L. Faino, M. Reverberi, A. Baglieri, I. Puglisi, M. Milani, G. Longo Minnolo and S. Barbagallo, Sustainability, 2023, 15, 2482 CrossRef CAS.
- O.-S. Mariana, C. Alzate and C. Ariel, J. Cleaner Prod., 2021, 322, 128814 CrossRef.
- N. E. Wedamulla, M. Fan, Y.-J. Choi and E.-K. Kim, J. Funct. Foods, 2022, 95, 105163 CrossRef CAS.
- S. A. Moreira, E. M. C. Alexandre, M. Pintado and J. A. Saraiva, Food Res. Int., 2019, 115, 177–190 CrossRef CAS PubMed.
- R. D. Khandare, P. D. Tomke and V. K. Rathod, Chem. Eng. Process., 2021, 159, 108181 CrossRef CAS.
- M. Velusamy, A. Rajan and M. Radhakrishnan, Int. J. Food Sci. Technol., 2023, 58, 2021–2041 CrossRef CAS.
- C. Perez-Pirotto, G. Moraga, A. Quiles, I. Hernando, S. Cozzano and P. Arcia, LWT, 2022, 166, 113765 CrossRef CAS.
- R. Bouaita, K. Derbal, A. Panico, F. Iasimone, L. Pontoni, M. Fabbricino and F. Pirozzi, Biomass Bioenergy, 2022, 160, 106421 CrossRef CAS.
- M. I. Ousaadi, F. Merouane, M. Berkani, F. Almomani, Y. Vasseghian and M. Kitouni, Environ. Res., 2021, 201, 111494 CrossRef CAS PubMed.
- L. Sepúlveda, E. Laredo-Alcalá, J. J. Buenrostro-Figueroa, J. A. Ascacio-Valdés, Z. Genisheva, C. Aguilar and J. Teixeira, Electron. J. Biotechnol., 2020, 43, 1–7 CrossRef.
- S. A. Almowallad, M. O. Aljobair, A. N. Alkuraieef, A. H. Aljahani, A. M. Alsuhaibani and M. M. Alsayadi, Saudi J. Biol. Sci., 2022, 29, 963–969 CrossRef CAS PubMed.
- M. Narayanan, N. Devarajan, S. H. Salmen, S. A. Alharbi, R. Lavarti, N. T. Lan Chi and K. Brindhadevi, Environ. Res., 2023, 216, 114734 CrossRef CAS PubMed.
- M. I. Skiba and V. I. Vorobyova, Adv. Mater. Sci. Eng., 2019, 2019, 8306015 Search PubMed.
- P. C. Morales, A. García-Martín and M. Ladero, Bioresour. Technol. Rep., 2023, 21, 101369 CrossRef CAS.
- Y. W. Jang, K. H. Lee and H. Y. Yoo, Processes, 2021, 9, 409 CrossRef CAS.
- B. R. Moser, C. Dorado, G. B. Bantchev, J. K. Winkler-Moser and K. M. Doll, Fuel, 2023, 342, 127727 CrossRef CAS.
- M. Davaritouchaee, I. Mosleh, Y. Dadmohammadi and A. Abbaspourrad, Polymers, 2023, 15, 697 CrossRef CAS PubMed.
- M. Ortiz-Sanchez, J.-C. Solarte-Toro, J.-A. González-Aguirre, K. E. Peltonen, P. Richard and C. A. Cardona Alzate, Biochem. Eng. J., 2020, 161, 107680 CrossRef CAS.
- A. Golberg, M. Sack, J. Teissie, G. Pataro, U. Pliquett, G. Saulis, T. Stefan, D. Miklavcic, E. Vorobiev and W. Frey, Biotechnol. Biofuels, 2016, 9, 94 CrossRef PubMed.
- C. Occhiuto, G. Aliberto, M. Ingegneri, D. Trombetta, C. Circosta and A. Smeriglio, Molecules, 2022, 27, 3431 CrossRef CAS PubMed.
- A. A. Prestes, M. H. M. Canella, C. V. Helm, A. Gomes da Cruz and E. S. Prudencio, Curr. Opin. Food Sci., 2023, 51, 101005 CrossRef CAS.
- K. Jedvert, A. Saltberg, H. Theliander, Y. Wang, G. Henriksson and M. E. Lindström, Nord. Pulp Pap. Res. J., 2012, 27, 828–835 CrossRef CAS.
- J. D. DeMartini, M. Foston, X. Meng, S. Jung, R. Kumar, A. J. Ragauskas and C. E. Wyman, Biotechnol. Biofuels, 2015, 8, 209 CrossRef PubMed.
- M. Borrega, K. Nieminen and H. Sixta, Bioresour. Technol., 2011, 102, 10724–10732 CrossRef CAS PubMed.
- P. Manzanares, I. Ballesteros, M. J. Negro, A. González, J. M. Oliva and M. Ballesteros, Renewable Energy, 2020, 145, 1235–1245 CrossRef CAS.
- J. M. Prado, D. Lachos-Perez, T. Forster-Carneiro and M. A. Rostagno, Food Bioprod. Process., 2016, 98, 95–123 CrossRef CAS.
- J. Huang, Y. Zhu, T. Liu, S. Sun, J. Ren, A. Wu and H. Li, Energy Convers. Manage., 2019, 194, 46–54 CrossRef CAS.
- W. Yuan, Z. Gong, G. Wang, W. Zhou, Y. Liu, X. Wang and M. Zhao, Bioresour. Technol., 2018, 265, 464–470 CrossRef CAS PubMed.
- M. Qian, H. Lei, E. Villota, W. Mateo, Y. Zhao, E. Huo, Q. Zhang, X. Lin and Z. Huang, Bioresour. Technol. Rep., 2019, 8, 100339 CrossRef.
- F. P. Bouxin, S. David Jackson and M. C. Jarvis, Bioresour. Technol., 2014, 151, 441–444 CrossRef CAS PubMed.
- Z. Zhang, M. D. Harrison, D. W. Rackemann, W. O. S. Doherty and I. M. O'Hara, Green Chem., 2016, 18, 360–381 RSC.
- D. W. K. Chin, S. Lim, Y. L. Pang, C. H. Lim and K. M. Lee, Bioresour. Technol., 2019, 292, 121967 CrossRef CAS PubMed.
- H. Q. Lê, Y. Ma, M. Borrega and H. Sixta, Green Chem., 2016, 18, 5466–5476 RSC.
- M. H. Studer, J. D. DeMartini, S. Brethauer, H. L. McKenzie and C. E. Wyman, Biotechnol. Bioeng., 2010, 105, 231–238 CrossRef CAS PubMed.
- R. P. Chandra, Q. Chu, J. Hu, N. Zhong, M. Lin, J.-S. Lee and J. Saddler, Bioresour. Technol., 2016, 199, 135–141 CrossRef CAS PubMed.
- R. Bocker and E. K. Silva, Food Chem.: X, 2022, 15, 100398 CAS.
- R. N. Arshad, Z. Abdul-Malek, A. Munir, Z. Buntat, M. H. Ahmad, Y. M. M. Jusoh, A. E.-D. Bekhit, U. Roobab, M. F. Manzoor and R. M. Aadil, Trends Food Sci. Technol., 2020, 104, 1–13 CrossRef CAS.
- A. T. Hoang, X. P. Nguyen, X. Q. Duong, Ü. Ağbulut, C. Len, P. Q. P. Nguyen, M. Kchaou and W.-H. Chen, Bioresour. Technol., 2023, 385, 129398 CrossRef CAS PubMed.
- A. Warghane, R. Saini, N. K. Dhiman, K. Khan, M. Koche, A. Sharma, R. M. Gade, P. Halami and A. Das, in Value-Addition in Agri-food Industry Waste Through Enzyme Technology, ed. M. Kuddus and P. Ramteke, Academic Press, 2023, pp. 177–190, DOI:10.1016/B978-0-323-89928-4.00024-9.
- E. Lainez-Cerón, N. Ramírez-Corona, A. López-Malo and A. Franco-Vega, Chem. Eng. Process., 2022, 178, 109032 CrossRef.
- J. Guerrero-Beltran and J. Welti-Chanes, Pulsed Electric Fields, Encyclopedia of Food and Health, 2016, pp. 561–565, DOI:10.1016/B978-0-12-384947-2.00579-1.
- X. Li and M. Farid, J. Food Eng., 2016, 182, 33–45 CrossRef.
- D. Redondo, M. E. Venturini, E. Luengo, J. Raso and E. Arias, Innovative Food Sci. Emerging Technol., 2018, 45, 335–343 CrossRef CAS.
- Z.-H. Zhang, L.-H. Wang, X.-A. Zeng, Z. Han and C. S. Brennan, Int. J. Food Sci. Technol., 2019, 54, 1–13 CrossRef CAS.
- Đ. Kovačić, S. Rupčić, D. Kralik, D. Jovičić, R. Spajić and M. Tišma, Waste Manage., 2021, 120, 467–483 CrossRef PubMed.
- J. Raso, W. Frey, G. Ferrari, G. Pataro, D. Knorr, J. Teissie and D. Miklavčič, Innovative Food Sci. Emerging Technol., 2016, 37, 312–321 CrossRef.
- S. Toepfl, Procedia Food Sci., 2011, 1, 776–779 CrossRef.
- A. Taha, F. Casanova, P. Šimonis, V. Stankevič, M. A. E. Gomaa and A. Stirkė, Foods, 2022, 11, 1556 CrossRef CAS PubMed.
- M. M. Poojary, S. Roohinejad, F. J. Barba, M. Koubaa, E. Puértolas, A. R. Jambrak, R. Greiner and I. Oey, in Handbook of Electroporation, ed. D. Miklavčič, Springer International Publishing, Cham, 2017, pp. 2573–2590, DOI:10.1007/978-3-319-32886-7_185.
- A. Priyadarshini, G. Rajauria, C. P. O'Donnell and B. K. Tiwari, Crit. Rev. Food Sci. Nutr., 2019, 59, 3082–3101 CrossRef PubMed.
- E. Vorobiev and N. Lebovka, in Handbook of Electroporation, ed. D. Miklavčič, Springer International Publishing, Cham, 2017, pp. 655–670, DOI:10.1007/978-3-319-32886-7_163.
- R. Fan, L. Wang, J. Fan, W. Sun and H. Dong, Front. Nutr., 2022, 9 Search PubMed.
- J. Thulasidas, G. Varadarajan and R. Sundararajan, Nov. Approaches Drug Des. Dev., 2019, 5, 26–31 Search PubMed.
- S. Dastangoo, M. T. Hamed Mosavian and S. Yeganehzad, Food Sci. Nutr., 2020, 8, 2025–2034 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |