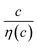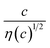Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Concentration dependence of resistance components in solutions containing dissolved Fe2+/Fe3+
Dai
Inoue
a and
Yutaka
Moritomo
 *abc
*abc
aGraduate School of Pure & Applied Science, University of Tsukuba, Tennodai 1-1-1, Tsukuba, Ibaraki 305-8571, Japan. E-mail: moritomo.yutaka.gf@u.tsukba.ac.jp
bFaculty of Pure & Applied Science, University of Tsukuba, Tennodai 1-1-1, Tsukuba, Ibaraki 305-8571, Japan
cTsukuba Research Center for Energy Materials Science (TREMS), University of Tsukuba, Tsukuba, Ibaraki 305-8571, Japan
First published on 20th February 2024
Abstract
Electrolyte solutions containing Fe2+/Fe3+ are suitable for liquid thermoelectric conversion devices (LTEs), because they are inexpensive materials and exhibit a high electrochemical Seebeck coefficient α. Here, we investigated the concentration (c) dependence of resistance components, i.e., solvent (Rs), charge-transfer (Rct), and diffusion (Rdif) resistances, of dissolved-Fe2+/Fe3+-containing aqueous, methanol (MeOH), acetone, and propylene carbonate (PC) solutions. We found that the c dependence of Rs and Rdif are well reproduced by empirical formulas, 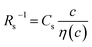 and
and 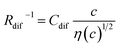 , where η(c) is viscosity at c. We further found that the magnitudes of Cs and Cdif are nearly independent of solvent, suggesting that η is one of the significant solution parameters that determine Rs and Rdif.
, where η(c) is viscosity at c. We further found that the magnitudes of Cs and Cdif are nearly independent of solvent, suggesting that η is one of the significant solution parameters that determine Rs and Rdif.
1 Introduction
Energy-harvesting devices are attracting the current attention of researchers from the viewpoint of a basic power source for the internet of things (IoT) society as well as sustainable development goals (SDGs). Thermoelectric conversion devices (TEs) are promising because they cover the field from bioelectric and wearable electronics to industrial power generation. In particular, flexible TEs using cellulose gel1 have excellent compatibility with various thermoelectric conversion materials. This is because cellulose can be used in the design of flexible and ion/electron conductive materials with robust mechanical properties. Flexible TEs are expected to be used not only for thermoelectric conversion but also for sensors and refrigeration units.Among TEs, liquid thermoelectric conversion devices (LTEs) are promising because they are made of inexpensive materials. There is already a long history of LTE research.2 Nevertheless, vigorous research has become more active in recent years and many research results have been reported.3–19 The performance of LTEs is governed by the electrochemical Seebeck coefficient α, effective electric conductivity σ, and effective thermal conductivity κ of the electrolyte.20 Unlike solid thermoelectric devices, σ is related to the charge transfer and diffusion processes of redox ions as well as the conventional ion migration. The magnitude of σ depends on the microscopic structure and material of the electrodes.16,17 In addition, effective σ and κ are influenced by convection of the electrolyte induced by ΔT. The dimensionless figure of merit (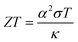 , where T is temperature) is a measure of the LTE performance. With the increase of ZT, the thermal efficiency η increases toward the Carnot efficiency
, where T is temperature) is a measure of the LTE performance. With the increase of ZT, the thermal efficiency η increases toward the Carnot efficiency 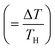 , which is the maximum efficiency of a heat engine.21 To enhance ZT, it is effective to increase (decrease) α and σ (κ).
, which is the maximum efficiency of a heat engine.21 To enhance ZT, it is effective to increase (decrease) α and σ (κ).
In recent years, research on LTEs using an organic electrolyte18,19 has begun to attract attention, as has research on conventional LTEs using an aqueous electrolyte.4–17 This is because the organic electrolytes exhibit both large α and small κ. In several organic solutions containing Fe2+/Fe3+, α is higher than the value (= 1.4 mV K−1) of an aqueous solution. For example, α is 3.6 mV K−1 in acetone solution and 1.8 mV K−1 in propylene carbonate (PC) solution.22 In addition, κ of a typical organic solvent is ≈ 0.2 W K−1 m−1 and is approximately 33% of the value (= 0.6 W K−1 m−1) of water. Recently, Wake et al.18,19 showed that LTEs composed of dissolved-Fe2+/Fe3+-containing methanol (MeOH) and acetone solutions exhibit a large power factor (PF = α2σ) comparable to that of the corresponding aqueous LTE. They also reported α and σ against solute concentration c. The disadvantage of organic electrolytes is a small σ value compared with that of an aqueous electrolyte. Except for aqueous electrolyte,7 there exists no detailed investigation on the resistance components. Therefore, the origin of the small σ in organic electrolytes is still unclear. Here, we will investigate the resistance components of several solutions containing dissolved Fe2+/Fe3+ against c to deeply understand σ and to obtain guidelines for increasing σ in organic electrolytes.
In general, the resistance R (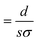 , where d and s are the electrode distance and area, respectively) of an electrolyte solution consists of the solution resistance Rs due to ion migration, charge transfer resistance Rct due to electron transfer, and diffusion resistance Rdif due to reactant/product diffusion.23 Among them, Rs is derived from the balance between the electric force (=|z|eEef; |z|, e, and Eef are the charge number, elementary charge, and electric field, respectively) and frictional force. According to Stokes’ law, the latter force is expressed as 6πηrv, where η, r, and v are the viscosity, effective radius, and velocity of the ion, respectively. Then, the mobility u
, where d and s are the electrode distance and area, respectively) of an electrolyte solution consists of the solution resistance Rs due to ion migration, charge transfer resistance Rct due to electron transfer, and diffusion resistance Rdif due to reactant/product diffusion.23 Among them, Rs is derived from the balance between the electric force (=|z|eEef; |z|, e, and Eef are the charge number, elementary charge, and electric field, respectively) and frictional force. According to Stokes’ law, the latter force is expressed as 6πηrv, where η, r, and v are the viscosity, effective radius, and velocity of the ion, respectively. Then, the mobility u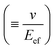 of an ion is expressed as
of an ion is expressed as 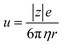 and is inversely proportional to η. On the other hand, Rct and Rdif are governed by the reaction kinetics in the vicinity of the electrode surface and are independent of d. The reaction rate k is expressed as
and is inversely proportional to η. On the other hand, Rct and Rdif are governed by the reaction kinetics in the vicinity of the electrode surface and are independent of d. The reaction rate k is expressed as 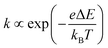 , where ΔE (= E − Eeq; E and Eeq are the electrode and equilibrium potentials, respectively) and kB are the overpotential and Boltzmann constant, respectively. In the region of
, where ΔE (= E − Eeq; E and Eeq are the electrode and equilibrium potentials, respectively) and kB are the overpotential and Boltzmann constant, respectively. In the region of 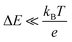 , the charge-transfer current Jct is expressed as
, the charge-transfer current Jct is expressed as 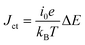 ,23 where i0 is the exchange current. Thus, Rct is proportional to
,23 where i0 is the exchange current. Thus, Rct is proportional to 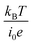 and is independent of d. The physical meaning of Rdif is as follows. As the reaction progresses, the concentration of reactants/products at the electrode surface changes in a way that prevents further reaction. For the reaction to continue, the reactants/products must diffuse into/from the bulk region. Note that the diffusion current of reactants/products is driven by the concentration gradient created by the reaction at the electrode surface and is independent of d.
and is independent of d. The physical meaning of Rdif is as follows. As the reaction progresses, the concentration of reactants/products at the electrode surface changes in a way that prevents further reaction. For the reaction to continue, the reactants/products must diffuse into/from the bulk region. Note that the diffusion current of reactants/products is driven by the concentration gradient created by the reaction at the electrode surface and is independent of d.
In this work, we investigated the c-dependence of Rs, Rct, and Rdif of dissolved-Fe2+/Fe3+-containing aqueous, MeOH, acetone, and PC solutions. We found that the c-dependence of Rs and Rdif is well reproduced by empirical formulas, 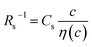 and
and 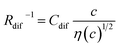 . We further found that their coefficients, Cs and Cdif, are nearly independent of solvent, suggesting that η is one of the significant solution parameters that determine Rs and Rdif.
. We further found that their coefficients, Cs and Cdif, are nearly independent of solvent, suggesting that η is one of the significant solution parameters that determine Rs and Rdif.
2 Experimental methods
2.1 Solution preparation
In this study, water, MeOH, acetone, and PC were selected as the solvents because they exhibit a high solubility of Fe(ClO4)2/Fe(ClO4)3. We prepared aqueous, MeOH, acetone, and PC solutions containing c M Fe(ClO4)2·6.0H2O and c M Fe(ClO4)3·7.1H2O. Distilled water, MeOH, acetone, PC, and solutes were purchased from FUJIFILM Wako Corp. and used as received. Table 1 shows the solubility s and critical concentration c* of Fe(ClO4)2/Fe(ClO4)3 in the four solvents. c* is defined as the concentration at which one Fe ion is dissolved per six solvent molecules. At c = c*, all solvent molecules are on average coordinated with Fe ions.| Solvent | s (M) | c* (M) |
|---|---|---|
| Water | 2.5 | 4.62 |
| MeOH | 2.5 | 2.06 |
| Acetone | 1.2 | 1.12 |
| PC | 1.5 | 0.97 |
2.2 Total resistance
The total resistance Rtot of the electrolyte was measured in a two-pole cell at 298 K.24 The electrodes were produced from a 220 μm graphite sheet (PREMA-FOIL, TOYO TANSO). The electrode distance d and area s are 1.0 cm and 0.42 cm2, respectively. The voltage drop V was measured against the current I (I ≤ 0.4 mA) with a multimeter. I was changed in a stepwise manner at intervals of several minutes. V was stable and no change over time was observed. The slope of the I–V plot corresponds to Rtot.2.3 Electrochemical impedance spectroscopy
R ct and Rdif were evaluated in the same cell with the same electrodes. d and s are 1.0 cm and 0.42 cm2, respectively. Electrochemical impedance spectroscopy (EIS) was performed at 298 K with use of a potentiostat (Vertex.one.EIS, Ivium Technologies). The frequency range was from 50 mHz to 100 kHz, and the amplitude was 10 mV. V was stable and no change over time was observed. It was confirmed that almost identical EIS data were obtained through multiple measurements.The EIS data were analyzed with a Randles equivalent circuit,23 which consists of Rs, Rct, double layer capacitance Cd, and Warburg impedance Zω. Zω is expressed as Zω = AW(ω−1/2 − iω−1/2), where AW and ω are the Warburg coefficient and angular velocity, respectively. It was difficult to evaluate the magnitude of Rdif from AW even though Zω describes the diffusion process of the reactants/products. In the present study, we tentatively evaluate the Rdif values by subtraction of Rs and Rct from Rtot. We confirmed a positive correlation between AW and Rdif (= Rtot − Rs − Rct), which strongly supports the correctness of our evaluation method of Rdif (vide infra).
3 Results
3.1 Total resistance
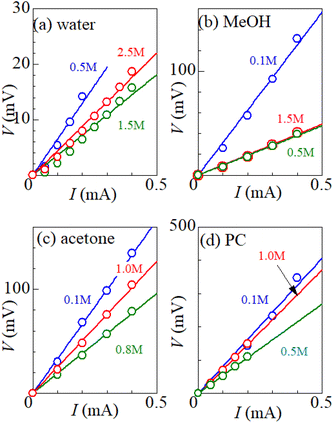
Fig. 1 shows examples of the I–V plot of several solutions containing dissolved Fe2+/Fe3+ at 298 K: (a) water, (b) MeOH, (c) acetone, and (d) PC. For all solutions, V increases in proportion to I. Rtot was evaluated from the slope of the plots, as indicated by the straight lines. The obtained Rtot values are listed in Table 2. In (a) the aqueous solution, Rtot decreases from 65.0 Ω at 0.5 M to 28.5 Ω at 1.5 M, and then increases to 37.9 Ω at 2.5 M. The decrease in Rtot in the low c region is due to the increase in the number (∝ c) of charge carriers, such as Fe2+ and Fe3+. This behavior is consistent with the literature.6 Similar local minima structures in the c–Rtot plot are also observed for the MeOH, acetone and PC solutions (Table 2).| Solvent | c (M) | R tot (Ω) | R s (Ω) | R ct (Ω) | R dif (Ω) | A W (Ω s−1/2) |
|---|---|---|---|---|---|---|
| Water | 0.10 | 351.0 | 56.0 | 32.0 | 263.0 | 86.2 |
| Water | 0.20 | 236.0 | 35.4 | 16.1 | 184.5 | 42.8 |
| Water | 0.50 | 65.0 | 19.8 | 5.3 | 39.9 | 15.7 |
| Water | 0.80 | 42.6 | 18.1 | 3.8 | 20.7 | 10.8 |
| Water | 1.00 | 39.4 | 18.1 | 2.9 | 18.4 | 10.0 |
| Water | 1.50 | 28.5 | 16.3 | 2.1 | 10.1 | 9.1 |
| Water | 2.00 | 27.7 | 19.6 | 1.9 | 6.2 | 7.0 |
| Water | 2.50 | 37.9 | 20.0 | 1.9 | 16.0 | 10.4 |
| MeOH | 0.05 | 906.0 | 221.7 | 65.2 | 619.1 | 176.7 |
| MeOH | 0.10 | 314.0 | 126.0 | 22.6 | 165.4 | 63.0 |
| MeOH | 0.50 | 95.6 | 54.6 | 4.4 | 36.6 | 16.2 |
| MeOH | 1.00 | 95.2 | 60.9 | 1.8 | 32.5 | 9.1 |
| MeOH | 1.50 | 98.2 | 67.4 | 1.6 | 29.2 | 8.4 |
| MeOH | 2.00 | 110.0 | 75.5 | 2.4 | 32,1 | 6.8 |
| MeOH | 2.50 | 115.0 | 83.3 | 3.2 | 28.6 | 7.5 |
| Acetone | 0.05 | 692.0 | 439.2 | 35.6 | 227.2 | 134.6 |
| Acetone | 0.10 | 333.0 | 187.0 | 18.0 | 128.0 | 72.0 |
| Acetone | 0.20 | 225.0 | 130.4 | 8.6 | 86.0 | 29.0 |
| Acetone | 0.40 | 172.0 | 100.3 | 5.0 | 66.7 | 14.7 |
| Acetone | 0.80 | 192.0 | 126.8 | 2.8 | 62.4 | 9.0 |
| Acetone | 1.00 | 254.0 | 160.5 | 4.5 | 89.0 | 9.2 |
| Acetone | 1.20 | 251.0 | 180.0 | 4.5 | 66.5 | 8.8 |
| PC | 0.05 | 1265.0 | 726.8 | 75.6 | 425.6 | 255.8 |
| PC | 0.10 | 813.0 | 615.0 | 27.0 | 171.0 | 70.0 |
| PC | 0.20 | 528.0 | 387.6 | 11.5 | 128.9 | 31.2 |
| PC | 0.50 | 537.0 | 447.9 | 11.4 | 77.7 | 17.5 |
| PC | 1.00 | 741.0 | 663.3 | 12.9 | 64.8 | 15.0 |
| PC | 1.50 | 726.0 | 674.1 | 13.2 | 38.7 | 13.9 |
Let us estimate the maximum value of ZT in the aqueous electrolyte at 300 K with the use of Rtot shown in Table 2. The maximum value of σ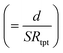 is 86.0 mS cm−1 at c = 2.0 M. Kim et al.6 reported c-dependence of α and κ in an aqueous solution containing Fe(ClO4)2/Fe(ClO4)3. From the extrapolation of the reported data, we evaluated α = 1.76 mV K−1 and κ = 0.4 W K−1 m−1 at 2.0 M. Then, we obtained ZT = 0.020 at 2.0 M. The ZT value is smaller than the value (= 0.036 (ref. 6) at 0.8 M) reported by Kim et al.,6 reflecting the smaller σ obtained in the present experiment. We note that effective σ of a LTE is influenced by the microscopic structure of the electrodes as well as the convection of the electrolyte.
is 86.0 mS cm−1 at c = 2.0 M. Kim et al.6 reported c-dependence of α and κ in an aqueous solution containing Fe(ClO4)2/Fe(ClO4)3. From the extrapolation of the reported data, we evaluated α = 1.76 mV K−1 and κ = 0.4 W K−1 m−1 at 2.0 M. Then, we obtained ZT = 0.020 at 2.0 M. The ZT value is smaller than the value (= 0.036 (ref. 6) at 0.8 M) reported by Kim et al.,6 reflecting the smaller σ obtained in the present experiment. We note that effective σ of a LTE is influenced by the microscopic structure of the electrodes as well as the convection of the electrolyte.
3.2 Electrochemical impedance spectroscopy
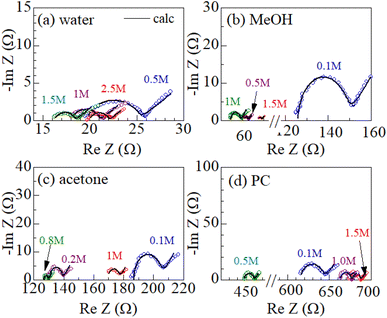
Fig. 2 shows examples of the Cole–Cole plots of complex impedance for several solutions containing dissolved Fe2+/Fe3+ at 298 K: (a) water, (b) MeOH, (c) acetone, and (d) PC. The Cole–Cole plot of 0.1 M MeOH solution (Fig. 2(b)) shows a prototypical shape. The plot shows a semicircle on the left side and a straight line with an inclination of 45° on the right side. The resistances on the left and right sides of the semicircle correspond to Rs and Rs + Rct, respectively. The intersection of the straight line with the horizontal axis corresponds to Rs + Rct − 2AW2Cd. Similar behaviors are also observed in the other solutions. The solid curves in Fig. 1 are the results of least-squares fits with a Randles equivalent circuit composed of Rs, Rct, Cd, and Zω. The Randles equivalent circuit well reproduces the observed complex impedance. Thus, we obtained Rs, Rct, Cd, and AW. We further evaluate Rdif (= Rtot − Rs − Rct) with the use of Rtot. The obtained Rs, Rct, Rdif, and AW values are listed in Table 2.A W is expected to have a strong correlation with Rdif because Zω [= AW(ω−1/2 − iω−1/2)] describes the diffusion process of the reactants/products. We calculated the correlation coefficient X between AW and Rdif (= Rtot − Rs − Rct) for each solution system; X = 0.976 for water, 0.995 for MeOH, 0.980 for acetone, and 0.988 for PC. The positive correlation (X ≥ 0.976) between AW and Rdif strongly supports the correctness of our evaluation method of Rdif.
3.3 Concentration dependence of resistivity components
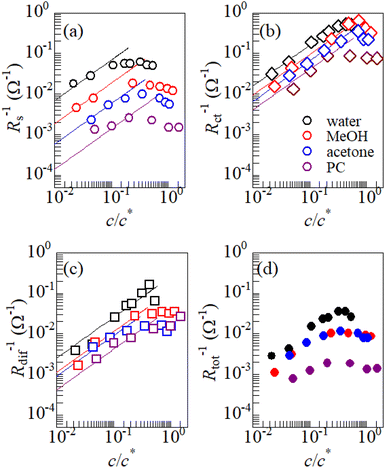
Fig. 3 shows the c-dependence of (a) Rs−1, (b) Rct−1, (c) Rdif−1, and (d) Rtot−1 in several solutions containing dissolved Fe2+/Fe3+ at 298 K. For the convenience of explanation, the horizontal axis is normalized by the critical concentration c* of each solvent. At c = c*, all solvent molecules are on average coordinated with Fe ions.First, let us examine the solvent dependence of Rs−1, Rct−1, and Rdif−1. Significant solvent dependence is observed for (a) Rs−1. Rs−1 values at 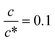 are 69.5 × 10−3 Ω−1 in water, 17.1 × 10−3 Ω−1 in MeOH, 6.7 × 10−3 Ω−1 in acetone, and 1.4 × 10−3 Ω−1 in PC. The Rs−1 values in organic solutions are much smaller than those in aqueous solutions. In contrast, Rct−1 and Rdif−1 have relatively small solvent dependence. In this sense, reducing Rs is effective to reduce Rtot in organic solution. Shortening d is especially effective because κ (≈ 0.2 W K−1 m−1) of an organic solvent is much smaller than κ (= 0.6 W K−1 m−1) of water. Reflecting the small κ in organic solvent, a sufficient ΔT is expected between the electrodes, even in the cell with smaller d.
are 69.5 × 10−3 Ω−1 in water, 17.1 × 10−3 Ω−1 in MeOH, 6.7 × 10−3 Ω−1 in acetone, and 1.4 × 10−3 Ω−1 in PC. The Rs−1 values in organic solutions are much smaller than those in aqueous solutions. In contrast, Rct−1 and Rdif−1 have relatively small solvent dependence. In this sense, reducing Rs is effective to reduce Rtot in organic solution. Shortening d is especially effective because κ (≈ 0.2 W K−1 m−1) of an organic solvent is much smaller than κ (= 0.6 W K−1 m−1) of water. Reflecting the small κ in organic solvent, a sufficient ΔT is expected between the electrodes, even in the cell with smaller d.
Next, let us investigate the 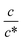 dependence of Rs−1, Rct−1, and Rdif−1. In the small
dependence of Rs−1, Rct−1, and Rdif−1. In the small 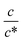 region, Rs−1 increases linearly with
region, Rs−1 increases linearly with 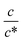 as indicated by the straight lines in Fig. 3(a). The increase in Rs−1 is due to the increase in the number (∝ c) of charge carriers, such as Fe2+ and Fe3+. Upon further increasing
as indicated by the straight lines in Fig. 3(a). The increase in Rs−1 is due to the increase in the number (∝ c) of charge carriers, such as Fe2+ and Fe3+. Upon further increasing 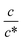 beyond ∼0.3, Rs−1 begins to decrease with
beyond ∼0.3, Rs−1 begins to decrease with 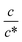 . Similarly, in the small
. Similarly, in the small 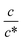 region, Rct−1 and Rdif−1 increase linearly with
region, Rct−1 and Rdif−1 increase linearly with 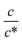 as indicated by the straight lines in Fig. 3(b) and (c), respectively. The increase in Rct−1 and Rdif−1 is due to the increase (∝ c) of reactant/product concentration, i.e., Fe2+/Fe3+. Upon further increasing
as indicated by the straight lines in Fig. 3(b) and (c), respectively. The increase in Rct−1 and Rdif−1 is due to the increase (∝ c) of reactant/product concentration, i.e., Fe2+/Fe3+. Upon further increasing 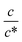 beyond ∼0.5, Rct−1 and Rdif−1 begin to saturate. The saturation of Rct−1 can be ascribed to the finite reaction number (Nreaction) per unit time at the electrode surface. The redox reaction cannot keep up with the supply of reactants when the number (Nreactant ∝ c) of reaching reactants per unit time exceeds Nreaction. In such a region, Nreaction becomes the rate-determining factor for the charge-transfer current Jct, and hence, Rct−1. As a result, Rct becomes constant at sufficiently large c.
beyond ∼0.5, Rct−1 and Rdif−1 begin to saturate. The saturation of Rct−1 can be ascribed to the finite reaction number (Nreaction) per unit time at the electrode surface. The redox reaction cannot keep up with the supply of reactants when the number (Nreactant ∝ c) of reaching reactants per unit time exceeds Nreaction. In such a region, Nreaction becomes the rate-determining factor for the charge-transfer current Jct, and hence, Rct−1. As a result, Rct becomes constant at sufficiently large c.
4 Discussion
4.1 Concentration dependence of Rs
Now, let us discuss the solution parameters that determine Rs.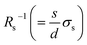 is expressed as
is expressed as 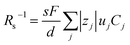 ,23 where F, zj, uj, and Cj are the Faraday constant, charge number, mobility, and molar concentration of the j-th ion, respectively. By substituting
,23 where F, zj, uj, and Cj are the Faraday constant, charge number, mobility, and molar concentration of the j-th ion, respectively. By substituting 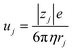 , we obtain
, we obtain 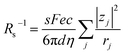 . Note that CFe2+ = CFe3+ = c in the present solutions. By assuming
. Note that CFe2+ = CFe3+ = c in the present solutions. By assuming 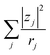 is independent of c in each solution, we obtain the simple relation
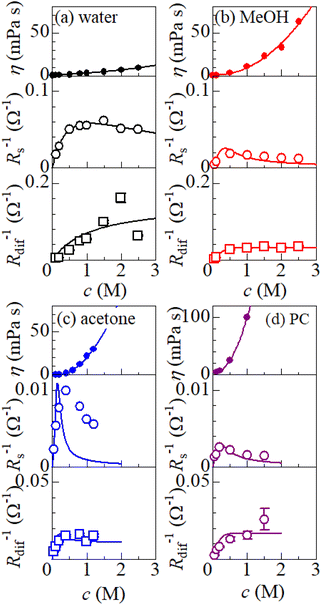
is independent of c in each solution, we obtain the simple relation 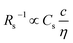 , where Cs is a constant. The top panels of Fig. 4(a)–(d) show η of each solution against c. The η values were evaluated at 298 K using a sine-wave vibro viscometer (SV-10; A&D Company Limited). In all solutions, η increases nonlinearly with c. The solid curves are the results of least-squares fits with a quadratic function. With use of the quadratic function η(c), the empirical formula,
, where Cs is a constant. The top panels of Fig. 4(a)–(d) show η of each solution against c. The η values were evaluated at 298 K using a sine-wave vibro viscometer (SV-10; A&D Company Limited). In all solutions, η increases nonlinearly with c. The solid curves are the results of least-squares fits with a quadratic function. With use of the quadratic function η(c), the empirical formula, 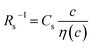 , can be calculated.
, can be calculated.
The middle panels of Fig. 4(a)–(d) show comparisons between observed Rs−1 (open circles) and empirical formula (solid curves) against c. We note that there is only one fitting parameter (Cs) to adjust the magnitude but no parameter to adjust the shape. Nevertheless, the curve reproduces the observed Rs−1 well, except for (c) acetone solution. In Table 3, we listed Cs. Except for the acetone solution, the solvent dependence of Cs is rather small, falling between 0.104 mPa s M−1 Ω−1 and 0.183 mPa s M−1 Ω−1. This is probably because the r value does not change greatly depending on the solvent.
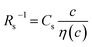 and
and 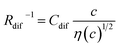 , determined by least-squares fits with the observed data. MeOH and PC represent methanol and propylene carbonate, respectively
, determined by least-squares fits with the observed data. MeOH and PC represent methanol and propylene carbonate, respectively
| Solvent | C s (mPa s M−1 Ω−1) | C dif (mPa1/2 s1/2 M−1 Ω−1) |
|---|---|---|
| Water | 0.183 | 0.125 |
| MeOH | 0.132 | 0.099 |
| Acetone | 0.019 | 0.053 |
| PC | 0.104 | 0.166 |
In (c) acetone solution, the shape of the c–Rs−1 plot (open circles) is qualitatively different from the shape of the empirical formula (solid curve). In the region of c ≥ 0.5, the empirical formula results decrease steeply while the observed Rs−1 decreases slowly. If Cs is set to ∼0.1, the agreement between the calculated and observed values is improved in the region of c ≥ 0.5 even though the calculated value is much larger in the region of c ∼ 0.3. This implies that an additional factor, e.g., the repulsive interaction between Fe ions, suppressed Rs in the region of c ∼ 0.3.
4.2 Concentration dependence of Rdif
Finally, let us consider the relationship between Rdif−1 and η. Rdif−1 is proportional to the diffusion current Jdif, which is expressed as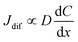 . Replacing the differential with the difference, we get
. Replacing the differential with the difference, we get 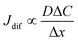 ,23 where Δx and ΔC are the diffusion length and concentration difference between electrode surface and bulk solution, respectively. In one-dimensional diffusion, Δx is expressed as
,23 where Δx and ΔC are the diffusion length and concentration difference between electrode surface and bulk solution, respectively. In one-dimensional diffusion, Δx is expressed as 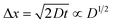 , where t is the elapsed time. Then, Jdif is proportional to cD1/2 because ΔC ∝ c. From the Stokes–Einstein equation, we obtain
, where t is the elapsed time. Then, Jdif is proportional to cD1/2 because ΔC ∝ c. From the Stokes–Einstein equation, we obtain 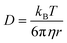 . Finally, we obtain an empirical relation
. Finally, we obtain an empirical relation 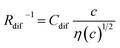 , where Cdif is a constant. We can calculate the empirical formula with use of the quadratic function η(c).
, where Cdif is a constant. We can calculate the empirical formula with use of the quadratic function η(c).
The bottom panels of Fig. 4(a)–(d) show comparisons between the observed Rdif−1 (open circles) and empirical formula (solid curves) against c. We note that there is only one fitting parameter (Cdif) to adjust the magnitude. Nevertheless, the curve reproduces the observed Rdif−1 well. In Table 3, we list the Cdif values. The solvent dependence of Cdif is rather small, falling between 0.053 mPa1/2 s1/2 M−1 Ω−1 and 0.166 mPa1/2 s1/2 M−1 Ω−1. This is probably because the r value does not change greatly depending on the solvent.
5 Conclusions
In conclusion, we investigated the c-dependence of Rs, Rct, and Rdif in dissolved-Fe2+/Fe3+-containing aqueous, MeOH, acetone, and PC solutions. We found that the c-dependence of Rs and Rdif is well reproduced by the empirical formulas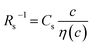 and
and 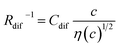 . We further found that the magnitudes of Cs and Cdif are nearly independent of the solvent, suggesting that η is one of the significant solution parameters that determine Rs and Rdif. Our findings suggest that σ of the electrolyte solution can be increased through reducing η.
. We further found that the magnitudes of Cs and Cdif are nearly independent of the solvent, suggesting that η is one of the significant solution parameters that determine Rs and Rdif. Our findings suggest that σ of the electrolyte solution can be increased through reducing η.
Author contributions
Dai Inoue: data curation, formal analysis, and investigation. Yutaka Moritomo: conceptualization, supervision, writing – original draft, and writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by JSPS KAKENHI (Grant No. 22KJ0413), Yazaki Memorial Foundation for Science and Technology, and joint research with Taisei Rotec Corporation.Notes and references
- Q. Long, G. Jiang, J. Zhou, D. Zhao, P. Jia and S. Nie, Nano Energy, 2024, 120, 109130 CrossRef CAS.
- T. Ikeshoji, Bull. Chem. Soc. Jpn., 1987, 60, 1505 CrossRef CAS; I. Quickenden and Y. Mua, J. Electrochem. Soc., 1995, 142, 3985 CrossRef; Y. Mua and T. I. Quickenden, J. Electrochem. Soc., 1996, 143, 2558 CrossRef; J. Kawamura, M. Shimoji and H. Hoshino, J. Phys. Soc. Jpn., 1981, 50, 194 CrossRef; A. Schiraldi, E. Pezzati and P. Baldini, J. Phys. Chem., 1985, 89, 1528 CrossRef; R. Hu, B. A. Cola, N. Haram, J. N. Barisci, S. Lee, S. Stoughton, G. Wallace, C. Too, M. Thomas, A. Gestos, M. Ed Cruz, J. P. Ferraris, A. A. Zakhidov and R. H. Baughman, Nano Lett., 2010, 10, 838 CrossRef; D. R. MacFarlane, N. Tachikawa, M. Forsyth, J. M. Pringle, P. C. Howlett, G. D. Elliott, J. F. Davis Jr, M. Watanabe, P. Simon and C. A. Angell, Energy Environ. Sci., 2014, 7, 232 RSC; M. Bonetti, S. Nakamae, M. Roger and P. Guenoun, J. Chem. Phys., 2011, 134, 114513 CrossRef PubMed; T. J. Abraham, D. R. MacFarlane and J. M. Pringle, Chem. Commun., 2011, 47, 6260 RSC; M. Romano, S. Gambhir, J. Razal, A. Gestos, G. Wallace, J. Chen and J. Therm, J. Therm. Anal. Calorim., 2012, 109, 1229 CrossRef; M. S. Romano, N. Li, D. Antiohos, J. M. Razal, A. Nattestad, S. Beirne, S. Fang, Y. Chen, R. Jalili, G. G. Wallace, R. Baughman and J. Chen, Adv. Mater., 2013, 25, 6602 CrossRef; T. J. Abraham, D. R. MacFarlane and J. M. Pringle, Energy Environ. Sci., 2013, 6, 2639 RSC; A. Gunawan, C.-H. Lin, D. A. Buttry, V. Mujica, R. A. Taylor, R. S. Prasher and P. E. Phelan, Nanoscale Microscale Thermophys. Eng., 2013, 17, 304 CrossRef; N. Jiao, T. J. Abraham, D. R. MacFarlane and J. M. Pringle, J. Electrochem. Soc., 2014, 161, D3061 CrossRef; S. Uhl, E. Laux, T. Journot, L. Jeandupeux, J. Charmet and H. Keppner, J. Electron. Mater., 2014, 43, 3758 CrossRef; A. Gunawan, H. Li, C.-H. Lin, D. A. Buttry, V. Mujica, R. A. Taylor, R. S. Prasher and P. E. Phelan, Int. J. Heat Mass Transfer, 2014, 78, 423 CrossRef; M. A. Lazar, D. Al-Masri, D. R. MacFarlane and J. M. Pringle, Phys. Chem. Chem. Phys., 2016, 18, 1404 RSC.
- Y. Liang, J. K.-H. Hui, M. Morikawa, H. Inoue, T. Yamada and N. Kimizuka, ACS Appl. Energy Mater., 2021, 4, 5326 CrossRef CAS.
- B. Yu, J. Duan, H. Cong, W. Xie, R. Liu, X. Ahuang, H. Wang, B. Qi, M. Xu and L. Wan, Science, 2020, 370, 342 CrossRef CAS.
- Y. Xaing, X. Guo, H. Zhu, Q. Zhang and S. Zhu, Chem. Eng. J., 2023, 461, 142018 CrossRef.
- J. H. Kim, J. H. Lee, E. E. Palen, M.-S. Suh, H. H. Lee and R. J. Kang, Sci. Rep., 2019, 9, 8706 CrossRef.
- M. A. Buckingham, F. Marken and L. Aldous, Sustainable Energy Fuels, 2018, 2, 2717 RSC.
- J. Duan, G. Feng, B. Yu, J. Li, M. Chen, P. Yang, J. Feng, K. Liu and J. Zhou, Nat. Commun., 2018, 9, 5146 CrossRef PubMed.
- T. Kim, J. S. Lee, G. Lee, H. Yoon, J. Yoon, T. J. Kangd and Y. H. Kim, Nano Energy, 2017, 31, 160 CrossRef CAS.
- M. Sindhuja, B. Lohith, V. Sudha, G. R. Manjunath and S. Harinipriya, Mater. Res. Express, 2017, 4, 075513 CrossRef.
- H. Im, T. Kim, H. Song, J. Choi, J. S. Park, R. Ovalle-Robles, H. D. Yang, K. D. Kihm, R. H. Baughman, H. H. Lee, T. J. Kang and Y. H. Kim, Nat. Commun., 2016, 7, 10600 CrossRef CAS PubMed.
- H. Zhou, T. Yamada and N. Kimizuka, J. Am. Chem. Soc., 2016, 138, 10502 CrossRef CAS.
- A. H. Kazim and B. A. Cola, J. Electrochem. Soc., 2016, 163, F867 CrossRef CAS.
- S. W. Lee, Y. Yang, H.-W. Lee, H. Ghasemi, D. Kraemer, G. Chen and Y. Cui, Nat. Commun., 2014, 5, 3942 CrossRef CAS.
- Y. Tanaka, A. Wake, D. Inoue and Y. Moritomo, Jpn. J. Appl. Phys., 2024, 63, 014002 CrossRef CAS.
- S.-M. Jung, S.-Y. Kang, B.-J. Lee, J. Kwon, D. Lee and Y.-T. Kim, Adv. Funct. Mater., 2023, 33, 2304067 CrossRef CAS.
- B. Yu, H. Xiao, Y. Zeng, S. Liu, D. Wu, P. Liu, J. Guo, W. Xie, J. Duan and J. Zhou, Nano Energy, 2022, 93, 106795 CrossRef CAS.
- A. Wake, D. Inoue and Y. Moritomo, Appl. Phys. Express, 2022, 15, 054002 CrossRef CAS.
- A. Wake, D. Inoue and Y. Moritomo, Jpn. J. Appl. Phys., 2023, 62, 014002 CrossRef CAS.
- J. Duan, B. Yu, L. Huang, B. Hu, M. Xu, G. Feng and J. Zhou, Joule, 2021, 5, 768 CrossRef CAS.
- S. W. Angrist, Direct Energy Conversion, Allyn and Bacon, Inc., Boston, 1982 Search PubMed.
- D. Inoue, H. Niwa, H. Nitani and Y. Moritomo, J. Phys. Soc. Jpn., 2021, 90, 033602 CrossRef.
- A. J. Bard, L. R. Faulkner, and H. S. White, Electrochemical Methods, Wiley, West Sussex, 2022 Search PubMed.
- Y. Fukuzumi, Y. Hinuma and Y. Moritomo, Jpn. J. Appl. Phys., 2019, 58, 065501 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |