Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Post-treatment strategies for pyrophoric KOH-activated carbon nanofibres†
Tom Fischer *ab,
Ansgar Kretzschmar
*ab,
Ansgar Kretzschmar a,
Victor Selmert
a,
Victor Selmert ab,
Sven Jovanovica,
Hans Kungla,
Hermann Tempel
ab,
Sven Jovanovica,
Hans Kungla,
Hermann Tempel a and
Rüdiger-A. Eichelab
a and
Rüdiger-A. Eichelab
aForschungszentrum Jülich GmbH, Institute of Energy and Climate Research (IEK-9) – Fundamental Electrochemistry, Jülich 52425, Germany
bRWTH Aachen University, Institute of Physical Chemistry, Aachen 52056, Germany
First published on 25th January 2024
Abstract
The effect of two atmospheric post-treatment conditions directly after the KOH activation of polyacrylonitrile-based nanofibres is studied in this work. As post-treatment different N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 flow conditions, namely high O2-flow and low O2-flow, are applied and their impact on occurring reactions and carbon nanofibres' properties is studied by thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), scanning electron microscopy (SEM), Raman spectroscopy, elemental analysis and CO2 and Ar gas adsorption. At high O2-flow conditions a pyrophoric effect was observed on the KOH-activated carbon nanofibers. Based on the obtained results from the TGA and DSC the pyrophoric effect is attributed to the oxidation reactions of metallic potassium formed during the KOH activation process and a consequent carbon combustion reaction. Suppression of this pyrophoric effect is achieved using the low O2-flow conditions due to a lower heat formation of the potassium oxidation and the absence of carbon combustion. Compared to the high O2-flow samples no partial destruction of the carbon nanofibers is observed in the SEM images. The determination of the adsorption isotherms, the surface area, the pore size distribution and the isosteric enthalpies of adsorption show the superior properties under low O2-flow conditions. The present micropore volume is increased from 0.424 cm3 g−1 at high O2-flow to 0.806 cm3 g−1 for low O2-flow samples, resulting in an increase of CO2 adsorption capacity of 38% up to 6.6 mmol g−1 at 1 bar. This significant improvement clearly points out the importance of considering highly exothermic potassium oxidation reactions and possible post-treatment strategies when applying KOH activation to electrospun carbon nanofiber materials.
O2 flow conditions, namely high O2-flow and low O2-flow, are applied and their impact on occurring reactions and carbon nanofibres' properties is studied by thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), scanning electron microscopy (SEM), Raman spectroscopy, elemental analysis and CO2 and Ar gas adsorption. At high O2-flow conditions a pyrophoric effect was observed on the KOH-activated carbon nanofibers. Based on the obtained results from the TGA and DSC the pyrophoric effect is attributed to the oxidation reactions of metallic potassium formed during the KOH activation process and a consequent carbon combustion reaction. Suppression of this pyrophoric effect is achieved using the low O2-flow conditions due to a lower heat formation of the potassium oxidation and the absence of carbon combustion. Compared to the high O2-flow samples no partial destruction of the carbon nanofibers is observed in the SEM images. The determination of the adsorption isotherms, the surface area, the pore size distribution and the isosteric enthalpies of adsorption show the superior properties under low O2-flow conditions. The present micropore volume is increased from 0.424 cm3 g−1 at high O2-flow to 0.806 cm3 g−1 for low O2-flow samples, resulting in an increase of CO2 adsorption capacity of 38% up to 6.6 mmol g−1 at 1 bar. This significant improvement clearly points out the importance of considering highly exothermic potassium oxidation reactions and possible post-treatment strategies when applying KOH activation to electrospun carbon nanofiber materials.
1. Introduction
Carbon-based materials are widely applied in research and industry, e.g. as a component of devices for energy storage or as adsorbent in gas separation processes.1–6 Amongst others, activated carbons are a frequently used material class due to their high micropore volume and high specific surface area. They are synthesized via a two-step process, comprised of carbonisation and activation. Typically, the precursor is first carbonised and subsequently activated, but also simultaneous carbonisation and activation processes are described.7–9 The applied process and activation method depends on the desired surface morphology and porosity of the carbon for the individual application. Therefore, a variety of activation processes have been developed to optimise the pore structure of carbons, which can be divided into physical activation and chemical activation methods. Physical activation is done using oxidising gases, e.g. CO2 or H2O, whereas chemical activation uses solid activating agents, e.g. KOH or H3PO4 to introduce porosity into the material.10–12 As a chemical activating agent for carbon-based materials, KOH is well-established and was patented by Wennerberg et al. in 1978.13 Since then, KOH activation has been widely used to activate many different precursor materials, e.g. coals, biomass and carbon fibres.1,12,14 KOH is mainly applied to obtain a high micropore volume and a high specific surface area of the activated materials. Frequently reported BET areas for KOH activated carbon fibres range from 1000 up to 3000 m2 g−1 and the total pore volume easily reaches up to 2 cm3 g−1.9,15 Despite the widespread application for decades, the exact mechanism of the KOH activation is still discussed. As overall reaction for the KOH activation, eqn (1) is proposed.1,15–17| 6KOH + 2C ↔ 2K + 3H2 + 2K2CO3, ΔHR = +428 kJ mol−1 | (1) |
Nevertheless, the actual occurring reactions are a sequence of several parallel and consecutive reactions and highly depend on the used precursor.1,10,18–20 The dehydration of KOH resulting in K2O and H2O (eqn (2)) is proposed as starting reaction for the KOH activation of petroleum coke.15,16 On elevating the temperature, the carbon and the H2O are transformed into H2 and CO via a coal gasification reaction (eqn (3)). Additional H2O reacts via the water gas shift reaction with the formed CO resulting in H2 and CO2 (eqn (4)). The obtained K2O (eqn (2)) and CO2 (eqn (4)) are transformed to K2CO3 at temperatures between 400 and 800 °C (eqn (5)). Furthermore, at reaction temperatures >700 °C K2O is reduced by H2 and carbon to metallic potassium (eqn (6) and (7)), which can intercalate into the carbon lattice.16
| 2KOH ↔ K2O + H2O, ΔHR = +245 kJ mol−1 | (2) |
| C + H2O ↔ H2 + CO, ΔHR = +131 kJ mol−1 | (3) |
| CO + H2O ↔ H2 + CO2, ΔHR = −41 kJ mol−1 | (4) |
| K2O + CO2 ↔ K2CO3, ΔHR = −393 kJ mol−1 | (5) |
| K2O + H2 ↔ 2K + H2O, ΔHR = +299 kJ mol−1 | (6) |
| K2O + C ↔ 2K + CO, ΔHR = +430 kJ mol−1 | (7) |
Similar reactions were proposed for the KOH activation of multi-walled carbon nanotubes with the formation of K2CO3 via redox reactions starting at 400 °C.17 In subsequent reactions the K2CO3 etches the carbon framework and K2O and CO are formed (eqn (8)).
| K2CO3 + C ↔ K2O + 2CO, ΔHR = +565 kJ mol−1 | (8) |
Similar to the activation reactions on petroleum coke the formation of elemental potassium is described for temperatures higher than 700 °C (eqn (7)). Summarising the proposed reactions involved in KOH activation it can be agreed on three types of pore-forming reactions:
(a) Etching of the carbon material via redox reactions (eqn (3), (7), and (8))
(b) Pore formation through gasification, i.e. H2O, CO, CO2, H2 (eqn (2)–(4), (6)–(8))
(c) Intercalation of elemental potassium (eqn (6) and (7)) into the carbon lattice, resulting in expansion of the carbon lattice.
Nevertheless, the actual reaction pathway highly depends on the applied activation parameters and the used precursor. Moreover, the structure of the precursor also affects the possibility of potassium intercalation. Overall, these impact factors render the assessment of the exact occurring reaction mechanisms a difficult task.1,20
Since the first patents, countless studies have been published on the impact of the different activation parameters, e.g. activation temperature, KOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) precursor ratio and activation duration.20,21 In contrast, the impact of the atmospheric post-treatment conditions directly subsequent to KOH activation has not been investigated so far. However, this factor may have a serious impact on the obtained activated carbon due to the high reactivity of elemental potassium formed during KOH activation. Elemental potassium is known to react vigorously in the presence of oxygen and water due to the formation of potassium oxides according to eqn (9)–(11).22,23
precursor ratio and activation duration.20,21 In contrast, the impact of the atmospheric post-treatment conditions directly subsequent to KOH activation has not been investigated so far. However, this factor may have a serious impact on the obtained activated carbon due to the high reactivity of elemental potassium formed during KOH activation. Elemental potassium is known to react vigorously in the presence of oxygen and water due to the formation of potassium oxides according to eqn (9)–(11).22,23
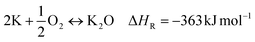 | (9) |
| 2K + O2 ↔ K2O2, ΔHR = −496 kJ mol−1 | (10) |
| K + O2 ↔ KO2, ΔHR = −285 kJ mol−1 | (11) |
The occurrence of such reactions in potassium-treated carbon materials has been frequently reported and was first observed by Fredenhagen and Cadenbach, who obtained pyrophoric potassium-graphite intercalation compounds in 1926.24,25 Additionally, a similar pyrophoric effect is described for the intercalation products of coals and amorphous carbons with K2CO3 during K2CO3-catalyzed gasification.26 Such an intercalation of potassium from melts into graphite and carbons was detailed studied in several publications.25,27,28
The present work investigates, the occurrence of such a pyrophoric effect after KOH activation by application of two different atmospheric post-treatments. Therefore, electrospun PAN-based carbon nanofibres are used, which exhibited carbonisation temperature dependent molecular sieve properties and a remarkable CO2/N2 adsorption selectivity in previous works.29–34 TGA-MS, DSC, SEM, elemental analysis, Raman spectroscopy and gas adsorption techniques are used to investigated occurring reactions and changes to the fibre morphology and porosity.
2. Experimental
2.1 Carbon nanofibres synthesis
For the synthesis of the carbon nanofibres, solutions of 10 wt.% PAN (150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, BOC Science, USA) in N,N-dimethyl formamide (VWR Chemicals, Germany) were prepared. All chemicals were used as received without further purification. To obtain a complete dissolution the mixtures were stirred for 48 h at room temperature. Subsequently, the solution was electrospun using an electrospinning device (IME Technologies, Netherlands). The polymer solution was supplied at a flow rate of 120 μL min−1 through a 4-tip spinning needle. The spinneret was moved laterally to the collector drum with a speed of 20 mm s−1 and a turn delay of 500 ms within a range of 120 mm. The spinning process was conducted at constant climate conditions of 25 °C and 30% relative humidity and the applied voltage was set to 25 kV. The tip-collector distance was 120 mm. The fibres were collected on a rotating drum with a diameter of 90 mm and a rotational speed of 1000 rpm. In total, the spinning was conducted for 3 h, which corresponds to 21.6 mL used spinning solution. Subsequently, oxidative stabilization at 250 °C in air for 15 h was performed at a heating rate of 5 K min−1 using a drying cabinet (Binder GmbH, Germany). In the following step, 200 mg of the stabilized nanofibres were impregnated with 10 mL aqueous KOH (c = 0.26 mol L−1, KOH
000 g mol−1, BOC Science, USA) in N,N-dimethyl formamide (VWR Chemicals, Germany) were prepared. All chemicals were used as received without further purification. To obtain a complete dissolution the mixtures were stirred for 48 h at room temperature. Subsequently, the solution was electrospun using an electrospinning device (IME Technologies, Netherlands). The polymer solution was supplied at a flow rate of 120 μL min−1 through a 4-tip spinning needle. The spinneret was moved laterally to the collector drum with a speed of 20 mm s−1 and a turn delay of 500 ms within a range of 120 mm. The spinning process was conducted at constant climate conditions of 25 °C and 30% relative humidity and the applied voltage was set to 25 kV. The tip-collector distance was 120 mm. The fibres were collected on a rotating drum with a diameter of 90 mm and a rotational speed of 1000 rpm. In total, the spinning was conducted for 3 h, which corresponds to 21.6 mL used spinning solution. Subsequently, oxidative stabilization at 250 °C in air for 15 h was performed at a heating rate of 5 K min−1 using a drying cabinet (Binder GmbH, Germany). In the following step, 200 mg of the stabilized nanofibres were impregnated with 10 mL aqueous KOH (c = 0.26 mol L−1, KOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAN weight ratio 3
PAN weight ratio 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) for 2 h. Afterwards, the sample was dried at 85 °C for 3 h to remove H2O.
4) for 2 h. Afterwards, the sample was dried at 85 °C for 3 h to remove H2O.
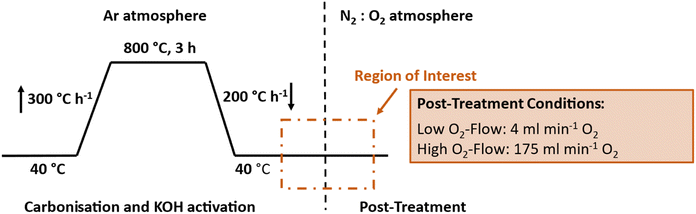
The entire carbonisation & activation process was conducted inside a thermogravimetric analyser (STA 449 F1 Jupiter, Netzsch GmbH, Germany) coupled to a mass spectrometer (QMS 403 D Aëlos, Netzsch GmbH, Germany) (TGA-MS). 200 mg of the obtained KOH impregnated nanofibres were transferred into the 5 mL TGA beaker to conduct the simultaneous carbonisation and activation. The TGA furnace was purged trifold with Ar to ensure an inert carbonisation atmosphere. Afterwards, the samples were heated at a rate of 300 K h−1 up to 800 °C and held for 3 h at this temperature in inert atmosphere at a flow rate of 40 mL min−1 Ar 5.2 (Air Liquide, France). Afterwards, the samples were cooled down to 40 °C at a rate of 200 K h−1. Once a temperature of 40 °C was reached, the atmosphere was changed to an O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N2 atmosphere. The used O2-flow rates were 4 mL min−1 for low O2-flow conditions and 175 mL min−1 for high O2-flow conditions. The respective N2 flow rates were 16 mL min−1 for low O2-flow conditions and 75 mL min−1 for high O2-flow conditions. After the switch to O2
N2 atmosphere. The used O2-flow rates were 4 mL min−1 for low O2-flow conditions and 175 mL min−1 for high O2-flow conditions. The respective N2 flow rates were 16 mL min−1 for low O2-flow conditions and 75 mL min−1 for high O2-flow conditions. After the switch to O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N2 atmosphere, the samples were kept for 45 minutes under the applied atmospheric conditions. After this process steps, the carbon nanofibres were obtained and neutralized using distilled water in several washing steps until a neutral pH value was achieved. Finally, the samples were dried at 100 °C. For each post-treatment, a fivefold determination was carried out.
N2 atmosphere, the samples were kept for 45 minutes under the applied atmospheric conditions. After this process steps, the carbon nanofibres were obtained and neutralized using distilled water in several washing steps until a neutral pH value was achieved. Finally, the samples were dried at 100 °C. For each post-treatment, a fivefold determination was carried out.
Additionally, similar experiments were conducted in a horizontal tube furnace (REST-E 400/6, Carbolite Gero GmbH & Co. KG, Germany). 200 mg of the KOH impregnated sample were transferred into an alumina boat and the furnace was trifold purged with Ar. Subsequently, the sample was heated up to 800 °C at a heating rate of 300 K h−1 in inert atmosphere at a flow rate of 105 L h−1 Ar 5.2 (Air Liquide, France). The temperature was maintained for 3 h and afterwards cooled down at a rate of 200 K h−1. At a temperature of 40 °C the furnace was opened and the samples were exposed directly to ambient air, which equals high O2-flow conditions.
2.2 Material characterization
Mass and temperature changes, as well as the gaseous reaction products were detected by the TGA-MS system. The MS measurements were conducted in multiple ion detection (MID) mode. Relevant gases were identified with a scan measurement for m/z 0–100 in an additional run prior to the actual measurements. The obtained MID runs were normalized to a reference run, to correct contamination of the device caused by the formation of soot particles during the reaction.For data evaluation, the peak area for the detected components was determined by integration. The obtained weight normalized peak area is converted into the molar amount of CO2 based on a calibration using CaC2O4 × H2O. For the calibration masses of 50, 75, 100, 125 and 150 mg of CaC2O4 × H2O were transferred into the TGA crucible and afterwards heated to 1000 °C. Based on the obtained results, a relation of peak area CO2 to molar amount could be drawn (S1).†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35
35
Additionally, differential scanning calorimetry (DSC) measurements were performed on a STA 449 F1 Jupiter (Netzsch GmbH, Germany) to measure the released reaction heats. Prior to the measurements, a calibration using a sapphire disc was performed. The DSC measurements were conducted with an equal heat treatment as for the carbonisation and activation process in the TGA beaker. The sample mass of KOH impregnated nanofibres for these measurements was 20–30 mg. For each post-treatment condition, a triple determination was conducted.
Elemental analysis was conducted using a varioELcube elemental analyser (Elementar, Germany). A triple determination with 2 mg for each sample was carried out to determine C, H and N content. The O content was determined as the difference between the CHN content to the total composition.
Inductively coupled plasma optical emission spectroscopy (ICP-OES) was performed on iCAP 7600 analyser (ThermoFisher Scientific, USA). Two parallel digestions of 100 mg sample each were prepared in a furnace with 250 mg lithium borate. The samples were heated to 1050 °C during 3 hours and maintained for 30 minutes at this temperature. The obtained melt was diluted in 50 mL 5% HNO3 and filled up to 100 mL.
A Quanta FEG 650 microscope (FEI, USA) was used to conduct the scanning electron microscopy (SEM) investigations. For image recording an acceleration voltage of 20 kV and an Everhart–Thornley detector was used. Small parts of the sample were fixed on the sample holders using copper strips.
Gas adsorption measurements were performed on a 3P micro 300 (3P Instruments, Germany). Argon (5.2, Air Liquide, France) adsorption measurements were conducted at 87 K and CO2 (4.5, Air Liquide, France) adsorption measurements at 273 K. Prior to the measurements the samples were outgassed at 150 °C for 12 h.
The obtained data was evaluated using Asiqwin 5.0 (Quantachrome Instruments, USA). The adsorption isotherms were fitted as a Tóth isotherm36 to calculate a mean isotherm for each parameter set. As error assessment the standard deviation was determined. A quenched solid state DFT (QSDFT) equilibrium model was applied to calculate the pore size distribution of the Argon adsorption data using a slit pore model on carbon. The pore size distribution of the CO2 adsorption isotherms was obtained by a Monte-Carlo model on carbon for slit pores.
Similar to the isotherm fits a mean value was calculated and the error indicated by the standard deviation. Additionally, the determination of the BET area, taking into account its limitations for microporous materials, was performed.37,38
Determination of the adsorption kinetics was performed using an Autosorb iQ 2 (Quantachrome, USA) by measurement of a single point isotherm at 50 mbar at 298 K using the VectorDose™ mode.
For calculation of the isosteric enthalpy of adsorption, CO2 adsorption isotherms were measured at 273 K, 283 K and 293 K on a QuadraSorb EVO (Quantachrome, USA) and fitted using a Tóth Fit. The calculation of the isosteric enthalpies of adsorption was conducted via the Clausius–Clapeyron approach.39
3. Results and discussion
In preliminary experiments KOH-activated carbon nanofibers were post-treated at ambient air as it is the usual procedure.1,11,12 During the exposure to ambient air, a strong red glowing of the carbon nanofibres was observed, which indicates vigorous potassium oxidation reactions (Fig. 1 and Video in the ESI†). | ||
| Fig. 1 Pyrophoric KOH-activated electrospun PAN-based carbon nanofibres during exposure to ambient air directly after the simultaneous carbonisation and KOH activation. A red glowing of the carbon nanofibres can be observed (a Video of the glowing is attached as ESI†). | ||
Detailed investigations on this pyrophoric effect using a TGA-MS following the heat treatment in Fig. 2 were conducted to get a deeper insight into the occurring reactions. Therefore, two different post-treatment conditions were applied subsequent to the simultaneous carbonisation and activation: one, labelled as ‘low O2-flow’ at an O2-flow rate of 4 mL min−1 (N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 80
O2 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) and a second one at 175 mL min−1 (N2
20) and a second one at 175 mL min−1 (N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 30
O2 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70), denoted as ‘high O2-flow’. Additionally, SEM, DSC, elemental analysis, Raman spectroscopy and gas adsorption were used to obtain detailed information on the observed glowing, the reaction process and its impact on the fibre morphology.
70), denoted as ‘high O2-flow’. Additionally, SEM, DSC, elemental analysis, Raman spectroscopy and gas adsorption were used to obtain detailed information on the observed glowing, the reaction process and its impact on the fibre morphology.
3.1 High O2-flow
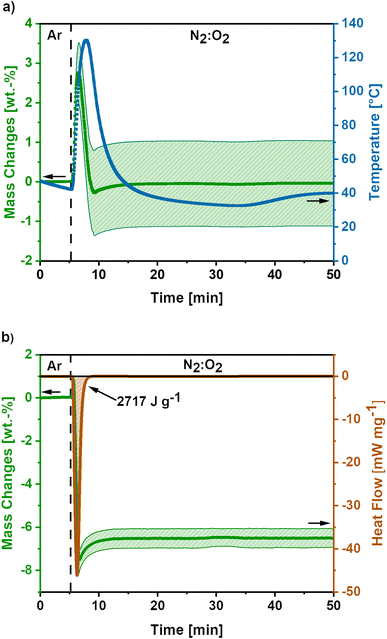
In Fig. 3a the mass and temperature changes of the freshly activated samples on switching the atmosphere from Ar to a mixture of N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 (30
O2 (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70) are shown for the high O2-flow. The vertical dashed line indicates the switch from Argon to N2
70) are shown for the high O2-flow. The vertical dashed line indicates the switch from Argon to N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 (30
O2 (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70) atmosphere at 175 mL min−1 O2-flow. Almost instantly after the change in atmosphere, a mass gain of 2.8 ± 0.7 wt.% is observed. Afterwards, a mass loss of 3.1 ± 1.1% occurs before the mass slightly increases again. Simultaneously, the temperature rises to 130 °C and cools down again to the temperature set point of 40 °C afterwards. However, it must be emphasized, that this is only an apparent temperature, as the reaction heat is partially consumed by the heat capacity of comparatively large TGA crucible. The actual temperature of the sample during the reaction is significantly higher.
70) atmosphere at 175 mL min−1 O2-flow. Almost instantly after the change in atmosphere, a mass gain of 2.8 ± 0.7 wt.% is observed. Afterwards, a mass loss of 3.1 ± 1.1% occurs before the mass slightly increases again. Simultaneously, the temperature rises to 130 °C and cools down again to the temperature set point of 40 °C afterwards. However, it must be emphasized, that this is only an apparent temperature, as the reaction heat is partially consumed by the heat capacity of comparatively large TGA crucible. The actual temperature of the sample during the reaction is significantly higher.
The mass gain and the temperature rise can be assigned to the oxidation reactions of elemental potassium, which was formed during the KOH activation reactions (eqn (6) and (7)).22,23,40 The formation of K2O (eqn (9)), K2O2 (eqn (10)) and KO2 (eqn (11)) releases high reaction enthalpies. The oxidation reactions most probably result in the formation of KO2 for conditions with high availability of oxygen, due to the higher lattice stability compared to K2O and K2O2.22,23
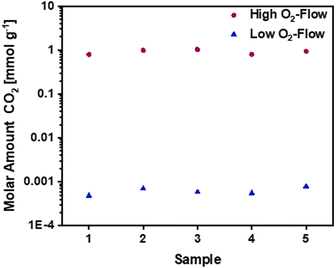
The mass gain of 2.8 ± 0.7 wt.% corresponds to the O2 uptake of 0.87 mmol g−1, which would require a potassium amount of 0.87 mmol g−1 according to eqn (11). This corresponds to 14% of the total potassium, present at the carbon nanofibre samples, which was determined via ICP-OES (S2).† The calculated reaction heat of the formation of 0.87 mmol g−1 KO2 is −248 J g−1 according to eqn (11), which is sufficient to cause a significant temperature rise.
However, these reactions cannot explain the subsequent mass loss of 3.1 ± 1.1 wt.%. An analysis of the gaseous products determined CO2 as main emission (S3),† suggesting a carbon partial combustion reaction caused by the reaction heat of the potassium oxidation. A total carbon loss of 2.62 mmol g−1 is calculated via eqn (12) accompanied by −1020 J g−1 reaction heat. In total potassium oxidation and carbon combustion reactions result in an emitted reaction heat of −1268 J g−1.
| C + O2 ↔ CO2, ΔHR = −393 kJ mol−1 | (12) |
Comparing the calculated reaction heats of KO2 and CO2 formation, the emitted reaction heat is caused to almost 80% by the carbon combustion to CO2. According to the basic heat equation of thermodynamics, the emitted heat could cause a temperature increase to a temperature higher than 700 °C, which would explain the observed glowing of the carbon nanofibres (Fig. 1).
Based on the calculated carbon loss the theoretical released amount of CO2 would be 2.58 mmol g−1, which equals a CO2 release of 114 mg g−1. The amount of CO2 determined via quantification of the gaseous products is lower, giving a value of 40.5 mg g−1. The significant difference could be explained by the emission of solid particles due to the vigorous reaction, which are not detected by the MS. Additionally, side reactions due to formed KO2 are possible during the reaction as listed in eqn (13) and (14). The possible side reactions mainly result in the formation of potassium carbonate and bicarbonate.41,42
| 2KO2 + CO2 ↔ K2CO3 + 1.5O2, ΔHR = −186 kJ mol−1 | (13) |
| 2KO2 + H2O + 2CO2 ↔ 2KHCO3 + 1.5O2, ΔHR = −328 kJ mol−1 | (14) |
Furthermore, combined TG-DSC measurements were conducted to obtain experimental data on the emitted reaction heats for high O2-flow (Fig. 3b). The simultaneous TG measurement was done to assess the comparability of both measurements since different sized crucibles had to be used for both measurements. An emitted reaction heat of −2717 J g−1 was determined for the post-treatment step at high O2-flow (Fig. 3b). This value is about twice as high as the calculated reaction heat of −1268 J g−1 from previous TG measurements (Fig. 3a).
The deviation of these two methods is explicable, when considering the shape of the TG signal of the DSC measurement. Similar to the first discussed TG signal, a mass gain is observed directly after the switch to the high O2-flow. Subsequently, a significant mass loss due to carbon combustion occurs. However, the mass loss during the DSC measurement is 8.2 wt.%, which is more than double the value of 3.1 wt.% during the TG measurement. Due to the increased carbon combustion during the DSC measurement the obtained experimental value and the calculated reaction enthalpy from the TG measurement may not be directly comparable. The increased carbon combustion is probably caused by denser packed carbon nanofibres in the smaller DSC crucible, resulting in a stronger heat accumulation. This is an important hint, as the extent of potassium oxidation and carbon combustion reactions depend on the packing density and, therefore, heat and oxygen transport properties of the material.
Interestingly, similar observations are not described for KOH activation of commonly used carbon precursors, e.g. PAN powder,43,44 carbon fibres,45–47 biomass48–50 and coals.18,51,52 Although, the occurrence of these potassium oxidation reactions could be expected based on the proposed mechanisms for KOH activation mentioned in eqn (6) and (7). Therefore, PAN powder was used as reference material and activated using the identical conditions as for activation of the nanofibre material. During high O2-flow post-treatment no pyrophoric effect was observed (S4).† Hence, the described pyrophoric effect is probably linked to the material structure induced by the electrospinning process and, possibly, the activation conditions.
Overall, the post-treatment at high O2-flow conditions results in the uncontrolled oxidation of metallic potassium to KO2 causing a carbon combustion under severe formation of heat and a strong glowing of the carbon nanofibres.
3.2 Low O2-flow
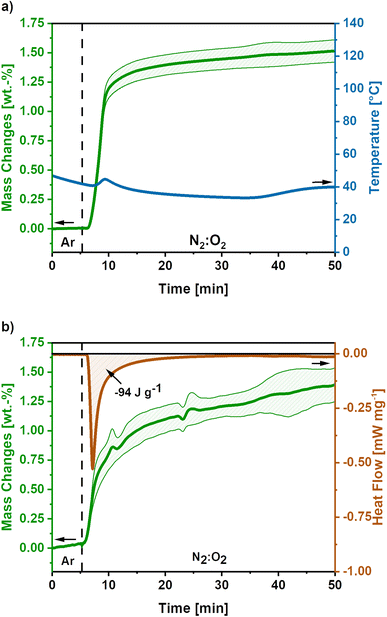
To develop a treatment strategy to suppress the sample glowing, similar experiments were conducted using an O2-flow of 4 mL min−1, ‘low O2-flow’. Fig. 4a shows the mass and temperature changes after the switch of atmosphere from Ar to a mixture of N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 (80
O2 (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) with an O2-flow rate of 4 mL min−1, indicated by the dashed line.
20) with an O2-flow rate of 4 mL min−1, indicated by the dashed line.
A mass gain of 1.5 ± 0.1 wt.% accompanied by a minor temperature rise to 45 °C after 8 minutes is observed after the atmosphere switch. Contrary to the high O2-flow, no weight loss is observed. The mass gain can be explained by the uptake of O2 due to the oxidation of metallic potassium according to eqn (9)–(11). As the temperature increase is insufficient to cause a carbon combustion, no mass loss is observed.
The obtained mass gain equals an O2 uptake of 0.44 mmol g−1, based on eqn (12) this results in the emission of −128 J g−1 reaction heat. This calculated reaction heat is comparable to the experimental value of −94 J g−1 obtained from additional DSC measurements (Fig. 4b) and causes a slight temperature rise on the carbon nanofibres. The slight deviation of the obtained reaction heats can be explained by the simple nature of the calculations used, which only considered one potassium formation reaction neglecting possible side reactions to the formation of other potassium oxide species.22,23,41
Summarising, the low O2-flow post-treatment results in the controlled oxidation reactions during exposure to oxygen. The emitted reaction heat of −94 J g−1 is significantly reduced compared to −2717 J g−1 for high O2-flow. Therefore, no indication for a carbon combustion or glowing was found.
3.3 Comparison of high O2-flow and low O2-flow
Besides the previously discussed mass and temperature changes during the sample treatment with high O2-flow and low O2-flow, this section addresses differences in the emitted CO2 amount, elemental composition and morphological changes observed in SEM.Additionally, CHNO elemental analysis was conducted to detect changes in the elemental composition of the obtained carbon nanofibres for high and low O2-flow (Table 1). The C content for low O2-flow is 69.6 wt.%, whereas it is at 59.8 wt.% for high O2-flow. This 10 wt.% decreased C content can be related to the carbon combustion and possibly also to oxidation of the carbon surface. For the H and N content no significant changes were obtained between high O2-flow and low O2-flow. Regarding the O content, an increase of 7 wt.% for high O2-flow compared to low O2-flow is observed. This is explained by the reduced amount of C relative to the O content and a partial oxidation of the carbon surface. However, the oxidation of the carbon surface would counteract the observed weight loss during the carbon combustion.
| Sample | Elemental composition (wt%) | |||
|---|---|---|---|---|
| C | H | N | O | |
| High O2-flow | 59.8 ± 1 | 2.2 ± 0.4 | 12.7 ± 0.5 | 25.3 ± 3.1 |
| Low O2-flow | 69.6 ± 0.9 | 1.7 ± 0.2 | 10.7 ± 0.6 | 18.0 ± 2.8 |
| Pristine fibres | 72.6 ± 0.1 | 1.4 ± 0.1 | 16.2 ± 0.2 | 9.8 ± 0.7 |
For further structural characterization and comparison, Raman spectroscopy was performed on low and high O2 flow samples. The results are shown and discussed in the ESI (S5).†
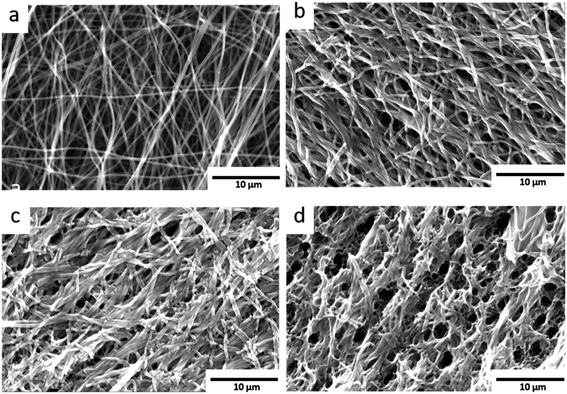
The pristine carbon nanofibres are randomly aligned and large void volumes are visible between the fibres. For the KOH activated carbon nanofibres smaller void volumes and fragmented fibres are observed. The low O2-flow samples exhibit a similar morphology as the pristine material (Fig. 6b, S6† vs. 6a). In contrast, high O2-flow samples (Fig. 6d) exhibit areas with significant destruction of surface morphology compared to the pristine material (Fig. 6a). The formation of voids in the size of up to 3 microns is clearly visible in the SEM images (Fig. 6d). These macropores are not homogeneously distributed on the surface of the high O2-flow samples as also areas with less severe destruction are visible (Fig. 6c). This observation could be explained by an inhomogeneous distribution of metallic potassium on the samples and, therefore, inhomogeneous severe heat formation due to the potassium oxidation reactions.
Summarising, the obtained SEM results prove that the control of post-treatment conditions significantly affects the morphology of the samples. The applied O2-flow affects the potassium oxidation reactions and a severe destruction of the carbon nanofibres can be avoided by application of a low O2-flow as post-treatment.
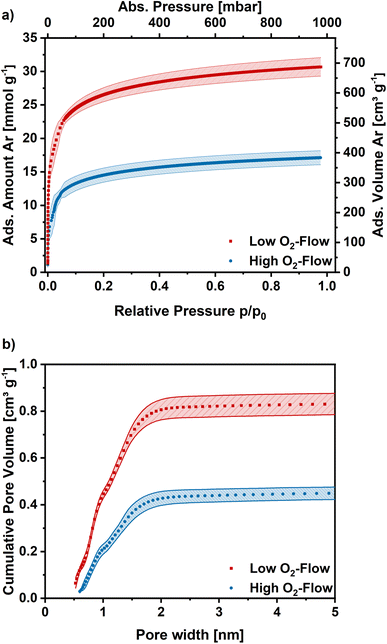
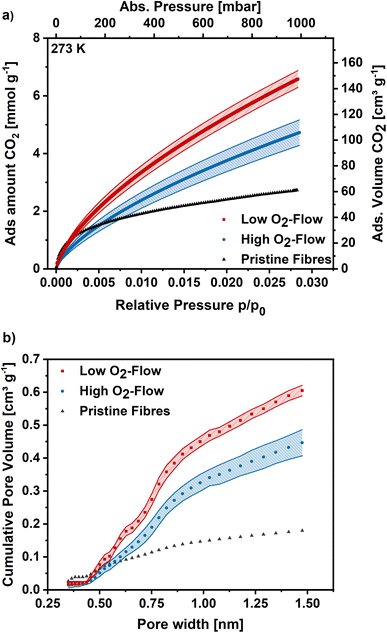
Argon adsorption measurements were performed at 87 K to assess the microporosity. The obtained adsorption isotherms exhibit a type I shape, which is typical for highly microporous adsorbents (Fig. 7a). In the low relative pressure range a steep increase is observed, which flattens for higher relative pressures and approaches a limiting value. The obtained isotherms exhibit similar shapes independent of post-treatment, although the obtained adsorption capacities are significantly higher on the low O2-flow samples. At 1 bar 17 mmol g−1 CO2 are adsorbed on the low O2-flow samples, whereas 31 mmol g−1 CO2 are obtained for high O2-flow. This equals an increase in adsorption capacity at 1 bar by 82%.
The pore size distribution was derived from the Ar adsorption isotherms using a density functional theory (DFT) kernel. The cumulative pore size distributions for low O2-flow and high O2-flow are shown in Fig. 7b. The comparison of the total pore volume VDFT(tot) and the pore volume of pores below 2 nm (VDFT<2nm) reveals that both samples have almost exclusively micropores (Table 2, VDFT<2nm). The obtained micropore volume is 0.806 cm3 g−1 for low O2-flow samples, which is an increase of 90% compared to 0.424 cm3 g−1 for the high O2-flow. Such values are comparable to values obtained by Im et al. and Chiang et al. for PAN-based activated carbon fibres.45,53
The BET area was determined at 1094 m2 g−1 for high O2-flow and at 2029 m2 g−1 for low O2-flow, which are typical BET areas of KOH-activated carbons.9,44,54 Especially the direct comparison to the BET area of the pristine fibres (13.4 m2 g−1) clearly proves a successful KOH activation of the electrospun PAN-based carbon nanofibres. Additionally, the high impact of the applied post-treatment conditions on the surface morphology and porosity of the carbon nanofibres is obvious.
For more detailed micropore characterization CO2 adsorption measurements at 273 K were performed. Fig. 8a shows the CO2 adsorption isotherms for the pristine,30 high O2-flow and the low O2-flow samples. The pristine fibres isotherm exhibits a high uptake at low relative pressures and turns more into a linear shape at higher relative pressures with a maximum CO2 uptake of 2.7 mmol g−1 at 1 bar. In comparison, the adsorption isotherm of the high O2-flow shows a lower CO2 uptake at pressures below 200 mbar.
| Sample | BET (m2 g−1) | VDFT(tot) (cm3 g−1) | VDFT<2nm (cm3 g−1) | VDFT<1.5nm (cm3 g−1) | VMC(tot) (cm3 g−1) | VDFT<0.7nm (cm3 g−1) | VMC<0.7nm (cm3 g−1) |
|---|---|---|---|---|---|---|---|
| Low O2-flow | 2029 | 0.831 | 0.806 | 0.696 | 0.605 | 0.173 | 0.208 |
| High O2-flow | 1094 | 0.461 | 0.424 | 0.365 | 0.446 | 0.058 | 0.145 |
| Pristine fibres | 13.4 | 0.016 | 0.004 | 0.003 | 0.180 | — | 0.101 |
 | ||
| Fig. 8 (a) CO2 adsorption isotherms at 273 K for high O2-flow, low O2-flow and the pristine fibres. (b) Determined pore size distribution via Monte-Carlo calculations for pores smaller 1.5 nm. | ||
At pressures above 200 mbar the adsorption isotherm of CO2 surpasses the adsorption isotherm of the pristine fibres and rises to 4.7 mmol g−1 at 1 bar (Fig. 8). Contrary, the low O2-flow samples exhibit a similar uptake as the pristine fibres at low relative pressures and already surpasses the adsorption capacity of the pristine fibres at pressures of 75 mbar. At 1 bar it reaches an CO2 adsorption capacity of 6.5 mmol g−1, which is 38% increase compared to the high O2-flow samples and even 140% increase compared to the pristine fibres.
In comparison to literature data the obtained CO2 adsorption capacities are among the highest for KOH-activated electrospun carbon nanofibres. Wang et al. reported CO2 adsorption capacities of 2.9 mmol g−1 at 1 bar and 273 K, which is significantly lower than the reported values in the present work.47 Comparable CO2 adsorption capacities were reported by Chiang et al. and Zainab et al. who obtained 3.5 mmol g−1 at 298 K, which is close to the obtained 4.2 mmol g−1 at 293 K on the high O2-flow sample in this study (S6).†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55,56
55,56
The pore size distribution was determined using Monte-Carlo calculations and the cumulative pore volume is shown in Fig. 8b. As described previously for DFT calculations based on Ar adsorption, the pore volume increases for low O2-flow samples over the full range of pore sizes compared to the high O2-flow samples. The total pore volume is 0.61 cm3 g−1 for low O2-flow and 0.45 cm3 g−1 for high O2-flow samples, resulting in an increase in pore volume of 36% for the low O2-flow samples. Regarding the ultramicropore volume (<0.7 nm), high O2-flow samples exhibit a value of 0.15 cm3 g−1, which is enhanced to 0.21 cm3 g−1 on low O2-flow samples. Overall, the obtained pore volumes from CO2 adsorption isotherms are comparable to those obtained from Argon isotherms, except for a significant difference of the obtained micropore volume below 1.5 nm for DFT (0.365 cm3 g−1) and Monte-Carlo (0.446 cm3 g−1) data for high O2-flow. This deviation could be caused by the different surface chemistry of samples prepared at high and low O2-flow. High O2-flow samples exhibit a higher oxygen content than low O2-flow samples, which could affect the interactions of adsorptive and adsorbent. Such effects are not accounted for in the standard calculation models used for the determination of the pore size distributions.
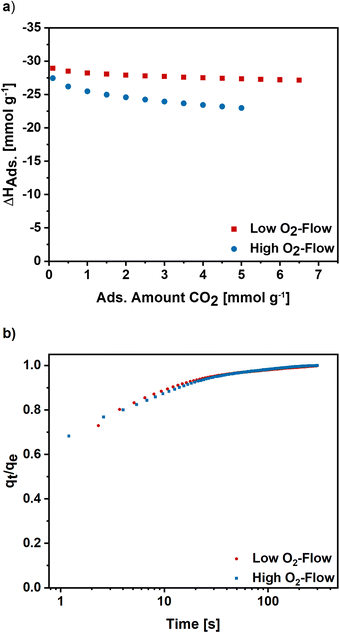
Furthermore, the isosteric enthalpy of adsorption was calculated for high and low O2-flow based on CO2 adsorption isotherms measured at 273 K, 283 K and 293 K (S7).† For low O2-flow a value of 28.9 kJ mol−1 was determined at a loading of 0.1 mmol g−1 which slightly decreases to 27.1 kJ mol−1 at a loading of 6.5 mmol g−1 (Fig. 9). For high O2-flow, the obtained enthalpy of adsorption is at 27.4 kJ mol−1 at a loading of 0.1 mmol g−1 and decreases to 23.0 kJ mol−1 at a loading of 5 mmol g−1. Both isosteric enthalpies of adsorption are comparable to adsorption enthalpies of activated electrospun carbon nanofibres in literature.57 The more distinctive decrease of the isosteric enthalpy of adsorption for high O2-flow can be linked to a higher degree of surface oxygen due to the partial carbon oxidation at high O2-flow (Table 1), which probably lowers the binding affinity towards CO2 due to the increased number of acidic groups on the surface.
To get an insight into the adsorption kinetics, equilibration curves of CO2 at 50 mbar and 298 K were measured (Fig. 9b). For high and low O2-flow the adsorption rate is fast, as the equilibrium loading is reached within the first 100 s with the steepest increase in the first 20 s. Comparing the two post-treatments, there are no significant changes of the adsorption kinetics. Therefore, the choice of post-treatment does not notably affect the adsorption rate of the carbon nanofibres.
Overall, the low O2-flow conditions result in improved gas adsorption properties, namely a higher adsorption capacity and a higher micropore volume. Furthermore, the isosteric enthalpy of adsorption is increased for low O2-flow, whereas the adsorption kinetics are very similar for both post-treatments. Based on the results, the importance the choice of post-treatment conditions after KOH activation of electrospun carbon nanofibres becomes evident.
4. Conclusion
The effect of two atmospheric post-treatments after the KOH activation of electrospun PAN-based carbon nanofibers was detailed studied with a focus on the occurring pyrophoric effect, chemical reactions during the post-treatment and changes of the morphology and adsorption properties.At high O2-flow conditions a significant formation of heat was observed and related to the oxidation reactions of metallic potassium, which was formed during the activation process prior to the post-treatment step. The reaction heat of the potassium oxidation reactions acts as igniter for a subsequent carbon combustion, which significantly changes the pore structure and surface chemistry of the material and destroys the original fibre structure. The comparability of high O2-flow to a usually applied ambient air post-treatment was shown in an additional experiment in which a significant pyrophoric effect was visible.
Control of the vigorous potassium oxidation reactions was enabled by the application of a low O2-flow as post-treatment. The low O2-flow limits the oxidation reactions, resulting in a reduced heat formation, which is insufficient to cause a carbon combustion. A significant increase in adsorption capacities and accessible pore volume as well as higher enthalpies of adsorption of CO2 were found for low O2-flow samples.
Summarising, this work clearly shows that vigorous potassium oxidation reactions can occur after the KOH activation of electrospun carbon nanofibres and alter the obtained material. By proper choice of the atmospheric post-treatment conditions this potassium oxidation reactions can be limited and a significant improvement of the obtained porosity and surface chemistry can be achieved.
Data availability
Data will be made available upon reasonable request.Conflicts of interest
The authors have no competing interest to declare.Acknowledgements
The authors acknowledge funding provided by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany's Excellence Strategy-Cluster of Excellence 2186 ‘The Fuel Science Center’ ID:390919832. The authors thank the Central Institute for Engineering, Electronics and Analytics (ZEA-3) of Forschungszentrum Jülich GmbH for performing CHN analysis and ICP-OES measurements.References
- J. Wang and S. Kaskel, KOH activation of carbon-based materials for energy storage, J. Mater. Chem., 2012, 22, 23710–23725, 10.1039/C2JM34066F.
- N. A. Rashidi and S. Yusup, An overview of activated carbons utilization for the post-combustion carbon dioxide capture, J. CO2 Util., 2016, 13, 1–16, DOI:10.1016/j.jcou.2015.11.002.
- X.-Q. Zhang, W.-C. Li and A.-H. Lu, Designed porous carbon materials for efficient CO2 adsorption and separation, New Carbon Mater., 2015, 30(6), 481–501, DOI:10.1016/s1872-5805(15)60203-7.
- M. Mohan, V. K. Sharma, E. A. Kumar and V. Gayathri, Hydrogen storage in carbon materials—A review, Energy Storage, 2019, 1(2), 1–26, DOI:10.1002/est2.35.
- A. Sharma, J. Jindal, A. Mittal, K. Kumari, S. Maken and N. Kumar, Carbon materials as CO2 adsorbents: a review, Environ. Chem. Lett., 2021, 19(2), 875–910, DOI:10.1007/s10311-020-01153-z.
- X.-Y. Li, Y. Yan, B. Zhang, T.-J. Bai, Z.-Z. Wang and T.-S. He, PAN-derived electrospun nanofibers for supercapacitor applications: ongoing approaches and challenges, J. Mater. Sci., 2021, 56(18), 10745–10781, DOI:10.1007/s10853-021-05939-6.
- U. Kamran, J. R. Choi and S. J. Park, A role of activators for efficient CO2 affinity on polyacrylonitrile-based porous carbon materials, Front. Chem., 2020, 8, 1–18, DOI:10.3389/fchem.2020.00710.
- M. Wu, Q. Zha, J. Qiu, Y. Guo, H. Shang and A. Yuan, Preparation and characterization of porous carbons from PAN-based preoxidized cloth by KOH activation, Carbon, 2004, 42(1), 205–210, DOI:10.1016/j.carbon.2003.10.025.
- W. Shen, S. Zhang, Y. He, J. Li and W. Fan, Hierarchical porous polyacrylonitrile-based activated carbon fibers for CO2 capture, J. Mater. Chem., 2011, 21(36), 14036–14040, 10.1039/c1jm12585k.
- J. Zhou, X. Wang and W. Xing, Carbon-based CO2 adsorbents, in Post-Combustion Carbon Dioxide Capture Materials, ed. Q. Wang, The Royal Society of Chemistry, 2019, pp. 1–75, 10.1039/9781788013352-00001.
- K. Kaneko and F. Rodriguez-Reinoso, Nanoporous Materials for Gas Storage, Springer, 2019, pp. 91–107, DOI:10.1007/978-981-13-3504-4.
- H. Marsh and F. Rodriguez-Reinoso, Activated Carbon, Elsevier, 2006, pp. 322–365, DOI:10.1016/B978-0-08-044463-5.X5013-4.
- A. N. Wennerberg and T. M. O'Grady, Active Carbon Process and Composition, US Pat. 4082694, 1978.
- N. E. Williams, O. A. Oba and N. P. Aydinlik, Modification, production, and methods of KOH-activated carbon, ChemBioEng Rev., 2022, 9(2), 164–189, DOI:10.1002/cben.202100030.
- T. Otowa, R. Tanibata and M. Itoh, Production and adsorption characteristics of MAXSORB: high surface-area active carbon, Gas Sep. Purif., 1993, 7, 241–245 CrossRef CAS.
- T. Otowa, Activation mechanism, surface properties and adsorption characteristics of KOH activated high surface area carbon, Fundam. Adsorpt., 1996, 709–716, DOI:10.1007/978-1-4613-1375-5_88.
- E. Raymundo-Piñero, P. Azaïs, T. Cacciaguerra, D. Cazorla-Amorós, A. Linares-Solano and F. Béguin, KOH and NaOH activation mechanisms of multiwalled carbon nanotubes with different structural organisation, Carbon, 2005, 43(4), 786–795, DOI:10.1016/j.carbon.2004.11.005.
- D. Lozano-Castelló, J. M. Calo, D. Cazorla-Amorós and A. Linares-Solano, Carbon activation with KOH as explored by temperature programmed techniques, and the effects of hydrogen, Carbon, 2007, 45(13), 2529–2536, DOI:10.1016/j.carbon.2007.08.021.
- M. Lillo-Ródenas, D. Cazorla-Amorós and A. Linares-Solano, Understanding chemical reactions between carbons and NaOH and KOH, Carbon, 2003, 41, 267–275, DOI:10.1016/S0008-6223(02)00279-8.
- B. Petrovic, M. Gorbounov and S. Masoudi Soltani, Influence of surface modification on selective CO2 adsorption: a technical review on mechanisms and methods, Microporous Mesoporous Mater., 2021, 312, 1–41, DOI:10.1016/j.micromeso.2020.110751.
- D. Lozano-Castelló, M. A. Lillo-Ródenas, D. Cazorla-Amorós and A. Linares-Solano, Carbon activation by alkaline hydroxides, in Chem. Phys. Carbon, ed. L. R. Radovic, CRC Press Taylor and Francis Group, 2008, pp. 1–56, DOI:10.1201/9781420042993.
- N.-G. Vannerberg, Peroxides, Superoxides, and Ozonides of the Metals of Groups Ia IIa and IIb, Prog. Inorg. Chem., 1962, 4, 125–297, DOI:10.1002/9780470166055.ch3.
- I. I. Vol'nov, Peroxides, Superoxides, and Ozonides of Alkali and Alkaline Earth Metals, Springer, New York, 1966, pp. 21–120 Search PubMed.
- K. Fredenhagen and G. Cadenbach, Die Bindung von Kalium dutch Kohlenstoff, Z. Anorg. Allg. Chem., 1926, 158(1), 249–263, DOI:10.1002/zaac.19261580122.
- H. Marsh, N. Murdie, I. A. S. Edwards and H. P. Boehm, Chemistry and Physics of Carbon, Marcel Dekker Inc., ed. P. A. Thrower, 1987, vol. 20, pp. 213–268, DOI:10.1201/9781003209034.
- F. Kapteijn, J. Jurriaans and J. A. Moulijn, Formation of intercalate-like structures by heat treatment of K2CO3-carbon in an inert atmosphere, Fuel, 1983, 62, 249–251, DOI:10.1016/0016-2361(83)90210-7.
- C. Herinckx, R. Perret and W. Ruland, Interstitial Compounds of Potassium with Carbon Fibres, Nature Volume, 1968, 220, 63–64 CrossRef CAS.
- W. Rüdorff, E. Schulze and Z. Anorg, Über Alkaligraphitverbindungen, Allg. Chem., 1954, 277(3–4), 156–171, DOI:10.1002/zaac.19542770307.
- V. Selmert, A. Kretzschmar, H. Weinrich, H. Kungl, H. Tempel and R. A. Eichel, CO2/N2 separation on highly selective carbon nanofibers investigated by dynamic gas adsorption, ChemSusChem, 2022, 15, 1–12, DOI:10.1002/cssc.202200761.
- A. Kretzschmar, V. Selmert, H. Weinrich, H. Kungl, H. Tempel and R. A. Eichel, Tailored gas adsorption properties of electrospun carbon nanofibers for gas separation and storage, ChemSusChem, 2020, 13(12), 3180–3191, DOI:10.1002/cssc.202000520.
- R. Schierholz, D. Kröger, H. Weinrich, M. Gehring, H. Tempel, H. Kungl, J. Mayer and R.-A. Eichel, The carbonization of polyacrylonitrile-derived electrospun carbon nanofibers studied by in situ transmission electron microscopy, RSC Adv., 2019, 9(11), 6267–6277, 10.1039/c8ra10491c.
- A. Kretzschmar, V. Selmert, H. Kungl, H. Tempel and R. A. Eichel, Application of a tailorable carbon molecular sieve to evaluate concepts for the molecular dimensions of gases, Microporous Mesoporous Mater., 2022, 343, 1–9, DOI:10.1016/j.micromeso.2022.112156.
- J. Park, A. Kretzschmar, V. Selmert, O. Camara, H. Kungl, H. Tempel, S. Basak and R. A. Eichel, Structural study of polyacrylonitrile-based carbon nanofibers for understanding gas adsorption, ACS Appl. Mater. Interfaces, 2021, 13(39), 46665–46670, DOI:10.1021/acsami.1c13541.
- J. Borowec, V. Selmert, A. Kretzschmar, K. Fries, R. Schierholz, H. Kungl, R. A. Eichel, H. Tempel and F. Hausen, Carbonization-temperature-dependent electrical properties of carbon nanofibers-from nanoscale to macroscale, Adv. Mater., 2023, 35(31), e2300936, DOI:10.1002/adma.202300936.
- G. Hotová and V. Slovák, Quantitative TG-MS analysis of evolved gases during the thermal decomposition of carbon containing solids, Thermochim. Acta, 2016, 632, 23–28, DOI:10.1016/j.tca.2016.03.012.
- J. Tòth, Uniform interpretation of gas/solid adsorption, Adv. Colloid Interface Sci., 1995, 55, 1–239, DOI:10.1016/0001-8686(94)00226-3.
- J. L. Rouquerol and P. F. Rouquerol, Is the BET equation applicable to microporous adsorbents?, Stud. Surface Sci. Caralysis, 2007, 160, 49–56, DOI:10.1016/S0167-2991(07)80008-5.
- M. Thommes, K. Kaneko, A. V. Neimark, J. P. Olivier, F. Rodriguez-Reinoso, J. Rouquerol and K. S. W. Sing, Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report), Pure Appl. Chem., 2015, 87(9–10), 1051–1069, DOI:10.1515/pac-2014-1117.
- A. Nuhnen and C. Janiak, A practical guide to calculate the isosteric heat/enthalpy of adsorption via adsorption isotherms in metal-organic frameworks, MOFs, Dalton Trans., 2020, 49(30), 10295–10307, 10.1039/d0dt01784a.
- M. W. J. Chase, NIST-JANAF thermochemical tables, J. Phys. Chem. Ref. Data, 1998, 1465–1489 Search PubMed.
- R. Bovard, Oxygen sources for space flights, Aerosp. Med., 1960, 407–412 CAS.
- C. B. Jackson and R. C. Werner, Manufacture and use of potassium superoxide, in Handling and Uses of the Alkali Metals, American Chemical Society, 1957, pp. 174–177, DOI:10.1021/ba-1957-0019.ch019.
- J. Singh, H. Bhunia and S. Basu, Adsorption of CO2 on KOH activated carbon adsorbents: effect of different mass ratios, J. Environ. Manage., 2019, 250, 1–9, DOI:10.1016/j.jenvman.2019.109457.
- Y. K. Kim, G. M. Kim and J. W. Lee, Highly porous N-doped carbons impregnated with sodium for efficient CO2 capture, J. Mater. Chem. A, 2015, 3(20), 10919–10927, 10.1039/c5ta01776a.
- J. S. Im, S. J. Park, T. J. Kim, Y. H. Kim and Y. S. Lee, The study of controlling pore size on electrospun carbon nanofibers for hydrogen adsorption, J. Colloid Interface Sci., 2008, 318(1), 42–49, DOI:10.1016/j.jcis.2007.10.024.
- S.-H. Yoon, S. Lim, Y. Song, Y. Ota, W. Qiao, A. Tanaka and I. Mochida, KOH activation of carbon nanofibers, Carbon, 2004, 42(8–9), 1723–1729, DOI:10.1016/j.carbon.2004.03.006.
- J. Wang, Y. K. Park and Y. M. Jo, Sequential improvement of activated carbon fiber properties for enhanced removal efficiency of indoor CO2, J. Ind. Eng. Chem., 2020, 89, 400–408, DOI:10.1016/j.jiec.2020.06.011.
- J. Romanos, M. Beckner, T. Rash, L. Firlej, B. Kuchta, P. Yu, G. Suppes, C. Wexler and P. Pfeifer, Nanospace engineering of KOH activated carbon, Nanotechnology, 2012, 23(1), 1–7, DOI:10.1088/0957-4484/23/1/015401.
- A.-N. A. El-Hendawy, An insight into the KOH activation mechanism through the production of microporous activated carbon for the removal of Pb2+ cations, Appl. Surf. Sci., 2009, 255(6), 3723–3730, DOI:10.1016/j.apsusc.2008.10.034.
- J. Wang, A. Heerwig, M. R. Lohe, M. Oschatz, L. Borchardt and S. Kaskel, Fungi-based porous carbons for CO2 adsorption and separation, J. Mater. Chem., 2012, 22(28), 13911–13913, 10.1039/c2jm32139d.
- D. Lozano-Castelló, M. A. Lillo-Ródenas, D. Cazorla-Amorós and A. Linares-Solano, Preparation of activated carbons from Spanish anthracite. Activation by KOH, Carbon, 2000, 39, 741–749, DOI:10.1016/S0008-6223(00)00185-8.
- N. Yoshizawa, K. Maruyama, Y. Yamada, E. K. M. Ishikawa, Y. Toda and M. Shiraishi, XRD evaluation of KOH activation process and influence coal rank, Fuel, 2002, 81, 1717–1722, DOI:10.1016/S0016-2361(02)00101-1.
- Y.-C. Chiang, C.-Y. Yeh and C.-H. Weng, Carbon dioxide adsorption on porous and functionalized activated carbon fibers, Appl. Sci., 2019, 9(10), 1–15, DOI:10.3390/app9101977.
- J. Singh, H. Bhunia and S. Basu, Adsorption of CO2 on KOH activated carbon adsorbents: effect of different mass ratios, J. Environ. Manage., 2019, 250, DOI:10.1016/j.jenvman.2019.109457.
- G. Zainab, A. A. Babar, N. Ali, A. A. Aboalhassan, X. Wang, J. Yu and B. Ding, Electrospun carbon nanofibers with multi-aperture/opening porous hierarchical structure for efficient CO(2) adsorption, J. Colloid Interface Sci., 2020, 561, 659–667, DOI:10.1016/j.jcis.2019.11.041.
- Y.-C. Chiang, C.-C. Huang and W.-T. Chin, Carbon dioxide adsorption on carbon nanofibers with different porous structures, Appl. Sci., 2021, 11(16), 7724, DOI:10.3390/app11167724.
- Y. C. Chiang, W. T. Chin and C. C. Huang, The application of hollow carbon nanofibers prepared by electrospinning to carbon dioxide capture, Polymers, 2021, 13(19), 3275, DOI:10.3390/polym13193275.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra07096d |
| This journal is © The Royal Society of Chemistry 2024 |