Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Highly efficient eco-friendly sodium titanate sorbents of Cs(I), Sr(II), Co(II) and Eu(III): synthesis, characterization and detailed adsorption study†
Monika Motlochová *ab,
Lórant Szatmáry*c,
Eva Pližingrováa,
Petra Salačovác,
Radek Fajgard,
Sven Lidin
*ab,
Lórant Szatmáry*c,
Eva Pližingrováa,
Petra Salačovác,
Radek Fajgard,
Sven Lidin b and
Jan Šubrta
b and
Jan Šubrta
aInstitute of Inorganic Chemistry of the Czech Academy of Sciences, 250 68 Řež, Czech Republic. E-mail: motlochova@iic.cas.cz
bCentre for Analysis and Synthesis, Lunds Universitet, Naturvetarvägen 14, Lund 222-61, Sweden
cFuel Cycle Chemistry Department, ÚJV Řež a.s., 250 68 Řež, Czech Republic. E-mail: lorant.szatmary@ujv.cz
dInstitute of Chemical Process Fundamentals of the Czech Academy of Sciences, Rozvojova 135, Prague 160 00, Czech Republic
First published on 5th January 2024
Abstract
Development of useful all-around materials which can quickly and efficiently adsorb radionuclides in response to environmental radioactive contamination is an urgent research objective. In response to this need, our team developed a simple preparation method for stable sodium titanates which can serve as efficient agents for removal of radionuclides from water. With an emphasis on an environmentally friendly synthesis, the resulting materials were defined by a range of means and methods measuring e.g. pH, ionic strength, contact time or metal ion concentration in order to assess their potential for use and applications as sorbents. The data obtained from measurements revealed rapid removal kinetics (up to 10 minutes), wide range of pH use and high equilibrium capacity. The maximum amount of adsorbed ions as calculated from the Langmuir isotherm was equal to 206.3 mg g−1 for Cs(I), 60.0 mg g−1 for Sr(II), 50.2 mg g−1 for Co(II) and 103.4 mg g−1 for Eu(III), significantly exceeding published data obtained with related materials. The removal mechanism is most likely ion exchange followed by complexation reactions, as indicated by TEM/EDS analyses. Given their extraordinary sorption capacity and facile synthesis under mild conditions, these materials are promising candidates for the efficient removal of radionuclides from aqueous solutions during the clean-up of radioactive pollution in the environment.
1. Introduction
Liquid wastes containing non-ferrous heavy metal ions and some radionuclides (137Cs and 90Sr in particular) represent potentially one of the most dangerous sources of environmental contamination. The remediation of wastewater containing such pollutants continues to be amongst the biggest challenges of sustainable development and environmental safety. Sorption-based technologies have proved their efficiency in reducing the radionuclide content in aqueous streams to low-level residual activity, with the concomitant decrease in the generated amount of ultimate solid waste.1,2Titanate based substances are known to be stable with respect to radiation, as well as chemical, thermal, and mechanical changes.3,4 Many solid nanostructured titanium oxide based materials showed excellent ability to absorb various radionuclides.1 Moreover, these compounds are characterized by their excellent ion-exchange capacity and fast sorption kinetics. Recent studies have also been focused on layered materials, i.e. layered titanate structures in different morphologies: nanotubes, nanowires, nanofibers or even nanoribbons.5–10 Effective radionuclide sorption was also observed with silica-titanates.11
The presence of different radioactive elements in water, e.g. Cs+, Sr2+, Eu3+ and Co2+ is of major concern because of their high toxicities and carcinogenic effects on human.12 Therefore, the accidental release of large amounts of radioactive elements into the aquatic environment has become a direct threat for human health.12,13 All these radionuclides are very hazardous because of their high solubility, high transferability and easy assimilation into living organisms.
Many physical and chemical methods have been proposed for the removal of Cs radionuclides from contaminated wastewater, such as solvent extraction, chemical precipitation, membrane processes, coagulation, electrochemical and ion exchange.14,15 Methods, such as solvent extraction, ion exchange and adsorption, could be widely used due to their ionic selectivity, and solvent extraction using the macrocyclic ligands such as crown ethers, calix-crowns and chlorinated cobalt dicarbollide can be initiated to remove these radionuclides.16–18 Sorption at the solid–liquid interface has attracted much scientific and technical interest mainly because of its high degree of control, flexibility, and ease of use together with either the reduction in the volume of solid waste to be stored in landfills or the reversibility of the sorption phenomenon allowing preservation of raw materials.
Titanate nanostructures are usually synthesized via a hydrothermal process. For this purpose, a precursor of titanium dioxide is mixed with a solution of NaOH or KOH to obtain a suspension, which is subsequently placed in a polytetrafluoroethylene autoclave under constant stirring at 200 °C for 3 days.
We have recently developed a new preparation method for the synthesis of hydrated titanate microrods in aqueous media starting with solid hydrated titanyl sulphate crystals with defined morphology. The particle size and morphology of the starting hydrated titanyl sulphate is closely preserved in the pseudomorphs of amorphous metatitanic acid or alkali obtained metatitanates including such details like the layered structure of the original hydrated titanyl sulphate crystals.19,20 The amorphous character of the material was documented by the electron diffraction pattern with only very broad signs of diffraction rings and by high-magnification image showing a porous character of the product.19 The rod-like particles of metatitanic acid possess excellent sorption properties toward heavy metals (e.g. Pb2+).21,22
Following the general demand for preparation of suitable adsorbents for radionuclides, the group efforts focused on suitable modifications of this material. In the present work, a sodium titanium oxide materials were synthesized in a fast and cost-effective way, the products were characterized by methods of electron microscopy, thermal analysis and surface area determination and then on the most suitable material, the effects of parameters such as contact time, pH, ionic strength and metal ion concentration were also investigated to assess the potential application of prepared titanium based sorbent in real applications.
2. Materials and methods
2.1. Synthesis
Based on alkaline controlled hydrolysis, a sodium titanium oxide was prepared according to the following procedure:27,28 a 100 ml of cooled distilled water was mixed with 50 g of ice and 0.07, 0.14 and 0.21 mol NaOH in form of a solution (50 g into 100 ml, Penta, Czech Republic), then 4.80 g of titanyl sulphate (monohydrate, provided by local supplier Precheza a.s.) was added. While the mixture was magnetically stirred for 2 hours, its temperature rose from 0 °C to room temperature. Then the solid residue was decanted twice and filtered off. The residue was dried on Petri dish and the samples were labelled accordingly as TSM-5ml-NaOH (corresponding to 0.07 mol), TSM-7.5ml-NaOH (corresponding to 0.14 mol), and TSM-10ml-NaOH (corresponding to 0.21 mol).Total content of alkali metals in prepared materials was determined by dissolving of 0.1 g of sample in 50 ml of concentrated HNO3. Then, the solution was analysed via atomic absorption spectroscopy (AAS, Varian AA240FS) and the total contents of the alkali metal cations are shown in Table 1.
| Sample name | Amount of alkali (g) | Amount of alkali (mol) | pH of resulting suspensions | Content of Na (mg l−1) | Content of Na (mmol l−1) |
|---|---|---|---|---|---|
| TSM-5ml-NaOH | 2.87 | 0.07 | 9 | 2.2 | 0.096 |
| TSM-7.5ml-NaOH | 4.31 | 0.14 | 10 | 133.8 | 5.817 |
| TSM-10ml-NaOH | 5.75 | 0.21 | 11 | 134.5 | 5.840 |
2.2. Characterization of the product
The following methods were used for morphological, structural, and chemical characterization of the product: scanning electron microscopy (SEM), transmission electron microscopy (TEM), surface area measurements (BET), thermal analysis (TG/DTA) and X-ray photoelectron spectroscopy (XPS). Details including experimental conditions are described in ESI – Characterization methods.†2.3. Sorption experiments
Sorption of Cs(I), Sr(II), Co(II) and Eu(III) on prepared titanates were investigated by batch sorption technique. The suspensions of 0.01 g of the materials and 1.5 ml of solutions of corresponding pH and ionic strength were first pre-equilibrated in test tubes for 24 h. The pH of solutions was adjusted with NaOH or HClO4 and the ionic strength was set to 0.01 or 0.1 mol l−1 by addition of NaClO4.Kinetics of the uptake of Cs(I), Sr(II),Co(II) and Eu(III) on the prepared samples were performed under following conditions: 1 × 10−3 mol l−1 CsCl at pH = 7.5, 1 × 10−3 mol l−1 SrCl2 at pH = 7.1, 1 × 10−3 mol l−1 CoCl2 at pH = 8.3 and 1 × 10−3 mol l−1 Eu(NO3)3 at pH = 8.4; I = 0.01 mol l−1 NaClO4, V/m = 150 ml g−1, T = 25 °C.
For sorption experiments, the radionuclide solutions labelled with 134Cs, 85Sr, 60Co and 152–154Eu radiotracer (134Cs, 60Co, 152–154Eu isotopes (POLATOM, Poland)), 85Sr isotope (PerkinElmer, USA) were added into the pre-equilibrated suspensions of materials and set for a specified time on a horizontal shaker (250 rpm, 25 ± 0.1 °C). A liquid-to-solid ratio was 150 ml g−1 for all experiments. The suspensions were filtered using mixed cellulose filters (pore size 0.2 μm) after shaking and aliquots of the filtrate (0.5 ml) were measured on the automatic gamma counter Wallac 1480 WIZARD 3 (PerkinElmer Life Sciences, Wallac Oy) with NaI(Tl) well-type detector. Each experiment was performed twice and the mean values are presented.
In order to investigate the influence of the pH on the sorption of selected radionuclides, the experiments were carried out in the pH range from ∼2 to ∼10 at ionic strength of 0.01 mol l−1. The pH of the solutions was measured using pH meter (Boeco BT-675) after equilibrium time of 24 h.
The sorption of radionuclides between the aqueous phase and the material was expressed as the uptake (%) and calculated by eqn (1):
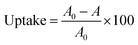 | (1) |
For the determination of the above mentioned sorption isotherms for radionuclides on prepared samples, solutions of various concentration of CsCl, SrCl2, CoCl2 and Eu (NO3)3 (2.5 × 10−5 to 4 × 10−2 mol l−1) labelled by radiotracers were prepared. Contact time was 24 h.
The sorption isotherms were plotted as dependencies of equilibrated concentrations of radionuclides on materials on their equilibrated concentrations in liquid phase. The equilibrium concentrations of radionuclides in liquid and solid phases, ceq (mol l−1) and qeq (mmol g−1), respectively, were calculated as follows:
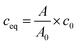 | (2) |
 | (3) |
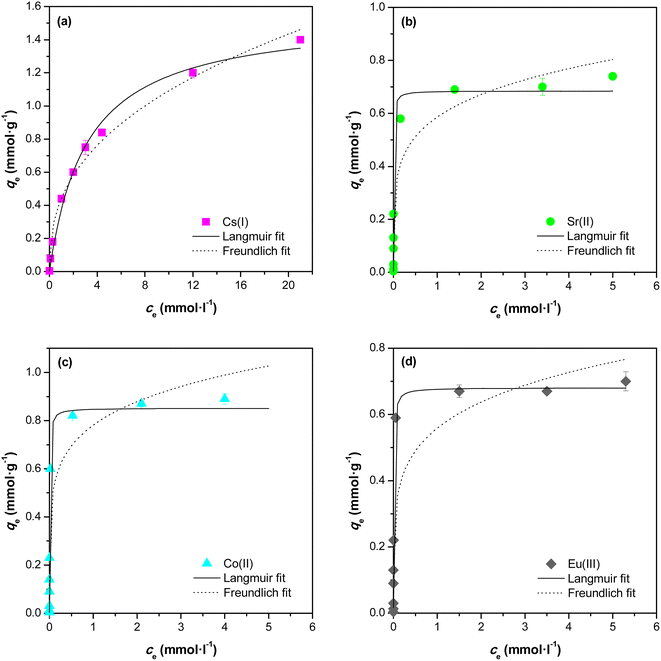
The adsorption equilibrium data were fitted by Freundlich and Langmuir adsorption isotherms. The Freundlich model can be expressed by eqn (4):
| qeq = KF × c1/neq | (4) |
The Langmuir model is a theoretical model use for monolayer adsorption and can be described by eqn (5):
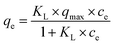 | (5) |
The pseudo-first-order kinetic equation is (6):
| Qt = Qe(1 − e−k1t) | (6) |
3. Results and discussion
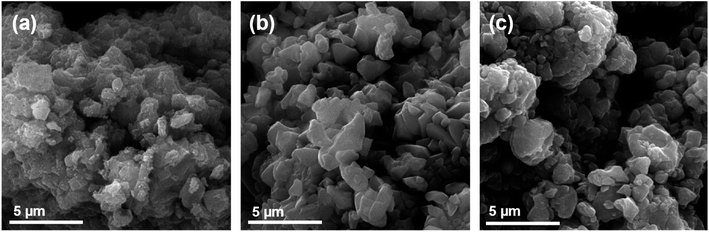
3.1. Prepared sorbents characterization
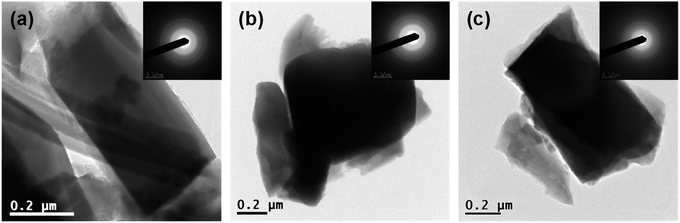
Detailed investigation of prepared materials was conducted via transmission electron microscopy where individual crystals were observed (Fig. 2). Only wide indefinite rings were discovered in the SAED patterns (inset Fig. 2) implying amorphous state of all the prepared samples.
| Element | Elemental composition (at%) | ||
|---|---|---|---|
| TIG-5ml-NaOH | TIG-7.5ml-NaOH | TIG-10ml-NaOH | |
| Ti 2p | 0.107 | 0.138 | 0.145 |
| O 1s | 0.457 | 0.510 | 0.457 |
| Na 1s | 0 | 0.056 | 0.062 |
| C 1s | 0.436 | 0.297 | 0.337 |
Titanium is present in the form of TiO2. Ti 2p3/2 peak is centred at 458.5 eV for the sample TIG-5ml-NaOH with no sodium observed in the superficial layer (Fig. 4). Separation of 2p3/2–2p1/2 is 5.75 eV, which is a typical value for titanium dioxide. In the samples TIG-10ml-NaOH and TIG-7.5ml-NaOH, the Ti 2p3/2 bands are centred at 458.4 and 458.3 eV respectively. Oxygen is present mainly in the metal-oxide lattice which is found at binding energies 530.0 eV in all samples. Contributions of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and M–OH (531.5 eV), C–O (532.7 eV) and adsorbed water (533.9 eV) were also revealed in all samples. Sodium is found in the superficial layer in both samples TIG-10ml-NaOH and TIG-7.5ml-NaOH, the band is relatively broad (FWHM = 1.9 eV) and positioned to 1071.9 eV.
O and M–OH (531.5 eV), C–O (532.7 eV) and adsorbed water (533.9 eV) were also revealed in all samples. Sodium is found in the superficial layer in both samples TIG-10ml-NaOH and TIG-7.5ml-NaOH, the band is relatively broad (FWHM = 1.9 eV) and positioned to 1071.9 eV.
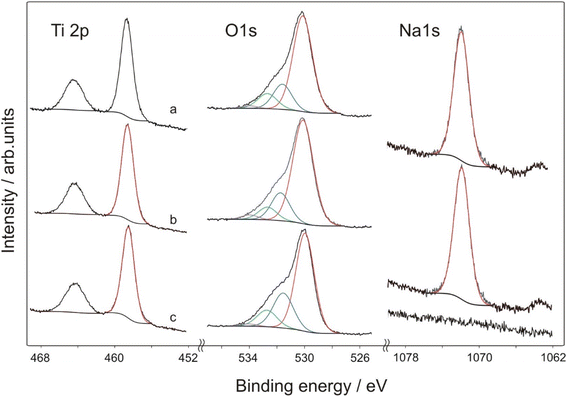 | ||
| Fig. 4 X-ray photoelectron spectroscopy (XPS) spectra of the prepared samples, (a) TIG-7.5ml-NaOH, (b) TIG-10ml-NaOH, (c) TIG-5ml-NaOH. | ||
After moderate sputtering, lower oxidation states Ti3+ oxide (2p3/2 = 457.1 eV) and Ti2+ oxide (2p3/2 = 455.6 eV) appeared. It is known, that argon ions reduce Ti4+ and lower oxidation states are observed as a result. Oxygen bands did not change by sputtering, while sodium bands were narrower by 0.2 eV. It is an evidence that sodium is present at least in two forms, with the most superficial one removed by sputtering.
Atomic ratios Ti/Na in the prepared samples were calculated to be 1.82 in the TIG-10ml-NaOH and 1.77 in the TIG-7.5ml-NaOH, while sputtered samples were sodium depleted (Ti/Na = 2.48 in the TIG-10ml-NaOH and 2.32 in the TIG-7.5ml-NaOH). It showed that sodium is present predominantly at the surface.
In the literature, the role of sodium doping is discussed. Some authors highlight the bigger atomic radius of Na+ (1.02 Å) in comparison with Ti4+ (0.68 Å) and demonstrate difficult substitutional doping and expect migration of Na+ to TiO2 surface.24,25 In our samples, sodium was found partially depleted after moderate argon ion sputtering, which supports expected separation of Na+ on TiO2 nanoparticles. In the recent paper,26 authors observed shift in the position of Ti4+ band to lower binding energies and explained it by substitutional doping of Na+ at Ti4+ site. In our samples we also observed shifted position of Ti 2p3/2 from 458.5 eV (no sodium present) to 458.4 (lower Na content) and 458.3 eV (higher Na content), which can be an evidence of partial sodium involvement in Ti–O network.
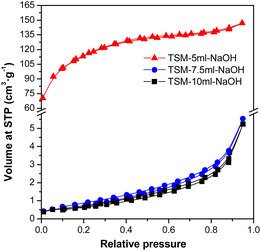
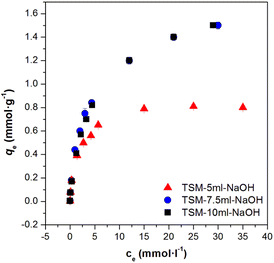
Samples with the addition of 7.5 ml, or 10 ml of NaOH had a significantly lower surface area than samples with the addition of 5 ml of NaOH, and according to the shape of the isotherm, the samples were completely mesoporous. A likely explanation for such a large reduction in the value of surface area could be the blockage of the micropores of the material by Na+ ions. This statement is also supported by the fact that for samples that were precipitated with another base (aqueous ammonia), such a rapid decrease in surface area was not observed (Table 3). Moreover, it is also evident from Fig. 6 that the samples with a low value of the measured surface area did not show lower sorption capacities and this is because the surface area is probably not that low, it is just effectively blocked by Na+ ions, which make correct surface area measurement impossible.
| Sample name | Surface area (m2 g−1) |
|---|---|
| TSM-5ml-NaOH | 411 |
| TSM-7.5ml-NaOH | 3 |
| TSM-10ml-NaOH | 2 |
| TSM-5ml-NH3 | 299 |
| TSM-7.5ml-NH3 | 189 |
The specific surface area and pore volume parameters characterizing various titanate structures have been collected in ref. 1. It was described that the layered compounds, and titanate nanotubes in particular, possess specific surface areas higher than those of silicotitanates. The specific surface areas of nanofibers, nanoribbons and nanowires are of a comparable magnitude but they are smaller than those of nanotubes.
The total content of alkali metal in the prepared materials was obtained as follows: 0.1 g of the sample was dissolved in the concentrated HNO3 under heating with the final solution volume at 50 ml. Solution analysis was then performed via atomic absorption spectroscopy with the detected total content of sodium in prepared samples being 0.05 mmol g−1 for TSM-5ml-NaOH, 2.90 mmol g−1 for TSM-7.5ml-NaOH and 2.92 mmol g−1 for TSM-5ml-NaOH.23 The significant differences in Na content could also justify the differences in surface area (of prepared samples) as the pores available for nitrogen adsorption could be blocked by the sodium ions.23
3.2. Application of prepared samples as sorbents of radionuclides
Based on these results, the adjusted preparation method of TSM-7.5ml-NaOH has been chosen as the most suitable and is used in the following experiments for water waste treatment.
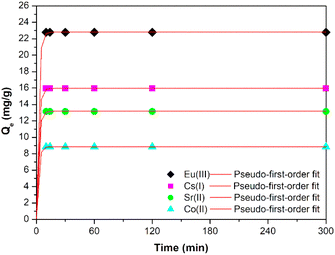 | ||
| Fig. 7 Kinetics of the uptake of Cs(I) and Sr(II), Co(II) and Eu(III) on the prepared sample; with pseudo-first-order model. | ||
| Radionuclide | Used salt | concentration (mmol l−1) | pH |
|---|---|---|---|
| Cs(I) | CsCl | 1 × 10−3 | 7.5 |
| Sr(II) | SrCl2 | 1 × 10−3 | 7.1 |
| Co(II) | CoCl2 | 1 × 10−3 | 8.3 |
| Eu(III) | Eu(NO3)3 | 1 × 10−3 | 8.4 |
| Radionuclide | k1 (min−1) | Qe (mg g−1) | R2 |
|---|---|---|---|
| Cs(I) | 0.486 | 15.967 | 0.984 |
| Sr(II) | 0.499 | 13.137 | 0.986 |
| Co(II) | 0.503 | 8.860 | 0.989 |
| Eu(III) | 0.484 | 22.822 | 0.985 |
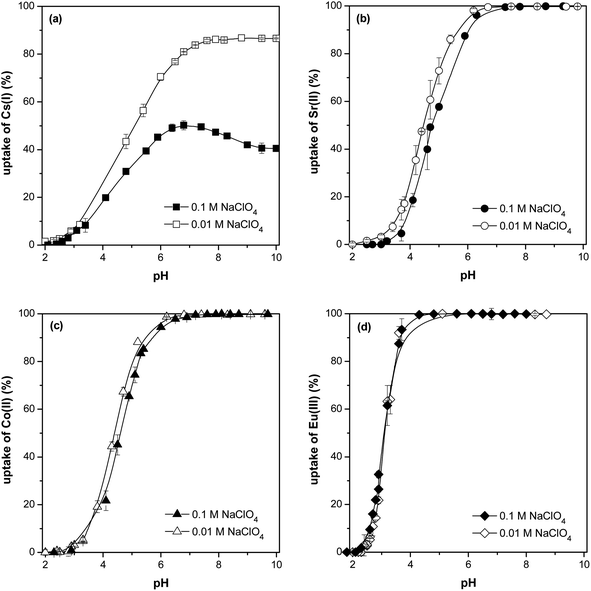
It is clear, based on the obtained data with I = 0.01 mol l−1 NaClO4, that the higher the pH, the higher the sorption of Cs(I), Sr(II), Co(II) a Eu(III) until a maximum is reached when the sorption stabilizes. Maximum sorption (quantitative ≥ 99.9%) was reached at pH ≥ 7.5 for Cs(I), pH ≥ 6.5 for Sr(II) and Co(II) and pH ≥ 4.5 for Eu(III). At pH = 6, the ion affinity towards the sorbent decreases in agreement with the decreasing ion potential in the following order: Eu(III) > Sr(II), Co(II) > Cs(I).
The ionic species of Co(II) and Eu(III) in solution with different pH values and ionic strengths are different. Co(II) and Eu(III) speciations were modelled by PHREEQC program. Speciation of cobalt is shown in Fig. 9. The majority species of free cobalt ions (Co2+) is present for pH < 8. Carbonate form CoCO3 appears at pH values above 8. At higher ionic strength (0.1 mol l−1), chloride phase CoCl+ occurs up to pH 9.5. The minor speciations (Co(OH)+, Co(HCO3)) are observed for pH values between 7.5 and 10.0 and hydroxide form Co(OH)2 is visible above pH 9.0. It is clear from the equilibrium pH on Co(II) uptake dependencies that the sorbent adsorbed all of the above cobalt speciations.
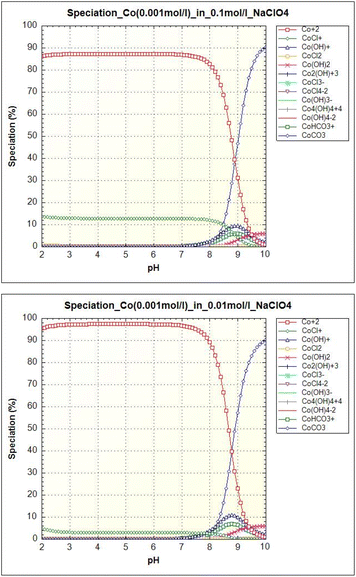 | ||
| Fig. 9 Cobalt (0.001 mol l−1) speciation as a function of pH in an aqueous solution with ionic strength 0.1 mol l−1 NaClO4 (left) and 0.01 mol l−1 NaClO4 (right). | ||
As we can see in Fig. 10, europium can be present in free form (Eu3+), complexed form with dissolved carbonates (Eu(CO3)+, Eu(CO3)2−, Eu(CO3)33−), chloride ions (EuCl2+), or in hydrolyzed form (Eu(OH)2+). Under our conditions, the majority species is the Eu3+ ion for pH < 6.5. For pH values between 6.5 and 8.5, Eu(CO3)+ species predominates. Other carbonate species Eu(CO3)2− and Eu(CO3)33− appear at pH values above 8.5. The smaller hydrolyzed form Eu(OH)2+ occurs at pH 6.5 to 8.0. In the case of higher ionic strength (0.1 mol l−1), chloride phase EuCl2+ occurs up to pH 7.5. On the basis of the equilibrium pH data on Eu(III) uptake, it can be concluded that the sorbent adsorbed all europium forms.
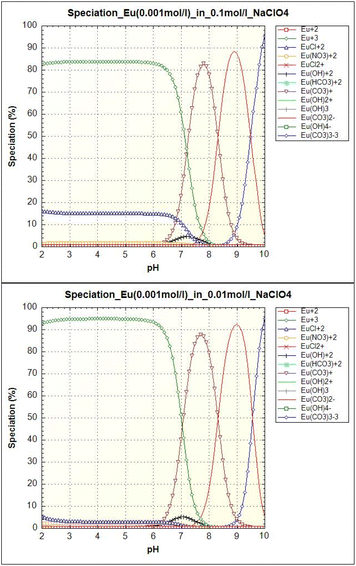 | ||
| Fig. 10 Europium (0.001 mol l−1) speciation as a function of pH in an aqueous solution with ionic strength 0.1 mol l−1 NaClO4 (left) and 0.01 mol l−1 NaClO4 (right). | ||
Fig. 8 also shows, that the effect of ion strength on sorption is most noticeable with Cs(I) ions, with approx. twofold sorption under low I (0.01 mol l−1 NaClO4) when compared to sorption under higher I (0.1 mol l−1 NaClO4) in range of pH 7–10 which is in good agreement with data published in ref. 28. The Sr(II) and Co(II) uptakes are lightly affected by the Na(I) ions in pH range 2–6.5, there is no I effect on sorption with higher pH. No effect of I on Eu(III) was observed at sorption throughout the pH range. Generally, the surface complexation and/or ion exchange is susceptible to exposure to ion forces, while the inner surface complexation is not influenced by the ion forces.
| radio-nuclide | Freundlich model | Langmuir model | ||||
|---|---|---|---|---|---|---|
| KF [mmol g−1] | n | R2 | qmax [mg g−1] | KL [l mmol−1] | R2 | |
| Cs(I) | 0.442 | 2.54 | 0.985 | 206.3 | 0.318 | 0.991 |
| Sr(II) | 0.586 | 5.10 | 0.930 | 60.0 | 203.0 | 0.988 |
| Co(II) | 0.781 | 5.87 | 0.922 | 50.2 | 164.3 | 0.970 |
| Eu(III) | 0.559 | 5.26 | 0.841 | 103.4 | 140.1 | 0.973 |
It can be seen in Fig. 12 that the radionuclides are homogenously distributed in the sample with no obvious location preference.
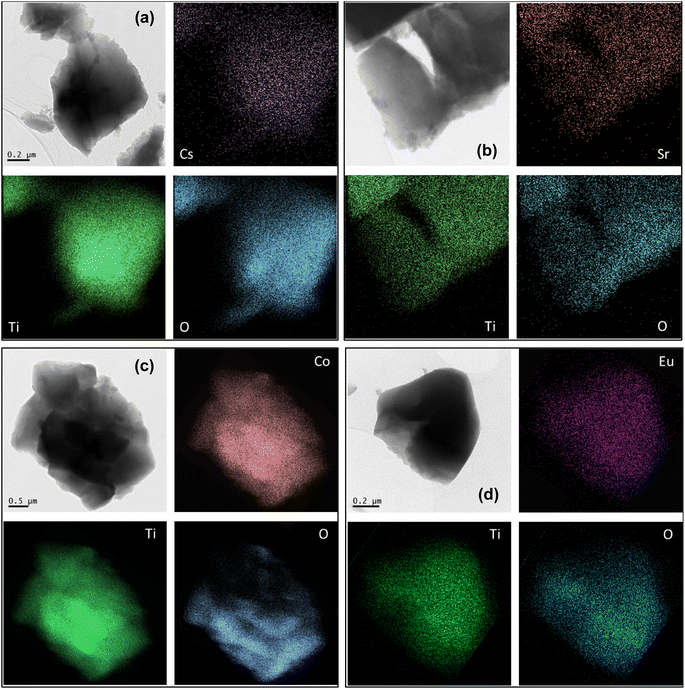 | ||
| Fig. 12 TEM/EDS mapping of O, Ti and radionuclide in prepared sample after adsorption of Cs(I) (a), Sr(II) (b), Co(II) (c) and Eu(III) (d). | ||
With the goal of proving an ion exchange theory, the samples after sorption were analyzed by SEM/EDS analysis and the results are summarized in Table 7. The variance in the adsorbed amount of Na ions would indicate that the mechanism of sorption is ion exchange as is in good agreement with data published earlier.8,11
| Sorption of radionuclide | Exchanged amount of radionuclide (wt%) | Remained amount of sodium ions (wt%) |
|---|---|---|
| Cs(I) | 6.4 | <0.01 |
| Sr(II) | 3.1 | <0.01 |
| Co(II) | 3.0 | <0.01 |
| Eu(III) | 2.2 | <0.01 |
We can summarize that the sorbent prepared according to the described procedure is highly effective and has all the prerequisites for technological application. The synthetic process itself does not use environmentally harmful raw materials and can be easily connected to the sulphate technology of titanium white production. Its price in commercial preparation should therefore not exceed the price of common types of white titanium pigments and should be significantly cheaper than zeolite based materials currently considered as very promising.
4. Conclusions
Three novel titanates were synthesized, characterized and studied for the removal of Cs(I), Sr(II), Co(II) and Eu(III) from aqueous environment. The comparison of sorption capacities of TIG-5ml-NaOH, TIG-7.5ml-NaOH and TIG-10ml-NaOH disproved the assumption that the higher the surface area the higher sorption capacity. The results showed a rapid removal kinetics (up to 10 minutes) and a high equilibrium capacity for selected sorbent, the maximum of adsorbed amounts were calculated from the Langmuir model, the maximum amount of adsorbed ions equalled to 206.3 mg g−1 for Cs(I), 60.0 mg g−1 for Sr(II), 50.2 mg g−1 for Co(II) and 103.4 mg g−1 for Eu(III). The removal mechanism is most likely ion exchange followed by complexation reactions. The results showed that the prepared materials exhibit much faster kinetics than commercial adsorbents.Combining the results of adsorption experiments with material characteristics leads to a conclusion that (for studied titania based adsorbents) the radionuclides adsorption efficiency increases with increasing amount of present exchangeable cations.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank for financial support provided by the Research Infrastructure NanoEnviCz, supported by the Ministry of Education, Youth and Sports of the Czech Republic under Project no. LM2015073, and by the Technological Agency of the Czech Republic, project TH02020110.References
- D. Alby, C. Charnay, M. Heran, B. Prelot and J. Zajac, Recent developments in nanostructured inorganic materials for sorption of cesium and strontium: Synthesis and shaping, sorption capacity, mechanisms, and selectivity-A review, J. Hazard. Mater., 2018, 344, 511–530 CrossRef CAS PubMed.
- M. M. Khin, A. S. Nair, V. J. Babu, R. Murugan and S. Ramakrishna, A review on nanomaterials for environmental remediation, Energy Environ. Sci., 2012, 5, 8075–8109 RSC.
- D. J. Yang, S. Sarina, H. Y. Zhu, H. W. Liu, Z. F. Zheng, M. X. Xie, S. V. Smith and S. Komarneni, Capture of Radioactive Cesium and Iodide Ions from Water by Using Titanate Nanofibers and Nanotubes, Angew. Chem., Int. Ed., 2011, 50, 10594–10598 CrossRef CAS PubMed.
- D. J. Yang, H. W. Liu, Z. F. Zheng, S. Sarina and H. Y. Zhu, Titanate-based adsorbents for radioactive ions entrapment from water, Nanoscale, 2013, 5, 2232–2242 RSC.
- B. Filipowicz, M. Pruszynski, S. Krajewski and A. Bilewicz, Adsorption of Cs-137 on titanate nanostructures, J. Radioanal. Nucl. Chem., 2014, 301, 889–895 CrossRef CAS PubMed.
- D. J. Yang, Z. F. Zheng, Y. Yuan, H. W. Liu, E. R. Waclawik, X. B. Ke, M. X. Xie and H. Y. Zhu, Sorption induced structural deformation of sodium hexa-titanate nanofibers and their ability to selectively trap radioactive Ra(II) ions from water, Phys. Chem. Chem. Phys., 2010, 12, 1271–1277 RSC.
- T. Möller, Selective crystalline inorganic materials as ion exchangers in the treatment of nuclear waste solutions, Kumpula Department of Chemistry, Faculty of Science of the University of Helsinki, Helsinky, 2002 Search PubMed.
- I. M. Ali, Synthesis and sorption behavior of semicrystalline sodium titanate as a new cation exchanger, J. Radioanal. Nucl. Chem., 2004, 260, 149–157 CrossRef CAS.
- A. J. Du, D. D. Sun and J. O. Leckie, Selective sorption of divalent cations using a high capacity sorbent, J. Hazard. Mater., 2011, 187, 96–100 CrossRef CAS PubMed.
- D. T. Hobbs, M. J. Barnes, R. L. Pulmano, K. M. Marshall, T. B. Edwards, M. G. Bronikowski and S. D. Fink, Strontium and actinide separations from high level nuclear waste solutions using monosodium titanate 1. Simulant testing, Sep. Sci. Technol., 2005, 40, 3093–3111 CrossRef CAS.
- L. Al-Attar, A. Dyer and R. Harjula, Uptake of radionuclides on microporous and layered ion exchange materials, J. Mater. Chem., 2003, 13, 2963–2968 RSC.
- Z. Majidnia and M. A. Fulazzaky, Photocatalytic reduction of Cs(I) ions removed by combined maghemite-titania PVA-alginate beads from aqueous solution, J. Environ. Manage., 2017, 191, 219–227 CrossRef CAS PubMed.
- H. Long, P. Wu and N. Zhu, Evaluation of Cs+ removal from aqueous solution by adsorption on ethylamine-modified montmorillonite, Chem. Eng. J., 2013, 225, 237–244 CrossRef CAS.
- C. Loos-Neskovic, S. Ayrault, V. Badillo, B. Jimenez, E. Garnier, M. Fedoroff, D. J. Jones and B. Merinov, Structure of copper-potassium hexacyanoferrate (II) and sorption mechanisms of cesium, J. Solid State Chem., 2004, 177, 1817–1828 CrossRef CAS.
- J.-K. Moon, E.-H. Lee and H.-T. Kim, Ion exchange of Cs ion in acid solution with potassium cobalt hexacyanoferrate, Korean J. Chem. Eng., 2004, 21, 1026–1031 CrossRef CAS.
- R. D. Ambashta, D. S. Deshingkar, P. K. Wattal and D. Bahadur, Application of magnetite hexacyanoferrate composites in magnetically assisted chemical separation of cesium, J. Radioanal. Nucl. Chem., 2006, 270, 585–592 CrossRef CAS.
- B. Gruner, M. Kvicalova, J. Plesek, V. Sicha, I. Cisarova, M. Lucanikova and P. Selucky, Cobalt bis(dicarbollide) ions functionalized by CMPO-like groups attached to boron by short bonds; efficient extraction agents for separation of trivalent f-block elements from highly acidic nuclear waste, J. Organomet. Chem., 2009, 694, 1678–1689 CrossRef.
- F. A. Shehata, M. F. Attallah, E. H. Borai, M. A. Hilal and M. M. Abo-Aly, Sorption reaction mechanism of some hazardous radionuclides from mixed waste by impregnated crown ether onto polymeric resin, Appl. Radiat. Isot., 2010, 68, 239–249 CrossRef CAS PubMed.
- M. Klementova, M. Motlochova, J. Bohacek, J. Kupcik, L. Palatinus, E. Plizingrova, L. Szatmary and J. Subrt, Metatitanic Acid Pseudomorphs after Titanyl Sulfates: Nanostructured Sorbents and Precursors for Crystalline Titania with Desired Particle Size and Shape, Cryst. Growth Des., 2017, 17, 6762–6769 CrossRef CAS.
- M. Palkovska, V. Slovak, J. Subrt, J. Bohacek, Z. Barbierikova, V. Brezova and R. Fajgar, Investigation of the thermal decomposition of a new titanium dioxide material, J. Therm. Anal. Calorim., 2016, 125, 1071–1078 CrossRef CAS.
- M. Gavrilescu, L. V. Pavel and I. Cretescu, Characterization and remediation of soils contaminated with uranium, J. Hazard. Mater., 2009, 163, 475–510 CrossRef CAS PubMed.
- M. Motlochova, V. Slovak, E. Plizingrova, S. Lidin and J. Subrt, Highly-efficient removal of Pb(ii), Cu(ii) and Cd(ii) from water by novel lithium, sodium and potassium titanate reusable microrods, RSC Adv., 2020, 10, 3694–3704 RSC.
- M. Motlochova, V. Slovak, E. Plizingrova, L. Szatmary, P. Bezdicka and J. Subrt, The influence of annealing temperature on properties of TiO2 based materials as adsorbents of radionuclides, Thermochim. Acta, 2019, 673, 34–39 CrossRef CAS.
- H. Xie, N. Li, B. S. Liu, J. J. Yang and X. J. Zhao, Role of Sodium Ion on TiO2 Photocatalyst: Influencing Crystallographic Properties or Serving as the Recombination Center of Charge Carriers?, J. Phys. Chem. C, 2016, 120, 10390–10399 CrossRef CAS.
- J. W. Liu, R. Han, H. T. Wang, Y. Zhao, W. J. Lu, H. Y. Wu, T. F. Yu and Y. X. Zhang, Degradation of PCP-Na with La-B co-doped TiO2 series synthesized by the sol-gel hydrothermal method under visible and solar light irradiation, J. Mol. Catal. A: Chem., 2011, 344, 145–152 CrossRef CAS.
- I. Singh and B. Birajdar, Synthesis, characterization and photocatalytic activity of mesoporous Na-doped TiO2 nano-powder prepared via a solvent-controlled non-aqueous sol-gel route, RSC Adv., 2017, 7, 54053–54062 RSC.
- A. M. El-Kamash, M. R. El-Naggar and M. I. El-Dessouky, Immobilization of cesium and strontium radionuclides in zeolite-cement blends, J. Hazard. Mater., 2006, 136, 310–316 CrossRef CAS PubMed.
- B. Filipowicz, M. Pruszyński, S. Krajewski and A. Bilewicz, Adsorption of 137Cs on titanate nanostructures, J. Radioanal. Nucl. Chem., 2014, 301, 889–895 CrossRef CAS PubMed.
- X. M. Sun and Y. D. Li, Synthesis and characterization of ion-exchangeable titanate nanotubes, Chem.–Eur. J., 2003, 9, 2229–2238 CrossRef CAS.
- S. Kasap, S. Piskin and H. Tel, Titanate nanotubes: preparation, characterization and application in adsorption of strontium ion from aqueous solution, Radiochim. Acta, 2012, 100, 925–929 CrossRef CAS.
- R. Chitrakar, Y. Makita and A. Sonoda, Cesium Ion Exchange on Synthetic Birnessite (Na0.35MnO2O), Chem. Lett., 2011, 40, 1118–1120 CrossRef CAS.
- J. Lehto, R. Harjula, H. Leinonen, A. Paajanen, T. Laurila, K. Mononen and L. Saarinen, Advanced separation of harmful metals from industrial waste effluents by ion exchange, J. Radioanal. Nucl. Chem., 1996, 208, 435–443 CrossRef CAS.
- W. E. Prout, E. R. Russell and H. J. Groh, Ion Exchange Absorption Of Cesium By Potassium Hexacyanocobalt (2) Ferrate (2), J. Inorg. Nucl. Chem., 1965, 27, 473 CrossRef CAS.
- S. Handley-Sidhu, J. C. Renshaw, S. Moriyama, B. Stolpe, C. Mennan, S. Bagheriasl, P. Yong, A. Stamboulis, M. Paterson-Beedle, K. Sasaki, R. A. D. Pattrick, J. R. Lead and L. E. Macaskie, Uptake of Sr2+ and Co2+ into Biogenic Hydroxyapatite: Implications for Biomineral Ion Exchange Synthesis, Environ. Sci. Technol., 2011, 45, 6985–6990 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra05663e |
| This journal is © The Royal Society of Chemistry 2024 |