Open Access Article
Open Access ArticleGeneral and versatile synthesis of highly recyclable chiral phosphoric acid organocatalysts†
Aitor
Maestro
 *ab,
Sándor B.
Ötvös
*ab,
Sándor B.
Ötvös
 bc,
Gerald
Auer
d and
C. Oliver
Kappe
bc,
Gerald
Auer
d and
C. Oliver
Kappe
 bc
bc
aDepartment of Organic Chemistry I, University of the Basque Country, UPV/EHU, Paseo de la Universidad 7, 01006 Vitoria-Gasteiz, Spain. E-mail: aitor.maestro@ehu.eus
bInstitute of Chemistry, University of Graz, NAWI Graz, A-8010 Graz, Austria
cCenter for Continuous Flow Synthesis and Processing (CC FLOW), Research Center Pharmaceutical Engineering GmbH (RCPE), A-8010 Graz, Austria
dDepartment of Earth Sciences, University of Graz, NAWI Graz Geocenter, A-8010 Graz, Austria
First published on 30th August 2024
Abstract
The development of recyclable catalysts has gained more attention in recent years in order to minimize the environmental effect and the overall cost of catalytic processes. Some of the most broadly used chiral organocatalysts are BINOL-derived chiral phosphoric acids, making it necessary to develop efficient recycling strategies. While literature reports require up to 13 synthetic steps to access recyclable chiral phosphoric acids, here we report a general and concise 9-step approach to anthracene decorated heterogeneous chiral phosphoric acids (PS-Anth), which have shown high performance either in batch or continuous flow without observing catalyst degradation.
Introduction
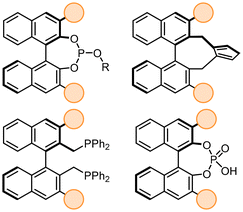
BINOL (1,1′-Bi-2-naphthol), is one of the most commonly used chiral scaffolds in asymmetric catalysis. It is the key starting material in the synthesis of broadly used chiral ligands such as BINAP or phosphoramidites.1 As the need for more sustainable methodologies steadily increases, the interest for developing easily recoverable and recyclable catalysts and ligands has grown in the last years.2–5 The immobilization of simple BINOL on solid supports is rather straightfoward as the selective bromination or nitration of the 6 and 6′ positions can be achieved by treating it with bromine or nitric acid respectively,6,7 leading to an ideal set point for further functionalization. However, immobilization of more complex 3,3′-substituted BINOL derivatives still represents a challenge, often requiring a large number of sequential steps.8–12 These structures have high importance in the field of asymmetric catalysis (Fig. 1), as they have applications as chiral ligands in metal-catalyzed reactions13–17 and chiral Brønsted acid organocatalysts.18–22Despite the effectiveness of their methodologies, 11–13 synthetic steps are needed, making them highly challenging. One of the most exploited heterogeneous CPAs is the polymer-supported TRIP (3,3′ substituent of BINOL derived CPA: 2,4,6-iPr3-C6H2) catalyst (PS-TRIP), developed by Pericàs (2016) (Fig. 2B).23 In particular, For this reason, several authors have made efforts to immobilize chiral phosphoric acids (CPA) on solid supports (Fig. 2A).24–29 For instance, Rueping30 (2010), Pericàs31 (2014), and, more recently Schneider (2024),11 reported different approaches to access polymer or silica-supported chiral phosphoric acids.
 | ||
| Fig. 2 (A) Highly challenging routes to heterogeneous CPAs. (B) Shorter synthetic approaches, strongly dependent on the aryl group (orange). (C) Aims of this manuscript. | ||
PS-TRIP has demonstrated high robustness and applications either in batch or continuous flow processes, allowing high productivity and no visible deactivation in most cases.32–35 Moreover, when deactivation was observed, PS-TRIP was easily reactivated by treating it with an acidic solution. However, the reported synthesis only allows immobilizing TRIP and closely related analogs, as the functionalization of 3,3′-aryl BINOLs has shown low selectivity to the 6,6′ positions for other BINOL derivatives.36–40 On the other hand, Blechert41 (2012) and You12 (2022) reported different approaches to hyper cross-linked CPAs (Fig. 2B). However, their strategy only proved to be effective in accessing anthryl-substituted immobilized BINOL derivatives. Therefore, to further explore the applications of heterogeneous CPA derivatives as recyclable variants of their homogeneous counterparts, general and more efficient strategies to immobilize a broad range of 3,3′-aryl BINOLs are needed. Based on the previous reports from Pericàs and You (Fig. 2B), the most challenging step is the bromination, since it results in different substitution patterns depending on the 3,3′-diaryl BINOL used in the reaction. In this manuscript, we present a general and concise route to overcome this limitation. To avoid potential interferences of the support in the catalytic activity, immobilization through the 6,6′ positions of BINOL is proposed. This way, the accessibility of the catalyst site is expected to be similar to the homogeneous one, allowing straightforward transfer of known homogeneous methodologies to the use of recyclable CPAs.
Results and discussion
Our initial hypothesis consisted of using BINOL 3 as a new key intermediate to heterogeneous chiral phosphoric acids, thus avoiding unexpected functionalization during the bromination step. BINOL 3 was reported by Kobayashi in 2002 through a 6-step synthesis.42 As one of our goals was minimizing the overall required steps to access immobilized CPAs, we explored more straightforward approaches to synthesize it. BINOL 2, a well-known intermediate for the synthesis of 3,3′-functionalized BINOL derivatives, was used as the starting material (Scheme 1). In the presence of molecular bromine, 2 undergoes a selective 6,6′-bromination, affording 3 in almost quantitative yield. Further optimization resulted in a one-pot iodination/bromination protocol that afforded BINOL 3 in 84% overall yield from 1, in contrast to the 72% yield obtained for the stepwise synthesis.After protection of 3, the initial Suzuki–Miyaura cross-coupling (SMCC) was carried out using 5 mol% of Pd(PPh3)4 as the catalyst (Scheme 1, A). To ensure high selectivity towards the iodine, only 2.5 equivalents of the boronic acid and temperatures not higher than 85 °C were used. Next, the crude reaction mixtures were stirred in MeOH in the presence of aqueous HCl to deprotect the BINOL moiety. The reaction was performed with some of the most commonly used aryl substituents in phosphoric acid-mediated enantioselective reactions such as anthryl or phenanthryl groups, as well as 3,5-bis(CF3)2 or para-substituted aryl groups. All of them were successful, overcoming the site-selectivity drawback of the previously reported brominations of 3,3′-substituted BINOL derivatives. Under these conditions, the desired BINOLs 4 were obtained in moderate to high yields (56–74%) over two-steps. Moreover, no 6,6′-cross-coupling product was observed in any of the performed reactions, and the main observed byproducts were identified as the protodeboronation of the boronic acid and 3-mono-coupled BINOL intermediates. Although preliminary experiments on the Kumada cross-coupling to access PS-TRIP intermediate23 showed promising results, the additional step required with our synthetic route discouraged us to further explore that direction for this particular case.
To demonstrate the versatility of our methodology to synthesize highly functionalized BINOL derivatives, 4a was used in sequential cross couplings. In contrast to the first cross-coupling, in this case, temperatures up to 110 °C and higher excess of the boronic acid was used to ensure high yields. For the 6,6′-position, boronic acids that have been previously used as binding points for immobilized chiral phosphoric acids were selected, introducing groups such as styryl (5aa),23,30 methyl benzoate (5ab),27,43,44 or 3,5-dimethoxyphenyl (5ac)12 in yields ranging from 76 to 91% (Scheme 1B). Finally, phosphorylation of 5aa afforded the chiral phosphoric acid 6aa in almost quantitative yield, which was immobilized through radical polymerization with styrene and divinylbenzene (Scheme 1C).
The as-prepared PS-Anth was fully characterized using the usual techniques for polymers and solid materials. The physical properties of the resulting catalyst beads were analyzed by optical and scanning electronic microscopy (SEM) to analyze the surface (Fig. 3). We observed the formation of spherical structures of around 300 μm diameter and the surface was covered with multiple small pores and cavities. The chemical characterization of the material was performed using FTIR, providing the characteristic bands of polystyrene. The catalyst loading of the material (0.07 mmol g−1) was determined based on the elemental analysis of P, which is only present on the phosphoric acid moiety.
Next, we evaluated our PS-Anth catalyst towards the synthesis of dihydroquinazolinones 8, previously reported by several authors in the presence of homogeneous chiral phosphoric acids (Scheme 2A).45–48 We compared the obtained data with Rueping's report,46 where a 3,3′-anthryl-BINOL derived phosphoric acid was used. Notably, the only difference between their optimal catalyst and our PS-Anth is the immobilization through the 6,6′-positions. To our delight, when benzaldehyde was used in the reaction, a 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 er was obtained, close to the 93
9 er was obtained, close to the 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 reported in the literature. The scope of aromatic aldehydes bearing electron donating and electron-withdrawing groups afforded products 8 in high yield and enantiomeric ratios comparable to the homogeneous catalyst. The main limitations in terms of the enantiocontrol were found when using 3-nitrobenzaldehyde, where the selectivity dropped to around 3
7 reported in the literature. The scope of aromatic aldehydes bearing electron donating and electron-withdrawing groups afforded products 8 in high yield and enantiomeric ratios comparable to the homogeneous catalyst. The main limitations in terms of the enantiocontrol were found when using 3-nitrobenzaldehyde, where the selectivity dropped to around 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and for 4-methylbenzaldehyde (4
1, and for 4-methylbenzaldehyde (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 er). Unfortunately, literature data is not available for comparison in those cases. On the other hand, sulfur-containing heteroaromatic aldehyde afforded almost exclusively the major enantiomer. All these experiments were carried out with the same sample of the catalyst, which was filtered and washed with CHCl3 between runs. Moreover, when the scope was finalized, the reaction of 7 with benzaldehyde was repeated to ensure the catalytic activity remained unaltered, obtaining identical results to the initial experiment.
1 er). Unfortunately, literature data is not available for comparison in those cases. On the other hand, sulfur-containing heteroaromatic aldehyde afforded almost exclusively the major enantiomer. All these experiments were carried out with the same sample of the catalyst, which was filtered and washed with CHCl3 between runs. Moreover, when the scope was finalized, the reaction of 7 with benzaldehyde was repeated to ensure the catalytic activity remained unaltered, obtaining identical results to the initial experiment.
To further evaluate the recyclability of PS-Anth, an adjustable column was filled with 0.3 g of the catalyst, and the resulting packed bed column was used in a flow setup. The asymmetric transfer hydrogenation of Hantzsch esters and 2-arylquinolines has already been reported in flow and represents an excellent model reaction to benchmark the catalytic activity of our PS-Anth due to the absence of reaction byproducts (Scheme 2B).11,34,49 After optimizing the process (ESI-Table S1†), we synthesized four 2-aryl tetrahydroquinolines in excellent yield and enantiocontrol under flow conditions. All the reaction mixtures were collected for 1 h in steady state. Then, a preparative run of 10a was performed, analyzing the reaction outcome every hour for 5 h after steady state, showing identical results over the analyzed period (Scheme 2C). The combined collected fractions yielded 1.05 g of tetrahydroquinoline 10a after purification, which represents a productivity of 3.35 mmol h−1 gPS-Anth−1. The turnover number (TON = 239.1) and space–time yield (STY = 71.99 kg m−3 h−3) were calculated based on the data obtained from the 5 h preparative run, and the overall catalyst loading for the same period was 0.4 mol%. It should be noted that during all the flow experiments, the catalyst was only washed with pure CHCl3 between runs, and no catalyst deactivation was observed in any case, demonstrating the high robustness of the new PS-Anth catalyst. To confirm the stability of PS-Anth beads, the used catalyst was fully characterized once again using FTIR, optical microscopy and SEM, observing no difference with respect to the as-prepared polymer-supported catalyst (ESI-Fig. S1–S4†).
Conclusions
In summary, we have developed a new synthetic approach to access highly functionalized BINOL derivatives through a sequence of two consecutive Suzuki–Miyaura cross-couplings and demonstrated their applicability for immobilizing catalysts such as chiral phosphoric acids. While most of the known methodologies to synthesize recyclable chiral phosphoric acids require 11–13 synthetic steps, our strategy allows obtaining them with a broad range of 3,3′-substituents in only 9 steps from commercially available BINOL, close to the 8-step syntheses developed by Pericàs and You, which represent the shortest routes known to date. Moreover, our methodology addresses the selectivity issues of the bromination step using a sequence of two consecutive Suzuki–Miyaura cross-couplings. This strategy represents a versatile approach to access a broad range of recyclable chiral phosphoric acids, being able to modulate not only the chirality directing group in 3,3′-positions but also the binding point that allows simple recycling of the catalyst.In order to demonstrate the applicability of our methodology to access recyclable catalysts, we have synthesized PS-Anth phosphoric acid, which represents the first back-side immobilization of this particular CPA. Moreover, we have explored the potential of PS-Anth in two enantioselective reactions, demonstrating high robustness either in batch or continuous flow processes. The flow recyclability tests resulted in an overall catalyst loading of 0.4 mol% (TON = 239.1), and the comparison of the used catalyst with the as-prepared one, showed no relevant differences either in the catalytic activity or its physicochemical properties that could affect the performance of PS-Anth in future applications.
Procedure and characterization
All relevant experimental procedures, data and characterization details are provided in the ESI.†Data availability
• Data for this article, including the detailed experimental procedures and characterization of organic molecules are available on the ESI.†• The data supporting this article have been included as part of the ESI.†
• A preprint of this article has been deposited on ChemRxiv (https://doi.org/10.26434/chemrxiv-2024-nqkmn-v2).
Conflicts of interest
There are no conflicts to declare.Acknowledgements
AM acknowledges the Postdoctoral Fellowship from the Basque Government. This research was funded in whole, or in part, by the Austrian Science Fund (FWF) [10.55776/P34397]. We thank Helmar Wiltsche from the TU Graz for the elemental analysis.References
- M. M. Pereira, M. J. F. Calvete, R. M. B. Carrilho and A. R. Abreu, Synthesis of binaphthyl based phosphine and phosphite ligands, Chem. Soc. Rev., 2013, 42, 6990–7027 RSC.
- Z. D. Susam and C. Tanyeli, Recyclable Organocatalysts in Asymmetric Synthesis, Asian J. Org. Chem., 2021, 10, 1251–1266 CrossRef CAS.
- Á. Molnár and A. Papp, Catalyst recycling—A survey of recent progress and current status, Coord. Chem. Rev., 2017, 349, 1–65 CrossRef.
- C. B. Watson, A. Kuechle and D. E. Bergbreiter, Fully recyclable Brønsted acid catalyst systems, Green Chem., 2021, 23, 1266–1273 RSC.
- J. Everaert, K. Leus, H. Rijckaert, M. Debruyne, K. Van Hecke, R. Morent, N. De Geyter, V. Van Speybroeck, P. Van Der Voort and C. V. Stevens, A recyclable rhodium catalyst anchored onto a bipyridine covalent triazine framework for transfer hydrogenation of N-heteroarenes in water, Green Chem., 2023, 25, 3267–3277 RSC.
- Y. Cui, O. R. Evans, H. L. Ngo, P. S. White and W. Lin, Rational design of homochiral solids based on two-dimensional metal carboxylates, Angew. Chem., Int. Ed., 2002, 41, 1159–1162 CrossRef CAS PubMed.
- H. Hu, J. Xiang, Y. Yang and C. Chen, A Helix − Turn − Helix Supersecondary Structure Based on Oligo (phenanthroline dicarboxamide)s, Org. Lett., 2008, 10, 69–72 CrossRef CAS PubMed.
- J. Bunzen, U. Kiehne, C. Benkhäuser-Schunk and A. Lützen, Immobilization of bis(bipyridine) BINOL ligands and their use in chiral resolution, Org. Lett., 2009, 11, 4786–4789 CrossRef CAS PubMed.
- S. Herres, P. Hesemann and J. J. E. Moreau, Polystyrene resins incorporating BINOL units: New materials for asymmetric catalysis, Eur. J. Org. Chem., 2003, 99–105 CrossRef CAS.
- J. Akai, S. Watanabe, K. Michikawa and T. Harada, Application of a Heterogeneous Chiral Titanium Catalyst Derived from Silica-Supported 3-Aryl H8-BINOL to Enantioselective Alkylation and Arylation of Aldehydes, Org. Lett., 2017, 19, 3632–3635 CrossRef CAS PubMed.
- M. Laue, M. Schneider, M. Gebauer, W. Böhlmann, R. Gläser and C. Schneider, General, Modular Access toward Immobilized Chiral Phosphoric Acid Catalysts and Their Application in Flow Chemistry, ACS Catal., 2024, 5550–5559 CrossRef CAS PubMed.
- X. Y. Huang, Q. Zheng, L. M. Zou, Q. Gu, T. Tu and S. L. You, Hyper-Crosslinked Porous Chiral Phosphoric Acids: Robust Solid Organocatalysts for Asymmetric Dearomatization Reactions, ACS Catal., 2022, 12, 4545–4553 CrossRef CAS.
- J. Margalef, M. Biosca, P. de la Cruz Sánchez, J. Faiges, O. Pàmies and M. Diéguez, Evolution in heterodonor P-N, P-S and P-O chiral ligands for preparing efficient catalysts for asymmetric catalysis. From design to applications, Coord. Chem. Rev., 2021, 446, 214120 CrossRef CAS.
- Y. G. Zhou and X. Zhang, Synthesis of novel BINOL-derived chiral bisphosphorus ligands and their application in catalytic asymmetric hydrogenation, Chem. Commun., 2002, 10, 1124–1125 RSC.
- B. Ye and N. Cramer, A tunable class of chiral Cp ligands for enantioselective rhodium(III)-catalyzed C-H allylations of benzamides, J. Am. Chem. Soc., 2013, 135, 636–639 CrossRef CAS PubMed.
- A. Maestro, E. Martinez De Marigorta, F. Palacios and J. Vicario, Enantioselective Aza-Reformatsky Reaction with Ketimines, Org. Lett., 2019, 21, 9473–9477 CrossRef CAS PubMed.
- Y. Yue, M. Turlington, X. Q. Yu and L. Pu, 3,3′-anisyl-substituted BINOL, H4BINOL, and H 8BINOL ligands: Asymmetric synthesis of diverse propargylic alcohols and their ring-closing metathesis to chiral cycloalkenes, J. Org. Chem., 2009, 74, 8681–8689 CrossRef CAS PubMed.
- X. del Corte, E. Martínez de Marigorta, F. Palacios, J. Vicario and A. Maestro, An overview of the applications of chiral phosphoric acid organocatalysts in enantioselective additions to C=O and C=N bonds, Org. Chem. Front., 2022, 9, 6331–6399 RSC.
- E. I. Jiménez, An update on chiral phosphoric acid organocatalyzed stereoselective reactions, Org. Biomol. Chem., 2023, 21, 3477–3502 RSC.
- G. Caballero-García and J. M. Goodman, N-Triflylphosphoramides: highly acidic catalysts for asymmetric transformations, Org. Biomol. Chem., 2021, 19, 9565–9618 RSC.
- L. Schreyer, R. Properzi and B. List, IDPi Catalysis, Angew. Chem., Int. Ed., 2019, 58, 12761–12777 CrossRef CAS PubMed.
- M. C. Benda and S. France, Chiral disulfonimides: a versatile template for asymmetric catalysis, Org. Biomol. Chem., 2020, 18, 7485–7513 RSC.
- L. Clot-Almenara, C. Rodríguez-Escrich, L. Osorio-Planes and M. A. Pericàs, Polystyrene-Supported TRIP: A highly recyclable catalyst for batch and flow enantioselective allylation of aldehydes, ACS Catal., 2016, 6, 7647–7651 CrossRef CAS.
- C. Bleschke, J. Schmidt, D. S. Kundu, S. Blechert and A. Thomas, A chiral microporous polymer network as asymmetric heterogeneous organocatalyst, Adv. Synth. Catal., 2011, 353, 3101–3106 CrossRef CAS.
- S. Li, J. Zhang, S. Chen and X. Ma, Semi-heterogeneous asymmetric organocatalysis: Covalent immobilization of BINOL-derived chiral phosphoric acid (TRIP) to polystyrene brush grafted on SiO2 nanoparticles, J. Catal., 2022, 416, 139–148 CrossRef CAS.
- X. Chen, H. Jiang, X. Li, B. Hou, W. Gong, X. Wu, X. Han, F. Zheng, Y. Liu, J. Jiang and Y. Cui, Chiral Phosphoric Acids in Metal–Organic Frameworks with Enhanced Acidity and Tunable Catalytic Selectivity, Angew. Chem., 2019, 131, 14890–14899 CrossRef.
- W. Gong, X. Chen, H. Jiang, D. Chu, Y. Cui and Y. Liu, Highly Stable Zr(IV)-Based Metal-Organic Frameworks with Chiral Phosphoric Acids for Catalytic Asymmetric Tandem Reactions, J. Am. Chem. Soc., 2019, 141, 7498–7508 CrossRef CAS PubMed.
- Y. Zhang, Z. Zhang, S. Ma, J. Jia, H. Xia and X. Liu, Hypercrosslinking chiral Brønsted acids into porous organic polymers for efficient heterogeneous asymmetric organosynthesis, J. Mater. Chem. A, 2021, 9, 25369–25373 RSC.
- Z. Zhang, Y. R. Ji, L. Wojtas, W. Y. Gao, S. Ma, M. J. Zaworotko and J. C. Antilla, Two homochiral organocatalytic metal organic materials with nanoscopic channels, Chem. Commun., 2013, 49, 7693–7695 RSC.
- M. Rueping, E. Sugiono, A. Steck and T. Theissmann, Synthesis and application of polymer-supported chiral Brønsted acid organocatalysts, Adv. Synth. Catal., 2010, 352, 281–287 CrossRef CAS.
- L. Osorio-Planes, C. Rodríguez-Escrich and M. A. Pericàs, Enantioselective continuous-flow production of 3-indolylmethanamines mediated by an immobilized phosphoric acid catalyst, Chem. – Eur. J., 2014, 20, 2367–2372 CrossRef CAS PubMed.
- M. B. Chaudhari, P. Gupta, P. Llanes and M. A. Pericàs, Polymer-Supported Phosphoric-Acid Catalysed Enantioselective Pictet-Spengler Cyclisation for the Synthesis of Quaternary Tryptolines in Batch/Continuous Flow, Adv. Synth. Catal., 2023, 365, 527–534 CrossRef CAS.
- A. Maestro, B. S. Nagy, S. B. Ötvös and C. O. Kappe, A Telescoped Continuous Flow Enantioselective Process for Accessing Intermediates of 1 - Aryl-1, 3-diols as Chiral Building Blocks, J. Org. Chem., 2023, 88, 15523–15529 CrossRef CAS PubMed.
- B. S. Nagy, A. Maestro, M. B. Chaudhari, C. O. Kappe and S. B. Ötvös, Enantioselective Flow Synthesis of a Tetrahydroquinoline SERM Enabled by Immobilized Chiral Phosphoric Acid Catalysis and Diboronic Acid Mediated Selective Nitro Reduction, Adv. Synth. Catal., 2024, 366, 1024–1030 CrossRef CAS.
- A. Maestro, B. K. Malviya, G. Auer, S. B. Ötvös and C. O. Kappe, A robust heterogeneous chiral phosphoric acid enables multi decagram scale production of optically active N,S-ketals, Green Chem., 2024, 26, 4593–4599 RSC.
- D. Wei, M. Y. Li, B. B. Zhu, X. D. Yang, F. Zhang, C. G. Feng and G. Q. Lin, Sequential Cross-Coupling/Annulation of ortho-Vinyl Bromobenzenes with Aromatic Bromides for the Synthesis of Polycyclic Aromatic Compounds, Angew. Chem., Int. Ed., 2019, 58, 16543–16547 CrossRef CAS PubMed.
- A. Nakazaki, A. Mori, S. Kobayashi and T. Nishikawa, A Divergent Approach to the Diastereoselective Synthesis of 3,3-Disubstituted Oxindoles from Atropisomeric N-Aryl Oxindole Derivatives, Chem. – Asian J., 2016, 11, 3267–3274 CrossRef CAS PubMed.
- D. M. E. Freeman, A. J. Musser, J. M. Frost, H. L. Stern, A. K. Forster, K. J. Fallon, A. G. Rapidis, F. Cacialli, I. McCulloch, T. M. Clarke, R. H. Friend and H. Bronstein, Synthesis and Exciton Dynamics of Donor-Orthogonal Acceptor Conjugated Polymers: Reducing the Singlet-Triplet Energy Gap, J. Am. Chem. Soc., 2017, 139, 11073–11080 CrossRef CAS PubMed.
- N. Negoro, S. Sasaki, S. Mikami, M. Ito, Y. Tsujihata, R. Ito, M. Suzuki, K. Takeuchi, N. Suzuki, J. Miyazaki, T. Santou, T. Odani, N. Kanzaki, M. Funami, A. Morohashi, M. Nonaka, S. Matsunaga, T. Yasuma and Y. Momose, Optimization of (2,3-Dihydro-1-benzofuran-3-yl)acetic Acids: Discovery of a Non-Free Fatty Acid-Like, Highly Bioavailable G Protein-Coupled Receptor 40/Free Fatty Acid Receptor 1 Agonist as a Glucose-Dependent Insulinotropic Agent, J. Med., 2012, 55, 3960–3974 CAS.
- X. Liao, D. Wang, Y. Huang, Y. Yang and J. You, Highly Chemo-, Regio- and E/Z-Selective Intermolecular Heck-Type Dearomative [2 + 2 + 1] Spiroannulation of Alkyl Bromoarenes with Internal Alkynes, Org. Lett., 2019, 21, 1152–1155 CrossRef CAS PubMed.
- D. S. Kundu, J. Schmidt, C. Bleschke, A. Thomas and S. Blechert, A microporous binol-derived phosphoric acid, Angew. Chem., Int. Ed., 2012, 51, 5456–5459 CrossRef CAS PubMed.
- Y. Yamashita, H. Ishitani, H. Shimizu and S. Kobayashi, Highly anti-selective asymmetric aldol reactions using chiral zirconium catalysts. Improvement of activities, structure of the novel zirconium complexes, and effect of a small amount of water for the preparation of the catalysts, J. Am. Chem. Soc., 2002, 124, 3292–3302 CrossRef CAS PubMed.
- M. Zheng, Y. Liu, C. Wang, S. Liu and W. Lin, Cavity-induced enantioselectivity reversal in a chiral metal-organic framework Brønsted acid catalyst, Chem. Sci., 2012, 3, 2623–2627 RSC.
- X. Chen, H. Jiang, X. Li, B. Hou, W. Gong, X. Wu, X. Han, F. Zheng, Y. Liu, J. Jiang and Y. Cui, Chiral Phosphoric Acids in Metal–Organic Frameworks with Enhanced Acidity and Tunable Catalytic Selectivity, Angew. Chem., Int. Ed., 2019, 58, 14748–14757 CrossRef CAS PubMed.
- X. Cheng, S. Vellalath, R. Goddard and B. List, Direct catalytic asymmetric synthesis of cyclic aminals from aldehydes, J. Am. Chem. Soc., 2008, 130, 15786–15787 CrossRef CAS PubMed.
- M. Rueping, A. P. Antonchick, E. Sugiono and K. Grenader, Asymmetric Brønsted acid catalysis: Catalytic enantioselective synthesis of highly biologically active dihydroquinazolinones, Angew. Chem., Int. Ed., 2009, 48, 908–910 CrossRef CAS PubMed.
- D. J. Cheng, Y. Tian and S. K. Tian, Catalytic asymmetric synthesis of dihydroquinazolinones from imines and 2-aminobenzamides, Adv. Synth. Catal., 2012, 354, 995–999 CrossRef CAS.
- D. Huang, X. Li, F. Xu, L. Li and X. Lin, Highly enantioselective synthesis of dihydroquinazolinones catalyzed by SPINOL-phosphoric acids, ACS Catal., 2013, 3, 2244–2247 CrossRef CAS.
- O. Zhelavskyi, Y. J. Jhang and P. Nagorny, Asymmetric Transfer Hydrogenation of Heterocyclic Compounds in Continuous Flow Using an Immobilized Chiral Phosphoric Acid as the Catalyst, Synthesis, 2023, 55, 2361–2369 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: General procedures and characterization of organic compounds. See DOI: https://doi.org/10.1039/d4qo01442a |
| This journal is © the Partner Organisations 2024 |