Open Access Article
Open Access ArticleChemoselective reaction of methoxyaminomethyl BODIPYs with unprotected carbohydrates: a powerful tool for accessing BODIPY neoglycosides†
Ana M.
Gómez
 *a,
Luis
García-Fernández
*a,
Luis
García-Fernández
 bc,
Andrés G.
Santana
bc,
Andrés G.
Santana
 a,
Clara
Uriel
a,
Leire
Gartzia-Rivero
a,
Clara
Uriel
a,
Leire
Gartzia-Rivero
 d,
Jorge
Bañuelos
d,
Jorge
Bañuelos
 *d,
Inmaculada
Garcia-Moreno
e,
Lourdes
Infantes
*d,
Inmaculada
Garcia-Moreno
e,
Lourdes
Infantes
 f,
María Rosa
Aguilar
f,
María Rosa
Aguilar
 bc and
J. Cristobal
Lopez
bc and
J. Cristobal
Lopez
 *a
*a
aInstituto de Química Orgánica General (IQOG-CSIC), Juan de la Cierva 3, 28006 Madrid, Spain. E-mail: ana.gomez@csic.es; jc.lopez@csic.es
bInstituto de Ciencia y Tecnología de Polímeros (ICTP-CSIC), Juan de la Cierva 3, 28006 Madrid, Spain
cCentro de Investigación Biomédica en Red de Bioingeniería, Biomateriales y Nanomedicina (CIBER-BBN), Instituto de Salud Carlos III, Monforte de Lemos 3-5, 28029 Madrid, Spain
dDepartamento de Química Física, Universidad del País Vasco (UPV/EHU), Apartado 644, 48080 Bilbao, Spain. E-mail: jorge.banuelos@ehu.eus
eDepartamento de Química-Física de Materiales, Instituto de Química-Física “Blas Cabrera” (IQF-CSIC), Serrano 119, 28006 Madrid, Spain
fDepartamento de Cristalografía y Biología Estructural, Instituto de Química-Física “Blas Cabrera” (IQF-CSIC), Serrano 119, 28006 Madrid, Spain
First published on 25th June 2024
Abstract
The neoglycosylation of methoxyaminomethyl-appended BODIPYs with unprotected reducing mono-, di-, and trisaccharides produces, in a regio- and stereoselective manner, cyclic N-glycosyl-N-methoxy–BODIPY conjugates, as a relevant class of neoglycosides that display excellent photophysical characteristics in pure water, even at high dye concentrations. In addition, the cellular uptake of some of the neoglycosylated BODIPYs has been confirmed via fluorescence microscopy, and a BODIPY–acarbose conjugate showed comparable enzymatic inhibitory activity to acarbose for two different α-amylases: A. oryzae α-amylase (AOA) and human salivary α-amylase (HSA).
Introduction
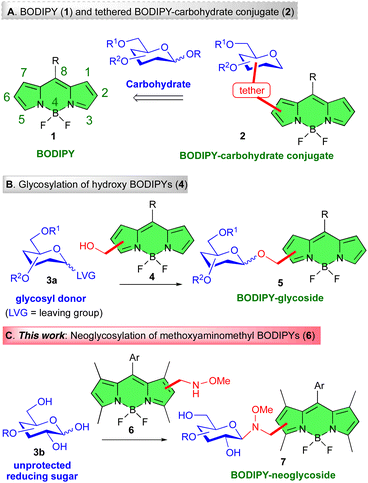
Carbohydrates, ubiquitous in Nature, play significant roles in many biological processes ranging from infection (viral and bacterial), cell recognition, triggering of immune responses, and cancer metastasis.1 In recent years, it has also become clear that carbohydrate–protein interactions involving cell surface proteins,2 or cell surface carbohydrates,3 are key to health and disease mechanisms.4,5 The investigation of these processes, which falls under the umbrella of Glycobiology6 is, therefore, a field attracting increasing interest. In this context, fluorescence imaging techniques have become powerful tools for the visualization of biomolecules, and the assessment of these phenomena. Such studies often require the derivatization of glycans by labeling with fluorophores7 or by attachment to surfaces.8 In this context, difluoroboron dipyrromethene (4,4-difluoro-4-bora-3a,4a-diaza-s-indacene) or BODIPY dyes, e.g., 1 (Fig. 1A),9 have arguably become one of the most popular fluorophores for saccharide tagging.7b,10 BODIPYs display relatively high photostability, neutral total charge, sharp absorption and emission spectra, notable chemical robustness, and high fluorescence quantum yield (ΦF).9 More interestingly, all of the above properties can be modulated by subtle postfunctional modifications of the dipyrromethene core.11,12 Thus, incorporating diverse functional groups to the BODIPY core can fine-tune the absorption and emission wavelengths of these dyes. This tunability enables the design of BODIPY derivatives with tailored fluorescence properties, making them suitable for various applications.13 BODIPY derivatives possess many ideal photosensitizer (PS) features, which makes them useful agents in photodynamic therapy (PDT),14 and, more recently, in photothermal therapy (PTT).15 They have also been used in optoelectronic devices, including organic light-emitting diodes (OLEDs)16 and organic photovoltaics (OPVs).17 Derivatives of BODIPY are frequently used in biological imaging.18 Their fluorescence makes them excellent for observing cellular structures, biomolecule distribution within cells, and dynamics.19In this regard, because of their wide range of uses, adjustable fluorescence characteristics, and photostability, luminescent BODIPY–sugar probes have gained the attention of researchers for the potential applications of such molecular systems in bio-imaging.20 The carbohydrate component of BODIPY–carbohydrate conjugates plays an important role since it confers remarkable properties to the BODIPY glycoprobes. Thus, the sugar component provides enhanced solubility in polar solvents (including water),21 biocompatibility,22 targeting ability,23 cell endocytosis,24 and, in some instances, reduced toxicity25 to the glyco-fluorophores.7,8
In a broad sense, BODIPY–carbohydrate conjugates can be divided into linker-free or tethered derivatives. Classically, synthetic routes to the former class have been less exploited,10,21,26 and attention has been mainly devoted to the synthesis of BODIPY–carbohydrate conjugates connected through a “linker”. Among the latter, synthetic approaches to tethered BODIPY–carbohydrate hybrids, e.g., 2 (Fig. 1A), often rely on the use of click reactions, in particular the copper(I)-catalyzed azide–alkyne cycloaddition (CuAAC)27 on azido- or alkynyl BODIPYs.28,29 On the contrary, methods based on carbohydrate transformations, for instance, the glycosylation of hydroxyl-appended BODIPYs, e.g., 4, with glycosyl donors 3a, leading to glycosyl BODIPYs 5 (Fig. 1B),30 have been scarcely employed.31
Seeking a powerful method to covalently link BODIPYs and carbohydrates, we were mindful of the seminal contribution by Peri, Dumy and Mutter,32 which reported the regio- chemo- and stereoselective formation of glycosidic bonds between unprotected reducing sugars, e.g.3b (Fig. 1C), and secondary methoxyamine-containing aglycons. This transformation provides access to cyclic N-glycosyl-N,O-dialkyl neoglycosides in the thermodynamically most stable configurations.33 These derivatives display conformational behavior similar to natural O-glycosides.34 This ligation method, further validated by Langenhan and Thorson,35 has proven useful in the preparation of bioactive probes and early-stage leads in drug discovery.36
Along these lines, we report in this manuscript the preparation of methoxyaminomethyl BODIPY dyes, i.e., 6, and their reaction with unprotected reducing sugar derivatives e.g., 3b, to afford BODIPY-neoglycosides, i.e., 7 (Fig. 1C). Specifically, the method is applied to different methoxyaminomethyl BODIPY compounds as well as to saccharides of different chain lengths. The ensuing BODIPY conjugates displayed excellent photophysical properties in water and at high dye concentrations. Furthermore, some of these conjugates were submitted to biological studies including cellular uptake, intracellular localization, and cytotoxicity in healthy and tumoral cells. One of the sugar derivatives employed in these studies has been acarbose. Acarbose, an α-amylase and α-glucosidase inhibitor, exerts a well-defined glucoregulatory effect. Cancer cells are known to exhibit a heightened dependence on glucose for ATP production compared to their non-malignant counterparts.37 Consequently, targeting this metabolic pathway by restricting glucose availability represents a well-established strategy in cancer therapy.38–40 Finally, the acarbose–BODIPY conjugate was also evaluated as a chromogenic inhibitor of α-amylases.
Results and discussion
To evaluate the feasibility, scope and limitations of this approach, we initiated our studies with the preparation of methoxyaminomethyl BODIPYs 6a–c (Fig. 2). These fluorescent dyes are derived from 8-aryl, 1,3,5,7 tetramethyl BODIPYs. In these compounds the methyl groups at C-1 and C-7 force the 8-aryl group to adopt an orthogonal orientation relative to the BODIPY core.31a This arrangement prevents unwanted aggregation and restricts the rotation of the aryl substituent, thereby preserving the chromophore's emission properties. In addition, the presence of the aryl and methyl groups in the BODIPY framework has a beneficial effect on its photostability and chemical robustness.31b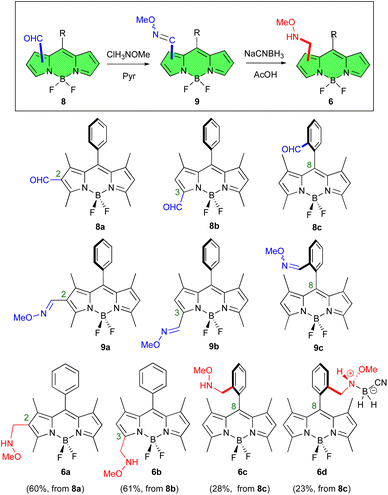 | ||
| Fig. 2 Methoxyaminomethyl BODIPY derivatives 6a–d, obtained from formyl-BODIPYs 8a–c, via BODIPY oximes 9a–c. | ||
Our synthetic strategy began by reacting previously described formyl BODIPYs 8a,418b,42 and 8c,31a with methoxyamine hydrochloride salt using pyridine as a mediator (Fig. 2). This produced the corresponding BODIPY-O-methyl oximes 9a–c in good yields (81%, 84%, and 74%, respectively).43 Compounds 9a and 9b were obtained as single stereoisomers while 9c exhibited a 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of isomers at the oxime double bond, as determined by 1H-NMR spectroscopy.
1 mixture of isomers at the oxime double bond, as determined by 1H-NMR spectroscopy.
Reduction of the O-methyl oximes 9a–c with NaCNBH3 in glacial acetic acid then gave the expected methoxyaminomethyl BODIPYs 6a, 6b and 6c in fair to good yields (74%, 73% and 38%, respectively, Fig. 2). In the latter case, the zwitterionic N-cyanoboronated-N-alkoxyamine derivative 6d was also isolated (32% yield) as a crystalline derivative (Fig. 2).44 This boronated species was characterized by NMR spectroscopy, mass spectrometry, and single crystal X-ray crystallography. The 1H-NMR spectrum of compound 6d showed benzylic protons that appeared as diastereotopic signals, due to the chiral nitrogen atom at the benzylic position. X-Ray diffraction confirmed that compound 6d crystallized as a racemic compound (Fig. S84 in ESI†).
Next, we tested the compared reactivity of methoxyaminomethyl BODIPY derivatives 6a–d in the neoglycosylation reaction using D-glucose as the glycosyl donor.45 Thus, treatment of methoxyaminomethyl BODIPYs 6a–c, under the reaction conditions initially recommended by Peri et al. (DMF/AcOH, r.t.),32 resulted in the synthesis of glucosyl derivatives 10a, 10b, and 10c in 37%, 25% and 40% yields, respectively (Fig. 3). Similarly, neoglycosylation of N-cyanoboronated-N-methoxyamine 6d, with D-glucose yielded derivative 10c in a slightly lower yield (25%, Fig. 3).
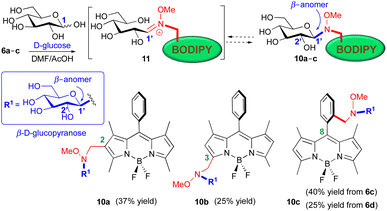 | ||
| Fig. 3 Stereoselective synthesis of BODIPY neoglucosides 10a–c, from the reaction of methoxyaminomethyl BODIPYs 6a–d with D-glucose (DMF/AcOH, r.t.). | ||
In agreement with literature precedents,32 the glycosylation of N,O-disubstituted secondary hydroxylamino BODIPYs (6) with D-glucose took place in a completely regio- and stereocontrolled manner, leading to the corresponding β-D-glucopyranosyl derivatives (10a–c), with the expected 1,2-trans stereoselectivity on the carbohydrate moiety (Fig. 3).
The β-configuration at the BODIPY-attached anomeric carbon was rigorously established for compounds 10a–c, on the basis of their observed J1′,2′ coupling constants in their 1H-NMR spectra. In the case of compound 10b, with no overlapping with other proton signals, the observed diagnostic coupling constant (4.16 ppm, J1′,2′ = 9.0 Hz) could be determined from its 1H-NMR spectrum. On the contrary, the stereochemical assignment of the C-1′ configuration in gluco-BODIPYs 10a and 10c had to be carried out in their corresponding per-O-acetyl derivatives, 10a-OAc and 10c-OAc (see ESI† for details), where the improved splitting of the proton signals allowed the unequivocal assignment of their anomeric protons.
The stereoselection of the process has been ascribed to a thermodynamic equilibrium between the open iminium intermediate, i.e., 11, and the closed ring isomer, i.e., 10, in its most stable form (Fig. 3).32,36
Having established that all three methoxyamino BODIPYs (6a–c) could be used as aglycons in the neoglycosylation reaction, we then set up to optimize the reaction conditions for glycosyl coupling using BODIPY 6a and D-glucose as partners. Compound 6a was selected owing to the easiness of its preparation and its observed improved reactivity toward D-glucose, under the assumption that the results obtained with this compound could be extended to isomeric BODIPYs 6b and 6c.
Accordingly, we examined a variety of reaction conditions for the transformation 6a → 10a (Table 1).36 In general, the different methods evaluated involved changes in the solvent system, temperature (T), and catalyst. Thus, the use of DMF/AcOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solvent mixtures led to modest yields of 10a, which could be slightly improved by increasing the reaction temperature (compare entries i and ii, r.t. vs. 40 °C, Table 1). The use of MeOH/CH2Cl2 (6
1) solvent mixtures led to modest yields of 10a, which could be slightly improved by increasing the reaction temperature (compare entries i and ii, r.t. vs. 40 °C, Table 1). The use of MeOH/CH2Cl2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a solvent system, in the absence of acid, produced a modest 25% yield of 10a (Table 1, entry iii). The use of a AcONa/AcOH buffer, as the reaction media, did not result in the formation of 10a (Table 1, entry iv). Better results were obtained when the neoglycosylation reaction was carried out in MeOH/AcOH (1
1) as a solvent system, in the absence of acid, produced a modest 25% yield of 10a (Table 1, entry iii). The use of a AcONa/AcOH buffer, as the reaction media, did not result in the formation of 10a (Table 1, entry iv). Better results were obtained when the neoglycosylation reaction was carried out in MeOH/AcOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solvent mixtures. Thus heating (60 °C) of the reaction for 48 h produced a 40% yield of 10a (Table 1, entry v). Interestingly, the use microwave irradiation (MW) allowed the reaction time to be reduced to 1 h, and the yield increased to 73% (Table 1, entry vi). Finally, best yields were obtained under microwave irradiation (MW, 60 °C, 1 h, 87% yield) in the presence of 5-methoxyanthranilic acid as nucleophilic catalyst, as suggested by Langenhan and coworkers (Table 1, entry vii).46
1) solvent mixtures. Thus heating (60 °C) of the reaction for 48 h produced a 40% yield of 10a (Table 1, entry v). Interestingly, the use microwave irradiation (MW) allowed the reaction time to be reduced to 1 h, and the yield increased to 73% (Table 1, entry vi). Finally, best yields were obtained under microwave irradiation (MW, 60 °C, 1 h, 87% yield) in the presence of 5-methoxyanthranilic acid as nucleophilic catalyst, as suggested by Langenhan and coworkers (Table 1, entry vii).46
| Entry | Solvent(s) | T (°C)/t | Catalyst | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 6a (1.0 mmol), D-glucose (3.0 mmol). b Isolated yields. | ||||
| i | DMF/AcOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
r.t./20 h | — | 37 |
| ii | DMF/AcOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
40 °C/20 h | — | 40 |
| iii | MeOH/CH2Cl2 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
60 °C/48 h | — | 25 |
| iv | AcONa/AcOH pH = 5 | r.t./24 h | — | — |
| v | MeOH/AcOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
60 °C/48 h | — | 40 |
| vi | MeOH/AcOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
60 °C/1 h (MW) | — | 73 |
| vii | MeOH/AcOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
60 °C/1 h (MW) | 5-Methoxy-anthranilic acid | 87 |
Likewise, the application of the optimized reaction conditions (MeOH/AcOH, 5-methoxyanthranilic acid cat., MW, 60 °C, 1 h) to methoxyamino BODIPYs 6b and 6c, allowed the preparation of glucoconjugates 10b and 10c in 76% and 84% yields, respectively (compare with yields displayed in Fig. 3).
To evaluate the scope of the method regarding the sugar donor, we studied the reaction of BODIPY 6a with a variety of commercially available unprotected reducing sugars, including: (i) cellobiose, lactose, and maltose disaccharides, which differ in the configuration of their interglycosidic bond (β- versus α-, e.g., cellobiose and lactose versus maltose); (ii) maltotriose as an example of a trisaccharide, and (iii) acarbose as a pseudotetrasaccharide. In each neoglycosylation reaction, the expected BODIPY-saccharides 12, 13, 14, 15, and 16, respectively, with β-anomeric configuration at the linking position, could be isolated (Fig. 4). The β-configuration at the BODIPY-attached anomeric carbon in compounds 15 and 16 was rigorously established from their corresponding per-O-acetyl derivatives, 15-OAc and 16-OAc (see ESI† for details) on the basis of their observed J1′,2′ coupling constants in their 1H-NMR spectra. On the other hand, the β-configuration at the BODIPY-attached anomeric carbon in compounds 12–14 was postulated in accordance with the literature precedents and the similarity with the related BODIPY glycosides prepared in this study, since only one isomer was isolated in each case.
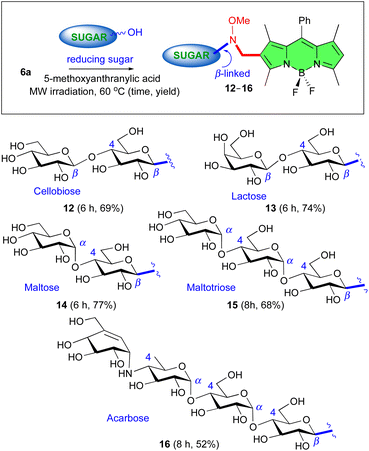 | ||
| Fig. 4 Screening of carbohydrate substrates: stereoselective synthesis of BODIPY saccharides 12–16 by reaction of unprotected reducing sugars with BODIPY 6a. | ||
Photophysical studies
The photophysical features of the novel BODIPY glycoconjugates were next studied. The attachment of progressively complex carbohydrate units at C-2 of the BODIPY core, facilitated by the methoxyaminomethyl spacer, enhanced the hydrophilicity of the dye significantly, ultimately rendering it ready soluble in water. Indeed, the intrinsic photophysical properties of representative BODIPY glycoconjugates 10a and 14–16 in diluted water solutions (Table 2) closely resembled those of their hydrophobic 8-phenyl BODIPY precursors (which lead to formyl dyes 8).32 Regardless of the number of carbohydrate units appended (ranging from one to four), these water-soluble dyes exhibited strong absorption and fluorescence bands in pure water (peaked at 502 nm and 513 nm, respectively, with molar extinction coefficients up to 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1 and 53% respectively, Table 2). Note that even at this low dye concentration, the parent 8-phenyl BODIPY was entirely insoluble and was prone to aggregate in aqueous solutions, resulting in the complete quenching of its fluorescent signal.47
000 M−1 cm−1 and 53% respectively, Table 2). Note that even at this low dye concentration, the parent 8-phenyl BODIPY was entirely insoluble and was prone to aggregate in aqueous solutions, resulting in the complete quenching of its fluorescent signal.47
| Dye | λ ab (nm) | ε max × 104 (M−1 cm−1) | λ fl (nm) | ϕ | τ (ns) |
|---|---|---|---|---|---|
| a Maximum absorption wavelength. b Maximum molar absorption. c Maximum fluorescence wavelength. d Fluorescence quantum yield. e Fluorescence lifetime. | |||||
| 10a | 502.5 | 2.7 | 513.5 | 0.52 | 3.80 |
| 14 | 502.5 | 2.8 | 513.5 | 0.53 | 3.60 |
| 15 | 502.5 | 4.2 | 513.5 | 0.49 | 3.70 |
| 16 | 502.5 | 5.0 | 513.5 | 0.50 | 3.70 |
To ensure the efficiency of these dyes as fluorescent bioprobes in physiological media, it is crucial to maintain their solubility and fluorescence response at higher concentrations. Thus, to assess the solubility and fluorescence performance of the BODIPY glycoconjugates at elevated concentrations in water, we studied the impact of dye concentration on their photophysical properties.
The incorporation of D-glucose or D-maltose to the BODIPY core, as in 10a or 14, respectively, facilitated the attainment of homogeneous water solutions up to 0.1 mM (Fig. 5). This water solubility was significantly enhanced by increasing the number of appended carbohydrate units, enabling the attainment of aqueous solution up to 1.9 mM in 15 with three sugar units (D-maltotriose), and even 2.4 mM grafting four sugar units (acarbose) in 16 (Fig. 5 and Fig. S85 in ESI†). However, at the highest concentration, the absorption profile of 15 and 16 became broader with a notable increase in the absorbance at shorter wavelengths (Fig. 5 and Fig. S85 in ESI†). According to the exciton model,48 this spectral trend is indicative of H-type aggregation. Such aggregation should be a consequence of a weak exciton coupling, since its spectroscopic contribution arises as a shoulder of the main absorption band, even at the highest optical density (dye concentration around 2 mM for BODIPY conjugates 15 and 16) herein tested in pure water. H-Aggregates are usually not fluorescent, as evidenced by both, a drastic decrease of the fluorescence efficiency (Fig. S87 in ESI†) and the absence of new emission bands in the fluorescence spectrum (Fig. 5). It is noteworthy that the observed increase of the long-wavelength shoulder in the fluorescence profile at this concentration was likely attributed to the reabsorption/reemission phenomena, which were not fully corrected solely by reducing the optical pathway in such highly concentrated media.49
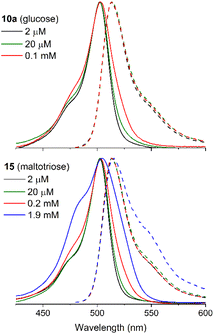 | ||
| Fig. 5 Normalized absorption (solid line) and fluorescence (dashed line) spectra of BODIPY glycoconjugates 10a and 15 bearing one and three carbohydrate units, respectively, as a function of the dye concentration in water using optically matched solutions (see ESI† for details). The spectra of BODIPY conjugates 14 and 16 are collected in Fig. S85 in ESI.† The recorded absorption spectra scaled by the molar absorption coefficient are collected in Fig. S86 in ESI.† | ||
To quantify the impact of the aggregation and/or reabsorption/reemission phenomena on the fluorescence response, the absolute fluorescence quantum yields were estimated as a function of dye concentration (Fig. S87 in ESI†). As expected, an increase in dye concentration led to a decrease in fluorescence efficiency due to reabsorption and reemission effects (the optical path length was maintained at 1 mm for all dye concentrations) and the promotion of H-aggregates. Remarkably, all the BODIPY glycoconjugates retained a substantial fluorescence response (higher than 20%) at 0.1–0.2 mM concentrations, which are typical for the biological assays. Beyond this dye concentration, the fluorescence efficiency sharply decreased due to an increase in reabsorption/reemission and aggregation probabilities, which were enhanced at high optical densities, as shown in Fig. 5. Despite these adverse conditions, dyes 15 and 16 (bearing maltotriose and acarbose residues, respectively) still were able to retain a measurable fluorescence signal. Furthermore, their molar absorption coefficients were the highest ones recorded (almost double than those recorded for 10a and 14, bearing glucose and maltose respectively, Table 2). Consequently, an enhancement in the hydrophilicity of the BODIPY derivative is also evident in a more efficient harvesting of incoming excitation light, likely because the dye is better solvated and stabilized in the aqueous environment. These photophysical properties are expected to enhance the potential of BODIPYs 15 and 16 as bioprobes. In particular, the good solubility in pure water, without any hint of aggregation, and the bright fluorescence signal up to 0.1 mM, a concentration high enough for bioimaging essays in the aqueous cellular media, support this notion.
Toxicity on mammalian cells (healthy and tumor)
To analyze the toxicity of the different compounds on healthy and tumor cells, we conducted a toxicity test using healthy human breast epithelial cells (HMEpiC) and human breast adenocarcinoma epithelial cells (MCF-7, ECACC) (Fig. 6A and B, respectively). We observed that the toxicity of BODIPY-saccharides increased with the number of sugar units in both healthy and tumor cells. Regarding cytotoxic derivatives 15 and 16, the former (maltotriose–BODIPY conjugate) displayed higher cytotoxicity against tumor cells (MCF-7) than against healthy human breast epithelial cells (HMEpiC) (Table 3). On the contrary, BODIPY–acarbose glycoconjugate (16), showed a different behavior, displaying similar LC50 against HMEpiC and MCF-7 cells (Fig. 7 and Table 3).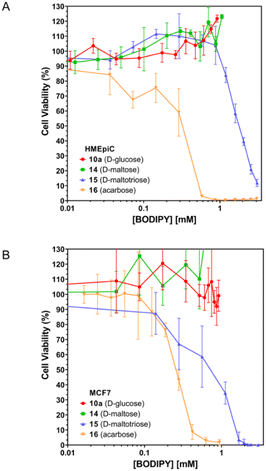 | ||
| Fig. 6 Toxicity assays of BODIPY conjugates 10a (D-glucose), 14 (D-maltose), 15 (D-maltotriose), and 16 (acarbose), on HMEpiC (A) and MCF-7 (B) cells. | ||
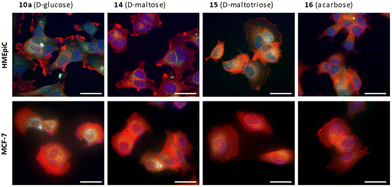 | ||
| Fig. 7 Epifluorescence imaging of HMEpiC and MCF-7 cells showing BODIPYs internalization in green. Actin is marked in red and the nucleus in blue. Scale bar: 50 μm. | ||
| LC50 (mM) | 15 (D-maltotriose) | 16 (acarbose) |
|---|---|---|
| HMEpiC | 1.8 | 0.32 |
| MCF7 | 0.75 | 0.24 |
Cancer cells exhibit heightened glucose absorption and rely on an aerobic glycolytic pathway to fulfill their metabolic requirements for growth and proliferation. Targeting the inhibition of aerobic glycolysis presents a strategic therapeutic avenue to impede cancer cell progression.40 In this sense, the use of acarbose or maltotriose could inhibit glucose uptake and promote glucose deprivation. In this way, both cell types showed the capacity to uptake the different BODIPYs derivatives (Fig. 7), which accumulated around the nucleus of the cell.
Cell internalization of BODIPY–acarbose conjugate 16
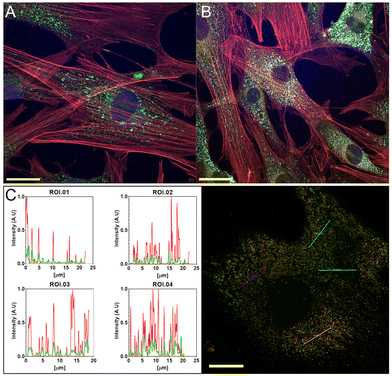
To evaluate cell internalization, BODIPY–acarbose conjugate 16 was selected due to its capacity to produce glucose deprivation.40 We conducted several staining experiments. Lysosomes and mitochondria stains were performed together with actin and nucleus (Fig. 8, and Fig. S88–S90† for full-size pictures).According to Fig. 8A, acarbose–BODIPY 16 is located near the nucleus but outside the mitochondria. The internalization process for 16 appears to be through the lysosomes as can be observed in Fig. 8B and C, and no accumulation was observed in the mitochondria. Lightning confocal microscopy (Fig. 8C) provides images to study spatiotemporal localization. The results showed a colocalization of the BODIPYs with the lysosomes and corroborate the capacity of cells to internalize acarbose–BODIPY 16.
Enzymatic studies
To validate the neoglycosylation tagging protocol from an enzymatic perspective, we decided to study how the newly incorporated BODIPY appendage could affect the binding affinity of a well-known amylase inhibitor, such as acarbose.50 The latter is a pseudo-tetrasaccharide composed of an acarvosine unit, responsible for the inhibitory activity, which has been derivatized with D-maltose at the reducing end. With this purpose in mind, we tackled enzymatic inhibition studies of two different α-amylases: A. oryzae α-amylase (AOA) and human salivary α-amylase (HSA). The obtained IC50 values (Table 4) are in accordance with reported data,51 confirm a dose-dependent competitive inhibition mode and reveal that the incorporation of the methoxyamino-BODIPY aglycon to acarbose, not only does not interfere with the inhibitory activity for AOA, but improves by two-fold the binding affinity for HSA. Overall, these results indicate that the neoglycosylation of selective enzymatic ligands with BODIPY dyes could be a convenient way to turn them into chromogenic probes with biological applications without affecting their inhibitory potency.| IC50 (mM) | Acarbose | BODIPY–acarbose (16) |
|---|---|---|
| AOA | 0.351 | 0.361 |
| HSA | 0.023 | 0.011 |
Conclusions
The neoglycosylation protocol used for the conjugation of BODIPYs to unprotected carbohydrate derivatives is a versatile and highly selective coupling method, which occurs under mild reaction conditions. The method can be applied to a variety of carbohydrate derivatives, including those with complex structures. The attachment of a sufficient number of saccharide units to the BODIPY core led to completely water-soluble dyes, retaining a high fluorescence signal even at high concentrations and hence suitable for use as fluorescent probes in physiological media. The biological studies of the BODIPY-neoglycosides showed excellent biocompatibilities and no cytotoxicity up to 0.1 mM of the probes. The cell's ability to uptake various BODIPY derivatives within a non-toxic range could have applications in various biological fields including bio-imaging. Specifically, the fluorescent acarbose–BODIPY conjugate 16 demonstrated the capability to be taken up by cells and localized within lysosomes, as evidenced by lighting confocal microscopy. This derivative also showed a binding affinity for α-amylases that is comparable to or better than that of acarbose alone, according to enzymatic activity studies. Accordingly, the uptake capacity combined with the PS features of BODIPY conjugates show promise in future biological applications in photodynamic and photothermal therapies.Author contributions
A. M. G.: conceptualization, project administration, funding acquisition, writing – original draft, writing – review & editing. L. G. F.: investigation and writing – original draft. A. G. S.: investigation. C. U.: investigation and writing – original draft. L. G.-R.: investigation. J. B.: funding acquisition, supervising and writing. I. G.-M.: funding acquisition, supervising and writing. L. I.: investigation and funding acquisition. M. R. A.: investigation and funding acquisition. J. C. L.: conceptualization, funding acquisition, writing – original draft, writing – review & editing. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research received financial support from the Spanish Ministerio de Ciencia e Innovación (MCIN)/Agencia Estatal de Investigación (AEI) Grants: PID2020-114755GB-C31 and -C33, and PID2021-122504NB-I00 funded by MCIN/AEI/10.13039/501100011033 and by ERDF A way of making Europe. LGR and JB acknowledge Gobierno Vasco for financial support (IT1639-22). MRA and LGF acknowledge financial support from MCIN (PID2020-114086RB-I00) and Comunidad de Madrid (P2022/BMD-7406). MRA and LGF are members of the SusPlast platform from Consejo Superior de Investigaciones Científicas (CSIC). This research work was performed in the framework of the Nanomedicine CSIC HUB (ref 202180E048). The authors thank Ms. Rosa Ana Ramírez (ICTP-CSIC) for assistance in the biological experiments. We are also indebted to Ms. Marina Rodríguez and Mr Diego Raúl Pozas (IQOG-CSIC) for skilful technical support.References
- (a) A. Varki, Biological roles of oligosaccharides: all of the theories are correct, Glycobiology, 1993, 3, 97–130 CrossRef CAS PubMed; (b) A. Varki, R. Cummings, J. Esko, H. Freeze, G. Hart and J. Marth, Essentials of Glycobiology, Cold Spring Harbor Laboratory Press, New York, 2nd edn, 2009 Search PubMed; (c) A. Varki, Biological Roles of Glycans, Glycobiology, 2017, 27, 3–49 CrossRef CAS PubMed.
- K. Villadsen, M. C. Martos-Maldonado, K. J. Jensen and M. B. Thygesen, Chemoselective Reactions for the Synthesis of Glycoconjugates from Unprotected Carbohydrates, ChemBioChem, 2017, 18, 574–612 CrossRef CAS PubMed.
- J. Ramos-Soriano, M. Ghirardello and M. C. Galan, Carbon-based glyco-nanoplatforms: towards the next generation of glycan-based multivalent probes, Chem. Soc. Rev., 2022, 51, 9960–9985 RSC.
- J. E. Hudak and C. R. Bertozzi, Glycotherapy: New Advances Inspire a Reemergence of Glycans in Medicine, Chem. Biol. Rev., 2014, 21, 16–37 CrossRef CAS PubMed.
- S. Leusmann, P. Menova, E. Shanin, A. Titz and C. Rademacher, Glycomimetics for the inhibition and modulation of lectins, Chem. Soc. Rev., 2023, 52, 3663–3740 RSC.
- C. R. Bertozzi and L. L. Kiessling, Chemical Biology, Science, 2001, 291, 2357–2364 CrossRef CAS PubMed.
- (a) X.-P. He, Y. Zang, T. D. James, J. Li, G.-R. Chen and J. Xie, Fluorescent glycoprobes: a sweet addition for improved sensing, Chem. Commun., 2017, 53, 82–90 RSC; (b) B. Thomas, K.-C. Yan, X.-L. Hu, M. Donnier-Marechal, G.-R. Chen, X.-P. He and S. Vidal, Fluorescent glycoconjugates and their applications, Chem. Soc. Rev., 2020, 49, 593–641 RSC; (c) X.-P. He and H. Tian, Lightening Up Membrane Receptors with Fluorescent Molecular Probes and Supramolecular Materials, Chem, 2018, 4, 246–268 CrossRef CAS; (d) M. Singh, M. Watkinson, E. M. Scanlan and G. J. Miller, Illuminating glycoscience: synthetic strategies for FRET-enabled carbohydrate active enzyme probes, RSC Chem. Biol., 2020, 1, 352–368 RSC.
- Y. Chen, A. Star and S. Vidal, Sweet carbon nanostructures: carbohydrate conjugates with carbon nanotubes and graphene and their applications, Chem. Soc. Rev., 2013, 42, 4532–4542 RSC.
- (a) A. Loudet and K. Burgess, BODIPY Dyes and Their Derivatives: Syntheses and Spectroscopic Properties, Chem. Rev., 2007, 107, 4891–4932 CrossRef CAS PubMed; (b) R. Ziessel, G. Ulrich and A. Harriman, The chemistry of Bodipy: A new El Dorado for fluorescence tools, New J. Chem., 2007, 31, 496–501 RSC; (c) G. Ulrich, R. Ziessel and A. Harriman, The chemistry of fluorescent bodipy dyes: versatility unsurpassed, Angew. Chem., Int. Ed., 2008, 47, 1184–1201 CrossRef CAS PubMed.
- D. Kanyan, M. Horacek-Glading, M. J. Wildervanck, T. Söhnel, D. C. Ware and P. J. Brothers, O-BODIPYs as fluorescent labels for sugars: glucose, xylose and ribose, Org. Chem. Front., 2022, 9, 720–730 RSC.
- (a) N. Boens, B. Verbelen, M. J. Ortiz, L. Jiao and W. Dehaen, Synthesis of BODIPY dyes through postfunctionalization of the boron dipyrromethene core, Coord. Chem. Rev., 2019, 399, 213024 CrossRef CAS; (b) N. Boens, B. Verbelen and W. Dehaen, Postfunctionalization of the BODIPY Core: Synthesis and Spectroscopy, Eur. J. Org. Chem., 2015, 6577–6595 CrossRef CAS.
- Y. Ni and J. Wu, Far-red and near infrared BODIPY dyes: synthesis and applications for fluorescent pH probes and bio-imaging, Org. Biomol. Chem., 2014, 12, 3774–3791 RSC.
- Y. Fan, J. Zhang, Z. Hong, H. Qiu, Y. Li and S. Yin, Architectures and Applications of BODIPY-Based Conjugated Polymers, Polymers, 2021, 13, 75 CrossRef CAS PubMed.
- (a) S. Das, S. Dey, S. Patra, A. Bera, T. Ghosh, B. Prasad, K. D. Sayala, K. Maji, A. Bedi and S. Debnath, BODIPY-Based Molecules for Biomedical Applications, Biomolecules, 2023, 13, 1723 CrossRef CAS PubMed; (b) J. Wang, Q. Gong, L. Jiao and E. Hao, Research advances in BODIPY-assembled supramolecular photosensitizers for photodynamic therapy, Coord. Chem. Rev., 2023, 496, 215367 CrossRef CAS.
- (a) J. Zou, P. Wang, Y. Wang, G. Liu, Y. Zhang, Q. Zhang, J. Shao, W. Si, W. Huang and X. Dong, Penetration depth tunable BODIPY derivatives for pH triggered enhanced photothermal/photodynamic synergistic therapy, Chem. Sci., 2019, 10, 268–276 RSC; (b) L. Schneider, M. Kalt, S. Koch, S. Sithamparanathan, V. Villiger, J. Mattiat, F. Kradolfer, E. Slyshkina, S. Luber, M. Bonmarin, C. Maake and B. Spingler, BODIPY-Based Photothermal Agents with Excellent Phototoxic Indices for Cancer Treatment, J. Am. Chem. Soc., 2023, 145, 4534–4544 CrossRef CAS PubMed; (c) Z. Kang, W. Bu, X. Guo, L. Wang, Q. Wu, J. Cao, H. Wang, G. Yu, J. Gao, E. Hao and L. Jiao, Synthesis and Properties of Bright Red-to-NIR BODIPY Dyes for Targeting Fluorescence Imaging and Near-Infrared Photothermal Conversion, Inorg. Chem., 2024, 63, 3402–3410 CrossRef CAS PubMed; (d) S. Lee, S. Min, G. Kim and S. Lee, Recent advances in the design of organic photothermal agents for cancer treatment: A review, Coord. Chem. Rev., 2024, 506, 215719 CrossRef CAS.
- H. Nakamura, S. Sasabe, K. Abe, K. R. Kumada, T. Sugiyama, J. Hanayama and M. Kido, Highly efficient and stable green fluorescent OLEDs with high color purity using a BODIPY derivative, Mol. Syst. Des. Eng., 2023, 8, 866–873 RSC.
- D. Ho, R. Ozdemir, H. Kim, T. Earmme, H. Usta and C. Kim, BODIPY-Based Semiconducting Materials for Organic Bulk Heterojunction Photovoltaics and Thin-Film Transistors, ChemPlusChem, 2019, 84, 18–37 CrossRef CAS PubMed.
- S. Koleman and E. U. Akkaya, Reaction-based BODIPY probes for selective bio-imaging, Coord. Chem. Rev., 2018, 354, 121–134 CrossRef.
- T. Kowada, H. Maeda and K. Kikuchi, BODIPY-based probes for the fluorescence imaging of biomolecules in living cells, Chem. Soc. Rev., 2015, 44, 4953–4972 RSC.
- (a) A. Barattucci, S. Campagna, T. Papalia, M. Galletta, A. Santoro, F. Puntoriero and P. Bonaccorsi, BODIPY on Board of Sugars: A Short Enlightened Journey up to the Cells, ChemPhotoChem, 2020, 4, 647–658 CrossRef CAS; (b) A. Barattucci, C. M. A. Gangemi, A. Santoro, S. Campagna, F. Puntoriero and P. Bonaccorsi, Bodipy-carbohydrate systems: synthesis and bioapplications, Org. Biomol. Chem., 2022, 20, 2742–2763 RSC.
- A. M. Gomez, C. Uriel, A. Oliden-Sanchez, J. Bañuelos, I. Garcia-Moreno and J. C. López, A Concise Route to Water-Soluble 2,6-Disubstituted BODIPY-Carbohydrate Fluorophores by Direct Ferrier-Type C-Glycosylation, J. Org. Chem., 2021, 86, 9181–9188 CrossRef CAS PubMed.
- B. Kang, T. Opatz, K. Landfester and F. R. Wurm, Carbohydrate nanocarriers in biomedical applications: functionalization and construction, Chem. Soc. Rev., 2015, 44, 8301–8325 RSC.
- (a) L. Shi, C. Yan, Y. Ma, T. Wang, Z. Guo and W.-H. Zhu, In vivo ratiometric tracking of endogenous β-galactosidase activity using an activatable near-infrared fluorescent probe, Chem. Commun., 2019, 55, 12308–12311 RSC; (b) N. E. M. Kaufman, Q. Meng, K. E. Griffin, S. S. Singh, A. Dahal, Z. Zhou, F. R. Fronczek, J. M. Mathis, S. D. Jois and M. G. H. Vicente, Synthesis, Characterization, and Evaluation of Near-IR Boron Dipyrromethene Bioconjugates for Labeling of Adenocarcinomas by Selectively Targeting the Epidermal Growth Factor Receptor, J. Med. Chem., 2019, 62, 3323–3335 CrossRef CAS PubMed; (c) W. Feng, S. Zhang, Y. Wan, Z. Chen, Y. Qu, J. Li, T. D. James, Z. Pei and Y. Pei, Nanococktail Based on Supramolecular Glyco-Assembly for Eradicating Tumors In Vivo, ACS Appl. Mater. Interfaces, 2022, 14, 20749–20761 CrossRef CAS PubMed; (d) G. Duran-Sampedro, E. Y. Xue, M. Moreno-Simoni, C. Paramio, T. Torres, D. K. P. Ng and G. de la Torre, Glycosylated BODIPY- Incorporated Pt(II) Metallacycles for Targeted and Synergistic Chemo-Photodynamic Therapy, J. Med. Chem., 2023, 66, 3448–3459 CAS.
- T. Papalia, G. Siracusano, I. Colao, A. Barattucci, M. C. Aversa, S. Serroni, G. Zappala, S. Campagna, M. T. Sciortino, F. Puntoriero and P. Bonaccorsi, Cell internalization of BODIPY-based fluorescent dyes bearing carbohydrate residues, Dyes Pigm., 2014, 110, 67–71 CrossRef CAS.
- (a) L. Dong, Y. Zang, D. Zhou, X.-P. He, G.-R. Chen, T. D. James and J. Li, Glycosylation enhances the aqueous sensitivity and lowers the cytotoxicity of a naphthalimide zinc ion fluorescence probe, Chem. Commun., 2015, 51, 11852–11855 RSC; (b) F. Liu, P. Tang, R. Ding, L. Liao, L. Wang, M. Wang and J. Wang, A glycosylation strategy to develop a low toxic naphthalimide fluorescent probe for the detection of Fe3+ in aqueous medium, Dalton Trans., 2017, 46, 7515–7522 RSC.
- (a) B. Liu, N. Novikova, M. C. Simpson, M. S. M. Timmer, B. L. Stocker, T. Söhnel, D. C. Ware and P. J. Brothers, Lighting up sugars: fluorescent BODIPY–glucofuranose and –septanose conjugates linked by direct B–O–C bonds, Org. Biomol. Chem., 2016, 14, 5205–5209 RSC; (b) L. J. Patalag, S. Ahadi, O. Lashchuk, P. G. Jones, S. Ebbinghaus and D. B. Werz, GlycoBODIPYs: Sugars Serving as a Natural Stock for Water-soluble Fluorescent Probes of Complex Chiral Morphology, Angew. Chem., Int. Ed., 2021, 60, 8766–8771 CrossRef CAS PubMed; (c) C. Sollert, D. Kocsi, R. T. Jane, A. Orthaber and K. E. Borbas, C-glycosylated pyrroles and their application in dipyrromethane and porphyrin synthesis, J. Porphyrins Phthalocyanines, 2021, 25, 741–755 CrossRef CAS; (d) C. Uriel, D. Grenier, F. Herranz, N. Casado, J. Bañuelos, E. Rebollar, I. Garcia-Moreno, A. M. Gomez and J. C. Lopez, De Novo Access to BODIPY C-Glycosides as Linker-Free Nonsymmetrical BODIPY-Carbohydrate Conjugates, J. Org. Chem., 2024, 89, 4042–4065 CrossRef CAS PubMed.
- M. Meldal and C. W. Tornoe, Cu-catalyzed azide-alkyne cycloaddition, Chem. Rev., 2008, 108, 2952–3015 CrossRef CAS PubMed.
- M. R. Martinez-Gonzalez, A. Urias-Benavides, E. Alvarado-Martínez, J. C. Lopez, A. M. Gomez, M. del Rio, I. Garcia, A. Costela, J. Bañuelos, T. Arbeloa, I. Lopez Arbeloa and E. Peña-Cabrera, Convenient Access to Carbohydrate–BODIPY Hybrids by Two Complementary Methods Involving One-Pot Assembly of “Clickable” BODIPY Dyes, Eur. J. Org. Chem., 2014, 5659–5663 CrossRef CAS.
- A. M. Gomez and J. C. Lopez, Bringing Color to Sugars: The Chemical Assembly of Carbohydrates to BODIPY Dyes, Chem. Rec., 2021, 21, 3112–3130 CrossRef CAS PubMed.
- X. Zhu and R. R. Schmidt, New Principles for Glycoside-Bond Formation, Angew. Chem., Int. Ed., 2009, 48, 1900–1934 CrossRef CAS PubMed.
- (a) M. del Rio, F. Lobo, J. C. Lopez, A. Oliden, J. Bañuelos, I. Lopez-Arbeloa, I. Garcia-Moreno and A. M. Gomez, One-Pot Synthesis of Rotationally Restricted, Conjugatable, BODIPY Derivatives from Phthalides, J. Org. Chem., 2017, 82, 1240–1247 CrossRef CAS PubMed; (b) C. Uriel, C. Permingeat, J. Ventura, E. Avellanal-Zaballa, J. Bañuelos, I. Garcia-Moreno, A. M. Gomez and J. C. Lopez, BODIPYs as Chemically Stable Fluorescent Tags for Synthetic Glycosylation Strategies towards Fluorescently Labeled Saccharides, Chem. – Eur. J., 2020, 26, 5388–5399 CrossRef CAS PubMed; (c) J. Ventura, C. Uriel, A. M. Gomez, E. Avellanal-Zaballa, J. Bañuelos, I. García-Moreno and J. C. Lopez, A Concise Synthesis of a BODIPY-Labeled Tetrasaccharide Related to the Antitumor PI-88, Molecules, 2021, 26, 2909 CrossRef CAS PubMed.
- F. Peri, P. Dumy and M. Mutter, Chemo- and Stereoselective Glycosylation of Hydroxylamino Derivatives: A Versatile Approach to Glycoconjugates, Tetrahedron, 1998, 54, 12269–12278 CrossRef CAS.
- F. Peri, A. Deutman, B. La Ferla and F. Nicotra, Solution and solid-phase chemoselective synthesis of (1–6)-amino(methoxy) di- and trisaccharide analogues, Chem. Commun., 2002, 1504–1505 RSC.
- F. Peri, J. Jimenez-Barbero, V. Garcia-Aparicio, I. Tvaroska and F. Nicotra, Synthesis and Conformational Analysis of Novel N(OCH3)-linked Disaccharide Analogues, Chem. – Eur. J., 2004, 10, 1433–1444 CrossRef CAS PubMed.
- J. M. Langenhan, N. R. Peters, I. A. Guzei, F. M. Hoffmann and J. S. Thorson, Enhancing the anticancer properties of cardiac glycosides by neoglycorandomization, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 12305–12310 CrossRef CAS PubMed.
- R. D. Goff and J. S. Thorson, Neoglycosylation and neoglycorandomization: enabling tools for the discovery of novel glycosylated bioactive probes and early stage leads, MedChemComm, 2014, 5, 1036–1047 RSC.
- S. Romero-Garcia, J. S. Lopez-Gonzalez, J. L. Baez-Viveros, D. Aguilar-Cazares and H. Prado-Garcia, Tumor cell metabolism, Cancer Biol. Ther., 2011, 12, 939–948 CrossRef CAS PubMed.
- A. Gatenby and R. J. Gillies, Glycolysis in cancer: A potential target for therapy, Int. J. Biochem. Cell Biol., 2007, 39, 7–8 CrossRef PubMed.
- R. M. Orlandella, W. J. Turbitt, J. T. Gibson, S. K. Boi, P. Li, D. L. Smith Jr. and L. A. Norian, The Antidiabetic Agent Acarbose Improves Anti-PD-1 and Rapamycin Efficacy in Preclinical Renal Cancer, Cancers, 2020, 12, 2872 CrossRef CAS PubMed.
- Q. A. Obaid, A. M. Al-Shammari and K. K. Khudair, Glucose Deprivation Induced by Acarbose and Oncolytic Newcastle Disease Virus Promote Metabolic Oxidative Stress and Cell Death in a Breast Cancer Model, Front. Mol. Biosci., 2022, 9, 816510 CrossRef CAS PubMed.
- L. Jiao, C. Yu, J. Li, Z. Wang, M. Wu and E. Hao, β-Formyl-BODIPYs from the Vilsmeier-Haack Reaction, J. Org. Chem., 2009, 74, 7525–7528 CrossRef CAS PubMed.
- A. Ramos-Torres, E. Avellanal-Zaballa, A. Prieto-Castañeda, F. García-Garrido, J. Bañuelos, A. R. Agarrabeitia and M. J. Ortiz, FormylBODIPYs by PCC-Promoted Selective Oxidation of α-MethylBODIPYs. Synthetic Versatility and Applications, Org. Lett., 2019, 21, 4563–4566 CrossRef CAS PubMed.
- H. Ilhan and Y. Cakmak, Functionalization of BODIPY dyes with additional C-N Double Bonds and their applications, Top. Curr. Chem., 2023, 381, 28 CrossRef CAS PubMed.
- J. M. Márquez, E. Martínez-Castro, S. Gabrielli, O. López, I. Maya, M. Angulo, E. Álvarez and J. G. Fernández-Bolaños, Alkoxyamine-cyanoborane adducts: efficient cyanoborane transfer agents, Chem. Commun., 2011, 47, 5617–5619 RSC.
- S. van der Vorm, T. Hansen, J. M. A. van Hengst, H. S. Overkleeft, G. A. van der Marel and J. D. C. Codee, Acceptor reactivity in glycosylation reactions, Chem. Soc. Rev., 2019, 48, 4688–4706 RSC.
- S. A. Loskot, J. Zhang and J. M. Langenhan, Nucleophilic Catalysis of MeON-Neoglycoside Formation by Aniline Derivatives, J. Org. Chem., 2013, 78, 12189–12193 CrossRef CAS PubMed.
- A. B. Descalzo, P. Ashokkumar, Z. Shen and K. Rurack, On the aggregation behaviour and spectroscopic properties of alkylated and annelated Boron Dipyrromethene (BODIPY) dyes in aqueous solution, ChemPhotoChem, 2020, 4, 120–131 CrossRef CAS.
- S. C. J. Meskers, The exciton model for molecular materials; past, present and future?, ChemPhysChem, 2023, 24, e202300666 CrossRef CAS PubMed.
- H. B. Rodriguez, M. Mirenda, M. G. Lagorio and E. San Román, Photophysics at unusually high dye concentrations, Acc. Chem. Res., 2019, 52, 110–118 CrossRef CAS PubMed.
- (a) J.-L. Chiasson, R. G. Josse, R. Gomis, M. Hanefeld, A. Karasik and M. Laakso, Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM ransomised trial, Lancet, 2002, 359, 2072–2077 CrossRef CAS PubMed; (b) M. Qian, R. Haser, G. Buisson, E. Duée and F. Payan, The Active Center of a Mammalian α-Amylase. Structure of the Complex of a Pancreatic α-Amylase with a Carbohydrate Inhibitor Refined to 2.2-A Resolution, Biochemistry, 1994, 33, 6284–6294 CrossRef CAS PubMed.
- M. Yilmazer-Musa, A. M. Griffith, A. J. Michels, E. Schneider and B. Frei, Grape Seed and Tea Extracts and Catechin 3-Gallates Are Potent Inhibitors of α-Amylase and α-Glucosidase Activity, J. Agric. Food Chem., 2012, 60, 8924–8929 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2351271. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4qo00886c |
| This journal is © the Partner Organisations 2024 |