Open Access Article
Open Access ArticleConcise syntheses of (+)-maximumins B and C and (+)-ottensinin†
Qing-Xin
Li‡
ab,
Lin-Lin
Wang‡
cd,
Yue-Ming
Zheng
bc,
Zhao-Bing
Gao
 *bcd,
Jin-Xin
Zhao
*bcd,
Jin-Xin
Zhao
 *ab and
Jian-Min
Yue
*ab and
Jian-Min
Yue
 *abd
*abd
aEthnomedicine and Biofunctional Molecule Research Center, State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zuchongzhi Road, Shanghai 201203, China. E-mail: jxzhao@simm.ac.cn; jmyue@simm.ac.cn
bUniversity of Chinese Academy of Science, No. 19A Yuquan Road, Beijing 100049, China
cCenter for Neurological and Psychiatric Research and Drug Discovery, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 501 Haike Road, Shanghai 201203, China. E-mail: zbgao@simm.ac.cn
dSchool of Chinese Materia Medica, Nanjing University of Chinese Medicine, 138 Xianlin Road, Nanjing 210023, China
First published on 26th July 2024
Abstract
Maximumin B (1), maximumin C (2), and ottensinin (3) are three rearranged labdane diterpenoids featuring a unique 3-substituted γ-pyrone motif. Herein, we report the first syntheses of (+)-1 and (+)-2 and an improved synthesis of (+)-3 in 4–12 steps. Key features for the syntheses of 1 and 2 include a Pd-catalyzed decarboxylative coupling reaction establishing the 3-substituted γ-pyrone and a Baran decarboxylative coupling enabling ketone formation in the final step. A Ni-catalyzed C(sp2)–C(sp3) cross-electrophile coupling reaction was applied to accomplish the synthesis of 3. Furthermore, in-depth pharmacological screening unveiled ottensinin (3) as a potent KCNQ2 agonist exhibiting comparable potency to retigabine, making it a promising lead for the development of a novel class of anti-epileptic drugs.
1. Introduction
Rearranged labdane diterpenoids represent a rare class of natural products that are biogenetically derived via the skeletal rearrangement of intact labdane diterpenoid precursors, and often exhibit a diverse range of bioactivities,1–6 thereby drawing considerable interest from the synthetic community.7–9 Maximumins B (1) and C (2) are two rearranged labdane diterpenoids with novel carbon skeletons that were isolated from Amomum maximum by our group in 2019 (Fig. 1).10 A co-isolated known 16(13 → 12)-abeo-labdane diterpenoid ottensinin (3),11–14 sharing a common 3-substituted γ-pyrone motif, was considered to be their hypothetically biosynthetic precursor. Compared to 3, compounds 1 and 2 feature a distinctively modified B-ring. Of note, compounds 1–3 demonstrated significant inhibition against nuclear factor kappa B (NF-κB), a promising target in regulating immune and inflammatory dysfunction.15,16The unusual structural features and promising pharmacological properties of 1–3 attracted our attention. To overcome limited accessibility for further biological studies, chemical synthesis towards this diterpenoid class is highly desired.17 Previously, the research groups of Katoh,18,19 Yang,20 and Li21 successfully employed 3-lithio-γ-pyrone as the primary reactant for the syntheses of diterpenoids with a fully substituted γ-pyrone (Scheme 1A). However, there are very few methods22,23 available for the synthesis of 3-substituted γ-pyrone as in 1–3. In particular, Boukouvalas’ group reported the structure revision and first synthesis of 3 in 27% overall yield over 9 steps by using 6-endo-dig cyclization to construct the 3-substituted γ-pyrone (Scheme 1B),22 whereas the chemical syntheses of maximumins B (1) and C (2) have not been reported yet.
The Kv7/KCNQ family of voltage-gated K+ channels crucially regulates neuronal signaling and human disease states in the brain and peripheral nerves.24 Among the KCNQ subtypes, KCNQ2 is an important drug target for treating epilepsy and a range of other neurological disorders associated with neuronal hyper-excitability.25 Its significance has garnered immense interest, culminating in the approval by the FDA of retigabine (RTG),26 a KCNQ2-targeting medication approved for use as an adjunctive treatment for partial-onset seizures in adults with epilepsy. However, RTG had been withdrawn from the market in 2017 due to concerns regarding its poor stability and potential toxicity,27 prompting the search for new drugs targeted on KCNQ2. In the present study, a screening program to identify KCNQ2 activators led to the identification of ottensinin (3) as a potent agonist. As part of our continuing synthetic efforts towards bioactive natural products,28–30 we report herein the first syntheses of (+)-maximumins B and C, and a more concise synthesis of (+)-ottensinin, as well as their biological evaluation.
2. Results and discussion
2.1 Retrosynthetic analyses of maximumin B, maximumin C, and ottensinin
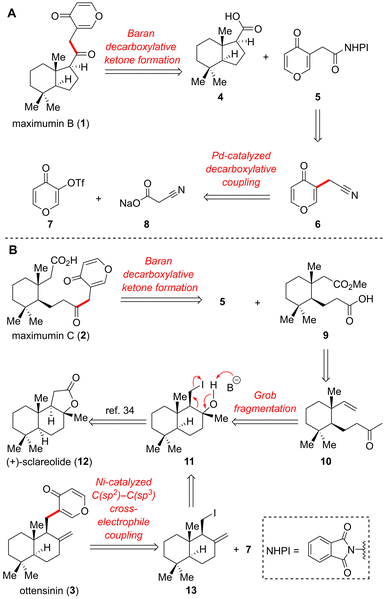
Our retrosynthetic analyses for 1–3 are outlined in Scheme 2. We envisioned that maximumin B (1) could be prepared by assembly of the known bicyclic carboxylic acid 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 and redox-active ester (RAE) 5via the Baran decarboxylative ketone formation (Scheme 2A).32 RAE 5 could be readily made from nitrile 6, which could be accessed through Pd-catalyzed decarboxylative coupling of triflate 7 with cyanoacetate salt 8 developed by the Liu group.33 Likewise, maximumin C (2) could be synthesized via the Baran decarboxylative ketone formation32 from RAE 5 and acid 9 followed by ester hydrolysis, and the latter could be obtained from methyl ketone 10via multistep functional group manipulation (Scheme 2B). Ketone 10 could be conveniently prepared from iodide 11via Grob fragmentation. Compound 11 could be obtained from commercially available (+)-sclareolide (12) through a known ring-opening process.34 For the preparation of ottensinin (3), we envisaged that it could be produced by the Ni-catalyzed C(sp2)–C(sp3) cross-electrophile coupling35 of iodide 13 with triflate 7 (Scheme 2B). The former could be synthesized from tertiary alcohol 11 by a dehydration process. Such a modular approach would not only improve overall synthetic efficiency, but also facilitate further structural optimization and structure–activity relationship (SAR) analysis.
31 and redox-active ester (RAE) 5via the Baran decarboxylative ketone formation (Scheme 2A).32 RAE 5 could be readily made from nitrile 6, which could be accessed through Pd-catalyzed decarboxylative coupling of triflate 7 with cyanoacetate salt 8 developed by the Liu group.33 Likewise, maximumin C (2) could be synthesized via the Baran decarboxylative ketone formation32 from RAE 5 and acid 9 followed by ester hydrolysis, and the latter could be obtained from methyl ketone 10via multistep functional group manipulation (Scheme 2B). Ketone 10 could be conveniently prepared from iodide 11via Grob fragmentation. Compound 11 could be obtained from commercially available (+)-sclareolide (12) through a known ring-opening process.34 For the preparation of ottensinin (3), we envisaged that it could be produced by the Ni-catalyzed C(sp2)–C(sp3) cross-electrophile coupling35 of iodide 13 with triflate 7 (Scheme 2B). The former could be synthesized from tertiary alcohol 11 by a dehydration process. Such a modular approach would not only improve overall synthetic efficiency, but also facilitate further structural optimization and structure–activity relationship (SAR) analysis.
2.2 Synthesis of (+)-maximumin B (1)
Our synthesis commenced with the preparation of two decarboxylative cross-coupling precursors, acid 4 and RAE 5 (Scheme 3). The former was readily prepared in 51% overall yield over 3 steps from the well-documented Wieland–Miescher ketone derivative 14 according to the known method reported by the Liang group.31 The synthesis of RAE 5 initially began with the Pd-catalyzed decarboxylative coupling33 of triflate 7 (obtained from 3-hydroxy-γ-pyrone 15 in 75% yield) with potassium cyanoacetate 8′. After carefully screening the reaction conditions, it was observed that employing sodium cyanoacetate 8 as the reactant instead led to an increase in the yield. Furthermore, 2-dicyclohexylphosphino-2′,6′-dimethoxybiphenyl (S-Phos) served as a superior ligand compared to 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene (Xant-Phos). Under the optimal reaction conditions, the desired nitrile 6 was obtained in 54% yield. Notably, it represents the first successful utilization of pyrone–triflate as a substrate in this Pd-catalyzed decarboxylative coupling reaction. In our attempt to hydrolyze nitrile 6, we unexpectedly found that various basic conditions (NaOH, LiOH, KOH) proved fruitless. This could potentially be attributed to the reactive α,β-unsaturated ketone moiety present in 6. Finally, we achieved the desired γ-pyrone-3-acetic acid 16 under acidic conditions in 77% yield. Interestingly, compound 16 is a natural product exhibiting selective and irreversible inhibition of interleukin-1β converting enzyme.36With the two acid fragments 4 and 16 securely in hand, we attempted to combine them together through the Baran decarboxylative ketone formation.32 Given the likelihood of inversion at the C-8 stereocenter in compound 4, we opted to transform acid 16 into RAE 5 by using N,N-diisopropylcarbodiimide (DIC) as the condensing agent. Unfortunately, RAE 5 decomposed spontaneously during separation by column chromatography. Thus, we decided to use a one-pot method to circumvent the challenging purification of 5. Pleasingly, the coupling proceeded smoothly under the standard conditions, delivering the target maximumin B (1) in 56% yield.
2.3 Synthesis of (+)-maximumin C (2)
Upon successful completion of the concise synthesis of (+)-maximumin B, we then moved forward to synthesize (+)-maximumin C (2) starting from commercial (+)-sclareolide (12), which was converted to iodide 11 based on a three-step sequence reported by the Baran group (Scheme 4).34 Subsequently, compound 11 was subjected to Grob fragmentation37,38 under the basic conditions to achieve the ring-opening product 10. To avoid Prins cyclization, the keto carbonyl group of 10 was strategically protected with (CH2OTMS)2. Then, hydroboration–oxidation of the terminal alkene resulted in the alcohol 17. Exposure of 17 to Jones reagent efficiently transformed the hydroxymethyl group into a carboxylic acid, concomitantly eliminating the ketal protecting group. The resulting acid was methylated with trimethylsilyl diazomethane (TMSCHN2) to afford ester 18. Since the ester group was incompatible with the harsh basic conditions of the haloform reaction, a two-step approach utilizing ozonolysis of the enol triflate intermediate was developed to transform methyl ketone 18 to acid 9. This mild alternative approach represents an improved variation of Heathcock's two-step method,39 employing a silyl enol ether as an intermediate and delivering a one-carbon less silyl ester instead. The following one-pot decarboxylative coupling32 of acid 9 with RAE 5 took place smoothly to yield ketone 19, which underwent acidic hydrolysis to readily achieve the desired maximumin C (2).2.4 Synthesis of (+)-ottensinin (3)
Henceforward, we began the synthesis of ottensinin (3) (Scheme 4). Initially, our attempts to synthesize ottensinin via Suzuki cross-coupling of borate ester with vinyl triflate were unsuccessful. Therefore, we opted to employ the Ni-catalyzed C(sp2)–C(sp3) cross-electrophile coupling35 to attach iodide 13 to vinyl triflate 7, and the former could be easily synthesized from the advanced intermediate 11 using the method previously reported by the Li group.40 To our delight, the coupling reaction resulted in the desired ottensinin (3) with good conversion.2.5 Biological evaluation
With adequate quantity of maximumin B (1), maximumin C (2), and ottensinin (3) in hand, we advanced to a broader spectrum of pharmacological screening studies. Epilepsy is a chronic neurological disorder arising from abnormal discharges of brain neurons.41 The KCNQ2 channel, a pivotal member of potassium voltage-gated channels, is closely related to neuronal excitatory disorders, serving as a significant target for anti-epileptic drugs.42 Compounds 1–3 and two synthetic intermediates 16 and 19 were evaluated for their activation effects on KCNQ2. It was found that ottensinin (3) possesses the ability to efficiently activate KCNQ2 (Idrug/Icontrol = 1.77 ± 0.07), which is even stronger than RTG (Idrug/Icontrol = 1.44 ± 0.13) (Table 1). The dose–effect relationship of ottensinin and RTG on KCNQ2 channel was further examined, and the median effective concentration (EC50) was determined by a dose–response curve analysis (Fig. 2). The results revealed that ottensinin could dose-dependently activated KCNQ2 channel, with an EC50 value of 6.30 ± 2.55 μM, which is comparable to retigabine (3.52 ± 1.27 μM). In order to reveal how ottensinin activates KCNQ2 channel activity, the effect of 10 μM ottensinin on channel dynamics was investigated. Activation curves were fitted with the Boltzmann equation and showed that the half-activation voltages of KCNQ2 before and after treatment with 10 μM ottensinin were −9.53 ± 1.91 mV and −12.26 ± 3.23 mV, respectively (Fig. 3A). Before and after treatment with 10 μM ottensin, the activation constant was 247.75 ± 35.03 and 237.56 ± 30.32, and the deactivation constant was 12.81 ± 30.32 and 14.92 ± 0.18, there were no significant change (Fig. 3B). Therefore, we suspect that ottensinin does not generate activity by changing KCNQ2 channel dynamics, and its specific mechanism of action is still under investigation.| Compound |
I
drug/Icontrol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
n |
|---|---|---|
| a The amplitude change of the outward current: Icontrol, amplitude of the outward current without the compound; Idrug, amplitude of the outward current with the compound; Idrug/Icontrol, effect of 10 μM compound on the amplitude of the outward current of KCNQ2 channel. b RTG (retigabine) was used as the positive control. | ||
| 1 | 1.01 ± 0.01 | 3 |
| 2 | 0.98 ± 0.03 | 3 |
| 3 | 1.77 ± 0.07 | 3 |
| 16 | 1.14 ± 0.09 | 3 |
| 19 | 1.03 ± 0.09 | 3 |
| RTGb | 1.44 ± 0.13 | 4 |
3. Conclusions
In summary, we have accomplished the first asymmetric total synthesis of maximumin B (1) from a well-documented Wieland–Miescher ketone derivative 14 and 3-hydroxy-γ-pyrone 15 in 17% yield over 4 steps. Moreover, starting from commercially available (+)-sclareolide, (+)-maximumin C (2) and (+)-ottensinin (3) have been effectively synthesized in 12 and 5 linear longest steps with overall yields of 6.6% and 30%, respectively. The prominent features of the present chemical syntheses include Baran decarboxylative ketone formation, Pd-catalyzed decarboxylative coupling, and Ni-catalyzed C(sp2)–C(sp3) cross-electrophile coupling. Of the same importance, our robust synthesis built the foundation for deeper biological studies. The in-depth pharmacological screening revealed ottensinin (3) to be a potent KCNQ2 agonist, thereby holding the potential to be further developed into a completely novel class of antiepileptic drug. Further structural optimization of ottensinin is currently underway in our laboratory, and related progress will be disclosed in due course.Data availability
Data for this article, including experimental procedures, 1H NMR spectra, 13C NMR spectra, physical states, high-resolution mass spectroscopy (HRMS) data of all products, specific rotations of new chiral compounds 9, 10, 17–19, S4–S6, the melting point range of compound 3, representative assay results of compounds 1–3, 16, 19, and effects of compound (3) on current of KCNQ2 channel at different concentrations are available in the ESI.†Crystallographic data for compound 3 has been deposited at the Cambridge Crystallographic Data Centre (deposition number: CCDC 2355511†).
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The financial support from the National Key Research and Development Program of China (No. 2023YFE0206100), the National Natural Science Foundation of China (No. 22237007 and T2192972), and the Youth Innovation Promotion Association of Chinese Academy of Sciences (No. 2022282) is gratefully acknowledged.References
- Q. Zhao, L.-G. Xiao, L.-S. Bi, Y. Si, X.-M. Zhang, J.-H. Chen and H.-Y. Liu, Hedychins E and F: Labdane-Type Norditerpenoids with Anti-Inflammatory Activity from the Rhizomes of Hedychium forrestii, Org. Lett., 2022, 24, 6936–6939 CrossRef CAS PubMed.
- C. Zhang, Y. Li, Z. Chu, S. Yuan, Y. Qiao, J. Zhang, L. Li, Y. Zhang, R. Tian, Y. Tang and H. Lou, Rearranged 19-nor-7,8-seco-labdane diterpenoids and Diels–Alder cycloadducts from the Chinese liverwort Pallavicinia ambigua: Structural elucidation, photoinduced rearrangement, and cytotoxic activity, Chin. Chem. Lett., 2024, 35, 108206 CrossRef CAS.
- M. Zhu, Y. Gao, Y. Li, F. Xie, J. Zhou, L. Xu, D. Lv, X. Zhang, Z. Xu, T. Dong, T. Shen, J. Zhang and H. Lou, Novel Diterpenoids Incorporating Rearranged Labdanes from the Chinese Liverwort Anastrophyllum joergensenii and Their Anti-inflammatory Activity, J. Agric. Food Chem., 2023, 71, 19551–19567 CrossRef CAS PubMed.
- J. Zhou, J. Zhang, R. Li, J. Liu, P. Fan, Y. Li, M. Ji, Y. Dong, H. Yuan and H. Lou, Hapmnioides A–C, Rearranged Labdane-Type Diterpenoids from the Chinese Liverwort Haplomitrium mnioides, Org. Lett., 2016, 18, 4274–4276 CrossRef CAS PubMed.
- X.-D. Wu, D. Luo, W.-C. Tu, Z.-T. Deng, X.-J. Chen, J. Su, X. Ji and Q.-S. Zhao, Hypophyllins A–D, Labdane-Type Diterpenoids with Vasorelaxant Activity from Hypoestes phyllostachya “Rosea”, Org. Lett., 2016, 18, 6484–6487 CrossRef CAS PubMed.
- H. Yin, J.-G. Luo, S.-M. Shan, X.-B. Wang, J. Luo, M.-H. Yang and L.-Y. Kong, Amomaxins A and B, Two Unprecedented Rearranged Labdane Norditerpenoids with a Nine-Membered Ring from Amomum maximum, Org. Lett., 2013, 15, 1572–1575 CrossRef CAS PubMed.
- L. M. T. Frija, R. F. M. Frade and C. A. M. Afonso, Isolation, Chemical, and Biotransformation Routes of Labdane-type Diterpenes, Chem. Rev., 2011, 111, 4418–4452 CrossRef CAS PubMed.
- P. S. Grant and M. A. Brimble, seco-Labdanes: A Study of Terpenoid Structural Diversity Resulting from Biosynthetic C–C Bond Cleavage, Chem. – Eur. J., 2021, 27, 6367–6389 CrossRef CAS PubMed.
- S. C. Philkhana and D. S. Reddy, Total synthesis of natural fregenedadiol and its diacetate, rearranged labdanes with aromatized B ring, Tetrahedron Lett., 2017, 58, 1262–1264 CrossRef CAS.
- K.-L. Ji, Y.-Y. Fan, Z.-P. Ge, L. Sheng, Y.-K. Xu, L.-S. Gan, J.-Y. Li and J.-M. Yue, Maximumins A–D, Rearranged Labdane-Type Diterpenoids with Four Different Carbon Skeletons from Amomum maximum, J. Org. Chem., 2019, 84, 282–288 CrossRef CAS PubMed.
- H. Yin, C. Guo and J.-M. Gao, Four new pyrrole alkaloids from the rhizomes of Amomum koenigii, J. Nat. Med., 2021, 75, 173–177 CrossRef CAS PubMed.
- K. Akiyama, H. Kikuzaki, T. Aoki, A. Okuda, N. H. Lajis and N. Nakatani, Terpenoids and a Diarylheptanoid from Zingiber ottensii, J. Nat. Prod., 2006, 69, 1637–1640 CrossRef CAS PubMed.
- J.-G. Luo, H. Yin, B.-Y. Fan and L.-Y. Kong, Labdane Diterpenoids from the Roots of Amomum maximum and Their Cytotoxic Evaluation, Helv. Chim. Acta, 2014, 97, 1140–1145 CrossRef CAS.
- Y. Sivasothy, H. Ibrahim, A. S. Paliany, S. A. Alias, N. R. Md Nor and K. Awang, A New Bis-Labdanic Diterpene from the Rhizomes of Alpinia pahangensis, Planta Med., 2013, 79, 1775–1780 CrossRef CAS PubMed.
- G.-Y. Kim, K.-H. Kim, S.-H. Lee, M.-S. Yoon, H.-J. Lee, D.-O. Moon, C.-M. Lee, S.-C. Ahn, Y. C. Park and Y.-M. Park, Curcumin Inhibits Immunostimulatory Function of Dendritic Cells: MAPKs and Translocation of NF-κB as Potential Targets1, J. Immunol., 2005, 174, 8116–8124 CrossRef CAS PubMed.
- E. Pikarsky, R. M. Porat, I. Stein, R. Abramovitch, S. Amit, S. Kasem, E. Gutkovich-Pyest, S. Urieli-Shoval, E. Galun and Y. Ben-Neriah, NF-κB functions as a tumour promoter in inflammation-associated cancer, Nature, 2004, 431, 461–466 CrossRef CAS PubMed.
- J.-X. Zhao and J.-M. Yue, Frontier studies on natural products: moving toward paradigm shifts, Sci. China: Chem., 2023, 66, 928–942 CrossRef CAS.
- K. Watanabe, K. Iwasaki, T. Abe, M. Inoue, K. Ohkubo, T. Suzuki and T. Katoh, Enantioselective Total Synthesis of (−)-Candelalide A, a Novel Blocker of the Voltage-Gated Potassium Channel Kv1.3 for an Immunosuppressive Agent, Org. Lett., 2005, 7, 3745–3748 CrossRef CAS PubMed.
- T. Abe, K. Iwasaki, M. Inoue, T. Suzuki, K. Watanabe and T. Katoh, Convergent and enantioselective total synthesis of (−)-nalanthalide, a potential Kv1.3 blocking immunosuppressant, Tetrahedron Lett., 2006, 47, 3251–3255 CrossRef CAS.
- Z. Li and D. Yang, Construction of 9,10-syn–trans-decalin skeleton via semipinacol rearrangement: asymmetric synthesis of (+)-syn-copalol and a candelalide analog, Tetrahedron Lett., 2015, 56, 3667–3669 CrossRef CAS.
- Y. Ji, Y. Liu, W. Guan, C. Guo, H. Jia, B. Hong and H. Li, Enantioselective Divergent Syntheses of Diterpenoid Pyrones, J. Am. Chem. Soc., 2024, 146, 9395–9403 CrossRef CAS PubMed.
- J. Boukouvalas and J.-X. Wang, Structure Revision and Synthesis of a Novel Labdane Diterpenoid from Zingiber ottensii, Org. Lett., 2008, 10, 3397–3399 CrossRef CAS PubMed.
- L. Klier, T. Bresser, T. A. Nigst, K. Karaghiosoff and P. Knochel, Lewis Acid-Triggered Selective Zincation of Chromones, Quinolones, and Thiochromones: Application to the Preparation of Natural Flavones and Isoflavones, J. Am. Chem. Soc., 2012, 134, 13584–13587 CrossRef CAS PubMed.
- C. M. Carver, S. D. Hastings, M. E. Cook and M. S. Shapiro, Functional responses of the hippocampus to hyperexcitability depend on directed, neuron-specific KCNQ2 K+ channel plasticity, Hippocampus, 2020, 30, 435–455 CrossRef CAS PubMed.
- X. Li, Q. Zhang, P. Guo, J. Fu, L. Mei, D. Lv, J. Wang, D. Lai, S. Ye, H. Yang and J. Guo, Molecular basis for ligand activation of the human KCNQ2 channel, Cell Res., 2021, 31, 52–61 CrossRef CAS PubMed.
- M. Y. Splinter, Ezogabine (Retigabine) and Its Role in the Treatment of Partial-Onset Seizures: A Review, Clin. Ther., 2012, 34, 1845–1856 CrossRef CAS PubMed.
- M. R. Groseclose and S. Castellino, An Investigation into Retigabine (Ezogabine) Associated Dyspigmentation in Rat Eyes by MALDI Imaging Mass Spectrometry, Chem. Res. Toxicol., 2019, 32, 294–303 Search PubMed.
- J.-X. Zhao, Y.-Y. Yu, S.-S. Wang, S.-L. Huang, Y. Shen, X.-H. Gao, L. Sheng, J.-Y. Li, Y. Leng, J. Li and J.-M. Yue, Structural Elucidation and Bioinspired Total Syntheses of Ascorbylated Diterpenoid Hongkonoids A–D, J. Am. Chem. Soc., 2018, 140, 2485–2492 CrossRef CAS PubMed.
- G.-Z. Yang, L. Wang, Y.-Y. Fan, Z.-W. Lai, X.-N. Yu, L.-G. Lou, K. Gao and J.-M. Yue, Concise Total Synthesis of Dysoxylactam A and a Simplified Analog, Chin. J. Chem., 2022, 40, 2027–2034 CrossRef CAS.
- C.-Y. Zheng, J.-X. Zhao, C.-H. Yuan, X. Peng, M. Geng, J. Ai, Y.-Y. Fan and J.-M. Yue, Unprecedented sesterterpenoids orientanoids A–C: Discovery, bioinspired total synthesis and antitumor immunity, Chem. Sci., 2023, 14, 13410–13418 RSC.
- T. Qiao, Y. Wang, S. Zheng, H. Kang and G. Liang, Total Syntheses of Norrisolide-Type Spongian Diterpenes Cheloviolene C, Seconorrisolide B, and Seconorrisolide C, Angew. Chem., Int. Ed., 2020, 59, 14111–14114 CrossRef CAS PubMed.
- S. Ni, N. M. Padial, C. Kingston, J. C. Vantourout, D. C. Schmitt, J. T. Edwards, M. M. Kruszyk, R. R. Merchant, P. K. Mykhailiuk, B. B. Sanchez, S. Yang, M. A. Perry, G. M. Gallego, J. J. Mousseau, M. R. Collins, R. J. Cherney, P. S. Lebed, J. S. Chen, T. Qin and P. S. Baran, A Radical Approach to Anionic Chemistry: Synthesis of Ketones, Alcohols, and Amines, J. Am. Chem. Soc., 2019, 141, 6726–6739 CrossRef CAS PubMed.
- R. Shang, D.-S. Ji, L. Chu, Y. Fu and L. Liu, Synthesis of α-Aryl Nitriles through Palladium-Catalyzed Decarboxylative Coupling of Cyanoacetate Salts with Aryl Halides and Triflates, Angew. Chem., Int. Ed., 2011, 50, 4470–4474 CrossRef CAS PubMed.
- D. D. Dixon, J. W. Lockner, Q. Zhou and P. S. Baran, Scalable, Divergent Synthesis of Meroterpenoids via “Borono-sclareolide”, J. Am. Chem. Soc., 2012, 134, 8432–8435 CrossRef CAS PubMed.
- K. A. Johnson, S. Biswas and D. J. Weix, Cross-Electrophile Coupling of Vinyl Halides with Alkyl Halides, Chem. – Eur. J., 2016, 22, 7399–7402 CrossRef CAS PubMed.
- M. J. Salvatore, O. D. Hensens, D. L. Zink, J. Liesch, C. Dufresne, J. G. Ondeyka, T. M. Jürgens, R. P. Borris, S. Raghoobar, E. McCauley, L. Kong, S. E. Gartner, G. E. Koch, F. Pelaéz, M. T. Diez, C. Cascales, I. Martin, J. D. Polishook, M. J. Balick, H. T. Beck, S. R. King, A. Hsu and R. B. Lingham, L-741,494, a Fungal Metabolite That Is an Inhibitor of Interleukin-1β Converting Enzyme, J. Nat. Prod., 1994, 57, 755–760 CrossRef CAS PubMed.
- A. F. Barrero, E. J. Alvarez-Manzaneda, R. Chahboun and M. C. Páiz, A new enantiospecific route toward monocarbocyclic terpenoids: Synthesis of (−)- caparrapi oxide, Tetrahedron Lett., 1998, 39, 9543–9544 CrossRef CAS.
- S.-K. Li and X. Wang, China Pat, CN114751814, 2022 Search PubMed.
- R. D. Clark and C. H. Heathcock, Synthesis of a cytotoxic vernolepin prototype. Ozonization of silyloxyalkenes, J. Org. Chem., 1976, 41, 1396–1403 CrossRef CAS PubMed.
- Y. Sun, R. Li, W. Zhang and A. Li, Total Synthesis of Indotertine A and Drimentines A, F, and G, Angew. Chem., Int. Ed., 2013, 52, 9201–9204 CrossRef CAS PubMed.
- T. Hu, J. Zhang, J. Wang, L. Sha, Y. Xia, T. C. Ortyl, X. Tian and L. Chen, Advances in Epilepsy: Mechanisms, Clinical Trials, and Drug Therapies, J. Med. Chem., 2023, 66, 4434–4467 CrossRef CAS PubMed.
- S. Shi, J. Li, F. Sun, Y. Chen, C. Pang, Y. Geng, J. Qi, S. Guo, X. Wang, H. Zhang, Y. Zhan and H. An, Molecular Mechanisms and Structural Basis of Retigabine Analogues in Regulating KCNQ2 Channel, J. Membr. Biol., 2020, 253, 167–181 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2355511 (3). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4qo00878b |
| ‡ Contributed equally. |
| This journal is © the Partner Organisations 2024 |