Open Access Article
Open Access ArticlePalladium-catalyzed and ligand-controlled divergent cycloadditions of vinylidenecyclopropane-diesters with para-quinone methides enabled by zwitterionic π-propargyl palladium species†
Jia-Hao
Shen
a,
Yong-Jie
Long
b,
Min
Shi
 b and
Yin
Wei
b and
Yin
Wei
 *ab
*ab
aSchool of Materials and Chemistry, University of Shanghai for Science and Technology, 516 Jungong Road, Shanghai, 200093, China
bState Key Laboratory of Organometallic Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032, China. E-mail: weiyin@sioc.ac.cn
First published on 16th April 2024
Abstract
A palladium-catalyzed divergent synthesis of spiro-cyclohexadienones with a five- or a six-membered ring by a cycloaddition reaction of vinylidenecyclopropane-diesters (VDCP-diesters) with p-quinone methides (p-QMs) was disclosed. This protocol features a switchable process between [3 + 2] and [4 + 2] cycloadditions tuned by subtle choice of the phosphine ligand. The substrate scopes have been investigated and the reaction mechanism has been clarified by mechanistic studies; and DFT calculations also revealed that the coordination modes of the ligands with the substrates and the bite angle of the ligands play critical roles in the product regioselectivity.
Introduction
Spiro-cyclohexadienones are widely found as natural molecular backbones in bioactive natural products and have been important sources of medicines and organic synthetic building blocks (Fig. 1).1 Thus, great efforts have been devoted to the synthesis of functionalized spiro-cyclohexadienones.2 Several strategies for the efficient construction of spiro-cyclohexadienones have been reported in recent years.3 The p-QM consists of a cyclohexadiene moiety that is in para-conjugation with a carbonyl group and an exo-methylene component, which serves as an important intercalated allylic Michael receptor.1c,4 The ability of the para double bond to undergo nucleophilic addition with a wide range of nucleophilic reagents enables [2 + n] spirocyclization products to be provided.5–7 Spirocyclopropanation reaction of p-QMs with α-keto carbonyls,5d carbocyclic spiro[5,5]undeca-1,4-dien-3-ones via 1,6-conjugate addition initiated formal [4 + 2] annulation sequences,7a and Pd-catalyzed annulated coupling of spirovinyl-cyclopropyl oxindoles with p-QMs (Scheme 1a) were reported in recent years.3c Very recently, the metal-free cycloaddition reactions of p-QMs with halo alcohols to generate divergent types of total carbon spiro-cyclohexadienone compounds were also reported.7eOn the other hand, cycloaddition reactions via amphiphilic palladium species have been intensely studied in the past few years,8 wherein palladium-catalyzed reactions of propargyl species are challenging due to the greater complexity in achieving regioselective and chemoselective transformations.9 In recent years, our group has developed and explored a series of reactions employing vinylidenecyclopropane (VDCP) derivatives,9b,10 which are small cyclic compounds containing a highly reactive cyclopropane ring attached to an allene moiety. In 2021, our group reported the first reaction of zwitterionic π-propargyl palladium species derived from VDCP-diesters,11 which realized the regio-divergent synthesis of spirooxindoles fused with a five- or a six-membered ring by switching the phosphine ligand (Scheme 1b). The zwitterionic π-propargyl palladium species was proposed as the key reaction intermediate (Scheme 1b); however, the demonstration of the mechanism of the reaction was not revealed clearly in previous work.
Based on the previous research and the progress of our group's research on zwitterionic π-propargyl palladium species from vinylidenecyclopropane-diesters (VDCP-diesters),11 we anticipated the palladium-catalyzed cycloaddition of p-QMs with VDCP-diesters to be an effective and facile method to provide a series of spiro-cyclohexadienone compounds. Herein, we wish to report the palladium-catalyzed cycloadditions of p-QMs with VDCP-diesters which were tuned by phosphorus ligands to generate diverse spiro-products, and explore the reaction mechanism involving the zwitterionic π-propargyl palladium species as the key reaction intermediates and critical factors influencing the cycloaddition modes (Scheme 1c).
To explore the proposed synthesis of spiro-cyclohexadienones, we started with a model reaction employing p-quinone methide 1a and VDCP-diester 2a as the substrates, and optimized the reaction conditions using Pd2dba3 as a palladium catalyst, DPEphos as a ligand, Yb(OTf)3 as a Lewis acid additive, 4 Å MS as an additive and Cs2CO3 as an inorganic base additive in tetrahydrofuran (THF) at 65 °C for 22 h. Gratifyingly, the corresponding [3 + 2] spiro-cyclohexadienone product 3a was obtained as a single product in a yield of 24% (Scheme S1 in the ESI†). The structure was unambiguously determined by X-ray diffraction. The ORTEP drawing is shown in Scheme 2 and the CIF data are summarized in the ESI.† First, we examined the temperature influence and determined that the optimal reaction temperature was 45 °C (Table 1, entries 1–4). To enhance the nucleophilicity of the substrate p-QMs, DMAP (50 mol%) was added into the reaction system, and we identified that the reaction produced 3a in 68% yield under otherwise identical conditions (Table 1, entry 5). Then, we further examined the solvent effects, and using MeCN as the solvent, the desired product was obtained in 85% yield and that served as the optimal reaction condition. The use of other solvents, such as toluene, DCE, dioxane, DMF and DMSO, furnished the desired products in 27%–82% yields (Table 1, entries 6–11). Using Binap and Xantphos as ligands gave 3a in lower yields (32% yield with Xantphos and 55% yield with Binap) than that using DPEphos (Table 1, entries 12 and 13). Moreover, we examined the base additives and found that using both organic and inorganic bases did not work as well as Cs2CO3 (Table 1, entries 14 and 15). Changing the palladium source from Pd2dba3 to Pd(OAc)2 and Pd(PPh3)4 did not affect the yield significantly, giving 3a in 77% and 79% yields, respectively (Table 1, entries 16 and 17).
| Entrya | 2a (equiv.) | [Pd] | Ligand | Solvent | Base | DMAP (mol%) | T, °C | t (h) | Yieldb (%) |
|---|---|---|---|---|---|---|---|---|---|
| a Reaction conditions: a solution of 1a (0.1 mmol) and 2a (0.25 mmol), [Pd] (5.0 mol%), ligand (7.5 mol%), 4 Å MS (100 mg), Yb(OTf)3 (10.0 mol%) and base (0.1 mmol) in solvent (1.5 mL) was heated at 45 °C for 12 h. b Isolated yield. | |||||||||
| 1 | 2.5 | Pd2dba3 | DPEphos | THF | Cs2CO3 | — | 40 | 22 | 29 |
| 2 | 2.5 | Pd2dba3 | DPEphos | THF | Cs2CO3 | — | 45 | 22 | 46 |
| 3 | 2.5 | Pd2dba3 | DPEphos | THF | Cs2CO3 | — | 50 | 22 | 36 |
| 4 | 2.5 | Pd2dba3 | DPEphos | THF | Cs2CO3 | — | 55 | 22 | 31 |
| 5 | 2.5 | Pd2dba3 | DPEphos | THF | Cs2CO3 | 50 | 45 | 12 | 68 |
| 6 | 2.5 | Pd2dba3 | DPEphos | Dioxane | Cs2CO3 | 50 | 45 | 12 | 45 |
| 7 | 2.5 | Pd 2 dba 3 | DPEphos | MeCN | Cs 2 CO 3 | 50 | 45 | 12 | 85 |
| 8 | 2.5 | Pd2dba3 | DPEphos | Toluene | Cs2CO3 | 50 | 45 | 12 | 27 |
| 9 | 2.5 | Pd2dba3 | DPEphos | DCE | Cs2CO3 | 50 | 45 | 12 | 44 |
| 10 | 2.5 | Pd2dba3 | DPEphos | DMF | Cs2CO3 | 50 | 45 | 12 | 57 |
| 11 | 2.5 | Pd2dba3 | DPEphos | DMSO | Cs2CO3 | 50 | 45 | 12 | 82 |
| 12 | 2.5 | Pd2dba3 | Binap | MeCN | Cs2CO3 | 50 | 45 | 12 | 55 |
| 13 | 2.5 | Pd2dba3 | Xantphos | MeCN | Cs2CO3 | 50 | 45 | 12 | 32 |
| 14 | 2.5 | Pd2dba3 | DPEphos | MeCN | DBU | 50 | 45 | 12 | 54 |
| 15 | 2.5 | Pd2dba3 | DPEphos | MeCN | KH2PO4 | 50 | 45 | 12 | Trace |
| 16 | 2.5 | Pd(OAc)2 | DPEphos | MeCN | Cs2CO3 | 50 | 45 | 12 | 77 |
| 17 | 2.5 | Pd(PPh3)4 | DPEphos | MeCN | Cs2CO3 | 50 | 45 | 12 | 79 |
Surprisingly, we obtained a mixture of [4 + 2] cycloaddition product 4a and [3 + 2] cycloaddition product 3a employing the Ph-PHOX ligand, during optimization of the phosphorous ligands. Next, we turned our attention to further optimizing the reaction conditions for generation of the [4 + 2] cycloaddition product 4a. We also examined the reaction under similar conditions to those mentioned above. Temperature and solvents were primarily examined, and the yield of 4a increased to 74% and the total yield was 97% when 1,4-dioxane was used as the solvent (Table 2, entry 3). In the case of using the DPEphos ligand, the total yield of products decreased with increasing reaction temperature. Overall, the optimal conditions for the production of 4a were to carry out the reaction in 1,4-dioxane at 45 °C for 12 h with Ph-PHOX as a ligand. The X-ray crystal structure of 4a is shown in Scheme 3.
| Entrya | 2a (equiv.) | Solvent | Base | T (°C) | t (h) | Total yieldb (%) |
3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4a, yieldb (%) 4a, yieldb (%) |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: a solution of 1a (0.1 mmol) and 2a (0.25 mmol), [Pd] (5.0 mol%), PH = PHOX (7.5 mol%), Yb(OTf)3 (10.0 mol%), 4 Å MS (100 mg), DMAP (50 mol%) and base (0.1 mol) in solvent (1.5 mL) was heated at 45 °C for 12 h. b Yields were determined by 1H NMR using dibromomethane as an internal standard. | |||||||
| 1 | 2.5 | THF | Cs2CO3 | 45 | 12 | 84 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 63 63 |
| 2 | 2.5 | MeCN | Cs2CO3 | 45 | 12 | 88 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 64 64 |
| 3 | 2.5 | 1,4-Dioxane | Cs 2 CO 3 | 45 | 12 | 97 |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 74 74 |
| 4 | 2.5 | DMF | Cs2CO3 | 45 | 12 | 91 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 67 67 |
| 5 | 2.5 | DCM | Cs2CO3 | 45 | 12 | 95 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 67 67 |
| 6 | 2.5 | Toluene | Cs2CO3 | 45 | 12 | 91 | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 56 56 |
| 7 | 2.5 | DMSO | Cs2CO3 | 45 | 12 | 73 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47 47 |
| 8 | 2.5 | DCE | Cs2CO3 | 45 | 12 | 34 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 27 |
| 9 | 2.5 | 1,4-Dioxane | Cs2CO3 | 55 | 12 | 91 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 63 63 |
| 10 | 2.5 | 1,4-Dioxane | Cs2CO3 | 65 | 12 | 85 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 58 58 |
On the basis of the above optimized conditions for this divergent cycloaddition reaction, the substrate scope for the [3 + 2] cycloaddition reactions between p-QMs 1 and the zwitterionic π-propargyl palladium species generated from VDCP-diesters 2 was investigated, and the results are shown in Scheme 2. Fixing the R2 group as a t-butyl group, whether an electron-withdrawing or -donating substituent was introduced at the para-position of the benzene ring on the p-QM, the reactions proceeded smoothly, and the target products 3a–3j were obtained in 42%–85% yields. The low yield of the nitro-substituted product 3i was probably due to the low stability of the product. Employing substrates having substituents at the meta-position of the benzene ring, the target products 3k–3m were also obtained in 70% to 83% yields. In addition, substrates 1n–1p having substituents at the ortho-position of the benzene ring were tolerated in this reaction, affording the desired products 3n–3p in 63% to 85% yields. R1 groups that were a heterocycle or a fused aromatic ring also gave the desired products 3q–3u in 65% to 83% yields. However, the reaction did not occur when R1 was a methyl group; this result indicated that the aryl ring structure was necessary (Scheme S3 in the ESI†). In addition, we tested the reaction of substrate VDCP-diester 1v having an ethyl group at the terminal position of the allene moiety, and the corresponding product 3v was obtained in 30% yield with 2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, probably due to steric effects. Methyl- and benzyl-substituted VDCP were also reacted with p-QMs to produce the desired products, giving the desired products 3w and 3x in 63% and 78% yields, respectively. However, when the VDCP-diester had an isopropyl group at R4, the corresponding product was not obtained (Scheme S3 in the ESI†). We then examined the R2 group and found that when the t-butyl group was replaced with a phenyl group, the reaction proceeded smoothly with DMF as the solvent and the target product 3y was obtained in 72% yield. Subsequently, keeping R2 as a phenyl group, variation of R1 produced 3z–3ae in moderate yields under the same conditions. However, when the R2 group was changed from t-butyl to isopropyl and methyl groups, a significant decrease in yield ensued, with 3af and 3ag produced in 40% and 23% yields, respectively.
1 dr, probably due to steric effects. Methyl- and benzyl-substituted VDCP were also reacted with p-QMs to produce the desired products, giving the desired products 3w and 3x in 63% and 78% yields, respectively. However, when the VDCP-diester had an isopropyl group at R4, the corresponding product was not obtained (Scheme S3 in the ESI†). We then examined the R2 group and found that when the t-butyl group was replaced with a phenyl group, the reaction proceeded smoothly with DMF as the solvent and the target product 3y was obtained in 72% yield. Subsequently, keeping R2 as a phenyl group, variation of R1 produced 3z–3ae in moderate yields under the same conditions. However, when the R2 group was changed from t-butyl to isopropyl and methyl groups, a significant decrease in yield ensued, with 3af and 3ag produced in 40% and 23% yields, respectively.
Next, the scope of the [4 + 2] cycloaddition reaction was also investigated under the optimal conditions, and the results are displayed in Scheme 3. Note that the yields were determined by 1H NMR using dibromomethane as an internal standard due to the difficulty of separating the reaction products. Substrates 1 bearing different substituents at the para- and meta-positions on the benzene ring could be converted to the corresponding products 4a–4i in 49–74% yields. However, the yield was lower when the ortho-position was methyl substituted, with 4j afforded in 37% yield, and 4k and 4l in which the same position had methoxy and chlorine substituents were obtained in 50% and 70% yields, respectively. Moreover, p-QMs containing fused aromatic rings and heterocycles were also tolerated in this reaction, affording the corresponding products 4m–4q in 33% to 60% yields. In addition, on changing the ester group in VDCP-diester 2 from an ethyl group to a methyl group or a benzyl group, the reaction took place smoothly, delivering the corresponding spiro-cyclohexadienones 4r and 4s in 27% and 50% yields, respectively. A p-QM with t-butyl replaced by i-propyl also underwent this reaction smoothly, giving the product 4t in 50% yield. Meanwhile, the product 4u with vinyl substitution at the alkene terminal position was also obtained in 44% yield.
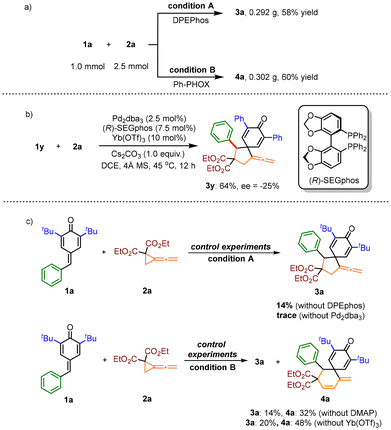
Subsequently, a scale-up synthesis was performed with 1.0 mmol of substrate 1a and 2.5 mmol of substrate 2a under both conditions A and conditions B to verify the utility of the reaction, affording the corresponding products 3a and 4a in 58% and 60% yields, respectively (Scheme 4a). The catalytic asymmetric version of this divergent cycloaddition was also attempted (for details, see Schemes S3 and S4 in the ESI†), and we found that the use of chiral phosphine ligand (R)-SEGphos delivered 3y in 64% yield with 25% ee value (Scheme 4b). In order to validate the reaction mechanism, we performed the following control experiments (Scheme 4c). The reactions of 1a and 2a were first allowed to proceed under standard conditions A without DPEphos as the ligand, affording the product 3a in only 14% yield; only a trace amount of product 3a was obtained in the absence of the Pd catalyst. These results indicate that the palladium catalyst and phosphorous ligand play critical roles in this reaction. In addition, under conditions B with Ph-PHOX as ligand, the yield decreased and the ratio of the two products did not change in the absence of DMAP or Yb(OTf)3 as a Lewis acid, indicating that the Lewis acid and DMAP have no effect on the selectivity of the reaction.
In order to further understand the mechanistic details for these divergent palladium-catalyzed cycloadditions of VDCP-diesters with p-QMs, we conducted a series of DFT calculations to gain further insights. For the reaction with Ph-PHOX as the ligand, the DFT calculations were conducted at the SMD(solvent)/M06-D3/def2-tzvp/SDD//B3LYP(D3BJ)/6-31g(d,p)/SDD level using the Gaussian 16 program.12 The possible reaction pathways were investigated and are shown in Schemes 5 and 6 (for computational details, see the ESI†).
 | ||
| Scheme 6 (a) DFT calculations on the possible reaction pathways under conditions B; (b) DFT calculations on the proton transfer process assisted by base. | ||
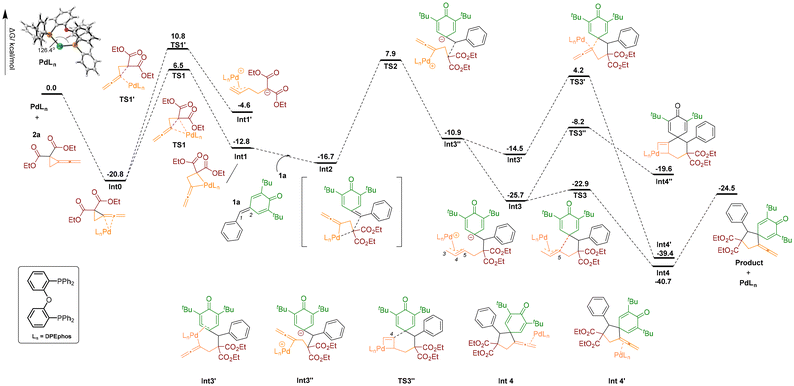
For the generation of product 3a using DPEphos, we investigated the reaction pathway starting from a stable palladium complex Int0 (shown in Scheme 5), in which the allene unit of 2a is coordinated to the palladium catalyst combined with the DPEphos ligand. The complex Int0 undergoes the ring-opening process to form the allenyl palladium intermediate Int1 passing through transition state TS1 with an energy barrier of 27.3 kcal mol−1. Another possible zwitterionic π-propargyl palladium intermediate Int1′ was also investigated; however, the energy of Int1′ is higher than that of Int1 by 8.2 kcal mol−1; the complex Int0 has to overcome an energy barrier of 31.6 kcal mol−1 through transition state TS1′ to obtain zwitterionic π-propargyl palladium Int1′. Thus, the formation of allenyl palladium intermediate Int1 is more favourable thermodynamically and kinetically. Next, the intermediate Int1 is associated to the alkene moiety of the p-QM to afford an intermediate Int2, which is an exothermic process (ΔG = −3.9 kcal mol−1). The nucleophilic attack at the C1 position of 1a generates the intermediate Int3′′viaTS2 with an energy barrier of 24.6 kcal mol−1. Isomerization of Int3′′ to zwitterionic π-propargyl palladium intermediate Int3 is exothermic by 14.8 kcal mol−1, where the carbon anion of Int3 selectively attacks the internal carbon atom C5 of the Pd-π-propargyl unit viaTS4 to afford the complex Int4 of the Pd(0) catalyst with the [3 + 2] cycloaddition product. The energy barrier for [3 + 2] cycloaddition is 2.8 kcal mol−1, which is much lower than that of the competitive pathway involving attacking the Pd-π-propargyl unit at its central carbon C4 to generate the corresponding pallada-cyclobutene intermediate Int4′′ (17.5 kcal mol−1viaTS3′′), which is the key intermediate for generation of the [4 + 2] cycloaddition product 4a. This calculation result indicates that the formation of the [3 + 2] cycloaddition product is favorable thermodynamically and kinetically; this is also consistent with the fact that we only obtained product 3a in experiments using DPEphos as the ligand. Subsequently, another possible isomerization intermediate Int3′, in which both the allyl of 1a and the allene unit are coordinated to the palladium catalyst, is also investigated. However, the intermediate Int3′ undergoes the ring-closing step to produce an intermediate Int4′′via transition state TS3′, this energy barrier of 18.7 kcal mol−1 is higher than the competitive pathway passing through transition state TS3, therefore, this pathway is ruled out. Finally the intermediate Int4 dissociates to the final product 3a and the catalyst is regenerated.
We also investigated the reaction pathway for conditions B, in which Ph-PHOX is utilized as the ligand (shown in Scheme 6a).13 It should be mentioned here that the stabilized structure of Ph-PHOX-Pd(0) involves palladium coordinated to the phosphorus atom and benzene ring of the ligand (PdLn). The energy of PdLn is lower than that of the Ph-PHOX-Pd(0) catalyst (PdLn′) by 13.2 kcal mol−1, in which palladium is coordinated to the ligand's phosphorus atom and nitrogen atom; this result may be due to the hemilability of Ph-PHOX. The coordination angle of Ph-PHOX with palladium (PdLn′) is only 96.3°, which is much smaller than the angle of 126.4° for DPEphos, and may affect the site of attack in the ring closure step. Next, a stable palladium complex Int0-B, in which allene units of 2a are coordinated to Ph-PHOX-Pd(0), is generated. Int0-B can undergo an oxidative ring cleavage reaction to give a zwitterionic π-propargyl palladium intermediate Int1-B through TS1-B with an energy barrier of 24.0 kcal mol−1. Subsequently, the palladium intermediate Int1-B is associated to 1a to form a complex Int2-B. Alternatively, the zwitterionic π-propargyl palladium intermediate Int1-B is isomerized to another intermediate Int1′-B due to a different coordination mode, which is an exothermic process (ΔG = −14.6 kcal mol−1). Passing through transition state TS2-B with an energy barrier of 5.4 kcal mol−1, the intermediate Int3-B is formed in an exothermic process (ΔG = −11.2 kcal mol−1). Although the energies of Int1′-B and Int2′-B are lower than those of Int1-B and Int2-B, respectively, a higher energy barrier of 20.0 kcal mol−1 has to be overcome to access the intermediate Int3′-B in a highly endothermic process (ΔG = 14.3 kcal mol−1). Thus, the formation of intermediate Int3-B is thermodynamically and kinetically favorable. Next, the pathway with the lower energy barrier of 11.4 kcal mol−1viaTS3-B in the next process involves the carbon anion of Int3-B attacking the Pd-π-propargyl unit at its central carbon C4 to generate the pallada-cyclobutene intermediate Int4-B, which is the key intermediate for generation of the [4 + 2] product 4a. Another competitive path involving the carbon anion of Int3-B attacking the internal carbon atom C5 of the Pd-π-propargyl unit has an energy barrier of 16.7 kcal mol−1viaTS3′′-B to give an intermediate Int4′-B, which further dissociates to the [3 + 2] product 3a and palladium catalyst. Another possible isomerization intermediate Int3′-B passing through transition state TS3′-B with an energy barrier of 14.8 kcal mol−1 also can generate intermediate Int4′-B; however, the barrier is still higher than that for generation of intermediate Int4-B. These calculation results show that the formation of intermediate Int4-B is the most kinetically favorable, indicating the generation of the [4 + 2] product 4a is kinetically favorable. These results may account for why product 4a is mainly obtained under reaction conditions B.
Next, we continued to investigate the following reaction steps involving the proton transfer assisted by base from intermediate Int4 (Scheme 6b).14 At this stage, two alternative pathways are investigated, respectively. One of them is path a involving the base coordinated to the palladium and the other is path b involving the direct proton transfer and no coordination between the base and the palladium. As depicted in path a, the bicarbonate, which is generated by trace amounts of water in the system and the base, is coordinated to Int4-B, affording the intermediate Int5 in a process that is exothermic by 1.8 kcal mol−1, due to the hemilability of the ligand, wherein bicarbonate undergoes ligand exchange with the nitrogen atom in the ligand. The generated intermediate Int5 then protonates the allyl alpha position via transition state TS5 to form Int6 with a small energy barrier of 8.7 kcal mol−1. The hydrogen in the allylic β-site of Int6 then approaches the undissociated carbonate to undergo β-hydrogen elimination viaTS6 with an energy barrier of 12.7 kcal mol−1. Finally, the product complex Int7 dissociates to give the [4 + 2] product 4a in a 9.7 kcal mol−1 endothermic process. In contrast, due to the higher energy barrier of 27.7 kcal mol−1 for direct proton transfer by bicarbonate and the large ring tension of the four-membered ring transition state TS8 during β-hydrogen elimination, the reaction mechanism viapath b is energetically unfavorable, and is excluded.
On the basis of DFT calculation results and the previously reported processes, a plausible mechanism for the [3 + 2] and [4 + 2] cycloadditions is depicted in Scheme 7. Pd(0) complex I and VDCP-diester 2a undergo an oxidative addition and generate the zwitterionic Pd species II or II′. The nucleophilic carbon anion of intermediate II or II′ attacks 1a to afford an intermediate III. Then the carbon anion selectively attacks the inner carbon atom to generate the corresponding five-membered [3 + 2] cycloaddition product 3avia transition state VI′ and simultaneously regenerate the catalyst Pd(0) with DPEphos with a large bite angle. On the other hand, when using Ph-PHOX as a small bite angle ligand, the carbon anion selectively attacks the central carbon of the allene moiety to generate pallada-cyclobutene intermediate IV, then IV undergoes base-induced protonation and β-hydride elimination to obtain the six-membered [4 + 2] cycloaddition product 4a and regenerate the Pd(0) catalyst; simultaneously product 3a was obtained through intermediate VI. Additives and bases play auxiliary roles in this reaction. Yb(OTf)3 as a Lewis acid additive probably promotes the C–C bond cleavage of the cyclopropane ring through coordination with the ester moieties;15 4 Å MS as an additive get rid of the ambient moisture; DMAP and Cs2CO3 as base additives probably stabilize the zwitterionic palladium species and assist the proton transfer process.
Conclusions
In summary, a regioselective divergent cycloaddition of p-QMs with zwitterionic palladium intermediates generated from VDCP-diesters has been developed, and two different types of spiro-cyclohexadienone products, namely [3 + 2] and [4 + 2] cycloaddition products, can be synthesized by switching ligands in good to excellent total yields with good functional group compatibility. DFT calculations reveal that the [3 + 2] cycloaddition product is favorable thermodynamically and kinetically using DPEphos as a ligand, probably due to the large bite angle of DPEphos which makes the [3 + 2] cycloaddition mode preferred. The hemilability and the small bite angle of Ph-PHOX probably lead to the key [4 + 2] cycloaddition intermediate being kinetically favourable; thus, the [4 + 2] cycloaddition product was mainly obtained in experiments. Our group will further develop highly enantioselective cycloadditions of VDCPs with p-QMs catalyzed by palladium in the future and apply this reaction to the synthesis of biologically active molecules.Author contributions
Yong-Jie Long discovered the reactions. Min Shi and Yong-Jie Long designed the experiments. Jia-Hao Shen performed the experiments and analysed the results. Min Shi, Yin Wei and Jia-Hao Shen wrote the manuscript. Yin Wei and Jia-Hao Shen performed and described the computational section.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for the financial support from the National Key R & D Program of China (2023YFA1506700), the National Natural Science Foundation of China (21372250, 21121062, 21302203, 21772037, 21772226, 21861132014, 91956115 and 22171078) and the Fundamental Research Funds for the Central Universities 222201717003.References
- (a) L. K. Smith and I. R. Baxendale, Total syntheses of natural products containing spirocarbocycles, Org. Biomol. Chem., 2015, 13, 9907–9933 RSC; (b) R. Chen, S. Jia, Y. Man, H.-G. Cheng and Q. Zhou, A Concise Total Synthesis of (±)-Stepharine and (±)-Pronuciferine, Synthesis, 2023 DOI:10.1055/a-1984-0755; (c) G. Blay, L. Cardona, A. M. Collado, B. García, V. Morcillo and J. R. Pedro, Synthesis of Spirovetivane Sesquiterpenes from Santonin. Synthesis of (+)-Anhydro-β-rotunol and All Diastereomers of 6,11-Spirovetivadiene, J. Org. Chem., 2004, 69, 7294–7302 CrossRef CAS PubMed; (d) C.-P. Chung, C.-Y. Hsu, J.-H. Lin, Y.-H. Kuo, W. Chiang and Y.-L. Lin, Antiproliferative Lactams and Spiroenone from Adlay Bran in Human Breast Cancer Cell Lines, J. Agric. Food Chem., 2011, 59, 1185–1194 CrossRef CAS PubMed; (e) A. R. Díaz-Marrero, G. Porras, Z. Aragón, J. M. de la Rosa, E. Dorta, M. Cueto, L. D'Croz, J. Maté and J. Darias, Carijodienone from the Octocoral Carijoa multiflora. A Spiropregnane-Based Steroid, J. Nat. Prod., 2011, 74, 292–295 CrossRef PubMed; (f) A. B. Petersen, M. H. Rønnest, T. O. Larsen and M. H. Clausen, The Chemistry of Griseofulvin, Chem. Rev., 2014, 114, 12088–12107 CrossRef CAS PubMed; (g) W. Li, X. Xu, P. Zhang and P. Li, Recent Advances in the Catalytic Enantioselective Reactions of para-Quinone Methides, Chem. – Asian J., 2018, 13, 2350–2359 CrossRef CAS PubMed.
- (a) X. Liu, Y. Ren, L. Zhu, T. Li, W. Xu, Y. Liu, K.-W. Tang and B. Xiong, Recent advances in the cyclization of para-quinone methides, Tetrahedron, 2023, 148, 133655 CrossRef CAS; (b) W. Baik, H. J. Lee, J. M. Jang, S. Koo and B. H. Kim, NBS-Promoted Reactions of Symmetrically Hindered Methylphenols via p-Benzoquinone Methide, J. Org. Chem., 2000, 65, 108–115 CrossRef CAS PubMed; (c) W. R. Hatchard, Syntheses by Free-radical Reactions. VII. The Reaction of 2,6-Di-t-butyl-4-methylphenol and 2,6-Di-t-butyl-4-isopropylphenol with Chloroprene, J. Am. Chem. Soc., 1958, 80, 3640–3642 CrossRef; (d) J. D. McClure, Synthesis of Spiroundecatrienones from 2,6-Di-t-butylquinone Methide and Butadienes, J. Org. Chem., 1962, 27, 2365–2368 CrossRef CAS.
- (a) R. Pan, L. Hu, C. Han, A. Lin and H. Yao, Cascade Radical 1,6-Addition/Cyclization of para-Quinone Methides: Leading to Spiro[4.5]deca-6,9-dien-8-ones, Org. Lett., 2018, 20, 1974–1977 CrossRef CAS PubMed; (b) X.-Z. Zhang, Y.-H. Deng, K.-J. Gan, X. Yan, K.-Y. Yu, F.-X. Wang and C.-A. Fan, Tandem Spirocyclopropanation/Rearrangement Reaction of Vinyl p-Quinone Methides with Sulfonium Salts: Synthesis of Spirocyclopentenyl p-Dienones, Org. Lett., 2017, 19, 1752–1755 CrossRef CAS PubMed; (c) B. Debnath, T. Sarkar, P. Karjee, S. K. Purkayastha, A. K. Guha and T. Punniyamurthy, Palladium-Catalyzed Annulative Coupling of Spirovinylcyclopropyl Oxindoles with p-Quinone Methides, J. Org. Chem., 2023, 88, 9704–9719 CrossRef CAS PubMed.
- (a) W.-D. Chu, L.-F. Zhang, X. Bao, X.-H. Zhao, C. Zeng, J.-Y. Du, G.-B. Zhang, F.-X. Wang, X.-Y. Ma and C.-A. Fan, Asymmetric Catalytic 1,6-Conjugate Addition/Aromatization of para-Quinone Methides: Enantioselective Introduction of Functionalized Diarylmethine Stereogenic Centers, Angew. Chem., Int. Ed., 2013, 52, 9229–9233 CrossRef CAS PubMed; (b) K. Zhao, Y. Zhi, A. Wang and D. Enders, Asymmetric Organocatalytic Synthesis of 3-Diarylmethine-Substituted Oxindoles Bearing a Quaternary Stereocenter via 1,6-Conjugate Addition to para-Quinone Methides, ACS Catal., 2016, 6, 657–660 CrossRef CAS; (c) P. Goswami, S. Sharma, G. Singh and R. V. Anand, Bis(amino)cyclopropenylidene Catalyzed Rauhut–Currier Reaction between α,β-Unsaturated Carbonyl Compounds and para-Quinone Methides, J. Org. Chem., 2018, 83, 4213–4220 CrossRef CAS PubMed; (d) X.-Z. Zhang, Y.-H. Deng, X. Yan, K.-Y. Yu, F.-X. Wang, X.-Y. Ma and C.-A. Fan, Diastereoselective and Enantioselective Synthesis of Unsymmetric β,β-Diaryl-α-Amino Acid Esters via Organocatalytic 1,6-Conjugate Addition of para-Quinone Methides, J. Org. Chem., 2016, 81, 5655–5662 CrossRef CAS PubMed; (e) F.-S. He, J.-H. Jin, Z.-T. Yang, X. Yu, J. S. Fossey and W.-P. Deng, Direct Asymmetric Synthesis of β-Bis-Aryl-α-Amino Acid Esters via Enantioselective Copper-Catalyzed Addition of p-Quinone Methides, ACS Catal., 2016, 6, 652–656 CrossRef CAS.
- Three-membered ring: (a) S. B. Kale, P. K. Jori, T. Thatikonda, R. G. Gonnade and U. Das, 1,6-Conjugate-Addition-Induced [2 + 1] Annulation of para-Quinone Methides and Pyrazolones: Synthesis of Bis-Spiro Compounds with Contiguous Quaternary Spiro-Centers, Org. Lett., 2019, 21, 7736–7740 CrossRef CAS PubMed; (b) Z. Yuan, X. Fang, X. Li, J. Wu, H. Yao and A. Lin, 1,6-Conjugated Addition-Mediated [2 + 1] Annulation: Approach to Spiro[2.5]octa-4,7-dien-6-one, J. Org. Chem., 2015, 80, 11123–11130 CrossRef CAS PubMed; (c) L. Roiser and M. Waser, Enantioselective Spirocyclopropanation of para-Quinone Methides Using Ammonium Ylides, Org. Lett., 2017, 19, 2338–2341 CrossRef CAS PubMed; (d) Y.-H. Deng, W.-D. Chu, Y.-H. Shang, K.-Y. Yu, Z.-L. Jia and C.-A. Fan, P(NMe2)3-Mediated Umpolung Spirocyclopropanation Reaction of p-Quinone Methides: Diastereoselective Synthesis of Spirocyclopropane-Cyclohexadienones, Org. Lett., 2020, 22, 8376–8381 CrossRef CAS PubMed; (e) X.-Z. Zhang, J.-Y. Du, Y.-H. Deng, W.-D. Chu, X. Yan, K.-Y. Yu and C.-A. Fan, Spirocyclopropanation Reaction of para-Quinone Methides with Sulfonium Salts: The Synthesis of Spirocyclopropanyl para-Dienones, J. Org. Chem., 2016, 81, 2598–2606 CrossRef CAS PubMed; (f) K. Gai, X. Fang, X. Li, J. Xu, X. Wu, A. Lin and H. Yao, Synthesis of spiro[2.5]octa-4,7-dien-6-one with consecutive quaternary centers via 1,6-conjugate addition induced dearomatization of para -quinone methides, Chem. Commun., 2015, 51, 15831–15834 RSC; (g) J.-R. Zhang, H.-S. Jin, J. Sun, J. Wang and L.-M. Zhao, Time-Economical Synthesis of Bis-Spiro Cyclopro-panes via Cascade 1,6-Conjugate Addition/Dearomatization Reaction of para-Quinone Methides with 3-Chlorooxindoles, Eur. J. Org. Chem., 2020, 4988–4994 CrossRef CAS.
- Five-membered ring: (a) Y. Su, Y. Zhao, B. Chang, X. Zhao, R. Zhang, X. Liu, D. Huang, K.-H. Wang, C. Huo and Y. Hu, [3 + 2] Cycloaddition of para-Quinone Methides with Nitrile Imines: Approach to Spiro-pyrazoline-cyclohexadienones, J. Org. Chem., 2019, 84, 6719–6728 CrossRef CAS PubMed; (b) M. Soleymani and S. Jahanparvar, A computational study on the [3 + 2] cycloaddition of para-quinone methides with nitrile imines: a two-stage one-step mechanism, Monatsh. Chem., 2020, 151, 51–61 CrossRef CAS; (c) Z. Yuan, W. Wei, A. Lin and H. Yao, Bifunctional Organo/Metal Cooperatively Catalyzed [3 + 2] Annulation of para-Quinone Methides with Vinylcyclopropanes: Approach to Spiro[4.5]deca-6,9-diene-8-ones, Org. Lett., 2016, 18, 3370–3373 CrossRef CAS PubMed; (d) L. Roiser, K. Zielke and M. Waser, Formal (4 + 1) Cyclization of Ammonium Ylides with Vinylogous para-Quinone Methides, Synthesis, 2018, 50, 4047–4054 CrossRef CAS PubMed; (e) G.-T. Song, Y. Liu, X.-Y. Hu, S.-T. Li, J.-B. Liu, Y. Li and C.-H. Qu, Microwave-assisted copper catalyzed decarboxylative reductive coupling of para-quinone methides with 3-indoleacetic acids: rapid access to polycyclic spiroindolequinone derivatives, Org. Chem. Front., 2023, 10, 1512–1520 RSC; (f) Z.-L. Jia, X.-T. An, Y.-H. Deng, H.-B. Wang, K.-J. Gan, J. Zhang, X.-H. Zhao and C.-A. Fan, Palladium-Catalyzed Asymmetric (2 + 3) Annulation of p-Quinone Methides with Trimethyleneme-thanes: Enantioselective Synthesis of Functionalized Chiral Spirocyclopentyl p-Dienones, Org. Lett., 2020, 22, 4171–4175 CrossRef CAS PubMed; (g) Z. Yuan, L. Liu, R. Pan, H. Yao and A. Lin, Silver-Catalyzed Cascade 1,6-Addition/Cyclization of para-Quinone Methides with Propargyl Malonates: An Approach to Spiro[4.5]deca-6,9-dien-8-ones, J. Org. Chem., 2017, 82, 8743–8751 CrossRef CAS PubMed; (h) C. Ma, Y. Huang and Y. Zhao, Stereoselective 1,6-Conjugate Addition/Annulation of para-Quinone Methides with Vinyl Epoxides/Cyclopropanes, ACS Catal., 2016, 6, 6408–6412 CrossRef CAS.
- Six-membered ring: (a) G. S. Ghotekar, S. R. Shirsath, A. C. Shaikh and M. Muthukrishnan, 1,6-Conjugate addition initiated formal [4 + 2] annulation of p-quinone methides with sulfonyl allenols: a unique access to spiro[5.5]undeca-1,4-dien-3-one scaffolds, Chem. Commun., 2020, 56, 5022–5025 RSC; (b) Z.-L. Jia, X.-T. An, Y.-H. Deng, L.-H. Pang, C.-F. Liu, L.-L. Meng, J.-K. Xue, X.-H. Zhao and C.-A. Fan, Palladium-Catalyzed Asymmetric (4 + 2) Annulation of γ-Methylidene-δ-valerolactones with Alkenes: Enantioselective Synthesis of Functionalized Chiral Cyclohexyl Spirooxindoles, Org. Lett., 2021, 23, 745–750 CrossRef CAS PubMed; (c) Z. Yuan, R. Pan, H. Zhang, L. Liu, A. Lin and H. Yao, Palladium-catalyzed Oxa-[4 + 2] Annulation of para-Quinone Methides, Adv. Synth. Catal., 2017, 359, 4244–4249 CrossRef CAS; (d) L. Cao, F. Hu, J. Dong, X.-M. Zhang and S.-S. Li, Aromatization-driven cascade [1,5]-hydride transfer/cyclization for synthesis of spirochromanes, Org. Chem. Front., 2023, 10, 1796–1802 RSC; (e) R. A. Gaikwad, A. T. Savekar and S. B. Waghmode, Metal-Free Approach for Oxa-spirocyclohexadienones through [3 + 2]/[4 + 2] ipso-Cyclization of para-Quinone Methides with Halo Alcohols, J. Org. Chem., 2023, 88, 9987–10001 CrossRef CAS PubMed.
- (a) B. Niu, Y. Wei and M. Shi, Recent advances in annulation reactions based on zwitterionic π-allyl palladium and propargyl palladium complexes, Org. Chem. Front., 2021, 8, 3475–3501 RSC; (b) J. Du, Y.-F. Li and C.-H. Ding, Recent advances of Pd-π-allyl zwitterions in cycloaddition reactions, Chin. Chem. Lett., 2023, 34, 108401 CrossRef CAS; (c) Z. Han, Y. Xue, X. Li, X. Hu, X.-Q. Dong, J. Sun and H. Huang, Studies on the [4 + 2] cycloaddition and allylic substitution of indole-fused zwitterionic π-allylpalladium, Org. Biomol. Chem., 2023, 21, 8162–8169 RSC.
- (a) A. E. Nibbs, T. D. Montgomery, Y. Zhu and V. H. Rawal, Access to Spirocyclized Oxindoles and Indolenines via Palladium-Catalyzed Cascade Reactions of Propargyl Carbonates with 2-Oxotryptamines and Tryptamines, J. Org. Chem., 2015, 80, 4928–4941 CrossRef CAS PubMed; (b) Z. Yang, B. Zhang, Y. Long and M. Shi, Palladium-catalyzed hydroamination of vinylidenecyclopropane-diester with pyrroles and indoles: an approach to azaaromatic vinylcyclopropanes, Chem. Commun., 2022, 58, 9926–9929 RSC; (c) R.-D. Gao, C. Liu, L.-X. Dai, W. Zhang and S.-L. You, Pd(0)-Catalyzed Alkenylation and Allylic Dearomatization Reactions between Nucleophile-Bearing Indoles and Propargyl Carbonate, Org. Lett., 2014, 16, 3919–3921 CrossRef CAS PubMed; (d) A. Iwata, S. Inuki, S. Oishi, N. Fujii and H. Ohno, Synthesis of fused tetracyclic spiroindoles via palladium-catalysed cascade cyclisation, Chem. Commun., 2013, 50, 298–300 RSC.
- (a) Z. Meng, J. Yan, C. Ning, M. Shi and Y. Wei, Construction of pyrroles, furans and thiophenes via intramolecular cascade desulfonylative/dehydrogenative cyclization of vinylidenecycl-opropanes induced by NXS (X = I or Br), Chem. Sci., 2023, 14, 7648–7655 RSC; (b) S. Yang and M. Shi, Recent Advances in Transition-Metal-Catalyzed/Mediated Transformations of Vinylidenecyclopropanes, Acc. Chem. Res., 2018, 51, 1667–1680 CrossRef CAS PubMed; (c) Z. Meng, X. Zhang and M. Shi, Visible-light mediated cascade cyclization of ene-vinylidenecyclopropanes: access to fluorinated heterocyclic compounds, Org. Chem. Front., 2021, 8, 3796–3801 RSC.
- B. Niu, Y. Wei and M. Shi, Palladium catalyzed divergent cycloadditions of vinylidenecyclopropane-diesters with methyleneindolinones enabled by zwitterionic π-propargyl palladium species, Chem. Commun., 2021, 57, 4783–4786 RSC.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 16, Revision C.01, Gaussian, Inc., Wallingford CT, 2016 Search PubMed.
- C. Y. Legault, CYLview, 1.0b, Université de Sherbrooke, 2009 (https://www.cylview.org) Search PubMed.
- G. Zhang, Y.-K. Song, F. Zhang, Z.-J. Xue, M.-Y. Li, G.-S. Zhang, B.-B. Zhu, J. Wei, C. Li, C.-G. Feng and G.-Q. Lin, Palladium-catalyzed allene synthesis enabled by β-hydrogen elimination from sp2-carbon, Nat. Commun., 2021, 12, 728 CrossRef CAS PubMed.
- L. Wu and M. Shi, Ring-Opening Reaction of Vinylidenecyclopropanediesters Catalyzed by Re2(CO)10 or Yb(OTf)3, Eur. J. Org. Chem., 2011, 1099–1105 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures; characterization data for new compounds. CCDC 2225338 and 2288733. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4qo00368c |
| This journal is © the Partner Organisations 2024 |