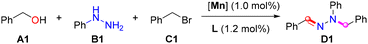Open Access Article
Open Access ArticleFormation of ylidenehydrazines enabled by manganese-catalyzed acceptorless dehydrogenative coupling†
Fangchao
Wang‡
bc,
Ding
Ding‡
bc,
Chunyan
Zhang
 *a and
Guoying
Zhang
*a and
Guoying
Zhang
 *bc
*bc
aCollege of Ecology, Taiyuan University of Technology, Taiyuan 030001, China. E-mail: zhangchunyan@tyut.edu.cn
bState Key Laboratory of Coal Conversion, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan 030001, China
cUniversity of Chinese Academy of Sciences, Beijing 100049, China. E-mail: zhanggy@sxicc.ac.cn
First published on 9th January 2024
Abstract
Catalytic dehydrogenation, which exhibits highly atom-economical and chemo-selective properties, converts multiple green sustainable alcohols to critical molecules and is a highly desirable and elusive process; furthermore, the dehydrogenation of alcohols with hydrazines produces ylidenehydrazines, which are versatile building blocks in the formation of numerous pharmaceuticals. However, the syntheses of functionalized ylidenehydrazines involve multi-step reactions that require harsh conditions. Herein, we report a practical one-pot catalytic tandem dehydrogenative condensation coupling of hydrazines with alcohols and halides to form ylidenehydrazines via an acceptorless dehydrogenative coupling strategy, overcoming the challenge of selectivity in multicomponent reactions. The system is based on the Earth-abundant metal Mn, which is stabilized by a novel bench-stable PNN pincer ligand derived from aminoindazole. A large series of functionalized ylidenehydrazines is obtained in high yields with excellent selectivities, and gram-scale ylidenehydrazines are prepared using this protocol. Notably, using this protocol, several pharmaceuticals may be easily synthesized in a one-pot manner. This strategy significantly broadens the scope of Mn-catalyzed dehydrogenative condensation coupling for synthesizing unsaturated molecules.
Introduction
Ylidenehydrazines, which exhibit remarkable biological activities, are frequently used in pharmaceuticals, agrochemicals, and functional materials (Fig. 1A).1 They also serve as attractive precursors in a series of transformations that form various complex, functional compounds.2 Owing to their scientific applications, considerable effort has been devoted to synthesizing these privileged scaffolds, particularly via metal-catalyzed dehydrogenative condensation and/or nucleophilic substitution strategies.3 However, most approaches require super stoichiometric amounts of external additives or harsh reaction conditions, particularly multi-step reactions, which undeniably result in decreased atom efficiency owing to complex operation processes and the formation of undesired waste byproducts (Fig. 1D).4 Therefore, a novel and direct method is required for one-pot synthesis of hydrazones using bulk raw materials.The efficient and highly selective conversion of alcohols to significant compounds is highly attractive because alcohols may be obtained from abundant lignocellulose biomass, which may reduce CO2 emissions and conserve finite fossil carbon resources.5 An atom economical dehydrogenative reaction is a green method of transforming alcohols to produce H2 or H2O byproducts.6 Dehydrogenative transformations have been successfully developed over the past decades.7 Early catalytic dehydrogenation reactions were mostly based on the use of rare noble metals (Ir, Pt, Rh, and Ru) as catalysts (Fig. 1B).8 Recently, the use of abundant 3d metals (Fe, Co, and Ni) as efficient catalysts in these transformations has emerged.9 Mn metal catalytic systems have also been developed for use in catalytic dehydrogenative transformations, displaying novel patterns of selectivity compared to those of rare noble metals.10 Additionally, the sustainable generation of H2via dehydrogenation is highly attractive because of its central role as an energy carrier and reducing agent. Therefore, novel reactions mediated by 3d metal catalysts that transform green or sustainable starting materials into significant classes of complex molecules via the dehydrogenation of alcohols and H2 generation are highly desirable.
To the best of our knowledge, transition metal catalyzed dehydrogenative transformation of alcohols with hydrazines and halides remains unexplored. The evident challenge of this reaction is that the accelerator or catalyst is surrounded by a large excess of active substrates under the reaction conditions, and thus, undesired byproducts are easily generated. To overcome this challenge, improving the affinity between intermediate I (in situ-formed hydrazine with halides) and the aldehyde species (in situ dehydrogenation of the alcohol) is critical. Additionally, ensuring that (i) intermediate I is sufficiently stable in the reactive system and (ii) both dehydrogenation of the alcohol and condensation with I occur via similar rapid processes such that the formation of byproducts is largely suppressed is crucial. In this study, we report an efficient catalytic system that combines the Earth-abundant Mn metal with a readily available PN3N pincer ligand. The catalytic system exhibits an unprecedented, practical catalytic condensation coupling of hydrazines with alcohols and halides to form ylidenehydrazines via an acceptorless dihydrogen coupling strategy; the challenge of selectivity in a one-pot multicomponent reaction is thus overcome (Fig. 1C).
Furthermore, dehydrogenative condensation cross-coupling reactions may add to the existing synthesis concepts used in generating H2 in chemical reactions, such as alcohol dehydrogenation,11 dehydrogenative condensation,12 rearrangement,13 and coupling reactions (Fig. 1B).14
Results and discussion
Based on our experience with Mn-promoted dehydrogenative coupling reactions,15 phenyl methanol (A1), phenylhydrazine (B1), and (bromomethyl)benzene (C1) were used as model substrates, and dehydrogenative condensation was performed in the presence of Mn(CO)5Br with bi- or tridentate ligands (Table 1). The desired product was obtained in a trace amount using a P–N-based accessory ligand (L1), and the reaction efficiency was improved when using L2 as a ligand (entries 1 and 2). The use of other tridentate ligands (L3–L5) functionalized on the pyridyl moiety did not improve the reactivity under similar reaction conditions (entries 3–5). Similar results were obtained using other P–N–N (L6–L9)-based ligands with different indazole phenyl groups, indicating that the conversion is insensitive to the electrical properties of the indazole backbone (entries 6–9). Evaluation of the P–N–N ligands with different phosphine groups on the amine of the indazole revealed that the conversion is sensitive to the electrostatic and steric hindrance of the ligand, with L10–L11 providing optimal regioselectivity toward the condensation product. L11 is the most effective, affording the desired D1 in 91% yield and with excellent selectivity (E1, 1,2-dibenzyl-1-phenylhydrazine). D1 is obtained in 96% yield after a prolonged reaction time, and only a trace amount of the desired product is obtained in the absence of the Mn catalyst or ligand. Furthermore, the desired product D1 is not obtained when MnBr2 is stabilized using L11 (entry 14). The optimal results in terms of the hydrazone adducts are obtained after the final optimization of the reaction parameters, with L11 being the most active ligand (ESI†).16| Entry | Pre-catalyst | Ligand | D1 (%) |
D1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) E1a E1a |
|---|---|---|---|---|
| a Conditions: A1 (0.8 mmol), B1 (0.5 mmol), C1 (0.7 mmol), Mn(CO)5Br (1.0 mol%), L1–L11 (1.2 mol%), tBuOK (1.0 mmol), THF (3.0 mL), 100 °C, 6 h. The yields of D1 were determined via GC using n-cetane as the internal standard. b 12 h. | ||||
| 1 | Mn(CO)5Br | L1 | <5 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | Mn(CO)5Br | L2 | 62 (66)b | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | Mn(CO)5Br | L3 | 41 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | Mn(CO)5Br | L4 | 54 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | Mn(CO)5Br | L5 | 22 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | Mn(CO)5Br | L6 | 60 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | Mn(CO)5Br | L7 | 63 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | Mn(CO)5Br | L8 | 51 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9 | Mn(CO)5Br | L9 | 47 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10 | Mn(CO)5Br | L10 | 82 (85)b | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 11 | Mn(CO)5Br | L11 | 91 (96)b | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 12 | Mn(CO)5Br | — | 0 | — |
| 13 | — | L11 | 0 | — |
| 14 | MnB2 | L11 | 0 | — |

|
||||
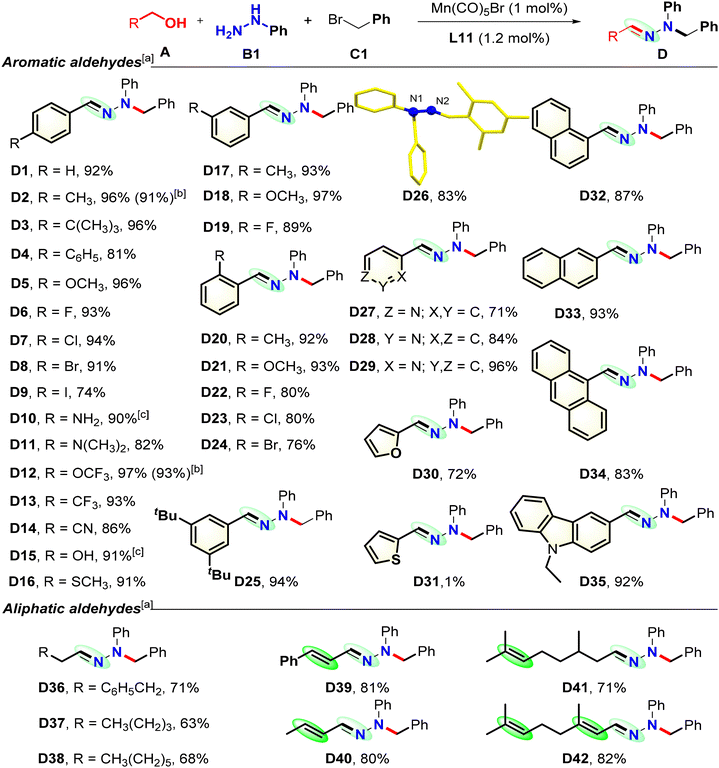
The generality and limitations of the dehydrogenative condensation were investigated. Initially, C1 and B1 were used as model reagents with various primary alcohols (Scheme 1). Fortunately, benzyl alcohols bearing electron-donating and -withdrawing groups are tolerated well under the reaction conditions, affording the desired corresponding products in high to excellent yields (D1–D16). The electronic properties of the phenyl rings in the benzyl alcohols do not affect the reaction. Critically, benzyl alcohols with various substituents at their ortho-positions are transformed smoothly under the standard conditions, but hindrance at the phenyl ring affects the transformation efficiencies (D20–D24). The dehydrogenative transformations with halo-substituted alcohols proceed smoothly to generate the corresponding desired hydrazones in 74–94% yields (D6–D9, D22–D24), thus enabling further possible complex transformations, such as metal catalyzed carbonylation or cross-coupling reactions. Notably, the use of an alcohol with a free amino or hydroxyl moiety on the phenyl group, respectively, produces D10 or D15 in a high yield. Amino, trifluoromethoxy, trifluoromethyl, and cyano groups tolerate this conversion, and their corresponding products are obtained in high yields. Meanwhile, heterocyclic moieties, including pyridine, furan, and thiofuran, may be smoothly incorporated into the hydrazone molecules using the corresponding alcohols (D27–D31). Naphthyl, anthryl, and carbazolyl groups are tolerated under the standard catalytic conditions, yielding the desired products D32–D35 in high to excellent yields. To further explore the synthetic potential of this transformation, more challenging aliphatic alcohol substrates were used under the standard reaction conditions. Alkyl alcohols are good dehydrogenation partners, providing D36–D38 in good yields. Alkyl alcohols bearing various types of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds are also good coupling partners, providing D39–D42 in good to high yields, while the internal double bonds remain intact. The structures of D4 and D26 were confirmed using single-crystal X-ray diffraction.17 Gram-scale reactions using this Mn catalytic system are successful, producing D2 and D12 in high yields.16 Disappointingly, secondary alcohols did not work under the standard reaction conditions.
C bonds are also good coupling partners, providing D39–D42 in good to high yields, while the internal double bonds remain intact. The structures of D4 and D26 were confirmed using single-crystal X-ray diffraction.17 Gram-scale reactions using this Mn catalytic system are successful, producing D2 and D12 in high yields.16 Disappointingly, secondary alcohols did not work under the standard reaction conditions.
The scope of tandem dehydrogenation condensation with respect to alkyl halide substrates is shown in Scheme 2. A1 and B1 were employed with a series of alkyl halide derivatives, leading to the efficient production of various hydrazones and demonstrating the excellent tolerance of different functional groups. Generally, the yields obtained using benzyl bromides containing electron-withdrawing groups (D43, D44, and D49) are higher than those obtained using benzyl bromides containing electron-donating groups (D46–D48). Additionally, compared to the results obtained using para- and meta-substituted substrates, steric hindrance is observed when the ortho-position of the benzyl bromide is substituted (D54 andD56). The halide groups, in particular, survive well under the standard reaction conditions, leading to the formation of the corresponding desired products in good to excellent yields, which may be used in further transformations to synthesize complicated molecules. The substitution of F3C− and F3CO− has no significant influence on the transformation (D48 andD49), and the naphthyl group is smoothly incorporated into D57, resulting in high yields. Substrates containing two bromide groups are successfully transformed into the corresponding bis-hydrazones D64 andD65 in isolated yields of 74% and 76%, respectively. The allyl and cinnamyl groups also survive the standard procedure, leading to the formation of D60–D63 in high yields, while the internal double bonds remain intact. Additionally, the desired hydrazones D44–D63 are obtained in good to high yields when using more challenging benzyl chloride substrates as reaction partners. Scaled-up reactions (10 mmol scale) proceed easily, furnishing 84% and 83% yields of the hydrazones D43 and D46, respectively.
As tandem dehydrogenative condensation is compatible with a wide range of alkyl halides and alcohols, the scope with respect to the hydrazine was investigated (Scheme 3). With most aromatic hydrazines, the condensation proceeds smoothly to furnish the corresponding desired hydrazones in good to excellent yields. Although numerous hydrazines are in the form of hydrochlorides, hydrazones may be obtained in good to high yields in the presence of 1.5 equivalents of the base. The electronic properties of the phenyl rings of the hydrazines exhibit negligible effects on the reaction. The positions of the substituted functional groups on the phenyl ring clearly influence the efficacy of the transformation, and the corresponding hydrazones (D74–D77) are generated in moderate to good yields. Gram-scale reactions are successful, yielding D67 and D81 in high amounts.16 The transformations with halo-substituted hydrazines proceed smoothly to produce the corresponding desired products in 67–92% yields, thus enabling further complex reactions. Additionally, the F3C− group survives well the standard procedure, with the desired adducts D73 and D79 formed in good yields, and the naphthyl moieties may be smoothly incorporated into hydrazones. Meanwhile, several heterocyclic moieties, including pyridine and benzothiazole, are smoothly incorporated into the hydrazone molecules (D80–D81) using the corresponding hydrazines. Benzo hydrazide is less reactive with D82 isolated in a moderate yield. In addition, the desired hydrazone adducts, D84–D89, are obtained in moderate to good yields when using more challenging aliphatic hydrazine substrates. These results confirm the viability of the transformation for use in preparing highly functionalized hydrazone derivatives. Furthermore, various allyl halides are compatible with this catalytic dehydrogenation system with various hydrazines, and the desired adducts are isolated in moderate to high yields with excellent regioselectivities (D90–D92). Using this tandem dehydrogenation–condensation procedure, gram-scale reactions were successful, yielding D67 and D81 in high amounts.16
Several mechanistic investigations were performed under the optimal reaction conditions to yield additional data regarding this dehydrogenative condensation, and the results are shown in Fig. 2. Initially, H2 was analyzed using gas chromatography under the standard conditions, and D1 could be obtained in an excellent yield, with H2 detected in 81% yield [eqn (1)], and thus, dehydrogenation may be involved in the catalytic cycle. Additionally, the transformation of A1 under similar conditions produces D93 in a moderate yield, and H2 is detected in 42% yield, with similar results obtained in the presence of a catalytic amount of base [eqn (2)]. These results suggest that (i) dehydrogenation is not only promoted by the Mn catalyst and ligand but also by the catalytic amount of base and (ii) the base may accelerate the condensation process. As 1-benzyl-1-phenylhydrazine (D94) is detected when the reaction parameters are optimized, D94 is employed in combination with A1. A high yield of the desired product D1 is obtained under the standard conditions, but the reaction shuts down in the absence of the Mn catalyst or ligand, and D94 may be recovered in significant quantity. Similar results can be observed in the presence of a catalytic amount of base, and hydrogen is detected in 78% yield [eqn (3)].
Additionally, D93 may be employed as a partner for smooth condensation with D94 in the presence or absence of a catalyst, ligand, or base [eqn (4)]. However, only trace amounts of D1 are detected with a benzyl bromide partner, and D98 is obtained in a high yield [eqn (5)]. Hence, (i) D94 (in situ-generated) may be the intermediate because its transformation to D1 is distinctly observed in the time conversion plot (Fig. 3b); (ii) additives are unnecessary for the condensation process; and (iii) benzyl bromide does not condense under the standard conditions but undergoes nucleophilic substitution in the presence of a base. Furthermore, only trace amounts of D1 are detected with 1-benzylidene-2-phenylhydrazine (D95) as the coupling partner, and H2 is detected in a moderate yield. This is attributed to the lower nucleophilic attack activity of the NH condensation site of D95 towards an aldehyde group, and dehydrogenation is unaffected [eqn (6)]. Notably, benzyl bromide as a substrate reacts with D95, generating 87% yield of D1. D1 is obtained in a high yield in the absence of a Mn catalyst or a ligand, but the reaction stops in the absence of a base [eqn (7)]. Thus, (i) D95 could be the intermediate, as its transformation to D1 is undetected in the time conversion plot (Fig. 3b); (ii) a base is necessary for nucleophilic substitution; and (iii) A1 is involved in the hydrazone backbone that should undergo dehydrogenation.
Additionally, a Hg drop experiment was performed, resulting in acceptable yields of the desired product D1 without causing a significant decrease in yields. These results demonstrate the significant role of the Mn catalyst in promoting the dehydrogenative reaction. Thereafter, TEMPO was added as the radical scavenger under the standard conditions, and D1 was obtained in good yield, suggesting that this dehydrogenation could not be a free radical process [eqn (8)]. The time conversion plot reveals that the concentration of the intermediate D94 initially increases and then decreases with increasing reaction time. The concentration of D93 is maintained at trace levels, indicating that the condensation of the aldehyde with D94 is relatively rapid. These results further confirm that the formation of the intermediate D94 and the dehydrogenation are the rate-determining steps in the transformation reaction. Furthermore, only a trace amount of the product D98 was obtained when using D1 as the partner in the presence of H2 or iPrOH as the solvent under the standard conditions [eqn (9)]. These results show that the catalytic system is not sufficiently active for the catalytic reductive hydrogenation of ylidenehydrazines.
Although the precise mechanism remains unclear, a possible mechanism of the tandem dehydrogenation condensation is shown in Fig. 3a. Initially, the Mn species I is produced from Mn(CO)5Br and L11 in the presence of a base under catalytic reaction conditions. The oxidative addition of the alcohol to I generates the key alkoxy-Mn species II. The putative Mn species III may be generated via β-hydrogen elimination and release the aldehyde. Subsequent reductive elimination releases I and H2. Thereafter, I is re-oxidized to regenerate the active Mn catalyst for use in the next catalytic cycle. Aldehyde condensation with in situ-generated D94 from hydrazine and benzyl bromide produces the desired product. Another pathway involves the direct condensation of the aldehyde with hydrazine to produce D95, which yields the product via nucleophilic substitution with benzyl bromide in the presence of a base.
To further demonstrate the robustness of the tandem dehydrogenation condensation reaction, we conducted a reaction on a gram scale to produce D1 in a high yield (11.88 g) (Fig. 3).16 Ylidenehydrazines are attractive reactive building blocks that may be readily utilized in synthesizing functionalized complex products (Fig. 3c). D1 may be easily converted to functional nitrogenous heterocyclic compounds, such as benzimidazole (D97) and tetrazole (D99), via cyclization reactions. D98 is obtained in an excellent yield via reductive conversion; furthermore, a high yield of D96 is obtained via sulfonamidation. The CVB-5 pharmaceutical (D100) could be synthesis from D97.18 Furthermore, D98 could be produced into the desired inhibitory activity adrenoceptor pharmaceutical (D101).19 Additionally, with the newly developed catalytic system, the desired product (D102) was isolated in 86% yield, which could be transformed into the KDM4C inhibitor pharmaceutical toxoflavin (D103) in an acceptable isolated yield (Fig. 3d).20
Conclusions
In summary, we have initiated the unprecedented transition metal Mn-catalyzed tandem dehydrogenative condensation coupling of hydrazines with alcohols and halides to generate trisubstituted hydrazones, overcoming the challenge of selectivity in multicomponent reactions. Based on the complexation of the Earth-abundant Mn with a readily available PNN pincer ligand, this catalytic system not only provides an efficient method for preparing gram-scale (11.88 g) hydrazones but also facilitates the preparation of various novel hydrazone derivatives. Using this protocol, several pharmaceuticals may be easily synthesized. These are the first direct one-pot syntheses of hydrazones via dehydrogenation, liberating H2. This strategy significantly broadens the scope of Mn-catalyzed dehydrogenative condensation coupling for synthesizing unsaturated molecules. Studies aimed at obtaining a detailed mechanistic understanding of these tandem dehydrogenative condensation coupling reactions and the application of this strategy in other transformations are currently ongoing.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China (22002067 and 22202228), the Hundred-Talent Program of the Chinese Academy of Sciences, the Fund Program for the Scientific Activities of Selected Returned Overseas Professionals in Shanxi Province (20220052), the Science and Technology Project of Shanxi Province (202103021223457), and the State Key Laboratory of Coal Conversion, Institute of Coal Chemistry, Chinese Academy of Sciences (2021BWZ011).References
- (a) A. Raja, J. Lebbos and P. Kirkpatrick, Atazanavir sulphate, Nat. Rev. Drug Discovery, 2003, 2, 857–858 CrossRef CAS PubMed; (b) R. Chingle, C. Proulx and W. D. Lubell, Azapeptide synthesis methods for expanding sidechain diversity for biomedical applications, Acc. Chem. Res., 2017, 50, 1541–1556 CrossRef CAS PubMed; (c) Z. Ko-kan and M. J. Chmielewski, A photoswitchable heteroditopic ion-pair receptor, J. Am. Chem. Soc., 2018, 140, 16010–16014 CrossRef CAS PubMed; (d) M. Li, L. P. Cheng, W. Pang, Z. J. Zhong and L. L. Guo, Design, synthesis, and bio-logical evaluation of novel acylhydrazone derivatives as potent neuraminidase inhibitors, ACS Med. Chem. Lett., 2020, 11, 1745–1750 CrossRef CAS PubMed; (e) G. Bagnolini, B. Balboni, F. Schipani, D. Gioia, M. Veronesi, F. D. Franco, C. Kaya, R. P. Jumde, J. A. Ortega, S. Girotto, A. K. H. Hirsch, M. Roberti and A. Cavalli, Identification of RAD51–BRCA2 inhibitors using N-acylhydrazone-based dynamic combinatorial chemistry, ACS Med. Chem. Lett., 2022, 13, 1262–1269 CrossRef CAS PubMed.
- (a) J. W. Cahn and R. E. Powell, The Raschig synthesis of hy-drazine, J. Am. Chem. Soc., 1954, 76, 2565–2567 CrossRef CAS; (b) B. M. Trost and M. L. Crawley, Asymmetric transition-metal-catalyzed allylic alkylations: applications in total synthesis, Chem. Rev., 2003, 103, 2921–2944 CrossRef CAS PubMed; (c) M. Kilian and N. Martin, Catalytic activation of N-N multiple bonds: a homogeneous palladium catalyst for mechanistically unprecedented reduction of azo compounds, Angew. Chem., Int. Ed., 2006, 45, 2305–2308 CrossRef PubMed; (d) S. Shirakawa, P. J. Lombard and J. L. Leighton, A simple and general chiral silicon lewis acid for asymmetric synthesis: highly enantioselective [3 + 2] acylhydrazoneenol ether cycloadditions, J. Am. Chem. Soc., 2015, 127, 9974–9975 CrossRef PubMed; (e) A. J. Waldman, T. L. Ng, P. Wang and E. P. Balskus, Heteroatom–heteroatom bond formation in natural product biosynthesis, Chem. Rev., 2017, 117, 5784–5863 CrossRef CAS PubMed; (f) S. Shahi, H. Roghani-Mamaqani, R. Hoogenboom, S. Talebi and H. Mardani, Stimuli-responsive covalent adaptable hydrogels based on homolytic bond dissociation and chain transfer reactions, Chem. Mater., 2022, 34, 468–498 CrossRef CAS.
- (a) J. G. De Vries and C. J. Elsevier, The handbook of homogeneous hydrogenation, Wiley-VCH, Weinhem, 2007 Search PubMed; (b) J. F. Sonnenberg, N. Coombs, P. A. Dube and R. H. Morris, Iron nanoparticles catalyzing the asymmetric transfer hydrogenation of ketones, J. Am. Chem. Soc., 2012, 134, 5893–5899 CrossRef CAS PubMed.
- (a) F. Li, C. Sun and N. Wang, Catalytic acceptorless dehy-drogenative coupling of arylhydrazines and alcohols for the synthesis of arylhydrazones, J. Org. Chem., 2014, 79, 8031–8039 CrossRef PubMed; (b) L. Hao, G. Wang, J. Sun, J. Xu, H. Li, G. Duan, C. Xia and P. Zhang, From phenylhydrazone to 1H-1,2,4-triazoles via nitrification, reduction and cyclization, Adv. Synth. Catal., 2020, 362, 1657–1662 CrossRef CAS.
- (a) M. J. Hülsey, H. Yang and N. Yan, Sustainable routes for the synthesis of renewable heteroatom-containing chemicals, ACS Sustainable Chem. Eng., 2018, 6, 5694–5707 CrossRef; (b) S. Michlik and R. Kempe, A sustainable catalytic pyrrole synthesis, Nat. Chem., 2013, 5, 140–144 CrossRef CAS PubMed; (c) K. Weissermel and H.-J. Arpe, Industrial organic chemistry, Wiley-VCH, Weinheim, 2003 CrossRef.
- (a) A. Kumar, P. Daw and D. Milstein, Homogeneous catalysis for sustainable energy: hydrogen and methanol economies, fuels from biomass, and related topics, Chem. Rev., 2022, 122, 385–441 CrossRef CAS PubMed; (b) K. Barta and P. C. Ford, Catalytic conversion of nonfood woody biomass solids to organic liquids, Acc. Chem. Res., 2014, 47, 1503–1512 CrossRef CAS PubMed; (c) T. P. Vispute, H. Zhang, A. Sanna, R. Xiao and G. W. Huber, Renewable chemical commodity feedstocks from integrated catalytic processing of pyrolysis oils, Science, 2010, 330, 1222–1227 CrossRef CAS PubMed.
- (a) R. H. Crabtree, Deactivation in homogeneous transition metal catalysis: causes, avoidance, and cure, Chem. Rev., 2015, 115, 127–150 CrossRef CAS PubMed; (b) F. Kallmeier, T. Irrgang, T. Dietel and R. Kempe, Highly active and selective manganese C=O bond hydrogenation catalysts: the importance of the multidentate ligand, the ancillary ligands, and the oxidation state, Angew. Chem., Int. Ed., 2016, 55, 11806–11809 CrossRef CAS PubMed; (c) S. Elangovan, C. Topf, S. Fischer, H. Jiao, A. Spannenberg, W. Baumann, R. Ludwig, K. Junge and M. Beller, Selective catalytic hydrogenations of nitriles, ketones, and aldehydes by well-defined manganese pincer complexes, J. Am. Chem. Soc., 2016, 138, 8809–8814 CrossRef CAS PubMed; (d) M. Garbe, K. Junge, S. Walker, Z. Wei, H. Jiao, A. Spannenberg, S. Bachmann, M. Scalone and M. Beller, Manganese(I)-catalyzed enantioselective hydrogenation of ketones using a defined chiral PNP pincer ligand, Angew. Chem., Int. Ed., 2017, 56, 11237–11241 CrossRef CAS PubMed; (e) M. B. Widegren, G. J. Harkness, A. M. Z. Slawin, D. B. Cordes and M. L. Clarke, A highly active manganese catalyst for enantioselective ketone and ester hydrogenation, Angew. Chem., Int. Ed., 2017, 56, 5825–5828 CrossRef CAS PubMed.
- (a) G. Guillena, D. J. Ramon and M. Yus, Hydrogen autotrans-fer in the N-alkylation of amines and related compounds using alcohols and amines as electrophiles, Chem. Rev., 2010, 110, 1611–1641 CrossRef CAS PubMed; (b) J. Choi, A. H. MacArthur, M. Brookhart and A. S. Goldman, Dehydrogenation and related reactions catalyzed by iridium pincer complexes, Chem. Rev., 2011, 111, 1761–1779 CrossRef CAS PubMed; (c) Y. Obora, Recent advances in α-alkylation reactions using alcohols with hydrogen borrowing methodologies, ACS Catal., 2014, 4, 3972–3981 CrossRef CAS; (d) Q. Yang, Q. Wang and Z. Yu, Substitution of alcohols by N-nucleophiles via transition metal-catalyzed dehydrogenation, Chem. Soc. Rev., 2015, 44, 2305–2329 RSC; (e) A. Quintard and J. Rodriguez, A step into an eco-compatible future: iron- and cobalt-catalyzed borrowing hydrogen transformation, ChemSusChem, 2016, 9, 28–30 CrossRef CAS PubMed; (f) Y. Xie, Y. Ben-David, L. J. W. Shimon and D. Milstein, Highly efficient process for production of biofuel from ethanol catalyzed by ruthenium pincer complexes, J. Am. Chem. Soc., 2016, 138, 9077–9080 CrossRef CAS PubMed; (g) S. Lv, X. Han, J.-Y. Wang, M. Zhou, Y. Wu, L. Ma, L. Niu, W. Gao, J. Zhou, W. Hu, Y. Cui and J. Chen, Angew. Chem., Int. Ed., 2020, 59, 11583–11590 CrossRef CAS PubMed; (h) P. Zhang, B. Li, L. Niu, L. Wang, G. Zhang, X. Jia, G. Zhang, S. Liu, L. Ma, W. Gao, D. Qin and J. Chen, Scalable Electrochemical Transition-Metal-Free Dehydrogenative Cross-Coupling Amination Enabled Alkaloid Clausines Synthesis, Adv. Synth. Catal., 2020, 362, 2342–2347 CrossRef CAS.
- (a) C. O. Tuck, E. Perez, I. T. Horvath, R. A. Sheldon and M. Poliakoff, Valorization of biomass: deriving more value from waste, Science, 2012, 337, 695–699 CrossRef CAS PubMed; (b) W. Zuo, A. J. Lough, Y. F. Li and R. H. Morris, Amine(imine)diphosphine iron catalysts for asymmetric transfer hydrogenation of ketones and imines, Science, 2013, 342, 1080–1083 CrossRef CAS PubMed; (c) G. Zhang, K. V. Vasudevan, B. L. Scott and S. K. Hanson, Understanding the mechanisms of cobalt-catalyzed hydrogenation and dehydrogenation reactions, J. Am. Chem. Soc., 2013, 135, 8668–8681 CrossRef CAS PubMed; (d) C. Gunanathan and D. Milstein, Applications of acceptorless dehydrogenation and related transformations in chemical synthesis, Science, 2013, 341, 1229712 CrossRef PubMed; (e) S. Fleischer, S. Zhou, K. Junge and M. Beller, General and highly efficient iron-catalyzed hydrogenation of aldehydes, ketones, and α, β-unsaturated aldehydes, Angew. Chem., Int. Ed., 2013, 52, 5120–5124 CrossRef CAS PubMed; (f) T. Yan, B. L. Feringa and K. Barta, Iron catalysed direct alkylation of amines with alcohols, Nat. Commun., 2014, 5, 5602 CrossRef CAS PubMed; (g) S. Qu, Y. Dang, C. Song, M. Wen, K.-W. Huang and Z.-X. Wang, Catalytic mechanisms of direct pyrrole synthesis via dehydrogenative coupling mediated by PNP-Ir or PNN-Ru pincer complexes: crucial role of proton-transfer shuttles in the PNP-Ir system, J. Am. Chem. Soc., 2014, 136, 4974–4991 CrossRef CAS PubMed; (h) S. Chakraborty, H. Dai, P. Bhattacharya, N. T. Fairweather, M. S. Gibson, J. A. Krause and H. Guan, Iron-based catalysts for the hydrogenation of esters to alcohols, J. Am. Chem. Soc., 2014, 136, 7869–7872 CrossRef CAS PubMed; (i) D. Gärtner, A. Welther, B. R. Rad, R. Wolf and A. J. von Wangelin, Heteroatom-free arene-cobalt and arene-iron catalysts for hydrogenations, Angew. Chem., Int. Ed., 2014, 53, 3722–3726 CrossRef PubMed; (j) T. J. Korstanje, J. Ivar van der Vlugt, C. J. Elsevier and B. de Bruin, Hydrogenation of carboxylic acids with a homogeneous cobalt catalyst, Science, 2015, 350, 298–302 CrossRef CAS PubMed; (k) N. Deibl and R. Kempe, General and mild cobalt-catalyzed C-alkylation of unactivated amides and esters with alcohols, J. Am. Chem. Soc., 2016, 138, 10786–10789 CrossRef CAS PubMed; (l) L. De Luca and A. Mezzetti, Base-free asymmetric transfer hydrogenation of 1,2-di- and monoketones catalyzed by a (NH)2P2-macrocyclic iron(II) hydride, Angew. Chem., Int. Ed., 2017, 56, 11949–11953 CrossRef CAS PubMed; (m) W. Yang, I. Yu. Chernyshov, M. Weber, E. A. Pidko and G. A. Filonenko, Switching between hydrogenation and olefin transposition catalysis via Silencing NH cooperativity in Mn(I) pincer complexes, ACS Catal., 2022, 12, 10818–10825 CrossRef CAS PubMed; (n) Y. F. Gu, T. Y. Wang, M. Gao and Z. J. Yao, Scalable Cu(II)-mediated intramolecular dehydrogenative phenol-phenol coupling: Concise synthesis of enantiopure axially chiral homo- and hetero-diphenols, Chin. Chem. Lett., 2021, 32, 380–384 CrossRef CAS.
- (a) S. Elangovan, M. Garbe, H. Jiao, A. Spannenberg, K. Junge and M. Beller, Hydrogenation of esters to alcohols catalyzed by defined manganese pincer complexes, Angew. Chem., Int. Ed., 2016, 55, 15364–15368 CrossRef CAS PubMed; (b) M. Mastalir, M. Glatz, E. Pittenauer, G. Allmaier and K. Kirchner, Sustainable synthesis of quinolines and pyrimidines catalyzed by manganese PNP pincer complexes, J. Am. Chem. Soc., 2016, 138, 15543–15546 CrossRef CAS PubMed; (c) D. Milstein, A. Kumar, N.-A. Espinosa-Jalapa, G. Leitus, Y. Diskin-Posner and L. Avram, Direct synthesis of amides by dehydrogenative coupling of amines with either alcohols or esters: manganese pincer complex as catalyst, Angew. Chem., Int. Ed., 2017, 56, 14992–14996 CrossRef PubMed; (d) S. Chakraborty, U. K. Das, Y. Ben-David and D. Milstein, Manganese catalyzed α-olefination of nitriles by primary alcohols, J. Am. Chem. Soc., 2017, 139, 11710–11713 CrossRef CAS PubMed; (e) A. Zirakzadeh, S. R. M. M. de Aguiar, B. Stöger, M. Widhalm and K. Kirchner, Enantioselective transfer hydrogenation of ketones catalyzed by a manganese complex containing an unsymmetrical chiral PNP’ tridentate ligand, ChemCatChem, 2017, 9, 1744–1748 CrossRef CAS; (f) A. Dubey, L. Nencini, R. R. Fayzullin, C. Nervi and J. R. Khusnutdinova, Bio-inspired Mn(I) complexes for the hydrogenation of CO2 to formate and formamide, ACS Catal., 2017, 7, 3864–3868 CrossRef CAS; (g) A. Bruneau-Voisine, D. Wang, V. Dorcet, T. Roisnel, C. Darcel and J.-B. Sortais, Transfer hydrogenation of carbonyl derivatives catalyzed by an inexpensive phosphinepree manganese precatalyst, Org. Lett., 2017, 19, 3656–3659 CrossRef CAS PubMed; (h) O. Martínez-Ferraté, C. Werlé, G. Franciò and W. Leitner, Aminotriazole Mn(I) complexes as effective catalysts for transfer hydrogenation of ketones, ChemCatChem, 2018, 10, 4514–4518 CrossRef PubMed; (i) K. Ganguli, S. Shee, D. Panjaa and S. Kundu, Cooperative Mn(i)-complex catalyzed transfer hydrogenation of ketones and imines, Dalton Trans., 2019, 48, 7358–7366 RSC; (j) N. V. Shvydkiy, O. Vyhivskyi, Y. V. Nelyubina and D. S. Perekalin, Design of manganese phenol pi-complexes as shvo-type catalysts for transfer hydrogenation of ketones, ChemCatChem, 2019, 11, 1602–1605 CrossRef CAS; (k) S. Waiba, M. Maiti and B. Maji, Manganese-catalyzed reformation of vicinal glycols to α-hydroxy carboxylic acids with the liberation of hydrogen gas, ACS Catal., 2022, 12, 3995–4001 CrossRef CAS; (l) Q. Liang, C. Zhang, F. Wang, Z. Luo, W. Yang, G. Zhang, D. Ding and G. Zhang, Triazole backbone ligand in an unprecedented efficient manganese catalyst for use in transfer hydrogenation, Sci. China: Chem., 2023, 66, 2028–2036 CrossRef CAS.
- (a) Y. Zhou, X. Shan, R. Mas-Balleste, M. R. Bukowski, A. Stubna, M. Chakrabarti, L. Slominski, J. A. Halfen, E. Muenck and Q. Lawrence Jr., Contrasting cis and trans effects on the reactivity of nonheme oxoiron(IV) complexes, Angew. Chem., Int. Ed., 2008, 47, 1896–1899 CrossRef CAS PubMed; (b) B. Tu, Q. Pang, H. Xu, X. Li, Y. Wang, Z. Ma, L. Weng and Q. Li, Reversible redox activity in multicomponent meta-organic frameworks constructed from trinuclear copper pyrazolate building blocks, J. Am. Chem. Soc., 2017, 139, 7998–8007 CrossRef CAS PubMed; (c) K. Warm, G. Tripodi, E. Andris, S. Mebs, U. Kuhlmann, H. Dau, P. Hildebrandt, J. Roithová and K. Ray, Spectroscopic characterization of a reactive [Cu2(μ-OH)2]2+ intermediate in Cu/TEMPO catalyzed aerobic alcohol oxidation reaction, Angew. Chem., Int. Ed., 2021, 60, 23018–23024 CrossRef CAS PubMed.
- (a) Y. Wang, D. Zhu, L. Tang, S. Wang and Z. Wang, Highly efficient amide synthesis from alcohols and amines by virtue of a water-soluble gold/DNA catalyst, Angew. Chem., Int. Ed., 2011, 50, 8917–8921 CrossRef CAS PubMed; (b) M. Tamura and K. Tomishige, Redox properties of CeO2 at low temperature: the direct synthesis of imines from alcohol and amine, Angew. Chem., Int. Ed., 2015, 54, 864–867 CrossRef CAS PubMed; (c) C. Zhang, Q. Liang, W. Yang, G. Zhang, M. Hu and G. Zhang, Nickel(I)-catalyzed (de)hydrogenative coupling of amines and alkyl het-eroarenes with alcohols, Green Chem., 2022, 24, 7368–7375 RSC.
- G. Zhang, T. Irrgang, M. Schlagbauer and R. Kempe, Synthesis of 1,3-diketones from esters via liberation of hydrogen, Chem. Catal., 2021, 1, 681–690 CrossRef CAS.
- (a) N. Deibl and R. Kempe, Manganese-Catalyzed Multicom-ponent Synthesis of Pyrimidines from Alcohols and Ami-dines, Angew. Chem., Int. Ed., 2017, 56, 1663–1666 CrossRef CAS PubMed; (b) G. Zhang, T. Irrgang, T. Dietel, F. Kallmeier and R. Kempe, Manganese-catalyzed dehydrogenative alkylation or α-olefination of alkyl-substituted N-heteroarenes with alcohols, Angew. Chem., Int. Ed., 2018, 57, 9131–9135 CrossRef CAS PubMed.
- (a) E. Balaraman, E. Khaskin, G. Leitus and D. Milstein, Catalytic transformation of alcohols to carboxylic acid salts and H2 using water as the oxygen atom source, Nat. Chem., 2013, 5, 122–125 CrossRef CAS PubMed; (b) S. Elangovan, J. Neumann, J.-B. Sortais, K. Junge, C. Darcel and M. Beller, Efficient and selective N-alkylation of amines with alcohols catalysed by manganese pincer complexes, Nat. Commun., 2016, 7, 12641 CrossRef PubMed; (c) M. Perez, S. Elangovan, A. Spannenberg, K. Junge and M. Beller, Molecularly defined manganese pincer complexes for selective transfer hydrogenation of ketones, ChemSusChem, 2017, 10, 83–86 CrossRef CAS PubMed; (d) M. Mastalir, E. Pittenauer, G. Allmaier and K. Kirchner, Manganese-catalyzed aminomethylation of aromatic compounds with methanol as a sustainable C1 building block, J. Am. Chem. Soc., 2017, 139, 8812–8815 CrossRef CAS PubMed; (e) G. A. Filonenko, R. van Putten, E. J. M. Hensen and E. A. Pidko, Catalytic (de)hydrogenation promoted by non-precious metals – Co, Fe and Mn: recent advances in an emerging field, Chem. Soc. Rev., 2018, 47, 1459–1483 RSC; (f) Y. Wang, M. Wang, Y. Li and Q. Liu, Homogeneous manganese-catalyzed hydrogenation and dehydrogenation reactions, Chem, 2021, 7, 1180–1223 CrossRef CAS; (g) A. E. Owen, A. Preiss, A. McLuskie, C. Gao, G. Peters, M. Bühl and A. Kumar, Manganese-catalyzed dehydrogenative synthesis of urea derivatives and polyureas, ACS Catal., 2022, 12, 6923–6933 CrossRef CAS.
- See the ESI† for details.
- D4 (CCDC 2288831), D26 (CCDC 2288839).†.
- (a) M. Tonelli, F. Novelli, B. Tasso, I. Vazzana, A. Sparatore, V. Boido, F. Sparatore, P. L. Colla, G. Sanna, G. Giliberti, B. Busonera, P. Farci, C. Ibba and R. Loddo, Antiviral activity of benzimidazole derivatives. III. novel anti-CVB-5, anti-RSV and anti-Sb-1 agents, Bioorg. Med. Chem., 2014, 22, 4893–4909 CrossRef CAS PubMed; (b) V. Boido, G. Paglietti, M. Tonelli and G. Vitale, Nonnucleoside benzimidazoles as antiviral drugs against HIV infections, in RNA-Viruses. Enzymatic and Receptoral Inhibitors, ed. A. Carta, Research Signpost, Kerala, 2009, pp. 1–39 Search PubMed.
- C. H. Boehringer, Sohn AG & Co., KG-US2009/12161, A9, 2009.
- (a) J. Zeller, A. J. Turbiak, I. A. Powelson, S. Lee, D. Sun, H. D. Hollis Showalter and E. R. Fearon, Investigation of 3-aryl-pyrimido[5,4-e] [1,2,4] triazine-5,7-diones as small molecule antagonists of β-catenin/TCF transcription, Bioorg. Med. Chem., 2013, 23, 5814–5820 CrossRef CAS PubMed; (b) V. Letfus, D. Jelić, A. Bokulić, A. Petrinić Grba and S. Koštrun, Rational design, synthesis and biological profiling of new KDM4C inhibitors, Bioorg. Med. Chem., 2020, 28, 115128 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2288831 and 2288839. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3qo01890c |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2024 |