Facile and practical access to chiral benzofused oxa-heterocycles via an asymmetric hydrogenation and intramolecular SNAr cascade†
Abstract
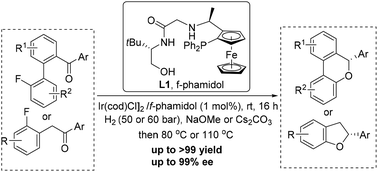
Development of efficient protocols for the synthesis of chiral benzofused oxa-heterocycles has attracted tremendous interest due to the ubiquitous presence of these structural motifs in numerous bioactive compounds and antibiotics. Herein, we report a tandem asymmetric hydrogenation and intramolecular SNAr reaction, providing a facile and practical access to valuable six- and five-membered chiral benzofused cyclic ethers with high yields and enantioselectivities (up to 99% yield and up to 99% ee). Furthermore, the synthetic potential of the reaction was demonstrated by a gram-scale experiment.
- This article is part of the themed collection: Organic Chemistry Frontiers Emerging Investigator Series 2024–2025


 Please wait while we load your content...
Please wait while we load your content...