Copper-catalyzed crosscoupling of vinyl nitrenes and CF3-carbenes to synthesize CF3-2-azadienes†
Yongjuan
Jiao
a,
Tao
Wu
a,
Xinyu
Zhang
a and
Yaojia
Jiang
 *ab
*ab
aInstitute of Advanced Synthesis, School of Chemistry and Molecular Engineering, Nanjing Tech University, Nanjing 211816, China. E-mail: iamyjjiang@njtech.edu.cn
bLaboratory Breeding Base of Green Pesticide and Agricultural Bioengineering, Key Laboratory of Green Pesticide and Agricultural Bioengineering Ministry of Education, Guizhou University, Huaxi, Guiyang 550025, China
First published on 7th November 2023
Abstract
A unique and efficient copper catalytic system has been established to synthesize fluorinated 2-azadienes from isoxazoles and CF3-N-tosylhydrazones via crosscoupling reactions of vinyl nitrenes with CF3-carbenes. A proposed reaction pathway involves the tandem cleavage of N–O/C–N bonds and formation of a C–N double bond. The developed method features a broad substrate scope, easy handling, step economy, and well-defined regio- and stereoselectivities.
Introduction
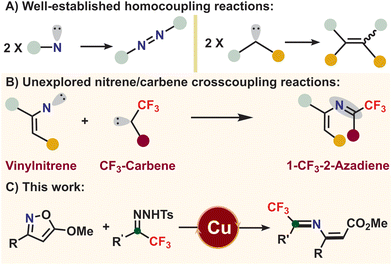
The eternal pursuit of organic chemists is the construction of aggregated functional molecules from simple and easily available substrates with high chemo- and regioselectivities.1 The past few decades have witnessed remarkable progress in reactions involving reactive species such as carbenes and nitrenes to design key skeletons owing to well-established catalytic systems based on transition metals.2 Notably, while carbenes and nitrenes are employed to participate in various unique and efficient transformations, including C–X bond (X = H, N, O, etc.) insertions,3 ylide formations,4 and cycloadditions,5 they are prone to undergo homocoupling reactions yielding undesired products (Fig. 1A).6 Although several researchers have applied this homocoupling strategy to prepare highly functionalized alkenes,7 crosscoupling reactions of carbenes with nitrenes have received limited exploration, perhaps due to chemo- and regioselective issues of these highly reactive species.8 We have envisioned that suitable precursors might be essential for realizing such nonhomocoupling reactions of carbenes with nitrenes.Inspired by recent works of Wang and Bi and spurred on by our previous results, we have developed interest in α-fluoroalkyl-N-tosylhydrazones. This interest is also driven by the balance between the reactivity and stability of their carbene precursors, attributed to the unique effect of the fluoride group.9 Furthermore, employing α-CF3-N-tosylhydrazones as carbene precursors offers the possibility of not only coupling them with nitrenes but also introducing CF3 groups into molecules, which are difficult to achieve using other available methods.10 Conversely, several representative nitrene precursors, such as azides,11 arylsulfonyl hydroxylamines,12 amines,13 and nitro compounds,14 take part in amination reactions through denitrogenation, α-elimination, oxidation, and deoxygenation reduction processes. For instance, the Chiba and Jiao groups employed vinyl azides as vinyl nitrene precursors to synthesize aza-heterocycles and N-containing molecules.15 Despite these achievements, many of these reactions involve toxic substances, harsh reaction conditions, and high potential risk of explosion.16 In our continuing interest in 2H-azirine and isoxazole chemistry,17 we envisioned that isoxazoles, as easily available and bench-stable heterocycles, could be used as vinyl nitrene precursors to react with α-CF3-N-tosylhydrazones through the tandem cleavage of N–O/C–N bonds and formation of a C–N double bond (Fig. 1B).18 Herein, we disclose a unique and efficient copper catalytic system for synthesizing fluorinated 2-azadienes from isoxazoles and CF3-N-tosylhydrazones via crosscoupling reactions of fluoroalkyl carbenes with vinyl nitrenes (Fig. 1C). Notably, CF3-2-azadienes are key skeletons that can not only be applied in diverse organic transformations, including nucleophilic addition, aziridination, and aza-Diels–Alder cyclization reactions,19 but also be potentially applied in biological and materials science.20
Results and discussion
We employed isoxazole 1a and CF3-N-tosylhydrazone 2a as model substrates to synthesize fluorinated aza-containing molecules (Table 1). Initially, various copper catalysts were screened with K2CO3 as the base in toluene at 90 °C; the results showed copper catalyst to be essential for this transformation, and Cu(I) and Cu(II) performed well at generating 1-CF3-2-aza-1,3-diene 3aa with yields of 29%–57% (Table 1, entries 1–8). NMR spectroscopy and single-crystal X-ray data were collected to determine the structure and geometry of 3aa (CCDC 2246278†). As CuCl2 worked more efficiently than other catalysts, various solvents were investigated in the presence of CuCl2 and K2CO3 (Table 1, entries 9–13). This investigation revealed that aromatic solvents such as xylene and PhCF3 were more compatible with this reaction than DCM, THF, and hexane. PhCF3 stood out with a high product yield of 68% occurring in its presence (Table 1, entry 13). To our delight, modifying the reaction temperature to 110 °C further significantly increased the reaction yield to 82% (Table 1, entries 14–16). Examination of other bases, however, failed to improve the reaction efficiency (Table 1, entries 17–20), causing retention of the starting material in the absence of the base (Table 1, entry 20).| Entry | Catalyst | Solvent | Base | Temp. (°C) | Yieldb (%) |
|---|---|---|---|---|---|
| a Unless otherwise specified, the reactions were performed using 1a (0.20 mmol), 2a (0.40 mmol), copper catalyst (10 mol%), base (2.5 equiv.), and solvent (2.0 mL) at 110 °C in a sealed tube until 1a was consumed completely according to thin later chromatography analysis. b Isolated yield. Tol. = toluene, Hex. = hexane. | |||||
| 1 | CuCl | Tol. | K2CO3 | 90 | 45 |
| 2 | CuBr | Tol. | K2CO3 | 90 | 50 |
| 3 | CuI | Tol. | K2CO3 | 90 | 33 |
| 4 | Cu(OAc)2 | Tol. | K2CO3 | 90 | 29 |
| 5 | Cu(acac)2 | Tol. | K2CO3 | 90 | 44 |
| 6 | Cu(OTf)2 | Tol. | K2CO3 | 90 | 29 |
| 7 | CuBr2 | Tol. | K2CO3 | 90 | 54 |
| 8 | CuCl2 | Tol. | K2CO3 | 90 | 57 |
| 9 | CuCl2 | DCM | K2CO3 | 90 | 37 |
| 10 | CuCl2 | THF | K2CO3 | 90 | 0 |
| 11 | CuCl2 | Hex. | K2CO3 | 90 | 16 |
| 12 | CuCl2 | Xylene | K2CO3 | 90 | 56 |
| 13 | CuCl2 | PhCF3 | K2CO3 | 90 | 68 |
| 14 | CuCl2 | PhCF3 | K2CO3 | 80 | 40 |
| 15 | CuCl2 | PhCF3 | K2CO3 | 100 | 74 |
| 16 | CuCl 2 | PhCF 3 | K 2 CO 3 | 110 | 82 |
| 17 | CuCl2 | PhCF3 | Na2CO3 | 110 | 41 |
| 18 | CuCl2 | PhCF3 | Cs2CO3 | 110 | 30 |
| 19 | CuCl2 | PhCF3 | t BuOK | 110 | 25 |
| 20 | CuCl2 | PhCF3 | — | 110 | 0 |
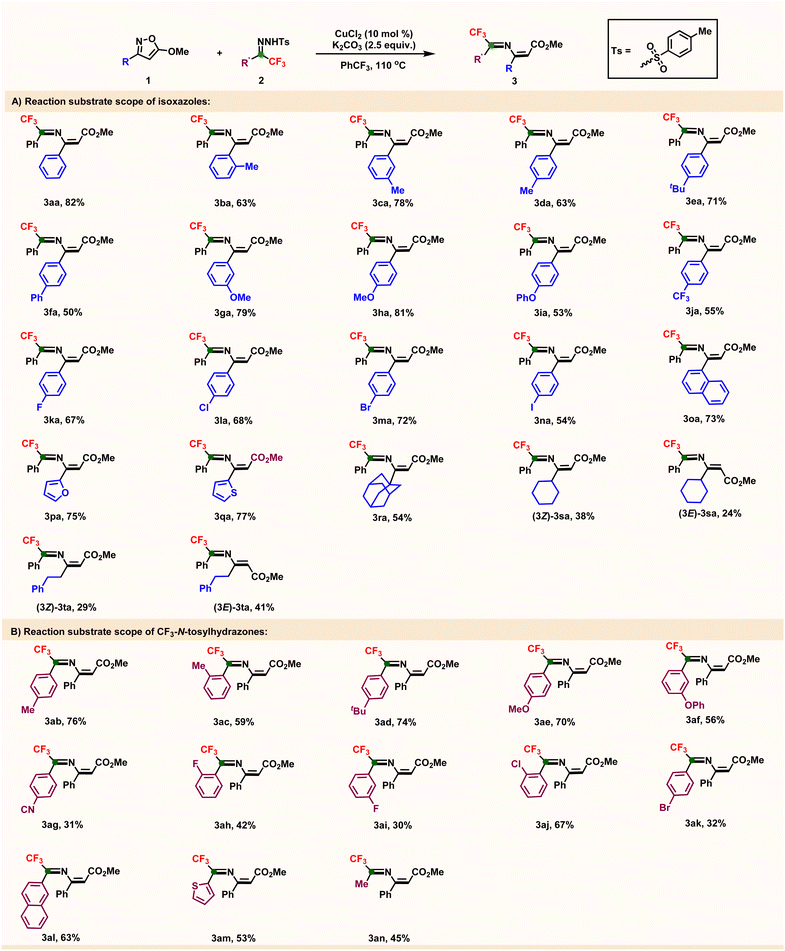
With the established catalytic system in hand, we then investigated the reaction substrate scope, specifically testing various substituted 5-methoxyl-isoxazoles 1 and CF3-N-tosylhydrazones 2 (Table 2). At the outset, diverse functional isoxazoles 1 were examined (Table 2-A). The results showed that reactions with these substrates proceeded smoothly under the standard reaction conditions to afford 2-aza-1,3-dienes in moderate-to-good yields. Aryl-substituted isoxazoles with different alkyl groups inserted into the aromatic ring all worked well, generating desired product yields of 63%–82% (3aa–3ea); a phenyl-substituted substrate also reacted to afford the corresponding product, but in a lower yield (3fa, 50%). Subsequently, we investigated electronic effects by examining various substituted isoxazoles including electron-donating (–OMe and –OPh) and electron-withdrawing (–CF3) substituents. Electron-rich substrates generally gave higher yields (3ga–3ia, 53%–81%) than electron-poor ones (3ja, 55%). Aromatic-substituted isoxazoles bearing halides (–F, –Cl, –Br, and –I) were quite compatible with this modular catalytic system and delivered the corresponding fluorinated azadienes in moderate-to-good yields (3ka–3na, 54%–72%). Notably, these halogenic functionalities are widely employed in transition-metal-catalyzed coupling reactions.21 Herein, naphthyl-substituted isoxazole 1o also worked well and afforded a product with 73% yield (3oa). Additionally, the furyl and thienyl heterocyclic groups survived this mild reaction system and offered the corresponding 2-aza-1,3-dienes in 75% and 77% yields, respectively (3pa and 3qa). Alkyl-substituted isoxazoles including bulky, cyclic, and linear groups also reacted smoothly and provided desired products with yields of 54%–70% (3ra–3ta). Notably, E and Z isomers of the C–C double bonds were isolated, indicating the loss of the steric effect (see ESI†). Subsequently, the scope of the CF3-N-tosylhydrazones 2 substrate was investigated under the same catalytic conditions (Table 2-B). Various aromatic rings with alkyl-substituted CF3-N-tosylhydrazones reacted efficiently with isoxazole 1a, yielding the construction of fluorinated 2-azadienes in moderate-to-good yields (3ab–3ad). Substituted CF3-N-tosylhydrazones with electron-rich and electron-poor groups performed well, though the tested electron-poor substituted N-tosylhydrazone gave a lower yield (3ag, 31%) than electron-rich functionalities (3ae and 3af). Reactions with halogenic substitutions (–F, –Cl, and –Br) at different positions on the aryl ring proceeded smoothly under standard conditions, although with quite different yields (3ah–3ak). Furthermore, naphthyl and thienyl substrates afforded the corresponding azadiene products in 63% and 53% yields, respectively (3al–3am). Finally, methyl-substituted N-tosylhydrazone 2an also reacted smoothly and provided the desired product, albeit in a relatively low yield (3an, 45%).
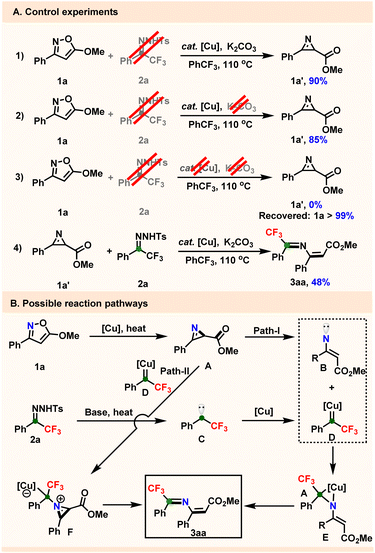
Several control experiments were conducted to derive possible reaction mechanisms (Scheme 1). Initially, isoxazole 1a was deployed under the standard reaction conditions in the absence of trifluoromethyl N-tosylhydrazone 2a to produce carboxylic 2H-azirine 1a′ in 90% yield (Scheme 1A-1). This result prompted us to further study whether the 2H-azirine 1a′ is the key intermediate of the catalytic cycle. We found that the copper catalyst is essential to the ring contraction of isoxazole 1a to 2H-azirine 1a′ even without the base (Scheme 1A-2 and A-3). Notably, the reaction of 1a′ with 2a under standard reaction conditions generated corresponding CF3-2-azadiene 3aa, albeit in a relatively low yield (48%). Based on these obtained results, a plausible mechanism is proposed taking a model reaction as an example. Initially, the isoxazole 1a ring contracts to 2H-azirine A, following previous mechanism-study results, with cleavage of one of the C–N bonds of A, further generating vinyl nitrene B. Conversely, the conversion of CF3-N-tosylhydrazone 2a to fluoroalkyl carbene C under an external heating source was promoted by K2CO3, with carbene C then directly getting converted into carbenoid D with copper catalyst.22 We envisioned that there might be two possible pathways here. For path-I, coupling of the free nitrene of B with carbenoid D generates copper complex E, with this intermediate E releasing the copper catalyst and isomerizing to give the corresponding azadiene 3aa. In path-II, carbene complex D is directly attacked by the nitrogen lone pair of 2H-azirine A to provide copper counterion intermediate F,23 with 3aa then obtained as a result of a C–N bond cleavage in the small ring.
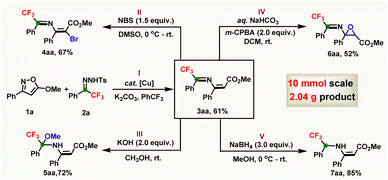
Notably, we were able to extend this method to a scale of 10 mmol for 1a in the standard catalytic system, here delivering the corresponding azadiene 3aa in 61% yield (2.03 g) (Scheme 2-I). Late-stage functionalization of fluorinated azadiene 3aa was then investigated. For instance, 3aa was easily brominated under mild conditions via reaction with N-bromo succinimide in dimethyl sulfoxide, and this reaction provided 4-bromo-2-aza-1,3-diene 4aa in 67% yield (Scheme 2-II).24 Addition of the nucleophile methanol to the very active C–N double bond of 2-aza-1,3-diene 3aa provided 5aa in 72% yield under basic conditions (Scheme 2-III).25 Conversely, the C–C double bond of 3aa smoothly to afford epoxide 6aa with retention of the C–N double bond; here, 6aa was afforded in only moderate yield (Scheme 2-IV).26 Furthermore, by employing NaBH4, the C–N double bond of azadiene 3aa was chemoselectively reduced to generate fluorinated enamine 7aa in 85% yield (Scheme 2-V).27 These obtained results showed the potential for application of the designed strategy in industry and academic areas.
Conclusions
A straightforward and efficient method for synthesizing CF3-substituted 2-azadienes was described. The reaction transformation was concluded to proceed through the tandem cleavage of N–O/C–N bonds and formation of a C–N double bond in the presence of a copper catalyst with well-defined regio- and stereoselectivities. This unique strategy can be accomplished using an easy-handling procedure and mild catalytic system that has tolerance to diverse functionalities.Author contributions
Y. Jiao and T. Wu contributed equally to this work. Y. Jiao, T. Wu and X. Zhang performed the experiments. Y. Jiang conceived and directed the project and wrote the manuscript. All authors discussed the results.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the funding received from the National Natural Science Foundation of China (21971112 and 22361010) and the Starting Funding of Research from Guizhou University.References
-
(a) X. Zhu and S. Chiba, Copper-catalyzed Oxidative Carbon–Heteroatom Bond Formation: A Recent Update, Chem. Soc. Rev., 2016, 45, 4504–4523 RSC
; (b) R. Blieck, M. Taillefer and F. Monnier, Metal-Catalyzed Intermolecular Hydrofunctionalization of Allenes: Easy Access to Allylic Structures via the Selective Formation of C–N, C–C, and C–O Bonds, Chem. Rev., 2020, 120, 13545–13598 CrossRef CAS PubMed
; (c) M.-Z. Lu, J. Goh, M. Maraswami, Z. Jia, J.-S. Tian and T.-P. Loh, Recent Advances in Alkenyl sp2 C–H and C–F Bond Functionalizations: Scope, Mechanism, and Applications, Chem. Rev., 2022, 122, 17479–17646 CrossRef CAS PubMed
.
-
(a) D. Zhu, L. Chen, H. Fan, Q. Yao and S. Zhu, Recent Progress on Donor and Donor–Donor Carbenes, Chem. Soc. Rev., 2020, 49, 908–950 RSC
; (b) C. Wentrup, Carbenes and Nitrenes: Recent Developments in Fundamental Chemistry, Angew. Chem., Int. Ed., 2018, 57, 11508–11521 CrossRef CAS
; (c) Y.-C. Wang, X.-J. Lai, K. Huang, S. Yadav, G. Qiu, L. Zhang and H. Zhou, Unravelling Nitrene Chemistry from Acyclic Precursors: Recent Advances and Challenges, Org. Chem. Front., 2021, 8, 1677–1693 RSC
.
-
(a) J. Rong, H. Li, R. Fu, W. Sun, T.-P. Loh and Y. Jiang, Cleavage and Reassembly C≡C Bonds of Ynones to Access Highly Functionalized Ketones, ACS Catal., 2020, 10, 3664–3669 CrossRef CAS
; (b) M.-Y. Huang and S.-F. Zhu, Catalytic Reactions for Enantioselective Transfers of Donor-Substituted Carbenes, Chem. Catal., 2022, 2, 3112–3139 CrossRef CAS
; (c) Z. K. Liu, Q. Q. Zhao, Y. Gao, Y. X. Hou and X. Q. Hu, Organic Azides: Versatile Synthons in Transition Metal–Catalyzed C(sp2)−H Amination/Annulation for N–Heterocycle Synthesis, Adv. Synth. Catal., 2021, 363, 411–424 CrossRef CAS
.
- S. Dong, X. Liu and X. Feng, Asymmetric Catalytic Rearrangements with α-Diazocarbonyl Compounds, Acc. Chem. Res., 2022, 55, 415–428 CrossRef CAS PubMed
.
-
(a) M. Mato, A. Franchino, C. García-Morales and A. M. Echavarren, Gold-Catalyzed Synthesis of Small Rings, Chem. Rev., 2021, 121, 8613–8684 CrossRef CAS PubMed
; (b) J. L. Jat, M. P. Paudyal, H. Gao, Q.-L. Xu, M. Yousufuddin, D. Devarajan, D. H. Ess, L. Kürti and J. R. Falck, Direct Stereospecific Synthesis of Unprotected N-H and N-Me Aziridines from Olefins, Science, 2014, 343, 61–65 CrossRef CAS PubMed
; (c) A. Fanourakis, N. J. Hodson, A. R. Lit and R. J. Phipps, Substrate-Directed Enantioselective Aziridination of Alkenyl Alcohols Controlled by a Chiral Cation, J. Am. Chem. Soc., 2023, 145, 7516–7527 CrossRef CAS PubMed
.
-
(a) D. S. Davas, S. Bhardwaj, R. Sen, D. K. Gopalakrishnan and J. Vaitla, Synthesis of Olefins by Formal Carbene Coupling, Adv. Synth. Catal., 2022, 364, 3122–3142 CrossRef CAS
; (b) S. S. Kurup and S. Groysman, Catalytic Synthesis of Azoarenes via Metal-Mediated Nitrene Coupling, Dalton Trans., 2022, 51, 4577–4589 RSC
.
-
(a) Y. Xia, Z. Liu, Q. Xiao, P. Qu, R. Ge, Y. Zhang and J. Wang, Rhodium(II)-Catalyzed Cyclization of Bis(N-tosylhydrazone)s: An Efficient Approach towards Polycyclic Aromatic Compounds, Angew. Chem., Int. Ed., 2012, 51, 5714–5717 CrossRef CAS PubMed
; (b) Q. Song, Y. Zhao, S. Liu, Y. Wu and Z. Liu, Stereoselective Synthesis of trans-Stilbenes through Silver-Catalyzed Self-Coupling of N-Triftosylhydrazones: An Experimental and Theoretical Study, Org. Lett., 2023, 25, 3461–3465 CrossRef CAS PubMed
.
-
(a) N. S. Y. Loy, A. Singh, X. Xu and C.-M. Park, Synthesis of Pyridines by Carbenoid-Mediated Ring Opening of 2H-Azirines, Angew. Chem., Int. Ed., 2013, 52, 2212–2216 CrossRef CAS
; (b) X.-P. Wu, Y. Su and P. Gu, Catalytic Enantioselective Desymmetrization of 1,3-Diazido-2-propanol via Intramolecular Interception of Alkyl Azides with Diazo(aryl)acetates, Org. Lett., 2014, 16, 5339–5341 CrossRef CAS PubMed
; (c) Z. Fang, Y. Gong, B. Liu, J. Zhang, X. Han, Z. Liu and Y. Ning, Rh-Catalyzed Coupling Reactions of Fluoroalkyl N-Sulfonylhydrazones with Azides Leading to α-Trifluoroethylated Imines, Org. Lett., 2022, 24, 8920–8924 CrossRef CAS PubMed
.
-
(a) X. Wang, Y. Xu, Y. Deng, Y. Zhou, J. Feng, G. Ji, Y. Zhang and J. Wang, Pd-Carbene Migratory Insertion: Application to the Synthesis of Trifluoromethylated Alkenes and Dienes, Chem. – Eur. J., 2014, 20, 961–965 CrossRef CAS PubMed
; (b) Z. Zhang, W. Yu, Q. Zhou, T. Li, Y. Zhang and J. Wang, Rh(I)-Catalyzed Reaction of Trifluoromethylketone N-Tosylhydrazones and Arylboronates, Chin. J. Chem., 2016, 34, 473–476 CrossRef CAS
; (c) X. Zhang, Z. Liu, X. Yang, Y. Dong, M. Virelli, G. Zanoni, E. A. Anderson and X. Bi, Use of Trifluoroacetaldehyde N-Tfsylhydrazone as a Trifluorodiazoethane Surrogate and Its Synthetic Applications, Nat. Commun., 2019, 10, 284 CrossRef PubMed
; (d) X. Zhang, Y. Ning, Z. Liu, S. Li, G. Zanoni and X. Bi, Defluorinative Carboimination of Trifluoromethyl Ketones, ACS Catal., 2022, 12, 8802–8810 CrossRef CAS
; (e) X. Liang, P. Guo, W. Yang, M. Li, C. Jiang, W. Sun, T.-P. Loh and Y. Jiang, Stereoselective Synthesis of Trifluoromethyl-Substituted 2H-Furan-Amines from Enaminones, Chem. Commun., 2020, 56, 2043–2046 RSC
; (f) X. Zhang, J. Zhang, J. Chen, B. Zhou, J. Zhang, S. Chen, J. Wu and Y. Jiang, Modular Synthesis of Fluorinated 2H-Thiophenes via, [4+1] Cyclization of Enaminothiones, Org. Biomol. Chem., 2023, 21, 3345–3349 RSC
.
-
(a) P. Sivaguru and X. Bi, Fluoroalkyl N-Sulfonyl Hydrazones: An Efficient Reagent for the Synthesis of Fluoroalkylated Compounds, Sci. China: Chem., 2021, 64, 1614–1629 CrossRef CAS
; (b) Z. Liu, P. Sivaguru, G. Zanoni and X. Bi, N-Triftosylhydrazones: A New Chapter for Diazo-Based Carbene Chemistry, Acc. Chem. Res., 2022, 55, 1763–1781 CrossRef CAS PubMed
.
-
(a) J. Fu, G. Zanoni, E. A. Anderson and X. Bi,
α-Substituted Vinyl Azides: an Emerging Functionalized Alkene, Chem. Soc. Rev., 2017, 46, 7208–7228 RSC
; (b) B. Plietker and A. Röske, Recent Advances in Fe-Catalyzed C–H Aminations using Azides as Nitrene Precursors, Catal. Sci. Technol., 2019, 9, 4188–4197 RSC
.
-
(a) Y. Gao, H. Li, Y. Zhao and X.-Q. Hu, Nitrene Transfer Reaction with Hydroxylamine Derivatives, Chem. Commun., 2023, 59, 1889–1906 RSC
; (b) U. Todorović, R. M. Romero and L. Anthore-Dalion, Activation of N−O σ Bonds with Transition Metals: A Versatile Platform for Organic Synthesis and C−N Bonds Formation, Eur. J. Org. Chem., 2023, 26, e202300391 CrossRef
.
-
(a) J. L. Roizen, M. E. Harvey and J. D. Bois, Metal-Catalyzed Nitrogen-Atom Transfer Methods for the Oxidation of Aliphatic C-H Bonds, Acc. Chem. Res., 2012, 45, 911–922 CrossRef CAS PubMed
; (b) J. M. Alderson, J. R. Corbin and J. M. Schomaker, Chemo- and Site-Selective Nitrene Transfer Reactions through the Rational Design of Silver(I) Catalysts, Acc. Chem. Res., 2017, 50, 2147–2158 CrossRef CAS PubMed
.
-
(a) T. V. Nykaza, A. Ramirez, T. S. Harrison, M. R. Luzung and A. T. Radosevich, Biphilic Organophosphorus-Catalyzed Intramolecular Csp2−H Amination: Evidence for a Nitrenoid in Catalytic Cadogan Cyclizations, J. Am. Chem. Soc., 2018, 140, 3103–3113 CrossRef CAS PubMed
; (b) S. Suárez-Pantiga, R. Hernández-Ruiz, C. Virumbrales, M. R. Pedrosa and R. Sanz, Reductive Molybdenum-Catalyzed Direct Amination of Boronic Acids with Nitro Compounds, Angew. Chem., Int. Ed., 2019, 58, 2129–2133 CrossRef PubMed
.
-
(a) Y. F. Wang and S. Chiba, Mn(III)-Mediated Reactions of Cyclopropanols with Vinyl Azides: Synthesis of Pyridine and 2-Azabicyclo[3.3.1]non-2-en-1-ol Derivatives, J. Am. Chem. Soc., 2009, 131, 12570–12572 CrossRef CAS PubMed
; (b) Y. F. Wang, K. K. Toh, E. P. J. Ng and S. Chiba, Mn(III)-Mediated Formal [3+3]-Annulation of Vinyl Azides and Cyclopropanols: A Divergent Synthesis of Azaheterocycles, J. Am. Chem. Soc., 2011, 133, 6411–6421 CrossRef CAS PubMed
; (c) Y.-F. Wang, K. K. Toh, J.-Y. Lee and S. Chiba, Synthesis of Isoquinolines from α-Aryl Vinyl Azides and Internal Alkynes by Rh-Cu Bimetallic Cooperation, Angew. Chem., Int. Ed., 2011, 50, 5927–5931 CrossRef CAS PubMed
; (d) Y.-F. Wang, G. H. Lonca and S. Chiba, PhI(OAc)2-M ediated Radical Trifluoromethylation of Vinyl Azides with Me3SiCF3, Angew. Chem., Int. Ed., 2014, 53, 1067–1071 CrossRef CAS PubMed
; (e) F.-L. Zhang, Y.-F. Wang, G. H. Lonca, X. Zhu and S. Chiba, Amide Synthesis by Nucleophilic Attack of Vinyl Azides, Angew. Chem., Int. Ed., 2014, 53, 4390–4394 CrossRef CAS PubMed
; (f) F. Chen, T. Shen, Y. X. Cui and N. Jiao, 2,4- vs 3,4-Disubsituted Pyrrole Synthesis Switched by Copper and Nickel Catalysts, Org. Lett., 2012, 14, 4926–4929 CrossRef CAS PubMed
.
- F. Gholami, F. Yousefnejad, B. Larijani and M. Mahdavi, Vinyl Aides in Organic Synthesis: An Overview, RSC Adv., 2023, 13, 990–1018 RSC
.
-
(a) Y. Jiang, W. C. Chan and C.-M. Park, Expedient Synthesis of Highly Substituted Pyrroles via Tandem Rearrangement of α-Diazo Oxime Ethers, J. Am. Chem. Soc., 2012, 134, 4104–4107 CrossRef CAS PubMed
; (b) Y. Jiang and C.-M. Park, A Catalyst-Controlled Selective Synthesis of Pyridines and Pyrroles, Chem. Sci., 2014, 5, 2347–2351 RSC
; (c) Y. Jiang, C.-M. Park and T.-P. Loh, Transition-Metal-Free Synthesis of Substituted Pyridines via Ring Expansion of 2-Allyl-2H-azirines, Org. Lett., 2014, 16, 3432–3435 CrossRef CAS PubMed
; (d) Y. Ge, W. Sun, B. Pei, J. Ding, Y. Jiang and T.-P. Loh, Hoveyda–Grubbs II Catalyst: A Useful Catalyst for One-Pot Visible-Light-Promoted Ring Contraction and Olefin Metathesis Reactions, Org. Lett., 2018, 20, 2774–2777 CrossRef CAS PubMed
; (e) Y. Ge, W. Sun, Y. Chen, Y. Huang, Z. Liu, Y. Jiang and T.-P. Loh, Reactions of 5-Aminoisoxazoles with α-Diazocarbonyl Compounds: Wolff Rearrangement vs N–H Insertion, J. Org. Chem., 2019, 84, 2676–2688 CrossRef CAS PubMed
; (f) Y. Chen, W. Yang, J. Wu, W. Sun, T.-P. Loh and Y. Jiang, 2H-Azirines as Potential Bifunctional Chemical Linkers of Cysteine Residues in Bioconjugate Technology, Org. Lett., 2020, 22, 2038–2043 CrossRef CAS PubMed
.
-
(a) F. Hu and M. Szostak, Recent Developments in the Synthesis and Reactivity of Isoxazoles: Metal Catalysis and Beyond, Adv. Synth. Catal., 2015, 357, 2583–2614 CrossRef CAS
; (b) S. Madhavan, S. K. Keshri and M. Kapur, Transition Metal–Mediated Functionalization of Isoxazoles: A Review, Asian J. Org. Chem., 2021, 10, 3127–3165 CrossRef CAS
.
-
(a) K. Li, X. Shao, L. Tseng and S. J. Malcolmson, 2-Azadienes as Reagents for Preparing Chiral Amines: Synthesis of 1,2-Amino Tertiary Alcohols by Cu-Catalyzed Enantioselective Reductive Couplings with Ketones, J. Am. Chem. Soc., 2018, 140, 598–601 CrossRef CAS PubMed
; (b) P. E. Daniel, C. I. Onyeagusi, A. A. Ribeiro, K. Li and S. J. Malcolmson, Palladium-Catalyzed Synthesis of α-Trifluoromethyl Benzylic Amines via Fluoroarylation of gem-Difluoro-2-azadienes Enabled by Phosphine-Catalyzed Formation of an Azaallyl–Silver Intermediate, ACS Catal., 2019, 9, 205–210 CrossRef CAS PubMed
; (c) X. Shao and S. J. Malcolmson, Catalytic Enantio- and Diastereoselective Cyclopropanation of 2-Azadienes for the Synthesis of Aminocyclopropanes Bearing Quaternary Carbon Stereogenic Centers, Org. Lett., 2019, 21, 7380–7385 CrossRef CAS PubMed
; (d) J.-C. M. Monbaliu, K. G. R. Masschelein and C. V. Stevens, Electron-Deficient 1- and 2-Azabuta-1,3-Dienes: a Comprehensive Survey of Their Synthesis and Reactivity, Chem. Soc. Rev., 2011, 40, 4708–4739 RSC
; (e) G. Masson, C. Lalli, M. Benohoud and G. Dagousset, Catalytic Enantioselective [4+2]-Cycloaddition: a Strategy to Access Aza-Hexacycles, Chem. Soc. Rev., 2013, 42, 902–923 RSC
.
-
(a) A. Caballero, A. Espinosa, A. Tarraga and P. Molina, Ferrocene-Based Small Molecules for Dual-Channel Sensing of Heavy- and Transition-Metal Cations, J. Org. Chem., 2008, 73, 5489–5497 CrossRef CAS PubMed
; (b) R. Martínez, A. Espinosa, A. Tárraga and P. Molina, A New Bis(pyrenyl)azadiene-Based Probe for the Colorimetric and Fluorescent Sensing of Cu(II) and Hg(II), Tetrahedron, 2010, 66, 3662–3667 CrossRef
.
-
(a) R. F. Heck, Palladium-Catalyzed Reactions of Organic Halides with Olefins, Acc. Chem. Res., 1979, 12, 146–151 CrossRef CAS
; (b) P. Knochel and R. D. Singer, Preparation and Reactions of Polyfunctional Organozinc Reagents in Organic-Synthesis, Chem. Rev., 1993, 93, 2117–2188 CrossRef CAS
; (c) N. Miyaura and A. Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds, Chem. Rev., 1995, 95, 2457–2483 CrossRef CAS
.
-
(a) M. Ni, J. Zhang, X. Liang, Y. Jiang and T.-P. Loh, Directed C–C Bond Ceavage of a Cyclopropane Intermediate Generated from N-Tosylhydrazones and Stable Enaminones: Expedient Synthesis of Functionalized 1,4-Ketoaldehydes, Chem. Commun., 2017, 53, 12286–12289 RSC
; (b) J. Huo, K. Zhong, Y. Xue, M. Lyu, Y. Ping, Z. Liu, Y. Lan and J. Wang, Palladium-Catalyzed Enantioselective Carbene Insertion into Carbon–Silicon Bonds of Silacyclobutanes, J. Am. Chem. Soc., 2021, 143, 12968–12973 CrossRef CAS PubMed
.
- N. S. Y. Loy, A. Singh, X. Xu and C.-M. Park, Synthesis of Pyridines by Carbenoid-Mediated Ring Opening of 2H-Azirines, Angew. Chem., Int. Ed., 2013, 52, 2212–2216 CrossRef CAS PubMed
.
- I. Galve, R. Ondoño, C. de Rocafiguera, R. P. de la Bellacasa, X. Batllori, C. Puigjaner, M. Font-Bardia, O. Vallcorba, J. Teixidó and J. I. Borrell, A Captured Room Temperature Stable Wheland Intermediate as a Key Structure for the Orthogonal Decoration of 4-Amino-Pyrido[2,3-d]Pyrimidin-7(8H)-Ones, Org. Biomol. Chem., 2020, 18, 9810–9815 RSC
.
- M. Ikeda, T. Matsuzawa, T. Morita, T. Hosoya and S. Yoshida, Synthesis of Diverse Aromatic Ketones through C-F Cleavage of Trifluoromethyl Group, Chem. – Eur. J., 2020, 26, 12333–12337 CrossRef CAS PubMed
.
- B. Solaja, J. Huguet, M. Karpf and A. S. Dreiding, The Synthesis of (±)-Isoptychanolide by Application of the α-Alkynone Cyclisation, Tetrahedron, 1987, 43, 4875–4886 CrossRef CAS
.
- H. Suzuki, S. Yoshioka, A. Igesaka, H. Nishioka and Y. Takeuchi, Palladium-Catalyzed Hydrogenation with Use of Ionic Liquid bis(2-Hydroxyethyl)Ammonium Formate [BHEA][HCO2] as a Solvent and Hydrogen Source, Tetrahedron, 2013, 69, 6399–6403 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2246278. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3qo01537h |
| This journal is © the Partner Organisations 2024 |