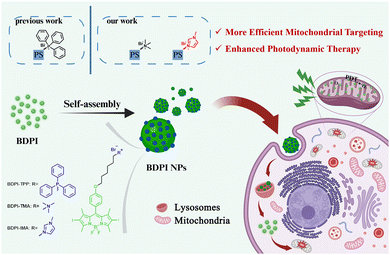
Optimizing mitochondrial-targeting groups of positively-charged BODIPY nanoparticles for enhanced photodynamic therapy†
Huixuan
Qi‡
 a,
Ruobing
Qu‡
a,
Jiaping
Shen
a,
Hui
Wen
b,
Chunyu
Yuan
c,
Wenhai
Lin
a,
Ruobing
Qu‡
a,
Jiaping
Shen
a,
Hui
Wen
b,
Chunyu
Yuan
c,
Wenhai
Lin
 *a,
Tingting
Sun
*a,
Tingting
Sun
 *b and
Min
Li
*c
*b and
Min
Li
*c
aBiomedical Polymers Laboratory, College of Chemistry, Chemical Engineering and Materials Science, and State Key Laboratory of Radiation Medicine and Protection, Soochow University, Suzhou 215123, P. R. China. E-mail: whlin@suda.edu.cn
bState Key Laboratory of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, Jilin 130022, P. R. China. E-mail: suntt@ciac.ac.cn
cDepartment of Dermatology, The Fourth Affiliated Hospital of Soochow University (Suzhou Dushu Lake Hospital, Medical Center of Soochow University), Suzhou, Jiangsu 215125, China. E-mail: lmpfdoctor@163.com
First published on 2nd October 2024
Abstract
Mitochondria play an important role in regulating programmed cell death and various available mitochondrial-targeting photosensitizers are modified by cationic groups, especially triphenylphosphine (TPP). However, it's still a big challenge to develop novel mitochondrial-targeting photosensitizers, especially those that possess better performance than traditional TPP-modified photosensitizers. In this work, three cationic boron-dipyrromethene (BODIPY) nanoparticles with different mitochondrial-targeting groups (triphenylphosphine, trimethylamine and 1-methylimidazole) were designed and synthesized for enhanced photodynamic therapy. These BODIPY nanoparticles (BDPI NPs) could be endocytosed by various cancer cells and dissociated in the lysosomes. Subsequently, they escaped from the lysosomes due to the “proton-sponge” effect and were enriched on the inner membrane of mitochondria for enhanced photodynamic therapy. BDPI NPs could generate not only singlet oxygen (1O2) but also superoxide anions (O2−˙), showing great type I and II photodynamic activity. Compared with TPP and the trimethylamine substitution, the 1-methylimidazole-modified nanoparticles (BDPI-IMA NPs) exhibited the most efficient mitochondrial-targeting capability and the most excellent photodynamic activity. This work highlights the great potential of 1-methylimidazole-modified photosensitizers and nanoparticles as highly efficient mitochondrial-specific probes and phototherapy agents.
Introduction
Mitochondria, a double-membrane organelle, are the key energy source of cells and play an important role in the maintenance of cellular functions.1–3 The electron transport chain occurs on the inner membrane of mitochondria, leading to an asymmetry of protons (H+) and other ions across the membrane and creating a negative membrane potential distinct from other organelles.4–6 This unique structural feature of negative potential makes it possible to design theranostic reagents that could target mitochondria. Moreover, mitochondria, known as the “powerhouses” of cells, are the primary sites for aerobic respiration and ATP production in cells.7,8 They play a crucial role in providing energy for various cellular activities.9 Additionally, mitochondria are also responsible for the generation of most reactive oxygen species (ROS) and are involved in regulating intracellular redox homeostasis, apoptosis, necrosis and pyrotosis.7,10–14 However, the overexpression of ROS in mitochondria can lead to mitochondrial oxidative stress, resulting in mitochondrial dysfunction and cell death.15 Therefore, mitochondria serve as an ideal target organelle for oncotherapy.16,17Photodynamic therapy (PDT), which can utilize photosensitizers to generate ROS, has been widely applied in the treatment of various diseases such as cancer and skin diseases due to its spatiotemporal selectivity, non-invasiveness and minimal side effects.18–24 Mitochondria are ideal target organelles for PDT, which can produce a large amount of ROS leading to mitochondrial dysfunction.25,26 Mitochondrial dysfunction can directly inhibit the energy supply, and afterwards trigger processes such as apoptosis and pyroptosis, which has excellent anti-cancer effects and low toxicity.27–33 However, the drawback of the short lifespan of ROS (<2 ms) exists in PDT.34,35 This limitation results in restricted action time and diffusion distance for ROS, which may not effectively eliminate all target cells, thus impacting therapeutic efficacy.36,37 Therefore, the mitochondrial-targeting effect of photosensitizers determines the effect of PDT.38 Currently, some positively-charged photosensitizers have been designed, which could be specifically anchored to the mitochondria through electrostatic interaction.39 Cationic groups, especially triphenylphosphine (TPP) as the “gold standard”, are introduced to construct photosensitizers with mitochondrial-targeting capabilities.40 Guo et al. reported a mitochondrial-targeting porphyrin-based photosensitizer containing TPP cations for efficient PDT of human cervical carcinoma cells (HeLa cells).41 Two meso-TPP-BODIPY were reported by Kim's group for mitochondria-specific imaging and PDT of HeLa cells and breast cancer MCF-7 cells.42 Ong et al. developed a probe containing TPP as the mitochondrial-targeting functional group for real-time monitoring of mitochondrial platinum accumulation in living cells.43 Therefore, we wondered whether there were more effective mitochondrial-targeting groups than TPP. It is still a big challenge to develop more efficient mitochondrial-targeting photosensitizers for PDT.44,45
In this work, we introduced three lipophilic delocalized cationic groups, named triphenylphosphine (TPP), trimethylamine (TMA) and 1-methylimidazole (IMA) into BODIPY, to make them possess mitochondrial-targeting capabilities and photodynamic activity. And these amphiphilic compounds (BDPI) could self-assemble into nanoparticles (BDPI NPs) in water (Scheme 1). BDPI NPs had high positive potential and could be efficiently attached to the inner mitochondrial membrane. After the nanoparticles were endocytosed by the cancer cells, they first were trapped in the lysosomes. However, due to the “proton-sponge” effect, the nanoparticles dissociated and were released from lysosomes. Subsequently, the positively-charged photosensitizers (BDPI) were enriched on the negatively charged inner mitochondrial membrane, and the fluorescence was observed. Moreover, BDPI could produce ROS under green light irradiation, which could be used for mitochondrial-targeting type I/II PDT. Compared with TPP and TMA modifications, the 1-methylimidazole-modified nanoparticles (BDPI-IMA NPs) have the most excellent mitochondrial-targeting capability and the most efficient photodynamic activity.
Results and discussion
Preparation and characterization of BODIPY derivatives and nanoparticles
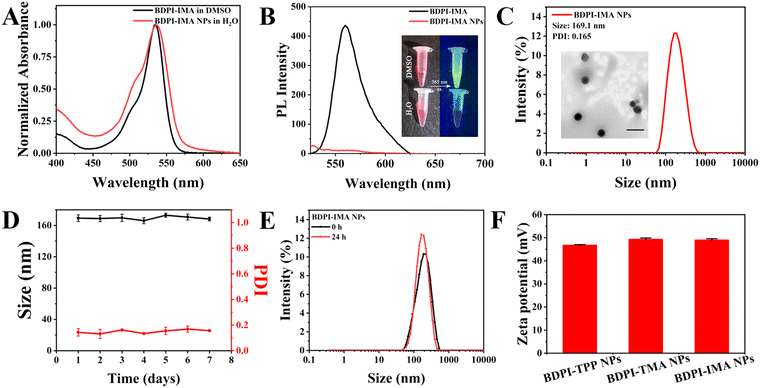
To obtain BODIPY derivatives with mitochondrial-targeting functions, TPP, TMA and IMA were introduced into BODIPY to construct BDPI-TPP, BDPI-TMA and BDPI-IMA, respectively (Fig. S1, ESI†). BDPI-TPP and BDPI-TMA were synthesized according to previous methods.36,46 Their successful synthesis was confirmed by proton nuclear magnetic resonance (1H NMR, Fig. S2 and S3, ESI†). In addition, BDPI-IMA was obtained by refluxing BDPI-Br and IMA in acetonitrile. The structure of BDPI-IMA was confirmed by 1H NMR (Fig. S4, ESI†) and matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS, Fig. S5, ESI†), showing the successful synthesis of BDP-IMA. Then, BDPI-TPP, BDPI-TMA and BDPI-IMA self-assembled into BDPI-TPP nanoparticles (BDPI-TPP NPs), BDPI-TMA nanoparticles (BDPI-TMA NPs) and BDPI-IMA nanoparticles (BDPI-IMA NPs) in water via a nanoprecipitation method, respectively.As shown in Fig. 1(A) and (B), the maximum absorption wavelength (λabs) and the maximum emission wavelength (λem) of free BDPI-IMA in DMSO were 535 nm and 561 nm, respectively. In addition, the λabs values of BDPI-TPP and BDPI-TMA were 536 nm and 535 nm, respectively (Fig. S6 and S7, ESI†). The λem values of BDPI-TPP and BDPI-TMA were 556 nm and 561 nm, which are listed in Table S1 (ESI†). Thus, the modification of different targeting groups did not change the λabs and λem of BDPI. As shown in Fig. 1(A) and Fig. S6, S7 (ESI†), BDPI NPs in water showed broad absorption spectra compared with the free BDPI in DMSO due to the aggregation of BDPI.47 Due to the aggregation-caused quenching effect (ACQ), there was no fluorescence for BDPI NPs (Fig. 1(B) and Fig. S6, S7, ESI†).48 The diameters of BDPI-TPP NPs, BDPI-TMA NPs and BDPI-IMA NPs measured by dynamic light scattering (DLS, Fig. 1(C) and Fig. S8 ESI†) were 155 nm, 155 nm and 169 nm, respectively. In addition, transmission electron microscopy (TEM) was used to characterize the morphology of BDPI NPs (Fig. 1(C) and Fig. S9, ESI†), which further confirmed that they formed spherical nanoparticles. The sizes measured by TEM were consistent with those measured by DLS. The size and PDI of the nanoparticles were barely altered for a week, demonstrating that they possessed high colloidal stabilities (Fig. 1(D) and Fig. S10, ESI†). The stabilities of BDPI NPs in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS) were also measured. As shown in Fig. 1(E) and Fig. S11 (ESI†), BDPI NPs were stable in DMEN with 10% FBS for 24 h, which suggested that they didn’t dissociate under physiological conditions and could be used for biological applications. The photostability of BDPI NPs was subsequently measured. As shown in Fig. S12 (ESI†), the absorbance of BDPI-TPP NPs, BDPI-TMA NPs and BDPI-IMA NPs only dropped a little under green light irradiation (530 nm, 10 mW cm−2) for 20 min, evaluating the excellent photostability of BDPI NPs. Moreover, the zeta potentials of the BDPI-TPP NPs, BDPI-TMA NPs and BDPI-IMA NPs were +46.1 mV, +49.2 mV, and +48.9 mV, respectively (Fig. 1(F)), which confirmed that positively-charged cation groups were distributed on the surface of the nanoparticles.
Generation of reactive oxygen species
The energy band gaps of the photosensitizers were calculated by density functional theory (DFT). As shown in Fig. S13 (ESI†), BDPI-IMA possesses the smallest energy gap (ΔEST) between the singlet and triplet states (S1 and T1), which suggested that it was easiest to process the intersystem crossing (ISC) to generate ROS.49 Therefore, BDPI-IMA might possess the best ROS-generating capability among BDPI. Afterwards, the ROS generation capabilities of BDPI-TPP, BDPI-TMA and BDPI-IMA were evaluated by using 1,3-diphenylisobenzofuran (DPBF) as an indicator. As shown in Fig. 2(A) and (B) and Fig. S14 (ESI†), under green light irradiation (530 nm, 10 mW cm−2), the absorbance of DPBF gradually decreased in the presence of BDPI-TPP, BDPI-TMA or BDPI-IMA. Under green light irradiation for 70 s, the absorbance of DPBF decreased by 70% and 67% in the presence of BDPI-TPP and BDPI-TMA, respectively. But the absorbance of DPBF decreased by 96% in the presence of BDPI-IMA, which dropped fastest among BDPI (Fig. 2(B)). In contrast, the DPBF in the control group showed negligible changes in the absorption spectra (Fig. S14C, ESI†). The singlet oxygen quantum yields of BDPI-TPP, BDPI-TMA and BDPI-IMA were 78.63%, 86.01% and 100.00%, respectively (Table S1, ESI†). Moreover, the ROS generation capabilities of the BDPI NPs were also studied, the results of which were consistent with those of BDPI (Fig. S15, S16, and Table S1, ESI†). BDPI NPs all could generate ROS efficiently, and the ROS generation capability of BDPI-IMA NPs was the most excellent among BDPI NPs.We further investigated the types of ROS generated by BDPI. Singlet oxygen sensor green (SOSG) was selected as a fluorescent probe of 1O2. As shown in Fig. S17 and S18 (ESI†), the fluorescence intensity of SOSG gradually increased in the presence of BDPI. Under green light irradiation for 75 s, the fluorescence intensity of SOSG was increased to 7, 9 and 14-fold of the initial level in the presence of BDPI-TPP, BDPI-TMA and BDPI-IMA, respectively. These results demonstrated that BDPI-TMA had a better 1O2 generation capacity than BDPI-TPP, but BDPI-IMA is the best among the three photosensitizers. Type I photosensitizers generate free radicals, such as superoxide radicals (O2−˙), through electron transfer mechanisms to reduce the dependence on oxygen. In the hypoxic microenvironment of tumors, type I photosensitizers show greater therapeutic potential.50 Dihydrorhodamine 123 (DHR123) was used for the determination of O2−˙. The fluorescence intensity of DHR123 gradually increased by 3.3, 3.5 and 4.7-fold within 80 s in the presence of BDPI-TPP, BDPI-TMA and BDPI-IMA, respectively (Fig. 2(C), (D) and Fig. S19, ESI†). BDPI-IMA exhibited the best O2−˙ generation capabilities and type I photodynamic potential among BDPI. Subsequently, we examined the ROS production capacities of BDPI NPs in HeLa cells using 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) as a ROS probe. As shown in Fig. 2(E), green fluorescence was observed in HeLa cells treated with BDPI NPs under green light irradiation. However, negligible fluorescence in cells was observed under dark conditions. Moreover, the cells treated with BDPI-IMA NPs showed the brightest fluorescence, with fluorescence intensities 1.7 and 1.8-fold higher than those of the BDPI-TPP NPs group and BDPI-TMA NPs group, respectively (Fig. S20, ESI†). The above results proved that BDPI-IMA NPs exhibited the best photodynamic activity among BDPI NPs.
Cellular uptake and mitochondrial targeting
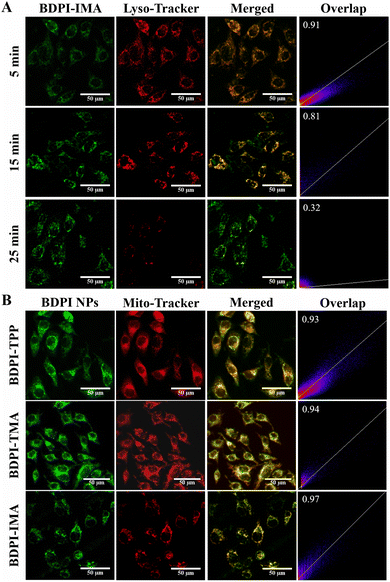
Endocytosis is a prerequisite for mitochondrial targeting and PDT, so the internalization of BDPI NPs in HeLa cells was investigated. As shown in Fig. 3(A), the green fluorescence of BDPI-IMA coexisted well with the red fluorescence of lysosomes after HeLa cells were incubated with BDPI-IMA NPs for 5 min, indicating that nanoparticles were first trapped in lysosomes and dissociated to fluoresce. As time went on, the green fluorescence of BDPI-IMA gradually diffused while the red fluorescence of lysosomes decreased (Fig. S24, ESI†). And the Pearson correlation coefficients (PCCs) of BDPI-IMA and lysosomes decreased from 0.91 to 0.32. In addition, similar phenomena were observed in HeLa cells treated with BDPI-TPP NPs and BDPI-TMA NPs (Fig. S25–S28, ESI†). These results demonstrated that the BDPI NPs were endocytosed and dissociated in lysosomes, and lysosomal escape was achieved driven by the “proton-sponge” effect.Afterwards, mitochondrial localization in HeLa cells was studied by confocal laser scanning microscopy (CLSM). As shown in Fig. 3(B), the fluorescence of BDPI NPs overlapped well with Mito-Tracker Deep Red 633 after HeLa cells were incubated with BDPI NPs for 30 min. The PCCs of the mitochondria and BDPI-TPP, BDPI-TMA and BDPI-IMA were 0.93, 0.94 and 0.97, respectively. BDPI-IMA NPs possessed the best mitochondrial localization capability and the greatest mitochondrial-targeting accuracy among BDPI NPs. Additionally, the mitochondrial localization capabilities of BDPI NPs in mouse breast cancer cells (4T1 cells) and mouse melanoma cells (B16-F10 cells) were measured. The PCCs were all higher than 0.9 (Fig. S29 and S30, ESI†), which showed that BDPI NPs also had excellent mitochondrial-targeting capabilities in various cancer cells.
These results demonstrated the universality of the mitochondrial-targeting capability of the BDPI NPs. It is worth noting that the targeting accuracy of BDPI-TMA NPs was better than that of BDPI-TPP NPs, while BDPI-IMA NPs were consistently the best.
The appropriate lipophilic/hydrophilic character of the molecule has a significant impact on targeting effectiveness to mitochondria.51,52 The positive-charge of TPP is surrounded by phenyl groups which increase the local steric hindrance. Besides that, the polarity of TPP is very low so BDPI-TPP is relatively more lipophilic. Whereas the small molecular weight of TMA makes it easy to interact with water molecules so TMA is more hydrophilic. However, the positive charge of BDPI-IMA is distributed on the heterocycle and the positive-charge is relatively exposed. IMA is a polar molecule and can form hydrogen bonds with water, which makes it hydrophilic. But the planar conjugated structure and the modification of a methyl group increase the lipophilicity. Thus, BDPI-IMA is well balanced between lipophilic and hydrophilic characters. Consequently, BDPI-IMA might show the best mitochondrial targeting capacity.
The mitochondrial membrane potential (MMP) of HeLa cells was subsequently measured using a JC-1 fluorescent probe. The decline of MMP is a hallmark event in the early stages of apoptosis.53 The drop in MMP can be easily detected by the transition of JC-1 fluorescence from red to green. As shown in Fig. 4, bright red fluorescence was observed in untreated cells but the fluorescence of JC-1 in the cells treated with BDPI NPs changed from red to green under green light irradiation, indicating a decrease of MMP. Carbonyl cyanide 3-chlorophenylhydrazone (CCCP) treatment was used as the positive control. Bright green fluorescence of JC-1 monomers was observed after the HeLa cells were treated by CCCP, the mitochondria of which were severely damaged. Among BDPI NPs, the cells incubated with BDPI-IMA NPs exhibited the brightest green fluorescence (Fig. S31, ESI†), suggesting that BDPI-IMA NPs could effectively induce mitochondrial dysfunction and lead to early apoptosis under green light irradiation.
Photodynamic therapy
To evaluate the photocytotoxicity and biocompatibility of BDPI NPs, HeLa cells, 4T1 cells and B16-F10 cells were incubated with BDPI NPs and irradiated by green light. The cell cytotoxicity was determined by thiazolyl blue tetrazolium bromide (MTT) analysis. As shown in Fig. S32 (ESI†), the viabilities of cells incubated with BDPI NPs were all above 85% without light irradiation, confirming the good biocompatibility of BDPI NPs. However, BDPI NPs exhibited a concentration-dependent cytotoxic effect on any HeLa cells, 4T1 cells or B16-F10 cells under green light irradiation for 25 min (Fig. 5). For HeLa cells, the median inhibition concentrations (IC50) of BDPI-TPP NPs, BDPI-TMA NPs and BDPI-IMA NPs were 10.34 nmol L−1, 8.16 nmol L−1 and 3.81 nmol L−1, respectively. When the concentration of BDPI-IMA NPs was as low as 10 ng mL−1, less than 10% of cells survived under green light irradiation (Fig. 5), suggesting that BDPI-IMA NPs had excellent capability for PDT. Moreover, the IC50 values of BDPI-IMA NPs on various cells were only one-third to one-half of those of BDPI-TPP NPs and BDPI-TMA NPs (Table 1). Thus, BDPI-TPP NPs and BDPI-TMA NPs had a weaker inhibitory effect on tumor cells than BDPI-IMA NPs. BDPI-IMA NPs showed the greatest potential for PDT among BDPI NPs due to the best biocompatibility and the most excellent photocytotoxicity.| IC50 (nmol L−1) | |||
|---|---|---|---|
| BDPI-TPP NPs | BDPI-TMA NPs | BDPI-IMA NPs | |
| HeLa | 10.34 | 8.16 | 3.81 |
| 4T1 | 11.06 | 13.28 | 6.54 |
| B16-F10 | 15.50 | 13.78 | 5.17 |
Conclusions
In summary, we designed and synthesized three BDPI NPs with different cationic groups (TPP, TMA and IMA), and discovered that the 1-methylimidazole-modified nanoparticles exhibited the most efficient mitochondrial-targeting capability. The positive charge of cations enhanced the interaction of BDPI NPs with mitochondria, allowing them to be precisely targeted to mitochondria for enhanced PDT. BDPI NPs were endocytosed by cancer cells and dissociated in the lysosomes. Then, the BDPI escaped from the lysosomes due to the “proton-sponge” effect and were enriched in the inner membrane of mitochondria. BDPI that could generate 1O2 and O2−˙ under green light irradiation possessed potential for use in both type I and II PDT. Compared with BDPI-TPP NPs and BDPI-TMA NPs, BDPI-IMA NPs had the best mitochondrial-targeting capability. After various cancer cells (HeLa cells, 4T1 cells and B16-F10 cells) were incubated with BDPI-IMA NPs for 30 min, the PCCs of BDPI-IMA and mitochondria were all above 0.96. Moreover, BDPI-IMA NPs possessed the best generating ROS capacity and the smallest IC50 value (only 3.81 nmol L−1 on HeLa cells, 6.54 nmol L−1 on 4T1 cells and 5.17 nmol L−1 on B16-F10 cells) under green light irradiation, compared to the BDPI-TPP NPs and BDPI-TMA NPs. Therefore, IMA might be a better mitochondrial-targeting group than the “gold standard” TPP. This work provides new insights into the design of cationic photosensitizers and nanoparticles as effective mitochondrial-targeting diagnostic reagents.Author contributions
H. Q. and R. Q. designed the work, participated in all experiments and wrote the draft. J. S., H. W. and C. Y. provided the help for synthesis and cell experiments. W. L., T. S. and M. L. supervised the work.Data availability
All data are available from the corresponding authors by request. The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (project no. 52003267 and 51973214). We thank Jiangsu Key Laboratory of Advanced Functional Polymer Design and Application, Soochow University for the instrument support.Notes and references
- H. Liu and H. Wang, From cells to subcellular organelles: Next-generation cancer therapy based on peptide self-assembly, Adv. Drug Delivery Rev., 2024, 209, 115327 CrossRef CAS PubMed.
- A. K. Dey, S. Das, S. M. Jose, S. Sreedharan, N. Kandoth, S. Barman, A. Patra, A. Das and S. K. Pramanik, Surface functionalized perovskite nanocrystals: a design strategy for organelle-specific fluorescence lifetime multiplexing, Chem. Sci., 2024, 15, 10935–10944 RSC.
- P. E. Porporato, N. Filigheddu, J. M. B.-S. Pedro, G. Kroemer and L. Galluzzi, Mitochondrial metabolism and cancer, Cell Res., 2018, 28, 265–280 CrossRef CAS PubMed.
- Y. Wang, J.-S. Yang, M. Zhao, J.-Q. Chen, H.-X. Xie, H.-Y. Yu, N.-H. Liu, Z.-J. Yi, H.-L. Liang, L. Xing and H.-L. Jiang, Mitochondrial endogenous substance transport-inspired nanomaterials for mitochondria-targeted gene delivery, Adv. Drug Delivery Rev., 2024, 211, 115355 CrossRef CAS PubMed.
- K. Birsoy, T. Wang, W. W. Chen, E. Freinkman, M. Abu-Remaileh and D. M. Sabatini, An Essential Role of the Mitochondrial Electron Transport Chain in Cell Proliferation Is to Enable Aspartate Synthesis, Cell, 2015, 162, 540–551 CrossRef CAS PubMed.
- S. Dong, Y. Dong, Z. Zhao, J. Liu, S. Liu, L. Feng, F. He, S. Gai, Y. Xie and P. Yang, “Electron Transport Chain Interference” Strategy of Amplified Mild-Photothermal Therapy and Defect-Engineered Multi-Enzymatic Activities for Synergistic Tumor-Personalized Suppression, J. Am. Chem. Soc., 2023, 145, 9488–9507 CrossRef CAS.
- J. B. Spinelli and M. C. Haigis, The multifaceted contributions of mitochondria to cellular metabolism, Nat. Cell Biol., 2018, 20, 745–754 CrossRef CAS.
- I. Vercellino and L. A. Sazanov, The assembly, regulation and function of the mitochondrial respiratory chain, Nat. Rev. Mol. Cell Biol., 2022, 23, 141–161 CrossRef CAS PubMed.
- R. Zhang, Y. Teng, G. Shao, Y. Wang, H. Yang and Y. Tian, Tetrazine-derived chromones as conditionally activated solvatochromic fluorescent probes for dual imaging of lipid droplets and mitochondria, Mater. Chem. Front., 2024, 8, 2341–2349 RSC.
- J. Cao, Q. Wu, X. Liu, X. Zhu, C. Huang, X. Wang and Y. Song, Mechanistic insight on nanomaterial-induced reactive oxygen species formation, J. Environ. Sci., 2025, 151, 200–210 CrossRef.
- H. Yuan, Z. Han, Y. Chen, F. Qi, H. Fang, Z. Guo, S. Zhang and W. He, Ferroptosis Photoinduced by New Cyclometalated Iridium(III) Complexes and Its Synergism with Apoptosis in Tumor Cell Inhibition, Angew. Chem., Int. Ed., 2021, 60, 8174–8181 CrossRef CAS PubMed.
- C. Ouyang, L. Chen, T. W. Rees, Y. Chen, J. Liu, L. Ji, J. Long and H. Chao, A mitochondria-targeting hetero-binuclear Ir(III)–Pt(II) complex induces necrosis in cisplatin-resistant tumor cells, Chem. Commun., 2018, 54, 6268–6271 RSC.
- Kanika and L. Singh, Mitigating cognitive deficits with teriflunomide: unraveling PI3K-modulated behavioral outcomes in mice, Mol. Biol. Rep., 2024, 51, 572 CrossRef CAS PubMed.
- T.-Z. Ma, L.-Y. Liu, Y.-L. Zeng, K. Ding, H. Zhang, W. Liu, Q. Cao, W. Xia, X. Xiong, C. Wu and Z.-W. Mao, G-quadruplex-guided cisplatin triggers multiple pathways in targeted chemotherapy and immunotherapy, Chem. Sci., 2024, 15, 9756–9774 RSC.
- M. He, M. Wang, T. Xu, M. Zhang, H. Dai, C. Wang, D. Ding and Z. Zhong, Reactive oxygen species-powered cancer immunotherapy: Current status and challenges, J. Controlled Release, 2023, 356, 623–648 CrossRef CAS PubMed.
- Z. Sun, W. Chen, J. Liu, B. Yu, C. Jiang and L. Lu, Mitochondria-Targeting Enhanced Phototherapy by Intrinsic Characteristics Engineered “One-for-All” Nanoparticles, ACS Appl. Mater. Interfaces, 2021, 13, 35568–35578 CrossRef CAS.
- Y. Li, X. Li, X. Cao, J. Xu, X. Zhao and H. Lu, Single laser activated photodynamic/photothermal cancer therapy using a single mitochondria-targeted phototherapeutic agent with aggregation-induced emission characteristics, Mater. Chem. Front., 2024, 8, 2897–2904 RSC.
- Y. Xiong, Z. Yong, Q. Zhao, A. Hua, X. Wang, X. Chen, X. Yang and Z. Li, Hydroxyethyl starch-based self-reinforced nanomedicine inhibits both glutathione and thioredoxin antioxidant pathways to boost reactive oxygen species-powered immunotherapy, Biomaterials, 2024, 311, 122673 CrossRef CAS.
- T. C. Pham, M. Cho, V.-N. Nguyen, V. K. T. Nguyen, G. Kim, S. Lee, W. Dehaen, J. Yoon and S. Lee, Charge Transfer-Promoted Excited State of a Heavy-Atom-Free Photosensitizer for Efficient Application of Mitochondria-Targeted Fluorescence Imaging and Hypoxia Photodynamic Therapy, ACS Appl. Mater. Interfaces, 2024, 16, 21699–21708 CrossRef CAS.
- K. Deng, C. Li, S. Huang, B. Xing, D. Jin, Q. Zeng, Z. Hou and J. Lin, Recent Progress in Near Infrared Light Triggered Photodynamic Therapy, Small, 2017, 13, 1702299 CrossRef PubMed.
- W. Tang, Z. Zhen, M. Wang, H. Wang, Y.-J. Chuang, W. Zhang, G. D. Wang, T. Todd, T. Cowger, H. Chen, L. Liu, Z. Li and J. Xie, Red Blood Cell-Facilitated Photodynamic Therapy for Cancer Treatment, Adv. Funct. Mater., 2016, 26, 1757–1768 CrossRef CAS PubMed.
- L. Tian, X. Li, L. Guo, L. Huang, X. Wu and W. Gao, Visualized photodynamic nanomaterials activating tumor-associated immune landscape as a next-generation anticancer strategy, Coord. Chem. Rev., 2024, 517, 216027 CrossRef CAS.
- Y. Xu, S.-Y. Liu, L. Zeng, H. Ma, Y. Zhang, H. Yang, Y. Liu, S. Fang, J. Zhao, Y. Xu, C. R. Ashby Jr, Y. He, Z. Dai and Y. Pan, An Enzyme-Engineered Nonporous Copper(I) Coordination Polymer Nanoplatform for Cuproptosis-Based Synergistic Cancer Therapy, Adv. Mater., 2022, 34, 2204733 CrossRef CAS PubMed.
- V. Juvekar, Y. Cao, C. W. Koh, D. J. Lee, S. Y. Kwak, S. M. Kim, T. J. Park, S. Park, Z. Liu and H. M. Kim, Overcoming melanin interference in melanocyte photodynamic therapy with a pyrene-derived two-photon photosensitizer, Chem. Eng. J., 2024, 493, 152796 CrossRef CAS.
- M. Tian, W. Chen, Y. Wu, J. An, G. Hong, M. Chen, F. Song, W.-H. Zheng and X. Peng, Liposome-Based Nanoencapsulation of a Mitochondria-Stapling Photosensitizer for Efficient Photodynamic Therapy, ACS Appl. Mater. Interfaces, 2022, 14, 12050–12058 CrossRef CAS.
- B.-K. Liu, J. Zheng, H. Wang, L.-Y. Niu and Q.-Z. Yang, BODIPY-based photosensitizers with simultaneous photodynamic antitumor and antibacterial effects, Mater. Chem. Front., 2023, 7, 5879–5890 RSC.
- Y. Ouyang, Y. Li, C. Chen, S. Zhao, M. Wu, B. Zhou, Y. Cao and H. Liu, Copper phosphate-rotenone nanocomposites for tumor therapy through autophagy blockage-enhanced triphosadenine supply interruption and lipid peroxidation accumulation, Chem. Eng. J., 2024, 495, 153435 CrossRef CAS.
- N. Haga, N. Fujita and T. Tsuruo, Mitochondrial aggregation precedes cytochrome c release from mitochondria during apoptosis, Oncogene, 2003, 22, 5579–5585 CrossRef CAS.
- P. Zheng, B. Ding, G. Zhu, C. Li and J. Lin, Biodegradable Ca2+ Nanomodulators Activate Pyroptosis through Mitochondrial Ca2+ Overload for Cancer Immunotherapy, Angew. Chem., Int. Ed., 2022, 61, e202204904 CrossRef CAS.
- Z. Yi, X. Qin, L. Zhang, H. Chen, T. Song, Z. Luo, T. Wang, J. Lau, Y. Wu, T. B. Toh, C.-S. Lee, W. Bu and X. Liu, Mitochondria-Targeting Type-I Photodrug: Harnessing Caspase-3 Activity for Pyroptotic Oncotherapy, J. Am. Chem. Soc., 2024, 146, 9413–9421 CrossRef CAS PubMed.
- R. Miao, C. Jiang, W. Y. Chang, H. Zhang, J. An, F. Ho, P. Chen, H. Zhang, C. Junqueira, D. Amgalan, F. G. Liang, J. Zhang, C. L. Evavold, I. Hafner-Bratkovič, Z. Zhang, P. Fontana, S. Xia, M. Waldeck-Weiermair, Y. Pan, T. Michel, L. Bar-Peled, H. Wu, J. C. Kagan, R. N. Kitsis, P. Zhang, X. Liu and J. Lieberman, Gasdermin D permeabilization of mitochondrial inner and outer membranes accelerates and enhances pyroptosis, Immunity, 2023, 56, 2523–2541 CrossRef CAS PubMed.
- X. Li, Y. Zhao, T. Zhang and D. Xing, Mitochondria-Specific Agents for Photodynamic Cancer Therapy: A Key Determinant to Boost the Efficacy, Adv. Healthcare Mater., 2021, 10, 2001240 CrossRef CAS PubMed.
- J. Liu, X. Liu, M. Wu, G. Qi and B. Liu, Engineering Living Mitochondria with AIE Photosensitizer for Synergistic Cancer Cell Ablation, Nano Lett., 2020, 20, 7438–7445 CrossRef CAS.
- Z. Zhou, J. Song, L. Nie and X. Chen, Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy, Chem. Soc. Rev., 2016, 45, 6597–6626 RSC.
- X. Mu, Y. Chang, Y. Bao, A. Cui, X. Zhong, G. B. Cooper, A. Guo and G. Shan, Core-satellite nanoreactors based on cationic photosensitizer modified hollow CuS nanocage for ROS diffusion enhanced phototherapy of hypoxic tumor, Biomater. Adv., 2023, 145, 213263 CrossRef CAS.
- B. Chu, Y. Qu, X. He, Y. Hao, C. Yang, Y. Yang, D. Hu, F. Wang and Z. Qian, ROS-Responsive Camptothecin Prodrug Nanoparticles for On-Demand Drug Release and Combination of Chemotherapy and Photodynamic Therapy, Adv. Funct. Mater., 2020, 30, 2005918 CrossRef CAS.
- B. Yang, Y. Chen and J. Shi, Reactive Oxygen Species (ROS)-Based Nanomedicine, Chem. Rev., 2019, 119, 4881–4985 CrossRef CAS.
- T. Luo, Y. Fan, J. Mao, X. Jiang, L. Albano, E. Yuan, T. Germanas and W. Lin, Metal–Organic Layer Delivers 5-Aminolevulinic Acid and Porphyrin for Dual-Organelle-Targeted Photodynamic Therapy, Angew. Chem., Int. Ed., 2023, 135, e202301910 CrossRef.
- H. Wang, C. Li, Q. Wu, H. Wen, T. Sun and Z. Xie, A cationic BODIPY photosensitizer decorated with quaternary ammonium for high-efficiency photodynamic inhibition of bacterial growth, J. Mater. Chem. B, 2022, 10, 4967–4973 RSC.
- E. R. H. Walter, L. C.-C. Lee, P. K.-K. Leung, K. K.-W. Lo and N. J. Long, Mitochondria-targeting biocompatible fluorescent BODIPY probes, Chem. Sci., 2024, 15, 4846–4852 RSC.
- X. Guo, H. Wu, W. Miao, Y. Wu, E. Hao and L. Jiao, Mitochondria-targeted porphyrin-based photosensitizers containing triphenylphosphonium cations showing efficient in vitro photodynamic therapy effects, J. Porphyrins Phthalocyanines, 2019, 23, 1505–1514 CrossRef CAS.
- T. P. Vales, S. Cho, J. Lee, H. T. Bui, D. K. Mai, I. W. Badon, H. Lim, W. Jeong, J.-L. Kim, H.-K. Kim and H.-J. Kim, Functionalization of 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY)-based photosensitizers with Triphenylphosphonium (TPP) for mitochondria-targeted fluorescence bioimaging and photodynamic therapy, J. Mol. Struct., 2021, 1246, 131284 CrossRef CAS.
- J. X. Ong, H. V. Le, V. E. Y. Lee and W. H. Ang, A Cisplatin-Selective Fluorescent Probe for Real-Time Monitoring of Mitochondrial Platinum Accumulation in Living Cells, Angew. Chem., Int. Ed., 2021, 60, 9264–9269 CrossRef CAS.
- G. Battogtokh, Y. S. Choi, D. S. Kang, S. J. Park, M. S. Shim, K. M. Huh, Y.-Y. Cho, J. Y. Lee, H. S. Lee and H. C. Kang, Mitochondria-targeting drug conjugates for cytotoxic, anti-oxidizing and sensing purposes: current strategies and future perspectives, Acta Pharm. Sin. B, 2018, 8, 862–880 CrossRef PubMed.
- L. Zhang, Y.-F. Zhang and Y.-F. Han, A perylene diimide-based fluorescent probe for the selective detection of hypochlorite in living cells, Mater. Chem. Front., 2022, 6, 2266–2273 RSC.
- H. Wen, Q. Wu, C. Li, T. Sun and Z. Xie, 4,4-Difluoro-4-bora-3a,4a-diaza-s-indacene (BDPI)-Triphenylphosphine Nanoparticles as a Photodynamic Antibacterial Agent, ACS Appl. Nano Mater., 2022, 5, 1500–1507 CrossRef CAS.
- N. Joudeh and D. Linke, Nanoparticle classification, physicochemical properties, characterization, and applications: a comprehensive review for biologists, J. Nanobiotechnol., 2022, 20, 262 CrossRef.
- X. Zhang, H. Liu, G. Zhuang, S. Yang and P. Du, An unexpected dual-emissive luminogen with tunable aggregation-induced emission and enhanced chiroptical property, Nat. Commun., 2022, 13, 3543 CrossRef CAS.
- Y. Tang, X. Wang, G. Zhu, Z. Liu, X.-M. Chen, H. K. Bisoyi, X. Chen, X. Chen, Y. Xu, J. Li and Q. Li, Hypoxia-Responsive Photosensitizer Targeting Dual Organelles for Photodynamic Therapy of Tumors, Small, 2023, 19, 2205440 CrossRef CAS.
- K. Wen, H. Tan, Q. Peng, H. Chen, H. Ma, L. Wang, A. Peng, Q. Shi, X. Cai and H. Huang, Achieving Efficient NIR-II Type-I Photosensitizers for Photodynamic/Photothermal Therapy upon Regulating Chalcogen Elements, Adv. Mater., 2022, 34, 2108146 CrossRef CAS.
- J. S. Armstrong, Mitochondrial medicine: pharmacological targeting of mitochondria in disease, Br. J. Pharmacol., 2007, 151, 1154–1165 CrossRef CAS.
- Z. Chen, M. Li, L. Zhang, J. He, L. Wu, Y. Xiao, J. Duan, T. Cai and W. Li, Mitochondria-targeted drug delivery system for cancer treatment, J. Drug Targeting, 2015, 24, 492–502 CrossRef.
- B. J. Berry, A. Vodičková, A. Müller-Eigner, C. Meng, C. Ludwig, M. Kaeberlein, S. Peleg and A. P. Wojtovich, Optogenetic rejuvenation of mitochondrial membrane potential extends C. elegans lifespan, Nat. Aging, 2023, 3, 157–161 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4qm00725e |
| ‡ H. Q. and R. Q. contributed equally. |
| This journal is © the Partner Organisations 2024 |