Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Colloidal fluorine-doped ZnO quantum dots: the synergistic action of atomic doping and growth conditions directs fluorescence and photoactivity†
Noemi
Gallucci
ab,
Alessandro
Cangiano
ab,
Simone
Russo
c,
Giulio
Pota
c,
Rocco
Di Girolamo
 a,
Eugénie
Martinez
d,
Nicolas
Vaxelaire
d,
Luigi
Paduano
ab and
Giuseppe
Vitiello
a,
Eugénie
Martinez
d,
Nicolas
Vaxelaire
d,
Luigi
Paduano
ab and
Giuseppe
Vitiello
 *bc
*bc
aDepartment of Chemical Sciences, University of Naples Federico II, Via Cintia 4, 80126 Naples, Italy. E-mail: giuseppe.vitiello@unina.it
bCSGI-Center for Colloid and Surface Science, Via della Lastruccia 3, 50019 Sesto Fiorentino, Italy
cDepartment of Chemical, Materials and Production Engineering, University of Naples Federico II, P.le Tecchio 80, 80125 Naples, Italy
dUniversity of Grenoble Alpes, CEA-LETI, 17 Av. des Martyrs, 38054 Grenoble, France
First published on 11th October 2024
Abstract
Quantum dots are nano-sized semiconductor particles showing peculiar optical properties due to the quantum confinement effect. They can efficiently absorb photons and generate excitons, leading to a stable fluorescence emission decisive to designing light-sensitive devices, or they can exert a pronounced photoactivity that favors their use in photocatalysis and photodynamic fields. Among the inorganic quantum dots, ZnO ones show unique optical and electronic properties together with low toxicity, good biocompatibility, and excellent photochemical stability. These features can be deeply influenced by tuning their size, surface, and/or bulk defects as well as by doping. Doping with anionic atoms represents an intriguing alternative to cationic metals to improve ZnO activity. Here, the emission behaviour and photoactivity of fluorine-doped ZnO quantum dots were simultaneously studied as a function of fluorine content and synthesis conditions (e.g., wet-precipitation or solvothermal) adopted for the fabrication. The obtained results demonstrated that a low fluorine content (<5 nominal at%) was pivotal to induce a significant enhancement of the relative emission quantum yield of quantum dots from the wet-precipitation route, while a high photocatalytic activity was guaranteed for those obtained by a solvothermal strategy due to the bulk distribution of atomic defects.
Introduction
Quantum dots (QDs) are nano-sized semiconductor particles that exhibit unique properties due to their quantum confinement effects in all dimensions.1,2 These structures, typically ranging from 2 to 15 nm in diameter, can emit or absorb light at specific wavelengths depending on their size, shape, and composition. Due to their unique electronic structure, QDs can efficiently absorb photons and generate excitons, leading to compositions with bright and stable fluorescence emissions.2,3 Moreover, their size-dependent bandgap enables light absorption across a broad range of wavelengths, making them valuable components for diverse applications such as light-emitting diodes,4 solar cells,5 and photodetectors.6 Also, the photoactivity of QDs extends beyond conventional optics, with potential applications in photocatalysis and photodynamic therapy, where their ability to generate reactive oxygen species under light irradiation holds promise for environmental remediation and cancer treatment.7–9The properties of QDs are profoundly influenced by synthesis conditions, which encompass parameters such as reaction temperature, precursor concentration, reaction time, solvent choice, surface ligands, and doping agents.10,11 These factors directly impact the size, shape, composition, crystallinity, and surface chemistry of the QDs, consequently determining their optical, electronic, and structural properties.12–15 For example, doping agents can play a crucial role in amplifying the optical and photoactivity properties of QDs by introducing controlled impurities and defects into their crystal lattice.16,17 By incorporating doping agents with appropriate energy levels, such as transition metal ions or rare earth elements into QDs, it is possible to effectively tune the energy bandgap and to extend the range of absorbed and emitted wavelengths, thus broadening the final optical response.12,17 Additionally, doping can modify the charge carrier dynamics within the QD structure, enhancing the photoactivity and photoresponsivity, which are crucial for applications such as photocatalysis and photovoltaics.12,18 Moreover, doping can enhance the stability and quantum efficiency of QDs, making them more suitable for long-term and high-performance applications in fields like display technologies, sensing, and biomedical imaging.19–23
Among the inorganic materials used to realize photoresponsive QDs, zinc oxide (ZnO) shows fascinating and unique optical and electronic properties.24,25 Compared with the traditionally II–VI group quantum dots, such as CdSe and CdTe, ZnO-QDs are cheap and biocompatible with biological systems and have attracted great attention in biological labeling and photocatalytic applications thanks to low toxicity and excellent photochemical stability.26 In this way, atomic doping emerges as a valuable strategy for fine-tuning many features of ZnO-QDs, and in particular the fluorescence and photoactivity properties (especially their visible emission).27,28 By substituting Zn and O atoms and/or replacing the atomic vacancies within the bulk structure or on the surface of nanocrystals with specific dopants, such as aluminum, indium, or nitrogen,29 it is possible to finely control the crystalline structure and the photoinduced performances of QDs to suit specific nanotechnological applications.30,31 The doping with fluorine (F) was proposed as an interesting strategy to improve the activity of ZnO.32 Indeed, bringing one electron more than an oxygen atom, fluorine atoms may represent a shallow donor state by exploiting their tendency to fill oxygen vacancies (Vo), which are the prevalent defects in the ZnO crystalline structure. As proposed by theoretical calculations, F atoms could increase the distance between neighbouring Zn, leading to the formation of deep localized electron states, and can act as donors when they fill Vo or as acceptors if they remain interstitials.33,34
In this context, the role of synthesis conditions appears decisive in finely tuning the growth and properties of undoped and doped ZnO. In particular, wet-chemical syntheses belonging to the bottom-up approach were widely used to produce ZnO nanomaterials offering a high degree of controllability and reproducibility.35 Among them, wet-precipitation, solvothermal, templated, self-assembly, and interface-mediated syntheses are the main ones used to produce semiconductor nanomaterials. All of them deal with chemical reactions in the solution phase using precursors under suitable conditions. However, each wet-chemical synthesis method differs from the others, meaning that one cannot find a general rule for these kinds of synthesis approaches. To the best of our knowledge, although several F-doped ZnO-based functional materials have been prepared in various shapes and morphologies, such as nano/meso/microcrystals or thin films, the effect of a synergistic action of synthesis conditions and fluorine doping on the properties of ZnO-QDs was not well-proposed yet.
Our study focused on exploring the optical behaviour and photoactivity of colloidal F/ZnO-QDs. We achieved this by adjusting the doping agent content (between 0 and 20 nominal atomic %) and by implementing two different wet-chemical synthetic routes – wet-precipitation and solvothermal methods, which allowed us to control the surface, morphological, dimensional and structural properties of the nanocrystals. A combined approach of physicochemical techniques allowed us to assess how the photoluminescence and photoactive performances of the resulting F/ZnO-QDs were tuned with the aim of favoring a specific application in sensing and/or photocatalysis.
Experimental section/methods
Materials
Zinc acetate dihydrate (ZAD) Zn(CH3COO2)·2H2O (≥99% purity), ammonium hydrogen difluoride NH4HF2 (≥99.999% purity), sodium hydroxide KOH (≥85% purity), triethylamine (TEA) C6H15N (>99% purity), quinine sulfate monohydrate C20H24N2O2·H2SO4·H2O (90% purity), methanol (≥99.8% purity), and ethanol (96% vol.) were purchased from Merck (Germany) and used without further purification.Synthesis of fluorine/doped ZnO nanoparticles
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 minutes, to separate the supernatant from ZnO nanocrystals. The obtained nanocrystals were finally dispersed and stored in ethanol.
000 rpm for 5 minutes, to separate the supernatant from ZnO nanocrystals. The obtained nanocrystals were finally dispersed and stored in ethanol.
Physicochemical analysis
![[t with combining macron]](https://www.rsc.org/images/entities/char_0074_0304.gif) e) for charge compensation. The pass energy was set at 23.5 eV, leading to an overall energy resolution of 0.6 eV. Photoelectrons were collected at a take-off angle of 45°, which means a sampling depth of approximately 5 nm. The decomposition of the spectra was done using Voigt functions after Shirley's background subtraction with the Multipak software.38
e) for charge compensation. The pass energy was set at 23.5 eV, leading to an overall energy resolution of 0.6 eV. Photoelectrons were collected at a take-off angle of 45°, which means a sampling depth of approximately 5 nm. The decomposition of the spectra was done using Voigt functions after Shirley's background subtraction with the Multipak software.38
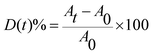 | (1) |
Results and discussion
Morphological and dimensional features of F/ZnO
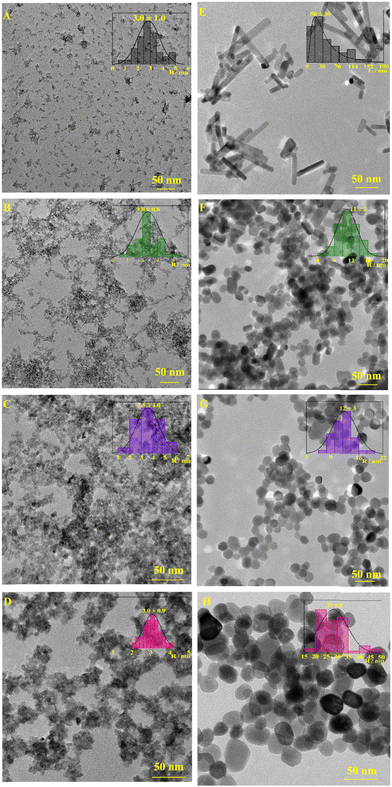
The formation of F/ZnO through both the adopted synthetic routes was proven by morphological and dimensional analyses. As reported in Fig. 1 and Fig. S1 (ESI†), TEM images clearly showed dramatic changes in the morphology and size of undoped and doped ZnO depending on the synthesis conditions and as a function of the amount of doping salt used during the syntheses. In the case of the ZnO-QDs synthesized through the wet-precipitation method, undoped ZnO-QDs (F0/ZnO-QDs) showed a pseudo-spherical morphology with a mean size of ∼3 nm in radius (Fig. 1A). These morphological and dimensional properties were obtained for F/ZnO-QDs with a nominal F content in the range 1–7.5 at% (Table S3, ESI†), although they showed an increasing tendency to form disordered nanoclusters (Fig. 1A–C and Fig. S1A–E, ESI†). By increasing the fluorine content, QDs with an irregular morphology and a slightly increased size were obtained (Fig. 1D and Fig. S1F–H, ESI†). Moreover, it gradually became almost impossible to distinguish the borders of the single QDs, which resulted in assembled and strongly interconnected as demonstrated by the formation of very irregular clusters with a final morphology like small bushes. This effect was directly related to F content and indicated that the relative composition of the synthesis mixtures drove differently the growth of ZnO-QDs, favoring self-aggregation.On the other hand, ZnO-NCs synthesized through the solvothermal method showed many differences in morphology and size as a function of doping. Specifically, undoped ZnO nanocrystals (F0/ZnO-NCs) were characterized by a rod-like shape with a rod length 〈L〉 equal to about 50 nm (Fig. 1E). This evidence indicated that the solvothermal synthesis, although realized for the same time as the wet-precipitation route, hindered the formation of confined quantum dots thus favoring the growth of nanocrystals into nanorods with higher dimensions (Table S3, ESI†), similar to what was previously achieved for a comparable synthesis strategy.44 The presence of the doping salt drove the formation of pseudo-spherical nanocrystals with a mean radius of ∼30 nm for the F1/ZnO-NCs sample (Fig. S1J, ESI†) and of ∼10 nm with the increase of the fluorine content (Fig. 1F, G and Fig. S1K–M, ESI†). By increasing the atomic percentage of the doping agent, the morphology of nanoparticles varies further, allowing the statistical determination of a length of about 30 nm for F10/ZnO-NCs (Fig. S1N, ESI†) and F15/ZnO-NCs (Fig. 1H) samples. Finally, F20/ZnO-NCs systems showed a heterogeneous character with the prevalence of spheroidal nanoparticles of ∼50 nm in radius (Fig. S1P, ESI†) together with microstructures.
The obtained results confirmed that the incorporation of F− ions into ZnO seeds influenced the growth of the host lattices. Also, the relative concentration of dopant precursor in the reagents represented a key factor that resulted in initial growth seeds with different crystallographic phases and shapes, leading to doped nanocrystals with different structures and morphologies.45,46 This evidence agreed with those of mechanistic reports describing the effect of the mostly studied doping with cationic species on the primary stage of semiconductor nanocrystal growth.47,48
The colloidal behavior of F/ZnO systems in alcohol suspension was investigated by DLS measurements (Fig. S2, ESI†) with the aim to verify if the clustering process was only due to the solvent evaporation occurring during TEM analysis or if F/ZnO exerted a natural tendency to self-aggregate as a function of their morphology and composition. Hydrodynamic radius distributions revealed the presence of a single population in all the samples, except for the F0/ZnO-NCs system, which showed two populations with Rh of ∼100 and 600 nm, respectively. This behavior can be explained as due to the very different morphology and size of such nanocrystals, which favored their clustering and the subsequent increased colloidal instability. At greater F contents, Rh increased moving from F0/ZnO-QDs to F2.5/ZnO-QDs and remained constant for F5/ZnO-QDs and F7.5/ZnO-QDs. Rh values of F10/ZnO-QDs and F15/ZnO-QDs were smaller than those of samples with less fluorine, being about 28 nm, while for a population with a hydrodynamic radius value of about 62 nm was observed F20/ZnO-QDs sample. In contrast, for samples obtained through the solvothermal method, the self-aggregation process was not controllable with the formation of aggregates with Rh ranging in 75–300 nm, not following any specific trend as a function of fluorine content and confirming that the process of self-aggregation was not a controllable process.
Structural and chemical features of F/ZnO
As shown in Fig. 2A, B and Fig. S3A, B (ESI†), XRD patterns of all F/ZnO nanosystems indicated the presence of typical peaks of wurtzite crystalline structure (JCPDS 36-1451). This was quantitatively confirmed by the values of the diffraction angles (2θ), the cell dimensions (a, b, c, α, β, and γ), and Miller indices (hkl) of the peaks, as listed in Table S4 (ESI†). Any shift of the diffraction peaks resulted from the experimental data of doped versus undoped samples, suggesting that any distortion of the crystalline lattice of ZnO occurred because of the presence of the doping agent and the synthesis method. However, the presence of diffraction peaks corresponding to a second different crystalline phase was detected in the diffraction spectra of samples obtained using a high amount of doping salt (from F7.5/ZnO-QDs to F20/ZnO-QDs). It could be ascribable to the presence of ZnF2 (indicated by stars in Fig. 2A) in the tetragonal crystalline structure with spatial group P42/mnm was also observed. The formation of ZnF2 may be a direct consequence of the dissociation of the fluorine-based doping salt, NH4FHF, into H+, HF2−, and HF species occurring in the reaction mixture. The HF specie was able to react with Zn(CH3COO)2 salt, inducing a competitive reaction Zn(CH3COO)2 + 2HF → ZnF2 + CH3COOH which led to the formation of ZnF2.49 At the same time, these species could exert a corrosive action toward the nanocrystal surface exposed to the solution, probably contributing to the definition of the irregular morphology of QDs. Although these phenomena were secondary concerning the formation of the ZnO phase, they became very decisive at the highest nominal content of the doping agent (F10/ZnO-QDs to F20/ZnO-QDs) in causing the deepest morphological differences and highest tendency to form disordered clusters, as indicated by TEM and DLS. These results confirmed that both the synthesis conditions and the relative amount of doping agent strictly influenced the chemical process of nanocrystal growth, simultaneously tuning the crystalline structure, shape, and size of F/ZnO.The XPS analysis led to the description of the surface chemical composition of doped ZnO nanocrystals compared to the undoped ones. The spectra reported in Fig. 2C, D and Fig. S3C, D (ESI†) showed the characteristic peaks of ZnO (Zn 2p, Zn 3s, Zn 3p, Zn 3d, and O 1s) for all the samples, while in those with the highest nominal F concentration (F15/ZnO-QDs and F20/ZnO-QDs), the F 1s peak was visible. The very small C 1s peak was ascribable to carbon contamination, probably due to the presence of unconverted reagents and/or of reaction by-products not completely removed during the purification step. A quantitative determination of the chemical species was also realized by a deeper analysis of the main XPS peaks, such as Zn 3p, O 1s, F 1s, and C 1s (Fig. S3, ESI†). The spectrum of Zn 3p (Fig. S3A and B, ESI†) showed two peaks related to the two components of the Zn3p3/2–Zn3p1/2 doublet located at 87 and 90 eV respectively. The XPS spectrum of O 1s (Fig. S3C and D, ESI†) also presented two peaks: (i) one between 529 and 530 eV, due to the oxygen bound to zinc, and (ii) the other one at about 532 eV, due to the oxygen bound to carbon and fluorine. In all the cases, the intensity of the O–Zn peak was higher than that of the O–C/O–F peak, except for the F15/ZnO-QDs and F20/ZnO-QDs samples. The peak of F was visible and, therefore, its concentration was quantifiable only for the highest nominal concentration samples obtained through the wet synthesis (Fig. S3E and F, ESI†). Finally, as previously indicated, the C 1s signal (Fig. S3G and H, ESI†) was due to the presence of an unconverted reagent as well as to the K 2p signal (Fig. S3G, ESI†) which was detected only in the samples with the highest F amount. By integration of these peaks, the atomic composition of the different F/ZnO was estimated, as summarized in Table 1.
| Sample | Zn at% | O (O–Zn) at% | F at% | C at% | O (O–C) at% |
|---|---|---|---|---|---|
| Wet-precipitation synthesis | |||||
| F0/ZnO-QDs | 31 ± 6 | 31 ± 6 | 14 ± 3 | 24 ± 5 | |
| F1/ZnO-QDs | 33 ± 6 | 27 ± 5 | 18 ± 3 | 22 ± 4 | |
| F2.5/ZnO-QDs | 33 ± 7 | 25 ± 5 | 18 ± 4 | 23 ± 5 | |
| F5/ZnO-QDs | 36 ± 7 | 27 ± 5 | 1.5 ± 0.3 | 17 ± 3 | 19 ± 4 |
| F7.5/ZnO-QDs | 39 ± 8 | 27 ± 5 | 1.7 ± 0.3 | 15 ± 3 | 17 ± 3 |
| F10/ZnO-QDs | 40 ± 8 | 29 ± 6 | 7 ± 1 | 15 ± 3 | 16 ± 3 |
| F15/ZnO-QDs | 31 ± 6 | 13 ± 3 | 8 ± 2 | 14 ± 3 | 24 ± 5 |
| F20/ZnO-QDs | 35 ± 7 | 19 ± 4 | 7 ± 1 | 17 ± 3 | 23 ± 5 |
| Solvothermal synthesis | |||||
| F0/ZnO-NCs | 41 ± 8 | 28 ± 5 | 15 ± 3 | 15 ± 3 | |
| F1/ZnO-NCs | 37 ± 7 | 24 ± 5 | 21 ± 4 | 18 ± 4 | |
| F2.5/ZnO-NCs | 42 ± 8 | 27 ± 5 | 11 ± 2 | 20 ± 4 | |
| F5/ZnO-NCs | 38 ± 8 | 31 ± 6 | 15 ± 3 | 16 ± 3 | |
| F7.5/ZnO-NCs | 39 ± 8 | 29 ± 6 | 17 ± 3 | 15 ± 3 | |
| F10/ZnO-NCs | 41 ± 8 | 26 ± 5 | 4.2 ± 0.8 | 9 ± 2 | 19 ± 4 |
| F15/ZnO-NCs | 40 ± 8 | 18 ± 3 | 9 ± 2 | 6 ± 1 | 26 ± 5 |
| F20/ZnO-NCs | 38 ± 8 | 10 ± 2 | 13 ± 3 | 7 ± 1 | 32 ± 6 |
For all F/ZnO nanocrystals, no significant changes were observed in the surface atomic concentration of Zn as well as of C and both C-bonded and Zn-bonded O species. Only in the case of F15 and F20 samples for both syntheses, a lower concentration of O–Zn species with respect to the other samples was detected, suggesting that the content of oxygen vacancies (Vo) on the nanocrystal surface was greater. The presence of F atoms was not detectable below the XPS sensitivity (∼1 at%). Indeed, the presence of F atoms on the surface of F/ZnO-QDs synthesized through the wet method (up to F5/ZnO-QDs) resulted in approximately equal to ∼1.5 at% for F5 and F7.5, ∼7–8% from F10 to F20 samples. Instead, for F/ZnO-NCs samples synthesized through the solvothermal method (up to F10/ZnO-NCs), the F content was estimated to be ∼4 at% for F10, ∼9–13 at% for F15 and F20 nanocrystals. These results indicated an increasing amount of surface defects. This probably indicated that a concentration of 7/8 at% (wet-precipitation method) and of 9/13 at% (solvothermal method) is the maximum concentration of dopant that ZnO may exhibit on the surface. However, in the case of ZnO-QDs from wet-precipitation synthesis, it is plausible to consider that the F content estimated from the XPS analysis may represent the effective one, given that the QDs diameter (∼5 nm) was compatible with the penetration depth of the beam used for analyses. Instead, in the case of solvothermal ZnO-NCs, these results could indirectly suggest that the monovalent anionic species were partially included in the crystalline structure by replacing the oxygen vacancies,33 and not only localizing on the surface.
Optical features of F/ZnO: absorption, emission, and relative quantum yield
UV-Vis and fluorescence spectroscopy measurements were carried out to define the optical properties of F/ZnO as a function of size and fluorine relative content.All F/ZnO samples showed an absorption behavior in the UV region exerting with a peak ranging between 332 and 361 nm, with changes directly related to the preparation methods, and consequently to nanocrystal size and fluorine content. Indeed, the quantum dots synthesized by the wet-precipitation (Fig. 3A) showed a maximum absorption centered between 332 and 347 nm, while a redshift was observed for nanocrystals obtained through the solvothermal method (Fig. 3D), with a maximum at ∼360 nm. This effect is ascribable to the greater size of such nanocrystals in agreement with previous results.50,51
Fig. 3B, E and Fig. S5 (ESI†) reported the Tauc plot reflectance data for the different F/ZnO samples. They were deduced using Tauc formulae and Kubelka–Munk function F® and expressed in eqn (2) and (3) respectively,
| (α × hν)n = B(hν − Eg) | (2) |
 | (3) |
 | ||
| Fig. 4 Band-gap energy values obtained from the Tauc-plot and relative quantum yield for all F/ZnO systems. | ||
By the inspection of Fig. 4, it is possible to note that the relative doping concentration strongly affected the optical properties of F/ZnO. Indeed, the maximum absorption wavelength of F0/ZnO-QDs, F1/ZnO-QDs and F2.5/ZnO-QDs, for which XPS had not detected the fluorine presence, increased as the Rh of suspended aggregates increased, as also observed by Xu et al. for undoped ZnO-QDs with a dimension between 15 and 40 nm.52 F5/ZnO-QDs and F7.5/ZnO-QDs (with an estimated F amount of 1.5 and 1.7 at%, respectively) had a maximum at about 343 nm, as well as the same F concentration shifted the maximum peak in F10, F15 and F20 ZnO-QDs at about 333 nm. It is interesting to note that the increased concentration of the doping agent caused a decrease in the absorption maximum, as already observed by Singh et al. doping ZnO nanoparticles with Mn and observing a blueshift from 366 to 352 nm by increasing the Mn concentration from 1 to 4%.53 The same trend was also observed by Hammad et al. for Co-doped ZnO nanoparticles.54
Instead, a redshift can be observed for samples synthesized through the solvothermal method. This shift is typical of doped zinc oxide nanoparticles and increases with the amount of doping agent.19,20,55 The presence of defects and doping agent also affected the bandgap values of F/ZnO. For undoped ZnO, it was 3.31 eV for F0/ZnO-QDs and about 3.24 eV for F0/ZnO-NCs, which was slightly lower than that of ZnO bulk (3.37 eV) and can be explained as due to the presence of defects in the crystalline structure of nanocrystals.56,57 Then, F1/ZnO-QDs and F2.5/ZnO-QDs samples showed values comparable to that of F0/ZnO-QDs, which confirmed the results of XPS that do not detect the presence of F in these samples. In the case of F5/ZnO-QDs and F7.5/ZnO-QDs samples, Eg was equal to 3.27 eV; this decrease, if compared to the values of previous samples, was due to the presence of the doping agent at low amounts (1.5 and 1.7 at%, respectively) as indicated by XPS analysis. Indeed, the presence of the fluorine doping agent can create a new valence band causing a decrease in the bandgap value.58 Finally, the bandgap of F10/ZnO-QDs, F15/ZnO-QDs, and F20/ZnO-QDs samples was even lower, probably due to a greater concentration of fluorine anions. These values agreed with the shift of the maximum absorption peaks observed for F/ZnO-QDs in suspension. Instead, the bandgap of doped ZnO-NCs synthesized by the solvothermal method was lower than that of the undoped ones but, in all cases, the value was equal to about 3.17 eV, except for F10/ZnO-NCs, F15/ZnO-NCs, and F20/ZnO-NCs samples prepared with a greater nominal amount of fluorine doping agent. This evidence confirmed that the changes in the electron structures depended not only on the dopant amount but also on the size and shape of nanocrystals as driven by preferential growth way due to the specific synthesis method.34,59
Fluorescence spectra were recorded in the 400–700 nm range by exciting each sample at the maximum absorption wavelength as determined by UV-Vis analysis. All emission spectra of F/ZnO-QDs synthesized by wet-precipitation (Fig. 5A, B and Fig. S7, ESI†) showed a single band centered at about 530 nm that can be attributed to the presence of point defects, such as oxygen vacancies or interstitial oxygen, directly related to emission peaks and affecting the fluorescence intensity. An emission band between 500 and 520 nm was ascribable to oxygen vacancies VO; a band between 550 and 560 nm to interstitial oxygen Oi; a band between 400 and 440 nm to zinc vacancies VZn and interstitial zinc Zni.60–63 The green emission could be associated with a transition between VO and holes.64 The intensity of these broad bands depends on the number of luminescence centers and the recombination of the electron–hole pairs.20,33 In the range of 1 to 7.5%, the fluorescence intensity decreases with increasing dopant, due to the decrease in the Vo number, since the F atoms fill Vo sites.33 The trend is not linear for the last three samples of the series, probably due to the presence of another crystalline species, as demonstrated by XRD data. Instead, the emission spectra of F/ZnO nanocrystals synthesized through the solvothermal method (from F0/ZnO-NCs to F7.5/ZnO-NCs) resulted in more complex with the presence of multiple peaks which were identified by a Gaussian peak deconvolution as shown in Fig. S8 (ESI†).
This approach highlighted that the emission of F/ZnO-NCs consists of several contributions. For all analyzed systems (F0/ZnO-NCs–F7.5/ZnO-NCs), four different peaks were identified, together contributing to the fluorescence behavior of these nanocrystals. The comparison of experimental and fitted spectra indicated that the doping agent did not influence the optical properties. Consequently, it is plausible to consider that a key role was played by the structural characteristics of ZnO nanocrystals given by the synthetic protocol.65 Indeed, ZnO nanocrystals are characterized by various kinds of defects, which are directly related to the features of their fluorescence emission spectra. Especially, defects such as zinc vacancies (VZn) and interstitial zinc (Zni) were widely recognized as responsible for the emission in the region of 400–440 nm.60 However, the origin of the broad emission band in the 540–560 nm region was still the subject of debate in the scientific community. In particular, two kinds of defects, such as oxygen vacancies (VO) and interstitial oxygen (Oi), can be considered as possibly responsible for this emission band,61,63 acting as acceptor and donor punctual defects, respectively. Carefully analyzing the experimental data obtained in this work and the scientific literature can be inferred that the VO was pointed out as responsible for the “green” emission band (large emission band between 500 and 520 nm) of ZnO-NPs; hence, the emission band observed in the systems here (emission band centered at 550 nm) can be attributed to Oi occupying octahedral sites,66,67 as more thermodynamically stable than tetrahedral Oi.61
To quantitatively estimate the changes in fluorescence emission, the relative quantum yield (Φ) with respect to the quinine sulfate chosen as a standard was determined by following the analytical procedure as described in the ESI.† The trends of the experimental values are reported in Fig. 5C and D. In the case of ZnO-NCs, the relative quantum yield is not affected by the presence of the doping agent, in fact, in all cases, it is less than 3%. Conversely, relative quantum yield increases with the fluorine content up to the sample F5/ZnO-QDs, which by XPS analysis has an atomic percentage of F on the QDs surface of 1.5% at, then decreases by further increasing the fluorine content, with quantum yields of F15/ZnO-QDs and F20/ZnO-QDs lower than that of the undoped sample. So, for QDs with a size less than 4 nm, a minimum amount of dopant was sufficient and optimal to improve the optical properties of F/ZnO-QDs.
Photoactivity under UVA-light irradiation
The photoactivity of F/ZnO was evaluated by monitoring their ability to degrade Rhodamine B (RhB) selected as a model dye.68 First, the pro-oxidant activity of nanocrystals was assessed in the dark and no RhB degradation was recorded. This result was in agreement with what was observed for preliminary pro-oxidative peroxidase (POD) and oxidase (OXD) mimicking assays, during which all the samples were inactive. This multiple evidence led to suppose that the production of free radicals had to be light-triggered.Fig. 6 summarizes the results of photodegradation tests for undoped and F/ZnO at two significantly different fluorine contents (F5 and F15), as obtained from both synthetic routes, while Table 2 reports a summary of the estimated kinetic constants obtained by fitting the experimental trends.
| Samples | 0 order kinetics | 1st order kinetics |
|---|---|---|
| k 0 (min−1) | k 1 (μmol L−1 min−1) | |
| F0/ZnO-NCs | 0.097 ± 0.007 | |
| F0/ZnO-QDs | 0.10 ± 0.01 | |
| F5/ZnO-NCs | 0.053 ± 0.001 | |
| F5/ZnO-QDs | 0.082 ± 0.008 | |
| F15/ZnO-NCs | 0.17 ± 0.02 | |
| F15/ZnO-QDs | 0.028 ± 0.004 |
By comparing the general trends, NCs produced by the solvothermal method were way more active than those produced through the wet-precipitation approach. In particular, the degradation of RhB was more intense for F/ZnO-NCs and this could be ascribable to a higher presence of defects in the crystalline structure. Indeed, as reported in the literature, the presence of intrinsic defects may intensify the photo(electro)chemical properties.69,70 Specifically, oxygen vacancies VO and zinc interstitials Zni could act as charge-carrier traps which delay the charge recombination. In addition, these defects may behave as surface sites facilitating the degradation of organic molecules through charge transfer interactions between the site and the analyte.71 These results could be also explained by considering that the defects in the ZnO-NCs decreased the recombination of the excitons which turned into a lower emission intensity at higher F content, and also in agreement with luminescence results previously reported in literature.24 It must be pointed out that the degradation efficiency of the nanocrystals with a higher content of fluoride decreased, this occurred because the fluoride had almost the same atomic radius as oxygen, so it could occupy the VO decreasing the number of defects present in the structure. Finally, RhB concentration profiles over time were fitted to linear and exponential decay equations to identify the kinetic model suiting the most the photocatalytic behaviour of each nanosystem. In detail, F15/ZnO-NCs and all the photoactive nanosystems produced through wet-chemistry route exhibited 0-order kinetics, with the solvothermal one outperforming the others since exhibiting the highest k0 (0.17 ± 0.02). Moreover, k0 constant decreased with increasing nominal fluorine content. On the other hand, the best-performing F0/ZnO-NCs and F5/ZnO-NCs samples exhibited 1st order kinetics, with k1 constant being again dependent on nominal fluorine content. Definitely, kinetic analysis was effective in further clarifying that the (i) solvothermal approach provided higher number of defects and active sites; (ii) wet-chemistry approach resulted in less photoactive nanomaterials, with saturated active sites even at such low RhB concentration (16.7 μM); and (iii) fluorine was particularly able in replacing oxygen in the lattices, reducing the number of defects and thus the charge transfer efficiency.
Conclusions
The study aimed to prepare colloidal fluorine-doped ZnO nanocrystals using two different synthesis strategies – wet-precipitation and solvothermal methods – with varying amounts of doping agent. The focus was to investigate how atomic doping, nanocrystal growth, and surface defects affect the optical behavior and photoactivity of F/ZnO. The research was conducted with a wide range of nominal atomic% of the doping agent (0–20) to understand how the properties of the nanocrystals could be simultaneously tuned as a function of the fluorine content and synthesis strategy employed. The wet-precipitation route led to the formation of very small quantum dots of about 3 nm in radius, showing good colloidal stability in suspension which was reduced by increasing the fluorine content as a consequence of the significant changes in the morphological and surface features induced by a greater amount of doping agent used during the synthesis. F/ZnO-QDs, obtained through the wet-precipitation method, exhibited scarce photoactivity and a significant fluorescence emission with the highest value of the relative quantum yield (Φ = 22%) for F/ZnO-QDs doped with a nominal concentration of fluorine of 5 at%. During solvothermal synthesis, nanocrystals are formed with varying shapes (from rod-like to spherical), sizes (ranging from 10 to 50 nm), and clustering tendencies that are strongly influenced by the presence of fluorine. These conditions result in low fluorescence emission, but high photoactivity is observed in F/ZnO with a low F content (5 at%), small size (approximately 10 nm in radius), and high surface defects. The photoactive efficiency of these nanocrystals can reach up to 95% after 60 minutes. This suggests a different growth of the crystalline structure and highlights the importance of controlling the fluorine content during the synthesis process. Definitely, this study allowed us to define the optimal conditions to alternatively design fluorescent F/ZnO-QDs for sensing and optoelectronic fields or photoactive F/ZnO-NCs for photocatalytic applications.Data availability
Data for this article, including XRD and XPS data, are available at Zenodo repository at https://doi.org/10.5281/zenodo.13144846. The data supporting this article have been also included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work was supported by Programma per il Finanziamento della Ricerca di Ateneo (FRA) 2020, funded by the University of Naples Federico II, project title “LPS-targeting nanostructured aptasensors for bacterial pathogens detection – LipAptaNanoSens” (Cod. 000008 ALTRI_CdA_75_2021_FRA_LINEA_B-VITIELLO) and Fondo per il Programma Nazionale della Ricerca (PNR) e Progetti di Ricerca di Rilevante Interesse Nazionale (PRIN 2022), project title “Glycolipids-coated colloidal quantum dots as optical biosensing platform for selective molecular recognition – GLYBIOSENS”, funded by the Italian Ministry of University and Research (MUR) and European Union – Next Generation EU, Mission 4, Component 2, Investment 1.1 – D.D. n. 104 del 02.02.2022, C.I. 2022PJP24F. This work has also received funding from the European Union's Horizon 2020 Research and Innovation Program under grant agreement No. 101007417, having benefitted from the access provided for experimental analyses by the CEA-LETI (Grenoble, France) within the framework of NFFA-Europe Pilot Transnational Access Activity (nffa.eu), proposal ID071.References
- S. Kargozar, S. J. Hoseini, P. B. Milan, S. Hooshmand, H.-W. Kim and M. Mozafari, Biotechnol. J., 2020, 15, 2000117 CrossRef CAS PubMed.
- H. Park, D. J. Shin and J. Yu, J. Chem. Educ., 2021, 98, 703–709 CrossRef CAS.
- N. X. Ca, N. T. Hien, X. Fan, P. V. Do, V. H. Yen, P. V. Hao, L. K. Quynh, T. T. T. Huong and V. X. Quang, RSC Adv., 2023, 13, 27292–27302 RSC.
- H. Moon, C. Lee, W. Lee, J. Kim and H. Chae, Adv. Mater., 2019, 31, 1804294 CrossRef PubMed.
- G. S. Selopal, H. Zhao, Z. M. Wang and F. Rosei, Adv. Funct. Mater., 2020, 30, 1908762 CrossRef CAS.
- W. Zhou, Y. Shang, F. P. García de Arquer, K. Xu, R. Wang, S. Luo, X. Xiao, X. Zhou, R. Huang, E. H. Sargent and Z. Ning, Nat. Electron., 2020, 3, 251–258 CrossRef CAS.
- P. Juzenas, W. Chen, Y.-P. Sun, M. A. N. Coelho, R. Generalov, N. Generalova and I. L. Christensen, Adv. Drug Delivery Rev., 2008, 60, 1600–1614 CrossRef CAS PubMed.
- A. C. S. Samia, X. Chen and C. Burda, J. Am. Chem. Soc., 2003, 125, 15736–15737 CrossRef CAS PubMed.
- P. Sun, Z. Xing, Z. Li and W. Zhou, Chem. Eng. J., 2023, 458, 141399 CrossRef CAS.
- A. Luchini, R. K. Heenan, L. Paduano and G. Vitiello, Phys. Chem. Chem. Phys., 2016, 18, 18441–18449 RSC.
- A. Luchini, G. Vitiello, F. Rossi, O. R. D. Ballesteros, A. Radulescu, G. D’Errico, D. Montesarchio, C. de, J. Fernández and L. Paduano, Phys. Chem. Chem. Phys., 2015, 17, 6087–6097 RSC.
- S. Brichkin and V. Razumov, Russ. Chem. Rev., 2016, 85(12), 1297–1312 CrossRef CAS.
- F. Chen, Q. Lin, H. Shen and A. Tang, Mater. Chem. Front., 2020, 4, 1340–1365 RSC.
- A. Luchini, G. D’Errico, S. Leone, Z. Vaezi, A. Bortolotti, L. Stella, G. Vitiello and L. Paduano, Colloids Surf., B, 2018, 168, 2–9 CrossRef CAS PubMed.
- A. Luchini, C. Irace, R. Santamaria, D. Montesarchio, R. K. Heenan, N. Szekely, A. Flori, L. Menichetti and L. Paduano, Nanoscale, 2016, 8, 10078–10086 RSC.
- R. Jiang, H. Wu, D. Manzani, W. Zhang and C. Liu, Appl. Surf. Sci., 2023, 622, 156931 CrossRef CAS.
- L. Zhang, L. Yin, C. Wang, N. Lun, Y. Qi and D. Xiang, J. Phys. Chem. C, 2010, 114, 9651–9658 CrossRef CAS.
- J. Zhu, K. Lu, J. Li, Z. Liu and W. Ma, Mater. Chem. Front., 2024, 8, 1792–1807 RSC.
- V. Kumar, J. Prakash, J. P. Singh, K. H. Chae, C. Swart, O. M. Ntwaeaborwa, H. C. Swart and V. Dutta, Colloids Surf., B, 2017, 159, 191–199 CrossRef CAS PubMed.
- W. Li, G. Wang, C. Chen, J. Liao and Z. Li, Nanomaterials, 2017, 7, 20 CrossRef PubMed.
- K. Pradeev Raj, K. Sadaiyandi, A. Kennedy, S. Sagadevan, Z. Z. Chowdhury, M. R. B. Johan, F. A. Aziz, R. F. Rafique, R. Thamiz Selvi and R. Rathina Bala, Nanoscale Res. Lett., 2018, 13, 229 CrossRef CAS PubMed.
- J. Sowik, M. Miodyńska, B. Bajorowicz, A. Mikolajczyk, W. Lisowski, T. Klimczuk, D. Kaczor, A. Zaleska Medynska and A. Malankowska, Appl. Surf. Sci., 2019, 464, 651–663 CrossRef CAS.
- A. Arivarasan, S. Bharathi, S. Ezhil Arasi, S. Arunpandiyan, M. S. Revathy and R. Jayavel, J. Lumin., 2020, 219, 116881 CrossRef CAS.
- D. W. Bahnemann, Claudius Kormann and M. R. Hoffmann, J. Phys. Chem., 1987, 91, 3789–3798 CrossRef CAS.
- L. Spanhel, J. Sol-Gel Sci. Technol., 2006, 39, 7–24 CrossRef CAS.
- S. Das, P. Somu, A. K. Yadav, P. K. Hopke and S. Paul, Environ. Sci.: Nano, 2024, 11, 739–765 RSC.
- L. K. Jangir, Y. Kumari, A. Kumar, M. Kumar and K. Awasthi, Mater. Chem. Front., 2017, 1, 1413–1421 RSC.
- X. Gong, H. Jiang, M. Cao, Z. Rao, X. Zhao and A. Vomiero, Mater. Chem. Front., 2021, 5, 4746–4755 RSC.
- A. Agrawal, I. Kriegel and D. J. Milliron, J. Phys. Chem. C, 2015, 119, 6227–6238 CrossRef CAS.
- M. Carofiglio, S. Barui, V. Cauda and M. Laurenti, Appl. Sci., 2020, 10(15), 5194 CrossRef CAS PubMed.
- A. Grala, M. Wolska-Pietkiewicz, Z. Wróbel, T. Ratajczyk, J. Kuncewicz and J. Lewiński, Mater. Chem. Front., 2018, 2, 1104–1111 RSC.
- J. Huo, Y. Zhang, W. Kang, Y. Shen, X. Li, Z. Yan, Y. Pan and W. Sun, Nanoscale Adv., 2023, 5, 2846–2864 RSC.
- G. P. Papari, B. Silvestri, G. Vitiello, L. De Stefano, I. Rea, G. Luciani, A. Aronne and A. Andreone, J. Phys. Chem. C, 2017, 121, 16012–16020 CrossRef CAS.
- G. Vitiello, G. Iervolino, C. Imparato, I. Rea, F. Borbone, L. De Stefano, A. Aronne and V. Vaiano, Sci. Total Environ., 2021, 762, 143066 CrossRef CAS PubMed.
- Solution Methods for Metal Oxide Nanostructures – 1st Edition|Elsevier Shop, https://shop.elsevier.com/books/solution-methods-for-metal-oxide-nanostructures/mane/978-0-12-824353-4, (accessed 3 April 2024).
- W. J. E. Beek, M. M. Wienk, M. Kemerink, X. Yang and R. A. J. Janssen, J. Phys. Chem. B, 2005, 109, 9505–9516 CrossRef CAS PubMed.
- V. Venezia, M. Verrillo, N. Gallucci, R. Di Girolamo, G. Luciani, G. D’Errico, L. Paduano, A. Piccolo and G. Vitiello, J. Environ. Chem. Eng., 2023, 11(1), 108973 CrossRef CAS.
- N. Gallucci, M. Hmoudah, E. Martinez, A. El-Qanni, M. Di Serio, L. Paduano, G. Vitiello and V. Russo, J. Environ. Chem. Eng., 2022, 10, 107866 CrossRef CAS.
- N. Gallucci, G. Vitiello, R. Di Girolamo, P. Imbimbo, D. M. Monti, O. Tarallo, A. Vergara, I. Russo Krauss and L. Paduano, Nanomaterials, 2021, 11, 542 CrossRef CAS PubMed.
- N. Gallucci, M.-S. Appavou, N. Cowieson, G. D’Errico, R. Di Girolamo, S. Lettieri, F. Sica, G. Vitiello and L. Paduano, J. Colloid Interface Sci., 2024, 659, 926–935 CrossRef CAS PubMed.
- G. Pota, N. Gallucci, D. Cavasso, I. Russo Krauss, G. Vitiello, F. Lopez-Gallego, A. Costantini and V. Califano, Langmuir, 2023, 39(4), 1482–1494 CrossRef CAS PubMed.
- I. Romano, G. Vitiello, N. Gallucci, R. Di Girolamo, A. Cattaneo, A. Poli and P. Di Donato, Microorganisms, 2022, 10, 1885 CrossRef CAS PubMed.
- S. Russo, M. Muscetta, P. Amato, V. Venezia, M. Verrillo, R. Rega, S. Lettieri, M. Cocca, R. Marotta and G. Vitiello, Chemosphere, 2024, 346, 140605 CrossRef CAS PubMed.
- P. Amato, M. Muscetta, V. Venezia, M. Cocca, G. Gentile, R. Castaldo, R. Marotta and G. Vitiello, J. Environ. Chem. Eng., 2023, 11, 109003 CrossRef CAS.
- P. Wainer, O. Kendall, A. Lamb, S. J. Barrow, A. Tricoli, D. E. Gomez, J. van Embden and E. D. Gasparra, Chem. Mater., 2019, 31(23), 9604–9613 CrossRef CAS.
- A. Dakhlaoui, M. Jendoubi, L. S. Smiri, A. Kanaev and N. Jouini, J. Cryst. Grow., 2009, 311, 3989–3996 CrossRef CAS.
- Y. Yang, Y. Jin, H. He, A. Wang, Y. Tu, H. Lu and Z. Ye, J. Am. Chem. Soc., 2010, 132(38), 13381–13394 CrossRef CAS PubMed.
- R. Buonsanti and D. J. Milliron, Chem. Mater., 2013, 25(8), 1305–1317 CrossRef CAS.
- Y. Guo, S. Wuttke, A. Vimont, M. Daturi, J.-C. Lavalley, K. Teinz and E. Kemnitz, J. Mater. Chem., 2012, 22, 14587–14593 RSC.
- A. Zuber, M. Purdey, E. Schartner, C. Forbes, B. van der Hoek, D. Giles, A. Abell, T. Monro and H. Ebendorff-Heidepriem, Sens. Actuators, B, 2016, 227, 117–127 CrossRef CAS.
- N. Ahmed, J. Singh, H. Chauhan, P. Gupta, A. Anjum and H. Kour, Int. J. Food Nutr. Saf., 2013, 4, 34–42 Search PubMed.
- E. G. Goh, X. Xu and P. G. McCormick, Scr. Mater., 2014, 78–79, 49–52 CrossRef CAS.
- J. Singh, A. Rathi, M. Rawat, V. Kumar and K.-H. Kim, Composites, Part B, 2019, 166, 361–370 CrossRef CAS.
- T. M. Hammad, J. K. Salem and R. G. Harrison, Appl. Nanosci., 2013, 3, 133–139 CrossRef CAS.
- H. Saadi, F. I. H. Rhouma, Z. Benzarti, Z. Bougrioua, S. Guermazi and K. Khirouni, Mater. Res. Bull., 2020, 129, 110884 CrossRef CAS.
- E. Y. Shaba, J. O. Jacob, J. O. Tijani and M. A. T. Suleiman, Appl. Water Sci., 2021, 11, 48 CrossRef CAS.
- I. H. Ifijen, M. Maliki and B. Anegbe, OpenNano, 2022, 8, 100082 CrossRef.
- M. Samadi, M. Zirak, A. Naseri, E. Khorashadizade and A. Z. Moshfegh, Thin Solid Films, 2016, 605, 2–19 CrossRef CAS.
- P. K. Samanta, Optik, 2020, 221, 165337 CrossRef CAS.
- J. Oliva, L. Diaz-Torres, A. Torres-Castro, P. Salas, L. Perez-Mayen and E. D. la Rosa, Opt. Mater. Express, 2015, 5, 1109–1121 CrossRef.
- A. Sahai and N. Goswami, Ceram. Int., 2014, 40, 14569–14578 CrossRef CAS.
- G. Sico, M. Montanino, M. Ventre, V. Mollo, C. T. Prontera, C. Minarini and G. Magnani, Scr. Mater., 2019, 164, 48–51 CrossRef CAS.
- O. W. Kennedy, E. R. White, A. Howkins, C. K. Williams, I. W. Boyd, P. A. Warburton and M. S. P. Shaffer, J. Phys. Chem. Lett., 2019, 10, 386–392 CrossRef CAS PubMed.
- H. Parangusan, D. Ponnamma and M. A. A. Al-Maadeed, Bull. Mater. Sci., 2019, 42, 179 CrossRef.
- W. Yang, H. Yang, W. Ding, B. Zhang, L. Zhang, L. Wang, M. Yu and Q. Zhang, Ultrason. Sonochem., 2016, 33, 106–117 CrossRef CAS PubMed.
- R. Raji and K. G. Gopchandran, J. Sci.: Adv. Mater. Devices, 2017, 2, 51–58 Search PubMed.
- A. B. Djurišić, Y. H. Leung, K. H. Tam, L. Ding, W. K. Ge, H. Y. Chen and S. Gwo, Appl. Phys. Lett., 2006, 88, 103107 CrossRef.
- V. Venezia, M. Verrillo, N. Gallucci, R. Di Girolamo, G. Luciani, G. D’Errico, L. Paduano, A. Piccolo and G. Vitiello, J. Environ. Chem. Eng., 2023, 11, 108973 CrossRef CAS.
- F. Liu, Y. H. Leung, A. B. Djurisic, A. M. C. Ng and W. K. Chan, J. Phys. Chem. C, 2013, 117(23), 12218–12228 CrossRef CAS.
- F. Kayaci, S. Vempati, I. Donmez, N. Biyikli and T. Uyar, Nanoscale, 2014, 6, 10224–10234 RSC.
- J. Kegel, V. Z. Zubialevich, M. Schmidt, I. A. Povey and M. E. Pemble, ACS Appl. Mater. Interfaces, 2018, 10(21), 17994–18004 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4qm00655k |
| This journal is © the Partner Organisations 2024 |