A hypochlorite-activatable persistent luminescence nanoprobe for assisted tumor resection†
Zhouyu
Zhang
a,
Zi-Jin
Wei
a,
Mengjie
Sun
a,
Zichao
Yan
a,
Chang
Yin
a,
Wei
Wang
 *a and
Zhi
Yuan
*a and
Zhi
Yuan
 *ab
*ab
aKey Laboratory of Functional Polymer Materials of the Ministry of Education, Institute of Polymer Chemistry, College of Chemistry, Nankai University, Tianjin 300071, China. E-mail: duruo@nankai.edu.cn; zhiy@nankai.edu.cn
bCollaborative Innovation Center of Chemical Science and Engineering, Nankai University, Tianjin 300071, China
First published on 13th February 2024
Abstract
Surgical resection is widely recognized as one of the most effective treatments for cancer. However, it requires precise and convenient visualization of tumor boundaries to assist surgeons. Persistent luminescence nanoparticles (PLNPs) exhibit bright and long-lasting afterglow, non-autofluorescence interference, and excellent photostability, making them ideal candidates for surgical navigation. Nevertheless, the currently used persistent luminescence tumor imaging method suffers from the issue of ambiguous tumor boundaries. Hence, a hypochlorite-responsive near-infrared (NIR) absorber A690 was bonded to ZnGa2O4:Cr3+ (ZGC) PLNPs coated with DSPE-mPEG2000 (ZGC@P&A). In normal tissue, ZGC@P&A was quenched due to the Förster resonance energy transfer from ZGC PLNPs to A690 molecules. Upon uptake by cancer cells, ZGC@P&A was turned “on” through the oxidation of A690 by high levels of hypochlorite. Compared to non-responsive ZGC@P, ZGC@P&A not only effectively eliminated interference from peripheral normal tissue afterglow but also accurately aligned with the tumor boundary within 30 minutes of peritumoral injection. Under the guidance of ZGC@P&A, the tumor was completely excised with minimal removal of surrounding tissue. Hypochlorite-activatable PLNPs greatly enhance the credibility of imaging, opening up new perspectives for a variety of clinical diagnostic applications.
1. Introduction
Despite the continuous development of innovative therapeutic methods for cancer, surgical resection remains the most effective approach for solid tumors.1 However, the outcomes of these therapies are still unsatisfactory. One major challenge lies in the difficulty for surgeons to accurately define the boundaries of tumors and ensure complete removal of tumor tissues and tiny metastases.2,3 Although widely used imaging modalities such as computed tomography (CT) and magnetic resonance imaging (MRI) are valuable for preoperative diagnosis, they are not suitable for surgical procedures due to their bulky equipment and complex operations.4 Therefore, there is an urgent need to develop a real-time technique that can assist surgeons in accurately identifying and completely resecting tumors.Fluorescence imaging technology has garnered significant interest due to its notable advantages of high sensitivity, rapid imaging, and convenient operation.5 Currently, several fluorescent molecular probes, such as indocyanine green and methylene blue, have been approved by the Food and Drug Administration for clinical use. However, these probes possess certain disadvantages, including poor photostability, susceptibility to bleaching, and interference from tissue autofluorescence.6 These limitations can result in unsatisfactory image quality during the complex process of tumor resection. In contrast, persistent luminescence nanoparticles (PLNPs) exhibit a unique energy trapping mechanism, allowing them to emit persistent luminescence (PersL) for minutes, hours, and even days after the cessation of excitation light irradiation.7,8 Thus, PersL signals can be captured for imaging after the excitation is terminated, effectively avoiding autofluorescence interference caused by biomolecules that are capable of emitting fluorescence during the irradiation process.9–11 Benefiting from their durable PersL, repeatable excitation capability, non-autofluorescence, and excellent photostability, PLNPs hold immense potential for application in surgical navigation fields.12
ZnGa2O4:Cr3+ (ZGC) is one of the most commonly used near-infrared (NIR) PLNPs that can be excited by UV and visible light, and ZGC PLNPs have been widely applied in cancer diagnosis and treatment systems.13,14 However, surgical navigation requires more precise imaging of tumor boundaries, and current PLNPs generally exhibit insufficient signal-to-noise ratios (SNRs) and ambiguous tumor boundaries. To address this challenge, two primary solutions have recently been proposed. (a) Increasing the intensity of persistent luminescence and (b) designing a tumor microenvironment-responsive system. The former has been studied in depth,15–17 while the tumor microenvironment-responsive PLNP systems are mainly applied in the field of therapy. In 2016, Yan et al. reported an activatable PLNP/CuS-based nanoprobe for luminescence imaging-guided photothermal therapy.18 This system utilized CuS as a photothermal agent and a quencher, coupled with a matrix metalloproteinases (MMPs)-specific peptide substrate to build an MMP-activatable system. In 2022, Yang et al. designed a PLNP coated with a shell of MnO2 that could be decomposed by the highly expressed GSH in cancer cells, enabling laser-excitation-free photodynamic therapy.19 These activatable systems have demonstrated excellent tumor-specific therapeutic outcomes, shedding light on the application of activatable persistent luminescence probes in the field of surgical navigation. The tumor microenvironment is characterized by regional hypoxia, low pH, high levels of reactive oxygen species (ROS), and highly expressed glutathione (GSH).20–22 Hypochlorite (ClO−) is one of the important ROS, serving as an oxidation substance bearing a 10-fold quantity in cancer cells compared to normal cells, making it a general characteristic not specific to a particular cancer type.23–25 Therefore, hypochlorite could serve as a potential biomarker for tumor detection.
Herein, we present the design and synthesis of a novel hypochlorite-activatable persistent luminescence nanoprobe ZGC@P&A (P: DSPE-mPEG2000, A: NIR absorber A690) for assisting in cancer surgery (Fig. 1). The absorption spectrum of A690 and the emission spectrum of ZGC PLNPs exhibited a high degree of overlap, enabling efficient Förster resonance energy transfer (FRET). Under normal conditions, where hypochlorite levels are scanty, ZGC@P&A remained in the “off” state. Instead, in tumor tissues, A690 was oxidized by overexpressed ClO−. Hence, FRET was interrupted and ZGC PLNPs were selectively turned “on”. Additionally, DSPE-mPEG2000 not only acted as a vehicle for A690 to provide the appropriate distance for FRET but also improved the biocompatibility. ZGC@P&A accurately delineated the tumor boundary without interference from peripheral normal tissue. These properties make activatable PLNPs promising candidates for using in cancer surgery.
 | ||
| Fig. 1 Schematic illustration of the construction and mechanism of the hypochlorite-activatable persistent luminescence nanoprobe ZGC@P&A. | ||
2. Experimental
2.1. Materials
Oleic acid (OA), Zn(NO3)2·6H2O, Ga(NO3)3·9H2O, Cr(NO3)3·9H2O, ethanol, sodium hydroxide (NaOH), lipopolysaccharide (LPS), and sodium hypochlorite were purchased from Aladdin Industrial Corporation (Shanghai, China). Cyclohexanone, 2-hydroxy-4-methoxybenzaldehyde, Fischer aldehyde, piperidine, acetic acid, acetic anhydride, and taurine were purchased from Heowns Biochem Technology Co., Ltd (Tianjin, China). Petroleum ether, dichloromethane, ethyl acetate, methanol, and cyclohexane were purchased from Tianjin chemical reagent company (Tianjin, China). DSPE-mPEG2000 was purchased from Mreda Biochemical Co., Ltd (Beijing, China). Phosphate buffer (PBS, pH 7.4), cell counting kit-8 (CCK-8), and 4′,6-diamidino-2-phenylindole (DAPI) were acquired from Beyotime Biotechnology Co., Ltd (Shanghai, China). All chemicals were used without further purification.2.2. Characterization
The morphologies of ZGC PLNPs were characterized using a transmission electron microscope (TEM, HITACHI HT7700 Exalens, 120 kV, Japan). The crystal structure of the prepared powders was characterized using a powder Xray diffractometer (XRD, MimFlex 600, Rigaku, Japan) with Cu Kα radiation over a step scan mode of 4°/min in the angular range of 2θ = 10–80°. The hydration diameters were measured using a Zetasizer (Nano-ZS 90, Malvern, UK). 1H NMR spectra were measured using a Bruker AVANCE III (400MHz, Germany). The molecular weight was measured with a time-of-flight mass spectrometer (MALDI-TOF, Autoflex III LRF200-CID, Bruker Daltonics, Germany). The UV-vis absorbance and infrared spectra were measured with a UV spectrophotometer (SHIMADZU, TCC240A, Japan) and Fourier transform infrared spectrometer (FTIR, TENSOR II, Bruker, Germany), respectively. The persistent luminescence excitation and emission spectra were recorded using a fluorescence spectrometer (FLS5, Edinburgh, UK). The thermal gravimetric loss was measured using a thermal gravimetric analyzer (NETZSCH TG 209, Germany) in the air atmosphere, with a heating rate of 10 °C min−1 from room temperature (RT) to 600 °C. The PersL images and decay curves were obtained using an in vivo imaging system (IVIS Lumina II, Xenogen, USA) in the bioluminescence mode without any filter.2.3. Synthesis of ZGC PLNPs
ZGC PLNPs were synthesized using a typical solvothermal method as previously reported with modifications.26 5.3 g OA, 14.2 g ethanol, and 8.6 g aqueous NaOH (7% w/w) were mixed at room temperature and left to stir for 1 h, and the resulting solution was denoted as Solution A. Then, 0.2975 g (1 mmol) of Zn(NO3)2·6H2O, 0.8375 g (2 mmol) of Ga(NO3)3·9H2O, and 0.0024 g (0.006 mmol) of Cr(NO3)3·9H2O were dissolved in 2 mL of water, and the resulting solution was denoted as Solution B. Solution B was slowly added dropwise to Solution A under vigorous stirring. After stirring for 30 min, the mixed solution was transferred to a Teflon-lined autoclave for solvothermal treatment at 180 °C for 16 h. ZGC PLNPs were collected by centrifugation at 5000 rpm, followed by three cycles of washing with cyclohexane and ethanol. The end products were dried at 60 °C in a vacuum oven for 12 h. The ZGC PLNPs had their surface coated with oleic acid molecules, making them hydrophobic. The measurement of the hydrodynamic diameter was conducted in cyclohexane, while the surface potential of ZGC PLNPs in aqueous solution needed to be rapidly tested after vigorous ultrasonication.2.4. Synthesis of A690
A690 was synthesized according to the method reported by Yuan with modifications.27 Briefly, 1 g (6.6 mmol) 2-hydroxy-4-methoxybenzaldehyde and 1.3 g (13.2 mmol) cyclohexanone were dissolved in 50 mL absolute ethanol, followed by the addition of a catalytic agent containing 0.6 mL piperidine and 0.2 mL acetic acid. The reaction mixture was heated and stirred at 80 °C for 12 h under a nitrogen atmosphere. After the reaction was completed, the ethanol was removed by vacuum distillation, and 10 mL acetic anhydride was added to redissolve the reaction mixture. Then, 2.8 g (14.0 mmol) Fischer aldehyde was introduced, and the mixture was heated and stirred at 50 °C for 8 h under a nitrogen atmosphere. The product was further purified by silica gel flash chromatography using petroleum/ethyl acetate (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to dichloromethane/methanol (1
1) to dichloromethane/methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 to 15
0 to 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 200 mg of the above products was dissolved in 3 mL dichloromethane and cooled down to 0 °C under a nitrogen atmosphere. BBr3 (1 mL BBr3 dissolved in 3 mL CH2Cl2) was slowly added dropwise to the mixture, and the reaction mixture was warmed to room temperature and stirred for 24h. The product was further purified by silica gel flash chromatography using petroleum/ethyl acetate (2
1). 200 mg of the above products was dissolved in 3 mL dichloromethane and cooled down to 0 °C under a nitrogen atmosphere. BBr3 (1 mL BBr3 dissolved in 3 mL CH2Cl2) was slowly added dropwise to the mixture, and the reaction mixture was warmed to room temperature and stirred for 24h. The product was further purified by silica gel flash chromatography using petroleum/ethyl acetate (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to dichloromethane/methanol (1
1) to dichloromethane/methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 to 15
0 to 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The turquoise solid powder obtained was A690 (yield 9.5%). 1H NMR (400 MHz, Chloroform-d, δ): 8.10 (d, J = 13.3 Hz, 1H), 7.35–7.27 (m, 3H), 7.22 (d, J = 9.1 Hz, 1H), 7.06 (t, J = 7.4 Hz, 1H), 6.85 (d, J = 7.9 Hz, 1H), 6.79 (dd, J = 9.0, 2.0 Hz, 1H), 6.62 (s, 1H), 5.61 (d, J = 13.4 Hz, 1H), 3.37 (s, 3H), 2.67 (d, J = 6.1 Hz, 2H), 2.62 (t, J = 6.2 Hz, 2H), 1.93–1.87 (m, 2H), 1.67 (s, 6H). HRMS (ESI) m/z: calcd for C26H26NO2+ (M+): 384.1958. Found: 384.1958.
1). The turquoise solid powder obtained was A690 (yield 9.5%). 1H NMR (400 MHz, Chloroform-d, δ): 8.10 (d, J = 13.3 Hz, 1H), 7.35–7.27 (m, 3H), 7.22 (d, J = 9.1 Hz, 1H), 7.06 (t, J = 7.4 Hz, 1H), 6.85 (d, J = 7.9 Hz, 1H), 6.79 (dd, J = 9.0, 2.0 Hz, 1H), 6.62 (s, 1H), 5.61 (d, J = 13.4 Hz, 1H), 3.37 (s, 3H), 2.67 (d, J = 6.1 Hz, 2H), 2.62 (t, J = 6.2 Hz, 2H), 1.93–1.87 (m, 2H), 1.67 (s, 6H). HRMS (ESI) m/z: calcd for C26H26NO2+ (M+): 384.1958. Found: 384.1958.
2.5. Preparation of ZGC@P&A
60 mg ZGC PLNPs was dispersed in 1 mL chloroform, and 30 mg DSPE-mPEG2000 (dissolved in 3 mL chloroform) was added. After 1 h of ultrasonic treatment, the chloroform dissolvent was evaporated by nitrogen sweeping. Next, the product was redispersed in 10 mL water and centrifuged to remove excessive DSPE-mPEG2000. The final product was collected by vacuum freeze-drying and denoted as ZGC@P. A690 molecules can be immobilized on the DSPE layer through hydrophobic interactions. 1 mL A690 solution (1 mg mL−1) was added to 10 mL ZGC@P (2 mg mL−1) suspension, and the mixture was stirred at room temperature for 2 h. The product ZGC@P&A was collected by centrifugation at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm and washed with water three times. The resulting stable dispersion was stored at 4 °C for further studies.
000 rpm and washed with water three times. The resulting stable dispersion was stored at 4 °C for further studies.
2.6. Cell culture and cytotoxicity
HepG2 and L929 mouse fibroblast cells were cultured in DMEM medium supplemented with 10% fetal bovine serum (FBS, Hyclone). All cells were cultured in an atmosphere of 5% CO2 and 95% relative humidity at 37 °C.The assessment of cell viability was performed using the CCK-8 method. HepG2 and L929 cells were seeded into a 96-well plate at 8 × 103 cells per well and incubated overnight. The cells were then treated with various concentrations of ZGC@P&A (100, 200, 400, 600, and 800 μg mL−1) for 24 and 48 h. Next, the PLNPs were removed by PBS washing and CCK-8 solution was added. After incubation for 4 h, the absorbance was measured using a microplate reader (Spark, Tecan Group Ltd., Switzerland) at 450 nm. The cell viability (CV) was calculated according to eqn (1):
 | (1) |
2.7. Animal model
All animal experiments were approved and supervised by the Laboratory Animal Ethics Committee of Nankai University. Six-week-old female BALB/c mice were purchased from SPF Biotechnology Co., Ltd (Beijing). The H22 tumor model was established by subcutaneously injecting H22 ascites (1 × 106 cells per mouse). The tumor-bearing mice were used when the tumor reached a size of 50–100 mm3 (7–10 days after implant).2.8. In vivo PersL imaging of mice
All imaging mice were anaesthetized with intraperitoneally injected pentobarbital sodium to minimize suffering. ZGC@P&A was dispersed in physiological saline at a concentration of 2 mg mL−1 and the tumor-bearing mouse was peritumorally injected with 100 μL. The mice were imaged at different time points post injection with an acquisition time of 60 s. The PLNPs were in situ re-irradiated with a 405 nm LED (irradiance = 90 mW cm−2) for 5 min before NIR PersL imaging.2.9 In vivo biosafety analyses
Blood samples were collected from the fundus artery of healthy mice at 14 days after intravenous injection of 200 μL of a 2 mg mL−1 ZGC@P&A physiological saline solution. The mice in the control group were injected with the same volume of physiological saline.A 100 μL blood sample was collected into an anticoagulant for routine blood analysis. 1.0 mL of blood was collected into a centrifuge tube. After stewing 2 h, the serum was separated by centrifugation (3000 rpm, 15 min) for blood biochemistry and hematology analyses. The heart, liver, spleen, lung and kidneys of each mouse were excised, fixed in 4% paraformaldehyde, embedded in paraffin, cut into 4-μm-thickness cross sections, and finally stained with H&E.
2.10. Statistical analysis
All data were presented as mean ± standard deviation. The comparison of the data was conducted using a Student's t-test (*: P < 0.05, **: P < 0.01, and ***: P < 0.001).3. Results and discussion
3.1 Synthesis and characterization of ZGC@P
The OA (oleic acid) coated ZGC PLNPs (ZGC-OA) were prepared by a solvothermal method in a mixture of OA and ethanol.26 The size and crystal structure of the nanoparticles were characterized using transmission electron microscopy (TEM) and X-ray powder diffraction (XRD) measurements. As shown in Fig. 2(a), ZGC PLNPs were regular sphere-like in morphology with a uniform size of 6.7 nm. XRD results (Fig. 2(d)) indicated that the ZGC PLNPs possessed a high degree of crystallinity, with a pure cubic spinel phase ZnGa2O4 (JCPDS No. 38-1240). The wide X-ray photoelectron spectra (XPS) of ZGC@P&A confirmed the presence of relevant elements, and the XPS O 1s spectra demonstrated the existence of metal oxides and C–O bonds (Fig. S1, ESI†). This finding was further supported by the clear lattice fingerprint observed in the (220) plane of the cubic spinel structure (Fig. 2(b)). A Zn/Ga molar ratio of 1/1.8 in the ZGC PLNPs, with a 4‰ chromium (Cr) doping, was confirmed by ICP analysis (Fig. S2, ESI†).To improve the dispersibility and biocompatibility of ZGC-OA, DSPE-mPEG2000 was introduced onto the surface through hydrophobic interactions.28 Compared to the TEM image of ZGC PLNPs, no significant changes were observed in the TEM image of ZGC@P&A (Fig. S3, ESI†), which may be attributed to the relatively small molecular weight of DSPE-mPEG2000 and A690. The changes in surface potential (Fig. S4, ESI†) confirmed the successful encapsulation of DSPE-mPEG2000 and the effective adsorption of A690 molecules. Fourier transform infrared (FTIR) spectroscopy analysis (Fig. S5, ESI†) revealed characteristic bands associated with the functional groups present. The broad bands observed at 1553 cm−1 and 1409 cm−1 were assigned to the stretching vibration of the carboxylic group (COOH), while the bands at 2921 cm−1 and 2851 cm−1 corresponded to the stretching vibration of methylene (CH2) in the oleic acid molecules. Following the coating of DSPE-mPEG2000, the presence of the ether bond (C–O) antisymmetric stretching vibration (1102 cm−1) was observed. Compared to ZGC-OA, the hydrodynamic diameter of ZGC@P (Fig. 2(e)) increased from 13.3 nm (PDI of 0.22) to 15.7 nm (PDI of 0.20), confirming the successful coating of DSPE-mPEG2000, and the hydrodynamic diameter of ZGC@P&A was 15.8 nm (PDI of 0.22), which showed no significant change compared to ZGC@P. The thermogravimetric (TG) curves after 200 °C, which measured weight loss due to the decomposition of organic matter, indicated that the additional weight loss observed in ZGC@P compared to ZGC-OA was attributed to the presence of the coated DSPE-mPEG2000. Calculation results showed that the weight of the coated DSPE-mPEG2000 accounted for 11.6% of the total organic compound (Fig. S6, ESI†).
3.2. Spectral analyses of ZGC@P and A690
The photophysical properties of ZGC@P and A690 were further investigated. A690 was synthesized following the method reported by Yuan with some modifications,27 and its structures were characterized using 1H NMR and MS (Fig. S7–S9, ESI†). The excitation spectrum of ZGC@P revealed three peaks at 249 nm, 416 nm, and 568 nm (Fig. S10, ESI†), indicating that ZGC@P can be excited by UV light and LED light. Comparing the persistent luminescence (PersL) emission spectrum of ZGC@P with the absorption spectrum of A690 (Fig. 2(c)), a significant overlap was observed, ensuring efficient FRET between ZGC@P and A690.29 Furthermore, the re-activation performance of ZGC@P was also studied (Fig. 2(f)). The renewable PersL, activated by a 405 nm lamp, exhibited nearly the same intensity and lifetime in each cycle, with the PersL intensity returning to its original position.3.3. Response performance of ZGC@P&A toward ClO−
A690 was loaded onto the DSPE layer of ZGC@P through hydrophobic interactions and the composite was named as ZGC@P&A below. To achieve a favorable quenching effect of ZGC PersL, the loading amount of A690 was appropriately saturated, with a loading concentration of 49.8 μg A690 per 1 mg of ZGC@P. Subsequently, gradient concentrations of ClO− ranging from 0 to 25 μM were added to the A690 solution. As the ClO− concentration increased, the color of the A690 solution changed from turquoise to light red (Fig. S11, ESI†). Notably, this color change occurred immediately upon mixing, indicating the rapid response performance of A690. Concurrently, the characteristic absorption peak of A690 at 690 nm gradually disappeared (Fig. 3(a) and (b)), demonstrating the significant potential of A690 for ClO− detection. Consistent with the observations mentioned earlier, the PersL intensity of ZGC@P&A at 695 nm gradually increased as A690 underwent oxidation (Fig. 3(c)). The PersL image captured using the IVIS system also revealed brighter PersL with higher concentrations of ClO− (Fig. S12, ESI†). Furthermore, to verify the specificity of ZGC@P&A, various reactive oxygen species (ROS) and predominant physiological ions were added to the ZGC@P&A solution. None of these ions had a significant influence on the PersL activation of ZGC@P&A (Fig. 3(d)), indicating the excellent specificity of ZGC@P&A towards ClO−. These results collectively demonstrated that the persistent luminescence of ZGC@P&A could be selectively activated, and its intensity was proportional to the concentration of ClO−.3.4. Response performances of ZGC@P&A in vitro and cytotoxicity
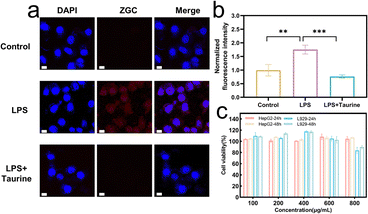
To further investigate the intracellular specific response of ZGC@P&A, fluorescence colocalization imaging was performed using confocal laser-scanning microscopy. HepG2 cells were pre-incubated with 1 mL (100 μg mL−1) ZGC@P&A, followed by the addition of gradient concentrations of ClO− solution ranging from 0 to 25 μM. The cells were then stained with DAPI, which labelled the nucleus with blue fluorescence.30 The signals from ZGC@P&A (red, λex = 408 nm) gradually increased to a maximum at 25 μM (Fig. S13, ESI†), indicating that exogenous ClO− can activate intracellular ZGC@P&A. Next, the response performance of ZGC@P&A toward endogenous ClO− was investigated. Lipopolysaccharide (LPS) was employed to induce cells to produce endogenous ClO−, while taurine was employed to clear ClO−.24,31,32 HepG2 cells were pre-incubated with LPS at a concentration of 10 μg mL−1, and then further incubated with ZGC@P&A (1 mL, 100 μg mL−1) as the experimental group. The empty control group was pre-incubated with PBS, and another control group was incubated with taurine after LPS incubation. In the representative images (Fig. 4(a)), no observable ZGC@P&A fluorescence signal was observed for HepG2 cells in the absence of the ClO− donor LPS. Conversely, intense ZGC@P&A fluorescence was observed in the presence of LPS. Importantly, no ZGC@P&A fluorescence signal was observed after taurine incubation. This result indicates that ClO− was the specific factor that activated ZGC@P&A in cells. The normalized fluorescence intensity was calculated using ImageJ software (Fig. 4(b)).33 The LPS group showed a 1.9-fold increase compared to the control group (**p < 0.01) and a 2.3-fold increase compared to the LPS-taurine group (***p < 0.001). These results demonstrated the impressive potential of ZGC@P&A for the specific detection of ClO−in vivo.To assess the potential biological applications, the cytotoxicity of ZGC@P&A was investigated via CCK-8 assay. HepG2 cells and L929 cells were incubated with various concentrations of ZGC@P&A for 24 h and 48 h. In the presence of 100–800 μg mL−1 of ZGC@P&A, the cellular viabilities of both HepG2 cells and L929 cells were above 80% after 24 h and 48 h of incubation, respectively (Fig. 4(c)). Based on these findings, it could be concluded that ZGC@P&A exhibited no significant cytotoxicity.
3.5. Response performances of ZGC@P&A in vivo and tumor visualization
The feasibility of ZGC@P&A for specific diagnosis of tumors in vivo was subsequently assessed. It is known that ClO− levels are significantly higher in tumor cells compared to normal cells, providing a basis for the diagnosis and visualization of tumors using ZGC@P&A.34,35 To evaluate this, a subcutaneous H22 tumor mouse model was utilized. After peritumoral injection of the activatable probe ZGC@P&A, the PersL signal was immediately detected at the tumor region within 10 min. However, the image obtained at this time point did not provide complete tumor visualization due to ongoing tumor cell uptake. 30 min post-injection, the tumor boundary information was accurately depicted, with no interference afterglow observed in other areas (Fig. 5(a)). As the activating reactions proceed, the signal-to-noise ratio (SNR) reached its peak within 2 h and then gradually decreased due to metabolism (Fig. 5(c)). In contrast, no significant signal was observed in the control group (healthy mice). This result demonstrated that ZGC@P&A could be specifically activated by endogenous ClO− in tumors to accurately visualize the tumor boundary. In contrast, after peritumoral injection of the always-on probe ZGC@P, the PersL signal was extensively distributed throughout the tumor and surrounding region, making it difficult to distinguish the tumor from normal tissue (Fig. 5(b)). This result highlighted the advantages of using the activatable probe ZGC@P&A for tumor boundary visualization.3.6. Tumor resection navigation and biosafety
Encouraged by the promising in vitro and in vivo results, the effects of ZGC@P&A in assisting tumor resection were evaluated. To simulate surgery, a subcutaneous H22 tumor mouse model was utilized. Based on the optimal tumor image quality and SNR observed within 1.5 to 2.5 h post-injection, the beginning time of surgical resection was set at 1.5 h. Using the PersL signal from ZGC@P&A as a guide, the skin around the tumor was incised (Fig. 6(a)). Subsequently, the tumor was completely removed with the assistance of a more detailed second imaging. Importantly, the bright PersL signals were observed exclusively in the tumor (Fig. 6(b)), indicating that ZGC@P&A could specifically illuminate solid tumors with high sensitivity. Standard H&E staining of excised tissue was performed (Fig. 6(c)), confirming that the excised tissue contained the entire solid tumor with a thin covering of muscular tissues at the outer edge. By utilizing ZGC@P&A for tumor navigation, the damage to healthy tissues was minimized while ensuring a negative margin, which would significantly improve the postoperative recovery rate.Furthermore, the biosafety of ZGC@P&A was also investigated.36,37 Blood samples and paraffin sections of organs were collected at 14 days after intravenous injection of ZGC@P&A (2 mg mL−1, 200 μL) and PBS (200 μL). Compared to the control group, ZGC@P&A had no adverse effects on main organs such as heart, liver, spleen, lung and kidneys (Fig. S14, ESI†). Blood biochemistry and hematology analyses shown negligible fluctuations in blood indicators, liver and kidney function indexes compared to the control group (Fig. S15 and S16, ESI†). Considering the high biocompatibility of the nanoprobe, ZGC@P&A holds great promise for clinical applications.
4. Conclusions
In summary, we have successfully developed a sensitive hypochlorite-activatable persistent luminescence nanoprobe ZGC@P&A for assisted cancer resection. By anchoring A690 on the surface of ZGC PLNPs, the nanoprobe could specifically detect ClO−. Both in vitro and in vivo experiments have demonstrated the specific activation of ZGC@P&A by ClO−. Utilizing the unique optical properties of ZGC PLNPs, the nanoprobe enabled high-quality in vivo imaging with autofluorescence-free, long-lasting PersL, and excellent photostability. In a simulated resection operation, the H22 solid tumor was precisely excised under the guidance of ZGC@P&A. To the best of our knowledge, this is the first FRET-based ClO− response PLNPs for in vivo imaging of tumors. Through this strategy, we effectively leverage the rapid and sensitive characteristics of organic responsive molecules to enhance the precision and accuracy of persistent luminescence images of tumors. We believe that the study will greatly inspire the development of activatable PLNPs in the fields of precise tumor navigation and rapid pathological diagnosis.Author contributions
This manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant 52073147) and the Natural Science Foundation of Tianjin (Grant 20JCYBJC01540). The authors greatly appreciate the assistance from Prof. Qiang Wu at Nankai University in material characterization.References
- N. E. Wojtynek and A. M. Mohs, Image-guided tumor surgery: The emerging role of nanotechnology, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2020, 12, e1624 Search PubMed.
- C. Chi, Y. Du, J. Ye, D. Kou, J. Qiu, J. Wang, J. Tian and X. Chen, Intraoperative Imaging-Guided Cancer Surgery: From Current Fluorescence Molecular Imaging Methods to Future Multi-Modality Imaging Technology, Theranostics, 2014, 4, 1072–1084 CrossRef PubMed.
- J. Dai, H. Xue, D. Chen, X. Lou, F. Xia and S. Wang, Aggregation-induced emission luminogens for assisted cancer surgery, Coord. Chem. Rev., 2022, 464, 214552 CrossRef CAS.
- J. Liu, T. Lecuyer, J. Seguin, N. Mignet, D. Scherman, B. Viana and C. Richard, Imaging and therapeutic applications of persistent luminescence nanomaterials, Adv. Drug Delivery Rev., 2019, 138, 193–210 CrossRef CAS PubMed.
- C. Li, G. Chen, Y. Zhang, F. Wu and Q. Wang, Advanced Fluorescence Imaging Technology in the Near-Infrared-II Window for Biomedical Applications, J. Am. Chem. Soc., 2020, 142, 14789–14804 CrossRef CAS PubMed.
- P. Y. Wang, Y. Fan, L. F. Lu, L. Liu, L. L. Fan, M. Y. Zhao, Y. Xie, C. J. Xu and F. Zhang, NIR-II nanoprobes in-vivo assembly to improve image-guided surgery for metastatic ovarian cancer, Nat. Commun., 2018, 9, 2898 CrossRef PubMed.
- Z. Pan, Y.-Y. Lu and F. Liu, Sunlight-activated long-persistent luminescence in the near-infrared from Cr3 + -doped zinc gallogermanates, Nat. Mater., 2012, 11, 58–63 CrossRef CAS PubMed.
- T. Maldiney, D. Scherman and C. Richard, in Functional Nanoparticles for Bioanalysis, Nanomedicine, and Bioelectronic Devices, Vol 2, eds. M. Hepel and C. J. Zhong, 2012, vol. 1113, pp. 1–25 Search PubMed.
- N. Chen, N. Du, W. J. Wang, T. G. Liu, Q. Yuan and Y. B. Yang, Real-Time Monitoring of Dynamic Microbial Fe(III) Respiration Metabolism with a Living Cell-Compatible Electron-Sensing Probe, Angew. Chem., Int. Ed., 2022, 61, e202115572 CrossRef CAS PubMed.
- N. Chen, X. M. Zhang, J. Xi, Y. B. Yang and Q. Yuan, Recent advances of microbial metabolism analysis: from metabolic molecules to environments, Sci. China: Chem., 2023, 2941–2950 CrossRef CAS.
- N. Chen, D. Cheng, T. P. He and Q. Yuan, Real-Time Monitoring of Dynamic Chemical Processes in Microbial Metabolism with Optical Sensors, Chin. J. Chem., 2023, 41, 1836–1840 CrossRef CAS.
- S. K. Sun, H. F. Wang and X. P. Yan, Engineering Persistent Luminescence Nanoparticles for Biological Applications: From Biosensing/Bioimaging to Theranostics, Acc. Chem. Res., 2018, 51, 1131–1143 CrossRef CAS PubMed.
- Z. Li, Y. Zhang, X. Wu, L. Huang, D. Li, W. Fan and G. Han, Direct Aqueous-Phase Synthesis of Sub-10 nm “Luminous Pearls” with Enhanced in Vivo Renewable Near-Infrared Persistent Luminescence, J. Am. Chem. Soc., 2015, 137, 5304–5307 CrossRef CAS PubMed.
- Z. J. Li, Y. W. Zhang, X. Wu, X. Q. Wu, R. Maudgal, H. W. Zhang and G. Han, In Vivo Repeatedly Charging Near-Infrared-Emitting Mesoporous SiO2/ZnGa2O4:Cr3+ Persistent Luminescence Nanocomposites, Adv. Sci., 2015, 2, 1500001 CrossRef PubMed.
- Z. Li, Y. Zhao, K. Huang, L. Huang, Y. Zhang, H. Yang and G. Han, Enhancing Rechargeable Persistent Luminescence via Organic Dye Sensitization, Angew. Chem., Int. Ed., 2021, 60, 15886–15890 CrossRef CAS PubMed.
- M. J. Sun, C. Yin, Z. C. Yan, Z. J. Wei, Z. Y. Zhang, W. Wang and Z. Yuan, Energy recruitment via lanthanide-chelate to boost the persistent luminescence of nanophosphor for contrast-enhanced tumor navigation, Chem. Eng. J., 2023, 468, 143814 CrossRef CAS.
- Z. Yan, C. Yin, M. Sun, W. Yuan, W. Wang, Q. Wu and Z. Yuan, A novel removable template method for the preparation of persistent luminescence nanoparticles with biocompatible size and high intensity, J. Mater. Chem. B, 2023, 11, 4076–4082 RSC.
- L.-J. Chen, S.-K. Sun, Y. Wang, C.-X. Yang, S.-Q. Wu and X.-P. Yan, Activatable Multifunctional Persistent Luminescence Nanoparticle/Copper Sulfide Nanoprobe for in Vivo Luminescence Imaging Guided Photothermal Therapy, ACS Appl. Mater. Interfaces, 2016, 8, 32667–32674 CrossRef CAS PubMed.
- H. X. Zhao, L. H. Li, F. Li, C. X. Liu, M. X. Huang, J. Li, F. Gao, X. H. Ruan and D. Y. Yang, An Energy-Storing DNA-Based Nanocomplex for Laser-Free Photodynamic Therapy, Adv. Mater., 2022, 34, 2109920 CrossRef CAS PubMed.
- Q. Chen, L. Feng, J. Liu, W. Zhu, Z. Dong, Y. Wu and Z. Liu, Intelligent Albumin-MnO2 Nanoparticles as pH-/H2O2-Responsive Dissociable Nanocarriers to Modulate Tumor Hypoxia for Effective Combination Therapy, Adv. Mater., 2016, 28, 7129–7136 CrossRef CAS PubMed.
- T. Wu and Y. Dai, Tumor microenvironment and therapeutic response, Cancer Lett., 2017, 387, 61–68 CrossRef CAS PubMed.
- F. Gong, N. L. Yang, X. W. Wang, Q. Zhao, Q. Chen, Z. Liu and L. Cheng, Tumor microenvironment-responsive intelligent nanoplatforms for cancer theranostics, Nano Today, 2020, 32, 100851 CrossRef CAS.
- L. Yuan, W. Lin and H. Chen, Analogs of Changsha near-infrared dyes with large Stokes Shifts for bioimaging, Biomaterials, 2013, 34, 9566–9571 CrossRef CAS PubMed.
- P. Chen, Z. Zheng, Y. Zhu, Y. Dong, F. Wang and G. Liang, Bioluminescent Turn-On Probe for Sensing Hypochlorite in Vitro and in Tumors, Anal. Chem., 2017, 89, 5694–5697 Search PubMed.
- Y. Zhang, Y. Ni, X. Zhao, T. Wang, X. Zhu, X. Sun, S. Wang, D. Li, J. Wang and H. Zhou, Tumor Stimulus-Activatable Pretheranostic Agent: One Key to Three Locks, Anal. Chem., 2023, 95, 15636–15644 CrossRef CAS PubMed.
- X. Wei, X. Huang, Y. Zeng, L. Jing, W. Tang, X. Li, H. Ning, X. Sun, Y. Yi and M. Gao, Longer and Stronger: Improving Persistent Luminescence in Size-Tuned Zinc Gallate Nanoparticles by Alcohol-Mediated Chromium Doping, ACS Nano, 2020, 14, 12113–12124 CrossRef CAS PubMed.
- L. Yuan, W. Y. Lin, S. Zhao, W. S. Gao, B. Chen, L. W. He and S. S. Zhu, A Unique Approach to Development of Near-Infrared Fluorescent Sensors for in Vivo Imaging, J. Am. Chem. Soc., 2012, 134, 13510–13523 CrossRef CAS PubMed.
- T. Maldiney, A. Bessiere, J. Seguin, E. Teston, S. K. Sharma, B. Viana, A. J. Bos, P. Dorenbos, M. Bessodes, D. Gourier, D. Scherman and C. Richard, The in vivo activation of persistent nanophosphors for optical imaging of vascularization, tumours and grafted cells, Nat. Mater., 2014, 13, 418–426 CrossRef CAS PubMed.
- B.-Y. Wu, H.-F. Wang, J.-T. Chen and X.-P. Yan, Fluorescence Resonance Energy Transfer Inhibition Assay for alpha-Fetoprotein Excreted during Cancer Cell Growth Using Functionalized Persistent Luminescence Nanoparticles, J. Am. Chem. Soc., 2011, 133, 686–688 CrossRef CAS PubMed.
- J. Wang, J. Li, J. Yu, H. Zhang and B. Zhang, Large Hollow Cavity Luminous Nanoparticles with Near-Infrared Persistent Luminescence and Tunable Sizes for Tumor Afterglow Imaging and Chemo-/Photodynamic Therapies, ACS Nano, 2018, 12, 4246–4258 CrossRef CAS PubMed.
- F. P. Kong, X. Y. Meng, R. R. Chu, K. H. Xu and B. Tang, A turn-on fluorescence probe for imaging iodide in living cells based on an elimination reaction, Chem. Commun., 2015, 51, 6925–6927 RSC.
- S. Carvajal, M. Perramon, G. Casals, D. Oro, J. Ribera, M. Morales-Ruiz, E. Casals, P. Casado, P. Melgar-Lesmes, G. Fernandez-Varo, P. Cutillas, V. Puntes and W. Jimenez, Cerium Oxide Nanoparticles Protect against Oxidant Injury and Interfere with Oxidative Mediated Kinase Signaling in Human-Derived Hepatocytes, Int. J. Mol. Sci., 2019, 20, 5959 CrossRef CAS PubMed.
- M. Wang, Z. Yan, M. Sun, X. Feng, W. Wang and Z. Yuan, Cuprous Oxide-Based Dual Catalytic Nanostructures for Tumor Vascular Normalization-Enhanced Chemodynamic Therapy, ACS Appl. Nano Mater., 2023, 6, 6911–6919 CrossRef CAS.
- S. A. Weitzman and L. I. Gordon, Inflammation and Cancer: Role of Phagocyte-Generated Oxidants in Carcinogenesis, Blood, 1990, 76, 655–663 CrossRef CAS PubMed.
- N. Güngör, A. M. Knaapen, A. Munnia, M. Peluso, G. R. Haenen, R. K. Chiu, R. W. L. Godschalk and F. J. van Schooten, Genotoxic effects of neutrophils and hypochlorous acid, Mutagenesis, 2009, 25, 149–154 CrossRef PubMed.
- G. Ramírez-García, S. Gutierrez-Granados, M. A. Gallegos-Corona, L. Palma-Tirado, F. d'Orlyé, A. Varenne, N. Mignet, C. Richard and M. Martínez-Alfaro, Long-term toxicological effects of persistent luminescence nanoparticles after intravenous injection in mice, Int. J. Pharm., 2017, 532, 686–695 CrossRef PubMed.
- T. Lécuyer, J. Seguin, A. Balfourier, M. Delagrange, P. Burckel, R. Lai-Kuen, V. Mignon, B. Ducos, M. Tharaud, B. Saubaméa, D. Scherman, N. Mignet, F. Gazeau and C. Richard, Fate and biological impact of persistent luminescence nanoparticles after injection in mice: a one-year follow-up, Nanoscale, 2022, 14, 15760–15771 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3qm01308a |
| This journal is © the Partner Organisations 2024 |