Open Access Article
Open Access ArticleNear-infrared-photoinduced metamagnet based on a layered cyanido-bridged Co–W assembly with π–π interactions†
Kazuki
Nakamura
ab,
Koji
Nakabayashi
 *ab,
Shota
Kobayashi
a and
Shin-ichi
Ohkoshi
*ab,
Shota
Kobayashi
a and
Shin-ichi
Ohkoshi
 *ab
*ab
aDepartment of Chemistry, School of Science, The University of Tokyo, 7-3-1, Hongo, Bunkyo-ku, Tokyo 113-0033, Japan. E-mail: ohkoshi@chem.s.u-tokyo.ac.jp
bDYNACOM IRL2015 University of Tokyo - CNRS - Universite de Rennes, Department of Chemistry, 7-3-1, Hongo, Bunkyo-ku, Tokyo 113-0033, Japan
First published on 9th April 2024
Abstract
A newly synthesized photomagnet based on a cyanido-bridged Co–W assembly, [{Co(isoquinoline)4}3{W(CN)8}2]·2EtOH (CoWisoq), exhibits a controllable photoinduced (PI) phase with a metamagnetic behavior. The PI phase shows a transition from paramagnetic to ferromagnetic with a coercive field of 550 Oe at 2 K. It has a monoclinic (P21/n) crystal structure and is comprised of two-dimensional cyanido-bridged Co–W coordination layers with two crystallographically independent Co sites (Co1, Co2) and one W site. Both Co sites adopt a pseudo-octahedral six-coordinated geometry involving four isoquinoline and two cyanide ligands. π–π interactions are induced at half of the isoquinoline ligands in Co1 and all of those in Co2 sites, suggesting that the coordination environment at the Co1 site has structural flexibility. CoWisoq displays a thermal phase transition around 140 K with a small thermal hysteresis due to the charge transfer–induced spin transition between CoII–WV of the high temperature (HT) phase and CoIII–WIV of the low temperature (LT) phase. This charge transfer, which is also responsible for a drastic color change between purple (HT) and yellow (LT), involves only the structurally flexible Co1 sites. The ligand field, which is composed of four isoquinoline and two cyanide ligands, contributes to a unique metal-to-metal charge transfer absorption band of the LT phase at 1025 nm. Irradiation of the LT phase with near–infrared light produces a PI phase with the CoII–WV state.
Introduction
Molecule-based magnets1–15 have been widely investigated because of versatile interesting functionalities by the combination of the components of metal ions, organic ligands, and crystalline solvent molecules.16–28 In particular, cyanido-bridged bimetal assemblies show a high structural designability and diverse physical properties e.g., barocaloric effect,29 low-frequency THz wave absorption,30 ionic conductivity.31 Octacyanidemetallates are good building blocks due to the variety of coordination geometries, including square antiprism, bicapped trigonal prism, and dodecahedron, and have a high coordination number. These characteristics realize various dimensional coordination structures and physical properties.32–35Appropriate combinations of redox-active transition metal ions and octacyanidotungstate with the redox activity between WV(S = 1/2) and WIV (S = 0) produce charge-transfer (CT) phase transition compounds. For example, cyanido-bridged Co–W assemblies exhibit a phase transition between the high temperature (HT) phase with a CoII–WV state and the low temperature (LT) phase with a CoIII–WIV state. Some cyanido-bridged Co–W assemblies display interesting features such as thermal phase transitions with thermal hysteresis loops or photoinduced (PI) magnetization based on CT-induced spin transitions.36–40
Photomagnetic behavior is initiated by CT from WIV to CoIII to produce the low-spin CoII state and subsequent spin transition to the high-spin CoII state. The wavelength used for the PI phase transition depends on the energy band of metal-to-metal charge transfer (MMCT) between the Co and W sites. MMCT usually occurs around 700–800 nm37–40 due to the similarity of their ligand field splitting on the Co sites provided by the four nitrogen atoms of the cyanide ligands in the equatorial position and the two nitrogen atoms of organic ligands in the axial position. In Co–W photomagnet systems, substitutions of the organic ligands may control the MMCT absorption band as it changes the ligand field splitting and coordination geometries.41–44 Furthermore, alternating the organic ligands should also affect the thermal and PI phase transitions based on a CT-induced spin transition because it not only modulates the MMCT band but also alters intermolecular interactions.45,46
Our research focuses on a slightly large aromatic ligand of isoquinoline (isoq) to control the photomagnetic properties of the Co–W system. Isoq should realize more effective intermolecular π–π interactions47–49 than pyridine, pyrimidine, and their previously reported derivatives.37,39 In this study, we synthesize a two-dimensional cyanido-bridged Co–W assembly with a near-infrared MMCT absorption band, [{Co(isoq)4}3{W(CN)8}2]·2EtOH (CoWisoq). CoWisoq exhibits PI magnetization by near–infrared light irradiation. Its PI phase shows metamagnetism because the deliberate introduction of isoq causes π–π interactions. Herein we report its crystal structure, thermal phase transition, optical properties, photomagnetism, and PI metamagnetism.
Results and discussion
Synthesis and crystal structure
A purple crystal of CoWisoq was obtained by the slow diffusion method and kept for 2–3 weeks by adding a mixed solution of ethanol and water containing CoCl2·6H2O and isoq into an aqueous solution of Cs3[W(CN)8]·2H2O at room temperature. Elemental analyses by inductively coupled plasma mass spectrometry (ICP-MS) for metal ions, standard method for C, H, and N, and thermogravimetry (TG) measurement revealed that the composition of the present compound is [{Co(isoq)4}3{W(CN)8}2]·2EtOH. A single crystal X-ray diffraction (XRD) measurement at 300 K indicated that this compound is monoclinic in the P21/n space group (a = 14.6601(4) Å, b = 27.5666(5) Å, c = 15.3341(4) Å, β = 112.706(3)°) (Fig. 1, S4, Table S1†). The asymmetric unit of CoWisoq is composed of a [WV(CN)8]3− anion, a [Co(isoq)4]2+ cation (Co1), one-half of [Co(isoq)4]2+ cation (Co2), and an EtOH molecule.The coordination geometry of the W site is close to that of an ideal dodecahedron (Table S2†). The three cyanide groups of [W(CN)8]3− are connected to three Co atoms (two Co1 and one Co2). The remaining groups are terminal. The Co1 and Co2 sites adopt a pseudo-octahedral six-coordinated geometry, which is occupied by four nitrogen atoms of isoq ligands in equatorial positions and two nitrogen atoms of [W(CN)8]3− in axial positions. The cyanido-bridged Co1–W connections create zigzag chains. These chains are bound by the Co2 sites, providing two-dimensional dodecanuclear hexagonal units of cyanido-bridged Co6W6 (4Co1–2Co2–W6) moieties. The two-dimensional layers are displaced from each other and stacked along the [101] direction. A non-coordinated EtOH molecule is in the interstitial site (central part) of the hexagonal units. The single crystal left in the air for more than one year still has the EtOH molecules in the crystal structure. The distance between the layers in the [10−1] direction is 12.47 Å, and the nearest W–W and Co–Co distances between the layers are 10.12 Å and 11.89 Å, respectively (Fig. S5†).
The four isoq ligands of the Co2 site realize π–π interactions with isoq ligands of the Co1 sites in the same layer and in the neighboring layer with arene–arene distances of 3.67 Å and 3.94 Å, respectively (Fig. 2, S7, Table S3†).50,51 The displacement angles formed between the ring-centroid vector and the pyridine-ring plane are at 17.47° within the layer and 15.94° between the layers (Table S4†). These π–π interactions may stabilize the crystal structure, including the characteristic coordination environment with four pyridine-based ligands (isoq) coordinated to the Co site. This is a representative structural feature of CoWisoq and differs from cyanido-bridged Co–W assemblies in which two pyridine-analogue ligands coordinate to the Co site.30,38 The Co–N distances are 2.10–2.18 Å for Co1–N and 2.12–2.21 Å for Co2–N, indicating that Co1 and Co2 have divalent states (Table S5†). The powder XRD measurement showed that the powder form has an identical crystal structure to that obtained by the single-crystal X-ray structural analysis (Fig. S9†).
Magnetic properties
The temperature (T) dependence of the molar magnetic susceptibility (χM) and T of CoWisoq indicated a thermal phase transition with the small thermal hysteresis loop (Fig. 3). The χMT value of 8.46 cm3 K mol−1 at 300 K corresponds well with the expected value of 8.52 cm3 K mol−1 for CoIIhs3WV2 (CoIIhs (hs: high spin): g = 2.35, SCo = 3/2, WV: gW = 2.00, SW = 1/2), and is denoted as the high temperature (HT) phase. When the temperature decreases to 180 K, the χMT value gradually increases due to spin–orbit coupling in the Co sites and CoII–WV ferromagnetic coupling in the cyanido-bridged coordination layers.52–54 From 180 K, the χMT value decreases and reaches 2.58 cm3 K mol−1 at 80 K. This observation agrees with that of 2.59 cm3 K mol−1 for CoIIIls2WIV2CoII (CoIIIls (ls: low spin): SCo = 0, WIV: SW = 0, CoII: g = 2.35, SCo = 3/2), named the low temperature (LT) phase, suggesting a CT-induced spin transition. Upon heating, the χMT value for the LT phase is maintained until 140 K, increases sharply around 150 K, and then returns to the value for the HT phase. The transition behaviors with sweep rates of 0.5, 1.0, and 2.0 K min−1 in the χMT–T plots are similar, whereas that with 5.0 K min−1 indicates partial quenching of the transition from the HT phase to the LT phase in the cooling process (Fig. S10†). The field-cooled magnetization (FCM) curve does not show a critical temperature, suggesting the LT phase is paramagnetic. The magnetization (M) vs. magnetic-field (H) plot represents the magnetization of the remaining paramagnetic CoII site in the LT phase (Fig. S11†).Crystal structure of the LT phase
Single crystal XRD analysis of the LT phase of CoWisoq at 90 K indicated the same space group of P21/n as that at 300 K. The cell parameters are a = 14.3610(4) Å, b = 26.9695(5) Å, c = 15.1714(4) Å, and β = 113.674(3) ° (Fig. S8, Table S1†). The color of the single crystal at 90 K was clear yellow changed from purple for the HT phase (Fig. 4a). The distances of Co–N bonds for Co1 and Co2 sites are 1.90–1.99 Å, and 2.09–2.19 Å, respectively (Table S5†). The Co1–N bond was significantly shortened in comparison with those at 300 K, indicating the valence state of the Co1 site was trivalent at 90 K. By contrast, the Co2–N bond distances are similar to those of the HT phase, indicating that the Co2 site remains in the divalent state.30,39 Overall, structural analyses and the magnetic susceptibility show that the LT phase of CoWisoq has a formula with the electronic state of [{Co1III(isoq)4}2{Co2II(isoq)4}{WIV(CN)8}2]·2EtOH. The CT-induced spin transition occurs between the Co1 and W sites in the Co1–W zigzag chains (Fig. 4b). The arene–arene distances of the isoq ligands within the layer and between the layers at 90 K become shorter from 3.67 Å and 3.94 Å for the HT phase to 3.54 Å and 3.82 Å for the LT phase, respectively, while arene–arene displacement angles increase from 17.47° to 20.89° within the layer and from 15.94° to 19.63° between the layers (Tables S3 and S4†). Janiak reported that the π–π interactions of pyridine and quinoline systems tend to have a smaller displacement as the distance decreases, indicating stabilization of the π–π interactions.51 Therefore, the significant increases of the displacement angles in CoWisoq should weaken the π–π interactions in the LT phase. These structural features, especially the π–π interactions, probably affect the CT phase transitions in the cooling and heating processes.Optical spectroscopy
Variable-temperature UV–vis–NIR absorption spectroscopy confirmed the CT phase transition between the LT and HT phases. At 300 K, CoWisoq shows a strong absorption at 530 nm, which is assigned to MMCT from CoII to WV (Fig. 5, S12†).37,38,55 In the cooling process, this peak gradually shifts to 550 nm until 160 K, and the intensity significantly decreases around 130 K. Absorption peaks at 500 nm and 1025 nm appear, which are attributed to the d–d transition of CoIII and the MMCT from WIV to CoIII, respectively. The change of the UV–vis–NIR spectrum corresponds to the color change of the crystal between the LT phase (yellow) and the HT phase (purple).The LT phases of cyanido-bridged Co–W assemblies reported to date have blue colors due to the MMCT from WIV to CoIII in 700–800 nm (Table S6†).30,36–40 However, CoWisoq has a MMCT band of the LT phase at 1025 nm, which is in the infrared light region. The difference of the MMCT absorption band is derived mainly from the ligand field around the CoIII site. That is, CoWisoq has a ligand field composed of four isoq ligands and two cyanides, while other cyanido-bridged Co–W assemblies have two pyridine analogues and four cyanides coordinated to CoIII. Since isoq and pyridine-analogues ligands provide a weaker ligand field than cyanides, the Co sites of CoWisoq have smaller ligand field splitting than those of other Co–W assemblies.41–44 MMCT occurs between the W site and the eg orbital of the Co site, and the smaller ligand field splitting of the Co site leads to the longer wavelength of the MMCT band. A further red-shift of the MMCT could be possible by using pyridine analogues with weaker ligand field, but the additional shift should be small. Ligands with weaker ligand field such as a water molecule and halogen ions would suppress the thermal CT phase transition.37,54
Photoinduced phase transition
The photoresponsivity for the LT phase of CoWisoq was examined using UV–vis spectroscopy. Irradiation with 980 nm laser light at 3.8 K decreases the MMCT absorption band from WIV to CoIII at 1025 nm and increases the MMCT absorption band from CoII to WV at 565 nm (Fig. S13a†). Therefore, the PI phase has the same electronic state of CoII3WV2 as that of the HT phase. After thermal annealing to 100 K, the MMCT bands at 1025 nm and 565 nm increase and decrease around 70 K (Fig. S13b†), indicating the LT phase is recovered. Further heating to 300 K induces a large decrease in the absorption at 1025 nm and 150 K, which corresponds to the return to the HT phase. In the cooling process, the absorption change traces the temperature dependence of the χMT vs. T plot (Fig. S13c†).The detailed UV–vis absorption spectra before and after the photoirradiation at 3.8 K contains some peaks. The LT phase before irradiation has absorption peaks at 408, 464, 500, 565, 585, and 662 nm, while the PI phase shows absorption peaks at 464, 565, and 662 nm. The peaks of the PI phase are assigned to d–d transitions of 4Eg ← 4A2g (464 nm), 4A2g(P) ← 4A2g(F) (565 nm, overlapping with the MMCT), and 4B1g ← 4A2g (662 nm) in CoII, considering the coordination geometry of the Co sites with the trans-CoX4Y2 type (D4h) (X = isoq, Y = cyanide) with longer Co–N distances from isoq ligands than those from cyanides (Fig. S14†).56–58 In addition to the peaks from the CoII site, the LT phase with both CoII and CoIII sites has absorption peaks at 408, 500, and 585 nm, which are attributed to the d–d transitions of to 1Ebg ← 1A1g, 1Eag ← 1A1g, and 1A2g ← 1A1g in CoIII, respectively.59–61
The photomagnetic effect of CoWisoq was studied by magnetic measurements before and after photoirradiation (Fig. 6, S15†). The LT phase displays a small magnetization value in the FCM curve and the magnetization (M) vs. external magnetic field (H) plots. After irradiation with 980 nm laser light at 3 K under an external magnetic field of 500 Oe, the magnetization value significantly increases below 12 K, suggesting long-range magnetic ordering. In the M–H plots at 2 K, the saturation magnetization value reaches 8.00μB at 50 kOe by irradiation (Fig. S15†), and a magnetic hysteresis loop with the coercive field (HC) of 550 Oe was observed.
The saturation magnetization value of the PI phase is close to 8.50μB, assuming ferromagnetic interactions between CoII (Kramers doublet: g = 13/3, SCo = 1/2) and WV (gW = 2.00, SW = 1/2) for the Co3W2 unit rather than that of 4.50μB assuming antiferromagnetic interactions. The magnetization value of the PI phase remains stable for at least three days. Thermal treatment at 100 K restores the PI phase to the LT phase due to the critical temperature of the phase transition from the PI phase to the LT phase at 70 K. Both the UV–vis spectra (Fig. S13†) and the χMT–T plot (Fig. S16†) support this transition. PI magnetization is repeatedly observed (Fig. 6c).
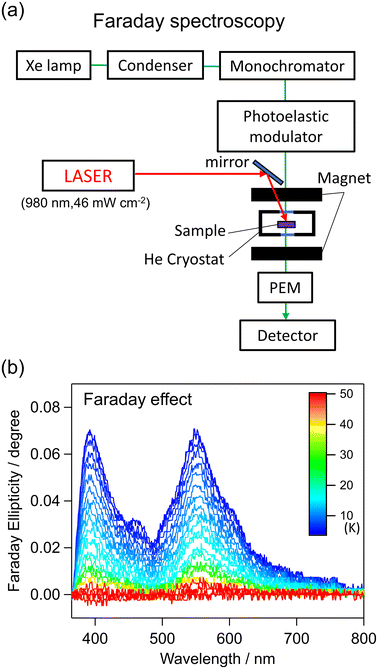
We then examined the photoresponsivity of the PI phase to confirm the photoreversibility from the PI phase to the LT phase using 473-, 532-, and 660 nm laser light. However, such a PI phase transition did not occur. To verify the ferromagnetic interaction between the CoII and WV in the PI phase, we measured the Faraday ellipticity after irradiation at low temperatures from 4 K to 80 K by applying a field of 10 kOe (Fig. 7, S7†). The Faraday ellipticities are attributed to the d–d transitions of CoII at 460 and 550 nm. Additionally, the ligand-to-metal CT of WV at 390 nm are parallel to each other, supporting the existence of a ferromagnetic interaction between CoII and WV.62,63 Furthermore, the magnetic property of the PI phase of CoWisoq was precisely examined. The PI phase is stable up to 70 K. Fig. 8 plots the temperature and magnetic field dependences of the magnetization of the PI phase in various conditions. The M–T curves in several external fields show spontaneous magnetizations under magnetic fields above 150 Oe and a small magnetization under magnetic fields below 100 Oe (Fig. 7a). The magnetic field dependences of the M–T curves indicate a spin-flip from an antiferromagnetic phase with a critical temperature like Néel temperature (TN) to a magnetic field–induced ferromagnetic phase. The initial magnetization in the M–H plots from 2 K to 8 K presents remarkable increments of the magnetization in the range of 0.1–0.6 kOe (Fig. 7b), signifying a spin–flip phenomenon.64–68 The spin–flip field (Hex) is expressed by the first derivative of the initial M–H plots (dM/dH), and Hex decreases upon heating. The temperature dependence of the Hex values provides the H–T phase diagram for the PI phase of CoWisoq (Fig. 7c). CoWisoq exhibits a metamagnetic behavior. The M–H measurements in the range of −50 to 50 kOe at 2, 3, 4, 6, and 8 K indicate temperature dependences of Hc. The remnant magnetization disappears around 6 K. Additionally, the M–H plots above 6 K resemble the typical curve for metamagnetic behavior (Fig. S18 and S19†). Thus, the ferromagnetic ordering in the PI phase is preserved in a zero magnetic field below 6 K. Considering these results for the magnetic measurements and the structural analyses, the metamagnetic behavior of the PI phase can be explained as follows. The cyanido-bridged CoII–WV layer shows ferromagnetic ordering due to ferromagnetic interactions between CoII and WV, as supported by the saturation magnetization value and the Faraday ellipsometry. Therefore, the antiferromagnetic interactions between the ferromagnetically ordered layers should be responsible for the antiferromagnetic alignment in the small magnetic field below Hex. In the magnetic field above Hex, the magnetic moments are parallelly ordered between the layers, resulting in the magnetic field–induced ferromagnetic phase. The ferromagnetic phase can be maintained even in a zero magnetic field due to the existence of Hc. Consequently, CoWisoq has an interesting PI phase with metamagnetism, including the ferromagnetic, antiferromagnetic, and paramagnetic phases.
Conclusions
We synthesized a new photomagnet based on the cyanido-bridged Co–W assembly. CoWisoq has a two-dimensional layered structure with crystallographically independent Co (Co1 and Co2) and W sites. Both the Co1 and Co2 sites have four isoq and two cyanide ligands. However, the π–π interactions between the isoq ligands differ. Two of the four isoq ligands on the Co1 site make π–π interactions, while all four isoq ligands on the Co2 site form π–π interactions. Magnetic and UV–vis spectroscopic studies reveal a thermal phase transition between the HT phase with the CoII3WV2 state and the LT phase with the CoIII2CoIIWIV2 state. The LT phase is derived from a CT-induced spin transition between the Co and W sites. Charge transfer occurs only on the Co1 and W sites since the Co1 site has a more flexible coordination environment than that of the Co2 sites as Co1 has fewer π–π interactions. The LT phase of the present compound exhibits an intriguing MMCT absorption band in the NIR region due to the weak ligand fields on the Co sites composed of four isoq and two cyanide ligands, which differs from the cyanido-bridged Co–W compounds reported previously. Irradiation with 980 nm light to the MMCT absorption band of the LT phase produces the PI phase with CoII3WV2 due to the photoinduced CT-induced spin transition.Interestingly, the PI phase shows a metamagnetic behavior in which the ferromagnetic and antiferromagnetic orderings can be controlled by the magnetic field. In the present system, we can choose the ferromagnetic PI phase when applying a magnetic field above 150 Oe or the antiferromagnetic PI phase when the magnetic field is below 100 Oe. Previous studies have not reported a photomagnetic system in which the ferromagnetically and antiferromagnetically ordered states are controlled by applying such a small magnetic field. Since photomagnetic properties are a representative feature of molecule-based magnets, the present study showcases a new aspect of photomagnets based on the cyanido-bridged metal assembly.
Experimental
Materials
Cobalt(II) chloride hexahydrate, CoIICl2·6H2O, and isoquinoline were purchased from FUJIFILM Wako and Tokyo Chemical Industry, respectively. All reagents were used without further purification. The cyanide precursor of Cs3[W(CN)8]·2H2O was prepared according to the literature.69,70Synthesis
[{Co(isoquinoline)4}3{W(CN)8}2]·2EtOH (CoWisoq) in the crystal form was prepared by reacting 2 mL of a mixed solution of ethanol/H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) including CoCl2·6H2O (0.300 mmol) and isoquinoline (1.200 mmol) with 2 mL of an aqueous solution containing Cs3[W(CN)8]·2H2O (0.200 mmol) inserting 20 mL of a buffer solution of ethanol/H2O (1
1) including CoCl2·6H2O (0.300 mmol) and isoquinoline (1.200 mmol) with 2 mL of an aqueous solution containing Cs3[W(CN)8]·2H2O (0.200 mmol) inserting 20 mL of a buffer solution of ethanol/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at room temperature in a dark, using a slow diffusion method. After 2–3 weeks, purple plate crystals were obtained by filtering and washing with ethanol. They were kept in the air for several days. The composition of CoWisoq was determined by CHN elemental analyses, ICP-MS spectrometry, and TG measurement (Fig. S1†). Elemental analyses: calcd for Co3W2C128H96N28O2 (CoWisoqMw = 2602.8): Co, 6.79%; W, 14.13%; C, 59.07%; H, 3.72%; N, 15.07%. Found: Co, 6.75%; W, 14.21%; C, 58.73%; H, 3.74%; N, 15.12%. IR spectra; cyanide stretching vibration: 2122 cm−1, 2141 cm−1, 2147 cm−1, and 2160 cm−1. CH stretching vibrations of isoquinoline: 2900 cm−1, 2925 cm−1, 2967 cm−1, 2977 cm−1, and 3059 cm−1 (Fig. S2†).37–40,71 Raman spectrum; cyanide stretching vibration: 2137 cm−1 (Fig. S3†).71
1) at room temperature in a dark, using a slow diffusion method. After 2–3 weeks, purple plate crystals were obtained by filtering and washing with ethanol. They were kept in the air for several days. The composition of CoWisoq was determined by CHN elemental analyses, ICP-MS spectrometry, and TG measurement (Fig. S1†). Elemental analyses: calcd for Co3W2C128H96N28O2 (CoWisoqMw = 2602.8): Co, 6.79%; W, 14.13%; C, 59.07%; H, 3.72%; N, 15.07%. Found: Co, 6.75%; W, 14.21%; C, 58.73%; H, 3.74%; N, 15.12%. IR spectra; cyanide stretching vibration: 2122 cm−1, 2141 cm−1, 2147 cm−1, and 2160 cm−1. CH stretching vibrations of isoquinoline: 2900 cm−1, 2925 cm−1, 2967 cm−1, 2977 cm−1, and 3059 cm−1 (Fig. S2†).37–40,71 Raman spectrum; cyanide stretching vibration: 2137 cm−1 (Fig. S3†).71
Measurements
Elemental analyses were performed using standard microanalytical methods for C, H, and N, and ICP-MS (Agilent 7700x) for Co and W. The TG properties were measured by a Rigaku Thermo plus Evo II TG8120. The magnetic properties were studied with a superconducting quantum interference device (SQUID) magnetometer (Quantum Design MPMS). The temperature-dependent magnetic susceptibility measurement was carried out under an externally applied magnetic field of 5000 Oe. The diamagnetic contributions from the samples were corrected on the basis of Pascal's constants.72 Powder XRD measurements for CoWisoq at 293 K were performed using a Rigaku Miniflex diffractometer equipped with Cu Kα radiation (λ = 1.5406 Å). The diffraction patterns were recorded within a diffraction angle range of 5–70° in 0.02° steps and an exposure time of 1° min−1. The infrared spectra at room temperature were measured by a spectrometer (JASCO FT/IR-4100). The measured samples were prepared by dispersing in KBr. The UV–vis spectrum at room temperature was measured using a spectrometer (JASCO V-670) and a sample dispersed in BaSO4. Variable-temperature UV–vis absorption spectra were measured using a Shimadzu UV-3600 plus spectrometer with an Oxford Instruments Microstate-He cryostat and a continuous wave (cw) diode laser of 980 nm. The sample was prepared by dispersing in paraffin oil sandwiched between quartz plates. Photomagnetism measurements were conducted by SQUID. An optical fiber with its edge connected to the sample and a diode laser of 980 nm and 1064 nm were employed (Fig. S20 and S21†). The sample for the photomagnetic measurements was prepared with grided crystals which were sandwiched between tapes because CoWisoq was sufficiently stable without liquid paraffin to prevent evaporation of EtOH molecules in the crystal structure under the vacuum condition in the sample chamber of the magnetic measurement system. The Faraday effect measurement was conducted using a JASCO E250 type magneto-optical spectrometer with an Oxford Instruments Microstate-He cryostat and a cw diode laser of 980 nm. The sample was prepared by dispersing in paraffin oil sandwiched between CaF2 plates. Single-crystal X-ray structures of CoWisoq at 300, 200, and 90 K were resolved on a Rigaku AFC10 diffractometer with a Rigaku Saturn Kappa CCD detector and a MicroMax-007 HF/VariMax rotating-anode X-ray generator. The single crystal was removed from the solution and placed inside the Paratone-N oil immediately. The crystal was placed on the cryoloop with a diameter of 100 μm and mounted on the diffractometer. The diffraction data were integrated after the experiment using Rigaku CrisAlisPro software. Crystal structures were solved by the direct method of SHELXS-97 program. Furthermore, using OLEX-2 1.5 software, these structures were refined and resolved to assign the missing electron density to the appropriate elements. The crystal structures at 90, 200, and 300 K are deposited in the Cambridge Crystallographic Data Centre of CCDC 2331970, 2331971, and 2331972,† respectively.Calculation
Continuous shape measure analyses were performed using SHAPE software (version 2.1) to determine the geometry of eight-coordinated [W(CN)8]n− (n = 3, 4) ions in CoWisoq at each temperature.73Author contributions
K. Nakamura, K. Nakabayashi, and S. O. designed the study. K. Nakamura performed most of the experiments and analyzed the data. K. Nakamura and S. K. investigated photomagnetic measurements. K. Nakamura and K. Nakabayashi wrote the manuscript in discussion with all the authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported in part by a Grant-in-Aid for Scientific Research (A) from JSPS KAKENHI (Grant Number 20H00369), Advanced Technologies for Carbon-Neutral(ALCA)-Next from JST (JPMJAN23A2), CNRS-University of Tokyo “Excellence Science” Joint Research Program, Second CNRS – University of Tokyo PhD Joint Program. The Cryogenic Research Center, The University of Tokyo, the Center for Nano Lithography & Analysis, The University of Tokyo, Quantum Leap Flagship Program (Q-LEAP, Grant Number JPMXS0118068681) by MEXT is also acknowledged for support. Kazuki Nakamura was supported by the World-Leading Innovative Graduate Study Program for Materials Research, Information, and Technology (MERIT-WINGS) and the Fellowship for Integrated Materials Science and Career Development. Koji Nakabayashi acknowledges the Iketani Science and Technology Foundation (Grant Number 0351111-A).References
- J. M. Manriquez, G. T. Yee, R. S. McLean, A. J. Epstein and J. S. Miller, A room-temperature molecular/organic-based magnet, Science, 1991, 252, 1415–1417 CrossRef CAS PubMed
.
- S. Ferlay, T. Mallah, R. Ouahes, P. Veillet and M. Verdaguer, A room-temperature organometallic magnet based on Prussian blue, Nature, 1995, 378, 701–703 CrossRef CAS
.
- L. Catala and T. Mallah, Nanoparticles of Prussian blue analogs and related coordination polymers: From information storage to biomedical applications, Coord. Chem. Rev., 2017, 346, 32–61 CrossRef CAS
.
- E. Coronado and P. Day, Magnetic Molecular Conductors, Chem. Rev., 2004, 104, 5419–5448 CrossRef CAS PubMed
.
- S. Ohkoshi, T. Iyoda, A. Fujishima and K. Hashimoto, Magnetic properties of mixed ferro-ferrimagnets composed of Prussian blue analogs, Phys. Rev. B: Condens. Matter Mater. Phys., 1997, 56, 11642–11652 CrossRef CAS
.
- S. M. Holmes and G. S. Girolami, Sol–Gel Synthesis of KVII[CrIII(CN)6]·2H2O: A Crystalline Molecule-Based Magnet with a Magnetic Ordering Temperature above 100 °C, J. Am. Chem. Soc., 1999, 121, 5593–5594 CrossRef CAS
.
- A. K. Bar, C. Pichon and J.-P. Sutter, Magnetic anisotropy in two- to eight-coordinated transition–metal complexes: Recent developments in molecular magnetism, Coord. Chem. Rev., 2016, 308, 346–380 CrossRef CAS
.
- M. Kurmoo, Magnetic metal-organic frameworks, Chem. Soc. Rev., 2009, 38, 1353–1379 RSC
.
- S. J. Blundell and F. L. Pratt, Organic and molecular magnets, J. Phys.: Condens.Matter, 2004, 16, R771–R828 CrossRef CAS
.
- S. Ohkoshi, K. Imoto, Y. Tsunobuchi, S. Takano and H. Tokoro, “Light-induced spin-crossover magnet, Nat. Chem., 2011, 3, 564–569 CrossRef CAS PubMed
.
- P. Perlepe, I. Oyarzabal, A. Mailman, M. Yquel, M. Platunov, I. Dovgaliuk, M. Rouzières, P. Négrier, D. Mondieig, E. A. Suturina, M.-A. Dourges, S. Bonhommeau, R. A. Musgrave, K. S. Pedersen, D. Chernyshov, F. Wilhelm, A. Rogalev, C. Mathonière and R. Clérac, Metal-organic magnets with large coercivity and ordering temperatures up to 242 °C, Science, 2020, 370, 587–592 CrossRef CAS PubMed
.
- S. Chorazy, J. J. Zakrzewski, M. Magott, T. Korzeniak, B. Nowicka, D. Pinkowicz, R. Podgajny and B. Sieklucka, Octacyanidometallates for multifunctional molecule-based materials, Chem. Soc. Rev., 2020, 49, 5945–6001 RSC
.
- K. Inoue, T. Hayamizu, H. Iwamura, D. Hashizume and Y. Ohashi, Assemblage and Alignment of the Spins of the Organic Trinitroxide Radical with a Quartet Ground State by Means of Complexation with Magnetic Metal Ions. A Molecule-Based Magnet with Three-Dimensional Structure and High TC of 46 K, J. Am. Chem. Soc., 1996, 118, 1803–1804 CrossRef CAS
.
- P. Gütlich, A. B. Gaspar, V. Ksenofontov and Y. Garcia, Pressure effect studies in molecular magnetism, J. Phys.: Condens.Matter, 2004, 16, S1087 CrossRef
.
- S. Ohkoshi, T. Hozumi and K. Hashimoto, Design and preparation of a bulk magnet exhibiting an inverted hysteresis loop, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 64, 132404 CrossRef
.
- A. Bleuzen, C. Lomenech, V. Escax, F. Villain, F. Varret, C. C. dit Moulin and M. Verdaguer, Photo-induced Ferrimagnetic Systems in Prussian Blue Analogues CIxCo4[Fe(CN)6]y (CI = Alkali Cation). 1. Conditions to Observe the Phenomenon, J. Am. Chem. Soc., 2000, 122, 6648–6652 CrossRef CAS
.
- D. M. Pajerowski, M. J. Andrus, J. E. Gardner, E. S. Knowles, M. W. Meisel and D. R. Talham, Persistent Photo-induced Magnetism in Heterostructures of Prussian Blue Analogues, J. Am. Chem. Soc., 2010, 132, 4058–4059 CrossRef CAS PubMed
.
- S. Ohkoshi and H. Tokoro, Photomagnetism in Cyano-Bridged Bimetal Assemblies, Acc. Chem. Res., 2012, 45, 1749–1758 CrossRef CAS PubMed
.
- S. Ohkoshi, S. Takano, K. Imoto, M. Yoshikiyo, A. Namai and H. Tokoro, 90-degree optical switching of output second-harmonic light in chiral photomagnet, Nat. Photonics, 2013, 8, 65–71 CrossRef
.
- M.-H. Zeng, Z. Yin, Y.-X. Tan, W.-X. Zhang, Y.-P. He and M. Kurmoo, Nanoporous Cobalt(II) MOF Exhibiting Four Magnetic Ground States and Changes in Gas Sorption upon Post-Synthetic Modification, J. Am. Chem. Soc., 2014, 136(12), 4680–4688 CrossRef CAS PubMed
.
- J. Zhang, W. Kosaka, Y. Kitagawa and H. Miyasaka, A metal–organic framework that exhibits CO2-induced transitions between paramagnetism and ferrimagnetism, Nat. Chem., 2021, 13, 19–199 Search PubMed
.
- J. Milon, M.-C. Daniel, A. Kaiba, P. Guionneau, S. Brandès and J.-P. Sutter, Nanoporous Magnets of Chiral and Racemic [{Mn(HL)}2Mn{Mo(CN)7}2] with Switchable Ordering Temperatures (TC = 85 K ↔ 106 K) Driven by H2O Sorption (L) N,N-Dimethylalaninol), J. Am. Chem. Soc., 2007, 129, 13872–13878 CrossRef CAS PubMed
.
- S. Ohkoshi, K. Arai, Y. Sato and K. Hashimoto, Humidity-induced magnetization and magnetic pole inversion in a cyano-bridged metal assembly, Nat. Mater., 2004, 3, 857–861 CrossRef CAS PubMed
.
- N. Yanai, W. Kaneko, K. Yoneda, M. Ohba and S. Kitagawa, Reversible Water-Induced Magnetic and Structural Conversion of a Flexible Microporous Ni(II)Fe(III) Ferromagnet, J. Am. Chem. Soc., 2007, 129, 3496–3349 CrossRef CAS PubMed
.
- S. Chorazy, K. Nakabayashi, S. Ohkoshi and B. Sieklucka, Green to red luminescence switchable by excitation light in cyanido-bridged TbIII–WV ferromagnet, Chem. Mater., 2014, 26, 4072–4075 CrossRef CAS
.
- S. Chorazy, B. Sieklucka and S. Ohkoshi, Near-Infrared Photoluminescence in Hexacyanid–Bridged Nd–Cr Layered Ferromagnet, Cryst. Growth Des., 2016, 16, 4918–4925 CrossRef CAS
.
- J. A. DeGayner, I.-R. Jeon, L. Sun, M. Dincă and T. D. Harris, 2D Conductive Iron-Quinoid Magnets Ordering up toTC = 105 K via Heterogenous Redox Chemistry, J. Am. Chem. Soc., 2017, 139, 4175–4184 CrossRef CAS PubMed
.
- J. Zhang, W. Kosaka, Y. Kitagawa and H. Miyasaka, A Host–Guest Electron Transfer Mechanism for Magnetic and Electronic Modifications in a Redox-Active Metal–Organic Framework, Angew. Chem., Int. Ed., 2022, 61, 1–9 Search PubMed
.
- S. Ohkoshi, K. Nakagawa, M. Yoshikiyo, A. Namai, K. Imoto, Y. Nagane, F. Jia, O. Stefanczyk, H. Tokoro, J. Wang, T. Sugahara, K. Chiba, K. Motodohi, K. Isogai, K. Nishioka, T. Momiki and R. Hatano, Giant adiabatic temperature change and its direct measurement of a barocaloric effect in a charge-transfer solid, Nat. Commun., 2023, 14, 8466 CrossRef CAS PubMed
.
- T. Yoshida, K. Nakabayashi, H. Tokoro, M. Yoshikiyo, A. Namai, K. Imoto, K. Chiba and S. Ohkoshi, Extremely low-frequency phonon material and its temperature- and photo-induced switching effects, Chem. Sci., 2020, 11, 8989–8998 RSC
.
- S. Ohkoshi, K. Nakagawa, K. Tomono, K. Imoto, Y. Tsunobuchi and H. Tokoro, J. Am. Chem. Soc., 2010, 132, 6620–6621 CrossRef CAS PubMed
.
- R. Garde, C. Desplanches, A. Bleuzen, P. Veillet and M. Verdaguer, New Molecule-Based Magnets: From Hexacyano to Octacyanometalates, Mol. Cryst. Liq. Cryst., 1999, 334, 587–595 CrossRef CAS
.
- P. M. Kiernan and W. P. Griffith, Studies on transition-metal cyano-complexes. Part I. Octacyanoniobates(III), -niobates(IV), -molybdates(V), and -tungstates(V), J. Chem. Soc., Dalton Trans., 1975, 2489–2494 RSC
.
- T. S. Venkatakrishnan, S. Sahoo, N. Bréfuel, C. Duhayon, C. Paulsen, A.-L. Barra, S. Ramasesha and J.-P. Sutter, Enhanced Ion Anisotropy by Nonconventional Coordination Geometry: Single-Chain Magnet Behavior for a [{FeIIL}2{NbIV(CN)8}] Helical Chain Compound Designed with Heptacoordinate FeII, J. Am. Chem. Soc., 2010, 132, 6047–6056 CrossRef CAS PubMed
.
- S. Chorazy, M. Rams, A. Hoczek, B. Czarnecki, B. Sieklucka, S. Ohkoshi and R. Podgajny, Structural anisotropy of cyanide-bridged {CoII9WV6} single-molecule magnets induced by bidentate ligands: towards the rational enhancement of an energy barrier, Chem. Commun., 2016, 52, 4772–4775 RSC
.
- Y. Arimoto, S. Ohkoshi, Z. J. Zhong, H. Seino, Y. Mizobe and K. Hashimoto, Photo-induced magnetization in a two-dimensional cobalt octacyanotungstate, J. Am. Chem. Soc., 2003, 125, 9240–9241 CrossRef CAS PubMed
.
- S. Ohkoshi, Y. Hamada, T. Matsuda, Y. Tsunobuchi and H. Tokoro, Crystal structure, charge-transfer-induced spin transition, and photoreversible magnetism in a cyano-bridged cobalt-tungstate bimetallic assembly, Chem. Mater., 2008, 20, 3048–3054 CrossRef CAS
.
- N. Ozaki, H. Tokoro, Y. Hamada, A. Namai, T. Matsuda, S. Kaneko and S. Ohkoshi, Photo-induced magnetization with a high Curie temperature and a large coercive field in a Co–W bimetallic assembly, Adv. Funct. Mater., 2012, 20, 2089–2093 CrossRef
.
- Y. Miyamoto, T. Nasu, N. Ozaki, Y. Umeta, H. Tokoro, K. Nakabayashi and S. Ohkoshi, Photo-induced magnetization and first-principles calculations of a two-dimensional cyanide-bridged Co–W bimetal assembly, Dalton Trans., 2016, 45, 19249–19256 RSC
.
- K. Nakamura, K. Nakabayashi, K. Imoto and S. Ohkoshi, Room-temperature bistability in a cobalt octacyanidotungstate framework showing a charge-transfer phase transition with a red-blue color change, Inorg. Chem. Front., 2023, 10, 850–859 RSC
.
- R. J. Deeth, D. L. Foulis and B. J. Williams-Hubbard, Molecular mechanics for multiple spin states of transition metal complex, Dalton Trans., 2003, 3949–3955 RSC
.
- T. Ishii, S. Tsuboi, G. Sakane, M. Yamashita and B. K. Breedlove, Universal spectrochemical series of six-coordinate octahedral metal complexes for modifying the ligand field splitting, Dalton Trans., 2009, 680–687 RSC
.
- J. Titis, J. Hudak, J. Kozisek, A. Krutosikova, J. Moncol, D. Tarabova and R. Boca, Structural, spectral and magnetic properties of carboxylato cobalt(II) complexes with heterocyclic N-donor ligands: Reconstruction of magnetic parameters from electronic spectra, Inorg. Chim. Acta, 2012, 388, 106–113 CrossRef CAS
.
- T. Oguma, D. Tsuneda, Y. Tsutsumi, Y. Fujikawa, M. Nakano, T. Ishii, G. Sakane and K. Ogasaware, The magnetism change by Jahn-Teller distortion in octahedral hexa-coordinate complex, IOP Conf. Ser.: Mater. Sci. Eng., 2020, 835, 012014 CAS
.
- R.-M. Wei, M. Kong, F. Cao, J. Li, T.-C. Pu, L. Yang, X.-L. Zhang and Y. Song, Water induced spin-crossover behaviour and magneto-structural correlation in octacyanotungstate(IV)-based iron(II) complexes, Dalton Trans., 2016, 45, 18463–18652 Search PubMed
.
- J. Qian, H. Yoshikawa, J. Hu, M. G. Humphrey, J. Zhang, K. Awaga and C. Zhang, Auxiliary ligand-induced structural diversities of octacyanometalate-based heterobimetallic coordination polymers towards diverse magnetic properties, Dalton Trans., 2019, 48, 7666–7676 RSC
.
- F. Setifi, A. Ota, L. Ouahab, S. Golhen, H. Yamochi and G. Saito, Charge Transfer Salts of BO with Paramagnetic Isothiocyanato Complex Anions: (BO)[M(isoq)2(NCS)4]; M=CrIII or FeIII, isoq = isoquinoline and BO=Bis(ethylenedioxo)tetrathiafulvalene, J. Solid State Chem., 2002, 168, 450–456 CrossRef CAS
.
- Y. Meng, Q. Q. Sheng, Md. N. Hoque, Y.-C. Chen, S.-G. Wu, J. Tucek, R. Zboril, T. Liu, Z.-P. Ni and M.-L. Tong, Two-Step Spin-Crossover with Three Inequivalent FeII Sites in a Two-Dimensional Hofmann-Type Coordination Polymer, Chem. – Eur. J., 2017, 23, 10034–10037 CrossRef CAS PubMed
.
- J. C. Rendón-Balboa, L. Villanueva-Sánchez, L. D. Rosales-Vázquez, J. Valdes-García, A. R. Vilchis-Nestor, D. Martínez-Otero, S. Martínez-Vargas and A. Dorazco-González, Structure of a luminescent 3D coordination polymer constructed with a trinuclear core of cadmium-trimesate and isoquinoline, Inorg. Chim. Acta, 2018, 483, 235–240 CrossRef
.
- S. Alvarez, A cartography of the van der Waals territories, Dalton Trans., 2013, 42, 8617–8636 RSC
.
- C. Janiak, A critical account on π-π stacking in metal complexes with aromatic nitrogen-containing ligands, J. Chem. Soc., Dalton Trans., 2000, 3885–3896 RSC
.
- F. Lloret, M. Julve, J. Cano, R. Ruiz-Garcia and E. Pardo, Magnetic properties of six-coordinated high-spin cobalt(II) complexes: Theoretical background and its application, Inorg. Chim. Acta, 2008, 361, 3432–3445 CrossRef CAS
.
- S. M. Ostrovsky, K. Falk, J. Pelikan, D. A. Brown, Z. Tomkowicz and W. Haase, Orbital Angular Momentum Contribution to the Magneto-Optical Behavior of a Binuclear Cobalt(II) Complex, Inorg. Chem., 2006, 45, 688–694 CrossRef CAS PubMed
.
- K. Nakabayashi, S. Chorazy, D. Takahashi, T. Kinoshita, B. Sieklucka and S. Ohkoshi, Cesium Cyano-Bridged CoII–MV (M = Mo and W) Layered Frameworks Exhibiting High Thermal Durability and Metamagnetism, Cryst. Growth Des., 2014, 14, 6093–6100 CrossRef CAS
.
- S. Chorazy, K. Nakabayashi, K. Imoto, J. Mlynarski, B. Sieklucka and S. Ohkoshi, Conjuction of chirality and slow magnetic relaxation in the supermolecular network constructed of crossed cyano-bridged CoII-WV molecular chains, J. Am. Chem. Soc., 2012, 134, 19989–19992 CrossRef PubMed
.
- D. M. L. Goodgame, M. A. Hitchman, D. F. Marsham and C. E. Souter, Complexes of Cobalt(II) Nitrite with Amine Ligands, J. Chem. Soc. A, 1969, 2464–2468 RSC
.
- B. J. A. Kakazal and G. A. Melson, Aromatic Diamine Complexes, II. Tetragonal Distortion in some Cobalt(II) Complexes, Inorg. Chim. Acta, 1970, 4, 360–364 CrossRef CAS
.
- R. W. Matthews, A. D. Hamer, D. L. Hoof, D. G. Tisley and R. A. Walton, Study on metal carboxylates. Part IV. Pyridine-2,6-dicarboxylate complexes of cobalt(II), nickel(II), rhodium(II), and rhodium(III). Synthesis, spectral and magnetic properties, and a study of rhodium 3d binding energies by X-ray photoelectron spectroscopy, J. Chem. Soc., Dalton Trans., 1973, 10, 1035–1038 RSC
.
- E. Ochiai, K. M. Long, C. R. Sperati and D. H. Busch, Bonded Organic Derivatives of a Cobalt(III) Macrocyclic Complex, J. Am. Chem. Soc., 1969, 91(12), 3201–3206 CrossRef CAS
.
- P. O. Whimp and N. F. Curtis, Some cyclic tetra-amines and their metal-ion complexes. Part V. Cobalt(III) complexes of two isomeric 5,7,7,12,12,14-hexamethyl-1,4,8,11-tetra-azacyclotetradecanes, J. Chem. Soc. A, 1968, 188–190 RSC
.
-
A. B. P. Lever, Inorganic Chemistry Spectroscopy, 1968 Search PubMed
.
- Y. Sato, S. Ohkoshi and K. Hashimoto, Photo-induced Faraday effect in a cobalt-iron polycyanide film, J. Appl. Phys., 2002, 92(8), 4834–4836 CrossRef CAS
.
- M. Ohba, T. Iwamoto and H. Okawa, Magneto-optical Properties of Two-dimensional Cyanide-Bridged MIIINiII Bimetallic Assemblies (M=Fe,
Co), Chem. Lett., 2002, 10, 1046–1047 CrossRef
.
- W. Kosaka, M. Itoh and H. Miyasaka, Metamagnetism with TN = 97 K in a layered assembly of paddlewheel [Ru2] units and TCNQ: an empirical rule for interlayer distances determining the magnetic ground state, Mater. Chem. Front., 2018, 2, 497–504 RSC
.
- J. Mandal, P. Brandão, S. Benmansour, C. J. Gómez-García and A. Saha, First Example of Chromone-Based Nickel(II) Coordination Polymers with Tunable Magnetic Properties, Cryst. Growth Des., 2022, 22, 7544–7554 CrossRef CAS
.
- S. Kaneko, Y. Tsunobuchi, S. Sakurai and S. Ohkoshi, Two-dimensional metamagnet composed of a cesium copper octacyanotungustate, Chem. Phys. Lett., 2007, 446, 292–296 CrossRef CAS
.
- S. Ohkoshi, Y. Arimoto, T. Hozumi, H. Seino, Y. Mizobe and K. Hashimoto, Two-dimensional metamagnet composed of cyano-bridged CuII-WV bimetallic assembly, Chem. Commun., 2003, 22, 2772–2773 RSC
.
- S. Chorazy, R. Podgajny, A. M. Majcher, W. Nitek, M. Rams, E. A. Suturina, L. Ungur, L. F. Chibotaru and B. Sieklucka, Magnetic anisotropy of CoII-WV ferromagnet: single crystal and ab initio study, CrystEngComm, 2013, 15, 2378–2385 RSC
.
- J. G. Leipoldt, L. D. C. Bok and P. J. Cilliers, The preparation of potassium octacyanotungstate(IV) dihydrate, Z. Anorg. Allg. Chem., 1974, 407, 350–352 CrossRef CAS
.
- L. D. C. Bok, J. G. Leipoldt and S. S. Basson, The preparation of Cs3Mo(CN)8·2H2O and Cs3W(CN)8·2H2O, Z. Anorg. Allg. Chem., 1975, 415, 81–83 CrossRef CAS
.
- M. Bolboaca, W. Kiefer and J. Popp, Fourier transform Raman and surface-enhanced Raman spectroscopy of some quinoline derivatives, J. Raman Spectrosc., 2002, 33, 207–212 CrossRef CAS
.
- G. A. Bain and J. F. Berry, Diamagnetic Corrections and Pascal's Constants, J. Chem. Educ., 2008, 85, 532–536 CrossRef CAS
.
-
M. Llunell, D. Casanova, J. Cirera, J. Bofill, P. Alemany, S. Alvarez, M. Pinsky and D. Avnir, SHAPE v. 2.1. Program for the Calculation of Continuous Shape Measures of Polygonal and Polyhedral Molecular Fragments, University of Barcelona, Barcelona, Spain, 2013 Search PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Product characterizations of optical and single-crystal XRD data and optical, magnetic, and photomagnetic studies. CCDC 2331970–2331972. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4qi00511b |
| This journal is © the Partner Organisations 2024 |