DOI:
10.1039/D3QI02008H
(Research Article)
Inorg. Chem. Front., 2024,
11, 462-469
Two solvent-dependent Al16 nanorings: design, synthesis and nonlinear optical limiting behavior†
Received
1st October 2023
, Accepted 14th November 2023
First published on 15th November 2023
Abstract
Cyclic compounds are of great interest to chemists for their adjustable organic shells and inorganic cores. Although the abundant bridges provide a bottom-up synthesis approach for the diversity and functionalization of ring compounds, the lack of suitable models still limits us in exploring the influence of bridge types on ring structures. Herein, we demonstrate two Al16 molecular nanorings synthesized via 2 × 8 and 4 × 4 strategies based on the “ligand-induced aggregation and solvent regulation” strategy and discuss the influence of surface bridge flexibility on molecular ring distortion and curvature. Rigid phenol endows AlOC-135 with unique intramolecular π⋯π interactions and higher curvature to increase the delocalization and transfer of electrons, resulting in a superior third-order nonlinear optical (NLO) response. This work provides a platform for explaining the relationship between the structure and optical properties of ring compounds at the molecular level.
Introduction
Macrocyclic molecules have attracted widespread attention due to their unique symmetry, adjustable supramolecular stacking, and central cavity for guest inclusion.1–3 Organic–inorganic hybrid macrocycles can be directionally assembled and functionalized, enabling expansion of the applications in specific fields by altering the composition of the organic shell and inorganic metal core.4–6 In recent decades, numerous innovative hybrid molecular ring models have emerged, such as the transition metal molecular rings Ti32, Fe36, Zr70, Mn84, Mo154 and Mo176.7–12 Another big family is rare earth molecular rings such as Ln42 (Yb42 and Sm42) bridged by acetoxy, Ce70 with a sulfate linker, and the Gd140 ring linked by 80 acetic acid molecules, 40 myo-inositol and 20 CO32− anions.13–15 Furthermore, heterometallic 3d–4f molecular rings are also the stars of hybrid molecular rings, such as Cr4Dy4, Sc8Gd8, and Mo128Ce4 with modified delta-chain structures, which are linked via benzoates.16–18 These macrocyclic molecules exhibit distinct benefits in the optics, magnetism and host–guest chemistry domains.19–23 Yang et al. reported a {Nd18} molecular ring exhibiting NIR luminescence sensing of antibiotics.24 Zheng et al. synthesized the {Cr8Dy8} wheel, which is a single-molecule magnet.25 Sun et al. reported the host–guest catalysis and self-assembly of Pd2L2 constructed from a pyridine-bonded macrocyclic ligand (L) and Pd2+.26 The excellent performance of macrocyclic molecules in these fields encourages us to further develop more cyclic compounds.
Linkers are essential components of cyclic compounds and can be divided into three main groups (Scheme S1†): (a) Chelating entirely by organic linkers with coordination sites such as Ocarboxyl, OH, S, and N. For example, Zhang et al. used N,N′-di[1-(2-hydroxyphenyl)-ethylidene]hydrazone ligand and DMF (N) as linkers to synthesize Ni12.27 Milios et al. synthesized Co16 by salicylaldehyde bridges.28 Besides, Baca et al. constructed a heterometallic ring Fe6Dy8 with N-butyl diethanolamine isobutyrate and methanol (Ohydroxyl).29 Yamamoto et al. reported a series of [Pt(μ-SC8H17)2]n (n = 5–13) clusters with the S atom as the connection point.30 (b) Bonding entirely with inorganic bridges. Su et al. synthesized [Nb48V8(OH)30O130]18− using μ2-OH− as linkers.31 Jacobson et al. and Wang et al. reported [M8]16− (M = Cr and Fe) and [Ti8]16− with μ2-OH and μ2-SO4 bridges.32,33 (c) The cooperation between organic connectors and inorganic components constitutes a ring structure. Zhao et al. reported on an Ln7 ring constructed from 1,1′-cyclopropane-dicarboxylic acid and μ3-OH inorganic linkers (Ln = Gd and Dy).34 Zheng et al. synthesized Ti32 constructed from acetic acid and μn-O (n = 2 or 3) linkers.35 Christou et al. used triethylamine and μ3-OH to synthesize an Fe36 cluster.12 Abundant organic/inorganic linkers provide a bottom-up synthesis approach for the diversity of molecular ring types and functionalization. However, exploring the influence of linker types on the ring structures is challenging.
Our group has been dedicated to the isolation of Earth-abundant element related aluminum-based molecular rings and developed the “ligand-induced aggregation and solvent regulation” strategy toward customizing ring compounds.36,37 In previous research, we successfully expanded the Al8 ring to the Al16 ring by tuning the carbon chain length of the flexible primary alcohols and developing the Al20 ring using a rigid phenol linker.38,39 As a continuous work, we herein report two rings with the same nuclearity but different degrees of conjugation and study their optical properties. Considering that a ligand with a high degree of conjugation will facilitate photo-related properties, we introduced 2-naphthoic acid (2-NA) instead of the previously used benzoic acid. To investigate the effects of different auxiliary ligands (solvents) on the ring structure, we chose flexible n-propanol and rigid phenol (melting point 43 °C). With this in mind, we successfully isolated two Al16 molecular nanorings constructed using a 2-NA ligand but with different auxiliary bridges. The introduction of rigid phenol brings intramolecular π–π interactions, increasing the ring curvature and further influencing the intermolecular stacking, resulting in photoluminescence and nonlinear optical (NLO) changes. This study provides a platform for explaining the structure–function relationship of macrocyclic molecules at the molecular level.
Results and discussion
Synthesis discussion
2-Naphthoic acid (2-NA) plays an essential role in inducing the aggregation of Al3+ to form molecular rings, while phenol can be viewed as a solvent and auxiliary bridge like n-propanol. A transparent square block crystal [Al16(OnPr)16(μ2-OH)8(2-NA)24] (AlOC-134) was prepared through the one-step self-assembly method by heating Al(OiPr)3 and 2-naphthoic acid (2-NA) under the regulation of a methylaminoethanol solution and hexamethylenetetramine in a mixture of n-propanol, 1,4-dioxane and a DMF solution. When we directly added 6 mL phenol and increased the reaction temperature (100 °C to 120 °C), a light yellow oval shaped block crystal [Al16(phenol)20(μ2-OH)8(2-NA)20] (AlOC-135) was successfully synthesized (Fig. 1). Although AlOC-134 and AlOC-135 are both 16-membered molecular rings, they exhibit different linkage modes (2 × 8 and 4 × 4 strategy).
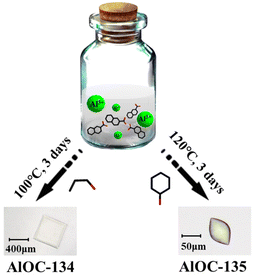 |
| | Fig. 1 “Ligand-induced aggregation and solvent regulation” synthesis of AlOC-134 and AlOC-135. | |
Aluminum molecular rings supported by flexible n-propanol (2 × 8 strategy)
Single-crystal X-ray diffraction (SCXRD) showed the compound AlOC-134 crystallizing in the monoclinic space group C2/c with a total diameter of 3.1 nm, a height of about 1.9 nm, and a central hole of 0.5 nm (Fig. 2a and c). The entire molecular ring includes 16 Al3+ ions, 24 2-NA ligands, 16 nOPr, and eight bridged hydroxyl connections (Fig. 2a). Each aluminum ion adopts a six-coordinated octahedral configuration with three carboxylate oxygens (OCOO) from three 2-NA ligands, two hydroxyl oxygens from four nOPr ligands and one μ2-OH (Fig. S1†), which is similar to the previously reported coordination mode of the Al16 ring bridged by alcohol.38 In AlOC-134, the 2-NA ligand adopts the μ2–η1:η1 coordination mode linking two neighbouring Al3+ ions to form an edge-sharing [Al2(nOPr)2(2-NA)3] (Al2) dimer (Fig. 2b and S2†). Eight Al2 dimers are connected with eight μ2-OH to form a coronal configuration ring through the vertex sharing mode (2 × 8, Fig. S3†).
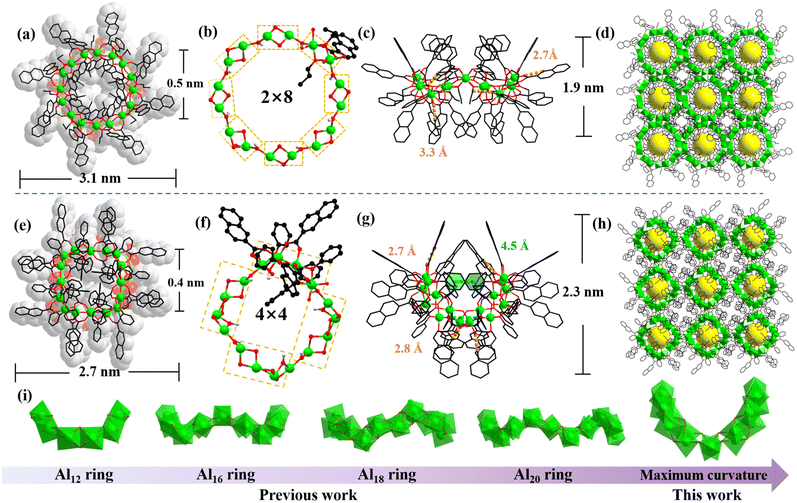 |
| | Fig. 2 Structure of AlOC-134 and AlOC-135. (a) The space-filling view of AlOC-134 (total diameter of 3.1 nm and central hole of about 0.5 nm); (b) the ball-and-stick view of the 2 × 8 ring of AlOC-134 (Al2 connected with μ2-OH); (c) the side view of the corona-shaped ring of AlOC-134 (presenting the intramolecular hydrogen bond interactions); (d) nanotube stacking diagram of AlOC-134; (e) the space-filling view of AlOC-135 (total diameter of 2.7 nm and central hole of about 0.4 nm); (f) the ball-and-stick view of the 4 × 4 ring of AlOC-135 (Al4 connected with μ2-OH); (g) the side view of the corona-shaped ring of AlOC-135 (presenting the intramolecular hydrogen bond interactions and π⋯π interaction); (h) nanotube stacking diagram of AlOC-135; and (i) curvature changes of the aluminum molecular ring; currently AlOC-135 has the largest curvature aluminum molecular ring. Color code: Al, green; O, red; C, black; H, grey; π⋯π interaction, blue dashed line in (g); and intramolecular hydrogen bonds, orange dashed lines in (c) and (g). | |
Aluminum molecular rings supported by rigid phenol (4 × 4 strategy)
When we replace the flexible n-propanol connector with rigid phenol, the Al16 crown-core is transformed into a twisted saddle-core (AlOC-135). AlOC-135 crystallizes in the tetragonal space group P42/n with a slightly smaller diameter (2.7 nm), a central hole of 0.4 nm and a height of about 2.3 nm (Fig. 2e and g). AlOC-135 consists of 16 Al3+ ions, 20 NA− ligands, 20 phenols and eight bridged hydroxyl groups (Fig. 2e). As shown in Fig. S1,† all Al3+ ions in this Al4 tetramer have a distorted octahedral geometry: for Al1, the coordination oxygen comes from two OCOO and four phenols; for Al2, the coordination comes from two OCOO, three phenols, and one μ2-OH; and for Al3, the coordination comes from three OCOO, one phenol, and two μ2-OH. Four 2-NA ligands adopt the same mode bridging bidentate μ2-linking four neighbouring Al3+ ions to construct an edge-sharing [Al4(phenol)5(2-NA)5] tetramer (Fig. 2f and S2†). Four independent μ2-OH connect two adjacent Al4 tetramers to form a saddle-shaped Al16 ring (4 × 4, Fig. S3†) with a bigger bending degree.
Ring curvature discussion
We tried to introduce rigid ligands with larger sizes to adjust the structural curvature, such as benzyl alcohol, 1-naphthol and 2-naphthol. Unfortunately, no related compounds were isolated, which may be attributed to their larger steric hindrance. The degree of curvature of the AlOC-134 (crown-type) is caused by significant intramolecular hydrogen bond interactions (C–H⋯O, 2.691 Å to 3.288 Å) (Fig. 2c and Table S3†). Compared to AlOC-134 and previous aluminum molecular rings, AlOC-135 exhibits the most significant curvature and a rare saddle structure (Fig. 2i).38–44 In solvent systems containing conjugated ligands, phenols with stronger polarity can easily interact with them via π⋯π interaction, thereby distorting the Al16 nucleus. This is also the reason why the amount of the linker phenol (20) is greater than that of n-propanol (16). Besides, the saddle-shaped conformation provides stronger supramolecular interactions within AlOC-135, manifested in strong hydrogen bonding interactions (C–H⋯O, 2.722–2.831 Å, Table S4†) and intramolecular π⋯π stacking interactions between phenols (4.549–4.562 Å, Fig. S4†). The two factors combine to cause the large curvature of AlOC-135. In both structures, all nanorings are connected to adjacent molecular rings by rich π⋯π interactions (4.129–4.869 Å, Fig. S4 and Table S5†) to form a dense array of supramolecular nanotubes. The Al–O bond lengths of two Al16 rings fell within the range of 1.837–1.943 Å (Table S2†), comparable with those reported in the literature.38,40,45 The conjugated system is instrumental in exploring the application of the two compounds in optics.
Characterization
The experimental PXRD peaks of AlOC-134 and AlOC-135 match well with the simulated peaks based on their space groups and cell parameters obtained using Berrar–Baldinozzi refinement. Negligible difference curves (Rwp and Rp values less than 5%) demonstrate the high purity of synthesized crystals (Fig. 3a and b). The energy dispersive spectroscopy (EDS) (Fig. S5 and S6†) and Fourier transform infrared spectroscopy (FT-IR) demonstrate the introduction of ligands (Fig. S7 and S8†). The vibration at 3054 cm−1 (AlOC-134) and the vibration at 3058 cm−1 (AlOC-135) are assigned to the C–H vibration of the aromatic ring. The absorption band of 3618 cm−1 (AlOC-135) is attributed to the ν(O–H) stretching on phenol; the bands at 1550 cm−1 (AlOC-134) and 1556 cm−1 (AlOC-135) can be ascribed to –CO2− asymmetric stretching. The bond valence sum (BVS) calculation shows that all Al ions have a +3 oxidation state (Tables S6 and S7†). The contact angle test reflects their excellent hydrophobicity and stability (Fig. S9†). Their thermal stability was evaluated by thermogravimetric analysis (TGA) (Fig. S10 and S11†). The solvent and air stability of the compounds are demonstrated by PXRD under different conditions, single crystal diffraction patterns and crystal pictures (Fig. 1 and S12–S14†). The excellent stability limited the dissolution of the compound in the organic solvent, which prevented further solution stability studies such as mass spectrometry.
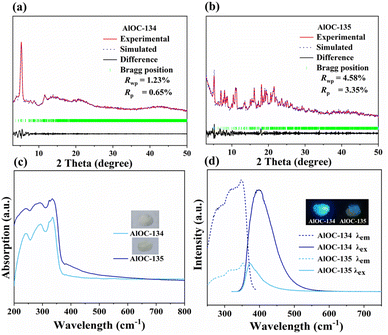 |
| | Fig. 3 (a) Simulated PXRD patterns (blue) and experimental PXRD patterns (red) of AlOC-134. (b) Simulated PXRD patterns (blue) and experimental PXRD patterns (red) of AlOC-135. (c) UV–vis diffuse reflectance spectra of AlOC-134 and AlOC-135 (insets are solid-state crystal samples). (d) Room-temperature photoluminescence spectra of AlOC-134 and AlOC-135 (inset is solid-state crystal samples under 365 nm UV light; λem is measured at an excitation wavelength of λex = 300 nm). | |
Luminescence properties
The large π-conjugated systems and abundant supramolecular forces encouraged us to investigate their optical properties. We first explored the light absorption properties of AlOCs by measuring the UV-vis diffuse reflectance spectra. As shown in Fig. 3c, the results show that the absorption spectra of both AlOC-134 and AlOC-135 exhibit broad profiles ranging from 250 nm to 450 nm (Fig. S15 and S16†), which are similar to the absorption bands of 2-NA ligands (Fig. S17†). Subsequently, the fluorescence properties of the solid-state samples were measured at room temperature. As shown in Fig. 3d and S18,† the maximum emission peaks show a red shift (28 nm for AlOC-134, 3 nm for AlOC-135) at the same excitation wavelength (300 nm). Besides, AlOC-134 has a longer lifetime and higher quantum yield (9.76 ns, 17.87%) than AlOC-135 (2.57 ns, 7.85%) (Fig. S19 and S20†). The comparative emission spectra show that the luminescence of the nanoring can be attributed to the coordination of the 2-NA ligand with inorganic aluminum atoms.46 The difference in their emission intensity may be related to the quantity of the 2-NA ligands (24 in AlOC-134vs. 20 in AlOC-135) (Fig. 3d).
Third-order nonlinear optical (NLO) properties
Third-order nonlinear optics has shown potential applications in fields such as laser protection and optical switches.47,48 The large conjugated system of Al16 rings encouraged us to combine crystalline materials with third-order NLO properties. The hydrophobic shell of the ring makes it less soluble and dispersible in solvents. Thus, we chose polydimethylsiloxane (PDMS, one chemically inert material with transmittance and excellent flexibility) as a solid dispersion substrate and made flexible AlOCs@PDMS films (30 mm in diameter).49,50 All sample films had similar thicknesses and high linear transmittance (inset pictures in Fig. 4a). Open aperture (OA) Z-scan measurements were performed on solid samples under a nanosecond laser at 532 nm and a pulse energy at 80 μJ (Fig. 4a). As shown in Fig. 4b, the Z-scan curves of AlOC-134 and AlOC-135 display typical reverse saturation absorption (RSA) responses. The minimum normalised transmittance (Tmin) values at the focal point were 0.84 and 0.79 for AlOC-134 and AlOC-135, respectively. Such responses were reproducible and presented consistent Tmin values, showing that the prepared samples featured excellent chemical and physical stability (Fig. S21 and S22†). According to eqn (4) and (5), the light fluence Fin(z) at the corresponding position z is calculated from the input laser pulse energy Ein. To compare the optical limiting (OL) performance, we extracted the representative OL parameter values from OL curves. Their optical limiting threshold values (FOL, defined as the input fluency at 50% linear transmittance) were 3.98 J cm−2 (AlOC-134) and 0.62 J cm−2 (AlOC-135) (Fig. 4c). The curves of output fluence versus input fluence of such samples showed that the output fluence linearly increased at low-incident fluence. At high-incident fluence, the output fluence deviated from linearity and showed a typical OL response (Fig. 4d). The nonlinear absorption coefficient (β) (eqn (1)–(3)) of AlOC-134 and AlOC-135 was 22 cm GW−1 and 41 cm GW−1, respectively. Usually, higher α and β values and lower OL values are key parameters for considering optical limiting performance. These values are not as good as those of some porphyrins and phthalocyanine materials, such as 2,9(10),16(17),23(24)-tetrakis-(4-pyridyloxy) phthalocyaninato (H2TPPc) with detonation nanodiamonds in DMSO and single-walled carbon nanotube-5,10,15,20-tetraphenylporphyrin (SWN7-TPP) in DMF, but superior to those of some known transition metal cluster materials, which include but are not limited to, KBr-based AgSn12 cluster thin films, graphene oxide (GO) in DMF and [(Tp*WS3Cu3)4(μ4-S)4(Ag)]OTf (3@PVBC) thin films (Fig. 4e and Table S9†).51–55
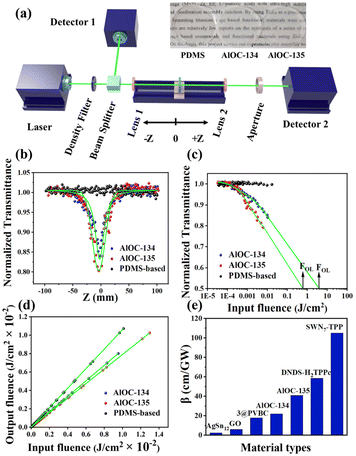 |
| | Fig. 4 (a) Schematic representation of the Z-scan setup (inset is a picture of the PDMS substrate and AlOCs@PDMS under natural light); (b) the OA Z-scan curves of input laser pulse energy 80 μJ of AlOCs@PDMS; (c) the plots of normalized transmittance versus input fluence; (d) curves of output fluence versus input fluence; and (e) the plots of comparisons of the nonlinear absorption coefficients. | |
The optical limiting properties of molecular rings are closely related to the supramolecular interactions in the structure. As shown in Table S8,†AlOC-135 has larger β and Im χ(3) (eqn (6) and (7)). To find their correlation, we compared the third-order NLO effects of aluminum molecular rings with different curvatures.39,40 However, there is insufficient evidence to prove that increasing the curvature increases the NLO effect. In this work, the introduction of phenol not only resulted in significant intramolecular π⋯π interactions but also affected intermolecular stacking, which resulted in the saddle-shaped configuration of AlOC-135. The synergistic interaction created by a richer conjugation interaction may directly affect the surrounding environment of the ring and its electron density, increase the delocalization and transfer of electrons, and thus produce a more obvious OL response, providing a new path for the research of enhanced light-limiting materials.
Conclusions
In summary, we demonstrated the specific application of the “ligand-induced aggregation and solvent regulation” strategy in nanoring-type regulation. Flexible n-propanol can generate a greater number of the components in the rings (2 × 8 strategy, AlOC-134). Increasing the rigidity of the solvent can reduce the crystalline symmetry and produce larger but fewer bricks (4 × 4 strategies, AlOC-135). Furthermore, their luminescence and third-order NLO were studied. AlOC-134 containing more chromophores has a stronger emission intensity. Replacing n-propanol with phenol regulates AlOC-135 intermolecular stacking, increases structural curvature and provides more intramolecular π⋯π interactions. These characteristics increase the delocalization and transfer of electrons, resulting in a more pronounced NLO response. This work provides a platform for us to further understand the binding mechanism of molecular rings and their structural influence on properties at the molecular level.
Experimental
Solvothermal synthesis of (Al16(nOPr)16(μ2-OH)8(2-NA)24) (AlOC-134)
A mixture of Al(OiPr)3 (153 mg, 0.75 mmol), 2-naphthoic acid (2-NA) (137 mg, 1.0 mmol), hexamethylenetetramine (50 mg, 0.35 mmol), methylamine solution in alcohol (50 μL), 1,4-dioxane (3 mL), n-propanol (5 mL), and DMF (2 mL) was sealed in a 20 mL vial and transferred to a preheated oven at 100 °C for 3 days. When cooled to room temperature, colorless transparent thin pieces of crystals were obtained (yield: 22% based on Al(OiPr)3). FT-IR (cm−1): 3058 (w), 2954 (w), 2360 (w), 1635 (m), 1556 (m), 1473 (m), 1421 (s) 1240 (w), 1070 (m), 965 (m), and 788 (s). The crystals were rinsed with n-propanol and preserved in a sealed and dry environment.
Solvothermal synthesis of (Al16(phenol)20(μ2-OH)8(2-NA)20) (AlOC-135)
A mixture of Al(OiPr)3 (550 mg, 2.7 mmol), 2-NA (200 mg, 1.16 mmol) and phenol (6 mL) was sealed in a 20 mL vial and transferred to a preheated oven at 120 °C for 3 days. When cooled to room temperature, colorless spheroidal crystals were obtained (yield: 13% based on Al(OiPr)3). FT-IR: 3618 (w), 3054 (w), 1550 (m), 2360 (w), 1550 (m), 1481 (m), 1423 (s), 1224 (m) 1002 (w), 833 (m), and 769 (m) cm−1. The crystals were rinsed with acetonitrile and preserved in a sealed and dry environment.
Caution: To avoid direct skin contact with corrosive phenol, we placed it at 80 °C for 60 minutes before use in the reaction as a solvent.
Single-crystal X-ray crystallography
Crystallographic data of AlOC-134 and AlOC-135 was collected on a Hybrid Pixel Array detector equipped with Ga Kα radiation (λ = 1.3405 Å) at about 298 K. The structures were determined by direct methods using Olex2 and refined with the full-matrix least-squares technique on F2 using SHELXL. Non-hydrogen atoms were refined anisotropically.
Contact angle measurements
Contact angles were measured on powder samples using a contact angle meter with a rotatable substrate holder. 10 mg powder samples of the clusters were pressed using a glass slide to make a flat surface. A 20 μL water droplet was released slowly on the flat surface of the powder samples. Later, the droplet image was captured using a high-performance charge-coupled device (CCD) sensor. Five-point simulation analysis was used to perform an analysis of the contact angles of all the powder samples.
Fluorescence spectroscopy measurement
Photoluminescence spectra (PL) and luminescence decay curves were performed on an Edinburgh FLS1000 fluorescence spectrometer at room temperature. All test samples were solid crystalline particles (5 mg). Emission wavelength, quantum yield and fluorescence lifetime were all measured at maximum excitation.
Preparation of AlOCs@PDMS samples
Sylgard 184 (Dow Corning) is a kit product consisting of liquid A and B components, including basic components and a curing agent. PDMS samples were fabricated using Sylgard 184 (Dow Corning) by thoroughly mixing ten parts base with one part curing agent. The fully ground AlOC samples were added to the PDMS mixture and stirred for 3 h to form AlOC dispersed PDMS suspension. Then the mixed suspension was added to a round mould, and the template was placed in a vacuum oven at 60 °C for six hours to obtain transparent and flexible AlOCs@PDMS films. The thickness of the films was measured using a digital caliper (MNT951101 manufactured by Shanghai Minette).
Third-order NLO measurement
The NLO properties of the samples were evaluated using the open-aperture (OA) Z-scan technique. The excitation light source was an Nd:YAG laser with a repetition rate of 10 Hz. The laser pulses (period, 8.5 ns; wavelength, 532 nm) were split into two beams with a mirror. The pulse energies at the front and back of the samples were monitored using energy detectors D1 and D2. All of the measurements were conducted at room temperature. The measured Z-scan curves were fitted using the following expressions:| | 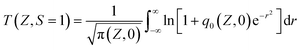 | (1) |
| | 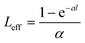 | (3) |
| | 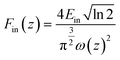 | (4) |
| | 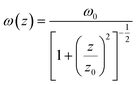 | (5) |
| | 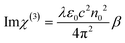 | (6) |
| | 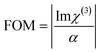 | (7) |
where T is the normalized transmittance, β is the nonlinear absorption coefficient, I0 is the peak on-axis irradiance of the laser beam at the focus, Leff is the effective thickness of the sample, α is the linear absorption coefficient at the laser wavelength 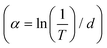 , z is the Z-scan displacement, ε0 is the permittivity of vacuum (8.85 × 10−12 F m−1), c is the speed of light and l denotes the thickness of the glass cell.
, z is the Z-scan displacement, ε0 is the permittivity of vacuum (8.85 × 10−12 F m−1), c is the speed of light and l denotes the thickness of the glass cell.
Data availability
The datasets supporting this article have been uploaded as part of the ESI.† CCDC reference numbers 2289659 (AlOC-134) and 2289660 (AlOC-135) contain the X-ray crystallographic data.†
Author contributions
All authors contributed extensively to the work presented in this paper. J. Zhang and W.-H. Fang conceived the research project. S.-T. Wang and X. Qi performed the synthesis, characterization, fluorescence property analysis and third-order NLO properties analysis. R.-Q. Chen assisted with the data collection and analysis. W.-H. Fang, X. Qi and S.-T. Wang wrote the manuscript and the ESI with input from the other authors.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22371278 and 92061104), Funding of Fujian Provincial Chemistry Discipline Alliance, the Natural Science Foundation of Fujian Province (2021J06035) and the Youth Innovation Promotion Association CAS (Y2018081).
References
- Y. Qin, X. Liu, P. P. Jia, L. Xu and H. B. Yang, BODIPY-based macrocycles, Chem. Soc. Rev., 2020, 49, 5678–5703 RSC.
- M. T. Chaudhry, S. Akine and M. J. MacLachlan, Contemporary macrocycles for discrete polymetallic complexes: Precise control over structure and function, Chem. Soc. Rev., 2021, 50, 10713–10732 RSC.
- Z. Liu, S. K. M. Nalluri and J. F. Stoddart, Surveying macrocyclic chemistry: From flexible crown ethers to rigid cyclophanes, Chem. Soc. Rev., 2017, 46, 2459–2478 RSC.
- W. Xuan, R. Pow, D. Long and L. Cronin, Exploring the molecular growth of two gigantic half-closed polyoxometalate clusters {Mo180} and {Mo130Ce6}, Angew. Chem., Int. Ed., 2017, 56, 9727–9731 CrossRef CAS PubMed.
- D. Lockey, C. Mathis, H. N. Miras and L. Cronin, Investigating the autocatalytically driven formation of Keggin-based polyoxometalate clusters, Matter, 2022, 5, 302–313 CrossRef CAS.
- L. Yue, K. Yang, X. Y. Lou, Y. W. Yang and R. Wang, Versatile roles of macrocycles in organic–inorganic hybrid materials for biomedical applications, Matter, 2020, 3, 1557–1588 CrossRef.
- S. Øien-Ødegaard, C. Bazioti, E. A. Redekop, Ø. Prytz, K. P. Lillerud and U. Olsbye, A toroidal Zr70 oxysulfate cluster and its diverse packing structures, Angew. Chem., Int. Ed., 2020, 59, 21397–21402 CrossRef PubMed.
- A. J. Tasiopoulos, A. Vinslava, W. Wernsdorfer, K. A. Abboud and G. Christou, Giant single-molecule magnets: A {Mn84} torus and its supramolecular nanotubes, Angew. Chem., Int. Ed., 2004, 43, 2117–2121 CrossRef CAS PubMed.
- C. Zhao, Y. Han, S. Dai, X. Chen, J. Yan, W. Zhang, H. Su, S. Lin, Z. Tang, B. K. Teo and N. Zheng, Microporous cyclic titanium–oxo clusters with labile surface ligands, Angew. Chem., 2017, 129, 16470–16474 CrossRef.
- M. A. Moussawi, M. Haouas, S. Floquet, W. E. Shepard, P. A. Abramov, M. N. Sokolov, V. P. Fedin, S. Cordier, A. Ponchel, E. Monflier, J. Marrot and E. Cadot, Nonconventional three-component hierarchical host–guest assembly based on Mo-blue ring-shaped giant anion, γ-cyclodextrin, and Dawson-type polyoxometalate, J. Am. Chem. Soc., 2017, 139, 14376–14379 CrossRef CAS PubMed.
- H. Imai, T. Akutagawa, F. Kudo, M. Ito, K. Toyoda, S. Noro, L. Cronin and T. Nakamura, Structure, magnetism, and ionic conductivity of the gigantic {Mo176} wheel assembly: Na15Fe3Co16[Mo176O528H3(H2O)80]Cl27·450H2O, J. Am. Chem. Soc., 2009, 131, 13578–13579 CrossRef CAS PubMed.
- K. H. K. Lee, L. Aebersold, J. E. Peralta, K. A. Abboud and G. Christou, Synthesis, structure, and magnetic properties of an Fe36 dimethylarsinate cluster: The largest “ferric wheel”, Inorg. Chem., 2022, 61, 17256–17267 CrossRef CAS PubMed.
- X. Y. Zheng, Y. H. Jiang, G. L. Zhuang, D. P. Liu, H. G. Liao, X. J. Kong, L. S. Long and L. S. Zheng, A gigantic molecular wheel of {Gd140}: A new member of the molecular wheel family, J. Am. Chem. Soc., 2017, 139, 18178–18181 CrossRef CAS PubMed.
- D. Shi, X. Yang, H. Chen, Y. Ma, D. Schipper and R. A. Jones, Self-assembly of luminescent 42-metal lanthanide nanowheels with sensing properties towards metal ions and nitro explosives, J. Mater. Chem. C, 2019, 7, 13425–13431 RSC.
- I. Colliard, J. C. Brown, D. B. Fast, A. K. Sockwell, A. E. Hixon and M. Nyman, Snapshots of Ce70 toroid assembly from solids and solution, J. Am. Chem. Soc., 2021, 143, 9612–9621 CrossRef CAS PubMed.
- J. Rinck, G. Novitchi, W. V. d. Heuvel, L. Ungur, Y. Lan, W. Wernsdorfer, C. E. Anson, L. F. Chibotaru and A. K. Powell, An octanuclear [CrIII4DyIII4] 3d–4f single-molecule magnet, Angew. Chem., Int. Ed., 2010, 49, 7583–7587 CrossRef CAS PubMed.
- H. L. Zhang, Y. Q. Zhai, H. Nojiri, C. Schröder, H. K. Hsu, Y. T. Chan, Z. Fu and Y. Z. Zheng, {ScnGdn} Heterometallic rings: Tunable ring topology for spin wave excitations, J. Am. Chem. Soc., 2022, 144, 15193–15202 CrossRef CAS PubMed.
- E. Garrido Ribó, N. L. Bell, W. Xuan, J. Luo, D. L. Long, T. Liu and L. Cronin, Synthesis, assembly, and sizing of neutral, lanthanide substituted molybdenum blue wheels {Mo90Ln10}, J. Am. Chem. Soc., 2020, 142, 17508–17514 CrossRef PubMed.
- Z. Li, Y. Ye, Z. Yao, J. Guo, Q. Lin, J. Zhang, Z. Zhang, F. Wei and S. Xiang, An antiferromagnetic metalloring pyrazolate (Pz) framework with [Cu12(μ2-OH)12(Pz)12] nodes for separation of C2H2/CH4 mixture, J. Mater. Chem. A, 2018, 6, 19681–19688 RSC.
- D. Gounden, N. Nombona and W. E. Van Zyl, Recent advances in phthalocyanines for chemical sensor, non-linear optics (NLO) and energy storage applications, Coord. Chem. Rev., 2020, 420, 213359 CrossRef CAS.
- W. Xuan, R. Pow, N. Watfa, Q. Zheng, A. J. Surman, D. L. Long and L. Cronin, Stereoselective assembly of gigantic chiral molybdenum blue wheels using lanthanide ions and amino acids, J. Am. Chem. Soc., 2019, 141, 1242–1250 CrossRef CAS PubMed.
- Y. Zou, W. Lv, Z. Z. Xue, J. H. Li, X. Y. Li and G. M. Wang, Solvent-controlled synthesis of an Al12-oxo molecular ring and Al24-oxo truncated metallo-cube, Inorg. Chem. Front., 2023, 10, 1614–1622 RSC.
- T. Wu, B. Han, J. X. Liu, J. Zhang, C. Xue, X. Wang and D. S. Li, A wheel-shaped gallium-sulfide molecular ring with enhanced photocatalytic activity via indium alloying, Inorg. Chem. Front., 2023, 10, 4147–4156 RSC.
- Y. Ma, X. P. Yang, D.-L. Shi, M.-Y. Niu and D. Schipper, Construction of a 18-metal neodymium(III) nanoring with NIR luminescent sensing to antibiotics, Inorg. Chem., 2020, 59, 17608–17613 CrossRef CAS PubMed.
- L. Qin, J. Singleton, W. Chen, H. Nojiri, L. Engelhardt, R. E. P. Winpenny and Y. Zheng, Quantum Monte Carlo simulations and high-field magnetization studies of antiferromagnetic interactions in a giant hetero-spin ring, Angew. Chem., 2017, 129, 16798–16801 CrossRef.
- D. Yan, L. Cai, S. Hu, Y. Zhou, L. Zhou and Q. Sun, An organo–palladium host built from a dynamic macrocyclic ligand: Adaptive self-assembly, induced-fit guest binding, and catalysis, Angew. Chem., Int. Ed., 2022, 61, e202209879 CrossRef CAS PubMed.
- Q. Liu, C. Li, B. Yao, Y. Cui, Y. Cheng, Y. Deng, Z. Chen and Y. Zhang, Self-assembly of a dodecanuclear [Ni12] wheel, Eur. J. Inorg. Chem., 2021, 2021, 1305–1310 CrossRef CAS.
- P. A. Tsami, T. G. Tziotzi, A. B. Canaj, M. K. Singh, S. J. Dalgarno, E. K. Brechin and C. J. Milios, A ferromagnetically coupled pseudo-calixarene [Co16] wheel that self-assembles as a tubular network of capsules, Dalton Trans., 2022, 51, 15128–15132 RSC.
- O. Botezat, J. Van Leusen, J. Hauser, S. Decurtins, S. X. Liu, P. Kögerler and S. G. Baca, A spontaneous condensation sequence from a {Fe6Dy3} wheel to a {Fe7Dy4} globe, Cryst. Growth Des., 2019, 19, 2097–2103 CrossRef CAS.
- T. Imaoka, Y. Akanuma, N. Haruta, S. Tsuchiya, K. Ishihara, T. Okayasu, W. J. Chun, M. Takahashi and K. Yamamoto, Platinum clusters with precise numbers of atoms for preparative-scale catalysis, Nat. Commun., 2017, 8, 688 CrossRef PubMed.
- Y. T. Zhang, C. Qin, X. L. Wang, P. Huang, B. Q. Song, K. Z. Shao and Z. M. Su, High-nuclear vanadoniobate {Nb48V8} multiple-strand wheel, Inorg. Chem., 2015, 54, 11083–11087 CrossRef CAS PubMed.
- C. Liu, C. Gao, A. Said, H. Niu, D. Wang, C. H. Tung and Y. Wang, Assembly of interlocked superstructures with a titanium oxide molecular ring in water, Inorg. Chem., 2021, 60, 14520–14524 CrossRef CAS PubMed.
- M. G. Sorolla, X. Wang, T. Makarenko and A. J. Jacobson, Sulfate-bridged, octanuclear chromic and ferric wheels, Inorg. Chem., 2019, 58, 9935–9940 CrossRef CAS PubMed.
- T. Q. Song, J. Dong, A. F. Yang, X. J. Che, H. L. Gao, J. Z. Cui and B. Zhao, Wheel-like Ln18 cluster organic frameworks for magnetic refrigeration and conversion of CO2, Inorg. Chem., 2018, 57, 3144–3150 CrossRef CAS PubMed.
- C. Zhao, Y. Han, S. Dai, X. Chen, J. Yan, W. Zhang, H. Su, S. Lin, Z. Tang, B. K. Teo and N. Zheng, Ti32 microporous cyclic titanium-oxo clusters with labile surface ligands, Angew. Chem., 2017, 129, 16470–16474 CrossRef.
- D. Luo, F. Wang, C. H. Liu, S. T. Wang, Y. Y. Sun, W. H. Fang and J. Zhang, Combination of aluminum molecular rings with chemical reduction centers for iodine capture and aggregation, Inorg. Chem. Front., 2022, 9, 4506–4516 RSC.
- J. B. Chen, S. T. Wang, S. H. Shen, Y. H. Yu, W. H. Fang and J. Zhang, In situ pyrazolylborate ligand synthesis and coordination behaviours in aluminum oxo clusters, Inorg. Chem. Front., 2023, 10, 1887–1893 RSC.
- L. Geng, C. Liu, S. Wang, W. Fang and J. Zhang, Designable aluminum molecular rings: ring expansion and ligand functionalization, Angew. Chem., Int. Ed., 2020, 59, 16735–16740 CrossRef CAS PubMed.
- S. T. Wang, Y. J. Liu, C. C. Feng, W. H. Fang and J. Zhang, The largest aluminum molecular rings: Phenol–thermal synthesis, photoluminescence, and optical limiting, Aggregate, 2023, 4, e264 CrossRef CAS.
- Y. Li, C. Zheng, S. Wang, Y. Liu, W. Fang and J. Zhang, Record aluminum molecular rings for optical limiting and nonlinear optics, Angew. Chem., Int. Ed., 2022, 61, e202116563 CrossRef CAS PubMed.
- C. Liu, W. Fang, Y. Sun, S. Yao, S. Wang, D. Lu and J. Zhang, Designable assembly of aluminum molecular rings for sequential confinement of iodine molecules, Angew. Chem., Int. Ed., 2021, 60, 21426–21433 CrossRef CAS PubMed.
- D. Luo, H. Xiao, M.-Y. Zhang, S.-D. Li, L. He, H. Lv, C.-S. Li, Q.-P. Lin, W.-H. Fang and J. Zhang, Accurate binding of porous aluminum molecular ring catalysts with the substrate, Chem. Sci., 2023, 14, 5396–5404 RSC.
- S. Yao, W.-H. Fang, Y. Sun, S.-T. Wang and J. Zhang, Mesoporous assembly of aluminum molecular rings for iodine capture, J. Am. Chem. Soc., 2021, 143, 2325–2330 CrossRef CAS PubMed.
- Y. Zhang, Q.-H. Li, W.-H. Fang and J. Zhang, Aluminum molecular rings bearing amino-polyalcohol for iodine capture, Inorg. Chem. Front., 2022, 9, 592–598 RSC.
- L. Geng, Q. H. Li, S. T. Wang, Y. J. Liu, W. H. Fang and J. Zhang, Aluminium nanorings: configuration deformation and structural transformation, Chem. Commun., 2021, 57, 2085–2088 RSC.
- X.-Z. Zhang, X.-F. Wang, W.-H. Fang and J. Zhang, Synthesis, structures, and fluorescence properties of dimeric aluminum oxo clusters, Inorg. Chem., 2021, 60, 7089–7093 CrossRef CAS PubMed.
- Y. Chen, T. Bai, N. Dong, F. Fan, S. Zhang, X. Zhuang, J. Sun, B. Zhang, X. Zhang, J. Wang and W. J. Blau, Graphene and its derivatives for laser protection, Prog. Mater. Sci., 2016, 84, 118–157 CrossRef CAS.
- K. A. Green, M. P. Cifuentes, M. Samoc and M. G. Humphrey, Metal alkynyl complexes as switchable NLO systems, Chem. Soc. Rev., 2011, 255, 2530–2541 CAS.
- W. Feng, K. Liu, J. Zang, G. Wang, R. Miao, L. Ding, T. Liu, J. Kong and Y. Fang, Flexible and transparent oligothiophene-o-carborane-containing hybrid films for nonlinear optical limiting based on efficient two photon absorption, ACS Appl. Mater. Interfaces, 2021, 13, 28985–28995 CrossRef CAS PubMed.
- D. J. Li, Q. Li, Z. R. Wang, Z. Z. Ma, Z. G. Gu and J. Zhang, Interpenetrated metal-porphyrinic framework for enhanced nonlinear optical limiting, J. Am. Chem. Soc., 2021, 143, 17162–17169 CrossRef CAS PubMed.
- Z. K. Wang, M. H. Du, P. Braunstein and J. P. Lang, A cut-to-link strategy for cubane-based heterometallic sulfide clusters with giant third-order nonlinear optical response, J. Am. Chem. Soc., 2023, 145, 9982–9987 CrossRef CAS PubMed.
- Z. B. Liu, J. G. Tian, Z. Guo, D. M. Ren, F. Du, J. Y. Zheng and Y. S. Chen, Enhanced optical limiting effects in porphyrin-covalently functionalized single-walled carbon nanotubes, Adv. Mater., 2008, 20, 511–515 CrossRef CAS.
- J. Zhu, Y. Li, Y. Chen, J. Wang, B. Zhang, J. Zhang and W. J. Blau, Graphene oxide covalently functionalized with zinc phthalocyanine for broadband optical limiting, Carbon, 2011, 49, 1900–1905 CrossRef CAS.
- R. Matshitse, S. Khene and T. Nyokong, Photophysical and nonlinear optical characteristics of pyridyl substituted phthalocyanine-Detonation nanodiamond conjugated systems in solution, Diamond Relat. Mater., 2019, 94, 218–232 CrossRef CAS.
- Y. Zhu, Z. Wang, D. Li, Y. Zhu, Q. Li, D. Li and L. Zhang, Silver-templated γ-Keggin alkyltin–oxo cluster: Electronic structure and optical limiting effect, Angew. Chem., Int. Ed., 2022, 61, e202202853 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2289659 and 2289660. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3qi02008h |
| ‡ These authors contributed equally to this work. |
|
| This journal is © the Partner Organisations 2024 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *a and
Jian
Zhang
*a and
Jian
Zhang
 a
a
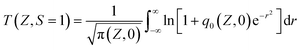
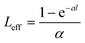
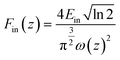
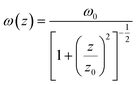
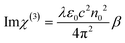
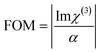
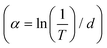 , z is the Z-scan displacement, ε0 is the permittivity of vacuum (8.85 × 10−12 F m−1), c is the speed of light and l denotes the thickness of the glass cell.
, z is the Z-scan displacement, ε0 is the permittivity of vacuum (8.85 × 10−12 F m−1), c is the speed of light and l denotes the thickness of the glass cell.



