Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Insights into hydrophobic (meth)acrylate polymers as coating for slow-release fertilizers to reduce nutrient leaching†
Asma
Sofyane
 a,
Salima
Atlas
a,
Salima
Atlas
 ab,
Mohammed
Lahcini
ac,
Elvira
Vidović
d,
Bruno
Ameduri
ab,
Mohammed
Lahcini
ac,
Elvira
Vidović
d,
Bruno
Ameduri
 *e and
Mustapha
Raihane
*e and
Mustapha
Raihane
 *af
*af
aIMED-Lab. Faculty of Sciences and Techniques, Cadi-Ayyad University, Av. A. Khattabi. BP 549, 40000 Marrakech, Morocco. E-mail: m.raihane@uca.ma
bERSIC, FPBM, Sultan Moulay Slimane University, PO. Box. 592, Mghila, 23000, Beni Mellal, Morocco
cMohammed VI Polytechnic University, 43150 Ben Guérir, Morocco
dFaculty of Chemical Engineering and Technology, University of Zagreb, Marulićevtrg 19, 10000 Zagreb, Croatia
eICGM, University of Montpellier, CNRS, ENSCM, 34095 Montpellier, France. E-mail: bruno.ameduri@enscm.fr
fApplied Chemistry and Engineering Research Centre of Excellence (ACER CoE), Mohammed VI Polytechnic University, Lot 660, Hay Moulay Rachid Ben Guérir, 43150, Morocco
First published on 17th July 2024
Abstract
To solve the problem of the low utilization rate of conventional fast-release water-soluble fertilizers and to minimize their negative impact on the environment, slow-release fertilizers (SRFs) have emerged as a sustainable solution to limit their losses, reduce fertilizer dosage and improve crop production. In this study, new hydrophobic (meth)acrylate polymers (poly(2,2,2-trifluoroethyle methacrylate) (PTFEMA) and poly(2-(perfluorohexyl)ethyl acrylate) (PPFEHEMA)) with different fluorinated side chains were synthesized by free radical polymerization and used as coatings for SFRs. These polymers were characterized by 1H and 19F NMR, FTIR, WCA, TGA and DSC. Compared to PTFEMA, PPFEHEMA with a higher content of F atoms displayed improved thermal stability and an elastomer property (Tg = −10 °C) leading to satisfactory film formation. Indeed, water contact angle (WCA) measurements were carried out on films of both materials: PPFEHEMA with WCA = 109° indicated a highly hydrophobic character with an excellent water-repellent surface, resulting in a coating layer. The use of these polymers as SFR coatings was explored using dip-coating. SEM and EDX mapping were performed to study the morphology of the coated fertilizer granules and showed the formation of a cohesive film with good adhesion between the DAP fertilizer and the coating films, limiting water diffusion. The release profiles of N and P nutrients were studied, and the corresponding release times increased with coating thickness (single layer: 1L or second layer: 2L). Compared to uncoated DAP granules which are totally solubilized after less than 2 h, DAP coated with 2L of PPFEHEMA shows the slowest release of N and P nutrients, and the times to reach maximum N and P releases were 30 and 38 times higher than those of uncoated DAP. The significant delay in the release of nutrients from DAP coated with PTFEMA or PPFEHEMA is consistent with nutrient demand during crop growth and increases the efficiency of fertilizer use and therefore enhances agricultural productivity.
1. Introduction
From the data of the population projections published by the United Nations, the world population will reach 9.5 billion people by 2050,1 with a planned increase in food supply of 70%.2 The forecast of the Food and Agriculture Organization of the United Nations (FAO) estimates that a quarter of this growing population could suffer from food insecurity. Around 30% of arable land will be lost due to soil degradation.3 In order to meet the growing global demand for food and to address food security challenges by promoting sustainable agriculture, the use of inorganic nitrogen (N), phosphorus (P) and potassium (K) fertilizers is expected to increase because they can improve crop productivity by about 60%.4 However, current conventional fertilizers are highly water-soluble, meaning that only 30–60% of N, 10–20% of P and 30–50% of K could be absorbed by plants. A large amount of these micronutrients is released into the environment through leaching, runoff, volatilization, etc., which has a negative impact on ecosystems and biodiversity, such as soil disturbance and groundwater contamination. These losses result not only in low absorption efficiency of the nutrients by plant roots,5 but also in financial losses due to the waste of energy associated with their production.6,7 Therefore, in order to maximize crop production one of the major challenges is to rationalize the use of fertilizers. Slow-release fertilizers (SRFs) are proposed as a promising technology to improve nutrient uptake by plants and to minimize environmental pollution.8 SRFs are designed to release nutrients slowly to meet their needs during crop growth.9 Polymers coated fertilizers are the most important candidates for SRFs because the polymer acts as a diffusion barrier membrane. Polyolefin, alkyd-resin and polyurethane-coated fertilizers are important commercially available synthetic polymer coatings for SRFs, manufactured by JCAM AGRI Co, ICL Specialty Fertilizers and Koch Agronomic Services, Inc. under the Nutricote®, Osmocote® and Polyon® trademarks10 (more details are given in ESI†). The synthetic polymer coatings can be divided into two classes: (i) hydrophobic polyolefins which are soluble in an organic solvent (e.g., polyethylene11 or polyacrylonitrile12), and (ii) superabsorbent hydrogels as three-dimensional or crosslinked matrices composed of linear or branched polymers with abundant hydrophilic groups,13 which in agriculture lead to increased water storage capacity, a limited amount of irrigation, and increased crop production in semi-arid and arid areas.14Poly(acrylate)s (PAs) have been widely used to produce SRFs to increase agricultural yields of corn and wheat15 and as superabsorbents.16 PA waterborne coatings using an aqueous solution in their preparation are known for their appropriate viscosity, good film-forming ability, and strong adhesion to substrates through polar groups.17 Polysaccharides such as starch or cellulose are used as biopolymers for the synthesis of bio-superabsorbents in which vinyl monomers such methacrylic acid, acrylamide, or acrylic acid are grafted onto their backbones to increase the hydrophilicity and swelling capacity of these superabsorbents.18 To elaborate these networks to give them enhanced water-retention capacity and regulated slow-release of nutrients, grafting reactions have been performed in an aqueous solution by free radical (co)polymerization of these monomers using ammonium persulfate and N,N‘-methylenebisacrylate (MBA) as initiator and crosslinking agent, respectively.16 Recently, Zhu et al.19 prepared superabsorbent hydrogel composites based on okara, a byproduct derived from soybean oil milk, grafted onto poly(acrylic acid), by in situ radical polymerization to improve vegetable cultivation through increasing the water holding capacity in soils. Jumpapaeng et al.20 prepared bionanocomposite hydrogels (BHMs) as a promising material by combining cassava starch, polyacrylamide, natural rubber, and various montmorillonite clay loadings. These low-cost biohydrogels exhibit high-strength properties and serve as coating membranes for slow-release urea fertilizers. However, these hydrogels present some defect pores when used as a coating on the surface of urea, increasing the solubility of the N nutrient and thus reducing the slow-release effect. To address this issue, a wax hydrophobic polymer solution was used to encapsulate the BHM hydrogel surfaces as an outer layer by filling in all cracks and defects detected on the surface. These hydrophobic and continuous wax layers improve the structural stability of the coating materials and enhance the slow-release performance by preventing water penetration into the fertilizer core.
With the above problems in the use of hydrophilic superabsorbent polymers, hence hydrophobic polymer coating films present an answer to this challenge by acting as good barrier membranes to limit the diffusion of water, and thus delay nutrient release from coated fertilizers. Among these polymers, fluorinated acrylate polymers are the most commonly proposed materials thanks to their remarkable properties, such as UV photo-chemical stability, remarkable weatherability, semi-permeable membranes, and self-cleaning surfaces.21–23 Homo- and copolymers of fluorinated (meth)acrylates with perfluoroalkyl side chains (CnF2n+1) are an important class of such materials that exhibit unexpected hydrophobicity in comparison to the corresponding n-alkyl chains (PAs). In fact, the fluorocarbons side chains pack less densely on the surfaces, leading to poorer van der Waals interactions with water and thus to good water-repellent properties.21,24–26 Their low surface energies, attributed to the properties of fluorine atoms, enable them to be widely used in high-performance coatings.25–29
To our knowledge, there have been only two papers reporting the use of hydrophobic fluorinated polymers as SRF coatings. To enhance the performance of polymer-encapsulated urea fertilizers, Chen et al.30 developed a novel waterborne hydrophobic polymer coating using nano-SiO2 and 1H,1H,2H,2H-perfluorooctyltriethoxysilane to modify water-based polyvinyl alcohol. More recently waterborne copolymers prepared by Pickering emulsion copolymerization of butyl methacrylate (BMA) with 2-(perfluorohexyl)ethyl acrylate (PFEHEMA) were reported by our team. The resulting waterborne latexes were tested as coating materials for granular water-soluble fast-release fertilizers.31 A P(BMA-co-PFEHEMA) copolymer containing 8 wt% starch nanocrystals and a low PFEHEMA percentage (6.5 mol%) showed better slow-release properties than those of non-fluorinated P(BMA), attributed to the presence of fluorinated units conferring improved hydrophobic properties on a P(BMA-co-PFEHEMA) copolymer coating.
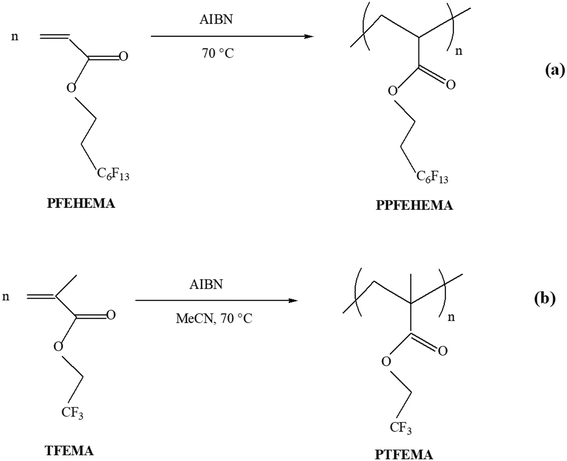
The aim of this work is the preparation of hydrophobic poly(meth)acrylates with different fluorinated side chains, such as poly(2,2,2-trifluoroethyl methacrylate) (PTFEMA) and poly(2-(perfluorohexyl)ethyl acrylate) (PPFEHEMA), by free radical polymerization. These polymers were characterized by 1H and 19F NMR and IR spectroscopy, WCA, DSC and TGA, and applied as coating materials to diammonium phosphate (DAP) fertilizers. The morphology and chemical composition of the coated fertilizer surfaces and cross-sections were investigated using SEM-EDX mapping, while a UV-visible spectrophotometer was utilized to monitor the release rates of phosphorus (P) and nitrogen (N) in water. Finally, the relationship between the structure of fluorinated polymers and the release profiles of N and P nutrients was studied to evaluate the performance in terms of slowing the release rate of nutrients through these fluorinated hydrophobic polymer coatings.
2. Experimental section
2.1. Materials
Diammonium phosphate (DAP) ((NH4)2HPO4) was chosen as a granular phosphate fertilizer to prepare SRFs. This commercial granular fertilizer, containing 46% phosphorus (P2O5) and 18% nitrogen (N), was generously provided by the OCP Group in Morocco. 2-(Perfluorohexyl)ethyl acrylate (PFEHEMA) (CAS: 17527-29-6) was kindly provided by Atofina (Pierre Bénite, France), while 2,2,2-trifluoroethyl methacrylate (TFEMA) was kindly supplied by Tosoh Finechemical Corp, Shunan, Yamaguchi (Japan). Azobisisobutyronitrile (AIBN) and all the solvents (hexafluorobenzene, acetonitrile, pentane, tetrahydrofuran, and methanol) were purchased from Sigma-Aldrich (France). Before use, TFEMA and PFEHEMA were purified by distillation under reduced pressure.2.2. Synthesis of fluorinated homopolymers
2.3. Coating technique
To provide a suitable viscosity for a coating process, we prepared PTFEMA and PPFEHEMA polymer solutions in THF and hexafluorobenzene, respectively (in 40% w/v ratio). The commercially available granular DAP fertilizers were coated by a dip-coating process, as described in previous studies.9,31 Dip-coating was achieved by immersing DAP granules (with diameters of 2–4 mm and a weight of ca. 35 mg) in the corresponding solutions for 10 min. The DAP pellets were then removed from solution and placed on a Teflon® film surface. Subsequently, the coated DAP granules were dried at room temperature, leading to a coated fertilizer with a single layer (1L). To create a coated DAP with a second layer (2L), this operation was repeated a second time on the coated DAP (1L) using the same coating solution.The percentage coating (CC) was calculated according to the equation:
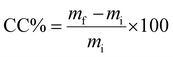 | (1) |
2.4. Characterizations
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of CDCl3/CF3CO2H were used as NMR solvents for PTFEMA and PPFEHEMA, respectively. Chemical shifts are given in ppm. 1H and 19F NMR spectra were performed under the following experimental conditions: a flip angle of 90° for 1H (30° for 19F), acquisition time of 4.5 s (0.7 s), pulse delay of 2 s (5 s), 16 scans (64 for 19F), and a pulse width of 5 μs for 19F NMR.
1 mixture of CDCl3/CF3CO2H were used as NMR solvents for PTFEMA and PPFEHEMA, respectively. Chemical shifts are given in ppm. 1H and 19F NMR spectra were performed under the following experimental conditions: a flip angle of 90° for 1H (30° for 19F), acquisition time of 4.5 s (0.7 s), pulse delay of 2 s (5 s), 16 scans (64 for 19F), and a pulse width of 5 μs for 19F NMR.
For this analysis, an axial rupture containing the fertilizer and the coating material was created using a razor blade. The coated granule and its cross-section were spread out on a carbon band and fixed to the surface of a metal disc using double-sided adhesive tape. Additionally, by examining the cross-sectional surface of coated DAP granular fertilizer, the coating thicknesses were determined.
3. Results and discussion
3.1. Preparation and characterization of PTFEMA and PPFEHEMA (coating materials)
For excellent weatherability, a semi-permeable membrane based on fluorinated acrylic polymers should be covered by as many fluorine-containing groups as possible.25,27,29,32 In contrast to some low-molar-mass per- and polyfluoroalkyl substances (PFASs), well established as being water soluble, toxic, persistent, bioaccumulative and mobile, fluoropolymers are insoluble in water and thus not mobile, are bio-inert, safe and have unique properties that are essential for our daily lives (coatings, electronics, internet of things, energy, transportation, etc.). Indeed, these materials are possibly irreplaceable, since suggested alternative products such as hydrocarbon polymers failed when used in similar conditions. Interestingly, these high-performance polymers satisfy the 13 polymer of low concern (PLC) criteria in their recommended conditions of use.33 Therefore, these specialty polymers must be separated from the PFAS family. Shirai et al.34 recently reported that poly(fluoroalkyl (meth)acrylate)s containing extended perfluoroalkyl groups (CnF2n+1) can degrade, leading to the formation of perfluorooctanoic acid (C7F15CO2H, PFOA). These authors also showed that polymers featuring short fluorinated side chains (n ≤ 6 fluorocarbons) present less bioaccumulative PFAS compared to those with n ≥ 7. Therefore, taking in account the hydrophobic coating performances with fluorine-containing groups and to address environmental concerns with less bioaccumulation of PFOA, we have chosen to use TFEMA (–CF3) and PFEHEMA (C6F13) as fluorinated monomers to prepare PTFEMA and PPFEHEMA polymers with high molar masses compared to those of PFASs, which can be applied as coating fertilizers to achieve the slow release of nutrients. These polymers were successfully synthesized by free radical polymerization (Scheme 1). The resulting polymers were then analyzed, and finally used as coating materials to cover diammonium phosphate (DAP) fertilizers.| Band | PTFEMA (cm−1) | PPFEHEMA (cm−1) |
|---|---|---|
| C–F symmetric stretching | 1225 | 1202 |
| C–F asymmetric stretching | 1176 | 1145 |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ester stretching O ester stretching |
1753 | 1737 |
| C–H: symmetric and asymmetric stretching | 2850 and 2960 | 2875 and 2972 |
| C–H (out of plane) | 973 | 844 |
| C–O stretching | 1176 | 1116 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of CDCl3/CF3CO2H, since PPFEHEMA is not soluble in organic solvents.
1 mixture of CDCl3/CF3CO2H, since PPFEHEMA is not soluble in organic solvents.
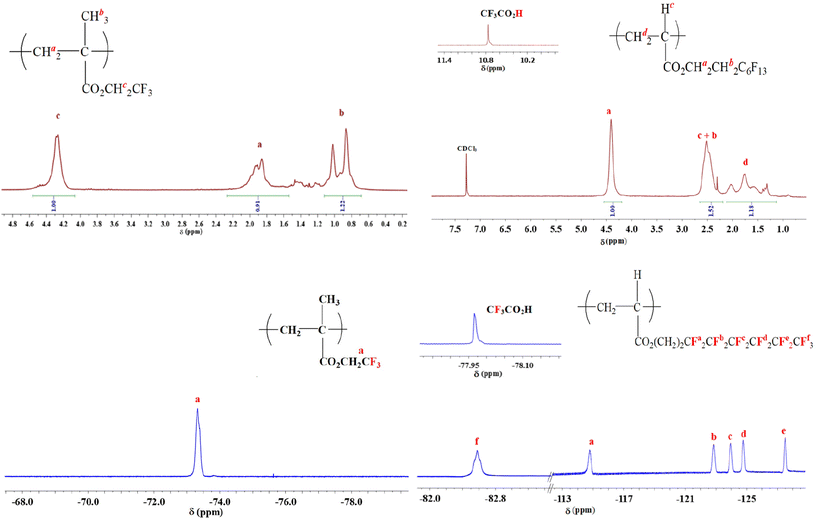
The assignments of the chemical shifts were derived by comparison with the values reported in the literature for TFEMA-based polymers38 and poly(perfluoro(meth)acrylate)s29 and are summarized in Table 2. For example, the 1H NMR spectrum of PTFEMA shows a signal of the methylene of ester group (–O-C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2-CF3) centered at 4.3 ppm. The methyl group of PTFEMA (–C
2-CF3) centered at 4.3 ppm. The methyl group of PTFEMA (–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3) was observed in the range 0.8–1.1 ppm, while the methylene protons of the backbone (C
3) was observed in the range 0.8–1.1 ppm, while the methylene protons of the backbone (C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2) appear between 1.8 and 2.1 ppm. The 19F NMR spectrum of PTFEMA exhibits the C
2) appear between 1.8 and 2.1 ppm. The 19F NMR spectrum of PTFEMA exhibits the C![[F with combining low line]](https://www.rsc.org/images/entities/char_0046_0332.gif) 3 peak at −73 ppm. The vinylic proton signal centred at 6.1 ppm for TFEMA and peaks at 6.5, 5.9 and 5.0 for PFEHEMA were not present in these spectra.
3 peak at −73 ppm. The vinylic proton signal centred at 6.1 ppm for TFEMA and peaks at 6.5, 5.9 and 5.0 for PFEHEMA were not present in these spectra.
| Type of proton | PTFEMA | PPFEHEMA |
|---|---|---|
| 1 H NMR | ||
| CH3 | 0.8–1.1 | — |
| CH2 (main chain) | 1.8–2.1 | 1.2–2.2 |
| CH (main chain) | — | 2.2–2.7 |
| OCH2CF3 | 4.3 | — |
| OCH2CH2C6F13 | — | 4.2–4.5 |
| OCH2CH2C6F13 | — | 2.2–2.7 |
| Type of fluorine | PTFEMA | PPFEHEMA |
|---|---|---|
| 19 F NMR | ||
| OCH2CF3 | −73.0 | — |
| O(CH2)2CF2CF2CF2CF2CF2CF3 | — | −114.8 |
| O(CH2)2CF2CF2CF2CF2CF2CF3 | — | −124.8 |
| O(CH2)2CF2CF2CF2CF2CF2CF3 | — | −122.8 |
| O(CH2)2CF2CF2CF2CF2CF2CF3 | — | −123.9 |
| O(CH2)2CF2CF2CF2CF2CF2CF3 | — | −127.5 |
| O(CH2)2CF2CF2CF2CF2CF2CF3 | — | −82.6 |
![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) n and
n and ![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) w and dispersity (Đ =
w and dispersity (Đ = ![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) w/
w/![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) n) values are equal to 26
n) values are equal to 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, 52
000 g mol−1, 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 and 2.0, respectively. Of course, these are relative values. However, it is not possible to determine the molar masses of poly(2-(perfluorohexyl)ethyl acrylate) (PPFEHEMA) as it is not soluble in organic solvents, but only in fluorinated solvents such as hexafluorobenzene or 1,1,1,3,3,3-hexafluoro-2-propanol. Actually, our SEC apparatus is not equipped with columns related to fluorinated solvents.
000 g mol−1 and 2.0, respectively. Of course, these are relative values. However, it is not possible to determine the molar masses of poly(2-(perfluorohexyl)ethyl acrylate) (PPFEHEMA) as it is not soluble in organic solvents, but only in fluorinated solvents such as hexafluorobenzene or 1,1,1,3,3,3-hexafluoro-2-propanol. Actually, our SEC apparatus is not equipped with columns related to fluorinated solvents.
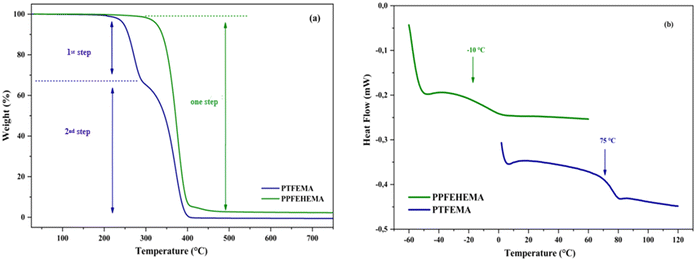
The degradation of PTFEMA takes place in two steps (Fig. 2a). The first one, in the range 200–300 °C, corresponds to the volatilization of side-chain fragments, including CO2, vinylidene fluoride and 2,2,2-trifluoroethanol, which are determined to be pyrolytic decomposition products (weight loss 26%). The second decomposition step, in the range 305–420 °C (weight loss 74%), is attributed to a depolymerization reaction.45,46 PPFEHEMA decomposes in a single step, in the range 280–420 °C, which is attributed to a random cleavage leading to a depolymerization mechanism (Fig. 2a). PPFEHEMA exhibits higher thermal stability than PTFEMA (Fig. 2a), which can be attributed to the better thermal stability of the C6F13pendant group in PPFEHEMA due to the strong C–F bond (EC–F = 450 kJ mol−1) that makes it possible to increase the heat resistance performance of the polymeric materials by adding more fluorinated components.31Table 3 lists the thermal characteristics of PTFEMA and PPFEHEMA.
The DSC second heating thermograms of both fluorinated polymers showed no melting temperature when the samples were heated from −60 °C to 120 °C (Fig. 2b). Only a sharp transition from the glassy state to the viscoelastic one was observed, as evidenced by the presence of a neat Tg, indicating that these fluorinated polymers exhibited amorphous behavior (Table 3); the Tg were close to −10 and 75 °C for PPFEHEMA and PTFEMA, respectively.
The decrease in Tg for PPFEHEMA compared to PTFEMA is related to the structure of the PFEHEMA units. In fact, the long alkyl dangling chains of the acrylate moiety (–CO2CH2CH2C6F13) serve as internal plasticizers, resulting in low Tg and giving PPFEHEMA a more elastomeric behavior at room temperature, as shown in Fig. S3.†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47 The decrease in Tg leads to excellent film-forming properties at room temperature for fertilizer coating. The PPFEHEMA coating films also help to improve the physical quality of granular fertilizer and are expected to have a positive effect on their compressive strength so that they do not break easily, preventing the generation of excessive dust during the handling and storage process.
47 The decrease in Tg leads to excellent film-forming properties at room temperature for fertilizer coating. The PPFEHEMA coating films also help to improve the physical quality of granular fertilizer and are expected to have a positive effect on their compressive strength so that they do not break easily, preventing the generation of excessive dust during the handling and storage process.
3.2. Morphological characterization of coated DAP fertilizers
Film forming from polymer solution coatings for DAP fertilizers was performed using the dip-coating method.9,30 The percentages of the different coating materials (calculated according to eqn (1)) are given in Table 4.| DAP coating | Weight coating percentage (%) | Average thickness (μm) |
|---|---|---|
| PTFEMA 1L | 4.5 | 51.0 |
| PTFEMA 2L | 10.7 | 90.0 |
| PPFEHEMA 1L | 7.7 | 27.0 |
| PPFEHEMA 2L | 16.0 | 73.0 |
To investigate the quality of the coating between the fertilizer and the coating, the morphology of the surface and the cross-section of uncoated and PTFEMA and PPFEHEMA coated DAP with a single layer (1L) and a second layer (2L) was investigated by SEM (Fig. 3).
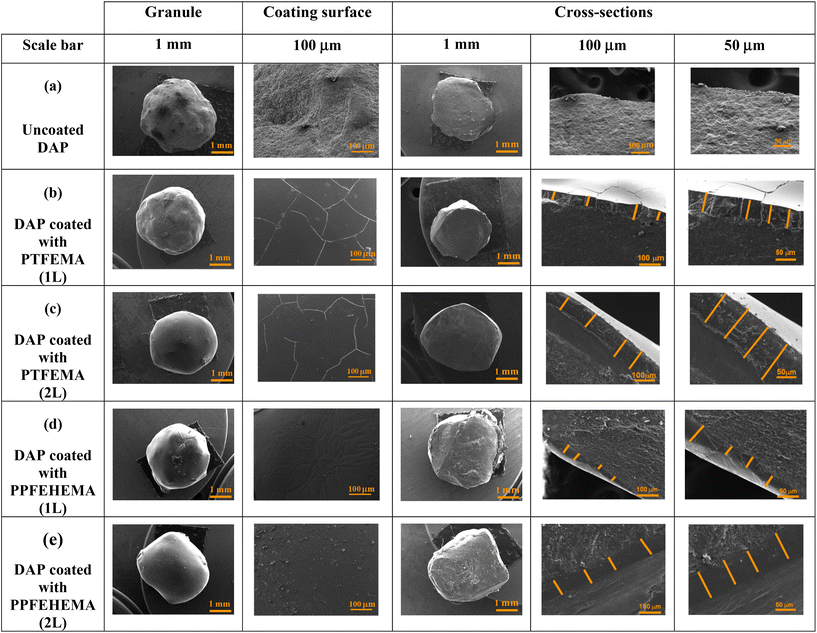 | ||
| Fig. 3 SEM analysis of a fertilizer granule and its cross-section containing the interface between DAP and tested PTFEMA and PPFEHEMA (single layer (1L) and second layer (2L)). | ||
A first overview of the SEM results showed that the surface of the uncoated DAP granule has an irregular and rough structure (Fig. 3a; scale bar: 1 mm).
The highly magnified surface (Fig. 3a; scale bar: 100 μm) showed some pinholes and an irregular morphology, due to the granulation process during the production of DAP fertilizers.40,48 When the DAP fertilizer was coated with the two PTFEMA and PPFEHEMA polymers, the coating surfaces exhibited a smoother and denser structure compared to uncoated DAP, especially when the fertilizers were covered with the second layer, as the content of the coating membrane on the surface of the fertilizers increased (Fig. 3b–d). This is in good agreement with our previous work.9,31,40,48,49
When analyzing the outer surface of the DAP granules coated with PTFEMA (Fig. 3b and c), we found that there are some microcracks in the surface compared to the granules coated with PPFEHEMA, which may be related to the structure of these polymers. PPFEHEMA has a Tg that is lower than the ambient temperature (−10 °C), so the PPFEHEMA coating has high flexibility and good film-formation, resulting in improved impact and crack resistance (Fig. S3†). In contrast, PTFEMA with a Tg of around 70 °C (Table 3) exhibits a glassy state at room temperature, which leads to some cracking when the solvent evaporates. These cracks could be reduced when the second layer was applied to the surface of the coated fertilizer. To eliminate these cracks or prevent their formation, Devassine et al.50 reported that controlling the rate of solvent evaporation or performing annealing could prevent the formation of cracks and pores. Yadavalli et al.51 observed some cracks in the SEM of the composite thin films and reported another explanation, which is the electron-beam-induced rapid volatilization of the organic species, such as residual solvent from the surface of these films during SEM analysis, leading to a buildup of tensile stress that causes cracks in the grain boundaries.
The cross-sectional images of coating materials observed by SEM with different magnitudes are shown in Fig. 3b–e. The contact surface between PTFEMA or PPFEHEMA (1L and 2L) coatings and the DAP core fertilizers is continuous with no gaps or voids present within it. In fact, the interaction between the hydrophilic inorganic DAP granules ((NH4)2HPO4) and the hydrophilic side (ester groups –CO2–) in the PTFEMA and PPFEHEMA coatings could be responsible for the good adhesion by both compounds.47 Indeed, the border lines between fertilizer and the film coatings are irregular due to the non-spherical irregular shape of the initial DAP granules (Fig. 3b and d; scale bar: 100 μm). Poly(fluorinated (meth)acrylate)s are a viable option for use in agriculture as coatings for SRFs, as confirmed by the formation of cohesive films.31
From the cross-section of core (fertilizer)–shell (coating) (Fig. 3b–e), the thicknesses of polymer coating were assessed by SEM at different points due to the irregular shape of DAP fertilizers, and the average thicknesses were calculated (Table 4). These values are a function of the type of coating (PTFEMA or PPFEHEMA) and their content (1L or 2L), as displayed in Fig. S4.†
The thicknesses of the different PTFEMA and PPFEHEMA coatings are also shown in Fig. S4.† The average thicknesses of DAP coated with PTFEMA (1L) and (2L) are close to 51 and 90 μm, respectively, while those achieved when PPFEHEMA is used as the coating are around 27 and 73 μm for 1L and 2L, respectively. The measured thicknesses of the two-layer (2L) coating are 1.5 and 2.7 times higher than those of the single-layer (1L) PTFEMA and PPFEHEMA coatings, respectively.
Energy dispersive X-ray analysis (EDX) was used to reveal the chemical compositions on the surface of the coated and uncoated DAP fertilizers to evaluate the quality of the coatings. The results are shown in Fig. 4 and Table 5.
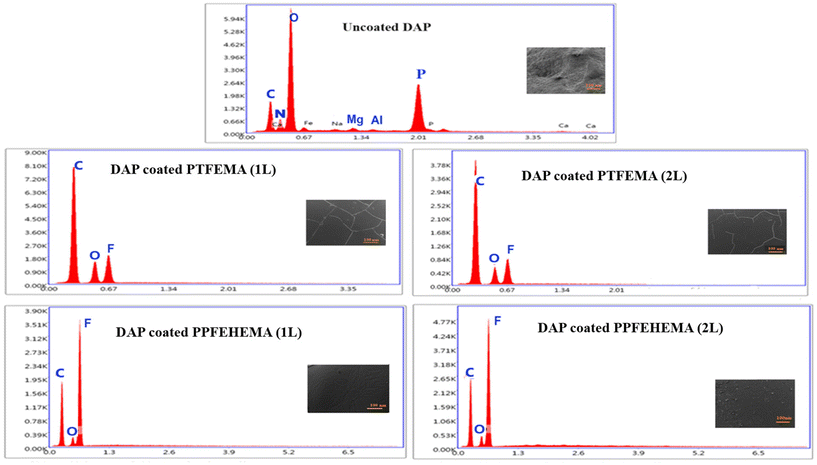 | ||
| Fig. 4 EDX analysis on the surface of uncoated DAP and DAP coated using PTFEMA and PPFEHEMA 1L and 2L. | ||
| Detected nutrients (wt%) | |||||||
|---|---|---|---|---|---|---|---|
| C | F | O | N | P | Other elements | ||
| Uncoated DAP | 19.54 | 0 | 46.98 | 10.10 | 21.55 | 0.67 | |
| DAP coated with PTFEMA | 1L | 67.03 | 15.42 | 17.55 | 0 | 0 | 0 |
| 2L | 73.41 | 14.37 | 12.21 | 0 | 0 | 0 | |
| DAP coated with PPFEHEMA | 1L | 44.28 | 50.12 | 05.59 | 0 | 0 | 0 |
| 2L | 44.79 | 49.57 | 05.64 | 0 | 0 | 0 | |
As essential macronutrients, the N and P signals of the DAP fertilizer were detectable only on the uncoated DAP surface. Their percentages were 21.55% and 10.10%, respectively (Table 5). Other microelements with a low content (0.67%), including Mg, Al and Ca, were also observed. The signal related to carbon (19.54%) was related to the metallization of DAP granules because the samples needed to be conductive to perform the SEM analysis.
The absence of N and P macronutrients on the outer surface of the coated DAP granules confirms that the PTFEMA and PPFEHEMA coatings covered the granular fertilizers successively with good adhesion and without any diffusion of the macronutrients N and P of the DAP fertilizer. These results are also corroborated by the SEM analyses. In the DAP coated with PTFEMA and PPFEHEMA membranes, the carbon content increases compared to that of the non-coated DAP fertilizer, which is attributed to the carbon atoms in the fluorinated (meth)acrylate units of the polymer coatings. As expected, the DAP coated with PPFEHEMA has a higher percentage of F-atoms than that coated with PTFEMA (Table 5 and Fig. 4).
The spatial distribution of the elements was investigated using the EDX technique. For example, Fig. 5 shows the element mapping (C, N, P, O and F) in the cross-section of DAP encapsulated with PPFEHEMA 2L. The C, N, P, O and F are the constituent elements of the core–shell that display a more homogeneous distribution on the cross-section of DAP coated with PPFEHEMA.
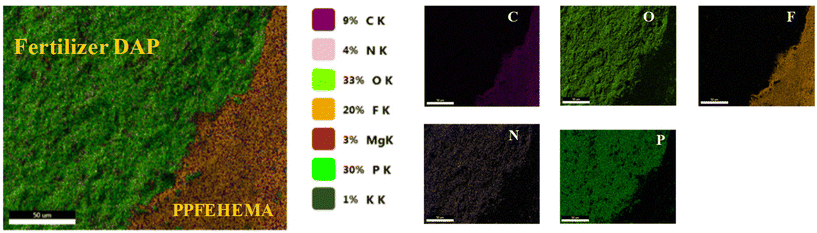 | ||
| Fig. 5 Chemical mapping obtained from the cross-sections of DAP coated with PPFEHEMA (2L) (scale bar: 10 μm). | ||
3.3. Phosphorous and nitrogen release behavior of coated and non-coated DAP fertilizers
To predict slow macronutrient releases for practical application, the nitrogen (N) and phosphorus (P) release patterns of uncoated and coated DAP fertilizer granules in water were studied according to the procedures described by Li et al.52 and Pereira et al.53 This allows an evaluation of the effects of the coating on the slow release and retarding performance of the coatings. The total percentage releases of P and N in water versus time for the uncoated and DAP coated with PTFEMA or PPFEHEMA polymers (1L or 2L) at pH 7 and ambient temperature are shown in Fig. 6.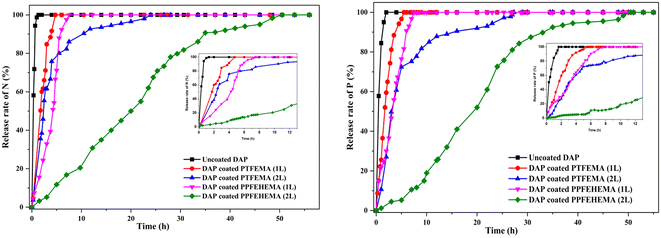 | ||
| Fig. 6 Release rate of P and N for uncoated DAP and coated DAP using PTFEMA and PPFEHEMA 1L and 2L in water at pH = 7 and ambient temperature. | ||
Fig. 6 shows that the uncoated DAP is completely dissolved in water in less than 2 h, whereas the rate of dissolution of nutrients in water is much slower with the encapsulated fertilizers than with uncoated DAP. For example, the times to reach the maximum percentage release of P are 3.3 and 14.5 times higher than for uncoated DAP when the fertilizer is covered with PTFEMA single-layer (1L) and double-layer (2L), respectively.
When the DAP was coated with PPFEHEMA 1L and 2L, respectively, the P release profiles of the coated granules reached the equilibrium stage at approximately 7.5 h and 50.5 h, indicating significantly slower P release or delaying performance properties of DAP fertilizers, and thus their potential applications as coating films in crop agriculture.31,40,47,48 DAP coated with 2L of PPFEHEMA presents the slowest macronutrient release: the times to reach the maximum N and P release are 30 and 38 times higher than those of uncoated DAP, respectively. Indeed, compared to PTFEMA, the PPFEHEMA coating shows significantly slower release of nutrients (Fig. 6 and Fig. S5†). In fact, the chemical structure of the coating is one of the key parameters determining the release rate of P nutrient from the coating. The presence of a larger number of F atoms and C–F bonds in the PFEHEMA monomer with hydrophobic properties, attributed to the -C6F13 side groups, gives the PPFEHEMA coating a very hydrophobic character, that acts as a physical barrier and reduces water diffusion, contributing to the slow release of P and N nutrients compared to PTFEMA-coated DAP.21,22,26 This hydrophobic character was confirmed by water contact measurement (WCA) (Fig. S2†), where the value of PPFEHEMA (WCA = 109°) is higher than that of PTFEMA (WCA = 79°). The soft structure of PPFEHEMA, which was confirmed by DSC (Fig. 2b and Table 3) gives the polymer good film-forming ability and good adhesion properties.31
Another important parameter that can contribute significantly to nutrient release is the thickness of the coating. A greater thickness of these coatings resulted in lower nutrient release, as the coating film creates diffusion resistance to water and hinders nutrient diffusion. According to da Cruz et al.,54 DAP coated with 3.0 and 4.5 wt% of polyurethane prepared from castor oil polyol showed a notable delay in phosphorus release. 80% of P was released in 50 h and 75 h when the coating percentage was close to 3.0 wt% and 4.5 wt%, respectively. In our case, the results indicated that thicker PPFEHEMA coatings may shift the maximum nutrient availability towards longer periods. The maximum release rate of P was reached after 7.5 h for DAP coated with a single layer (1L) (thickness = 27 μm), whereas that covered with a double-layer (2L) coating (thickness = 73 μm) resulted in a maximum release after 50.5 h (Fig. S5†).
To enhance the efficiency of fertilizer use and to minimize adverse environmental effects, the performance in terms of slow-release nutrients of fertilizer coatings is governed by extending nitrogen (N) and phosphorus (P) release properties by delaying the time to reach equilibrium, and therefore matching nutrient demand during crop growth. A comparison of P release times at equilibrium of PTFEMA and PPFEHEMA coatings with various previous studies using acrylate coating polymers is given in Table 6.
| Coating fertilizera | Coating process | Total release of (P2O5) in water (hours) | Ref. |
|---|---|---|---|
| a BMA: butyl methacrylate, PFEHEMA: 2-(perfluorohexyl)ethyl acrylate and TFEMA: 2,2,2-trifluoroethyl methacrylate and PMMA: poly(methyl methacrylate). | |||
| Uncoated DAP | 2.0 | — | |
| DAP coated with starch nanocrystal/PBMA | Immersion | 25.2 | 31 |
| DAP coated with starch nanocrystal/P(BMA-co-PFEHEMA) | Immersion | 32.5 | 31 |
| DAP coated with polymethyl methacrylate-g-carboxymethyl cellulose | Rotary pan | 30.0 | 49 |
| PMMA | Rotary pan | 23.0 | 49 |
| DAP coated with PTFEMA (double layer) | Immersion | 24.0 | This work |
| DAP coated with PPFEHEMA (double layer) | Immersion | 50.5 | This work |
Using the immersion method, the P release profiles of the fertilizers coated with PPFEHEMA reached the equilibrium stage after 50 h, longer than those encapsulated with poly(butyl methacrylate (BMA)-co-PFEHEMA) (ca. 32 h). This copolymer was synthesized by emulsion copolymerization from an initial ratio of [BMA]0/[PFEHEMA]0 = 90/10.31 Indeed, the molar incorporation of PFEHEMA in the copolymer, assessed by elemental analysis, was close to 6.5%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 which is much lower than that of PFEHEMA units in the PPFEHEMA homopolymer (100%). The structure and hydrophobic properties of PFEHEMA homopolymer and P(BMA-co-PFEHEMA) copolymer coatings could explain the difference in their slow-release performance. In fact, PPFEHEMA (i.e., with a high PFEHEMA molar percentage) compared to that incorporated in poly(BMA-co-PFEHEMA) (only 6.5%) exhibits a higher hydrophobic character, as confirmed by WCA measurements, of close to 110° and 80°, respectively.31Table 6 also reveals that DAP coated with PPFEHEMA exhibits a slower P release than that covered with PBMA. The time to reach the maximum P release of the granule encapsulated with PPFEHEMA was 2.0 times lower than that of DAP coated with PBMA. The increasing hydrophobicity of the fluorinated homopolymer (WCA = 110°, Fig. S2†) compared to that of non-fluorinated PBMA (WCA = 74°)31 suggests a reduction in water diffusion and contributes to the slow release of P nutrient due to the presence of the fluorinated comonomer bringing about better water repellency attributed to the –C6F13 side groups.
31 which is much lower than that of PFEHEMA units in the PPFEHEMA homopolymer (100%). The structure and hydrophobic properties of PFEHEMA homopolymer and P(BMA-co-PFEHEMA) copolymer coatings could explain the difference in their slow-release performance. In fact, PPFEHEMA (i.e., with a high PFEHEMA molar percentage) compared to that incorporated in poly(BMA-co-PFEHEMA) (only 6.5%) exhibits a higher hydrophobic character, as confirmed by WCA measurements, of close to 110° and 80°, respectively.31Table 6 also reveals that DAP coated with PPFEHEMA exhibits a slower P release than that covered with PBMA. The time to reach the maximum P release of the granule encapsulated with PPFEHEMA was 2.0 times lower than that of DAP coated with PBMA. The increasing hydrophobicity of the fluorinated homopolymer (WCA = 110°, Fig. S2†) compared to that of non-fluorinated PBMA (WCA = 74°)31 suggests a reduction in water diffusion and contributes to the slow release of P nutrient due to the presence of the fluorinated comonomer bringing about better water repellency attributed to the –C6F13 side groups.
This comparison shows that the release properties of PPFEHEMA 2L lead to better results thanks to its fluorinated structure, which improves the slow release of nutrients and avoids the loss of nutrients and their negative impact on the environment when uncoated fertilizer is used. Therefore, the better bioavailability of N and P nutrients is better for plants.
3.4. Mathematical modeling of release kinetics
To confirm the above interpretations of the behavior of nutrient release in water and to describe the release kinetics and the nutrient transport mechanism through the polymer coatings, data curves (Fig. 6) were fitted following the semi-empirical Ritger–Peppas model55 (eqn (S1) in ESI†). More details about this model and the corresponding mechanisms based on the diffusional exponent (n) characterizing the release mechanism are supplied in ESI.†The diffusion exponent (n), correlation coefficient (R2) and release factor (k) of each coating system were calculated by plotting ln(Mt/M∞) versus ln(t). Fig. S5† exhibits some of the curves (according to eqn (S2) in ESI†) while Table 7 supplies the resulting data for N and P nutrient releases from both polymers corresponding to the first and second layers for the first release step before reaching equilibrium.
| Release exponent, na | Release factor, k × 102![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (h−1) a (h−1) |
Correlation coefficient (R2) | Release mechanism | |||
|---|---|---|---|---|---|---|
| a Assessed from eqn (S2) (ESI†). | ||||||
| DAP coated | PTFEMA (1L) | P | 0.96 | 57 | 0.98 | Non-Fickian |
| N | 0.65 | 65 | 0.99 | Non-Fickian | ||
| PPFEHEMA (1L) | P | 0.78 | 51 | 0.97 | Non-Fickian | |
| N | 1.30 | 34 | 0.97 | — | ||
| DAP coated | PTFEMA (2L) | P | 0.52 | 51 | 0.97 | Fickian |
| N | 1.28 | 46 | 0.97 | — | ||
| PPFEHEMA (2L) | P | 0.48 | 46 | 0.98 | Fickian | |
| N | 1.29 | 32 | 0.98 | — | ||
PTFEMA and PPFEHEMA with a double layer (2L) present a Fickian release mechanism for P nutrient as their n values (0.52 and 0.48) are close to 0.5. However, those corresponding to N nutrient are greater than 1, indicating that the diffusion occurred from the pores in the coatings, which gradually become modified by the diffusion process itself. Furthermore, both PTFEMA and PPFEHEMA with 2L display a non-Fickian release mechanism for P nutrient as their n values range between 0.5 and 1.0 while for those corresponding to N release, the n values are greater than 1.
It is also noted that the k release factor values of P and N nutrients decreased for both PTFEMA and PPFEHEMA polymers when the percentage of coating or the layer coating number increased and vice versa. For example, when the DAP was coated with PTFEMA, k value of P release decreased from 57 × 10−2 to 51 × 10−2 h−1 while the percentage of coating increased from 4.5% to 10.7% for PTFEMA 1L and PTFEMA 2L, respectively. Compared to the PTFEMA coating, PPFEHEMA exhibits slower N and P release profiles when using the same number of layers, and the k value of P release decreased from 51 × 10−2 to 46 × 10−2 h−1, while those of N release decreased from 46 × 10−2 to 32 × 10−2 h−1 for PTFEMA 2L to PTFEMA 2L, respectively (Table 7). This attests to the nutrient releases being slower when DAP was coated with PPFEHEMA. This comparison is in good agreement with the release rates of N and P coated with both fluorinated polymers versus time (Fig. 6). Indeed, according to the WCA measurements, fluorinated PPFEHEMA is more hydrophobic than PTFEMA and therefore the swelling content is lower than that of PTFEMA leading to a low release factor k value.
4. Conclusions
Poorly bioaccumulative fluorinated (meth)acrylic polymers were used as hydrophobic coating materials for granular water-soluble fast-acting fertilizers. PTFEMA and PPFEHEMA were successfully synthesized, characterized, and applied to DAP fertilizers. PPFEHEMA, with a highly hydrophobic character due to the presence of a large number of fluorine atoms on the side chain (–C6F13), shows a highly water repellent surface with good film-forming properties. The characterization of the surface and cross-section of the DAP coating as well as the release rates of N and P nutrients were investigated. The following conclusions can be drawn: (i) there is good adhesion between the granules and the coating films; (ii) the total N and P nutrient release time of the coated DAP could be controlled by adjusting the thickness of the PTFEMA and PPFEHEMA coatings (1L and 2L); (iii) compared to uncoated DAP granules, which are totally solubilized after less than 2 h, coated DAP with 2L of PPFEHEMA shows the slowest N and P nutrient releases, and the P release profile of the granules coated with PPFEHEMA 2L reached the equilibrium stage after approximately 50.5 h. The applied strategy is a promising technology allowed the very slow release and long-term availability of nutrient sources with highly hydrophobic coatings. Therefore, the proposed SFR coatings exhibit promising applications for the development of modern agriculture by improving nutrient uptake by plants, minimizing nutrient losses, and reducing environmental pollution.The kinetic release of N and P nutrients in the soil and agronomic studies are under investigation.
Author contributions
Asma Sofyane: methodology, investigation, writing – original draft. Salima Atlas: visualization, formal analysis, writing – review, and editing. Mohammed Lahcini: formal analysis, review, and editing. Elvira Vidović: validation, review, and editing. Bruno Ameduri: visualization, formal analysis, validation. Mustapha Raihane: conceptualization, methodology, supervision, project administration, funding acquisition, validation, writing – review, and editing. All authors have read and agreed to the published version of the manuscript.Data availability
Industries of commercially available coated fertilizers by polymers, mathematical modelling of release kinetics, FTIR spectra of TFEMA, PTFEMA, PFEHEMA and PPFEHEMA; water contact angle (WCA) of: (a) PTFEMA and (b) PPFEHEMA; elastic behavior of PPFEHEMA after stretching; thickness of DAP coated with PTFEMA and PPFEHEMA 1L and 2L and the corresponding coating weight percentage % (red color); nutrient saturation time of uncoated DAP and coated DAP using PTFEMA and PPFEHEMA 1L and 2L; example of release kinetic plots ln(Mt/M∞) versus ln(t) of coated DAP with PTFEMA and PPFEHEMA. All the data have been supplied in the ESI.†Data provided in the manuscript are directly associated with the article.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank to the support through the R&D Initiative – Call project APPHOS (project ID: VAL-RAI-01/2017) for supporting this work. We also thank the Erasmus+ program between Cadi-Ayyad University of Marrakech (Morocco) and Zagreb University (Croatia), and the Center of Analysis and Characterization of Cadi Ayyad University (CAC-CAU). Tosoh Finechemical Corp, Yamaguchi (Japan) is also acknowledged for the free gift of 2,2,2-trifluoroethyl methacrylate (TFEMA).References
- N. Alexandratos and J. Bruinsma, WORLD AGRICULTURE TOWARDS 2030/2050 The 2012 Revision, Food Agric. Organ. United Nations, 2012, (no. 12), p. 146, DOI:10.1016/S0264-8377(03)00047-4.
- K. Pawlak and M. Kołodziejczak, Sustainability, 2020, 12, 5488 CrossRef.
- C. M. Nascimento, W. de Sousa Mendes, N. E. Quiñonez Silvero, R. R. Poppiel, V. M. Sayão, A. C. Dotto, N. Valadares dos Santos, M. T. Accorsi Amorim and J. A. M. Demattê, J. Environ. Manage., 2021, 277, 111316 CrossRef CAS PubMed.
- M. C. DeRosa, C. Monreal, M. Schnitzer, R. Walsh and Y. Sultan, Nat. Nanotechnol., 2010, 5, 91–91 CrossRef CAS PubMed.
- Z. Chen, T. Liu, J. Dong, G. Chen, Z. Li, J. Zhou and Z. Chen, ACS Sustainable Chem. Eng., 2023, 11, 1–12 CrossRef CAS.
- S. Sair, S. Aboulhrouz, O. Amadine, I. Ayouch, I. Jioui, Y. Essamlali, K. Danoun, B. Ouadil and M. Zahouily, Eur. Polym. J., 2023, 199, 112477 CrossRef CAS.
- Y. Shang, Md. K. Hasan, G. J. Ahammed, M. Li, H. Yin and J. Zhou, Molecules, 2019, 24, 2558 CrossRef CAS PubMed.
- I. Ganetri, Y. Essamlali, O. Amadine, K. Danoun, S. Aboulhrouz and M. Zahouily, Controlling factors of slow or controlled-release fertilizers, Elsevier Inc., 2021 Search PubMed.
- T. El Assimi, O. Lakbita, A. El Meziane, M. Khouloud, A. Dahchour, R. Beniazza, R. Boulif, M. Raihane and M. Lahcini, Int. J. Biol. Macromol., 2020, 161, 492–502 CrossRef CAS PubMed.
- M. E. Trenkel, Slow- and Controlled Release and Stabilized Fertilizers in Agriculture. An Option for Enhancing Nutrient Use Efficiency in Agriculture, International Fertilizer Industry Association (IFA), Paris, 2nd edn, 2010 Search PubMed.
- S. M. Al-Zahrani, Ind. Eng. Chem. Res., 2000, 39, 367–371 CrossRef CAS.
- A. Jarosiewicz and M. Tomaszewska, J. Agric. Food Chem., 2003, 51, 413–417 CrossRef CAS PubMed.
- A. Olad, M. Pourkhiyabi, H. Gharekhani and F. Doustdar, Carbohydr. Polym., 2018, 190, 295–306 CrossRef CAS PubMed.
- A. Rashidzadeh and A. Olad, Carbohydr. Polym., 2014, 114, 269–278 CrossRef CAS PubMed.
- Z. Zhou, Y. Shen, C. Du, J. Zhou, Y. Qin and Y. Wu, Land Degrad. Dev., 2017, 28, 2370–2379 CrossRef.
- E. L. Krasnopeeva, G. G. Panova and A. V. Yakimansky, Int. J. Mol. Sci., 2022, 23, 15134 CrossRef CAS PubMed.
- S. Qian, F. Zhang, B. Liu, H. Ren and G. Tong, BioResources, 2017, 12(3), 6607–6617 CrossRef CAS.
- T. G. Liu, Y. T. Wang, J. Guo, T. B. Liu, X. Wang and B. Li, J. Appl. Polym. Sci., 2017, 134, 45175 CrossRef.
- J. Zhu, W. K. Tan, X. Song, Z. Zhang, Z. Gao, Y. Wen, C. N. Ong, S. Swarup and J. Li, ACS Food Sci. Technol., 2023, 3, 553–561 CrossRef CAS.
- P. Jumpapaeng, P. Suwanakood, S. Nanan and S. Saengsuwan, J. Ind. Eng. Chem., 2023, 127, 191–209 CrossRef CAS.
- W. Yao, Y. Li and X. Huang, Polymer, 2014, 55, 6197–6211 CrossRef CAS.
- D. W. Smith, S. T. Iacono and S. S. Iyer, Handbook of Fluoropolymer Science and Technology, New York, 2014 Search PubMed.
- B. Ameduri and H. Sawada, Fluorinated Polymers Applications, Cambridge, UK, 2017th edn, 2017, vol. 2 Search PubMed.
- R. Ishige, T. Shinohara, K. L. White, A. Meskini, M. Raihane, A. Takahara and B. Ameduri, Macromolecules, 2014, 47, 3860–3870 CrossRef CAS.
- G. Alessandrini, M. Aglietto, V. Castelvetro, F. Ciardelli, R. Peruzzi and L. Toniolo, J. Appl. Polym. Sci., 2000, 76, 962–977 CrossRef CAS.
- I. Yamamoto, in Fluorinated Polymers: Volume 2: Applications, ed. B. Ameduri, H. Sawada, B. Ameduri and H. Sawada, The Royal Society of Chemistry, 2016, vol. 2 Search PubMed.
- V. Castelvetro, M. Aglietto, F. Ciardelli, O. Chiantore, M. Lazzari and L. Toniolo, J. Coat. Technol., 2002, 74, 57–66 CrossRef CAS.
- V. Sabatini, E. Pargoletti, V. Comite, M. A. Ortenzi, P. Fermo, D. Gulotta and G. Cappelletti, Polymers, 2019, 11, 1190 CrossRef PubMed.
- Z. Gu, M. Zhang, J. He and P. Ni, Colloids Surf., A, 2016, 502, 159–167 CrossRef CAS.
- S. Chen, Y. Han, M. Yang, X. Zhu, C. Liu, H. Liu and H. Zou, Prog. Org. Coat., 2020, 149, 105964 CrossRef CAS.
- A. Sofyane, E. Ben Ayed, M. Lahcini, M. Khouloud, H. Kaddami, B. Ameduri, S. Boufi and M. Raihane, Eur. Polym. J., 2021, 156, 110598 CrossRef CAS.
- M. Stone, T. G. Nevell and J. Tsibouklis, Mater. Lett., 1998, 37, 102–105 CrossRef CAS.
- B. Ameduri, J. Sales and M. Schlipf, Int. Chem. Regul. Law Rev., 2023, 6, 18–28 Search PubMed.
- T. Shirai, H. Fukumoto, Y. Kanno, T. Kubota and T. Agou, Polymer, 2021, 217, 123478 CrossRef CAS.
- V. Kumar, J. Pulpytel and F. Arefi-Khonsari, Plasma Processes Polym., 2010, 7, 939–950 CrossRef CAS.
- Q. Zhang, Q. Wang, J. Jiang, X. Zhan and F. Chen, Langmuir, 2015, 31, 4752–4760 CrossRef CAS PubMed.
- A. Kadimi, H. Kaddami, Z. Ounaies, Y. Habibi, R. Dieden, B. Ameduri and M. Raihane, Polym. Chem., 2019, 10, 5507–5521 RSC.
- M. Raihane and B. Ameduri, J. Fluorine Chem., 2006, 127, 391–399 CrossRef CAS.
- Y. Liu, Y. Higaki, M. Mukai and A. Takahara, Polymer, 2019, 182, 121846 CrossRef.
- A. Sofyane, E. Ablouh, M. Lahcini, A. Elmeziane, M. Khouloud, H. Kaddami and M. Raihane, Mater. Today: Proc., 2021, 36, 74–81 CAS.
- E. Barbu, R. A. Pullin, P. Graham, P. Eaton, R. J. Ewen, J. D. Smart, T. G. Nevell and J. Tsibouklis, Polymer, 2002, 43, 1727–1734 CrossRef CAS.
- J. Tsibouklis, P. Graham, P. J. Eaton, J. R. Smith, T. G. Nevell, J. D. Smart and R. J. Ewen, Macromolecules, 2000, 33, 8460–8465 CrossRef CAS.
- Y. Xu, W. Wang, Y. Wang, J. Zhu, D. Uhrig, X. Lu, J. K. Keum, J. W. Mays and K. Hong, Polym. Chem., 2016, 7, 680–688 RSC.
- R. W. Phillips and R. H. Dettre, J. Colloid Interface Sci., 1976, 56, 251–254 CrossRef CAS.
- M. Karamane, M. Raihane, M. A. Tasdelen, T. Uyar, M. Lahcini, M. Ilsouk and Y. Yagci, J. Polym. Sci., Part A: Polym. Chem., 2017, 55, 411–418 CrossRef CAS.
- V. Castelvetro, M. Raihane, S. Bianchi, S. Atlas and I. Bonaduce, Polym. Degrad. Stab., 2011, 96, 204–211 CrossRef CAS.
- B. Ameduri, Well Architectured Fluoropolymers: Synthesis, Properties and Applications, Elsevier, Amsterdam, 2004 Search PubMed.
- A. Sofyane, M. Lahcini, A. El Meziane, M. Khouloud, A. Dahchour, S. Caillol and M. Raihane, J. Am. Oil Chem. Soc., 2020, 97, 751–763 CrossRef CAS.
- E. H. Boutriouia, T. El Assimi, M. Raihane, R. Beniazza, H. Ben Youcef, M. Khouloud, M. H. V. Baouab, A. El Kadib and M. Lahcini, Prog. Org. Coat., 2022, 172, 107102 CrossRef CAS.
- M. Devassine, F. Henry, P. Guerin and X. Briand, Int. J. Pharm., 2002, 242, 399–404 CrossRef CAS PubMed.
- S. K. Yadavalli, M. Chen, M. Hu, Z. Dai, Y. Zhou and N. P. Padture, Scr. Mater., 2020, 187, 88–92 CrossRef CAS.
- T. Li, S. Lü, Y. Ji, T. Qi and M. Liu, New J. Chem., 2018, 42, 19129–19136 RSC.
- E. I. Pereira, F. B. Minussi, C. C. T. da Cruz, A. C. C. Bernardi and C. Ribeiro, J. Agric. Food Chem., 2012, 60, 5267–5272 CrossRef CAS PubMed.
- D. F. da Cruz, R. Bortoletto-Santos, G. G. F. Guimarães, W. L. Polito and C. Ribeiro, J. Agric. Food Chem., 2017, 65, 5890–5895 CrossRef CAS PubMed.
- P. L. Ritger and N. A. Peppas, J. Controlled Release, 1987, 5, 23–36 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4py00573b |
| This journal is © The Royal Society of Chemistry 2024 |