Open Access Article
Open Access ArticleCrosslinked siloxane–silsesquioxane elastomer with pyrene functionalization for rapid adsorption of benzene, toluene, and xylene (BTX) from water and sensing of charged species†
Teeraya
Bureerug
 a,
Chidchanok
Wannasiri
a,
Supphachok
Chanmungkalakul
a,
Chidchanok
Wannasiri
a,
Supphachok
Chanmungkalakul
 a,
Mongkol
Sukwattanasinitt
b,
Vuthichai
Ervithayasuporn
a,
Mongkol
Sukwattanasinitt
b,
Vuthichai
Ervithayasuporn
 *a and
Thanthapatra
Bunchuay
*a and
Thanthapatra
Bunchuay
 *a
*a
aDepartment of Chemistry, Center of Excellence for Innovation in Chemistry (PERCH-CIC), Faculty of Science, Mahidol University, Rama VI Road, Ratchathewi, Bangkok 10400, Thailand. E-mail: thanthapatra.bun@mahidol.edu; thanthapatra.bun@mahidol.ac.th
bDepartment of Chemistry, Faculty of Science, Chulalongkorn University, Bangkok, 10330, Thailand
First published on 5th July 2024
Abstract
A crosslinked siloxane/silsesquioxane elastomer with pyrene functionalization (Py-CSSE) was rapidly synthesised within 10 minutes via a one-pot multicomponent anionic ring-opening polymerization between octamethylcyclotetrasiloxane (methyl D4), octavinylsilsesquioxane (OVS) and a mono-pyrene functionalized SQ cage (Mono-PySQ). The structural characterization of Py-CSSE was conducted using MAS solid-state 29Si and 13C NMR, FTIR, and PXRD techniques. Furthermore, an examination of the mechanical and thermal characteristics was conducted using TGA, DSC, and compressive tests. Py-CSSE demonstrated significant elasticity (a strain of 60% and a pressure of 0.5 MPa) and quickly returned to its former shape without any cracking, even when subjected to a pressure range of up to 40% strain for 3 cycles. The pyrenyl moieties permitted a strong blue fluorescence emission in both the solid-state and in suspension in an organic solvent. The presence of silsesquioxanes, siloxanes, and pyrene moieties concomitantly facilitated its use in cation (Cu2+) and anion (F− and CN−) sensing in polar organic solvents. Importantly, Py-CSSE exhibited efficient adsorption of benzene, toluene, and xylene (BTX) by showing a remarkably high adsorption capacity in the range 28–34 mmol g−1. Py-CSSE exhibited ultrafast adsorption of BTX by showing complete removal of o-xylene within 90 seconds. Furthermore, Py-CSSE surpassed other porous materials in terms of BTX adsorption performance and showed exceptional recyclability for BTX removal, maintaining adsorption effectiveness for at least 5 cycles.
Introduction
Over the past few decades, rapid urbanization and industrialization have contributed to serious environmental problems such as water pollution. Aromatic hydrocarbons, particularly benzene, toluene, and xylene (BTX), are a major class of chemical contaminants as a result of their toxicity and carcinogenicity.1,2 Exposure to these compounds in even small amounts can cause adverse effects on both land-based and aquatic ecosystems. BTX water pollution is mostly caused by petroleum leakages from underground storage tanks, urban garbage, and the chemical industry. Various conventional methods can be used to remove BTX from water and wastewater, including adsorption, aeration, biological oxidation, and chemical oxidation.3,4 Undeniably, chemical adsorption utilising porous materials is a powerful and widely employed technique in practical applications.5–8Silica, or SiO2, makes up a significant proportion of the Earth's crust, accounting for approximately 60% of its composition. Because it is inexpensive and readily available, silica is commonly used as a chemical adsorbent and as a stationary phase in column chromatography.9,10 However, the strong Si–O covalent bonds make it insoluble in both common organic solvents as well as water, providing difficulties for mechanistic investigations of chemical reactions occurring on the silica surfaces and at the silicon center.11,12 As an alternative, attention has turned to employing silsesquioxanes (SQs), hybrid organic–inorganic silicon materials, as representative models for silica. The core structure of SQs comprises inorganic Si–O–Si units that are chemically functionalized with diverse organic residues (R groups), enabling subsequent modifications through various organic reactions.13–16 Furthermore, SQ-based materials with highly symmetrical cage-type structures, such as polyhedral oligomeric silsesquioxanes (POSSs), have shown enhanced physico-chemical characteristics compared to other silicon-based materials and have become promising contenders for various applications, for example, POSSs have been utilized as fillers in composite materials as a result of their nano-sized cage structures, thermally robust and elastic behavior,17–19 flame retardancy,20,21 and mechanical strength.22,23 These features have highlighted POSSs as potential high-performance electrode materials for supercapacitors24 and lithium-ion batteries.25–27 In addition, POSSs can be utilized to augment the hydrophobic properties and regulate the swelling capacity of hydrogels in the field of biomedical and coating applications.28–32
Recently, our group reported an ultrafast synthesis of a superhydrophobic SQ-based elastomer from the base catalysed condensation reaction of a cyclic siloxane (methyl D4) with octavinylsilsesquioxane (OVS).33 The reaction reached completion in a time span of only 15 minutes, resulting in the formation of homogeneous micro-ring elastomers that had exceptional mechanical properties and exhibited oil and non-polar solvent adsorption. The hydrophobicity of the elastomer presumably derived from the alkyl moieties appended on the silicon centers of both methyl D4 and OVS. In addition, functionalization of the POSS with the electron-withdrawing pyrenyl and anthracenyl fluorophores resulted in the creation of electron deficient silicon centers that showed strong affinity for the recognition and detection of fluoride, cyanide, and hydrogen phosphate in polar organic solvents.34–37 Importantly, the excimer emission mode of these fluorophores can be modulated in the presence of guest molecules, enabling their utility in detection of diverse chemical species including gases, organic compounds, and ions. Moreover, its high electron mobility and efficient light emission make it a promising candidate for fluorescent sensors.38 Taking inspiration from these previous results, our group aims to apply this established synthetic methodology to incorporate fractions of organic fluorophores into the elastomer in order to prepare fluorescent silicon-based hydrophobic elastomers capable of adsorption and sensing applications. A typical procedure utilized for retaining high elasticity and tunable photophysical and mechanical properties is the covalent cross-linking of fluorophores to elastomers.39 For instance, Craig and co-workers synthesised stress-responsive polysiloxanes by treating mechanoacid diene and rhodamine dye derivatives with poly(dimethylsiloxane) (PDMS).40 Brook and co-workers synthesised luminous silicon elastomers by coupling NH2-PDMS-NH2 with an aldehyde-modified tetraphenylene derivative via the imine condensation reaction.41
Herein, we present a one-pot synthetic methodology for preparing a fluorescent pyrene functionalized silicon-based hydrophobic elastomer (Py-CSSE) via the base catalysed anionic ring-opening polymerization reaction of three chemical components including methyl D4, OVS, and the mono-pyrene substituted OVS (Mono-PySQ).42 Under the specific reaction conditions, the crosslinked fluorescent elastomeric products were obtained within 10 minutes with the outstanding yield of 83%. The presence of pyrenyl substituents significantly modulated the chemical properties of the resulting materials for chemical adsorption applications and also turned-on fluorescent properties for sensing. Py-CSSE exhibited outstanding performance in BTX adsorption by showing adsorption capacities for benzene, toluene, o-xylene, m-xylene, and p-xylene of 2.65, 2.98, 3.23, 3.07, and 3.03 g g−1, respectively, outperforming previously reported adsorbents for BTX adsorption (Table S4†). In addition to chemical adsorption, Py-CSSE displayed fluorescent sensing properties towards dual detection of both Cu(II) and F− in polar organic solvents, enabling it to function as a hybrid adsorbent and fluorescent sensing material simultaneously.
Experimental
Chemicals
Octavinylsilsesquioxane (OVS) was prepared according to the literature report.43 Commercially available compounds including 1-bromopyrene, triphenylphosphine (PPh3), triethylamine (NEt3), tetrabutylammonium salts, all metal perchlorate salts, Pd(OAc)2, EDTA and octamethylcyclotetrasiloxane (methyl D4) were purchased from TCI chemicals and Sigma-Aldrich and used subsequently without further purification. The commercial-grade solvents including DCM, acetone, hexanes, ethyl acetate, and methanol were distilled prior to use. The analytical grade solvents including THF, toluene, isopropanol and DMF were purchased from RCI-Labscan and used without further purification. Deionized (DI) water was obtained from Ultra Clear SIEMENS under ASTM type 1 and 2.Synthesis
| Adsorption capacity (q) = (Ww − Wd)/Wd | (1) |
Time-dependent adsorption of Py-CSSE was investigated by using o-xylene and DI water as representative non-polar and polar solvents, respectively. A cube of Py-CSSE was added into a mixture of o-xylene (1.0 mL) and DI water (0.5 mL) and the adsorption capacities were calculated at different adsorption times ranging from 15 to 30, 45, 60, and 90 seconds. The maximum adsorption capacity was determined by varying the volume of o-xylene (0.25, 0.50, 0.75, 1.0, and 1.25 mL) in 0.5 mL of DI water and the adsorption capacity of each experimental set was calculated at the adsorption time of 90 seconds.
The recyclability of Py-CSSE was studied by adding the material into a mixture of o-xylene (1.0 mL) and DI water (0.5 mL). The adsorption process was performed for 90 seconds and the adsorption capacity was calculated. In the desorption process, the absorbed liquid was squeezed from the Py-CSSE and the Py-CSSE was dried at room temperature for 30 minutes before the next cycle.
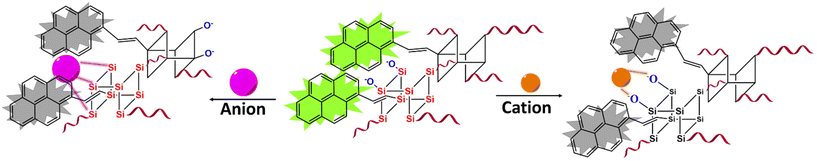
Fluoride and cyanide adsorption and recyclability of Py-CSSE
Anion adsorption experiments with Py-CSSE were carried out using fluoride and cyanide as model anions in this study. In the case of fluoride adsorption, Py-CSSE (10 mg) was suspended in THF (2 mL) and a solution of TBAF (0.96 mM) was added subsequently into the suspension. Each solution was collected after adsorption times of 5, 15, 30, 45, and 60 minutes by filtering the Py-CSSE residues off from each solution and the remaining solution was collected to determine the amount of fluoride.In order to determine the concentrations of the remaining fluoride, we used our previously reported hydrazone sensor for the spectrophotometric analysis of fluoride. First of all, a calibration curve was constructed by preparing a series of TBAF solutions in THF with concentrations of 0.2, 0.4, 0.8, 1.2, and 1.6 mM. Each TBAF solution (0.02 mL) was added subsequently into solutions of the sensor (2 mL, 2 × 10−5 M). The signals obtained from each absorption spectrum were plotted as a function of fluoride concentration to create a calibration curve. The sampling solutions collected from all samples were added into a vial containing the solution of the sensor (2 mL, 2 × 10−5 M). The absorption values were collected and the concentrations of fluoride were calculated using a linear equation obtained from the calibration curve. The removal efficiency (%RE) was calculated using the following eqn (2):
| %RE = (C0 − Ce)/C0 × 100 | (2) |
Results and discussion
Synthesis and characterization
Py-CSSE was synthesised from OVS (1 eq.), methyl D4 (6 eq.), and Mono-PySQ (2 mol%) via anionic ring-opening polymerization in the presence of a base (Scheme 1). The unreacted starting materials were removed by stepwise washing with solvents to afford Py-CSSE as the solid product in 83% yield. Py-CSSE was insoluble in organic solvents ranging from non-polar to polar solvents, which is in stark contrast to its synthetic precursors, confirming the successful crosslinking polymerization reaction.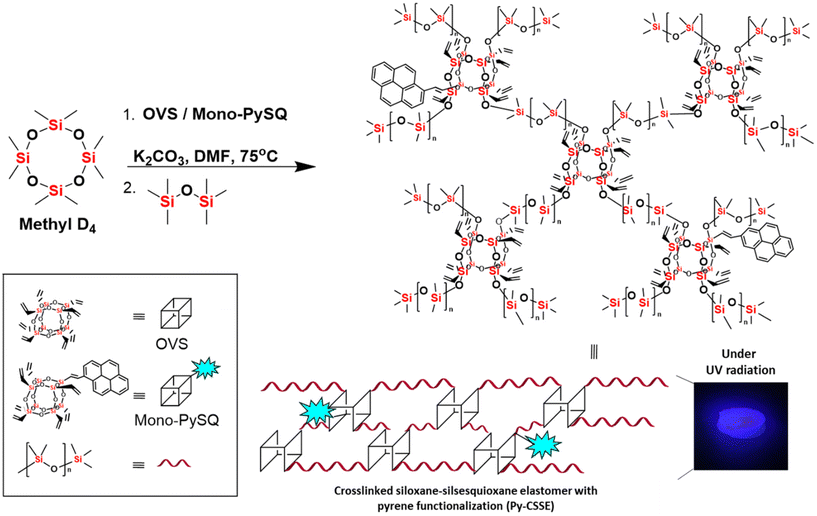 | ||
| Scheme 1 The synthesis of a crosslinked siloxane–silsesquioxane elastomer with pyrene functionalization (Py-CSSE). | ||
The MAS solid-state 29Si-NMR spectrum of Py-CSSE revealed a distinct silicon signal at −80 ppm, a characteristic silicon peak of the T3 species (Tn: CSi(OSi)n(OH)3−n) derived from the silsesquioxane cage. Furthermore, the spectrum also shows a sharp signal around −20 ppm suggesting the presence of the D2 species (Dn: (CH3)4−nSi(O1/2)n) derived from the polysiloxane chains. The shoulder signal at around −18 ppm potentially could be attributed to a signal of the D structures having different distance distributions to the T structures.44–49 The MAS solid-state 13C-NMR spectrum of Py-CSSE exhibits a signal at 0.9 ppm, corresponding to the sp3 methyl carbon of siloxane chains. The spectrum also showed broad multiple signals around 132–134 ppm, corresponding to sp2 carbons derived from the abundant vinyl groups of OVS (Fig. 1b). Importantly, the signals associated with pyrene are absent from the spectrum. This is due to the limited sensitivity of the MAS solid-state 13C-NMR technique, which inhibited the detection of signals resulting from the small amount of Mono-PySQ introduced into Py-CSSE (Fig. S2†).
 | ||
| Fig. 1 The MAS solid-state (a) 29Si-NMR spectrum and (b) 13C-NMR spectrum of Py-CSSE. (c and d) Comparative FTIR spectra of Mono-PySQ (blue), CSSE (orange) and Py-CSSE (black) samples. | ||
The FTIR spectrum of Py-CSSE displays the characteristics of a crosslinked siloxane–silsesquioxane elastomer (CSSE) and Mono-PySQ (Fig. 1c) by showing prominent vibrational peaks at 1072 cm−1 and 793 cm−1, which correspond to the stretching and bending frequencies of Si–O–Si, respectively. The results unequivocally verified the existence of Si moieties inside the polymeric network.50,51 The signal observed at a wavenumber of 968 cm−1 is attributed to the bending frequency of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in vinyl groups with a pyrene substituent. The absorption signal centred at 3023 cm−1 provides evidence for the existence of aromatic pyrenyl groups derived from Mono-PySQ and the intensity of the signal strongly correlates with the amount of Mono-PySQ additive, and the signal that emerges at 3064 cm−1 is attributed to vibrational stretching of C–H vinyl moieties.52,53 The susceptibility of POSS derivatives to bases may lead to a cage structure transformation into a partially-open cage structure.54–56 This was confirmed by the presence of weak and broad signals of O–H stretching in the range of 3200–3500 cm−1 (Fig. 1d and Table S1†).
C bond in vinyl groups with a pyrene substituent. The absorption signal centred at 3023 cm−1 provides evidence for the existence of aromatic pyrenyl groups derived from Mono-PySQ and the intensity of the signal strongly correlates with the amount of Mono-PySQ additive, and the signal that emerges at 3064 cm−1 is attributed to vibrational stretching of C–H vinyl moieties.52,53 The susceptibility of POSS derivatives to bases may lead to a cage structure transformation into a partially-open cage structure.54–56 This was confirmed by the presence of weak and broad signals of O–H stretching in the range of 3200–3500 cm−1 (Fig. 1d and Table S1†).
The crosslinking polymerization significantly changed the crystalline structure of OVS, from a well-defined structure to an amorphous solid, as determined by X-ray diffraction analysis.57Py-CSSE exhibited only two broad signals at 2θ = 11° and 22° (Fig. S4†). According to Bragg's Law, the d1-spacing for 2θ = 11° was approximately 0.81 nm, while the d2-spacing for 2θ = 22° was 0.41 nm, representing the distance between the Si–O–Si portions of the siloxane chains as amorphous silicone rubber and amorphous cluster, respectively.23,58 In addition, the d1-spacing of Py-CSSE is slightly shorter than that of CSSE (0.98 nm), indicating the strong interactions between each POSS layer as a result of stacking of pyrenyl moieties.
Thermogravimetric analysis (TGA) of Py-CSSE identified a weight loss occurring in two stages. Under a N2 atmosphere, an initial weight loss at 300 °C ascribed to the decomposition of organic constituents was observed. The second stage of weight loss took place within the temperature range of 487–531 °C, which can be explained by the decomposition of the Si–O inorganic core structure (Fig. S7†). Importantly, Py-CSSE exhibited superior thermal stability in comparison to OVS, which decomposed at approximately 274–303 °C (Fig. S5†).59 Under an O2 atmosphere, Py-CSSE was completely decomposed at 375 °C and gave the resulting ceramic yield of 78%, slightly higher than that of OVS itself (70%). This can be explained by the higher Si portions in Py-CSSE as a result of the extra siloxane chains from the methyl D4 monomer (Fig. S6 and S8†). The DSC analysis of Py-CSSE revealed Tg and Tm values at 179 °C and 317 °C, respectively, indicating that Py-CSSE changes from an amorphous solid to a rubbery state at 179 °C and is stable up to 300 °C (Fig. S9†).60
The morphology of Py-CSSE was studied via Field Emission Scanning Electron Microscopy (FESEM). The low magnification SEM image revealed the aggregation of microrod structures with a smooth surface. In addition, a uniform coral-like structure was observed in the higher magnification FESEM image (Fig. 2a and S10†). The micro-structures of Py-CSSE bear a resemblance to the hairy-structural surface of lotus leaves that contributes to their hydrophobic nature (Fig. 2b). Surprisingly, the contact angle of Py-CSSE is 114° (Fig. 2c) while CSSE, the previously reported analogous elastomer without the pyrenyl substituents, exhibited a contact angle value of 151°.61,62 This result is the opposite of our hypothesis that the addition of pyrenyl groups would increase the hydrophobicity of Py-CSSE. Py-CSSE demonstrates a shorter D1-spacing than CSSE (0.81 nm vs. 0.98 nm), indicating that the incorporation of pyrenyl moieties into Py-CSSE probably prevents the formation of long siloxane chains between each silsesquioxane unit during polymerization and potentially causes a collapsed structure as a result of the self-assembly disorder, resulting in a lower hydrophobicity.63 The energy dispersive X-ray (EDX) pattern showed the presence of Si, O, and C atoms which can imply the presence of both the SQ cage and pyrene aromatic ring within the polymeric networks (Fig. S11†).
 | ||
| Fig. 2 (a and b) FESEM images of a dried Py-CSSE. (c) The contact angle and wettability measurement of Py-CSSE. | ||
The determination of compressive strength was conducted in order to evaluate the mechanical properties of Py-CSSE. The observation that the maximum compressive strength of the sample increased up to 60% strain indicates that Py-CSSE is quite flexible presumably influenced by the presence of siloxane chains (Fig. S12b†).64,65 Furthermore, it exhibited notable flexibility without experiencing any cracking during three compressive tests conducted under an applied stress of 40% strain (Fig. S12c†).66,67
Photophysical properties of Py-CSSE
The solid-state fluorescence emission properties of Py-CSSE were preliminarily inspected under UV-light. Only Py-CSSE and Mono-PySQ exhibited fluorescence emission while OVS and CSSE did not show such a response. Subsequently, Py-CSSE was suspended in polar organic solvents including THF, DMF and DMSO and excited at wavelengths of 355, 360, and 370 nm, respectively (Fig. 3b). Py-CSSE showed a similar pattern of emission spectra which have emission signals of the monomeric pyrene centered at the wavelength around 397 nm and those of the excimeric pyrene centered at the wavelengths around 419 and 443 nm.68,69 Notably, it is obviously observed that the ratio of the emission intensity between the pyrene excimer and monomer (Iex/Imo) increased when the concentration of Py-CSSE increased. This observation suggests that the excimer formation of pyrene becomes more prevalent at high concentration due to the closer proximity of molecules (Fig. S13 and Table S2†).70 In addition, pyrene is non-polar and likely to aggregate in high polarity solvents.36Adsorption efficiency of Py-CSSE
Due to the remarkable hydrophobic properties of Py-CSSE, we proceeded to conduct more challenging studies on the adsorption of benzene, toluene, and xylenes (BTX). First of all, a cube-shaped piece of Py-CSSE (1 × 1 × 1 cm3) was immersed in an aqueous solution of rhodamine B dye that was layered with the organic solvent of interest (Fig. 4a). Py-CSSE demonstrated a maximum adsorption capacity of 34.02, 32.34, 30.46, 28.98, and 28.56 mmol g−1 for benzene, toluene, o-xylene, m-xylene, and p-xylene, respectively (Fig. 4c). Interestingly, despite showing less hydrophobicity than CSSE, Py-CSSE showed higher solvent and BTX adsorption capacities, highlighting the benefit of having the pyrenyl moieties within the material matrix.
Furthermore, the kinetics of o-xylene adsorption in the presence of DI water were also studied. Py-CSSE was exposed to a mixture of o-xylene and DI water, and the percentage of swelling was measured at several intervals in the adsorption experiments, specifically 15, 30, 45, 60, and 90 seconds. The results indicate that only o-xylene was absorbed, and the adsorption nearly reached saturation during the initial 30 seconds and almost complete (∼100%) removal of BTX within a timeframe of 90 seconds (Fig. 4a and S15†). Py-CSSE demonstrated a maximum adsorption capacity for o-xylene of 3.23 g g−1 or 30.46 mmol g−1. Additionally, it displayed a swelling percentage of 315% after attaining adsorption saturation (Fig. 5a). These results demonstrate that Py-CSSE had exceptional performance in BTX adsorption compared to other inorganic adsorbents and porous materials (i.e. SBA-15) previously documented (Table S4†). In order to be suitable for practical industrial use, it is necessary for the adsorbent to be capable of being recycled. We conducted multiple dynamic breakthrough experiments using the Py-CSSE material to study the adsorption of the mixture of o-xylene and DI water. The adsorption process lasted for 90 seconds, after which the sample was dried at room temperature for approximately 30 minutes. The recycling studies demonstrated consistent levels of both adsorption efficiency and quantity over five adsorption–desorption cycles (Fig. 5b).
In addition to anion sensing, the fluorescent cation sensing properties of Py-CSSE were explored in relation to specific transition metals, namely Cr2+, Mn2+, Fe2+, Co2+, Cu2+, Zn2+, and Cd2+ in the form of their perchlorate salts in THF. The presence of Cu2+ caused a substantial suppression of the fluorescence signal of Py-CSSE, as confirmed by both naked-eye observation and epifluorescent microscope images (Fig. 6b, d and S28†). According to previous reports on fluorescence sensors based on the pyrene fluorophore, fluorescence quenching is caused by the paramagnetic nature of Cu2+ and ligand-to-metal charge transfer (LMCT) processes that are highly selective and sensitive to Cu2+, particularly with ligands containing nitrogen and oxygen donor atoms.74 Furthermore, Cu2+ sensing yielded LOD and LOQ values of 2.72 and 8.23 nM, respectively (Table S5†). In order to examine the kinetics of Cu2+ adsorption, variations in fluorescence intensity in THF were monitored. The signals of fluorescence remained relatively constant after 15 minutes. Furthermore, the pseudo-first order kinetic rate constant was determined to be 5.1 × 10−3 s−1 (Fig. 6g inset and Table S6†).
Conclusions
A crosslinked siloxane/silsesquioxane elastomer with pyrene functionalization (Py-CSSE), a novel fluorescent elastomer, was effectively synthesised through the base catalysed one-pot anionic ring-opening polymerization affording the target elastomeric product in a high yield within 10 minutes. Py-CSSE displayed chemical characteristics similar to those of CSSE and Mono-PySQ confirmed by a suite spectroscopic techniques such as FTIR and MAS-NMR. In addition, Py-CSSE demonstrated significant elasticity and can quickly return to its initial shape without any cracking, even when subjected to a pressure range of up to 40% strain for 3 cycles. The solid state and suspension in organic solvent of Py-CSSE displayed intense fluorescence emissions, which can be attributed to the pre-functionalization of pyrene molecules as the fluorophore in this crosslinking elastomer. The simultaneous presence of silsesquioxane cages, siloxanes, and pyrene moieties in Py-CSSE permitted its use as a dual sensor for anions (F− and CN−) and cations (Cu2+) in polar organic media. Furthermore, Py-CSSE could also display a capability for cation and anion adsorption. Importantly, it is noteworthy that Py-CSSE exhibited a notable adsorption efficiency towards benzene, toluene, and xylenes (BTX) and can function as an adsorbent for solvent separation more than five times. Its distinctive structural attributes render it highly suitable for tackling obstacles encountered in the petrochemical sector, environmental remediation, and other analogous implementations.Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Data availability
Data for this article, including NMR, IR, SEM, XPS, compression testing, XRD, and adsorption data, are available at Open Science Framework at https://doi.org/10.17605/OSF.IO/RKB7N.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project was funded by the National Research Council of Thailand (NRCT) and Mahidol University (Basic Research Fund: fiscal year 2023). T. B. and V. E. thank the Center of Excellence for Innovation in Chemistry (PERCH-CIC), Ministry of Higher Education, Science, Research and Innovation and the Office of the Permanent Secretary, Ministry of Higher Education, Science, Research and Innovation (Grant No. RGNS 65-150) for financial support. M. S. is thankful for funding from the National Research Council of Thailand (NRCT, N42A661000).References
- M. K. S. Nascimento, S. Loureiro, M. R. d. R. Souza, M. d. R. Alexandre and J. Nilin, Mar. Pollut. Bull., 2020, 156, 111272 CrossRef CAS PubMed.
- A. Mirzaei, J.-H. Kim, H. W. Kim and S. S. Kim, J. Mater. Chem. C, 2018, 6, 4342–4370 RSC.
- J. C.-S. Wu, Z.-A. Lin, F.-M. Tsai and J.-W. Pan, Catal. Today, 2000, 63, 419–426 CrossRef CAS.
- E. R. L. Tiburtius, P. Peralta-Zamora and A. Emmel, J. Hazard. Mater., 2005, 126, 86–90 CrossRef CAS PubMed.
- A. Torabian, H. Kazemian, L. Seifi, G. N. Bidhendi, A. A. Azimi and S. K. Ghadiri, Clean: Soil, Air, Water, 2010, 38, 77–83 CAS.
- S. Štandeker, Z. Novak and Ž. Knez, J. Hazard. Mater., 2009, 165, 1114–1118 CrossRef PubMed.
- A. Aleghafouri, N. Hasanzadeh, M. Mahdyarfar, A. SeifKordi, S. M. Mahdavi and A. T. Zoghi, J. Nat. Gas Sci. Eng., 2015, 22, 618–624 CrossRef CAS.
- G. Hayase, K. Kanamori, M. Fukuchi, H. Kaji and K. Nakanishi, Angew. Chem., Int. Ed., 2013, 52, 1986–1989 CrossRef CAS PubMed.
- B. Buszewski, R. M. Gadzała-Kopciuch, M. Markuszewski and R. Kaliszan, Anal. Chem., 1997, 69, 3277–3284 CrossRef CAS.
- H. Qiu, X. Liang, M. Sun and S. Jiang, Anal. Bioanal. Chem., 2011, 399, 3307–3322 CrossRef CAS PubMed.
- V. Lenher and H. B. Merrill, J. Am. Chem. Soc., 1917, 39, 2630–2638 CrossRef CAS.
- L. Spitzmüller, F. Nitschke, B. Rudolph, J. Berson, T. Schimmel and T. Kohl, J. Nanopart. Res., 2023, 25, 40 CrossRef.
- J. R. Jagannathan, K. Targos and A. K. Franz, Angew. Chem., 2022, 134, e202110417 CrossRef.
- T. Jaroentomeechai, P.-k. Yingsukkamol, C. Phurat, E. Somsook, T. Osotchan and V. Ervithayasuporn, Inorg. Chem., 2012, 51, 12266–12272 CrossRef CAS PubMed.
- J. Kaźmierczak, D. Lewandowski and G. Hreczycho, Inorg. Chem., 2020, 59, 9206–9214 CrossRef PubMed.
- M. G. Mohamed and S.-W. Kuo, Soft Matter, 2022, 18, 5535–5561 RSC.
- T. Hamada, S. Takase, A. Tanaka, K. Okada, S. Mineoi, A. Uedono and J. Ohshita, ACS Appl. Polym. Mater., 2022, 5, 743–750 CrossRef.
- T. Hamada, Y. Nakanishi, K. Okada, S. Tsukada, A. Uedono and J. Ohshita, ACS Appl. Polym. Mater., 2021, 3, 3383–3391 CrossRef CAS.
- X. Chen, L. Li, T. Wei, D. C. Venerus and J. M. Torkelson, ACS Appl. Mater. Interfaces, 2018, 11, 2398–2407 CrossRef PubMed.
- S. Bourbigot, T. Turf, S. Bellayer and S. Duquesne, Polym. Degrad. Stab., 2009, 94, 1230–1237 CrossRef CAS.
- W. Zhang, G. Camino and R. Yang, Prog. Polym. Sci., 2017, 67, 77–125 CrossRef CAS.
- H. Chen, S. Wei, R. Wang and M. Zhu, ACS Biomater. Sci. Eng., 2021, 7, 1428–1437 CrossRef CAS PubMed.
- S. O. Hwang, J. Y. Lee and J.-H. Lee, Prog. Org. Coat., 2019, 137, 105316 CrossRef CAS.
- M. Ejaz, M. M. Samy, Y. Ye, S.-W. Kuo and M. G. Mohamed, Int. J. Mol. Sci., 2023, 24, 2501 CrossRef CAS PubMed.
- T. K. L. Nguyen, T. N. Phan, F. Cousin, D. Devaux, S. Mehan, F. Ziarelli, S. Viel, D. Gigmes, P. Soudant and R. Bouchet, Chem. Mater., 2022, 34, 6944–6957 CrossRef CAS.
- R. Yanagisawa, H. Endo, M. Unno, H. Morimoto and S.-i. Tobishima, J. Power Sources, 2014, 266, 232–240 CrossRef CAS.
- H. Zhang, S. Kulkarni and S. L. Wunder, J. Phys. Chem. B, 2007, 111, 3583–3590 CrossRef CAS PubMed.
- P. K. Behera, P. Mondal and N. K. Singha, Macromolecules, 2018, 51, 4770–4781 CrossRef CAS.
- Z. Zhao, Y. Ma, P. Ju, Y. Wu, L. Chen, H. Zhou and J. Chen, ACS Appl. Mater. Interfaces, 2022, 14, 36105–36115 CrossRef CAS PubMed.
- R. Y. Kannan, H. J. Salacinski, P. E. Butler and A. M. Seifalian, Acc. Chem. Res., 2005, 38, 879–884 CrossRef CAS PubMed.
- A. Birault, E. Molina, G. Toquer, P. Lacroix-Desmazes, N. Marcotte, C. Carcel, M. Katouli, J. R. Bartlett, C. Gerardin and M. W. C. Man, ACS Appl. Bio Mater., 2018, 1, 1787–1792 CrossRef CAS PubMed.
- T. Shimizu, K. Kanamori and K. Nakanishi, Chem. – Eur. J., 2017, 23, 5176–5187 CrossRef CAS PubMed.
- C. Wannasiri, S. Chanmungkalakul, T. Bureerug, M. Sukwattanasinitt and V. Ervithayasuporn, Chem. Commun., 2023, 59, 5471–5474 RSC.
- C. Wannasiri, S. Chanmungkalakul, T. Bunchuay, L. Chuenchom, K. Uraisin, V. Ervithayasuporn and S. Kiatkamjornwong, ACS Appl. Polym. Mater., 2020, 2, 1244–1255 CrossRef CAS.
- S. Chanmungkalakul, V. Ervithayasuporn, P. Boonkitti, A. Phuekphong, N. Prigyai, S. Kladsomboon and S. Kiatkamjornwong, Chem. Sci., 2018, 9, 7753–7765 RSC.
- S. Chanmungkalakul, V. Ervithayasuporn, S. Hanprasit, M. Masik, N. Prigyai and S. Kiatkamjornwong, Chem. Commun., 2017, 53, 12108–12111 RSC.
- Z. Gou, X. Zhang, Y. Zuo, M. Tian, B. Dong, Y. Tang and W. Lin, Sens. Actuators, B, 2021, 338, 129837 CrossRef CAS.
- P. Beyazkilic, A. Yildirim and M. Bayindir, ACS Appl. Mater. Interfaces, 2014, 6, 4997–5004 CrossRef CAS PubMed.
- Q. Wang, M. Unno and H. Liu, J. Mater. Chem. A, 2024, 12, 5679–5691 RSC.
- Y. Lin, T. B. Kouznetsova and S. L. Craig, J. Am. Chem. Soc., 2019, 142, 99–103 CrossRef PubMed.
- A. S. Fawcett, T. C. Hughes, L. Zepeda-Velazquez and M. A. Brook, Macromolecules, 2015, 48, 6499–6507 CrossRef CAS.
- P. Siripanich, T. Bureerug, S. Chanmungkalakul, M. Sukwattanasinitt and V. Ervithayasuporn, Organometallics, 2022, 41, 201–210 CrossRef CAS.
- L. Sun, Z. Liang and J. Yu, Polym. Chem., 2015, 6, 917–924 RSC.
- I. S. Protsak, Y. M. Morozov, W. Dong, Z. Le, D. Zhang and I. M. Henderson, Nanoscale Res. Lett., 2019, 14, 1–15 CrossRef CAS PubMed.
- S. Roualdes, R. Berjoan and J. Durand, Sep. Purif. Technol., 2001, 25, 391–397 CrossRef CAS.
- Y. Liu, N. Takeda, A. Ouali and M. Unno, Inorg. Chem., 2019, 58, 4093–4098 CrossRef CAS PubMed.
- Y. Liu, M. Kigure, K. Koizumi, N. Takeda, M. Unno and A. Ouali, Inorg. Chem., 2020, 59, 15478–15486 CrossRef CAS PubMed.
- J. Kaźmierczak, K. Kuciński and G. Hreczycho, Inorg. Chem., 2017, 56, 9337–9342 CrossRef PubMed.
- V. M. Litvinov, H. Barthel and J. Weis, Macromolecules, 2002, 35, 4356–4364 CrossRef CAS.
- T. Tokunaga, S. Koge, T. Mizumo, J. Ohshita and Y. Kaneko, Polym. Chem., 2015, 6, 3039–3045 RSC.
- D.-S. Ruan, Y.-L. Li, L. Wang, D. Su and F. Hou, J. Sol-Gel Sci. Technol., 2010, 56, 184–190 CrossRef CAS.
- M. Xue, X. Zhang, Z. f. Wu, H. Wang, X. Ding and X. y. Tian, Chin. J. Chem. Phys., 2013, 26, 445–450 CrossRef CAS.
- D. Chen, W. Sun, C. Qian, L. M. Reyes, A. P. Y. Wong, Y. Dong, J. Jia, K. K. Chen and G. A. Ozin, Adv. Funct. Mater., 2016, 26, 5102–5110 CrossRef CAS.
- S. Hanprasit, N. Tungkijanansin, A. Prompawilai, S. Eangpayung and V. Ervithayasuporn, Dalton Trans., 2016, 45, 16117–16120 RSC.
- F. Olivero, F. Renò, F. Carniato, M. Rizzi, M. Cannas and L. Marchese, Dalton Trans., 2012, 41, 7467–7473 RSC.
- M. Laird, P. Gaveau, P. Trens, C. Carcel, M. Unno, J. R. Bartlett and M. W. C. Man, New J. Chem., 2021, 45, 4227–4235 RSC.
- H. Liu and H. Liu, J. Mater. Chem. A, 2017, 5, 9156–9162 RSC.
- J. T. Leem, W. C. Seok, J. B. Yoo, S. Lee and H. J. Song, Polymers, 2021, 13, 1564 CrossRef CAS PubMed.
- H. Vakili, M. Mohseni and E. Mohajerani, J. Optoelectron. Adv. Mater., 2014, 16, 1031–1038 CAS.
- N. Prigyai, S. Chanmungkalakul, M. Sukwattanasinitt and V. Ervithayasuporn, New J. Chem., 2021, 45, 14141–14148 RSC.
- N. C. Escudé and E. Y.-X. Chen, Chem. Mater., 2009, 21, 5743–5753 CrossRef.
- W.-B. Zhang, Y. Li, X. Li, X. Dong, X. Yu, C.-L. Wang, C. Wesdemiotis, R. P. Quirk and S. Z. Cheng, Macromolecules, 2011, 44, 2589–2596 CrossRef CAS.
- A. A. Widati, M. Z. Fahmi, S. C. W. Sakti, T. A. Budiastanti and T. E. Purbaningtias, J. Manuf. Mater., 2022, 6, 110 CAS.
- G. Hayase, K. Kanamori, G. Hasegawa, A. Maeno, H. Kaji and K. Nakanishi, Angew. Chem., Int. Ed., 2013, 52, 10788–10791 CrossRef CAS PubMed.
- G. Hayase, K. Kanamori and K. Nakanishi, J. Mater. Chem., 2011, 21, 17077–17079 RSC.
- P. Wojciechowska, R. Cierpiszewski and H. Maciejewski, Appl. Sci., 2022, 12, 1258 CrossRef CAS.
- L. Wang, R. Guo, J. Ren, G. Song, G. Chen, Z. Zhou and Q. Li, Ceram. Int., 2020, 46, 10362–10369 CrossRef CAS.
- S. Pirouz and J. Duhamel, J. Polym. Sci., Part B: Polym. Phys., 2017, 55, 7–18 CrossRef CAS.
- S. Karuppannan and J. C. Chambron, Chem. – Asian J., 2011, 6, 964–984 CrossRef CAS PubMed.
- Z. Kowser, U. Rayhan, T. Akther, C. Redshaw and T. Yamato, Mater. Chem. Front., 2021, 5, 2173–2200 RSC.
- D. C. Santra, M. K. Bera, P. K. Sukul and S. Malik, Chem. – Eur. J., 2016, 22, 2012–2019 CrossRef CAS PubMed.
- G. G. Aloisi, F. Masetti and U. Mazzucato, Chem. Phys. Lett., 1974, 29, 502–505 CrossRef CAS.
- W. Thongyod, C. Buranachai, T. Pengpan and C. Punwong, Phys. Chem. Chem. Phys., 2019, 21, 16258–16269 RSC.
- Z. Kowser, U. Rayhan, T. Akther, C. Redshaw and T. Yamato, Mater. Chem. Front., 2021, 5, 2173–2200 RSC.
- M. Ronchi, S. Sulaiman, N. Boston and R. M. Laine, Appl. Organomet. Chem., 2010, 24, 551–557 CrossRef CAS.
- H. Oda, M. Sato, Y. Morizawa, K. Oshima and H. Nozaki, Tetrahedron, 1985, 41, 3257–3268 CrossRef CAS.
- Q. Shu, W. Qiu, M. Luo and L. Xiao, Mater. Today Sustainability, 2022, 17, 100094 CrossRef.
- A. Deptula, J. Rangel-Galera and R. M. Espinosa-Marzal, Adv. Funct. Mater., 2023, 33, 2300896 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4py00394b |
| This journal is © The Royal Society of Chemistry 2024 |