Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Electrospun dressings with a dual release functionality of two anti-inflammatory active ingredients†
Anna-Lena
Gürtler
 a,
Jonathan P.
Sirois
a,
Jonathan P.
Sirois
 a,
Julia C.
Lang
a,
Julia C.
Lang
 bc,
Keira
Melican
bc,
Keira
Melican
 bc,
Thomas
Rades
bc,
Thomas
Rades
 a and
Andrea
Heinz
a and
Andrea
Heinz
 *a
*a
aDepartment of Pharmacy, LEO Foundation Center for Cutaneous Drug Delivery, University of Copenhagen, Copenhagen, Denmark. E-mail: andrea.heinz@sund.ku.dk
bCenter for the Advancement of Integrated Medical and Engineering Sciences (AIMES), Karolinska Institutet and KTH Royal Institute of Technology, Stockholm, Sweden
cDepartment of Neuroscience Karolinska Institutet, Stockholm, Sweden
First published on 1st August 2024
Abstract
Inflammatory skin conditions are commonly treated using topical semi-solid formulations such as creams, ointments, or gels. However, the use of such formulations is often connected to poor patient adherence due to the greasiness of the formulations and the need to apply different products multiple times a day. To overcome this challenge, we aimed to develop an anti-inflammatory electrospun dressing containing two active ingredients with a reduced application frequency of once a day, enhancing the treatment comfort for the patients. Salicylic acid and hydrocortisone were combined in a polycaprolactone-based electrospun fiber dressing with a dual release functionality, featuring both a burst and sustained release of salicylic acid and hydrocortisone, respectively. While electrospun dressings have been extensively studied in terms of their material characteristics and drug release behavior, few studies have explored their release behavior in conjunction with their skin permeation performance. Our study bridges this gap by providing and comparing both drug release and skin permeation data. We found a rapid release of salicylic acid and a delayed release of hydrocortisone in our layer-by-layer system. Although significantly less hydrocortisone was released from the layer-by-layer system in the permeation studies compared to the release studies, hydrocortisone still permeated through different skin layers. Moreover, we verified the anti-inflammatory properties of the electrospun dressing in studies on human keratinocytes and human skin. Special emphasis was placed on comparing results with standard pharmaceutical formulations, namely a hydrocortisone cream and a salicylic acid ointment. Permeation studies showed higher hydrocortisone penetration into the skin from the layer-by-layer fiber dressing compared to standard formulations. Our findings highlight the feasibility of combining multiple drugs in a single electrospun fiber system, while achieving controlled release behavior through tuning the composition of the drug delivery system. Our study moreover confirms that conducting permeation experiments alongside release studies is crucial for correlating results and evaluating the effectiveness of a drug delivery system.
1. Introduction
Chronic inflammatory skin diseases such as psoriasis are common in the population, with 0.5–2% of all adults and children suffering from this disease.1 Psoriasis is characterized by both small papules and large, sharply defined scaly plaques, and it is typically treated with creams and ointments using topical agents such as keratolytics, corticosteroids, vitamin D analogues and calcineurin inhibitors.2–4 A combination of drugs can be beneficial for psoriasis treatment. For example, the keratolytic salicylic acid in combination with topical corticosteroids leads to a faster onset of action of the corticosteroids due to skin softening, descaling of the plaques and thus improved drug penetration.5 However, in topical skin therapy, patient adherence to the treatment is frequently a challenge,6 which can be attributed to the need for multiple daily applications, as well as the greasy and unpleasant sensation that many formulations impart on the skin. Patient surveys indicate that an ideal formulation should avoid leaving the skin greasy or sticky, should not have an unpleasant odor, require an application only once a day, provide a comfortable moisturization, and should generally be easy to apply.7,8 Electrospun skin dressings fulfil all these requirements and are, therefore, an ideal potential drug delivery system to treat inflammatory skin diseases.9,10 Electrospinning enables the production of dressings comprising polymer fibers ranging from nanometers to micrometers in size, which can contain one or multiple active ingredients and facilitate either a rapid, delayed, or on-demand release.9 The resulting fiber dressings provide the benefit of breathability, owing to their high porosity, and ensure adequate gas exchange and liquid permeability.11 The electrospinning process (uniaxial, coaxial or layer-by-layer electrospinning) can be used to tune the release of the active ingredient from the fibers. In uniaxial electrospinning, the active ingredient is homogeneously distributed in the fibers and is usually released quickly due to the distribution close to the fiber surface (Fig. 1).12 In contrast, in layer-by-layer electrospinning, consecutive electrospinning of multiple fiber layers leads to the formation of a diffusion barrier that affects the release of the active ingredient, hence enabling a sustained drug release (Fig. 1).13 To provide a more convenient option for topical psoriasis therapy compared with conventional semi-solid formulations, more precisely to reduce the application frequency and the number of formulations that need to be applied, we aimed to develop an electrospun skin dressing with dual release functionality, containing both salicylic acid and hydrocortisone. We used the hydrophobic polymer polycaprolactone (PCL) as the fiber basis, as it displays biocompatibility, slow degradation, and compatibility with multiple active ingredients.14 For salicylic acid, serving as a keratolytic to enhance the penetration of hydrocortisone, we aimed to achieve a rapid release, while hydrocortisone was to be released over 24 h in a delayed fashion. Additionally, we investigated the performance of the electrospun dressing in skin permeation studies and correlated the results with the findings from release studies – a comparison that is often neglected in studies on electrospun dressings, however, is of the utmost importance to predict the in vivo efficacy of drug delivery systems. Semi-solid formulations, namely a salicylic acid ointment and a hydrocortisone-containing cream from a retail pharmacy were used for comparison. One last goal of our study was to validate the anti-inflammatory activity and compatibility of the fiber dressing on human skin and in a cell model using human epidermal keratinocytes.2. Materials and methods
2.1 Materials
PCL with a molecular weight of approximately 80 kDa (Sigma Aldrich, Darmstadt, Germany) served as the polymer for electrospinning all fibers. The solvents utilized in the process were chloroform (CHL) with a purity of ≥99% and ethanol (EtOH) with a purity of 96%, both obtained from VWR Chemicals in Søborg, Denmark. Hydrocortisone (Euro OTC Pharma GmbH, Bönen, Germany) and salicylic acid (Sigma-Aldrich Chemie GmbH, Steinheim, Germany) were employed as the active ingredients. For release and permeation studies, phosphate-buffered saline (PBS) tablets (Medicago AB, Uppsala, Sweden) were used. These tablets were dissolved in ultra-pure water and pH-adjusted to 5.2 and 7.4 using formic acid (≥98%, Sigma-Aldrich Chemie GmbH, Steinheim, Germany). For the permeation studies, two standard pharmaceutical products were obtained from a retail pharmacy. The salicylic acid ointment was composed of salicylic acid (3% w/w), the additives cetylstearyl alcohol (emulsifying, type A), polyethylene glycol, lard, white vaseline, macrogol, and the hydrocortisone cream of hydrocortisone (1%, w/w), as well as the additives methylparahydroxybenzoate, propylparahydroxybenzoate, benzyl alcohol, cetylstearyl alcohol, citric acid, macrogol, sodium citrate, paraffin wax, purified water, and vaseline. Acetonitrile (with water content <30 ppm) obtained from VWR Chemicals (Søborg, Denmark) and ultra-pure water with the addition of 0.1% (v/v) formic acid were employed as solvents for the high-performance liquid chromatography (HPLC) experiments. Furthermore, methanol (VWR Chemicals, Søborg, Denmark) served as the extraction medium for the permeation studies.2.2 Electrospinning
A drug-free and two types of drug-loaded fiber dressings were fabricated either by uniaxial or layer-by-layer electrospinning from 20% (w/w) PCL dissolved in a mixture of CHL/EtOH (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) (Fig. 1). A Fluidnatek LE-50 (Bioinicia, Valencia, Spain) electrospinner set to a temperature of 25 °C and 40% relative humidity was used for preparing the fiber dressings. For the uniaxial fiber mats, drug-free fibers of pure PCL and drug-loaded fibers containing 6% (w/w, based on the total PCL weight) of salicylic acid in PCL were electrospun, respectively. The layer-by-layer fiber mats were composed of three uniaxially electrospun fiber layers, with the first layer consisting of pure PCL, the second containing 1% (w/w, based on total PCL weight) of hydrocortisone incorporated in PCL, and the third layer containing 6% (w/w, based on the total PCL weight) of salicylic acid in PCL.
1, v/v) (Fig. 1). A Fluidnatek LE-50 (Bioinicia, Valencia, Spain) electrospinner set to a temperature of 25 °C and 40% relative humidity was used for preparing the fiber dressings. For the uniaxial fiber mats, drug-free fibers of pure PCL and drug-loaded fibers containing 6% (w/w, based on the total PCL weight) of salicylic acid in PCL were electrospun, respectively. The layer-by-layer fiber mats were composed of three uniaxially electrospun fiber layers, with the first layer consisting of pure PCL, the second containing 1% (w/w, based on total PCL weight) of hydrocortisone incorporated in PCL, and the third layer containing 6% (w/w, based on the total PCL weight) of salicylic acid in PCL.
The active substance concentrations were determined based on review articles on inflammatory skin disease treatments,5,15 with salicylic acid used at a concentration of 6% for its keratolytic effect and hydrocortisone at a concentration of 1%,16 the highest over-the-counter concentration available in Danish pharmacies. For example, a 20 mg fiber mat sample contained 0.2 mg of hydrocortisone (1% w/w polymer) and 1.2 mg of salicylic acid (6% w/w polymer).
The collection of all samples took place on a drum collector covered with aluminum foil. The exact production parameters and sample abbreviations are shown in Table 1. For example, the sample name (PCL+S) indicates that the uniaxially electrospun PCL fibers contained salicylic acid (S). The electrospinning parameters are based on the insights of a previous study using PCL17 and were fine-tuned during an optimization phase, including adjustments of polymer concentration, injector, and collector voltage, as well as the distance between the needle and collector. Our goal was to ensure a consistent electrospinning process without the formation of droplets.
| Abbreviation | Fiber layers | PCL [% (w/v)] | Flow rate [μL h−1] | Injector voltage [kV] | Collector voltage [kV] | Distance injector collector [cm] |
|---|---|---|---|---|---|---|
| (PCL) | 1 | 20 | 1300 | 6.5 | −4.5 | 22.5 |
| (PCL+S) | 1 | 20 | 1300 | 6.5 | −4.5 | 22.5 |
| (PCL+H+S) layer | 3 | 20 | 1300 | 7.0 | −4.5 | 22.5 |
2.3 Fiber morphology, diameter, and diameter distribution
Fiber morphology, diameter, and diameter distribution were assessed using a scanning electron microscope (SEM) (TM3030 model from Hitachi, Tokyo, Japan). Prior to analysis, three samples per fiber dressing were coated with gold using a sputter coater (Cressington 108 auto from Ted Pella Inc., Redding, USA). Images were captured at 15 kV and analyzed using ImageJ software at a magnification of 2000x.2.4 Mechanical characteristics of electrospun fiber dressings
A texture analyzer TA.XT plus (Stable Micro Systems, Godalming, UK) was employed to assess the mechanical properties of the electrospun fiber dressings. Rectangular samples measuring 30 × 10 mm were utilized for the analysis. These samples were elongated to a total length of 200 mm by applying a force of 49.0 mN at a strain rate of 20 mm s−1. Young's moduli and tensile strengths were determined from the stress–strain curves. All measurements were carried out in triplicate.2.5 In vitro studies on drug release and drug loading
Circular fiber mat samples (d = 6 mm) were utilized for both drug loading and release experiments. To assess drug loading, the fiber mats were dissolved in a mixture of CHL/EtOH (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). For release studies, the samples were placed in 1.5 mL Eppendorf tubes filled with PBS pH 5.218 and then subjected to agitation at 32 °C and 300 rpm for 7 d. By testing the solubility of hydrocortisone (300 μg mL−1) and salicylic acid (3 mg mL−1) beforehand, we ensured that sink conditions were met. 50 μL samples were withdrawn at specific time points (5 min, 15 min, 30 min, 1 h, 3 h, 8 h, 24 h, 72 h, 7 d), and replaced with fresh PBS. Drug quantification of the samples was performed using HPLC on an Agilent 1260 Infinity system (Agilent Technologies, Santa Clara, CA, USA) equipped with a C18 column (Infinitylab Poroshell 120 C18 column, 4.6 × 150 mm, Agilent Technologies, Santa Clara, CA, USA) and a UV detector set at a wavelength of 254 nm (limit of detection: 0.35 μg mL−1 for hydrocortisone and 7.64 μg mL−1 for salicylic acid, limit of quantification: 1.17 μg mL−1 for hydrocortisone and 25.47 μg mL−1 for salicylic acid). The flow rate for all analyses was set to 1 mL min−1. Drug loading analysis utilized a gradient method involving a mixture of ultra-pure water with 0.1% (v/v) formic acid (A) and acetonitrile with 0.1% (v/v) formic acid (B). The gradient sequence was as follows: 80% A and 20% B to 60% A and 40% B within 8 min, followed by 10% A and 90% B for 4 min, and then maintenance at 80% A and 20% B for an additional 3 min. Release samples were quantified using an isocratic method with the mobile phases in a ratio of 60% (v/v) A and 40% (v/v) B.
1, v/v). For release studies, the samples were placed in 1.5 mL Eppendorf tubes filled with PBS pH 5.218 and then subjected to agitation at 32 °C and 300 rpm for 7 d. By testing the solubility of hydrocortisone (300 μg mL−1) and salicylic acid (3 mg mL−1) beforehand, we ensured that sink conditions were met. 50 μL samples were withdrawn at specific time points (5 min, 15 min, 30 min, 1 h, 3 h, 8 h, 24 h, 72 h, 7 d), and replaced with fresh PBS. Drug quantification of the samples was performed using HPLC on an Agilent 1260 Infinity system (Agilent Technologies, Santa Clara, CA, USA) equipped with a C18 column (Infinitylab Poroshell 120 C18 column, 4.6 × 150 mm, Agilent Technologies, Santa Clara, CA, USA) and a UV detector set at a wavelength of 254 nm (limit of detection: 0.35 μg mL−1 for hydrocortisone and 7.64 μg mL−1 for salicylic acid, limit of quantification: 1.17 μg mL−1 for hydrocortisone and 25.47 μg mL−1 for salicylic acid). The flow rate for all analyses was set to 1 mL min−1. Drug loading analysis utilized a gradient method involving a mixture of ultra-pure water with 0.1% (v/v) formic acid (A) and acetonitrile with 0.1% (v/v) formic acid (B). The gradient sequence was as follows: 80% A and 20% B to 60% A and 40% B within 8 min, followed by 10% A and 90% B for 4 min, and then maintenance at 80% A and 20% B for an additional 3 min. Release samples were quantified using an isocratic method with the mobile phases in a ratio of 60% (v/v) A and 40% (v/v) B.
2.6 Ex vivo permeation studies
Ex vivo permeation studies were performed on dorsal porcine skin (obtained from the Department of Veterinary and Animal Sciences, University of Copenhagen, Denmark) mounted on vertical Franz diffusion cells (Phoenix DB-6 Dry Heat Diffusion Cells, Teledyne Hanson Research, Chatsworth, CA, USA) with a diffusional surface area of 1 cm2. Fresh porcine skin was hair-trimmed and dermatomed (Zimmer Dermatome AN, Zimmer Biomet Denmark, Albertslund, Denmark) to a thickness of 500 μm, stored at −20 °C, and cut into circular pieces (d = 1.5 cm) just before the experiment. To ensure that the skin was intact, transepidermal water loss (TEWL) (AquaFlux™ AF200, Biox Systems Limited, London, UK) was measured after equilibration for 30 min on the Franz cell with the dermis being in contact with the acceptor compartment filled with 10 mL PBS, pH 7.4, at 35 °C ± 0.1 °C and shaken at 300 rpm. Intact skin with TEWL < 15 g m−2 h−1 was included in the experiments. Circular fiber dressing samples (d = 1.5 cm) were applied to the skin in contact with the stratum corneum and wetted on both sides with 100 μL PBS to ensure the release of the incorporated drugs. After 24 h of permeation, 1 mL of PBS was withdrawn from the acceptor compartment to determine the amount of drug that had permeated through the skin. To detect the amount of unreleased drug, fiber dressings were dissolved in CHL/EtOH (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). To investigate drug retention in the skin, skin samples were wrapped in aluminum foil and submerged in ultra-pure water at 60 °C for 2.5 min. Following this, the samples were carefully separated into epidermis and dermis using a scalpel, mixed with 2 mL methanol to extract the drugs, and placed in an ultrasonic bath and on a shaking board for 15 min each. Prior to quantification by HPLC, all samples were centrifuged for 15 min at 16
1, v/v). To investigate drug retention in the skin, skin samples were wrapped in aluminum foil and submerged in ultra-pure water at 60 °C for 2.5 min. Following this, the samples were carefully separated into epidermis and dermis using a scalpel, mixed with 2 mL methanol to extract the drugs, and placed in an ultrasonic bath and on a shaking board for 15 min each. Prior to quantification by HPLC, all samples were centrifuged for 15 min at 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 162g and cleaned using 0.2 μm PTFE filters.
162g and cleaned using 0.2 μm PTFE filters.
To compare the performance of the fiber dressings with standard semi-solid formulations, the experiments described above were carried out using both a 3% (w/w) salicylic acid ointment (approx. 20 mg, Lygal® Kopfsalbe N 3%, Almirall Hermal GmbH, Reinbek, Germany) and a 1% (w/w) hydrocortisone cream (approx. 10 mg, Mildison Lipid Creme®, Karo Pharma AB, Stockholm, Sweden).
2.7 Anti-inflammatory characteristics of the electrospun fiber dressings
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, and the collected supernatants were stored at −20 °C for further analysis. IL-6 levels were quantified from the supernatant as described above in section 2.7.1. Three individual skin donors were analyzed (n = 3), with two technical replicates per donor. For skin histology experiments, tissue was fixed after the 48 h incubation using 4% paraformaldehyde, washed with PBS, cryopreserved in Optimal Cutting Temperature embedding matrix (OCT, Cell Path, Newtown, UK), and stored at −80 °C. Sections of 12 μm were sliced using a CryoStarTM NX70 cryostat (Thermo Fisher Scientific, Waltham, USA) and collected on SuperFrost® Plus microscopy slides (Thermo Fisher Scientific, Waltham, USA). The sections were stained with hematoxylin and eosin (H&E) (Histolab, Askim, Sweden) following manufacturer's protocol, cleared with xylene (Histolab), and mounted with Pertex® (Histolab). Imaging was conducted using a LionHeart FX automated microscope (BioTek, AH Diagnostics, Sweden) with a 4× objective.
000g, and the collected supernatants were stored at −20 °C for further analysis. IL-6 levels were quantified from the supernatant as described above in section 2.7.1. Three individual skin donors were analyzed (n = 3), with two technical replicates per donor. For skin histology experiments, tissue was fixed after the 48 h incubation using 4% paraformaldehyde, washed with PBS, cryopreserved in Optimal Cutting Temperature embedding matrix (OCT, Cell Path, Newtown, UK), and stored at −80 °C. Sections of 12 μm were sliced using a CryoStarTM NX70 cryostat (Thermo Fisher Scientific, Waltham, USA) and collected on SuperFrost® Plus microscopy slides (Thermo Fisher Scientific, Waltham, USA). The sections were stained with hematoxylin and eosin (H&E) (Histolab, Askim, Sweden) following manufacturer's protocol, cleared with xylene (Histolab), and mounted with Pertex® (Histolab). Imaging was conducted using a LionHeart FX automated microscope (BioTek, AH Diagnostics, Sweden) with a 4× objective.
2.8 Statistical analysis
The analysis of all data was conducted using GraphPad Prism software version 10.1.2 (Dotmatics). To compare mean values, the one-way ANOVA test followed by Tukey's test was employed. Results are expressed as means with their corresponding standard deviations, and statistical significance is denoted by p-values (p < 0.0001 (****), p < 0.0002 (***), p < 0.0021 (**), p < 0.0332 (*)).3. Results and discussion
3.1 Fabrication and morphological characterization of electrospun fiber mats
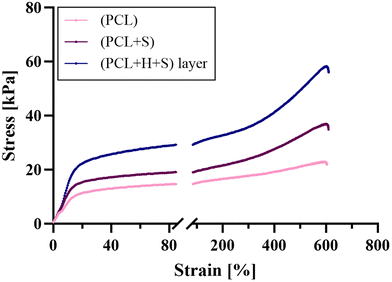
Uniaxial (with and without salicylic acid) and layer-by-layer PCL fibers loaded with hydrocortisone and salicylic acid consisting of three layers (Fig. 2) were produced using the settings described in Table 1. Homogeneous, artifact-free and tubular-shaped fibers with similar diameters between 3.2 μm and 3.6 μm were obtained (Fig. 3). In the case of the uniaxial fibers, the incorporation of 6% salicylic acid (w/w, based on PCL) had no effect on the fiber morphology and the fiber diameter (Fig. 3B). This was different for the layer-by-layer fiber system, where the inclusion of salicylic acid resulted in minor cracks on the fiber surface (Fig. 3C). In conclusion, we successfully produced uniform drug-loaded fibers. Such uniformity is crucial for ensuring consistent drug release, uniform mechanical properties, and reproducible in vivo efficacy.3.2 Mechanical characteristics of electrospun fiber dressings
The mechanical characteristics of electrospun fiber dressings primarily depend on factors such as the polymer used, the fiber diameter, and the manufacturing conditions.22 For a comfortable application and feel on the skin, the fiber dressings should be stretchy and flexible, easy to remove but still have enough firmness for a 24 h application.23 A significant difference in the mechanical characteristics was found between the layer-by-layer fibers and the uniaxial fibers (Table 2). The layer-by-layer dressing displayed a higher Young's modulus and higher tensile strength, indicating that the fibers are more ductile than the uniaxial fibers (Fig. 4). No change in the mechanical properties of the uniaxial fibers was observed upon incorporation of salicylic acid. In summary, the analysis demonstrated the stretchability of the fibers, which is reproducible and potentially implies a comfortable wearing sensation on the skin.| Sample | Mean diameter ± SD [μm] | Young's modulus ± SD [kPa] | Tensile strength ± SD [kPa] |
|---|---|---|---|
| (PCL) | 3.2 ± 1.0 | 3.0 ± 0.21 | 24.9 ± 3.19 |
| (PCL+S) | 3.2 ± 0.5 | 3.4 ± 0.34 | 30.2 ± 5.84 |
| (PCL+H+S) layer | 3.6 ± 0.6 (**) | 4.8 ± 0.94 (*) | 57.4 ± 8.53 (**) |
3.3 In vitro studies on drug release and drug loading
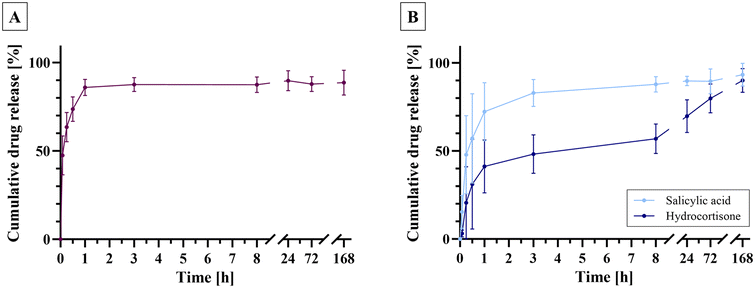
The objective of this research was to incorporate the keratolytic salicylic acid alongside the anti-inflammatory corticosteroid hydrocortisone into an electrospun dressing, aiming for rapid release of salicylic acid in a burst manner, while ensuring a delayed release of hydrocortisone over a 24 h period. To achieve this, salicylic acid was initially electrospun into uniaxial fibers (PCL+S) to confirm the intended burst release. Subsequently, it was electrospun as a third, bottom layer of a layer-by-layer electrospun dressing, with hydrocortisone positioned in the middle layer (sample “(PCL+H+S) layer”) (Fig. 2). The encapsulation efficiency exceeded 80% for all electrospun fiber types, with nearly 100% for hydrocortisone in the layer-by-layer electrospun dressing, indicating successful drug incorporation (Fig. 5).The release studies revealed that the uniaxially electrospun PCL+S fibers exhibited an initial burst release, with 80% of the encapsulated salicylic acid being released within the first hour (Fig. 6A). The layer-by-layer electrospun fibers demonstrated a comparable pattern, releasing over 70% of the salicylic acid content within the first hour (Fig. 6B). In contrast, as desired the release of hydrocortisone proceeded at a slower rate. Approximately 40% of the hydrocortisone content was released within the initial hour, followed by a delayed release, where 80% of the hydrocortisone content was released after 3 days, and 90% after 7 days. The delayed release of hydrocortisone can be attributed to the layered structure of the fiber dressing, where the outer layers serve as a diffusion barrier,13,24 requiring the release medium PBS to permeate the fiber dressing to dissolve the active ingredient before it can be released. In contrast, the salicylic acid present in the outermost layer of the fiber mat can be released rapidly, as it comes into contact with the release medium more quickly.24 This meets the requirements for the release of the incorporated drugs in two distinct phases: salicylic acid in an initial burst release and hydrocortisone in a sustained release over a 24 h period.
3.4 Ex vivo permeation studies
Semi-solid formulations such as creams and ointments are commonly used in the treatment of inflammatory skin conditions.6 To gain a more comprehensive understanding of the performance of electrospun fiber dressings, we compared them in ex vivo permeation studies with a standard cream and ointment obtained from a retail pharmacy. Dorsal porcine skin was utilized for this purpose, and its integrity was confirmed through TEWL measurements conducted before the study. Fig. 7 illustrates the results of the permeation study, including the percentage of drug content remaining (Residue) in either the electrospun fibers, cream, or ointment, and the drug percentage detected in the epidermis, dermis, or beneath the skin sample in the PBS medium (Acceptor). The “total” bars represent the total drug recovery. Notably, for the layer-by-layer electrospun fibers, it was found that a higher amount of hydrocortisone had penetrated into the skin compared to the combination of salicylic acid ointment and hydrocortisone cream in both the epidermis and dermis. In detail, 4.5% of hydrocortisone was found in the epidermis and dermis, respectively, for the electrospun fibers, as opposed to just under 1.5% found in the epidermis and dermis, respectively, for hydrocortisone cream. In contrast, there was only a minimal difference in the permeation of salicylic acid penetrated from the salicylic acid ointment or the electrospun fibers. To draw conclusions on a potential in vivo efficacy of the drug delivery systems, it is relevant to compare the permeation data with the in vitro release studies, with the latter clearly showing a burst release of salicylic acid and delayed release of hydrocortisone. Following the application of the layer-by-layer fiber dressing to the skin for 24 h, over 60% of the hydrocortisone remained within the electrospun fibers (Fig. 7), as opposed to just 30% after 24 h in the release study (Fig. 6B). This stands in contrast to the quantity of salicylic acid released and penetrated from both types of fibers. While the release studies indicated that nearly all salicylic acid was rapidly released from the fibers, only a negligible amount was detectable in the skin, which is desirable as salicylic acid is intended to act as a keratolytic on the top layer of the skin. This difference emphasizes the importance to conduct permeation studies, as skin permeation is a highly complex process25 that cannot be concluded on by only carrying out release studies.26,273.5 Anti-inflammatory characteristics of the electrospun fiber dressings
The anti-inflammatory properties of the electrospun fibers were evaluated in cell culture using human epidermal keratinocytes. After co-culturing the cells with the fiber dressings for 24 h and 48 h, a decrease in the pro-inflammatory cytokine IL-6, commonly secreted in inflammatory skin conditions,28 was seen in response to the electrospun fiber dressings (Fig. 8A). Notably, even the drug-free PCL electrospun fibers resulted in a reduction in IL-6 levels, which can be attributed to the binding of IL-6 from the cell culture medium by the fiber mats (ESI Fig. S1†). However, fibers containing either salicylic acid or both salicylic acid and hydrocortisone demonstrated a more pronounced reduction in released IL-6 compared to the baseline of untreated controls, confirming their anti-inflammatory efficacy. The primary anti-inflammatory action can be attributed to the hydrocortisone within the fibers, which effectively reduces inflammation in the skin by suppressing the release of pro-inflammatory substances such as IL-6 and by inhibiting the activity of immune cells implicated in inflammation.29 To further investigate this anti-inflammatory effect, the fiber dressings were incubated for 48 h on healthy human skin obtained from plastic surgery. This was to provide information on whether the fibers are compatible with human skin and confirm the reduction in the IL-6 from the cell studies. For this purpose, the slightly moistened fiber samples or the standard semi-solid formulations were applied to human skin biopsies, and after 48 h the levels of cytokine IL-6 were determined in skin homogenates. Interestingly, only the layer-by-layer electrospun fiber dressing, containing hydrocortisone alongside salicylic acid, exhibited a notable reduction in IL-6 compared to both the baseline and the control sample of drug-free PCL (Fig. 8B). Moreover, it was evident that the conventional therapy involving the combination of salicylic acid ointment and hydrocortisone cream did not demonstrate an anti-inflammatory effect comparable to that of the electrospun layer-by-layer fibers.The morphology of the skin after 48 h treatment with the different fiber dressings was validated by H&E staining (Fig. 9). While the stratum corneum was not obviously affected by the PCL control fiber mats (Fig. 9B), all the samples containing salicylic acid, either in electrospun fiber mats (Fig. 9C and D) or standard ointment (Fig. 9E and F), showed a disrupted stratum corneum and even epidermis (Fig. 9E). The disrupted stratum corneum could be seen by the loose tissue remnants above the stratum corneum. This indicates that salicylic acid displays its keratolytic effect by detaching the keratinized cells of the uppermost layer of the stratum corneum, consistent with another study in which a considerable amount of keratinized cells were removed after just 3 h of salicylic acid application.30 In addition, salicylic acid stimulates cell regeneration during long-term application, for example by leading to increased basal cell proliferation.31 This has been observed in other studies by a thickening of the epidermis following stratum corneum detachment,32,33 but was not significantly observed in our study.
4. Conclusions
This study was carried out to design an electrospun polycaprolactone-based fiber dressing against inflammatory skin diseases as an alternative to conventional semi-solid formulations. Specifically, we aimed to develop a drug delivery system with a dual release functionality containing salicylic acid as a keratolytic (to be released immediately) alongside hydrocortisone as an inflammatory agent (delivered continuously over a 24 h period), thus requiring an application of a single drug delivery system only once a day instead of different semi-solid formulations twice a day. Initially, we successfully manufactured uniform electrospun microfiber dressings without artifacts and similar diameters between 3.2 μm and 3.6 μm. Mechanical tests demonstrated favorable ductility, potentially allowing for a comfortable application on the skin. Our study further revealed that the layer-by-layer system fulfils the release requirements, exhibiting rapid release of salicylic acid from the bottom layer followed by a delayed release of hydrocortisone from the middle layer of the fiber dressing. Permeation studies revealed a higher penetration of hydrocortisone into the skin from the layer-by-layer fiber dressing compared to the standard formulations, a salicylic acid ointment and a hydrocortisone cream. HEKa cell studies demonstrated anti-inflammatory effects for both the uniaxial salicylic acid fibers and the layer-by-layer electrospun fibers containing salicylic acid and hydrocortisone. Moreover, skin studies revealed an anti-inflammatory effect of the hydrocortisone-containing fibers, while none of the standard therapies exhibited such an effect. H&E staining of the skin following 48 h of incubation with the electrospun fibers revealed that all formulations containing salicylic acid (both fiber dressings and ointment) exhibited a keratolytic effect, evidenced by the disruption and detachment of the superficial skin layer. Conversely, the layer-by-layer fibers containing hydrocortisone preserved the integrity of the skin barrier. Moistened and applied onto the skin, these electrospun fiber mats present a potential alternative to traditional semi-solid formulations requiring less frequent application, offering convenience and effectiveness, which is likely to enhance patient adherence.Abbreviations
| CHL | Chloroform |
| ELISA | Enzyme-linked immunosorbent assay |
| EtOH | Ethanol |
| H&E staining | Hematoxylin and eosin |
| HEKa | Human epidermal keratinocyte |
| HPLC | High-performance liquid chromatography |
| IL-6 | Interleukin-6 |
| PBS | Phosphate-buffered saline |
| PCL | Polycaprolactone |
| SEM | Scanning electron microscope |
| TEWL | Transepidermal water loss |
Author contributions
Anna-Lena Gürtler: conceptualization, data curation, formal analysis, investigation, methodology, validation, visualization, writing – original draft. Jonathan P. Sirois: formal analysis, investigation, validation, writing – review & editing. Julia C. Lang: data curation, formal analysis, investigation, methodology, visualization, writing – original draft. Keira Melican: funding acquisition, methodology, supervision, writing – review & editing. Thomas Rades: conceptualization, supervision, writing – review & editing. Andrea Heinz: conceptualization, funding acquisition, formal analysis, methodology, project administration, supervision, writing – review & editing.Declaration of generative AI and AI-assisted technologies in the writing process
During the preparation of this work, the authors used ChatGPT 3.5 in order to rephrase the methods part of the manuscript to avoid overlap with previous articles. After using this tool/service, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.Ethics statement
Healthy human skin was obtained as surgical excess from elective plastic surgery. Written informed consent was obtained and all procedures were performed according to Swedish National guidelines and approved by the Regionala etikprövningsnämd I Stockholm (ethical approval no: 2015/432-31 and 2023-00567-02).Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
Katerina Vangelofski is thanked for assistance in the laboratory. The work was supported by the LEO Foundation grant LF15007 (AH, ALG) and the Swedish Science Council, 2020-01790 (KM). KM and JCL acknowledge support from AIMES – Center for the Advancement of Integrated Medical and Engineering Sciences (https://www.aimes.se), Karolinska Institutet (1-249/2019), KTH Royal Institute of Technology (VF-2019-0110) and Getinge AB (4-1599/2018).References
- J. P. Rajguru, D. Maya, D. Kumar, P. Suri, S. Bhardwaj and N. D. Patel, J. Family Med. Prim. Care, 2020, 9, 20–24 CrossRef PubMed
.
- A. W. Armstrong and C. Read, J. Am. Med. Assoc., 2020, 323, 1945–1960 CrossRef CAS
.
- B. X. Yan, X. Y. Chen, L. R. Ye, J. Q. Chen, M. Zheng and X. Y. Man, Front. Med., 2021, 8, 649408 CrossRef PubMed
.
- A. Mitra and Y. Wu, Expert Opin. Drug Delivery, 2010, 7, 977–992 CrossRef CAS
.
- A. Jacobi, A. Mayer and M. Augustin, Dermatol. Ther., 2015, 5, 1–18 CrossRef
.
- R. Oliveira and I. F. Almeida, Pharmaceuticals, 2023, 16, 617 CrossRef CAS
.
- V. Vasconcelos, A. Teixeira, V. Almeida, M. Teixeira, S. Ramos, T. Torres, J. M. Sousa Lobo and I. F. Almeida, J. Dermatol. Treat., 2019, 30, 659–663 CrossRef CAS
.
- T. S. Housman, B. G. Mellen, S. R. Rapp, A. B. Fleischer Jr and S. R. Feldman, Cutis, 2002, 70, 327–332 Search PubMed
.
- A.-L. Gürtler, T. Rades and A. Heinz, J. Controlled Release, 2023, 363, 621–640 CrossRef PubMed
.
- Z. J. Krysiak and U. Stachewicz, Wiley Interdiscip Rev Nanomed Nanobiotechnol, 2022, 15, 1829 CrossRef
.
- I. R. Calori, G. Braga, P. D. C. C. de Jesus, H. Bi and A. C. Tedesco, Eur. Polym. J., 2020, 129, 109621 CrossRef CAS
.
- S. M. S. Shahriar, J. Mondal, M. N. Hasan, V. Revuri, D. Y. Lee and Y.-K. Lee, Nanomaterials, 2019, 9, 532 CrossRef CAS PubMed
.
- S.-M. Huang, S.-M. Liu, H.-Y. Tseng and W.-C. Chen, Membranes, 2023, 13, 564 CrossRef CAS PubMed
.
- M. J. Mochane, T. S. Motsoeneng, E. R. Sadiku, T. C. Mokhena and J. S. Sefadi, Appl. Sci., 2019, 9, 2205 CrossRef CAS
.
- C. A. Elmets, N. J. Korman, E. F. Prater, E. B. Wong, R. N. Rupani, D. Kivelevitch, A. W. Armstrong, C. Connor, K. M. Cordoro, D. M. R. Davis, B. E. Elewski, J. M. Gelfand, K. B. Gordon, A. B. Gottlieb, D. H. Kaplan, A. Kavanaugh, M. Kiselica, D. Kroshinsky, M. Lebwohl, C. L. Leonardi, J. Lichten, H. W. Lim, N. N. Mehta, A. S. Paller, S. L. Parra, A. L. Pathy, M. Siegel, B. Stoff, B. Strober, J. J. Wu, V. Hariharan and A. Menter, J. Am. Acad. Dermatol., 2021, 84, 432–470 CrossRef
.
- Z. D. Draelos, J. Cosmet. Dermatol., 2005, 4, 193–197 CrossRef
.
- A.-L. Gürtler, I. Linseisen, H. Grohganz and A. Heinz, Eur. Polym. J., 2024, 208, 112886 CrossRef
.
- S. P. Cannavò, F. Guarneri, R. Giuffrida, E. Aragona and C. Guarneri, Sking Res. Technol., 2017, 23, 41–47 CrossRef PubMed
.
- A. Martin, J. Cai, A. L. Schaedel, M. van der Plas, M. Malmsten, T. Rades and A. Heinz, Int. J. Pharm., 2022, 621, 121809 CrossRef CAS PubMed
.
- P. P. D. Kondiah, T. A. Rants'o, S. Mdanda, L. M. Mohlomi and Y. E. Choonara, Polymers, 2022, 14, 2633 CrossRef CAS PubMed
.
- J. Klebeko, P. Ossowicz-Rupniewska, E. Świątek, J. Szachnowska, E. Janus, S. G. Taneva, E. Krachmarova and M. Guncheva, Molecules, 2022, 27, 216 CrossRef CAS PubMed
.
- T. U. Rashid, R. E. Gorga and W. E. Krause, Adv. Eng. Mater., 2021, 23, 2100153 CrossRef
.
- B. Yan, Y. Zhang, Z. Li, P. Zhou and Y. Mao, SN Appl. Sci., 2022, 4, 172 CrossRef CAS
.
- E. B. Nauman, K. Patel and P. Karande, Drug Dev. Ind. Pharm., 2011, 37, 93–102 CrossRef CAS PubMed
.
- Y. Q. Yu, X. Yang, X. F. Wu and Y. B. Fan, Front. Bioeng. Biotechnol., 2021, 9, 646554 CrossRef
.
- R. N. Kamble, S. Gaikwad, A. Maske and S. S. Patil, J. Adv. Res., 2016, 7, 483–489 CrossRef CAS PubMed
.
- Y. Shi, S. Xu, A. Dong and J. Zhang, J. Mater. Sci.: Mater. Med., 2013, 24, 333–341 CrossRef CAS PubMed
.
- L. Uva, D. Miguel, C. Pinheiro, J. Antunes, D. Cruz, J. Ferreira and P. Filipe, Int. J. Endocrinol., 2012, 2012, 561018 Search PubMed
.
- R. Kirnbauer, A. Köck, P. Neuner, E. Förster, J. Krutmann, A. Urbanski, E. Schauer, J. C. Ansel, T. Schwarz and T. A. Luger, J. Invest. Dermatol., 1991, 96, 484–489 CrossRef CAS PubMed
.
- S. Imayama, S. Ueda and M. Isoda, Arch. Dermatol., 2000, 136, 1390–1395 CAS
.
- J.-H. Hwang, H. Jeong, N. Lee, S. Hur, N. Lee, J. J. Han, H. W. Jang, W. K. Choi, K. T. Nam and K.-M. Lim, Int. J. Mol. Sci., 2021, 22, 657 CrossRef CAS
.
- G. Sharma, N. Devi, K. Thakur, A. Jain and O. P. Katare, Drug Delivery Transl. Res., 2018, 8, 398–413 CrossRef CAS
.
- A. A. Abdel-Motaleb, E. E. Abu-Dief and M. R. Hussein, J. Cosmet. Dermatol., 2017, 16, 9–14 CrossRef
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4pm00147h |
| This journal is © The Royal Society of Chemistry 2024 |